Abstract
Artificial intelligence has been widely used in the field of dentistry in recent years. The present study highlights current advances and limitations in integrating artificial intelligence, machine learning, and deep learning in subfields of dentistry including periodontology, endodontics, orthodontics, restorative dentistry, and oral pathology. This article aims to provide a systematic review of current clinical applications of artificial intelligence within different fields of dentistry. The preferred reporting items for systematic reviews (PRISMA) statement was used as a formal guideline for data collection. Data was obtained from research studies for 2009–2022. The analysis included a total of 55 papers from Google Scholar, IEEE, PubMed, and Scopus databases. Results show that artificial intelligence has the potential to improve dental care, disease diagnosis and prognosis, treatment planning, and risk assessment. Finally, this study highlights the limitations of the analyzed studies and provides future directions to improve dental care.
Keywords: dentistry, artificial intelligence, deep learning, clinical applications
1. Introduction
Artificial intelligence (AI) has been widely utilized in the field of medicine since its conception over 60 years ago [1,2,3,4]. Although the maturity of AI in the field of dentistry has lagged in several subfields such as periodontology, endodontics, orthodontics, restorative dentistry, and oral pathology, there has been a large interest in the past few years as artificial intelligence has become increasingly accessible to researchers. AI has made substantial progress in the diverse disciplines of dentistry including dental disease diagnosis [5], localization [6], classification [7], estimation [8], and assessment of dental disease [9].
On a broader level, AI enables the creation of intelligent machines that can achieve tasks without requiring human intervention. Machine learning (ML) [10] is a subset of AI that utilizes computational algorithms to analyze datasets to make predictions without the need for explicit instructions. Towards a more sophisticated and increasingly independent approach for diagnosis, treatment planning, and risk assessment, there has been increased interest in deep learning (DL) applications [11,12]. To provide expert support to healthcare practitioners, artificial neural networks (ANNs) can be utilized as clinical decision support systems (CDSS) [13]. Moreover, such systems aid dental clinicians in producing improved dental health outcomes [14]. Similarly, fuzzy logic (FL)-based CDSS also provides effective means for dealing with uncertainties in the decision-making process [15,16].
1.1. Background
The related background of ANNs, machine learning, deep learning, CDSS, and Fuzzy logic is discussed in the below subsections.
1.1.1. Artificial Neural Networks
ANNs evolved after McCulloch and Pitts created a model in 1943 based on many simple processing units, such as neurons connected using weighted links. In 1958, Rosenbolt published neurodynamic principles containing research ideas related to brain modeling. Later, in the 1980s, researchers developed algorithms based on the idea of backpropagation to be applied in the medical field [1].
ANNs comprise data processing mechanisms inspired by the analytical processes of the human brain. The ANNs are used extensively to solve complex real-world problems [2]. Due to ANN’s remarkable learning, generalization, inherent contextual information processing, and fault and noise tolerance capabilities, they provide exciting alternatives and have many applications including medicinal science such as disease diagnosis, image and data analysis, and biomedical identification [3,4].
1.1.2. Machine Learning
ML is the scientific study of algorithms and statistical models [10] used for a vast array of processing tasks without requiring prior knowledge or hand-crafted rules. Recent years have witnessed the widespread of ML due to its superior performance for various healthcare applications such as dentistry. ML algorithms fall into two learning types: supervised and unsupervised. The amount of data generated by healthcare service providers is huge, making the data analysis process cumbersome. ML helps in effectively analyzing the data and gaining actionable insights. Additionally, different dentistry applications can benefit from ML techniques, including disease diagnosis, prognosis, treatment, and automating the clinical workflow. Moreover, ML for clinical applications has great potential to transform traditional healthcare service delivery [17].
1.1.3. Deep Learning
Recent years have seen a surge of interest in the DL field, a subfield of ML, as it allows machines to mimic human intelligence in increasingly independent and sophisticated ways [11,12]. DL uses multiple layers of non-linear units to analyze and extract useful knowledge from huge amounts of data. The extracted knowledge is then used to produce state-of-the-art prediction results. The neural network architectures used in DL provide the capability to perform automatic and accurate detection in healthcare. Based on the study, DL has enormous potential to bring genuinely impactful applications to the field of dentistry.
1.1.4. ANNs as Clinical Decision Support Systems
AI application technology is progressing remarkably in the field of dentistry; clinical decision support systems are one example in this context. CDSS provides expert support to a health practitioner [13]. Moreover, these systems have the capability of solving problems that are too complex to be solved by using conventional methods [14]. Moreover, CDSS provides valuable information to dental practitioners that aids in producing faster and superior dental health outcomes.
1.1.5. Fuzzy Logic
In the past decade, fuzzy logic has proven to be a powerful tool for decision-making systems [15]. The fuzzy set theory derives from the fact that the natural classes and concepts are vague. This makes fuzzy logic quite suitable for complex systems as they can summarize from massive information inputs with increased effectiveness and tolerance to imprecisions [16]. The study provides brief descriptions of the key contributions made by fuzzy technology in the field of dentistry.
1.2. Motivation and Contributions
Several surveys addressing the use of AI in dentistry have been published in recent years. Ossowoska et al. [18] discuss the possibilities of using neural networks in different fields of dentistry including restorative dentistry, endodontics, orthodontics, dental surgery, and periodontology. The study indicates that artificial intelligence has developed rapidly in recent years and has the potential to be applied in routine dentistry. However, the study lacks discussion related to the limitations of the included studies. This study aims to outline the clinical support decision systems developed in recent years possess the potential to be employed in different fields of dentistry. We also discuss the limitations and formulate future directions of such approaches to contribute to improving the current state of clinical dental practice. The contributions of this study are provided below
Identify the development of AI applications that are employed widely in dentistry for different diagnostic tasks such as disease diagnosis, risk assessment, treatment planning, and prognosis and evaluate their diagnostic performance,
Outline the limitations of the neural networks employed for different diagnostic tasks including disease diagnosis, landmark detection, risk assessment, treatment planning, and prognosis,
Provide future directions which may have a positive and stimulating impact on the future application of artificial intelligence-based techniques in the field of dentistry.
1.3. Article Organization
The rest of this survey is organized as follows. Survey methodology is explained in Section 2. Applications of AI approaches are discussed in Section 3 while the limitations and future directions are provided in Section 4. In the end, the survey is concluded in Section 5.
2. Methodology
For this article, the preferred reporting items for systematic reviews (PRISMA) guidelines are followed. PRISMA provides a standard peer-accepted methodology consisting of a guideline checklist to ensure the quality assurance of the work’s replicability. This section defines the article selection criteria, data sources, and the search strategy for data extraction and analysis procedure.
2.1. Research Questions
Following are the research questions formulated to analyze the relevant studies:
-
RQ1.
What are the main fields in dentistry in which AI is employed?
-
RQ2.
What are data modalities and features used for diagnosing dental diseases?
-
RQ3.
Which AI techniques (including subfields) are utilized for disease diagnosis in different fields of dentistry?
-
RQ4.
Which outcome measures are used for the performance assessment of models?
Furthermore, the research question was formatted using population, intervention, comparison, and outcome (PICO):
Population: Dental imagery related to radiographs (bitewing, periapical, occlusal, panoramic, cephalograms, cone-beam computed tomography (CBCT)), digital photographs, high-frequency ultrasound images, 3D CBCT images, domain, near-infrared light transillumination (NILT) images, extraoral/intraoral mold images, orthodontic scans, magnetic resonance imaging, water’s view radiographs, hyperspectral images and textual data including electronic health records.
Intervention: Artificial intelligence, machine learning, deep learning-based models for diagnosis, detection, classification, segmentation, risk assessment, treatment planning, and prognosis.
Comparison: Reference standard, expert opinions.
Outcome: Measurable and predictive outcomes that include accuracy, specificity, sensitivity, F1-score, intersection over union (IoU), dice co-efficient, regression co-efficient receiver operating characteristic curve (ROC), and area under the curve (AUC), successful detection rate (SDR).
2.2. Data Sources and Search Strategies
Different electronic databases were systematically searched including Google Scholar, Institute of Electrical and Electronics Engineers (IEEE) Xplore, PubMed, and Scopus. The search terms were broadened to identify as many eligible studies as possible. The search key strategy adopted for each database is given in Table 1.
Table 1.
Search strategy conducted in different databases.
| Search # | Topic | Terms |
|---|---|---|
| 1 | Artificial intelligence | artificial intelligence“[MeSH Terms] OR (“artificial” [All Fields] AND “intelligence”[All Fields]) OR “artificial intelligence”[All Fields] OR (“machine learning”[MeSH Terms] OR “machine learning”[All Fields]) OR (“deep learning”[MeSH Terms] OR (“deep”[All Fields] AND “learning”[All Fields]) OR “deep learning”[All Fields]) OR (“decision support systems, clinical”[MeSH Terms] OR “clinical decision support systems”[All Fields] OR (“fuzzy logic”[MeSH Terms] OR “fuzzy logic”[All Fields]) |
| 2 | Dentistry | “periodontics” OR “endodontics” OR pathology OR “dental health services”[All Fields] OR “dental”[All Fields] OR “dentally”[All Fields] OR “dentals”[All Fields]) AND (“surgery”[MeSH Subheading] OR “surgery”[All Fields] OR “surgical procedures, operative”[MeSH Terms] OR (“surgical”[All Fields] AND “procedures”[All Fields] AND “operative”[All Fields]) OR “maxillofacial”[All Fields] OR (“craniofacial“ [All Fields] OR (“restorative dent”[Journal] OR (“restorative”[All Fields] AND “dentistry”[All Fields]) OR “restorative dentistry”[All Fields]) OR (“dental implants”[MeSH Terms] OR (“dental”[All Fields] AND “implants”[All Fields]) OR “dental implants”[All Fields]) |
| 3 | Search #1 and #2 |
2.3. Selection of Studies
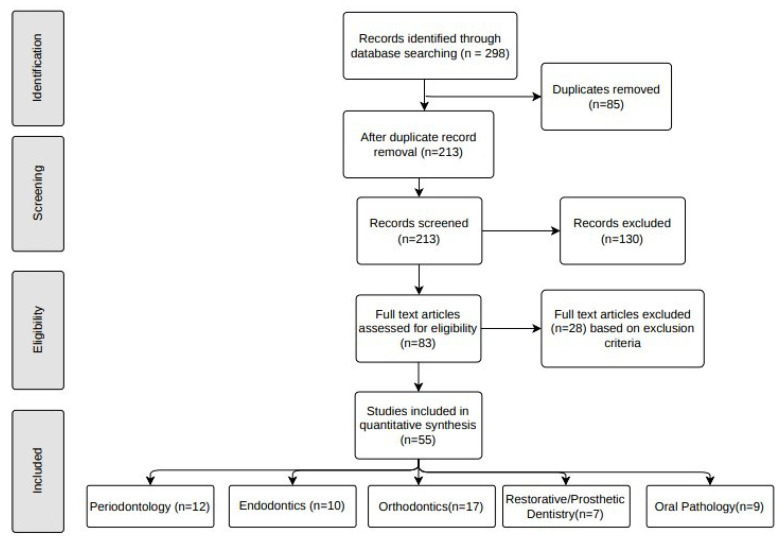
After an initial literature search was conducted, the title and abstract were screened for each study. Potentially relevant studies were further assessed for eligibility. Detailed information about the selection process is depicted in Figure 1.
Figure 1.
Relevant data about studies included for synthesis.
For risk assessment of bias for each study, sampling and measurement bias was assessed. The articles are identified based on the library searches. A total of 298 studies (Google Scholar, n = 128, PubMed, n = 66, IEEE Xplore, n = 58, and Scopus, n = 46) were identified via the initial search process. After examining the abstracts and titles of the identified studies, duplicates (n = 85) were excluded. The remaining 213 studies were screened, out of which 130 articles without full-text availability were eliminated. The full-text articles were assessed for eligibility and based on the exclusion criteria 28 articles were excluded. Following these procedure, 55 eligible studies (periodontology, n = 12, endodontics, n = 10, orthodontics, n = 17, restorative/prosthetic dentistry, n = 7, oral pathology, n = 9) were included in this review.
2.4. Eligibility Criteria
The following inclusion criteria were used in this study
Publications between 2009 and 2022 related to the application of artificial neural networks, machine learning, deep learning, fuzzy logic, expert systems, and clinical support decision systems (CDSS) in dentistry.
Studies in the English language and with full-text availability.
Published in a scholarly peer-reviewed journal.
Publications truly implementing AI including subfields in the context of dental topics.
The studies were excluded from the review if
Not published in a peer-reviewed journal.
Unpublished thesis or dissertation studies.
Studies that did not use artificial intelligence, review articles, and letter to the editor.
2.5. Risk Assessment
Each study was evaluated by the reviewers. To further minimize the risk of bias, studies dealing with AI implementation were included. Initial disagreements among the reviewers were noted while assessing the selected studies.
3. Applications of Artificial Intelligence in Dentistry
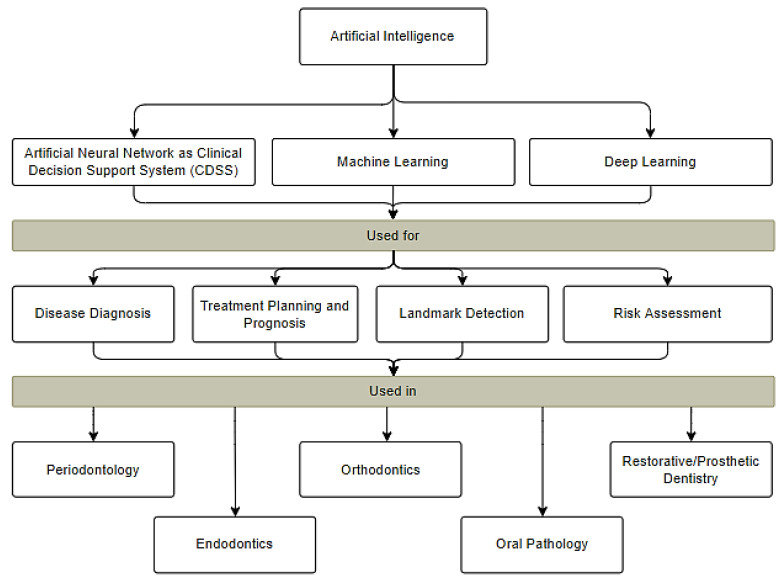
AI has made substantial progress in the diverse disciplines of medicine, specifically in the field of dentistry for diagnosis [5], localization [6], classification [7], estimation [8], and assessment of dental disease. With the recent rapid development of AI technologies designed for dental practitioners, dental clinicians make precise diagnoses and provide accurate recommendations. Figure 2 shows the areas within dentistry where AI can be used followed by a detailed description of AI applications in these fields.
Figure 2.
Applications of AI in different subfields of dentistry.
The progressive development of AI in dentistry will benefit both researchers and clinicians in integrating different fields of knowledge to improve patient care. There are different sub-disciplines of AI that are being used to provide precise, cost-effective, and user-friendly solutions to facilitate dentists and clinicians including ANNs and ANNs as CDSS, ML, and DL. The diagnostic activities performed using different input features in dentistry are shown in Table 2.
Table 2.
Summary of input features used for different diagnostic tasks.
| AI Technique | Diagnostic Tasks | Target Problem | Input Features |
|---|---|---|---|
| ANN as CDSS | Disease Diagnosis (Classification, Segmentation, Localization) | tooth decay detection [19], vertical root fracture [20], Cervical vertebra maturation degree [21,22] | Intraoral radiographs [19,20], cephalograms [21], orthodontic scans [22] |
| Risk Assessment | Bone loss assessment [23], periodontitis risk assessment [9] | Medical records [9,23] | |
| Treatment Planning and prognosis | Surgery type and extraction decision [24], tooth extraction therapy [25], occlusal and dental caries [26], temporomandibular joint disorders [27,28] | cephalograms [24], electronic health records [25,26,28], magnetic resonance images [27] | |
| Machine Learning | Disease Diagnosis (Classification, Segmentation, Localization) | Plaque segmentation [29], gingivitis identification [30], alveolar bone loss [6], scoring lesions [31], occlusal caries lesions [32], maxillary structure assessment [33], sagittal skeletal patterns [34], facial attractiveness [35], temporomandibular joint disorders [36] | Oral endoscopic images [29], digital photographs [30,35], intraoral radiographs [6], occlusal images [31,32], CBCT images [33], cephalograms [34], magnetic resonance images [36] |
| Treatment Planning and Prognosis | Facial and skin mould [37], teeth extraction [38], tooth prognosis [39], dental restorations [40] | Mould images [37], electronic records [38,39], panoramic images [40] | |
| Deep Learning | Disease Diagnosis (Classification, Segmentation, Localization) | Bone loss [5,8,41,42], odontogenic cystic lesion [43], periodontal bone destruction [7,26], bone recession and interradicular radiolucency [44], alveolar bone delineation [45], bone assessment [46], periapical lesions [47,48], vertical root fracture [49], apical lesions [50], interproximal caries [51], distal root assessment [52], proximal and occlusal caries [53], dental implants [54], preserve tooth boundary [23], maxillary sinusitis lesions [42,55,56,57], temporomandibular joint disorders [58], oral cancer [59] | Periapical radiographs [7,41,44,47], panoramic radiographs [5,26,42,43,49,50,54,56,57,58], CBCT images [8,43,48,52,55], high-frequency ultrasound images [45], intraoral ultrasound images [46], bitewing radiographs [51], NILT images [53], digital photographs [23], water’s view radiographs [42], hyperspectral images [59] |
| Landmark detection | Cephalometric landmarks [60,61] | cephalograms [60,61] | |
| Treatment Planning | Differential orthodontic diagnosis [62] | cephalograms [62] |
3.1. AI in Periodontology
In periodontology, AI has been used extensively to explore, understand and develop periodontal applications including periodontal bone loss detection, diagnosing gingivitis inflammation, and assessment of connective tissues and other periodontal caries. Table 3 shows different studies that are introduced in periodontology. These studies indicate that AI-assisted systems can be used in clinical practices to strengthen and improve dental treatment outcomes in a more sophisticated manner.
Table 3.
Summary of related studies for AI application in periodontology.
| AI Application | Author, Year (Ref) | Architecture | Data Modality | Dataset Size Split (Train/Val/Test or Train/Test) | Study Factor | Reference Standard (Ground Truth) | Validation Scheme | Results (Performance Metrics/Values) |
Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Disease segmentation |
Li et al., 2022 [29] | CNN | Oral endoscope images | 607 images Train: 320 images Test: 287 images 320/287 | Plaque segmentation | Dentist | NA | Acc: 0.864 IoU: 0.859 | The model is helpful in plaque segmentation on small dataset |
| Li et al., 2019 [30] | A method based on contrast limited adaptive histogram (CLAHE), gray-level co-occurrence matrix (GLCM), and extreme machine learning | Digital photographs | 93 images Train: 73 images Test: 20 images 73/20 | Gingivitis identification | NA | NA | Accuracy: 0.74, Sensitivity: 0.75, Specificity: 0.73, Precision: 0.74 | The method is helpful for gingivitis identification | |
| Disease localization | Lin et al., 2015 [6] | Level segmentation based on Bayesian or KNN or SVM classifier | Periapical Radiographs | 31 images | Alveolar bone loss | NA | Leave one-out | Mean SD True Positive Fraction (TPF): 0.925, True Positive Fraction (FPF): 0.14 | The model localizes bone loss areas with high classification effectiveness |
| Disease detection | Lee et al., 2022 [41] | VGG+Individual CNN | Periapical Radiographs | 1740 images Train: 1218 images, Valid: 417 images, Test: 105 images 1218/417/105 | Bone loss | Three dentists | NA | Acc: 0.99, AUC: 0.98 | The proposed algorithm is helpful in diagnosing periodontal bone loss |
| Krois et al., 2019 [5] | Seven layered deep CNN | Panoramic radiographs | 1750 images, Train: 1400 images, Valid: 350 images, 1400/350 | Bone loss | Three examiners | Ten-fold | Acc: 0.81 | The model shows discrimination ability similar to that of dentists | |
| Kim et al., 2019 [63] | Deep CNN + Transfer learning | Panoramic radiographs | 12,179 images, Train: 11,189 images, Valid: 190 images, Test: 800 images 1189/190/800 | Bone loss | Dental clinicians | NA | AUROC: 0.95, F1-score: 0.75, Sensitivity: 0.77, Specificity: 0.95, PPV: 0.73, NPV: 0.96 | The model is useful in tooth numbering and achieved detection performance superior to that of dental clinicians | |
| Lee et al., 2019 [43] | GoogleNet InceptionV3 + Transfer learning | Panoramic radiographs and CBCT images | 2126 images including 1140 panoramic and 986 CBCT images Train: 1700 images, Test: 426 images, 1700/426 | Odontogenic cyst lesion | NA | NA | Panoramic images: AUC—0.847, Sensitivity—0.882, Specificity—0.77, CBCT images AUC—0. 914, Sensitivity—0.961, Specificity—0.771 | The model provides higher diagnostic performance on CBCT images in effectively detecting and diagnosing cystic lesions | |
| Disease classification | Moran et al., 2020 [7] | ResNet Inception | Periapical radiographs | 467 images, Train: 415 images, Test: 52 images, 415/52 | Periodontal bone destruction | NA | NA | Acc: 0.81, Precision: 0.76, Recall: 0.92, Specificity: 0.71, NPV: 0.90 | The inception model classifies regions based on the presence of periodontal bone destruction with encouraging performance |
| Disease segmentation | Khan et al., 2021 [44] | UNet + DenseNet121 | Periapical radiographs | 200 images, Train: 160 images, Test: 40 images | Bone recession and inter-radicular radioulency | Three experts | NA | mIoU: 0.501, Dice score: 0.569 | Automates the process of detecting the presence and shape of caries |
| Zheng et al., 2021 [8] | Automatically constrained dense U-Net | CBCT images | 100 images | bone lesion identification | Three reviewers | Four-fold | Dice score for different categories: Background: 0.961, Lesion: 0.709, Material: 0.822, Bone: 0.877, Teeth: 0.801 | The model is helpful in detecting the correct shape of the lesion and the bone | |
| Duong et al., 2019 [45] | UNet | High frequency ultrasound images | 35 images, Train: 30 images, Test: 5 images, 30/5 | Alveolar bone assessment | Three experts | NA | Dice Coefficient: 0.75, Sensitivity: 0.77, Specificity: 0.99 | The method yields a higher performance in delineating alveolar bone as compared to experts | |
| Nguyen et al., 2020 [46] | U-Net with ResNet34 encoder | Intraoral ultrasound images | 1100 images, Train: 700 images, Valid: 200 images, Test: 200 images 700/200/200 | Alveolar bone assessment | Three examiners | NA | Dice Coefficient: 0.853, Sensitivity: 0.885, Specificity: 0.998 | The model has the potential to detect and segment alveolar bone automatically | |
| Disease diagnosis | Li et al., 2020 [64] | Mask RCNN + novel caliberation method | Panoramic radiographs | 298 images, Train: 270 images, Test: 28 images 270/28 | Periodontitis prediction | Junior dentist | NA | mAP: 0.826, Dice score: 0.868, F1-score: 0.454, Accuracy: 0.817 | The model is useful for diagnosing the severity degrees of periodontitis |
| Papantonopoulos et al., 2014 [23] | Multilayer Perceptron ANN | Textual | 29 subjects | Aggressive periodontitis | NA | Ten-fold | Accuracy: 0.981 | The model provides effective periodontitis classification | |
| Geetha et al., 2020 [19] | Back propagation Neural Network | Intraoral digital radiographs | 105 images | Dental caries detection | NA | Ten-fold | Accuracy: 0.971, FPR: 2.8%, ROC: 0.987 | The model is helpful for the detection of tooth decay and is independent of visual errors | |
| Risk assessment | Shankarapillai et al. 2012 [9] | Multilayer Feedforward Propagation | Textual | 230 subjects | Periodontitis risk assessment | NA | NA | MSE: 0.132 | The model can be used for effective periodontitis risk prediction |
Moreover, to minimize errors in diagnosis, authors have proposed methods involving machine learning techniques. Li et al. proposed a plaque segmentation method based on a convolutional neural network (CNN) using oral endoscopic images. The results provided performance superior to that of dentists with an accuracy of 86.42% [29]. An extreme machine learning method based on contrast limited adaptive histogram and gray-level covariance matrix was evaluated by Li and fellow authors, using digital photographs. The model provided an accuracy of 74% for gingivitis identification using a small dataset [30]. For localization of alveolar bone loss, Lin et al. evaluated a level segmentation method based on a support vector machine (SVM), k nearest neighbor (KNN), and Bayesian classifier. The model was able to localize alveolar bone loss with high classification effectiveness [6].
DL-based methods have gained immense popularity in recent years. Several authors have evaluated methods for bone loss detection in intraoral radiographs, Lee et al. proposed a VGG-based neural network for diagnosing periodontal bone loss using 1740 periapical radiographs. The model achieves an accuracy of 99% and AUC of 98% outperforming the performance of three dentists [41]. Another model using deep-feed forward CNN was evaluated by Krois and fellow authors using panoramic radiographs. The model showed discrimination ability similar to that of three examiners [5]. Kim et al. and Lee et al. suggested the use of transfer learning to improve the performance of bone loss and odontogenic cyst lesion detection using panoramic radiographs. The model was useful in tooth numbering with performance superior to that of dental clinicians [44,63]. For the classification of regions based on periodontal bone destruction, Moran et al. demonstrated a ResNet model achieving an accuracy of 82% using 467 periapical radiographs [7]. To automate the process of detecting bone lesions and detecting correct shapes, Khan et al. presented a disease segmentation method based on U-Net architecture. The model was able to detect the presence and shape of caries with performance higher than three experts [44]. Another anatomically constrained dense U-Net method was proposed by Zheng et al. for bone lesion identification. Using cone beam computed tomography (CBCT) images, the model was able to detect the correct shape of the bone and lesion [8]. Duong et al. proposed a U-Net-based network for alveolar bone delineation using high-frequency ultrasound images yielding performance higher than three experts [45]. Using ResNet34 as an encoder with U-Net, Nguyen et al. assessed 1100 intraoral images for alveolar bone segmentation. The model was able to identify alveolar bone with a dice coefficient of 85.3% [46]. For periodontitis detection, Li et al. utilized Mask R-CNN with a novel calibration method. Using panoramic radiographs, the model diagnosed the severity degree of periodontitis with an accuracy of 82% outperforming that of a junior dentist [64].
In dentistry, the interpretation of data and carrying out proper diagnosis are crucial. However, medical decision-making is cumbersome for doctors in a time-compressed environment. Thus, an intelligent tool is required to assist doctors in making accurate decisions. These systems come under the category of CDSS. ANNs have been used as CDSS for diagnosis, classification, and assessment. Several ANN architectures have been employed to assist doctors in making accurate decisions in periodontology. Recently, Geetha et al. proposed a back propagation neural network for tooth decay detection. The model achieved an accuracy of 97.1% and a false positive rate of 2.8% using intraoral radiographs. The study indicates that ANNs can be employed for precise decay detection compared to traditional dental examination methods [19]. Papantonopoulos et al. evaluated multilayer perceptron ANN for bone loss assessment on medical health records. The model provided effective periodontitis classification with an accuracy of 98.1% [23]. Using 230 textual subjects, Shankarapillai et al. proposed a multilayer feed-forward propagation network for effective periodontitis risk prediction [9].
3.2. AI in Endodontics
Endodontics is the branch of dentistry concerned with diseases of the dental pulp and tissues surrounding the root or tooth. AI has gained immense popularity in aiding treatment planning in endodontics. AI with different models can help dentists to diagnose, and manage endodontic problems with encouraging performance and ensuring enhanced and precise patient care. The review is focused on extracting and analyzing AI-based approaches for disease diagnosis and treatment planning. The summary of relevant studies for AI application in endodontics to provide better dental treatment outcomes is shown in Table 4.
Table 4.
Summary of related studies for AI application in endodontics.
| AI Application | Author, Year (Ref) | Architecture | Data Modality | Dataset Size Split (Train/Val/Test or Train/Test) |
Study Factor | Reference Standard (Ground Truth) | Validation Scheme | Results (Performance Metrics/Values) |
Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Disease detection and classification |
Ghaedi et al., 2014 [31] | Circular Hough Transform Based Segmentation | Intraoral optical occlusal tooth surface images | 88 images with blue background | Detect and score caries lesions | International Caries Detection and Assessment System (ICDAS) experts | Ten fold | Accuracy: 0.863, Specificity: 0.983, Sensitivity: 0.83 | The automated system was helpful in detecting and score caries lesions. |
| Berdouses et al., 2015 [32] | Random Forest | Photographic colored images | 103 digital images | Occlusal caries lesions | Two pediatric dentists | Ten-fold | Accuracy: 0.80, Precision: 0.86, Recall: 0.86, F1-score: 0.85, ROC: 0.98 | The model was able to provide detection performance similar to that of trained dentists | |
| Disease Detection | Pauwels et al., 2021 [47] | CNN + Transfer Learning | Intraoral radiographs | 280 images, Train: 168 images, Test 112 images 168/112 | Periapical lesions | 3 oral radiologists | Five-fold | Sensitivity: 0.79, Specificity: 0.88, ROC-AUC: 0.86 | The study explored the potential of CNN-based assessment of periapical lesions and achieved superior performance compared to human observers |
| Fukuda et al., 2020 [49] | DetectNet (CNN) | Panoramic radiographs | 300 images, Train: 240 images, Test: 60 images 240/60 | Vertical root fracture | Three observers | Five-fold | Recall: 0.75, Precision: 0.93, F1-score: 0.83 | The model was useful in identifying teeth with vertical root fracture | |
| Orhan et al., 2020 [48] | Deep CNN (U-Net) | 3D CBCT images | 153 images | Periapical lesions | Two Oral and maxillofacial radiologist | NA | Recall: 0.89, Precision: 0.95, F1-score: 0.93 | The model was able to detect periapical pathosis with 92.8% reliability | |
| Ekert et al. 2019 [50] | 7 layer feed forward CNN | Panoramic radiographs | 85 images, Train: 56 images, Valid: 29 images 56/29 | Apical lesions | Six independent and experienced dentists | Ten-fold | Avg AUC: 0.85, Confidence Interval (CI): 95% | A moderately deep CNN was helpful in detecting apical lesions and can be helpful in reducing dentists’ diagnostic efforts | |
| Disease diagnosis | Bayraktar & Ayan, 2022 [51] | Deep CNN (YOLO) pretrained using DarkNet-53 | Bitewing radiographs | 1000 images, Train: 800 images, Test: 200 images 800/200 | Interproximal caries lesions | Two experienced dentists | Hold-out | Accuracy: 0.945, Sensitivity: 0.722, Specificity: 0.981, PPV: 0.865, NPV: 0.954, AUC: 0.871 | The study shows promising outcomes for detecting caries in bitewing images achieving an accuracy above 90% |
| Disease classification | Hiraiwa et al., 2019 [52] | AlexNet GoogleNet | CBCT images and panoramic images | Training image patches: Single root group: 11,472, Extra root group: 11,004, Testing image patches: Single root group:32, Extra root group—32 | Assessing number of distal roots of mandibular first molars | Two radiologists | Five-fold | Accuracy: 87.4, Sensitivity: 77.3, Specificity: 97.1, PPV: 96.3, NPV: 81.8, AUC: 0.87 | The model achieved detection performance superior to that of dental radiologists in differentiating whether distal root was single or with an extra root |
| Disease segmentation | Casalegno et al., 2019 [53] | Symmetric Autoencoder with skip connections similar to U-Net and encoding path similar to VGG16 | Near Infrared transillumination (TI) images | 217 grayscale images of upper and lower molars and premolars, Train: 185 images, Test: 32 images 185/32 | Proximal and occlusal caries lesion | Two dentists | Monte Carlo | IoU score: Occlusal—0.49, Proximal—0.49, AUROC: Occlusal—0.83, Proximal—0.85 | The proposed system has the potential to support dentists by providing higher throughput in detecting occlusal and proximal lesions |
| Disease diagnosis | Kositbowornchai et al., 2013 [20] | Probabilistic Neural Network | Intraoral radiographs | 200 images (50 sound and 150 vertical root fractures), Train: 120, Test: 80 120/80 | Vertical root fracture detection | N/A | Three-fold | Sensitivity: 0.98, Specificity: 0.905, Accuracy: 0.957 | The model was helpful in diagnosing vertical root fractures using intraoral digital radiographs |
Ghaedi et al. presented a circular Hough transform-based segmentation method to detect and score caries lesions in intraoral occlusal tooth surface images. The model yielded an accuracy of 86.3% in detecting and scoring caries lesions [31]. A random forest-based machine learning algorithm has been proposed by Berdouses et al., evaluated on colored images, the model achieved an accuracy of 80% higher compared to two pediatric dentists [32].
Different DL models have also been employed in endodontics for disease detection, classification, and segmentation. Pauwels et al. evaluated a transfer learning-based CNN using intraoral radiographs to detect periapical lesions. The model achieved superior performance compared to three radiologists [47]. Fukuda et al. performed vertical root fracture detection using DetectNet on 300 panoramic radiographs. The model was found to be useful and provided better performance for fracture detection compared to three observers [49]. Orhan et al. proposed the U-Net model to diagnose periapical lesions using 3D CBCT images. The model was able to detect periapical lesions with 92.8% reliability [48]. A seven-layer feed-forward network was presented by Ekert et al. for diagnosing apical lesions using panoramic radiographs. Compared to six independent and experienced dentists, the model was able to detect lesions with an AUC of 85% [50]. Bayraktar and Aryan presented an interproximal caries detection method based on you look only once (YOLO) pre-trained using DarkNet-53 using bitewing radiographs. The authors achieved an accuracy of 94.59% higher compared to two experienced dentists [51]. For classification and assessment of distal roots, Hiraiwa et al. evaluated AlexNet achieving a detection performance of 87.4% superior to that of dental radiologists in differentiating whether the distal root was single or with an extra root [52]. Symmetric autoencoder with skip connections similar to U-Net and the encoding path was presented by Casalegno et al. to detect proximal occlusal caries using 217 grayscale near-infrared transilluminations (NILT) images. The model achieved higher throughput in detecting occlusal and proximal lesions compared to two dentists [53]. Kositbowornchai et al. presented a probabilistic neural network to be used as CDSS for diagnosing vertical root features. The model achieved an accuracy of 95.7% on intraoral digital radiographs [20].
3.3. AI in Orthodontics
Recent years have witnessed the immense popularity of AI in orthodontics due to its ability to make the diagnostic process more accurate and efficient. Orthodontic treatments are usually long procedures which is why more efficient solutions are required for effective and efficient planning. AI-based knowledge has the potential to automate disease diagnosis and treatment prognosis processes to help dental clinicians in making decisions more accurately and efficiently in a time-constrained environment. AI applications can further help in preventing human errors through their ability to learn and make automotive decisions. Different studies have been explored that incorporate the use of AI for orthodontic disease diagnosis and treatment planning which are shown in Table 5.
Table 5.
Summary of related studies for AI application in orthodontics.
| AI Application | Author, Year (Ref) | Architecture | Data Modality | Dataset Size Split (Train/Val/Test or Train/Test) | Study Factor | Reference Standard (Ground Truth) | Validation Scheme | Results (Performance Metrics/Values) | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Disease diagnosis |
Chen et al., 2020 [33] | ML-based algorithm based on multisource integration framework | CBCT images | 36 CBCT images, Train: 30 images, Test: 6 images 30/6 | Assess maxillary structure variation | NA | NA | Dice score of maxilla: 0.80 | The method is helpful in assessing maxillary structure variation in unilateral canine impaction |
| Nino-Sandoval et al., 2016 [34] | Support Vector Machine (SVM) | cephalograms | 229 cephalograms | Sagittal (skeletal) patterns | NA | Ten-fold | Accuracy: 0.741 | The non-parametric method has the potential to classify skeletal patterns using craniomaxillary variables | |
| Yu et al., 2014 [35] | Support Vector Regression (SVR) | Colored Photographs | 108 images | Facial attractiveness (most attractive to least attractive) | 69 Orthodontists | NA | Accuracy: 0.718 | The model was helpful in finding close correlation with facial attractiveness from orthodontic photographs | |
| Treatment planning |
Riri et al., 2020 [37] | Tree Based Classification | Extraoral intraoral and mould images | 1207 total images, Extraoral: 325 images, Intraoral: 812 images, Mould: 70 images | Facial and skin color features | NA | NA | Accuracy: 0.942, Sensitivity: 0.953, Specificity: 0.996, F1-score: 0.926 | The automatic approach was helpful in classification of orthodontic images with encouraging classification performance |
| Suhail et al., 2020 [38] | Random Forest Ensemble Learning Method | Patient records (medical charts, x-rays, facial photographs) | 287 patient records | Decision making for teeth extraction | Five experts | Five-Fold | Accuracy: 0.944 | The RF ensemble classifier was helpful in extraction and treatment planning | |
| Landmark detection in cephalograms |
Song et al., 2020 [60] | Pretrained ResNet-50 using transfer learning | X-Ray images | 400 cephalograms, Train: 150 images, Testing sets: Test set 1—150 images, Test set 2—100 images | Detect cephalometric landmarks | Two experienced doctors | NA | Successful Detection Rate (SDR): Test 1—0.862, Test 2—0.758 | The model was able to achieve satisfying SDR in detecting 19 landmarks |
| Kim et al., 2020 [65] | Two stage DNN with stacked hourglass network | Dataset 1: 2075 cephalograms, Dataset 2: 400 | cephalograms | Detect cephalometric landmarks | Two experts | NA | SDR: Test set 1—0.883, Test set 2—0.77 | The fully automated cephalometric analysis algorithm and web application help in the diagnosis of anatomic landmarks | |
| Gilmour and Ray, 2020 [66] | Pretrained ResNet-50 with foveated pyramid attention algorithm | cephalograms | 400 cephalograms | Detect cephalometric landmarks | NA | Four-fold | SDR: Test set 1—0.883, Test set 2—0.77 | The multiresolution approach was useful in learning features across all scales and is promising for large images | |
| Zhong et al., 2019 [67] | Attention guided deep regression model through 2 stage U-Net | cephalograms | 300 cephalograms Train: 150, images Test: 150 images 150/150 | Detect cephalometric landmarks | Two experienced doctors | Four-fold | SDR: 86.74% | The attention-guided mechanism ensures that smaller searching scopes and high data resolution with minimum information redundancy and the model is generalizable to other landmarks | |
| Park et al., 2019 [61] | YOLOv3 | cephalograms | 1311 cephalograms, Train: 1028 images, Test: 283 images 1028/283 | Detect cephalometric landmarks | One examiner | NA | SDR: 0.804 Computational time: 0.05 s | The model was effective in identifying 80 landmarks with 5% higher accuracy compared to top benchmarks | |
| Disease diagnosis |
Makaremi et al., 2019 [68] | Customized CNN | cephalograms | 1870 cephalograms | Cervical vertebra maturation (CVM) degree | Experts | Three-fold | Accuracy: 0.95 | The model was helpful in determining the degree of maturation of CVM and has the potential to be implemented in real world scenario |
| Yu et al., 2020 [69] | Modified DenseNet pretrained with ImageNet weights | Lateral cephalograms | 5890 cephalograms | skeletal classification | Five orthodontic specialists | NA | Sagittal Accuracy: 0.957, Vertical Accuracy: 0.964 | The model shows potential in skeletal orthodontic diagnosis using lateral cephalograms | |
| Treatment plannimng | Lee et al., 2020 [62] | Modified AlexNet | cephalograms | 333 cephalograms, Train: 220 images, Valid: 73 images, Test: 40 images | Differential orthodontic diagnosis | NA | Four-fold | Accuracy: 0.919, Sensitivity: 0.852, Specificity: 0.973, AUC: 0.969 | The study indicates that the DCNNs-based model can be applied for differential diagnosis in orthodontic surgery |
| Disease diagnosis |
Amasya et al., 2020 [21] | ANN | cephalograms | 647 cephalograms, Train: 498 images, Test: 149 images 498/149 | Cervical vertebra maturation degree | Two independent observers | NA | Accuracy: 0.869, Sagittal Sensitivity: 0.935, Vertical Specificity: 0.945 | The model was helpful in CVM staging and cervical vertebral morphology classification |
| Kok et al., 2019 [70] | ANN | cephalograms | 300 cephalograms | Cervical vertebrae stages | Orthodontists | Five-fold | AUC: CV1—0.99, CV2—0.96, CV3—0.94, CV4—0.90, CV5—0.91, CV6—0.96 | Compared with machine learning algorithms, ANN provides the most stable results with 2.17 average rank on hand-wrist radiographs | |
| Budiman et al., 2013 [22] | ANN | Orthodontic scans | 190 scanned dental casts | Shape of arch form | Three orthodonotics | NA | Accuracy: 0.763 | ANN has the potential to identify arch forms with encouraging accuracy | |
| Treatment planning and prognosis | Choi et al., 2019 [24] | ANN | cephalogram | 316 cephalograms, Train: 136 images, Valid: 68 images, Test: 112 images | Surgery type and extraction decision | One otthodontist | NA | Accuracy Surgery decision: 0.96, Surgery type and extraction decision: 0.91 | The model was helpful in diagnosing and making surgery type and extraction decision effectively and can be used as auxiliary reference when clinicians make a decision |
Several authors have adopted machine learning techniques for disease diagnosis and treatment planning in the field of orthodontics. Chen et al. proposed a machine learning algorithm based on a multisource integration framework for assessing maxillary structures using CBCT images. The model achieved encouraging performance with a dice ratio of 0.80 in assessing maxillary structure variation in unilateral canine impaction [33]. An SVM-based algorithm was evaluated by Nino-Sandoval to classify skeletal patterns using craniomaxillary variables using cephalograms. The model achieved an accuracy of 74.5% in analyzing sagittal skeletal patterns [34]. Yu et al. investigated support vector regression (SVR) to rate facial attractiveness from most attractive to least attractive using colored photographs. It was found that the model can be used in finding a close correlation with facial attractiveness from orthodontic photographs with an accuracy of 71.8% [35]. Different studies explored the use of ML in treatment planning. Riri et al. proposed a tree-based classification method to identify facial and skin molds using intraoral and extraoral mold images. The model yielded an accuracy of 94.28% [37]. Suhail et al. proposed a random forest ensemble method to automate the extraction and treatment planning process. Based on patients’ medical health records, the model was able to make decisions for teeth extraction with an accuracy of 94.4% [38].
Literature addressed landmark detection problems as well using DL techniques. Song et al. evaluated a ResNet-50 model pre-trained using transfer learning on 400 cephalograms to detect cephalometric landmarks. The model achieved a superior SDR for two test cases in detecting 19 landmarks compared to two experienced doctors [60]. Kim et al. presented a two-stage deep neural network (DNN) with a stacked hourglass network for landmark detection using 2075 cephalograms followed by a web application. The model provided SDR of 82.92% and 84.53% for dataset 1 and dataset 2, respectively [65]. Gilmour et al. proposed pre-trained ResNet-50 with foveated pyramid attention algorithm achieving an SDR of 88.32% and 77.05% for dataset 1 and dataset 2, respectively [66]. Park et al. performed landmark detection using YOLOv3 on 1311 cephalograms. The model was effective in identifying 80 landmarks with 5% higher accuracy compared to top benchmarks. Moreover, attention-based networks have also been explored actively in landmark detection [61]. Zheng et al. explored attention guided deep regression model through a two-stage U-Net using 300 cephalograms. The model achieved an SDR of 86.74% which was higher compared to that of two experienced doctors [8]. Different deep learning-based methods have been employed for disease diagnosis. Makeremi et al. proposed a customized CNN to determine cervical vertebra maturation degree using cephalograms. The model achieved an accuracy of 95% helpful in determining the degree of maturation of CVM and has the potential to be implemented in real-world scenarios [68]. Yu et al. presented modified DenseNet pre-trained with ImageNet weights using lateral cephalograms. The model yielded an accuracy of 95.70% superior to that of five orthodontic specialists [69]. Deep learning for treatment planning has also gained attention in recent years. Lee et al. proposed a modified AlexNet for differential diagnosis in orthodontic surgery achieving an accuracy of 91.9% [62]. The study indicated that deep CNNs-based models can be applied for differential diagnosis in orthodontic surgery.
Different studies have explored ANNs as CDSS in orthodontics. Amasya et al. proposed ANN to diagnose cervical vertebra maturation degree using cephalograms. The model achieved an accuracy of 86.93% in CVM staging and cervical vertebral morphology classification [21]. Kok et al. proposed an ANN-based network to classify cervical vertebrae stages. It was found that ANN provided the most stable results with a 2.17 average rank on hand-wrist radiographs compared to an orthodontist [70]. Another ANN-based method was evaluated by Budiman using orthodontic scans to detect the shape of the arch form. The model achieved an accuracy of 76.32% [22]. In terms of treatment planning and prognosis, different studies explored the use of ANNs. Choi et al. proposed an ANN-based method for diagnosing and making surgery type and extraction decisions effectively [24].
3.4. AI in Restorative Dentistry and Prosthodontics
The number of studies exploring the use of AI in restorative dentistry has increased considerably in recent years. Different studies explored the use of AI in assisting the diagnosis of caries, and vertical tooth fracture, predicting restoration failure, and planning treatment. Table 6 shows the relevant studies for AI application in restorative dentistry to provide better dental treatment outcomes.
Table 6.
Summary of related studies for AI application in prosthetic/restorative dentistry.
| AI Application | Author, Year (Ref) | Architecture | Data Modality | Dataset Size Split (Train/Val/Test or Train/Test) | Study Factor | Reference Standard (Ground Truth) | Validation Scheme | Results (Performance Metrics/Values) | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Treatment planning | Lee et al., 2022 [39] | Decision Tree | Electronic records | 94 clinical cases | Tooth prognosis | Three prosthodontists | NA | Accuracy: 0.841 | The model was helpful in determining tooth prognosis for effective treatment planning |
| Disease detection | Abdalla-Aslan et al., 2020 [40] | Cubic SVM based algorithm | Panoramic radiographs | 83 images | Dental restorations | Experienced practitioners | Five-fold | For detection Sensitivity: 0.94, For classification Sensitivity: 0.98 | The model has the potential to detect and classify dental restorations to promote patient’s health |
| Disease diagnosis | Lee et al. 2020 [54] | Fine Tuned and pretrained Deep CNN | Panoramic and periapical radiographs | 10,770 images, Train: 6462 images, Valid: 2154 images, Test: 2154 images 6462/2154/2154 | Dental implants | Periodontists | Ten-fold | AUC:0.971 | The model was helpful in the identification and classification of dental implants with performance similar to that of periodontist |
| Takahashi et al., 2021 [71] | Deep CNN (ResNet152) pretrained with ImageNet weights | Oral photographs | 1,184 images, maxilla: 748 images, mandible: 436 images | Partially edentulous arches | Clincian | NA | Maxilla: Accuracy: 0.995, Recall: 1.00, Precision: 0.25, AUC: 0.99, Mandible Accuracy: 0.997, Precision: 0.25, Recall: 1.00, AUC: 0.98 | The method was helpful in classification of dental arches and can be effective in designing removable partial dentures | |
| Disease segmentation | Xu et al., 2018 [72] | Two Hierarchical CNNs | 3D dental images | 1200 images, Train: 1000, Valid: 50, Testing: 150 1000/50/150 | Preserve teeth boundary | NA | NA | Accuracy: Upper dental model: 0.99, Lower dental model: 0.987 | The label-free mesh simplification method helped preserve the teeth boundary information using the 3D dental model. |
| Treatment Planningand Prognosis |
Cui et al., 2020 [25] | Triple Classification algorithm (Extreme Gradient Boost (XGBoost)) | Electronic health records | 4135 records | Tooth extraction therapy | Two prosthodontists | Five-fold | Binary Classification Accuracy: 0.962, Precision: 0.865, Recall: 0.830, Triple Classification Accuracy: 0.924, Precision: 0.879, Recall: 0.836 | The model was helpful in predicting tooth extracting therapy with performance superior to that of prosthodontists |
| Javed et al. 2020 [26] | ANN | Electronic records | 45 records of children | Occlusal caries lesions | NA | Leave one out | Regression co-efficient: 0.99 | The model was helpful in occlusal dentinal caries lesions and the study proposes an iOS app for meticulous prediction of caries |
The usefulness of AI in restorative dentistry has been investigated by several researchers. Lee et al. proposed a machine learning method based on a decision tree to determine tooth prognosis for effective treatment planning using clinical cases. The model achieved an accuracy of 84.1% [39]. A cubic SVM-based algorithm was proposed by Abdalla-Aslan et al. using panoramic radiographs. The model has the potential to detect and classify dental restorations to promote patients’ health [40].
Lee and Jeong proposed a fine-tuned and pre-trained deep CNN to diagnose dental implants using panoramic radiographs. The model yielded an AUC of 97.1% for the identification and classification of dental implants with performance similar to that of periodontists [54]. A deep CNN pre-trained with ImageNet weights was proposed by Takahashi et al. to diagnose partially edentulous arches achieving an accuracy of 99.5% for maxillar arches and 99.7% for mandible arches on oral photographs. The study showed that deep learning can be used effectively in designing removable partial dentures [71]. To preserve the teeth boundary, Xu et al. presented a two hierarchical CNN for segmentation of upper and lower teeth using 3D dental model images [72].
In terms of treatment planning and prognosis, ANNs have been utilized in different studies. Cui et al. proposed a triple classification algorithm based on extreme gradient boost (XGBoost). Using electronic health records, the model yielded an accuracy above 90% superior to that of prosthodontists in predicting tooth extracting therapy [25]. Another feed-forward ANN was presented by Javed et al. to identify occlusal dentinal caries lesions. The study proposed an iOS app for the meticulous prediction of caries using clinical cases [26].
3.5. AI in Oral Pathology
The relevant studies utilizing AI for disease diagnosis and prognosis are given in Table 7. Orhan et al. proposed a machine learning algorithm based on KNN and random forest using magnetic resonance images to diagnose temporomandibular disorders. The model achieved an accuracy of 77% in identifying condylar changes and 74% for disc displacement [36]. Hung et al. presented a three-step CNN-based on V-Net and SVR to identify maxillary sinusitis using CBCT images. The model yielded an AUC of 92% for mucosal thickening and 84% for mucous retention cyst identification [55].
Table 7.
Summary of related studies for AI application in oral pathology.
| AI Application | Author, Year (Ref) | Architecture | Data Modality | Dataset Size Split (Train/Val/Test or Train/Test) | Study Factor | Reference Standard (Ground Truth) | Validation Scheme | Results (Performance Metrics/Values) | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Disease diagnosis |
Orhan et al., 2021 [36] | ML (KNN and Random Forest (RF)) | Magnetic Resonance Imaging | Temporomandibular disorders | Pathologists | NA | NA | Accuracy: Condylar changes—0.77, Disk displacement—0.74 | The model was found to be optimal in predicting temporomandibular disorders |
| Hung et al., 2022 [55] | Three step CNN based on V-Net and SVR | CBCT images | 445 images, Train: 311 images, Valid: 62 images, Test: 249 images 311/62/249 | Maxillary sinusitis | NA | NA | AUC: Mucosal thickening—0.91, Mucous retention cyst—0.84 | The model helped detect and segment mucosal thickening and mucosal retention cyst using low-dosed CBCT scans | |
| Kuwana et al., 2021 [56] | CNN (DetectNet) | Panoramic radiographs | 1174 images | Maxillary sinus lesions | NA | NA | Maxillary sinusitis: Accuracy—0.90–0.91, Sensitivity—0.81–0.85, Specificity—0.91–0.96, Maxillary sinus cysts: Accuracy—0.97–1.00, Sensitivity—0.80–1.00, Specificity—1.00 | The model was helpful in detecting maxillary sinus lesions | |
| Choi et al., 2021 [58] | CNN (ResNet) | Panoramic radiographs | 1,189 images, Training: 951 images, Testing: 238 images 951/238 | Temporomandibular joint disorders (TMJ) osteoarthritis | Oral and maxillofacial radiologist (OMFR) | Five-fold | Temporal: AUC - 0.93, Geographical external: AUC—0.88 | The model achieved significantly higher diagnostic performance compared to that of radiologists | |
| Kim et al., 2019 [42] | CNN | Water’s view radiographs | 200 images | Maxillary sinusitis | Five radiologists | NA | Accuracy: Upper dental model: 0.99, Lower dental model: 0.987 | The label free mesh simplification method was helpful in preserving the teeth boundary information using 3D dental model | |
| Murata et al., 2019 [57] | AlexNet CNN | Panoramic radiographs | 120 images | Maxillary sinusitis | Two radiologists, Two dentists | NA | Accuracy: 0.875, Sensitivity: 0.867, Specificity: 0.883 | The model shows diagnostic performance similar to the radiologists and superior to the resident dentists | |
| Jeyaraj et al., 2019 [59] | Partitioned CNN (GoogleNet Inception V3) | Hyperspectral images | 600 images | Oral Cancer | Expert oncologist | Seven-fold | Benign tissue Accuracy—0.914, Malign tissue Accuracy—0.945 | The model helped predict cancerous or benign tumor and has the potential to be applied as a workbench for automated classification | |
| Disease prognosis |
Iwasaki et al. 2015 [27] | Bayesian Belief Network (BNN) | Magnetic Resonance Imaging | 590 images | Temporomandibular joint disorders (TMJ) | NA | Ten-Fold | Accuracy: 0.99 | The model has the potential to determine the progression of TMD in terms of bone changes, disc displacement and bony space and disc affect with encouraging diagnostic performance |
| Bas et al., 2012 [28] | Back Propagation ANN | Electronic records | 219 records | Clinical symptoms (Temporomandibular joint disorders (TMJ)) | Experienced oral and maxillofacial surgeon | NA | Unilateral with and without reduction: Sensitivity—0.80 & 0.95, Specificity—0.69 & 0.91 | The model was helpful in diagnosing the preliminary subtypes of TMJ and can be useful in the decision-making process |
In a similar fashion, Kuwana et al. evaluated DetectNet on panoramic radiographs to detect maxillary sinus lesions with an accuracy of 90 to 91% for maxillary sinusitis and 97 to 100% for maxillary sinus cysts [56]. Moreover, a deep CNN (ResNet) was presented by Choi et al. to diagnose temporomandibular joint disorders (TMJ) osteoarthritis. The model yielded an accuracy of 78% similar to that of Oral and maxillofacial radiologists (OMFR) [58].
Kim et al. investigated the performance of CNN on water’s view radiographs to identify maxillary sinusitis. The model achieved significantly higher diagnostic performance compared to that of five radiologists [42]. Murata et al. evaluated the deep learning model AlexNet to diagnose maxillary sinusitis on panoramic radiographs. The model showed diagnostic performance similar to the radiologists and superior to the resident dentists [57]. Jeyaraj et al. proposed a partitioned CNN based on GoogleNet and InceptionV3 for diagnosing oral cancer using hyperspectral images. The model achieved an accuracy of 91.4% and 94.5% for benign and malign tissue respectively [59].
Clinical support decision systems have also been used in oral pathology. Iwasaki et al. proposed a Bayesian belief network (BNN) using magnetic resonance images to diagnose TMJ disorders. The model achieved an accuracy of 99% and can be used to determine the progression of TMD in terms of bone changes, disc displacement, and bony space by dental clinicians [27]. Moreover, a backpropagation ANN has been proposed by Bas et al. to identify clinical symptoms of TMJ disorders. It was found that the model can help diagnose the preliminary subtypes of TMJ and can be useful in the decision-making process [28].
4. Limitations and Future Direction
This study confirms the growing presence of AI in dentistry and highlights the performance of AI-based techniques as clinical support decision systems. Although AI techniques have been integrated increasingly into different subfields of dentistry, the current evidence of AI in clinical practice is still very limited. This section discusses the limitations and provides some future directions which may have a positive and stimulating impact on the future application of AI-based techniques in the field of dentistry.
4.1. Limitations
Although AI techniques have been employed increasingly within the field of dentistry, certain limitations need to be addressed to improve the performance, reliability, and generalizability of AI-based models. These limitations are discussed here
Computational capability: AI-based techniques require a significant amount of parallel processing power to keep up with the demands. There are certain limitations of computational resources such as RAM and GPU cycles that are required to support such approaches.
Reliability: It is essential in a clinical setting and involves ethical fairness, the accuracy of the system, and patients’ trust/acceptance of such systems. Limited reliability causes spurious consequences.
Generalizability: Issues in generalizability are a dominant concern for clinical guidelines, and a precise level of generalizability is required for the application of AI-based models in clinical practices and ensuring that it performs well prospectively.
Class imbalance: The difference in the number of samples representing each class within the data is referred to as class imbalance. The lack of high-quality and labeled datasets limits the deployment of robust and accurate AI-based models.
Overfitting: Due to the high complexity in terms of parameters of deep learning models, the models are more likely to overfit with the training data, thus affecting the generalizability of the results.
The limitations of each relevant study covered in this review are given in Table 8 and Table 9. One of the most prevailing limitation in majority of the studies involving application of AI in subfields of dentistry is dataset limitation in terms of size for disease diagnosis [5,19,31,42,44,45,51,56,57,58,59,71], for treatment planning and prognosis [29,37,62], and for landmark detection [64]. Other limitations include relying on the reliability of examiners for accurate diagnosis [41], less flexibility to capture nonlinearities in data [23], limited practical use [54], poor performance on images that are not evenly illuminated [6], limited class discrimination [6], lack in terms of generalizability [29], lack in terms of performance [9,28,60,61,62,64,65,66,68,69], and computational capabilities [67].
Table 8.
Limitations of relevant studies employing AI for disease diagnosis.
| Diagnostic Task | Target Problem | Author, Year (Ref.) | Limitations |
|---|---|---|---|
| Disease diagnosis | bone recession and interradicular radiolucency | Khan et al., 2021 [44] | Limited in terms of size |
| temporomandibular joint disorders | Orhan et al., 2021 [36] | Further study is required for identification for the reduction state. | |
| Choi et al., 2021 [58] | The dataset size is rather small and more images are required to improve performance | ||
| maxillary sinusitis lesions | Hung et al., 2022 [55] | The performance can be further improved | |
| Kuwana et al., 2022 [56] | Dataset is small in terms of size. The study also did not include post-operative maxillary sinuses | ||
| Kim et al., 2019 [42] | Dataset is limited in terms of size and only includes maxillary sinus using water view radiographs | ||
| Murata et al., 2019 [57] | Limited dataset in terms of size | ||
| alveolar bone delineation | Duong et al., 2019 [45] | Limited dataset in terms of size | |
| bone assessment | Nguyen et al., 2020 [46] | The model is restricted to detection and segmentation in buccal surfaces | |
| periapical lesions | Orhan et al., 2020 [48] | The model can be influenced with variations i.e., presence of endo-perio lesions or other periodontal defects | |
| bone loss | Lee et al., 2022 [41] | The model is not able to detect vertical defect depth and angulation. The diagnosis also relies on the reliability of examiners | |
| Kim et al., 2019 [63] | Low resolution for the individual tooth in panoramic images as it captures a wide field of view | ||
| Krois et al., 2019 [5] | Limited dataset in terms of size | ||
| Zheng et al., 2021 [8] | The approach is not applicable on unlabeled data | ||
| Papantonopoulos et al., 2014 [23] | The model is not flexible enough to capture nonlinearities in data | ||
| oral cancer | Jeyaraj et al., 2019 [59] | Dataset is limited in terms of size | |
| odontogenic cystic lesion | Lee et al., 2019 [62] | Limited dataset in terms of size | |
| periodontal bone destruction | Moran et al., 2020 [7] | Poor performance in classifying healthy regions due to small dataset | |
| vertical root fracture | Fukuda et al., 2020 [49] | The model is trained on panoramic radiographs with clear vertical root fracture (VRF) lines which impacts the performance | |
| apical lesions | Ekert et al., 2019 [50] | Manually cropped image segments were used for training The sensitivity should be improved before clinical use | |
| interproximal caries | Bayraktar & Ayan, 2022 [51] | Dataset is limited in terms of size. Carious lesions were not classifiedas enamel caries or dental caries | |
| distal root assessment | Hiraiwa et al., 2019 [52] | Image patches are created by manual segmentation which is time-consuming. | |
| proximal and occlusal caries | Casalegno et al., 2019 [53] | Limited dataset in terms of size and ground truth labels | |
| dental implants | Lee et al., 2020 [43] | The proposed study only includes three types of dental implants which limit its practical use | |
| preserve tooth boundary | Takahashi et al., 2021 [71] | Limited dataset in terms of size of maxilla set | |
| Disease diagnosis | tooth decay detection | Geetha et al., 2018 [19] | Small dataset The model does not provide classification based on caries depth |
| alveolar bone loss detection | Lin et al. 2015 [6] | Poor performance on unevenly illuminated images | |
| occlusal caries lesion detection | Berdouses et al., 2015 [32] | The accuracy can be improved further | |
| sagittal skeletal patterns identification | Nino-Sandoval et al., 2016 [34] | Limited class discrimination | |
| vertical root fracture identification | Kositbowornchai et al., 2013 [20] | The proposed model is not applicable on other root fractures in clinical practice | |
| scoring lesions | Ghaedi et al. 2014 [31] | Small dataset Unbalanced number of images, histological verification of the diagnosis was not performed | |
| cervical vertebra maturation degree | Budiman, 2013 [22] | There is an overlap in data distribution that affects the models’ performance | |
| facial attractiveness | Yu et al., 2014 [35] | The model prediction is limited to certain angles and ratios | |
| plaque segmentation | Li et al., 2022 [29] | The model is not generalizable to other caries areas | |
| detect vertical vertebra maturation degree | Amasya et al. 2020 [21] | Hand-wrist radiographs are not considered | |
| maxillary structure assessment | Chen et al., 2020 [33] | The dataset is limited in terms of size |
Table 9.
Limitations of relevant studies employing AI for different treatment planning, landmark detection, and risk assessment.
| Diagnostic Task | Target Problem | Author, Year (Ref.) | Limitations |
|---|---|---|---|
| Treatment planning and prognosis | surgery type and extraction decision | Choi et al., 2019 [24] | The study does not include skeletal asymmetry cases |
| tooth extraction therapy | Cui et al., 2020 [24] | Other factors such as adjacent teeth, subsequent treatments are not considered in this study | |
| orthodontic diagnosis | Lee et al., 2020 [62] | Limited dataset in terms of size | |
| Landmark detection | cephalometric landmark identification | Song et al., 2020 [60] | The computational time still needs further improvement |
| Yu et al., 2020 [69] | Lacks in terms of performance | ||
| Li et al., 2020 [64] | Small dataset Poor performance on radiographs with severe periodontitis with abnormal shape of teeth | ||
| Kim et al., 2020 [65] | Not applicable to all landmarks in clinical situations | ||
| Gilmour and Ray, 2020 [66] | The bottleneck is in terms of storage as each iteration only loads small glimpse of image | ||
| Zhong et al., 2019 [67] | Limited in terms of computational capabilities | ||
| Park et al. 2019 [61] | Intra/inter-examiner reliability statistics and reproducibility comparisons are required | ||
| Risk Assessment | periodontitis risk assessment | Shankarapillai et al., 2012 [9] | Other metrics, i.e., accuracy have not been reported |
4.2. Future Directions
Keeping in view the current limitations of studies employing AI for disease diagnosis, treatment planning and prognosis, landmark detection, and risk assessment, the following are a few research directions that can help in elevating the deep learning model’s performance, reliability, and generalizability:
4.2.1. Data Augmentation
Class-imbalanced datasets are biased towards majority classes, hence the chances for misclassification are often higher. However, different techniques can be employed to deal with the class imbalance and improve the performance of deep learning models. Certain techniques can be utilized to minimize class imbalance, such as data augmentation. To extract the increased amount of information from medical images, non-linear transformations can be applied. Generative adversarial networks (GANs) can prove to be a good alternative for creating similar images using non-linear transformations inside the network. Particularly, GANs are being used increasingly for medical data augmentation such as on pulmonary computed tomography (CT) images to detect COVID-19 lesions [73] and on small-scale medical image datasets [74]. To get a powerful variation of the generative model that can be employed to overcome the class imbalance in a more sophisticated way, known as balancing generative adversarial networks (BAGANs). It focuses on generating minority-class images of high quality.
4.2.2. Cross Center Training
One of the limitations of deep learning application in dentistry is the uncertainty about the generalizability of the models. Limited generalizability may be related to differences in terms of population characteristics such as dental status, and image characteristics such as differences in data generation protocols involved. Thus, understanding the reasons behind generalizability limitations will help in formulating strategies to mitigate generalizability problems. Further research should be focused on employing cross-center training [75] to increase the generalizability of deep learning-based models.
4.2.3. Robotics in Dentistry
Similar to other fields, dentistry is also moving forward toward a new era of data-driven medicine assisted by robots. Robotic dental assistance [76] has the potential to be applied to different fields including orthodontics, implant dentistry, and prosthodontics. To improve the applicability of AI in dentistry, more flexible systems are needed to reach human-level performance and further improve the reliability of AI-based models in clinical practice.
4.2.4. Virtual Reality and Augmented Reality
For practicing safe and efficient dentistry and gaining simultaneous feedback, virtual reality (VR) can be adopted. To generate clinical information that can be visualized by patients, augmented reality (AR) can be adopted. The use of AR and VR is increasing gradually, and there is a limited number of studies that assessed their use [77]. From providing better visualization to reducing operative time and improving patient consultation and providing promising treatment outcomes, AR and VR have the potential to enable researchers in developing high-quality tools for clinical practice. Clinicians can make use of AR to enable patients to visualize expected outcomes before undergoing the procedure. AR and VR can be employed to provide improved dental education by enhancing students’ learning experiences during pre-clinical training [78].
5. Discussion and Conclusion
It is suggested that the AI-based systems developed in recent years possess the potential to be employed in different fields of dentistry to help dental clinicians and practitioners in making precise diagnoses and providing accurate recommendations. However, AI is still unlikely to replace the dentist-patient relationship in the foreseeable future as humane elements are also of utmost importance in decision-making to manage dental care. The AI technologies are intended to support dental clinicians in reducing misdiagnosis and work synergistically with the unique abilities of dentists to provide enhanced, accessible care by taking away routine parts of dentists’ work.
Artificial intelligence has contributed substantially to different subfields of dentistry over the past decade. This review discusses numerous applications of AI in periodontology, endodontics, orthodontics, restorative dentistry, and oral pathology including studies incorporating deep learning to effectively diagnose dental disease and plan treatments. However, the current evidence of AI is still sparse due to limitations in terms of dataset availability, size, performance, and generalizability. This study highlights the limitations of the relevant studies and provides future directions which may have a positive impact on the future application of AI-based networks within dentistry. This review indicates that more flexible systems are needed to reach human-level performance and increase the reliability of AI-based clinical decision support systems in dental practice.
Author Contributions
Conceptualization, A.F. and I.S.; Data curation, A.F. and J.B.; Formal analysis, I.S., H.A. and I.D.L.T.D.; Funding acquisition, I.D.L.T.D.; Investigation, H.A. and J.C.M.E.; Methodology, I.S.; Project administration, J.B. and J.C.M.E.; Resources, I.D.L.T.D. and D.R.-S.M.L.; Software, D.R.-S.M.L. and J.B.; Supervision, I.A.; Validation, J.C.M.E. and I.A.; Visualization, D.R.-S.M.L.; Writing—original draft, A.F. and H.A.; Writing—review & editing, I.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available from the authors on request.
Conflicts of Interest
The authors declare no conflict of interests.
Funding Statement
This research was supported by the European University of the Atlantic.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Haykin S. Neural Networks and Learning Machines, 3/E. Pearson Education India; Noida, India: 2009. [Google Scholar]
- 2.Basheer I.A., Hajmeer M. Artificial neural networks: Fundamentals, computing, design, and application. J. Microbiol. Methods. 2000;43:3–31. doi: 10.1016/S0167-7012(00)00201-3. [DOI] [PubMed] [Google Scholar]
- 3.Baxt W.G. Application of artificial neural networks to clinical medicine. Lancet. 1995;346:1135–1138. doi: 10.1016/S0140-6736(95)91804-3. [DOI] [PubMed] [Google Scholar]
- 4.Jain A.K., Mao J., Mohiuddin K.M. Artificial neural networks: A tutorial. Computer. 1996;29:31–44. doi: 10.1109/2.485891. [DOI] [Google Scholar]
- 5.Krois J., Ekert T., Meinhold L., Golla T., Kharbot B., Wittemeier A., Dörfer C., Schwendicke F. Deep learning for the radiographic detection of periodontal bone loss. Sci. Rep. 2019;9:8495. doi: 10.1038/s41598-019-44839-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin P., Huang P., Huang P., Hsu H. Alveolar bone-loss area localization in periodontitis radiographs based on threshold segmentation with a hybrid feature fused of intensity and the H-value of fractional Brownian motion model. Comput. Methods Progr. Biomed. 2015;121:117–126. doi: 10.1016/j.cmpb.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Moran M.B.H., Faria M., Giraldi G., Bastos L., da Silva Inacio B., Conci A. On using convolutional neural networks to classify periodontal bone destruction in periapical radiographs; Proceedings of the 2020 IEEE International Conference on Bioinformatics and Biomedicine (BIBM); Seoul, Korea. 16–19 December 2020; pp. 2036–2039. [Google Scholar]
- 8.Zheng Z., Yan H., Setzer F.C., Shi K.J., Mupparapu M., Li J. Anatomically constrained deep learning for automating dental CBCT segmentation and lesion detection. IEEE Trans. Autom. Sci. Eng. 2020;18:603–614. doi: 10.1109/TASE.2020.3025871. [DOI] [Google Scholar]
- 9.Shankarapillai R., Mathur L.K., Nair M.A., George R. Periodontitis risk assessment using two artificial neural network algorithms—A comparative study. Int. J. Dental Clin. 2012;4:17–21. [Google Scholar]
- 10.Carleo G., Cirac I., Cranmer K., Daudet L., Schuld M., Tishby N., Vogt-Maranto L., Zdeborová L. Machine learning and the physical sciences. Rev. Mod. Phys. 2019;91:045002. doi: 10.1103/RevModPhys.91.045002. [DOI] [Google Scholar]
- 11.Hinton G. Deep learning—A technology with the potential to transform health care. JAMA. 2018;320:1101–1102. doi: 10.1001/jama.2018.11100. [DOI] [PubMed] [Google Scholar]
- 12.Stead W.W. Clinical implications and challenges of artificial intelligence and deep learning. JAMA. 2018;320:1107–1108. doi: 10.1001/jama.2018.11029. [DOI] [PubMed] [Google Scholar]
- 13.Park W.J., Park J.B. History and application of artificial neural networks in dentistry. Eur. J. Dent. 2018;12:594–601. doi: 10.4103/ejd.ejd_325_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tundjungsari V., Sofro A.S.M., Sabiq A., Kardiana A. Investigating clinical decision support systems success factors with usability testing. Int. J. Adv. Comput. Sci. Appl. 2017;8 doi: 10.14569/IJACSA.2017.081168. [DOI] [Google Scholar]
- 15.Hajek P. Fuzzy logic from the logical point of view; Proceedings of the International Conference on Current Trends in Theory and Practice of Computer Science; Milovy, Czech Republic. 23 November–1 December 1995; pp. 31–49. [Google Scholar]
- 16.Trillas E., Eciolaza L. Fuzzy Logic. Volume 10. Springer International Publishing; Berlin, Germany: 2015. pp. 973–978. [Google Scholar]
- 17.Collins A., Yao Y. Applied Computational Genomics. Springer; Berlin/Heidelberg, Germany: 2018. Machine learning approaches: Data integration for disease prediction and prognosis; pp. 137–141. [Google Scholar]
- 18.Ossowska A., Kusiak A., Świetlik D. Artificial Intelligence in Dentistry—Narrative Review. Int. J. Environ. Res. Public Health. 2022;19:3449. doi: 10.3390/ijerph19063449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geetha V., Aprameya K., Hinduja D.M. Dental caries diagnosis in digital radiographs using back-propagation neural network. Health Inf. Sci. Syst. 2020;8:1–14. doi: 10.1007/s13755-019-0096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kositbowornchai S., Plermkamon S., Tangkosol T. Performance of an artificial neural network for vertical root fracture detection: An ex vivo study. Dental Traumatol. 2013;29:151–155. doi: 10.1111/j.1600-9657.2012.01148.x. [DOI] [PubMed] [Google Scholar]
- 21.Amasya H., Yildirim D., Aydogan T., Kemaloglu N., Orhan K. Cervical vertebral maturation assessment on lateral cephalometric radiographs using artificial intelligence: Comparison of machine learning classifier models. Dentomaxillofac. Radiol. 2020;49:20190441. doi: 10.1259/dmfr.20190441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budiman J.A. Neural network in developing software for Indentifying arch form. Int. J. Artif. Intell. Appl. 2013;4:1–11. doi: 10.5121/ijaia.2013.4301. [DOI] [Google Scholar]
- 23.Papantonopoulos G., Takahashi K., Bountis T., Loos B.G. Artificial neural networks for the diagnosis of aggressive periodontitis trained by immunologic parameters. PLoS ONE. 2014;9:e89757. doi: 10.1371/journal.pone.0089757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi H.I., Jung S.K., Baek S.H., Lim W.H., Ahn S.J., Yang I.H., Kim T.W. Artificial intelligent model with neural network machine learning for the diagnosis of orthognathic surgery. J. Craniofac. Surg. 2019;30:1986–1989. doi: 10.1097/SCS.0000000000005650. [DOI] [PubMed] [Google Scholar]
- 25.Cui Q., Chen Q., Liu P., Liu D., Wen Z. Clinical decision support model for tooth extraction therapy derived from electronic dental records. J. Prosthet. Dent. 2021;126:83–90. doi: 10.1016/j.prosdent.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Javed S., Zakirulla M., Baig R.U., Asif S., Meer A.B. Development of artificial neural network model for prediction of post-streptococcus mutans in dental caries. Comput. Methods Progr. Biomed. 2020;186:105198. doi: 10.1016/j.cmpb.2019.105198. [DOI] [PubMed] [Google Scholar]
- 27.Iwasaki H. Bayesian belief network analysis applied to determine the progression of temporomandibular disorders using MRI. Dentomaxillofac. Radiol. 2015;44:20140279. doi: 10.1259/dmfr.20140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bas B., Ozgonenel O., Ozden B., Bekcioglu B., Bulut E., Kurt M. Use of artificial neural network in differentiation of subgroups of temporomandibular internal derangements: A preliminary study. J. Oral Maxillofac. Surg. 2012;70:51–59. doi: 10.1016/j.joms.2011.03.069. [DOI] [PubMed] [Google Scholar]
- 29.Li S., Guo Y., Pang Z., Song W., Hao A., Xia B., Qin H. Automatic Dental Plaque Segmentation Based on Local-to-Global Features Fused Self-Attention Network. IEEE J. Biomed. Health Inform. 2022;26:2240–2251. doi: 10.1109/JBHI.2022.3141773. [DOI] [PubMed] [Google Scholar]
- 30.Li W., Chen Y., Sun W., Brown M., Zhang X., Wang S., Miao L. A gingivitis identification method based on contrast-limited adaptive histogram equalization, gray-level co-occurrence matrix, and extreme learning machine. Int. J. Imaging Syst. Technol. 2019;29:77–82. doi: 10.1002/ima.22298. [DOI] [Google Scholar]
- 31.Ghaedi L., Gottlieb R., Sarrett D.C., Ismail A., Belle A., Najarian K., Hargraves R.H. An automated dental caries detection and scoring system for optical images of tooth occlusal surface; Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; Chicago, IL, USA. 26–30 August 2014; pp. 1925–1928. [DOI] [PubMed] [Google Scholar]
- 32.Berdouses E.D., Koutsouri G.D., Tripoliti E.E., Matsopoulos G.K., Oulis C.J., Fotiadis D.I. A computer-aided automated methodology for the detection and classification of occlusal caries from photographic color images. Comput. Biol. Med. 2015;62:119–135. doi: 10.1016/j.compbiomed.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 33.Chen S., Wang L., Li G., Wu T.H., Diachina S., Tejera B., Kwon J.J., Lin F.C., Lee Y.T., Xu T., et al. Machine learning in orthodontics: Introducing a 3D auto-segmentation and auto-landmark finder of CBCT images to assess maxillary constriction in unilateral impacted canine patients. Angle Orthod. 2020;90:77–84. doi: 10.2319/012919-59.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niño-Sandoval T.C., Perez S.V.G., Gonzalez F.A., Jaque R.A., Infante-Contreras C. An automatic method for skeletal patterns classification using craniomaxillary variables on a Colombian population. Forens. Sci. Int. 2016;261:159-e1. doi: 10.1016/j.forsciint.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 35.Yu X., Liu B., Pei Y., Xu T. Evaluation of facial attractiveness for patients with malocclusion: A machine-learning technique employing Procrustes. Angle Orthod. 2014;84:410–416. doi: 10.2319/071513-516.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orhan K., Driesen L., Shujaat S., Jacobs R., Chai X. Development and validation of a magnetic resonance imaging-based machine learning model for TMJ pathologies. BioMed Res. Int. 2021;2021:6656773. doi: 10.1155/2021/6656773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riri H., Ed-Dhahraouy M., Elmoutaouakkil A., Beni-Hssane A., Bourzgui F. Extracted features based multi-class classification of orthodontic images. Int. J. Electr. Comput. Eng. 2020;10:3558–3567. doi: 10.11591/ijece.v10i4.pp3558-3567. [DOI] [Google Scholar]
- 38.Suhail Y., Upadhyay M., Chhibber A. Machine learning for the diagnosis of orthodontic extractions: A computational analysis using ensemble learning. Bioengineering. 2020;7:55. doi: 10.3390/bioengineering7020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee S.J., Chung D., Asano A., Sasaki D., Maeno M., Ishida Y., Kobayashi T., Kuwajima Y., Da Silva J.D., Nagai S. Diagnosis of Tooth Prognosis Using Artificial Intelligence. Diagnostics. 2022;12:1422. doi: 10.3390/diagnostics12061422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdalla-Aslan R., Yeshua T., Kabla D., Leichter I., Nadler C. An artificial intelligence system using machine-learning for automatic detection and classification of dental restorations in panoramic radiography. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020;130:593–602. doi: 10.1016/j.oooo.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Lee C.T., Kabir T., Nelson J., Sheng S., Meng H.W., Van Dyke T.E., Walji M.F., Jiang X., Shams S. Use of the deep learning approach to measure alveolar bone level. J. Clin. Periodontol. 2022;49:260–269. doi: 10.1111/jcpe.13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim Y., Lee K.J., Sunwoo L., Choi D., Nam C.M., Cho J., Kim J., Bae Y.J., Yoo R.E., Choi B.S., et al. Deep Learning in Diagnosis of Maxillary Sinusitis Using Conventional Radiography. Investig. Radiol. 2019;54:7–15. doi: 10.1097/RLI.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 43.Lee J.H., Kim D.H., Jeong S.N. Diagnosis of cystic lesions using panoramic and cone beam computed tomographic images based on deep learning neural network. Oral Dis. 2020;26:152–158. doi: 10.1111/odi.13223. [DOI] [PubMed] [Google Scholar]
- 44.Khan H.A., Haider M.A., Ansari H.A., Ishaq H., Kiyani A., Sohail K., Muhammad M., Khurram S.A. Automated feature detection in dental periapical radiographs by using deep learning. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021;131:711–720. doi: 10.1016/j.oooo.2020.08.024. [DOI] [PubMed] [Google Scholar]
- 45.Duong D.Q., Nguyen K.C.T., Kaipatur N.R., Lou E.H.M., Noga M., Major P.W., Punithakumar K., Le L.H. Fully Automated Segmentation of Alveolar Bone Using Deep Convolutional Neural Networks from Intraoral Ultrasound Images; Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); Berlin, Germany. 23–27 July 2019; pp. 6632–6635. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen K., Duong D., Almeida F., Major P., Kaipatur N., Pham T., Lou E., Noga M., Punithakumar K., Le L. Alveolar Bone Segmentation in Intraoral Ultrasonographs with Machine Learning. J. Dental Res. 2020;99:1054–1061. doi: 10.1177/0022034520920593. [DOI] [PubMed] [Google Scholar]
- 47.Pauwels R., Brasil D.M., Yamasaki M.C., Jacobs R., Bosmans H., Freitas D.Q., Haiter-Neto F. Artificial intelligence for detection of periapical lesions on intraoral radiographs: Comparison between convolutional neural networks and human observers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021;131:610–616. doi: 10.1016/j.oooo.2021.01.018. [DOI] [PubMed] [Google Scholar]
- 48.Orhan K., Bayrakdar I.S., Ezhov M., Kravtsov A., Özyürek T. Evaluation of artificial intelligence for detecting periapical pathosis on cone-beam computed tomography scans. Int. Endod. J. 2020;53:680–689. doi: 10.1111/iej.13265. [DOI] [PubMed] [Google Scholar]
- 49.Fukuda M., Inamoto K., Shibata N., Ariji Y., Yanashita Y., Kutsuna S., Nakata K., Katsumata A., Fujita H., Ariji E. Evaluation of an artificial intelligence system for detecting vertical root fracture on panoramic radiography. Oral Radiol. 2019;36:337–343. doi: 10.1007/s11282-019-00409-x. [DOI] [PubMed] [Google Scholar]
- 50.Ekert T., Krois J., Meinhold L., Elhennawy K., Emara R., Golla T., Schwendicke F. Deep Learning for the Radiographic Detection of Apical Lesions. J. Endod. 2019;45:917–922.e5. doi: 10.1016/j.joen.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 51.Bayraktar Y., Ayan E. Diagnosis of interproximal caries lesions with deep convolutional neural network in digital bitewing radiographs. Clin. Oral Investig. 2021;26:623–632. doi: 10.1007/s00784-021-04040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hiraiwa T., Ariji Y., Fukuda M., Kise Y., Nakata K., Katsumata A., Fujita H., Ariji E. A deep-learning artificial intelligence system for assessment of root morphology of the mandibular first molar on panoramic radiography. Dentomaxillofac. Radiol. 2019;48:20180218. doi: 10.1259/dmfr.20180218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Casalegno F., Newton T., Daher R., Abdelaziz M., Lodi-Rizzini A., Schürmann F., Krejci I., Markram H. Caries Detection with Near-Infrared Transillumination Using Deep Learning. J. Dental Res. 2019;98:1227–1233. doi: 10.1177/0022034519871884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee J.H., Jeong S.N. Efficacy of deep convolutional neural network algorithm for the identification and classification of dental implant systems, using panoramic and periapical radiographs. Medicine. 2020;99:e20787. doi: 10.1097/MD.0000000000020787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hung K.F., Ai Q.Y.H., King A.D., Bornstein M.M., Wong L.M., Leung Y.Y. Automatic detection and segmentation of morphological changes of the maxillary sinus mucosa on cone-beam computed tomography images using a three-dimensional convolutional neural network. Clin. Oral Investig. 2022;26:3987–3998. doi: 10.1007/s00784-021-04365-x. [DOI] [PubMed] [Google Scholar]
- 56.Kuwana R., Ariji Y., Fukuda M., Kise Y., Nozawa M., Kuwada C., Muramatsu C., Katsumata A., Fujita H., Ariji E. Performance of deep learning object detection technology in the detection and diagnosis of maxillary sinus lesions on panoramic radiographs. Dentomaxillofac. Radiol. 2020;50:20200171. doi: 10.1259/dmfr.20200171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murata M., Ariji Y., Ohashi Y., Kawai T., Fukuda M., Funakoshi T., Kise Y., Nozawa M., Katsumata A., Fujita H., et al. Deep-learning classification using convolutional neural network for evaluation of maxillary sinusitis on panoramic radiography. Oral Radiol. 2018;35:301–307. doi: 10.1007/s11282-018-0363-7. [DOI] [PubMed] [Google Scholar]
- 58.Choi E., Kim D., Lee J.Y., Park H.K. Artificial intelligence in detecting temporomandibular joint osteoarthritis on orthopantomogram. Sci. Rep. 2021;11:10246. doi: 10.1038/s41598-021-89742-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeyaraj P.R., Nadar E.R.S. Computer-assisted medical image classification for early diagnosis of oral cancer employing deep learning algorithm. J. Cancer Res. Clin. Oncol. 2019;145:829–837. doi: 10.1007/s00432-018-02834-7. [DOI] [PubMed] [Google Scholar]
- 60.Song Y., Qiao X., Iwamoto Y., wei Chen Y. Automatic Cephalometric Landmark Detection on X-ray Images Using a Deep-Learning Method. Appl. Sci. 2020;10:2547. doi: 10.3390/app10072547. [DOI] [Google Scholar]
- 61.Park J.H., Hwang H.W., Moon J.H., Yu Y., Kim H., Her S.B., Srinivasan G., Aljanabi M.N.A., Donatelli R.E., Lee S.J. Automated identification of cephalometric landmarks:Comparisons between the latest deep-learning methods YOLOV3 and SSD. Angle Orthod. 2019;89:903–909. doi: 10.2319/022019-127.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee K.S., Ryu J.J., Jang H.S., Lee D.Y., Jung S.K. Deep Convolutional Neural Networks Based Analysis of Cephalometric Radiographs for Differential Diagnosis of Orthognathic Surgery Indications. Appl. Sci. 2020;10:2124. doi: 10.3390/app10062124. [DOI] [Google Scholar]
- 63.Kim J., Lee H.S., Song I.S., Jung K.H. DeNTNet: Deep Neural Transfer Network for the detection of periodontal bone loss using panoramic dental radiographs. Sci. Rep. 2019;9:17615. doi: 10.1038/s41598-019-53758-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li H., Zhou J., Zhou Y., Chen J., Gao F., Xu Y., Gao X. Automatic and interpretable model for periodontitis diagnosis in panoramic radiographs; Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention; Lima, Peru. 4–8 October 2020; pp. 2454–2463. [Google Scholar]
- 65.Kim H., Shim E., Park J., Kim Y.J., Lee U., Kim Y. Web-based fully automated cephalometric analysis by deep learning. Comput. Methods Progr. Biomed. 2020;194:105513. doi: 10.1016/j.cmpb.2020.105513. [DOI] [PubMed] [Google Scholar]
- 66.Gilmour L., Ray N. Locating Cephalometric X-Ray Landmarks with Foveated Pyramid Attention; Proceedings of the Third Conference on Medical Imaging with Deep Learning; Montreal, QC, Canada. 6–8 July 2020; pp. 262–276. [Google Scholar]
- 67.Zhong Z., Li J., Zhang Z., Jiao Z., Gao X. An Attention-Guided Deep Regression Model for Landmark Detection in Cephalograms. arXiv. 20191906.07549 [Google Scholar]
- 68.Makaremi M., Lacaule C., Mohammad-Djafari A. Deep Learning and Artificial Intelligence for the Determination of the Cervical Vertebra Maturation Degree from Lateral Radiography. Entropy. 2019;21:1222. doi: 10.3390/e21121222. [DOI] [Google Scholar]
- 69.Yu H., Cho S., Kim M., Kim W., Kim J., Choi J. Automated Skeletal Classification with Lateral Cephalometry Based on Artificial Intelligence. J. Dental Res. 2020;99:249–256. doi: 10.1177/0022034520901715. [DOI] [PubMed] [Google Scholar]
- 70.Kök H., Acilar A.M., İzgi M.S. Usage and comparison of artificial intelligence algorithms for determination of growth and development by cervical vertebrae stages in orthodontics. Prog. Orthod. 2019;20:41. doi: 10.1186/s40510-019-0295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takahashi T., Nozaki K., Gonda T., Ikebe K. A system for designing removable partial dentures using artificial intelligence. Part 1. Classification of partially edentulous arches using a convolutional neural network. J. Prosthodont. Res. 2021;65:115–118. doi: 10.2186/jpr.JPOR_2019_354. [DOI] [PubMed] [Google Scholar]
- 72.Xu X., Liu C., Zheng Y. 3D Tooth Segmentation and Labeling Using Deep Convolutional Neural Networks. IEEE Trans. Vis. Comput. Graph. 2019;25:2336–2348. doi: 10.1109/TVCG.2018.2839685. [DOI] [PubMed] [Google Scholar]
- 73.Sedik A., Iliyasu A.M., El-Rahiem B.A., Samea M.E.A., Abdel-Raheem A., Hammad M., Peng J., El-Samie F.E.A., El-Latif A.A.A. Deploying Machine and Deep Learning Models for Efficient Data-Augmented Detection of COVID-19 Infections. Viruses. 2020;12:769. doi: 10.3390/v12070769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang G., Jafari A.H. Enhanced balancing GAN: Minority-class image generation. arXiv. 2021 doi: 10.1007/s00521-021-06163-8.2011.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krois J., Cantu A.G., Chaurasia A., Patil R., Chaudhari P.K., Gaudin R., Gehrung S., Schwendicke F. Generalizability of deep learning models for dental image analysis. Sci. Rep. 2021;11:6102. doi: 10.1038/s41598-021-85454-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grischke J., Johannsmeier L., Eich L., Griga L., Haddadin S. Dentronics: Towards robotics and artificial intelligence in dentistry. Dental Mater. 2020;36:765–778. doi: 10.1016/j.dental.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 77.Fahim S., Maqsood A., Das G., Ahmed N., Saquib S., Lal A., Khan A.A.G., Alam M.K. Augmented Reality and Virtual Reality in Dentistry: Highlights from the Current Research. Appl. Sci. 2022;12:3719. doi: 10.3390/app12083719. [DOI] [Google Scholar]
- 78.Monterubbianesi R., Tosco V., Vitiello F., Orilisi G., Fraccastoro F., Putignano A., Orsini G. Augmented, Virtual and Mixed Reality in Dentistry: A Narrative Review on the Existing Platforms and Future Challenges. Appl. Sci. 2022;12:877. doi: 10.3390/app12020877. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available from the authors on request.