Abstract
Transcriptional activation of vascular adhesion molecule expression, a major component of an inflammatory response, is regulated, in part, by the nuclear factor-κB/Rel (NF-κB) family of transcription factors. We therefore determined whether Trypanosoma cruzi infection of endothelial cells resulted in the activation of NF-κB and the induction or increased expression of adhesion molecules. Human umbilical vein endothelial cells (HUVEC) were infected with trypomastigotes of the Tulahuen strain of T. cruzi. Electrophoretic mobility shift assays with an NF-κB-specific oligonucleotide and nuclear extracts from T. cruzi-infected HUVEC (6 to 48 h postinfection) detected two major shifted complexes. Pretreatment with 50× cold NF-κB consensus sequence abolished both gel-shifted complexes while excess SP-1 consensus sequence had no effect. These data indicate that nuclear extracts from T. cruzi-infected HUVEC specifically bound to the NF-κB consensus DNA sequence. Supershift analysis revealed that the gel-shifted complexes were comprised of p65 (RelA) and p50 (NF-κB1). Northern blot analyses demonstrated both the induction of vascular cell adhesion molecule 1 and E-selectin and the upregulation of intercellular adhesion molecule 1 mRNA in HUVEC infected with T. cruzi. Immunocytochemical staining confirmed adhesion molecule expression in response to T. cruzi infection. These findings are consistent with the hypothesis that the activation of the NF-κB pathway in endothelial cells associated with T. cruzi infection may be an important factor in the inflammatory response and subsequent vascular injury and endothelial dysfunction that lead to chronic cardiomyopathy.
Chagas’ disease, a consequence of infection with the hemoflagellate parasite Trypanosoma cruzi, is a major cause of acute and chronic myocarditis and cardiomyopathy in areas of endemicity in Latin America (51). The pathogenesis of the cardiac damage associated with this infection is multifactorial. The clinical manifestations of T. cruzi infection may be the result of focal ischemia (13, 34, 50), autoimmune responses (18), and direct parasite invasion of cells of the myocardium, all of which may promote myocardial inflammation (31, 54, 62). Recent studies have underscored the primary role of inflammation in the pathogenesis of chagasic heart disease. The inflammatory response is composed of lymphocytes (predominately CD8+) (46), monocytes, macrophages, and eosinophils. This inflammatory process has been associated with the expression of cytokines and inducible nitric oxide synthase (3, 16). In addition, vascular adhesion molecules have been described both for the heart and for sera obtained from infected mice and humans (19, 20, 31, 61). More recently, Sunnemark et al. (47) described aortic vasculitis in T. cruzi-infected mice which was associated with inflammation and expression of cytokines and the adhesion molecule intercellular adhesion molecule 1 (ICAM-1).
The vascular endothelium is an important target of parasite invasion (48). T. cruzi infection of cultured endothelial cells results in expression and/or upregulation of important vasoactive molecules such as endothelin 1 (57, 59) and proinflammatory cytokines including interleukin-1β (IL-1β) and IL-6 (49, 52, 59). Murine T. cruzi infection is also associated with circulating tumor necrosis factor alpha (TNF-α) (53) and thromboxane A2 (48). All of these factors have been implicated in T. cruzi-associated microvascular compromise.
Activation of the nuclear factor κB/Rel (NF-κB) family of dimeric transcription factor complexes is regarded as an important initial event in the vascular response to a variety of infectious agents (10, 14, 17, 36, 37, 43, 60), toxins, cytokines, growth factors, and oxidant stress (5, 6). The inactive form of the best-characterized NF-κB heterodimer, consisting of a complex of p50 (NF-κB1) and p65 (RelA subunits), is retained in the cytoplasm either by association with IκBα or by association of the p65 subunit with p105, a precursor of p50. Multiple signal transduction pathways lead to phosphorylation, polyubiquitination, and degradation of IκBα or p105. Heterodimeric NF-κB enters and accumulates in the nucleus and contributes to the transcriptional activation of many genes relevant to endothelial pathophysiology, including those encoding vascular adhesion molecules (27, 28, 29, 38, 56).
Modulation of the transcriptional activity of NF-κB is critical to endothelial cell activation and the associated inflammatory response. Leukocyte accumulation at sites of local injury or endothelial cell infection is dependent on the interaction of circulating leukocytes with vascular adhesion molecules. The selectin family of adhesion molecules, including E-selectin (1, 2), mediates the rolling and initial tethering of leukocytes to vascular endothelium while firm adhesion and transmigration into subendothelial tissue are mediated by members of the immunoglobulin superfamily (44, 55), including vascular cell adhesion molecule 1 (VCAM-1) (4, 12, 32, 33) and ICAM-1 (45). NF-κB-like binding elements are present in the promoter regions of the E-selectin, VCAM-1, and ICAM-1 genes and play a pivotal role in the transcriptional regulation of these adhesion molecules (15, 25, 56). We therefore determined whether the inflammatory response elicited by T. cruzi infection is characterized by NF-κB activation and induction or increased expression of E-selectin, VCAM-1, and ICAM-1 in endothelial cells.
In the present study, we demonstrated that infection of cultured human umbilical vein endothelial cells (HUVEC) with T. cruzi is associated with activation of NF-κB and induction of endothelial cell adhesion molecule expression. These studies may provide a cellular basis for the inflammatory response to T. cruzi infection that is important in the pathogenesis of chagasic cardiomyopathy.
MATERIALS AND METHODS
Infection and TNF-α treatment of endothelial cell cultures.
Trypomastigotes of T. cruzi (Tulahuen strain) were harvested from the supernatants of infected myoblasts (35). HUVEC were isolated, cultured, and infected at a multiplicity of infection of 1.5 to 2.0:1 as previously described (49). The HUVEC had a characteristic cobblestone appearance and could be stained with antibody to von Willebrand factor (DAKO Corporation, Carpinteria, Calif.). The percent parasitism was determined by examination of fixed culture plates stained with May-Grunwald-Giemsa stain: parasitism was approximately <1% at 1 to 6 h, 10% at 24 h, 20 to 40% at 48 h, and >80% at 72 h postinfection. As a positive control for adhesion molecule expression and NF-κB activation, human recombinant TNF-α (Genzyme Diagnostics, Cambridge, Mass.) was added to uninfected cultured cells at a final concentration of 100 U/ml.
Nuclear isolation and extraction.
Extracts of infected and uninfected HUVEC were prepared as described by Read et al. (26). Briefly, cell monolayers (3 × 106 to 5 × 106 cells) were harvested by scraping, washed in cold phosphate-buffered saline (PBS), and incubated in 100 μl of buffer A (10 mM HEPES [pH 8.0], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, 200 mM sucrose, 0.5 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin per ml, 1 μg of aprotinin per ml, and 0.5% NP-40) for 10 min at 4°C. The crude nuclei released by lysis were collected by microcentrifugation, and the nuclear pellet was rinsed once in buffer A and resuspended in 100 μl of buffer B (20 mM HEPES [pH 8.0], 20% glycerol, 0.1 M KCl, 0.2 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 0.5 mM dithiothreitol, and 1 μg each of leupeptin and aprotinin per ml). Nuclei were sonicated for 10 s at 15% power output (Virsonic cell disrupter; Virtis, Gardner, N.Y.) and clarified by microcentrifugation for 30 s. The resulting supernatants contained 1 to 2 mg of protein per ml by Bio-Rad assay (Bio-Rad, Richmond, Calif.) with bovine serum albumin as the standard. Nuclear extracts were frozen on dry ice and stored at −80°C.
Electrophoretic mobility shift (gel shift) assay.
Assays were performed with the gel shift assay system (Promega, Valencia, Calif.) according to the manufacturer’s protocol, with 5 to 10 μg of nuclear protein. Sequences of double-stranded consensus oligonucleotides used in gel shift reactions were as follows: NF-κB (Promega), 5′-AGT TGA GGG GAC TTT CCC AGG C-3′; SP-1 (Promega), 5′-ATT CGA TCG GGG CGG GGC GAG C-3′. Probe labeling was carried out as specified by the manufacturer with [γ-32P]ATP (3,000 Ci/mmol; 10 mCi/ml) (Amersham, Arlington Heights, Ill.). Specificity studies were performed with a 50-fold molar excess of unlabeled oligonucleotide added to the reaction mixtures prior to the addition of radiolabeled oligonucleotides. Reaction mixtures were analyzed on 5% nondenatured polyacrylamide gels with 0.5× TBE (89 mM Tris-HCl [pH 8.0], 89 mM boric acid, 2 mM EDTA) as the running buffer. The gels were electrophoresed at 100 V for 3 h, dried (gel dryer), and subjected to autoradiographic exposure for 12 to 48 h.
Electrophoretic mobility supershift assays.
Polyclonal antibodies targeted to p50 and p65 (100 μg/0.1 ml) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Prior to the addition of radiolabeled oligonucleotide probe, 2 μg of antibody per gel shift reaction mixture was added and the mixtures were incubated at room temperature for 20 min. Each reaction mixture was analyzed by gel shift assays as described above.
RNA preparation.
HUVEC monolayers were washed briefly with cold PBS and immediately lysed with Trizol reagent. Total RNA was extracted as recommended by the manufacturer’s protocol (GIBCO BRL, Grand Island, N.Y.). For protection from RNase activity, the final RNA pellets were solubilized in Formazol (Molecular Research Center, Inc., Cincinnati, Ohio).
Northern blot analysis.
A high-efficiency hybridization system was purchased from Molecular Research Center, Inc. Northern blot analysis was performed as specified by the manufacturer. Briefly, equal amounts of total RNA (15 μg) were incubated in formaldehyde reaction solution at 55°C for 15 min and loaded onto 1% agarose-formaldehyde gels. After electrophoresis, RNA was transferred to nitrocellulose filters. Filters were prehybridized at 42°C for 6 to 12 h and hybridized at 42°C for 24 to 48 h in high-efficiency hybridization solution with appropriate random-primed labeled denatured cDNA probes for human VCAM-1, E-selectin (W. Newman, Otsuka Pharmaceuticals, Rockville, Md.), and ICAM-1 (Timothy Springer, Center for Blood Research, Harvard University, Boston, Mass.) at 1 × 106 to 3 × 106 dpm/ml. Hybridization with 18S (rRNA) cDNA was utilized to verify the loading equivalency of each lane. The filters were washed, and autoradiography was performed with X-ray film and an intensifying screen at −70°C.
Immunocytochemistry.
Fourth- or fifth-passage HUVEC were cultured in gelatin-coated 24-well plates for 3 days. Trypomastigotes were washed in PBS (pH 7.2) and resuspended in endothelial cell medium. Approximately 1.25 × 106 trypomastigotes were used to infect cells in each well. Supernatants from uninfected myoblasts were used for sham infections. At 24 and 48 h postinfection, the plates were washed gently three times with PBS and then fixed for 8 min in ice-cold methanol and 0.8% H2O2. After washing in PBS, wells were incubated at room temperature with blocking solution (PBS–4% fetal bovine serum [GIBCO]) for 30 min. Primary antibodies in blocking solution were added to the well and incubated overnight at 4°C. The primary monoclonal antibodies were used at the following dilutions: mouse anti-human VCAM-1 (immunoglobulin G1 [IgG1]) (Becton Dickinson, San Jose, Calif.) (1:500), mouse anti-human E-selectin or CD62E (ELAM-1) (IgG1) (1:500) (Becton Dickinson), human anti-ICAM-1 antibody (1:500) (DAKO Corporation), and anti-human von Willebrand factor (IgG1) (1:100) (DAKO Corporation). In addition, purified IgG1 mouse myeloma protein (Organon Teknika Corp., Durham, N.C.) was used as an isotype-matched negative control. Treatment of HUVEC with recombinant human TNF-α (100 U/well) (Genzyme Diagnostics) was used as a positive control for the induction or increased expression of adhesion molecules. After incubation with primary antibody, the wells were washed twice with PBS and incubated with biotinylated anti-mouse IgG (heavy plus light chains), avidin-biotin-coupled peroxidase (Vectastain ABC kit; Vector Laboratories, Inc., Burlingame, Calif.), and diaminobenzidine (peroxidase substrate kit; Vector Laboratories).
Statistical analysis.
The results of Northern blot analyses of adhesion molecule expression by HUVEC were quantified by densitometry and normalized to the corresponding 18S rRNA signal and expressed as a ratio. Data from three separate experiments were then analyzed by Student’s t test and were plotted as the means ± standard errors of the means.
RESULTS
T. cruzi infection of HUVEC upregulates or induces ICAM-1, VCAM-1, and E-selectin mRNA expression.
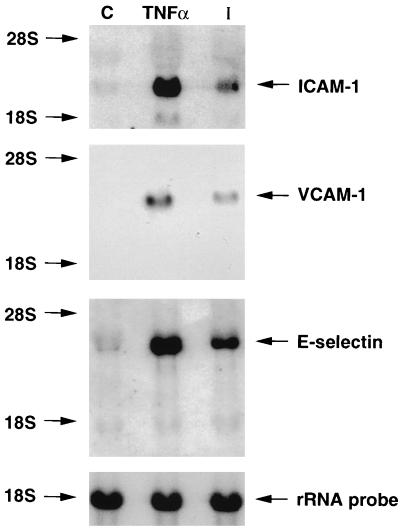
Northern blot analyses of adhesion molecule expression of HUVEC that were uninfected, TNF-α treated (24 h) (uninfected), and infected for 24 h are shown in Fig. 1. ICAM-1 was constitutively expressed in untreated HUVEC and was upregulated in infected and TNF-α-treated cultures (Fig. 1, top panel). VCAM-1 mRNA expression was undetectable in uninfected cells and was also significantly induced after infection with T. cruzi or TNF-α treatment (Fig. 1, middle panel). There was a significant increase in E-selectin mRNA expression in both T. cruzi-infected and TNF-α-treated HUVEC, while uninfected cells expressed a low basal level of E-selectin message (Fig. 1, bottom panel). These data indicate that T. cruzi infection of HUVEC for 24 h upregulates or induces ICAM-1, VCAM-1, and E-selectin mRNA expression.
FIG. 1.
T. cruzi infection induces or upregulates ICAM-1, VCAM-1, and E-selectin mRNA. Northern blot analyses of adhesion molecule expression by HUVEC that were uninfected, TNF-α treated, or infected for 24 h are shown (see Materials and Methods). ICAM-1 mRNA was constitutively expressed in untreated HUVEC and was upregulated in infected and TNF-α-treated cultures (top panel). VCAM-1 mRNA expression was undetectable in uninfected HUVEC and was induced after infection or TNF-α treatment (middle panel). There was a significant increase in E-selectin mRNA expression in both T. cruzi-infected and TNF-α-treated HUVEC, while uninfected cells expressed a low basal level of E-selectin message (bottom panel). An 18S rRNA probe was utilized to normalize the total RNA loading equivalency of each lane. Data shown are representative of three separate experiments. Lane C, uninfected cells as control; lane TNF-α, HUVEC treated with TNF-α; lane I, HUVEC infected with T. cruzi.
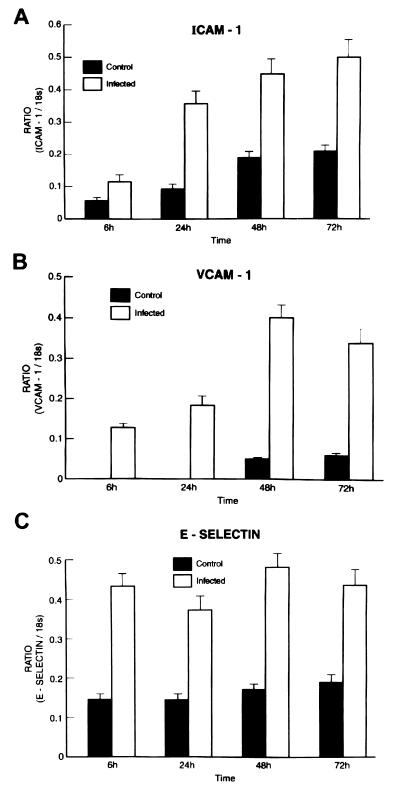
Time course of ICAM-1, VCAM-1, and E-selectin mRNA expression by T. cruzi-infected HUVEC.
Northern blot analyses of adhesion molecule expression by HUVEC that were uninfected or infected for 6, 24, 48, and 72 h were performed. Data from three separate blots were quantified by densitometry, normalized to the 18S rRNA signal, and expressed as a ratio as shown in Fig. 2. Student t test analysis indicated that the three adhesion molecules were significantly induced or upregulated from 6 to 72 h postinfection (Fig. 2). ICAM-1, constitutively expressed in uninfected cells, was upregulated from 6 to 72 h after infection (Fig. 2A). VCAM-1 was induced after 6 h, and message levels increased up to 72 h postinfection (Fig. 2B), the last time point analyzed. E-selectin expression was increased 6 h postinfection and remained upregulated until 72 h (Fig. 2C). These data suggest that infection of HUVEC by T. cruzi causes persistently elevated expression of adhesion molecules.
FIG. 2.
Time course of ICAM-1, VCAM-1, and E-selectin mRNA expression with T. cruzi infection. Northern blot analyses of adhesion molecule expression by HUVEC that were uninfected or infected for 6, 24, 48, and 72 h. Data from three separate blots were quantified by densitometry and normalized to the 18S rRNA signal and expressed as a ratio. Student t test analysis indicated that the three adhesion molecules were significantly induced or upregulated from 6 to 72 h postinfection (P < 0.001) (see Materials and Methods). (A) ICAM-1 was upregulated from 6 to 72 h and was constitutively expressed in uninfected cells. (B) VCAM-1 was induced from 6 to 72 h postinfection. (C) E-selectin expression was increased at 6 h postinfection and remained upregulated until 72 h, the last time point analyzed.
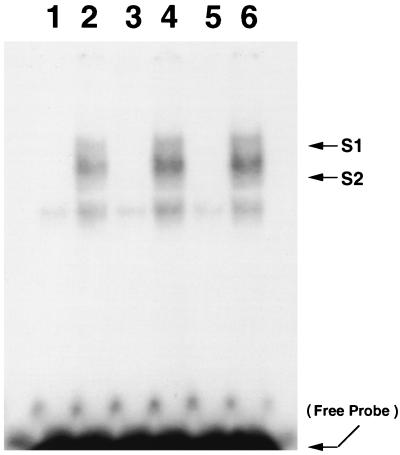
T. cruzi infection activates NF-κB in HUVEC.
NF-κB was assayed in nuclear extracts of infected HUVEC by electrophoretic mobility shift assays with a 32P-labeled, double-stranded consensus NF-κB oligonucleotide corresponding to the κB binding domain of the murine κ light chain gene enhancer (39). After 6 h of infection, two major gel-shifted protein-DNA complexes were evident, a faster-migrating lower complex (S2) and an upper complex (S1). Both of these bands were negligible in uninfected HUVEC. A time course study revealed that these complexes were also evident in nuclear protein extracts from HUVEC 24 and 48 h postinfection (Fig. 3).
FIG. 3.
T. cruzi infection activates NF-κB in HUVEC. NF-κB was assayed in nuclear extracts of HUVEC by electrophoretic mobility shift assays with a 32P-labeled, double-stranded consensus NF-κB oligonucleotide. Lanes 1, 3, and 5 represent nuclear extracts obtained from uninfected HUVEC after 6, 24, and 48 h in culture, respectively. Lanes 2, 4, and 6 represent nuclear extracts obtained from HUVEC infected for 6, 24, and 48 h, respectively. Two shifted complexes appeared in the nuclear protein-DNA interaction from infected HUVEC (S1, shifted complex 1; S2, shifted complex 2). Both shifted complexes were undetectable in uninfected HUVEC. The arrow at the bottom indicates free NF-κB probe.
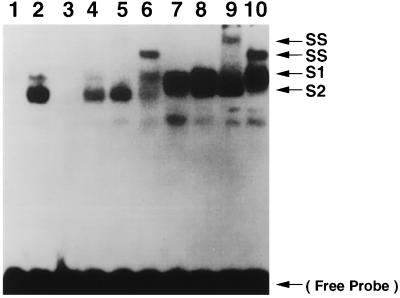
To confirm the specificity of nuclear protein binding to the NF-κB oligonucleotide, competition assays were performed (Fig. 4). Nuclear protein extracts from HUVEC at 6 h postinfection formed two major protein-DNA complexes (Fig. 4, lane 2) which were absent in uninfected cells (lane 1). Complex formation was specifically inhibited by incubation with a 50-fold molar excess of unlabeled NF-κB probe (Fig. 4, lane 3) but not by treatment with an unrelated oligonucleotide containing the SP-1 binding consensus sequence (lane 4). These data indicate that T. cruzi infection of HUVEC induces nuclear translocation of active NF-κB. To identify the subunit composition of NF-κB in the protein-DNA complexes induced after T. cruzi infection, supershift analyses were performed with polyclonal antibodies specific for p65 and p50. Changes in electrophoretic mobility resulting in supershifted complexes were detected with the addition of anti-p65 and anti-p50 to the reaction mixtures prior to the addition of the labeled NF-κB oligonucleotide. Anti-p65 induced a supershift of the upper protein-DNA complexes (Fig. 4, lane 5), while anti-p50 was specific for the lower protein-DNA complexes (lane 6). Nonimmunized rabbit serum did not cause any supershifted complexes (Fig. 4, lane 7). These data suggested that the upper complexes contain p65 and that the lower complexes contain p50. TNF-α-treated (5 min) HUVEC nuclear protein extract was used as a positive control and produced similarly shifted (Fig. 4, lane 8) and supershifted (lane 9, anti-p65, and lane 10, anti-p50) complexes.
FIG. 4.
Identification of the shifted complexes as a result of T. cruzi infection. To confirm the specificity of nuclear protein binding to the NF-κB oligonucleotide, competition assays were performed. Nuclear extracts from HUVEC at 6 h postinfection formed two major protein-DNA complexes (S1 and S2) (lane 2) which were absent in uninfected HUVEC (lane 1). Complex formation was specifically inhibited by incubation with a 50-fold molar excess of unlabeled NF-κB probe (lane 3) but not by a 50-fold molar excess treatment with an unrelated oligonucleotide containing the SP-1 binding consensus sequence (lane 4), indicating that these nuclear proteins specifically bound to the NF-κB consensus sequence. To identify the subunit composition of NF-κB in the protein-DNA complexes induced after infection, supershift (SS) analyses were performed with polyclonal antibodies specific for p65 and p50. Anti-p65 caused a supershift of S1 (lane 5), while anti-p50 induced a supershift of S2 (lane 6). Nonimmunized rabbit serum did not cause any supershifted complexes (lane 7). TNF-α-treated HUVEC nuclear protein extract was used as a positive control and produced similarly shifted (lane 8) and supershifted (lane 9, anti-p65 supershift; lane 10, anti-p50 supershift) complexes. Data are representative of three separate experiments. The arrow at the bottom indicates free NF-κB probe.
T. cruzi infection induces or upregulates E-selectin, VCAM-1, and ICAM-1 protein expression.
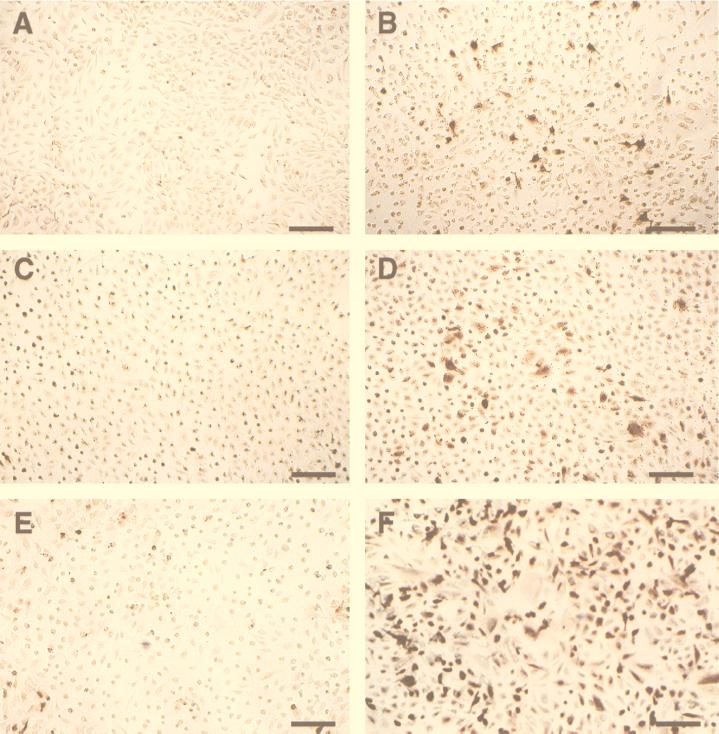
In order to examine the effects of T. cruzi infection on cell surface expression of E-selectin, VCAM-1, and ICAM-1, monoclonal antibodies specific for each adhesion molecule were used in an immunocytochemical analysis of infected HUVEC. Consistent with the results of Northern blot analysis, E-selectin protein was significantly upregulated in both T. cruzi-infected (Fig. 5B) and TNF-α-treated (4 h) HUVEC (data not shown). Untreated HUVEC were minimally reactive with the E-selectin antibody (Fig. 5A). Constitutive levels of ICAM-1 protein expression (Fig. 5C) were also upregulated with T. cruzi infection (Fig. 5D). Similarly, VCAM-1 was induced by T. cruzi infection (Fig. 5F), whereas normal reactivity was detectable on uninfected HUVEC (Fig. 5E). Untreated and infected HUVEC exhibited no reactivity with the isotype-matched negative-control IgG1 mouse myeloma protein (data not shown).
FIG. 5.
T. cruzi infection induces or upregulates E-selectin, VCAM-1, and ICAM-1 protein expression. Shown are photomicrographs of adhesion protein expression in infected HUVEC as demonstrated by immunocytochemistry. Uninfected HUVEC incubated with isotype-matched, negative-control, purified IgG1 mouse myeloma protein did not exhibit nonspecific staining (see Materials and Methods). Bar, 150 μm. (A) Uninfected HUVEC stained with anti-E-selectin antibody. There is minimal background staining and E-selectin expression. (B) HUVEC infected for 24 h and stained with anti-E-selectin antibody. E-selectin protein was upregulated. (C) Uninfected HUVEC stained with anti-ICAM-1 antibody. Cells constitutively expressed ICAM-1 protein. (D) HUVEC infected for 24 h and stained with anti-ICAM-1 antibody. ICAM-1 protein expression was upregulated. (E) Uninfected HUVEC stained with anti-VCAM-1 antibody. There is minimal VCAM-1 expression. (F) HUVEC infected for 24 h and stained with anti-VCAM antibody. VCAM-1 protein was upregulated.
DISCUSSION
We demonstrated that T. cruzi infection of endothelial cells is associated with activation of NF-κB and expression of the endothelial cell adhesion molecules E-selectin, VCAM-1, and ICAM-1. These findings suggest a possible mechanism for the recruitment of circulating leukocytes, a critical factor in the initiation of an inflammatory response, as a result of T. cruzi infection (50). The generation of an acute inflammatory response is important in the initial control of acute infection but may be detrimental if it persists for prolonged periods and may contribute to myocardial damage.
The vascular endothelium is an early target of T. cruzi invasion (48). We have demonstrated that infection of HUVEC results in alterations in host cell metabolism and signal transduction (23, 24, 48). In the murine model, infection causes vascular injury and altered function (13, 16, 49, 50, 52). Infection and ensuing injury to the vascular endothelium, as well as the ischemia-reperfusion damage to the heart, are important factors in the pathogenesis of chagasic heart disease. Amastigotes are not commonly found in endothelial cells in vivo on routine histopathology. This is most likely due to issues of timing and sampling.
In understanding the pathogenesis of Chagas’ disease, it is important to note that the infection is persistent throughout the lifetimes of patients and results in compromised microvasculature including vasospasm and decreased blood flow. During the chronic phase, antiparasitic therapy usually fails to attenuate the progression of the disease. Therefore, on the basis of observations made from experimental infections and human disease, we and others hypothesized that after the initial acute insult to the endothelium, endothelial cells undergo damage and regeneration. In other settings such as balloon angioplasty-induced endothelial removal (41) and Kawasaki disease (7), regenerated endothelial cells no longer function normally, resulting in endothelial dysfunction and cardiac pathology. Similarly, we believe that the upregulation of the inflammatory process and the subsequent ischemia after T. cruzi infection contribute to the development of chronic chagasic cardiomyopathy.
The mechanism of T. cruzi-associated expression of vascular adhesion molecules remains to be defined. We demonstrated previously that T. cruzi infection of HUVEC results in the expression of IL-1β and IL-6 (49). These cytokines are known to induce expression of vascular adhesion molecules. However, the primary event necessary for expression of vascular adhesion molecules requires the activation of NF-κB, which also plays a critical role in cytokine gene transcription and translation. Lockyer et al. (21) demonstrated that inhibition of NF-κB after transfection of a mutated IκB gene into human endothelial cells blocked the expression of adhesion molecules in response to TNF-α. Morishita et al. (22) introduced synthetic double-stranded oligodeoxynucleotides into rat hearts to block the nuclear translocation of NF-κB and found that the expression of cytokines and adhesion molecules was effectively inhibited during the ischemia-reperfusion event, thereby reducing the extent of myocardial infarction. Transfection of the same oligodeoxynucleotide into human endothelial cells also inhibited the expression of cytokines and adhesion molecules. These data indicate that NF-κB plays a pivotal role in the initiation of an inflammatory response. We believe that T. cruzi infection of HUVEC induces the activation of NF-κB, which leads to the production of IL-1β and IL-6. These cytokines also cause a positive feedback effect, thus further activating NF-κB.
Many extracellular signals trigger the activation of NF-κB by a number of signal transduction pathways. For example, TNF-α binds to its receptors and initiates second messenger and signaling cascades (42), resulting in the activation of the IκB kinase complexes including IKKα and IKKβ (8, 30, 61). These kinases are necessary for IκB phosphorylation and degradation and subsequent NF-κB activation. How parasite-endothelial cell interactions activate signaling pathways involved in NF-κB activation is unknown. The interaction and infection of endothelial cells with Rickettsia rickettsii also activate NF-κB (43) and induce the expression of adhesion molecules (9, 40). This organism shares many of the characteristics of T. cruzi, since they both invade and reside in endothelial cell cytoplasm. Currently, we are exploring alterations in intracellular signaling cascades during T. cruzi infection. The data presented in this report demonstrate that NF-κB is continuously activated from 6 to 48 h postinfection, indicating that parasitism can activate this pathway for extended periods. In addition, we also found that T. cruzi infection of endothelial cells (24) and vascular smooth muscle cells (23) activates phospholipase C, part of the signaling pathways involved in NF-κB activation (58). Infection with this parasite may also activate other kinases in host cells and cause the subsequent phosphorylation of the IκB subunit. Furthermore, T. cruzi is rich in secretory proteases (11) which may degrade the IκB subunit, thereby directly activating the NF-κB pathway. Currently, these cellular events are under investigation in our laboratory.
ACKNOWLEDGMENTS
This work was supported by a New Investigator Development Award from the American Heart Association, New York City Affiliate (H.H.); grants-in-aid from the American Heart Association (J.W.B. and H.H.); and Public Health Service grants AI-12770 (H.B.T.) AI-39454 (L.M.W.), and AI-41752 (M.W.).
REFERENCES
- 1.Bevilacqua M P, Pober J S, Mendrick D L, Cotran R S, Gimbrone M A. Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci USA. 1987;84:9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlos T, Kovach N, Schwartz B, Rosa M, Newman B, Wayner E, Benjamin C, Osborn L, Lobb R, Harlan J. Human monocytes bind to two cytokine-induced adhesive ligands on cultured human endothelial cells: endothelial-leukocyte adhesion molecule-1 and vascular cell adhesion molecule-1. Blood. 1991;77:2266–2271. [PubMed] [Google Scholar]
- 3.Chandrasekar B, Melby P C, Troyer D A, Coloston J T, Freeman G L. Temporal expression of pro-inflammatory cytokines and inducible nitric oxide synthase in experimental acute chagasic cardiomyopathy. Am J Pathol. 1998;152:925–934. [PMC free article] [PubMed] [Google Scholar]
- 4.Chuluyan H E, Issekutz A C. VLA-4 integrin can mediate CD11/CD18-independent transendothelial migration of human monocytes. J Clin Investig. 1993;92:2768–2777. doi: 10.1172/JCI116895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins T. Endothelial nuclear factor-κB and the initiation of the arteriosclerotic lesion. Lab Investig. 1993;68:499–508. [PubMed] [Google Scholar]
- 6.Collins T, Reed M A, Neish A S, Whitely M Z, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-κB and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 7.Dhillon R, Clarkson P, Donald A E, Powe A J, Nash M, Novelli V, Dillon M J, Deanfield J E. Endothelial dysfunction late after Kawasaki disease. Circulation. 1996;94:2103–2106. doi: 10.1161/01.cir.94.9.2103. [DOI] [PubMed] [Google Scholar]
- 8.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 9.Dignat-George F, Teysseire N, Mutin M, Bardin N, Lesaule G, Raoult D, Sampol J. Rickettsia conorii infection enhances vascular cell adhesion molecule-1 and intercellular adhesion molecule-1-dependent mononuclear cell adherence to endothelial cells. J Infect Dis. 1997;175:1142–1152. doi: 10.1086/520353. [DOI] [PubMed] [Google Scholar]
- 10.Dyer R B, Collaco C R, Niesel D W, Herzog N K. Shigella flexneri invasion of HeLa cells induces NF-κB DNA binding activity. Infect Immun. 1993;61:4427–4433. doi: 10.1128/iai.61.10.4427-4433.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eakin A E, Mills A A, Harth G, McKerrow J H, Craik C S. The sequence, organization, and expression of the major cysteine protease (cruzain) from Trypanosoma cruzi. J Biol Chem. 1992;267:7411–7420. [PubMed] [Google Scholar]
- 12.Elices M J, Osborne L, Takada Y, Crouse C, Luhowskyj S, Hemler M E, Lobb R R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 13.Factor S M, Cho S, Wittner M, Tanowitz H. Abnormalities of the coronary microcirculation in acute Chagas’ disease. Am J Trop Med Hyg. 1985;34:246–253. doi: 10.4269/ajtmh.1985.34.246. [DOI] [PubMed] [Google Scholar]
- 14.Hauf N, Goebel W, Serfling E, Kuhn M. Listeria monocytogenes infection enhances transcription factor NF-κB in P388D1 macrophage-like cells. Infect Immun. 1994;62:2740–2747. doi: 10.1128/iai.62.7.2740-2747.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou J, Baichwal V, Cao Z. Regulatory elements and transcription factors controlling basal and cytokine-induced expression of the gene encoding ICAM-1. Proc Natl Acad Sci USA. 1994;91:11641–11645. doi: 10.1073/pnas.91.24.11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang H, Chan J, Wittner M, Jelicks L A, Morris S A, Factor S M, Weiss L M, Braunstein V B, Bacchi C J, Yarlett N, Chandra M, Shirani J, Tanowitz H B. Expression of cardiac cytokines and inducible form of nitric oxide synthase (NOS2) in Trypanosoma cruzi-infected mice. J Mol Cell Cardiol. 1999;31:75–88. doi: 10.1006/jmcc.1998.0848. [DOI] [PubMed] [Google Scholar]
- 17.Ivanov V, Stein B, Baumann I, Dobbelaere A E, Herrlich P, Williams R O. Infection with the intracellular protozoan parasite Theileria parva induces constitutively high levels of NF-κB in bovine T lymphocytes. Mol Cell Biol. 1989;9:4677–4686. doi: 10.1128/mcb.9.11.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan D, Ferrari I, Bergami P L, Mahler E, Levitus G, Chiale P, Hoebeke J, Van Regenmortel M H, Levine M J. Antibodies to ribosomal P proteins of Trypanosoma cruzi in Chagas’ disease possess functional autoreactivity with heart tissue and differ from anti-P autoantibodies. Proc Natl Acad Sci USA. 1997;94:10301–10306. doi: 10.1073/pnas.94.19.10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laucella S, Salcedo R, Castanos-Velez E, Riarte A, De Titto E H, Patarroyo M, Orn A, Rottenberg M E. Increased expression and secretion of ICAM-1 during experimental infection with Trypanosoma cruzi. Parasite Immunol. 1996;18:227–239. doi: 10.1046/j.1365-3024.1996.d01-95.x. [DOI] [PubMed] [Google Scholar]
- 20.Laucella S, De Titto E H, Segura E L, Orn A, Rottenberg M E. Soluble cell adhesion molecules in human Chagas’ disease: association with disease severity and stage of infection. Am J Trop Med Hyg. 1997;55:629–634. doi: 10.4269/ajtmh.1996.55.629. [DOI] [PubMed] [Google Scholar]
- 21.Lockyer J M, Colladay J S, Alperin-Lea W L, Hammond T, Buda A J. Inhibition of nuclear factor-κB-mediated adhesion molecule expression in human endothelial cells. Circ Res. 1998;82:314–320. doi: 10.1161/01.res.82.3.314. [DOI] [PubMed] [Google Scholar]
- 22.Morishita R, Sugimoto T, Aoki M, Kida I, Tomita N, Moriguchi A, Maeda K, Sawa Y, Kaneda Y, Higaki J, Ogihara T. In vivo transfection of cis element “decoy” against nuclear factor-κB binding site prevents myocardial infarction. Nat Med. 1997;3:894–899. doi: 10.1038/nm0897-894. [DOI] [PubMed] [Google Scholar]
- 23.Morris, S. A. Unpublished data.
- 24.Morris S A, Bilezikian J P, Hatcher V, Weiss L M, Tanowitz H B, Wittner M. Trypanosoma cruzi: infection of cultured human endothelial cells alters inositol phosphate synthesis. Exp Parasitol. 1989;69:330–339. doi: 10.1016/0014-4894(89)90082-9. [DOI] [PubMed] [Google Scholar]
- 25.Neish A S, Read M A, Thanos D, Pine R, Maniatis T, Collins T. Endothelial interferon regulatory factor 1 cooperates with NF-κB as a transcriptional activator of vascular adhesion molecule 1. Mol Cell Biol. 1995;15:2558–2569. doi: 10.1128/mcb.15.5.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Read M A, Cordle A S R, Veach R A, Carlisle C D, Hawiger J. Cell-free pool of CD14 mediates activation of transcription factor NF-κB by lipopolysaccharide in human endothelial cells. Proc Natl Acad Sci USA. 1993;90:9887–9891. doi: 10.1073/pnas.90.21.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Read M A, Neish A S, Gerritsen M E, Collins T. Nuclear IκB-α and the post-induction transcriptional repression of the E-selectin and VCAM-1 genes. FASEB J. 1995;9:A126. . (Abstract.) [Google Scholar]
- 28.Read M A, Neish A S, Luscinskas F W, Palombella V J, Maniatis T, Collins T. The proteasome pathway is required for cytokine-induced endothelial-leukocyte adhesion molecule expression. Immunity. 1995;2:493–506. doi: 10.1016/1074-7613(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 29.Read M A, Whitley M Z, Williams A J, Collins T. NF-κB and Iκ-Bα: an inducible regulatory system in endothelial activation. J Exp Med. 1994;179:503–512. doi: 10.1084/jem.179.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Regnier C, Song H Y, Gao X, Goeddel D V, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 31.Reiss D D, Jones E M, Tostes S, Lopes E R, Chapadeiro E, Gazzinelli G, Colley D G, McCurley T L. Expression of major histocompatibility complex antigens and adhesion molecules in hearts of patients with chronic Chagas’ disease. Am J Trop Med Hyg. 1993;49:192–200. doi: 10.4269/ajtmh.1993.49.192. [DOI] [PubMed] [Google Scholar]
- 32.Rice G E, Bevilacqua M P. An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science. 1989;246:1303–1306. doi: 10.1126/science.2588007. [DOI] [PubMed] [Google Scholar]
- 33.Rice G E, Munro J M, Bevilacqua M P. Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes: a CD11/CD18-independent adhesion mechanism. J Exp Med. 1990;171:1369–1374. doi: 10.1084/jem.171.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossi M A, Ramos S G. Coronary microvascular abnormalities in Chagas’ disease. Am Heart J. 1996;132(1 Pt. 1):207–210. doi: 10.1016/s0002-8703(96)90417-2. [DOI] [PubMed] [Google Scholar]
- 35.Rowin K S, Tanowitz H B, Wittner M, Nguyen H T, Nadal-Ginard B. Inhibition of muscle differentiation by Trypanosoma cruzi. Proc Natl Acad Sci USA. 1983;80:6390–6394. doi: 10.1073/pnas.80.20.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruben S, Poteat H, Tan T-H, Kawakami K, Roeder R, Haseltine W, Rosen C A. Cellular transcription factors and regulation of IL-2 receptor gene expression by HTLV-1 tax gene product. Science. 1988;241:89–91. doi: 10.1126/science.2838905. [DOI] [PubMed] [Google Scholar]
- 37.Sambucetti L C, Cherrington J M, Wilkinson G W G, Mocarski E S. NF-κB activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J. 1989;8:4251–4258. doi: 10.1002/j.1460-2075.1989.tb08610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schindler U, Baichwal V R. Three NF-κB binding sites in the human E-selectin gene required for maximal tumor necrosis factor alpha-induced expression. Mol Cell Biol. 1994;14:5820–5831. doi: 10.1128/mcb.14.9.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. [PubMed] [Google Scholar]
- 40.Sessler C N, Schwartz M, Windsor A C, Fowler A A. Increased serum cytokines and intercellular adhesion molecule-1 in fulminant Rocky Mountain spotted fever. Crit Care Med. 1995;23:973–976. doi: 10.1097/00003246-199505000-00029. [DOI] [PubMed] [Google Scholar]
- 41.Shimokawa H, Flavahan N A, Vanhoutte P M. Natural course of the impairment of endothelium-dependent relaxations after balloon endothelial removal in porcine coronary arteries. Possible dysfunction of a pertussis toxin-sensitive G protein. Circ Res. 1989;65:740–753. doi: 10.1161/01.res.65.3.740. [DOI] [PubMed] [Google Scholar]
- 42.Smith C A, Farrah T, Goodwin R G. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 43.Sporn L A, Sahni S K, Lerner N B, Marder V J, Silverman D J, Turpin L C, Schwab A L. Rickettsia rickettsii infection of cultured human endothelial cells induces NF-κB activation. Infect Immun. 1997;65:2786–2791. doi: 10.1128/iai.65.7.2786-2791.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Springer T A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 45.Staunton D E, Marlin S D, Stratowa C, Dustin M L, Springer T A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988;52:925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- 46.Sun J, Tarleton R L. Predominance of CD8+ T lymphocytes in the inflammatory lesions of mice with acute Trypanosoma cruzi infection. Am J Trop Med Hyg. 1993;48:161–169. doi: 10.4269/ajtmh.1993.48.161. [DOI] [PubMed] [Google Scholar]
- 47.Sunnemark D, Frostegard J, Orn A, Harris R A. Cellular and cytokine characterization of vascular inflammation in CBA/J mice chronically infected with Trypanosoma cruzi. Scand J Immunol. 1998;48:480–484. doi: 10.1046/j.1365-3083.1998.00410.x. [DOI] [PubMed] [Google Scholar]
- 48.Tanowitz H B, Burns E R, Sinha A K, Kahn N N, Morris S A, Factor S M, Bilezikian J P, Wittner M. Enhanced platelet adherence and aggregation in Chagas’ disease: a potential pathogenic mechanism for cardiomyopathy. Am J Trop Med Hyg. 1990;43:274–281. doi: 10.4269/ajtmh.1990.43.274. [DOI] [PubMed] [Google Scholar]
- 49.Tanowitz H B, Gumprecht J P, Spurr D, Calderon T M, Ventura M C, Raventos-Suarez C, Factor S M, Hatcher V, Wittner M, Berman J W. Cytokine gene expression of endothelial cells infected with Trypanosoma cruzi. J Infect Dis. 1992;166:598–603. doi: 10.1093/infdis/166.3.598. [DOI] [PubMed] [Google Scholar]
- 50.Tanowitz H B, Kaul D K, Chen B, Morris S A, Factor S M, Weiss L M, Wittner M. Compromised microcirculation in acute murine Trypanosoma cruzi infection. J Parasitol. 1996;82:124–130. [PubMed] [Google Scholar]
- 51.Tanowitz H B, Kirchhoff L V, Simon D, Morris S A, Weiss L M, Wittner M. Chagas’ disease. Clin Microbiol Rev. 1992;5:400–419. doi: 10.1128/cmr.5.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanowitz H B, Wittner M, Morris S A, Zhao W, Weiss L M, Braunstein V L, Huang H, Douglas S A, Valcic M, Spektor M, Christ G J. The putative mechanistic basis for the modulatory role of endothelin-1 in the altered vascular tone induced by Trypanosoma cruzi. Endothelium. 1999;6:217–230. doi: 10.3109/10623329909053412. [DOI] [PubMed] [Google Scholar]
- 53.Tarleton R L. Tumour necrosis factor (cachectin) production during experimental Chagas’ disease. Clin Exp Immunol. 1988;73:186–190. [PMC free article] [PubMed] [Google Scholar]
- 54.Tarleton R L, Zhang L, Downs M O. “Autoimmune rejection” of neonatal heart transplants in experimental Chagas’ disease is a parasite-specific response to infected host tissue. Proc Natl Acad Sci USA. 1997;94:3932–3937. doi: 10.1073/pnas.94.8.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tedder T F, Steeber D A, Chen A, Engel P. The selectins: vascular adhesion molecules. FASEB J. 1995;9:866–873. [PubMed] [Google Scholar]
- 56.Whitley M Z, Thanos D, Read M A, Maniatis T, Collins T. A striking similarity in the organization of the E-selectin and beta interferon gene promoters. Mol Cell Biol. 1994;14:6454–6475. doi: 10.1128/mcb.14.10.6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wittner M, Christ G J, Huang H, Weiss L M, Hatcher V B, Morris S A, Orr G A, Berman J W, Zeballos G A, Douglas S A, Tanowitz H B. Trypanosoma cruzi induces endothelin release from endothelial cells. J Infect Dis. 1995;171:493–497. doi: 10.1093/infdis/171.2.493. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto H, Hanada K, Nishijima M. Involvement of diacylglycerol production in activation of nuclear factor kappa B by a CD14-mediated lipopolysaccharide stimulus. Biochem J. 1997;325:223–228. doi: 10.1042/bj3250223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstriction peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 60.Yurochko A D, Kowalik T F, Huong S M, Huang E S. Human cytomegalovirus upregulates NF-κB activity by transactivating the NF-κB p105/p50 and p65 promoters. J Virol. 1995;69:5391–5400. doi: 10.1128/jvi.69.9.5391-5400.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 62.Zhang L, Tarleton R L. Persistent production of inflammatory and anti-inflammatory cytokines and associated MHC and adhesion molecule expression at the site of infection and disease in experimental Trypanosoma cruzi infections. Exp Parasitol. 1996;84:203–213. doi: 10.1006/expr.1996.0106. [DOI] [PubMed] [Google Scholar]