Abstract
The influence of the microtubule-associated motor protein kinesin on Chlamydia psittaci inclusion development in epithelial and fibroblast cell lines was addressed. Kinesin was blocked early after chlamydial internalization (4 h postinfection [p.i.]) and before the initiation of active chlamydial multiplication (8 h p.i.). Chlamydia development was monitored by fluorescence and transmission electron microscopy at different times during the cycle. In both host cell lines, kinesin blockage restricted mitochondria from the chlamydial vacuole. The effects of kinesin blockage on the C. psittaci replication cycle included the presence of multiple inclusions up to late in the cycle, the presence of enlarged pleomorphic reticulate bodies, and a delayed reappearance of elementary bodies. The last effect seems to be greater when kinesin is blocked early after infection. Our results show that kinesin activity is required for optimal development of these microorganisms, most probably acting through the apposition of mitochondria to the C. psittaci inclusions.
Being an obligate intracellular parasite, Chlamydia psittaci interacts in a special way with the host cell in order to perpetuate itself in nature (14). Its unique biphasic development cycle starts when the infectious extracellular elementary body (EB) attaches to the host cell, inducing its own internalization within a vacuole, termed an inclusion, by evoking an invagination of the host cytoplasm membrane (24, 50). After 6 to 10 h, the EB transforms itself in the multiplicative intracellular reticulate body (RB), entering a stage of active binary fission at about 12 h after cell infection. At this time, RBs are found to circumscribe almost exclusively the inner margin of the inclusion, with few organisms observed free within its center (33), while mitochondria are progressively found closely associated with the inclusion membrane along with the reproductive activity of RBs (34, 35, 43, 47). After several divisions, RBs mature again into EBs in an asynchronous way. During this maturation, morphologically intermediate forms (intermediate bodies [IBs]) can be observed. Finally, in most cases, the host cell is lysed within 48 to 72 h, releasing the new progeny of infectious particles and thus completing the cycle. The duration of the developmental cycle may vary according to the chlamydial strain and the host cell involved (24, 47, 53).
Participation of the cytoskeleton during chlamydial infection has been reported (45). Eukaryotic cell processes such as cell division, organelle transport, and endocytic and exocytic pathways involve the action of motor proteins associated with the microtubules (MTs) in the eukaryotic cytoskeleton (49). Among these proteins, kinesin has been shown to associate with diverse eukaryotic organelles (8), to mediate Golgi-to-endoplasmic reticulum (ER) transport of escaped ER proteins (32), and to be responsible for mitochondrial movement in several cell types, including macrophages and fibroblasts (8, 30, 31). To study the influence of kinesin on the development of C. psittaci, we blocked the motor protein by using neutralizing antibodies and then studied the development of inclusions at different times during the chlamydial cycle. Our findings indicate that active kinesin plays a role in the development of Chlamydia, most probably through movement of mitochondria towards close apposition to the prokaryotic inclusion.
MATERIALS AND METHODS
Cells and medium.
The mouse fibroblast L-929 (kindly donated by Rudy Willebrords, Janssen Research Foundation) and buffalo green monkey epithelial (BGM) cell lines were cultured in minimal essential medium (Gibco, Paisly, United Kingdom) supplemented with 10% fetal bovine serum (Gibco), 1% l-glutamine (Gibco), 1% nonessential aminoacids (Gibco), 100 mg of streptomycin sulfate (Gibco) per ml, 100 mg of vancomycin (Gibco) per ml, and 100 mg of gentamicin (Gibco) per ml (hereafter called Chlamydia growth medium). For experiments involving fluorescence microscopy, 3 × 105 cells/well were seeded on 24-well microplates (Sterilin, Staffordshire, United Kingdom) containing 13-mm-diameter coverslips. For experiments involving electron microscopy, 1.5 × 106 cells/well were seeded as monolayers onto six-well microplates (Sterilin) without coverslips.
Chlamydia strain and growth conditions.
The avian C. psittaci strain 92/1293 (serovar D) (53) was used as the antigen. This chlamydial strain was propagated in L929 and BGM cells for 72 h. After this time, cells were lysed (by freezing and thawing followed by sonication), cell debris was removed by centrifugation, and the resulting supernatants were aliquoted and stored at −70°C. The chlamydial titer (inclusion-forming units per milliliter) was determined from one of the frozen aliquots as described previously (45).
During the course of the study, nonconfluent eukaryotic cell monolayers were infected with the chlamydial antigen grown in the homologous cell line at a multiplicity of infection (MOI) of 1. To ensure an even infection of the cells, plates were subjected to gentle agitation on a rocking platform for 2 h at 37°C. After the adsorption time, the inoculum was eliminated, fresh medium was added, and monolayers were then incubated at 37°C in a 5% CO2 humified atmosphere during the course of the experiments.
Antikinesin treatment.
An antikinesin monoclonal antibody (MAb) (Sigma Chemical Co., St. Louis, Mo.) was introduced into living cells after monolayers had been permeabilized by using a modification of the glass bead method of Fennell et al. (19). Briefly, 24-h cell monolayers of both infected and uninfected L and BGM cells were washed with Hanks balanced salt solution-Ca2+ buffer (pH 6.9) containing 10 mM HEPES, with 0.6 M sucrose to avoid lysis of the inclusion (51) (Perm buffer). The antikinesin antibody was diluted 1:400 in Perm buffer and placed on the monolayers in volumes of 100 μl/well for 24-well plates and 500 μl/well for 6-well plates. Sterile glass beads of 170 to 180 μm in diameter (B. Braun Biotech International, Melsungen, Germany) were poured and dispersed with gentle agitation to completely cover the monolayers with a single layer. The cells were incubated at 37°C for 10 min with gentle agitation every 2 min. After incubation, the plates were tilted and then washed several times thereafter with Perm buffer until all beads had been eliminated. Chlamydia growth medium was added to the monolayers, which were then incubated further at 37°C with 5% CO2.
To determine the direct effect that the permeabilization and antikinesin blockage treatments had on the eukaryotic cells, two sets of cell monolayers were used: one was infected with C. psittaci, while the other one remained uninfected. In both sets, 24-well microplates were divided into four groups according to the treatment to be performed: permeabilization treatment only, permeabilization treatment plus pristane-inoculated mouse ascites fluid, permeabilization treatment plus antikinesin MAb treatment (kinesin blockage), or no treatment (control). Permeabilization and blockage treatments were performed at 4 and 8 h postinfection (p.i.) as described above. In all cases monolayers were trypsinized, when applicable, at 8, 24, and 31 h p.i. Three wells were used for every treatment-time point combination mentioned: these were pooled after trypsinization, and viable cell counts per milliliter were determined by using trypan blue and a hemocytometer chamber.
To check the persistence of the antikinesin antibodies in the cells, cell monolayers were grown on 13-mm-diameter coverslips placed in 24-well microplates. Antikinesin MAb dilutions of 1:100, 1:200, 1:400, 1:500, 1:800, and 1:1,000 were prepared and introduced into the cells as described above. Monolayers were washed and fixed for 10 min with cold methanol at 2, 3, 4, 5, 6, 7, and 24 h after kinesin blockage and were kept at −20°C until further processing. Coverslips were incubated with biotinylated goat anti-mouse antibody (1:200) (Dako Diagnostics, Cambridgeshire, United Kingdom) for 30 min at room temperature and washed twice for 5 min each with phosphate-buffered saline (PBS). Avidin-biotin-peroxidase complex (Dako) was then added, followed by 30 min of incubation under the same conditions. Thereafter, coverslips were washed as before, and the brown chromogen diaminobenzidine (Sigma) was added in the presence of H2O2. Finally, coverslips were mounted on slides to be analyzed by light microscopy. As controls, cells were treated in the same way, but either ascites fluid or no antikinesin was added instead of the antikinesin MAb.
Fluorescence microscopy.
Kinesin was blocked in infected L and BGM cells at 4 and 8 h after inoculation of the cells with Chlamydia as described above. In each case, when applicable, infection was allowed to proceed for up to 8, 24, and 31 h p.i. As controls, three sets of chlamydia-infected cells were used: (i) permeabilized cells to which no antikinesin MAb was added, (ii) permeabilized cells to which pristane-inoculated mice ascites fluid was added (ascites fluid-treated cells), and (iii) untreated cells.
To visualize the mitochondria, a red-fluorescent molecular probe (Mitotracker CMXRos-H2; Molecular Probes Europe BV, Leiden, The Netherlands) was used to selectively stain these organelles. Half an hour before the end of the incubation period, the culture medium was replaced with 100 μl of prewarmed 1 μM Mitotracker diluted in Chlamydia growth medium. Cells were incubated for 30 min further at 37°C with 5% CO2. Immediately thereafter, the monolayers were washed three times with PBS. The cells were fixed for 10 min with cold methanol and air dried.
An antichlamydia fluorescein isothiocyanate (FITC)-conjugated genus-specific MAb mixture (lipopolysaccharide-specific MAb clone C5 plus major outer membrane protein-specific MAb clone C8) (reference no. 17-114; Argene Biosoft, Varilhes, France) was used to visualize the chlamydial inclusion. Thirty microliters of the antibody mixture was added to each coverslip on fixed cells, which were then incubated in the dark for 30 min at 37°C in a moist environment. The coverslips were carefully rinsed afterwards in distilled water for 30 s, and mounted on microscope slides with a mounting medium for immunofluorescence (Vectashield; Vector Laboratories, Burlingame, Calif.) to prevent rapid photobleaching of fluorochromes. Finally, the coverslips were sealed with clear nail polish and examined with a Leica DMRB microscope. Filters with excitation ranges of 515 to 560 nm and 450 to 490 nm were used to detect Mitotracker and FITC emissions, respectively. The number of inclusions per infected cell was counted in five fields (minimum of 380 cells) of every coverslip analyzed. A Zeiss LSM410 confocal laser microscope was also utilized, with a 100×/1.3NA plan-Neufluar lens. Images were typically recorded with a scanning time of 1 s and 8× line averaging, using a 1×, 1.5×, or 2× hard zoom. The excitation wavelengths and emission filters used for Mitotracker and FITC fluorochromes were 543 and 488 nm and LP 570 and BP 515-525 respectively.
Transmission electron microscopy (TEM).
Kinesin was blocked in infected L-929 cell monolayers at 4 and 8 h after chlamydial infection as described above. Incubation was allowed to proceed for up to 24, 31, and 48 h p.i. In each case, cell monolayers were washed three times with PBS, fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.6), and kept at 4°C until further processing. As negative controls, permeabilized but not kinesin-blocked infected cells were treated in the same way. For final processing, cells were scraped from the plates and washed several times with PBS to eliminate the glutaraldehyde. Postfixation was performed for 1 h in 1% osmium tetroxide in distilled water. Thereafter, cells were stained overnight with 2% uranyl acetate (pH 4.5). On the following day, the cells were progressively dehydrated in ethanol and then embedded in Epon 812 resin (Fluka Chemie AG, Buchs, Switzerland). Ultrathin sections were prepared (Ultracut; Reichert AG, Vienna, Austria), stained with lead citrate, and examined in a transmission electron microscope (model 2085; Philips, Eindhoven, The Netherlands). Pictures were taken with a plate camera (6 by 9 cm). Additionally, laser prints were obtained from images taken with a slow-scan camera (Kodak Megaplus model 1.6i) and the Image AnalySIS software for Windows 95 (Soft Imaging Software GmbH, Munster, Germany). Chlamydial particles inside the inclusions were counted from both the pictures and the prints, and analysis of variance was performed on the values obtained.
RESULTS
Permeabilization and blockage treatments have a detrimental effect on eukaryotic cells.
The effects of permeabilization and blockage treatments on the growth of BGM and L-929 cells were similar (data not shown). In uninfected cells, permeabilized monolayers showed a cell yield decrease of approximately 30% compared with nonpermeabilized cells. When kinesin was blocked, viable cell counts were even lower than those for permeabilized cells. In these cases, a decrease of about 70% compared with untreated cells was noted. Nevertheless, under all experimental conditions, cells continued to multiply at the same rate between every two time points analyzed, indicating that after the permeabilization treatment, recovered cells kept growing and dividing normally. Infection of C. psittaci in either type of host induced a further decrease in the viable cell counts in both permeabilized and kinesin-blocked cell monolayers at all times studied. As a consequence, the actual number of viable cells found at 8, 24, and 31 h after infection was always lower (∼40%) than the cell counts from uninfected monolayers. Uninfected and infected cells treated with ascites fluid behaved like permeabilized cells.
Antikinesin treatment restricts movement of mitochondria.
Mitochondria move along the cytoskeleton of eukaryotic cells by means of kinesin. To verify the presence of the antikinesin MAb inside the cells, immunocytochemistry was performed. Kinesin was revealed within the cells at all times studied (data not shown). The strength of the signal obtained was directly proportional to the concentration of the antibody used. A strong signal was found at up to a 1:500 antikinesin dilution, and it began to fade at 1:800, although it was still clearly discernible. In BGM cells, both negative controls, i.e., cells to which ascites fluid was added in place of antikinesin MAb and permeabilized cells not incubated with primary antibody, showed no positive signal. In L cells, however, a weak, nonspecific staining was also found in permeabilized samples. All cell monolayers showed a normal appearance at all times analyzed. By using CMXRos-H2, mitochondria were observed spread throughout the interior of the cell in permeabilized cells as well as in ascites fluid-treated cells, whereas in kinesin-blocked cells these organelles were observed as clusters mainly located in the perinuclear area.
Fluorescence microscopic analysis of the location of mitochondria and its effect on the chlamydial growth cycle.
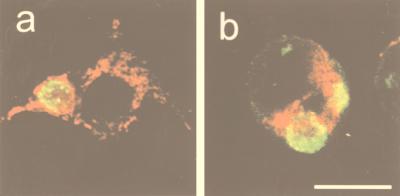
Throughout this study, mitochondria were seen surrounding the chlamydial inclusions both in permeabilized cells (Fig. 1a) and in ascites fluid-treated cells (data not shown). In contrast, in those cells where kinesin was blocked, mitochondria remained mostly in the perinuclear area, although a few of them could still be observed around the vacuole (Fig. 1b).
FIG. 1.
Double direct immunofluorescence of kinesin-blocked L-929 cells at 24 h after infection with C. psittaci 92/1293. Chlamydial inclusions (green) were stained with an FITC-labelled antichlamydial genus-specific MAb (clones C5 and C8), and mitochondria (red) were stained with Mitotracker CMXRos-H2. Permeabilization (control cells) and kinesin blockage treatments were performed at 4 h p.i. In permeabilized cells (a), mitochondria are distributed throughout the cell and surrounding the chlamydial inclusion. In kinesin-blocked cells (b), mitochondria cluster in the perinuclear area, and the chlamydial inclusion is not surrounded by these organelles. Bar, 25 μm.
Concerning the chlamydial morphology, only individual chlamydial particles and no inclusions were seen inside the host cell at 8 h p.i. in permeabilized and kinesin-blocked cells. Furthermore, multiple chlamydial inclusions per host cell could be seen in the monolayers analyzed at 24 and 31 h p.i., regardless of the treatment performed (Table 1). We noticed, however, that the evolution in the number of chlamydial inclusions present within the host cells between those times significantly differed (P < 0.05) for the different treatments. In permeabilized cells without kinesin blockage, a decrease in the number of inclusions per cell was observed from 24 to 31 h p.i., suggesting that fusion between them had occurred. In contrast, when mitochondria were immobilized by kinesin blockage, the number of the chlamydial inclusions found at 24 h p.i. was the same, or was even increased, by 31 h p.i. Chlamydial infection in untreated and ascites fluid-treated cells progressed as it did in permeabilized cells (data not shown).
TABLE 1.
Maximal number of inclusions per infected cell after C. psittaci 92/1293 infection
| Host cell line | Treatment | Treatment time p.i. (h) | Maximal no. of inclusionsa at the following fixation time p.i. (h)
|
||
|---|---|---|---|---|---|
| 8 | 24 | 31 | |||
| BGM | Kinesin blockage | 4 | 0 | 5 | 7 |
| 8 | NDb | 2 | 4 | ||
| Permeabilization | 4 | 0 | 6 | 2 | |
| 8 | ND | 2 | 2 | ||
| L-929 | Kinesin blockage | 4 | 0 | 8 | 8 |
| 8 | ND | 5 | 5 | ||
| Permeabilization | 4 | 0 | 8 | 5 | |
| 8 | ND | 5 | 2 | ||
Average for five fields.
ND, not done.
Electron microscopic analysis of mitochondrion immobilization and its effect on the chlamydial growth cycle.
Since no difference was observed between permeabilized and ascites fluid-treated cells for either the eukaryotic or prokaryotic cycle or for the mitochondrial position, as determined by viable cell counting and immunofluorescence, only permeabilized cells were used as negative controls during TEM studies. Immunofluorescence analysis suggested a delay in the C. psittaci growth cycle. For this reason, we also studied by TEM the chlamydial development at 48 h p.i., around the end of the cycle, for both permeabilized and kinesin-blocked cells.
Many mitochondria could be found around the chlamydial inclusions at 24, 31, and 48 h p.i. in permeabilized cells, regardless of the time at which treatment was performed. Some mitochondria, however, were also present adjacent to the inclusions in kinesin-blocked cells.
Multiple chlamydial vacuoles were observed in both permeabilized and kinesin-blocked host cells at all times analyzed, i.e., 24, 31, and 48 h p.i. Nevertheless, differences in the number and size of the inclusions in the two types of treated cells were seen. Non-kinesin-blocked cells permeabilized at both 4 and 8 h p.i., as well as kinesin-blocked cells at 8 h p.i., showed one or two inclusions at 31 and 48 h p.i. Conversely, when kinesin was blocked at 4 h p.i. and mitochondria were therefore immobilized early in the chlamydial growth cycle, infected cells containing large numbers of inclusions (more than five) could be observed up to 48 h p.i.
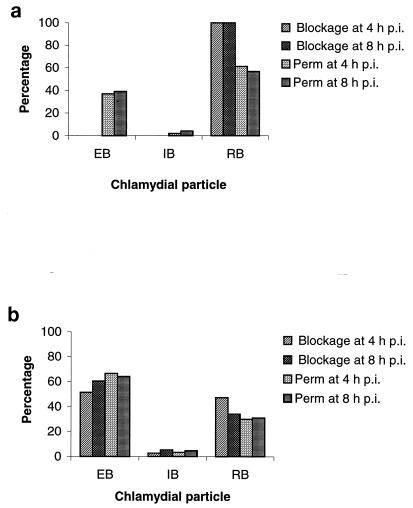
The chlamydial particles present within the inclusions in kinesin-blocked or permeabilized cells also showed differences. At 24 and 31 h p.i., both EBs and RBs could be seen in cells permeabilized at 4 and 8 h p.i. In contrast, when mitochondria were immobilized at 4 or 8 h p.i., the only chlamydial population observed at 24 and 31 h after chlamydial infection was RBs (P < 0.001) (Fig. 2). At 48 h p.i., the total chlamydial population found in control cells permeabilized shortly after C. psittaci had been internalized by the host cell, i.e., at 4 h p.i., consisted of 66.53% EBs, 3.46% IBs, and 30% RBs, whereas when mitochondria were blocked at 4 h p.i., 51.39% EBs, 2.79% IBs, and 47.20% RBs were found (P > 0.05). Conversely, when control cells were permeabilized close to the onset of the bacterial multiplication, i.e., at 8 h p.i., the proportions of chlamydial particles found at 48 h p.i. were 64.17% EBs, 4.72% IBs, and 31.10% RBs, while when kinesin was blocked at 8 h p.i., 60.51% EBs, 5.29% IBs, and 34.18% RBs were present (P > 0.05) (Fig. 3). In all kinesin-blocked cells, RBs showed abnormal, pleomorphic forms.
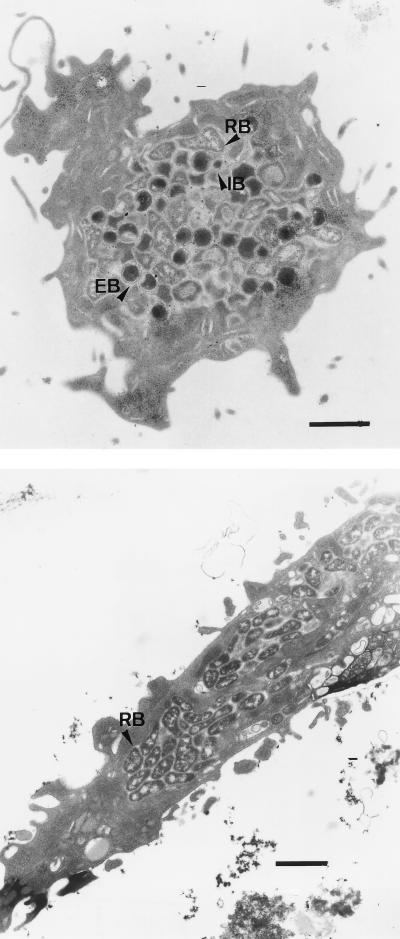
FIG. 2.
TEM of L-929 cells infected with C. psittaci 92/1293 for 31 h. Cells underwent either permeabilization treatment (top) or kinesin blockage (bottom) at 4 h p.i. Note the presence of only one large chlamydial inclusion containing EBs, IBs, and RBs in non-kinesin-blocked cells, whereas multiple smaller inclusions containing exclusively pleomorphic, elongated RBs are present in kinesin-blocked cells. Bars, 1 μm.
FIG. 3.
Chlamydial forms present in the inclusion after kinesin blockage. C. psittaci 92/1293-infected cells were subjected to either kinesin blockage or permeabilization (Perm) treatment at 4 and 8 h p.i. Infection was allowed to proceed for 31 h (a) and 48 h (b). Note that at 31 h p.i., in contrast to the case for permeabilized cells, only RBs, and no EBs or IBs, are present in kinesin-blocked cells (P < 0.001).
DISCUSSION
Besides its participation in organelle translocation and cell movement (8, 18, 20), kinesin is also involved in the mitotic process (4). All members of the kinesin ATPase family share conserved sequences within the kinesin motor domain, including binding sites for both nucleotides and MTs. These domains are located in what is known as the head of the kinesin molecules (7, 28), the target structure for the antikinesin MAb we used. As expected, introduction of this MAb altered the growth cycle of the eukaryotic cells, which might have led to the lower cell yields obtained in kinesin-blocked monolayers. When cells were infected with C. psittaci, the eukaryotic cell progeny also decreased. Previous investigations have demonstrated that with C. psittaci infections the duration of DNA synthesis is doubled, and thus the eukaryotic growth cycle is prolonged (1, 13), even at an MOI of ∼1 (27).
Two cell organelles have been found to be closely associated with C. psittaci vacuoles: mitochondria (34, 35, 47, 53) and the Golgi apparatus (21, 22, 45, 53). Such close associations might influence chlamydial development, being related to the acquisition of eukaryotic ATP (33, 48) and lipids (21, 22). Kinesin is known to associate with the two organelles (3, 8). Nevertheless, even if kinesin is implicated in the movement of mitochondria within the cell (38), it is not this MT motor protein but dynein that is responsible for the location, organization, and/or function of the Golgi and for the ER-to-Golgi transport in a number of cells, including fibroblast and epithelial cells (16, 26, 32, 52).
Our results support the idea that the close association of mitochondria with the chlamydial inclusions does exert an influence on the developmental cycle of C. psittaci. In previous studies (53), where the development of C. psittaci 92/1293 was monitored by using TEM, all sorts of chlamydial forms were visible from 30 h p.i. onwards, a feature that we found at 31 h p.i. only in permeabilized infected cells and not in those where kinesin had been blocked. Furthermore, the proportions of particles observed at 50 h p.i. by Vanrompay et al. (53) consisted of 65% EBs, 5% IBs, and 30% RBs. Such proportions are very much like the ones we obtained at 48 h p.i., when cells were permeabilized at 4 or 8 h after chlamydial infection, and when kinesin was blocked at 8 h p.i. However, when antikinesin was introduced early after chlamydiae had been internalized, i.e., at 4 h p.i., a larger proportion of RBs and a smaller proportion of EBs than those previously reported were found in mature inclusions. Our findings thus show a delayed reappearance of EBs in the inclusion when kinesin is blocked, and mitochondria are therefore immobilized, in the infected cells. It cannot be excluded, nevertheless, that an unknown additional effect of the antikinesin treatment other than mitochondrion immobilization may also influence the chlamydial growth.
By fluorescence microscopy we observed the immobilization effect of kinesin blockage on mitochondria up to 27 h after the antikinesin MAb had been introduced in the eukaryotic cells, i.e., up to 31 h after chlamydial infection. It could be possible that the activity of the MAb within the cell had decreased or disappeared after 40 to 44 h (i.e., at 48 h p.i.), the longest time analyzed by TEM. To investigate this, we used fluorescence microscopy as described above to verify whether mitochondria were still immobilized at those times. In both cases, mitochondria appeared to be immobilized in fewer than approximately 0.1% of the cells, indicating that at some time between 31 and 48 h p.i. these organelles could had come in close contact with the bacterial vacuole membrane. In that situation, chlamydiae could have been able to obtain more ATP, thus speeding up their development. It could have been expected, therefore, that we would observe all sorts of chlamydial particles at 48 h p.i. in kinesin-blocked cells, although significantly fewer than in permeabilized cells. Interestingly, by 48 h p.i. the proportions of chlamydial particles seen within the cells were very similar for permeabilized and kinesin-blocked cells at 8 h p.i. but not for kinesin-blocked cells at 4 h p.i. These features suggest that chlamydial energy requirements vary during the infection cycle, with more ATP being needed during the first stages of development than during the last ones. This is in agreement with recent studies demonstrating that C. psittaci infection induces an increase of high-energy metabolites in the host cells, where ATP enhancement reaches its peak when RBs are multiplying more actively while returning to near-normal levels after RBs have begun to differentiate back to EBs (41).
Chlamydial vacuoles within kinesin-blocked cells contained elongated, atypical RBs. Aberrant chlamydial developmental forms can be produced when the bacteria are grown under stressful conditions (12, 38, 42). For instance, increased production of the C. psittaci 57-kDa stress response protein (a protein in the hsp60 family, similar to GroEL of Escherichia coli) is associated with inefficient reorganization of RBs to EBs and the formation of large, aberrant RBs when the developmental cycle is blocked by penicillin (38). Antikinesin treatment, therefore, seems to constitute a stress for the chlamydial RB, most probably through a lack or restriction of energy availability.
When kinesin was blocked, and mitochondria were consequently immobilized, the number of bacterial inclusions per host cell at 24 h p.i. remained about the same at 31 h p.i. In contrast, a decrease in the number of chlamydial vacuoles per host cell was seen from 24 to 31 h p.i. in infected cells where mitochondria were not immobilized, suggesting that fusion of the inclusions had occurred. This presents us with two aspects to be considered. On the one hand, previous studies (25, 54) have indicated that fusion among C. psittaci inclusions does not occur. In those studies, other C. psittaci strains and a higher MOI were utilized. Therefore, it could be that C. psittaci vacuole fusion might be strain and/or infection dose dependent. On the other hand, it could be argued that blockage of kinesin might influence the vacuole fusion by not allowing them to approach. It has been demonstrated, however, that not kinesin but dynein is the motor protein involved in transport of endocytosed vesicles (2, 40). A more attractive possibility is that because of restriction of mitochondria from the chlamydial vacuole, not enough ATP could be found for fusion to take place. It has been confirmed recently that different proteins play an essential role in membrane fusion, among which are the N-ethylmaleimide-sensitive factor and the mammalian valosin-containing protein (p97) (37). These proteins, interestingly, are also ATPases. Future investigations in this area will be aimed at clarifying the precise events involved.
Kinesin blockage could not totally restrict mitochondria from the chlamydial inclusions, as observed in both the immunofluorescence and ultrastructural images obtained. This could be explained in three ways: (i) not all kinesin motor proteins were blocked, (ii) action of the antikinesin MAb decreased or disappeared in the course of the experiments, or (iii) movement of mitochondria within the cell does not depend exclusively on kinesin. In regard to the two first possibilities, even though we do not know the half-life of the antikinesin MAb once it is located inside the cells, the fact that a strong immunocytochemistry signal was obtained 24 h after kinesin was blocked, together with the constant appearance of mitochondrial clusters observed at 31 h p.i. by fluorescence, provides an argument that the motor proteins were effectively blocked for at least that period. Concerning the third possibility, intrinsic mitochondrial movement bringing these organelles toward zones of high energy demand has been described (5). Displacement of mitochondria to energy-consuming zones might be guided by ATP and/or ADP gradients. At sites of high ADP content, mitochondria are trapped by immobilization (5). RBs of C. psittaci have an ATP-ADP exchange mechanism that functionally acts as a reverse mitochondrion. They take up ATP while expelling ADP (23), a feature that might promote mitochondria to approach and/or to remain in close contact with the membrane vacuole. Nevertheless, since mitochondrial apposition does not occur during the early stages of Chlamydia trachomatis and Chlamydia pneumoniae infections (35), there might be intrinsic C. psittaci mechanisms that may induce or influence mitochondrial movement towards the inclusion. Moreover, while all chlamydiae are energy parasites (39, 46), the amount of ATP needed to develop might not necessarily be the same for each member of the group. Depending on the strain and host cell involved, there are differences in the time required for completion of their development cycles. Among other factors, this may be a reflection of the ATP available in the cell. Perhaps the ATP requirements of C. trachomatis and C. pneumoniae could be fulfilled without the need to be in close contact with the host energy-producing organelles. In this regard, C. trachomatis inclusions redistribute to the perinuclear area, where most mitochondria are located, before starting active multiplication, whereas inclusions of C. pneumoniae, which mature at later stages during the infection (10), can be found in both the perinuclear area and the host cell peripheral cytoplasm (11). In contrast, it could also be that these two species lack the chlamydial factor involved in the mitochondrial attraction towards and/or apposition to the chlamydial vacuole that might be present in C. psittaci.
There are reasons to believe that the redistribution of mitochondria in close apposition to the chlamydial vacuole may be orchestrated by the microorganisms themselves, possibly through the manipulation of kinesin phosphorylation. The interaction of kinesin with organelle membranes and its activity along MTs are known to be regulated by phosphorylation processes (29, 36). Phosphorylation of certain host cell proteins has been demonstrated in C. trachomatis infection (6, 17). Such phosphorylation is thought to be involved in the MT-dependent redistribution of C. trachomatis EBs to the perinuclear area (11). Furthermore, modulation of the host cell can take place in other cell compartments, at a distance from the microorganisms themselves. This has been shown for Listeria monocytogenes. This facultative intracellular bacterium produces a protein called ActA, which is translocated to the mitochondria (44) and phosphorylated during cell growth (9) and which subsequently changes the actin filaments. In addition, it has been reported that chlamydiae supress host cell apoptosis by blocking the release of mitochondrial cytochrome c (15), demonstrating that these microorganisms are capable of modifying mitochondrial function for their own benefit. One could speculate that C. psittaci could either produce a protein analogous to ActA or alter the phosphorylation patterns of the host cell by the production of some yet-unknown factor, therefore modulating the signal transduction pathway of the eukaryote, in order to bring the mitochondria in apposition to the vacuole membrane and thus be in an advantageous position to acquire ATP.
ACKNOWLEDGMENTS
We thank Karen Tilmant for her valuable assistance with the immunocytochemistry and Rudy Willebrords (Janssen Research Foundation) for providing us with the L-929 cells.
The National Council of Science and Technology (CONACYT), Mexico City, Mexico, is acknowledged for providing a grant to C. Escalante-Ochoa (register no. 93110). K. De Vos is a fellow with the Vlaams Instituut voor de Bevordering van het Wetenschappelijk-technologisch Onderzoek in de Industrie.
ADDENDUM IN PROOF
Since the preparation of the manuscript, an amended description of the order Chlamydiales has been published (K. D. Everett, R. M. Bush, and A. A. Andersen, Int. J. Syst. Bacteriol. 49:415–440, 1999). In accordance with this new classification, the bacterium Chlamydia psittaci, the subject of our study, should now be referred to as Chlamydophila psittaci.
REFERENCES
- 1.Alexander J J. Effect on infection with the meningopneumonitis agent of deoxyribonucleic acid and protein synthesis in its L-cell host. J Bacteriol. 1969;97:653–657. doi: 10.1128/jb.97.2.653-657.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aniento F, Emans N, Griffiths G, Guenberg J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J Cell Biol. 1993;123:1373–1378. doi: 10.1083/jcb.123.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball E H, Singer S J. Mitochondria are associated with microtubules and not with intermediate filaments in cultured fibroblasts. Proc Natl Acad Sci USA. 1982;79:123–126. doi: 10.1073/pnas.79.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton N R, Goldstein L S B. Going mobile: microtubule motors and chromosome segregation. Proc Natl Acad Sci USA. 1996;93:1735–1742. doi: 10.1073/pnas.93.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bereiter-Hahn J, Vöth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Technol. 1994;27:198–219. doi: 10.1002/jemt.1070270303. [DOI] [PubMed] [Google Scholar]
- 6.Birkelund S, Johnsen H, Christiansen G. Chlamydia trachomatis serovar L2 induces protein tyrosine phosphorylation during uptake by HeLa cells. Infect Immun. 1994;62:4900–4908. doi: 10.1128/iai.62.11.4900-4908.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloom G S, Wagner M C, Pfister K K, Brady S T. Native structure and physical properties of bovine brain kinesin and identification of the ATP-binding subunit polypeptide. Biochemistry. 1988;27:3409–3416. doi: 10.1021/bi00409a043. [DOI] [PubMed] [Google Scholar]
- 8.Brady S T, Pfister K K. Kinesin interactions with membrane bounded organelles in vivo and in vitro. J Cell Sci Suppl. 1991;14:103–108. doi: 10.1242/jcs.1991.supplement_14.21. [DOI] [PubMed] [Google Scholar]
- 9.Brundage R A, Smith G A, Camilli A, Theriot J A, Portnoy D A. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc Natl Acad Sci USA. 1993;90:11890–11894. doi: 10.1073/pnas.90.24.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christiansen G, Birkelund S. A review of Chlamydia pneumoniae. Eur Microbiol. 1992;1:24–29. [Google Scholar]
- 11.Clausen J D, Gunna C, Holst H U, Birkelund S. Chlamydia trachomatis utilizes the host cell microtubule network during early events of infection. Mol Microbiol. 1997;25:441–449. doi: 10.1046/j.1365-2958.1997.4591832.x. [DOI] [PubMed] [Google Scholar]
- 12.Coles T, Reynolds D J, Herper A, Devitt A, Pearce J H. Low-nutrient induction of abnormal chlamydial development: a novel component of chlamydial pathogenesis. FEMS Microbiol Lett. 1993;106:193–200. doi: 10.1111/j.1574-6968.1993.tb05958.x. [DOI] [PubMed] [Google Scholar]
- 13.Crocker T T, Pelc S R, Nielson B I, Eastwood J M, Banks J. Population dynamics and deoxyribonucleic acid synthesis in HeLa cells infected with an ornithosis agent. J Infect Dis. 1965;115:105–122. doi: 10.1093/infdis/115.2.105. [DOI] [PubMed] [Google Scholar]
- 14.Escalante-Ochoa C, Ducatelle R, Haesebrouck F. The intracellular life of Chlamydia psittaci: how do the bacteria interact with the host cell? FEMS Microbiol Rev. 1998;22:65–78. doi: 10.1111/j.1574-6976.1998.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 15.Fan T, Hang H, Hu H, Shi L, McClarty G A, Nance D M, Greenberg A H, Zhong G. Inhibition of apoptosis in Chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med. 1998;187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fath K R, Trimbur G M, Burgess D R. Molecular motors are differentially distributed on Golgi membranes from polarized epithelial cells. J Cell Biol. 1994;126:661–675. doi: 10.1083/jcb.126.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fawaz F S, Ooij C, Homola E, Mutka S C, Engel J N. Infection with Chlamydia trachomatis alters the tyrosine phosphorylation and/or localization of several host cell proteins including cortactin. Infect Immun. 1997;65:5301–5308. doi: 10.1128/iai.65.12.5301-5308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feiguin F, Ferreira A, Kosik K S, Caceres A. Kinesin-mediated organelle translocation revealed by specific cellular manipulations. J Cell Biol. 1994;4:1021–1039. doi: 10.1083/jcb.127.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fennell D F, Whatley R E, McIntyre T M, Prescott S M, Zimmerman G A. Endothelial cells reestablish functional integrity after reversible permeabilization. Arteriosclerosis Thrombosis. 1991;11:97–106. doi: 10.1161/01.atv.11.1.97. [DOI] [PubMed] [Google Scholar]
- 20.Fox L A, Sawin K E, Sale W S. Kinesin-related proteins in eukaryotic flagella. J Cell Sci. 1994;107:1545–1550. doi: 10.1242/jcs.107.6.1545. [DOI] [PubMed] [Google Scholar]
- 21.Hackstadt T, Rockey D D, Heinzen R A, Scidmore M A. Chlamydia trachomatis interrupts an exocytic pathway to acquire endogenously synthesized sphingomyelin in transit from the Golgi apparatus to the plasma membrane. EMBO J. 1996;15:964–977. [PMC free article] [PubMed] [Google Scholar]
- 22.Hackstadt T, Scidmore M A, Rockey D D. Lipid metabolism of Chlamydia trachomatis-infected cells: directed trafficking of Golgi derived sphingolipids to the chlamydial inclusion. Proc Natl Acad Sci USA. 1995;92:4877–4821. doi: 10.1073/pnas.92.11.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatch T P, Al-Hossainy E, Silverman J A. Adenine nucleotide and lysine transport in Chlamydia psittaci. J Bacteriol. 1982;150:662–670. doi: 10.1128/jb.150.2.662-670.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higashi N. Electron microscopic studies on the mode of reproduction of trachoma virus and psittacosis virus in cell cultures. Exp Mol Pathol. 1955;4:24–39. doi: 10.1016/0014-4800(65)90021-3. [DOI] [PubMed] [Google Scholar]
- 25.Hodinka R L, Davis C H, Choong J, Wyrick P B. Ultrastructural study of endocytosis of Chlamydia trachomatis by McCoy cells. Infect Immun. 1988;56:1456–1463. doi: 10.1128/iai.56.6.1456-1463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holzbaur E L F, Valle R B. Dyneins: molecular structure and cellular function. Annu Rev Cell Biol. 1994;10:339–372. doi: 10.1146/annurev.cb.10.110194.002011. [DOI] [PubMed] [Google Scholar]
- 27.Horoschak K D, Moulder J W. Division of single host cells after infection with chlamydiae. Infect Immun. 1978;19:281–286. doi: 10.1128/iai.19.1.281-286.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuntzentson S A, Basiberg E A, Shanina N A, Magretova N A, Chernyak N M, Gelfand V I. The quaternary structure of bovine brain kinesin. EMBO J. 1988;7:353–356. doi: 10.1002/j.1460-2075.1988.tb02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee K-D, Hollencbeck P J. Phosphorylation of kinesin in vivo correlates with organelle association and neurite outgrowth. J Biol Chem. 1995;270:5600–5605. doi: 10.1074/jbc.270.10.5600. [DOI] [PubMed] [Google Scholar]
- 30.Leopold P L, Pfister K K, Bloom G S, Brady S T. Association of kinesin heavy and light chains with purified membrane bounded organelles from mammalian cells. J Cell Biol. 1989;109:819. [Google Scholar]
- 31.Leopold P L, McDowall A W, Pfister K K, Bloom G S, Brady S T. Association of kinesin with characterized membrane-bounded organelles. Cell Motil Cytoskel. 1992;23:19–33. doi: 10.1002/cm.970230104. [DOI] [PubMed] [Google Scholar]
- 32.Lippincott-Schwartz J, Cole N B, Marotta A, Conrad P A, Bloom G S. Kinesin is the motor for microtubule-mediated Golgi-to-ER membrane traffic. J Cell Biol. 1995;128:293–306. doi: 10.1083/jcb.128.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louis C, Nicolas G, Eb F, Lefebvre J-F, Orfila J. Modifications of the envelope of Chlamydia psittaci during its developmental cycle: freeze-fracture study of complementary replicas. J Bacteriol. 1980;141:868–875. doi: 10.1128/jb.141.2.868-875.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsumoto A. Isolation and electron microscopic observations of intracytoplasmic inclusions containing Chlamydia psittaci. J Bacteriol. 1981;145:606–612. doi: 10.1128/jb.145.1.605-612.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsumoto A, Bessho H, Uehira K, Suda T. Morphological studies of the association of mitochondria with chlamydial inclusions and the fusion of chlamydial inclusions. J Electron Microsc. 1991;40:356–363. [PubMed] [Google Scholar]
- 36.McIlvain J M, Jr, Burkhardt J K, Hamm-Alvarez S, Argon Y, Sheetz M P. Regulation of kinesin activity by phosphorylation of kinesin-associated proteins. J Biol Chem. 1994;269:19176–19182. [PubMed] [Google Scholar]
- 37.Mellman I. Enigma variations: protein mediators of membrane fusion. Cell. 1995;82:869–872. doi: 10.1016/0092-8674(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 38.Morrison R P, Belland R J, Lyng K, Caldwell H D. Chlamydial disease pathogenesis: the 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989;170:1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moulder J W. Intracellular parasitism: life in an extreme environment. J Infect Dis. 1974;130:300–306. doi: 10.1093/infdis/130.3.300. [DOI] [PubMed] [Google Scholar]
- 40.Oda H, Stockert R J, Collins C, Wang H, Novikof P M, Satir P, Wolkoff A W. Interaction of the microtubule cytoskeleton with endocytic vesicles and cytoplasmic dynein in cultured rat hepatocytes. J Biol Chem. 1995;270:15242–15249. doi: 10.1074/jbc.270.25.15242. [DOI] [PubMed] [Google Scholar]
- 41.Ojcius D M, Degani H, Mispetter J, Dautry-Varsati A. Enhancement of ATP levels and glucose metabolism during an infection with Chlamydia. J Biol Chem. 1998;273:7052–7058. doi: 10.1074/jbc.273.12.7052. [DOI] [PubMed] [Google Scholar]
- 42.Paul T R, Knight S T, Raulston J E, Wyrick P B. Delivery of azithromycin to Chlamydia trachomatis-infected polarized human endometrial epithelial cells by polymorphonuclear leucocytes. J Antimicrob Chemother. 1997;39:623–630. doi: 10.1093/jac/39.5.623. [DOI] [PubMed] [Google Scholar]
- 43.Peterson E M, De la Maza L M. Chlamydia parasitism: ultrastructural characterization of the interaction between the chlamydial cell envelope and the host cell. J Bacteriol. 1988;170:1389–1392. doi: 10.1128/jb.170.3.1389-1392.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pistor S, Chakraborty T, Niebuhr K, Domann E, Wehland J. The ActA protein of Listeria monocytogenes acts as a nucleator inducing reorganization of the actin cytoskeleton. EMBO J. 1994;13:758–763. doi: 10.1002/j.1460-2075.1994.tb06318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schramm N, Wyrick P B. Cytoskeletal requirements in Chlamydia trachomatis infection of host cells. Infect Immun. 1995;63:324–332. doi: 10.1128/iai.63.1.324-332.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sinai A P, Joiner K A. Safe haven: the cell biology of non-fusogenic pathogen vacuoles. Annu Rev Microbiol. 1997;51:415–462. doi: 10.1146/annurev.micro.51.1.415. [DOI] [PubMed] [Google Scholar]
- 47.Storz J, Spears P. Chlamydiales: properties, developmental cycle and effect on eukaryotic host cells. Curr Top Microbiol Immunol. 1978;76:165–212. doi: 10.1007/978-3-642-66653-7_5. [DOI] [PubMed] [Google Scholar]
- 48.Storz J, Todd W, Schnorr K L. Chlamydial infection: breach of host cellular barriers. In: Roth A J, editor. Virulence mechanisms of bacterial pathogens. Washington, D.C: American Society for Microbiology; 1988. pp. 161–183. [Google Scholar]
- 49.Sweney H L, Holzbaur E L F. Mutational analysis of motor proteins. Annu Rev Physiol. 1996;58:751–792. doi: 10.1146/annurev.ph.58.030196.003535. [DOI] [PubMed] [Google Scholar]
- 50.Tamura A A, Matsumoto A, Manure G P, Higashi N. Electron microscopic observations on the structure of the envelopes of mature elementary bodies and development reticulate forms of Chlamydia psittaci. J Bacteriol. 1971;105:355–360. doi: 10.1128/jb.105.1.355-360.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taraska T, Ward D M, Ajioka R S, Wyrick P B, Davis-Kaplan S R, Davis C H, Kaplan J. The late chlamydial inclusion membrane is not derived from the endocytic pathway and is relatively deficient in host proteins. Infect Immun. 1996;64:3713–3727. doi: 10.1128/iai.64.9.3713-3727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaisberg E A, Grissom P M, McIntosh J R. Mammalian cells express three distinct dynein heavy chains that are localized to different cytoplasmic organelles. J Cell Biol. 1996;133:831–842. doi: 10.1083/jcb.133.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vanrompay D, Charlier G, Ducatelle R, Haesebrouck F. Ultrastructural changes in avian Chlamydia psittaci serovar A-, B-, and D-infected buffalo green monkey cells. Infect Immun. 1996;64:1265–1271. doi: 10.1128/iai.64.4.1265-1271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wyrick P B, Richmond S J. Biology of chlamydiae. Am Vet Med Assoc. 1989;195:1507–1512. [PubMed] [Google Scholar]