Abstract
Our past work has shown that long, flexible type IV pili (single or in bundles) are the predominant pili expressed on fecal isolates of diarrhea-associated species of Aeromonas (Aeromonas veronii biovar sobria and A. caviae). They represent a family of type IV pili which we have designated Bfp (for bundle-forming pili). Reports from Japan suggest that Bfp are intestinal colonization factors. This study presents compelling evidence to support this conclusion. Aeromonas bacteria and/or Bfp purified from a strain of A. veronii biovar sobria were shown to adhere to epithelial and intestinal cell lines, freshly isolated human enterocytes, and fresh and fixed human and rabbit intestinal tissues, as determined by light and electron microscopy and immunohistochemical detection. Removal of Bfp by mechanical means decreased adhesion to cell lines by up to 80%. Purified Bfp blocked adhesion of the test strain to intestinal cells in a dose-dependent manner. Adhesion was also blocked by the Fab fraction of anti-Bfp immunoglobulin G. Moreover, ultrastructural studies (ruthenium red staining and transmission and scanning electron microscopy) demonstrated for the first time that Aeromonas adhesion to human enterocytes is pilus mediated and suggested that Bfp may also promote colonization by forming bacterium-to-bacterium linkages. Bfp-positive isolates examined for type IV pilus-mediated twitching motility in agar and slide culture assays developed for Pseudomonas aeruginosa did not, however, exhibit this function.
Some strains of Aeromonas bacteria (aeromonads) are a significant, yet often underrated, cause of gastroenteritis, particularly in children under 5 years old and older persons in the summer (6, 18). They can also cause life-threatening infections, such as septicemia and meningitis (some of which may be acquired by the oral route) in immunocompromised individuals (38). Aeromonads are ubiquitous in water and a wide range of foods. Many strains possess an impressive array of putative virulence determinants. Such strains may also grow at refrigeration temperatures, increasing concern about their potential to emerge as an important public health threat (15, 20).
Approximately 85% of diarrhea-associated isolates belong to the species Aeromonas hydrophila (HG1 and HG3), A. veronii biovar sobria (HG8/10) (formerly A. sobria), and A. caviae (HG4) (17, 18). However, it is not yet possible to identify virulent strains definitively. Clinical manifestations vary from a mild, self-limiting diarrhea to severe or persistent diarrhea or dysentery (6, 18). These different clinical manifestations suggest that, as for Escherichia coli pathotypes, Aeromonas virulence is multifactorial (36). A critical step in pathogenesis for all virulent strains is likely to be adhesion to and colonization of the intestinal mucosa.
Intestinal colonization is a complex process, and a number of putative adhesins have been described for Aeromonas species (5, 11–14, 16, 33, 41). The best studied have been pilus adhesins. However, confusion has arisen concerning Aeromonas flexible pilus types in recent years. A plasmid-encoded flexible pilus characterized by Ho and colleagues (9, 10) may in fact be a filamentous phage (46). Other flexible pili, which we have designated Bfp, for bundle-forming pili, have now been purified from all Aeromonas species commonly associated with diarrheal infection. These purified pili represent a family of type IV pili with N-terminal amino acid sequence homology and pilin molecular masses of 19 to 23 kDa. Despite their tendency for bundle formation, the pilins of this family have closer N-terminal amino acid homology to the classical type IVA pilins, such as that of the mannose-sensitive hemagglutin pilus of Vibrio cholerae, than they do to the type IVB pilins of the Bfp of enteropathogenic E. coli and the toxin-coregulated pilus of V. cholerae (11–14, 16, 26, 27, 44). As yet, there has been no published genetic characterization of this pilus type. However, genetic analysis of Aeromonas species has identified a second type IV pilus family. A pilus biogenesis gene cluster, tapABCD, was originally cloned from a strain of A. hydrophila, and the product was designated Tap, for type IV Aeromonas pili (37). We subsequently cloned this cluster from a strain of A. veronii biovar sobria from which we had purified Bfp. We established that this strain and those from which Bfp had been purified all had the potential to produce this second type IV pilus (2). Tap pilins (predicted molecular mass of ∼17 kDa) are more closely related to the classical type IVA pilin proteins of Dichelobacter nodosus, Pseudomonas aeruginosa, and Neisseria gonorrhoeae than they are to Aeromonas Bfp pilin proteins (2).
The functional significance of the above type IV pilus families (Bfp and Tap) for Aeromonas species has been studied little. Type IV pili are recognized as key virulence determinants for a wide variety of other gram-negative bacterial pathogens. Their key functions are considered to be mediation of adhesion to epithelial cells and involvement in flagellum-independent cell movement across solid surfaces, known as twitching motility (44). Both of these activities are thought to contribute to colonization at mucosal surfaces. Studies with P. aeruginosa have led to the suggestion that twitching motility may be the primary function of type IV pili, as mutants of this organism which retain pili but have lost twitching motility are noninfectious (8, 32). However, not all type IV pili of enteropathogenic bacteria have been reported to mediate adherence to enterocytes (43), and there are virtually no studies of twitching motility in Aeromonas species.
Very little is yet known about the possible role of Tap in Aeromonas virulence. Strains from which Bfp have been purified do not appear to express Tap when shed from feces or when grown under standard in vitro conditions and examined by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) analysis and immunogold electron microscopy (11–14, 16, 26). Unpublished results from our laboratory show that a mutation in the structural pilin gene, tapA, does not decrease Aeromonas adhesion to epithelial and intestinal cell lines when bacteria are grown under standard in vitro conditions (2a). Further studies of Tap function(s) await elucidation of the factors influencing Tap expression.
There is some evidence from in vitro studies in Japan that Bfp are intestinal adhesins. We have demonstrated that the expression of Bfp correlates with adhesive ability (22, 23, 26). We have confirmed the findings of Carrello and colleagues (4) that the removal of flexible pili from HEp-2 cell-adherent strains by mechanical shearing can reduce bacterial adherence by up to 80% for some strains (22, 27). Japanese studies have shown that purified pili and antipilus antibody (Fab fraction) blocked Aeromonas adhesion to formalin-fixed rabbit intestinal tissue (11–14, 16). To date, however, there have been very few ultrastructural analyses of Aeromonas-host interactions. Such studies have provided valuable insights into the adhesive mechanisms of enteropathogens such as E. coli and V. cholerae (28, 29, 39) and are required to demonstrate that Bfp are intestinal adhesins.
The overall aim of this study was to investigate the functions of Aeromonas Bfp. Using ultrastructural and immunohistochemical analyses and Bfp that we had purified and characterized (26), we wished to prove the current hypothesis that these type IV pili are important intestinal colonization factors and show that they mediate attachment to human enterocytes. The ability of Aeromonas Bfp to exhibit twitching motility in assays developed for P. aeruginosa type IV pili was also examined.
MATERIALS AND METHODS
Bacterial strains.
Full details of A. veronii biovar sobria BC88 and A. caviae 195, from which we purified and characterized Bfp, are given elsewhere (26, 27). In brief, both strains were isolated from the feces of children with severe diarrhea or dysentery. The strains had the characteristic phenotype of their respective species (3, 40). Species identification was also confirmed by genetic typing (rRNA gene restriction patterns) (31). Both strains were highly adherent to epithelial and intestinal cell lines (HEp-2, Caco-2, and Henle 407 cells). P. aeruginosa PAO1 and an isogenic mutant of this strain lacking a functional pilin structural gene, strain PAO1pilA, were kindly provided by Richard Alm, Centre for Molecular and Cellular Biology, The University of Queensland, Brisbane, Queensland, Australia. Long-term storage of strains was done with glycerol-peptone (1:4; glycerol–1% [wt/vol] bacteriological peptone L37 [Oxoid, Basingstoke, United Kingdom]) at −70°C. Short-term storage was done with minimal maintenance medium at room temperature (24).
Growth conditions.
As Bfp are best expressed when bacteria are grown in liquid media at environmental temperatures (23), bacterial cultures for pilus purification studies and some adhesion assays were grown in tryptone soy broth supplemented with yeast extract L21 (Oxoid) (TSBY) at 22°C under static conditions for 48 h. In other experiments, isolates were plated from storage onto tryptone soy agar with yeast extract L21 (Oxoid) (TSAY) or brain heart infusion agar (Oxoid) (BHIA) and grown at 37°C for 18 to 24 h. Isolated colonies were then inoculated into 10 ml of TSBY and grown statically for 18 h at 37°C. Log-phase cultures were prepared by adding 0.5 ml of these overnight cultures to 10 ml of TSBY, followed by static incubation for 3.5 h at 37°C.
For the twitching motility agar assay, Aeromonas strains were grown under a variety of conditions before being used in the standard motility assay (see below). These included growth on TSAY with altered incubation conditions (temperature, anaerobiosis, and elevated CO2 levels), growth on TSAY containing supplements (the iron chelator deferoxamine mesylate [100 μM final concentration] or glucose [24 mM final concentration]), and growth in liquid media (TSBY or Luria-Bertani broth [Oxoid]) under a variety of conditions, as for TSAY. Repeated passage in broth, pH, osmolarity, and bacterial growth phase were other variables examined for their possible effects on results obtained in the twitching motility agar assay. For the slide culture assay of twitching motility, Aeromonas was tested after growth on TSAY at 37°C for 18 h and in TSBY at 22°C for 48 h.
Purification of pili.
Bfp were isolated as previously described (26, 27). Briefly, the organisms from 2 to 4 liters of a TSBY culture (22°C, 48 h, static) were harvested, suspended in 80 ml of 0.05 M Tris-hydrochloride (pH 7.4), and chilled on ice. Pili were removed from the bacterial cell surface by mechanical shearing (Omni-mixer; Omni International, Waterbury, Conn.) (speed 5 for 5-s intervals with intermittent cooling for 10 min). Bacteria were removed by centrifugation (26,000 × g, 30 min, 4°C) and filtration through a 0.2-μm-pore-size membrane. Pilin protein was recovered by precipitation with 5.8% sodium chloride and 2% polyethylene glycol 6000 (Rhône-Polenc, Manchester, United Kingdom). Precipitated protein was collected by centrifugation (26,000 × g, 30 min, 4°C) and resuspended in Tris buffer. Pili purified from seven preparations (26.5 liters of bacterial culture in total) were pooled in ∼5 ml of Tris buffer containing 0.02% sodium azide and stored in 500-μl aliquots at −20°C. Protein concentration was estimated with the Bio-Rad (Hercules, Calif.) DC protein assay system to be ∼2 mg per ml. This preparation, designated purified pili, was examined by electron microscopy and SDS-PAGE analysis (26).
Electrophoresis.
SDS-PAGE analysis of pilus preparations was performed as described by Laemmli (30) with a discontinuous 15% acrylamide gel (Mini-PROTEAN II; Bio-Rad).
N-terminal amino acid sequence analysis.
Proteins were transferred from SDS-polyacrylamide gels to polyvinylidene difluoride membranes (Mini-Transblot cell; Bio-Rad) and visualized with amido black (1% [wt/vol] in 40% [vol/vol] methanol–1% [vol/vol] acetic acid). They were sequenced by automated Edman degradation by use of a Porton P 12090 apparatus equipped for on-line phenylthiohydantion amino acid analysis at the Australian Proteome Analysis Facility, Macquarie University, Sydney, New South Wales, Australia.
Preparation of antibody.
Antiserum to the Bfp of A. veronii biovar sobria BC88 was prepared by immunizing New Zealand White rabbits subcutaneously with the 21-kDa pilin band (∼100 μg of protein) purified from this strain (26). The immunoglobulin G (IgG) fraction of this antiserum and normal rabbit serum from a control animal were obtained by precipitation with 33% ammonium sulfate. The Fab fraction of the IgG was prepared by papain (Sigma, St. Louis, Mo.) digestion and carboxymethyl cellulose (ICN Biomedicals Inc., Costa Mesa, Calif.) column chromatography.
Cell cultures.
HEp-2 cells (American Type Culture Collection [ATCC] CCL 23), Caco-2 cells (ATCC HTB 37), and Henle 407 human intestinal epithelial cells (ATCC CCL 6) are routinely maintained in our laboratory. Cells were grown in Eagle’s minimal essential medium (ICN) containing 5 to 10% fetal calf serum (CSL, Parkville, Victoria, Australia) (MEM+FCS). For the standard adhesion assay, semiconfluent monolayers of these cell lines were grown on 12-mm glass coverslips (Vitromed, Basel, Switzerland) in 24-well plates (Corning, New York, N.Y.) or on ACLAR plastic film (ProSciTech, Thuringowa, Queensland, Australia) cut to fit the wells. For transmission electron microscopy (TEM), cell monolayers were grown on the latter and/or on Thermanox plastic coverslips (Nunc Inc., Naperville, Ill.) to facilitate thin sectioning. Cell monolayers were washed twice with phosphate-buffered saline (PBS) before the addition of bacterial cells.
Enterocyte isolation.
The isolation procedure of Knutton et al. (29) was used to prepare fresh populations of intestinal epithelial cells which retained their characteristic columnar morphology and had clearly defined brush borders. In brief, duodenal and colonic mucosal biopsy samples were taken with informed consent from adult patients undergoing diagnostic endoscopies and colonoscopies. Bowel samples were taken from individuals undergoing colorectal surgery. Most of these samples were from the colon or terminal ileum. Biopsy and tissue samples were transported to the laboratory on ice in Ham F-10 medium (Sigma) containing gentamicin (100 μg/ml) and 5% fetal calf serum. They were washed twice in ice-cold PBS to remove erythrocytes and debris. Bowel samples were trimmed of fat and dissected into ∼1-cm2 portions. To prepare isolated enterocytes, tissue specimens or up to eight pooled biopsy samples were incubated in 5 ml of EDTA buffer (96 mM NaCl, 8 mM KH2PO4, 5.6 mM Na2HPO4, 1.5 mM KCl, 10 mM EDTA [pH 6.8]) for 5 min at room temperature with gentle shaking. They were transferred to fresh ice-cold Ham F-10 medium, and epithelial cells were released by mild shearing with a wide-bore Pasteur pipette. Enterocytes were sedimented at 100 × g for 2 min. Viability was assessed by trypan blue (∼0.2%) dye exclusion and was generally between 60 and 70%.
Rabbit intestinal tissue.
Samples (∼1 cm2) of rabbit ileum from three consecutive sites were washed three times in PBS and used directly for adhesion assays (fresh tissue) or fixed in formalin (fixed tissue) for immunohistochemical pilus-binding studies. Formalin-fixed tissue was thoroughly washed in PBS for 6 h before use in adhesion assays.
Adhesion to cell lines.
Adhesion to cells lines was accomplished as described elsewhere (7, 22). In brief, 1 ml of bacteria (∼1 × 106 to 5 × 106 CFU) was inoculated onto cell monolayers and incubated for 60 to 90 min at 37°C in 5% CO2. Nonadherent bacteria were removed by four washes (PBS), and the monolayers were fixed with methanol-acetic acid (3:1, 1 ml, 5 min), stained with May- Grünwald and Giemsa stains (BDH, Poole, United Kingdom), and mounted for assessment of adhesion by light microscopy.
Adhesion to human enterocytes.
A 100-μl portion of bacterial culture (∼5 × 106 CFU per ml) was added to 1 ml of enterocyte suspension (∼105 cells) prepared as described above, and the mixture was incubated (37°C, 10 to 90 min, 5% CO2). The suspension was centrifuged (100 × g, 1 min), and nonadherent bacteria were removed by three washes (PBS). Brush border adhesion of bacteria was assessed by light microscopy of hematoxylin-eosin-stained specimens.
Adhesion to intestinal tissue.
Human or rabbit intestinal tissue (fresh or fixed) was placed in microtiter plates containing MEM+FCS. Bacteria (1 × 106 to 5 × 106 CFU) were added, and the samples were incubated (90 min, 37°C, 5% CO2). After four washes in PBS to remove nonadherent bacteria, specimens were fixed in formalin, embedded in paraffin, and sectioned. Sections (∼10 μm) on glass slides were deparaffinized, hydrated, and stained with hematoxylin-eosin for light microscopy examination.
Adhesion inhibition tests.
Adhesion inhibition assays were performed with purified pili, antipilus antibody, and the Fab fraction of this antibody. (At low immune serum dilutions, the intact antibody agglutinates organisms.) In brief, cell monolayers were pretreated (30 min, room temperature [RT]) with increasing amounts (0.1 to 0.6 mg per ml) of purified pili prior to the standard adhesion assay. Alternatively, for antibody blocking experiments, 1-ml aliquots of bacteria (∼5 × 106 CFU per ml) were incubated (30 min, RT) with equal volumes of high (nonagglutinating) dilutions (1:500 to 1:2,500) of immune antipilus serum or preimmune control serum prior to the adhesion assay. In separate experiments, bacterial cells were pretreated (30 min, RT) with a single concentration (2 mg/ml) of the antipilus antibody Fab fraction or with a Fab fraction from normal rabbit serum prior to the adhesion assay.
Data were expressed as the means ± standard deviations. Student’s t test was use to evaluate the data.
Immunological techniques.
Purified pili adherent to cell lines (HEp-2 and Henle 407 cells) and to fresh and fixed rabbit intestinal tissue were detected immunohistochemically with antipilus serum (1:500 to 1:2,500) and horseradish peroxidase-conjugated goat anti-rabbit IgG serum (Vectastain; Vector Laboratories, Burlingame, Calif.). Semiconfluent cell monolayers or intestinal tissue samples were incubated with the purified pilus suspension (200 μg/ml) for 30 min at RT. After four washes in PBS, cell monolayers were stained directly according to the manufacturer’s instructions (Vectastain). Tissue sections were prepared for routine histological examination. Immunohistochemical reactions were processed on glass slides. After being washed in tap water, the slides were counterstained with hematoxylin-eosin. Control cell monolayers and tissue samples were processed identically, except that pili were omitted from the incubation step or a control protein, bovine serum albumin (Sigma) at 200 μg/ml, was substituted.
TEM.
Bacterial cells and purified pilus preparations were adsorbed onto Formvar-coated copper grids and negatively stained (1% uranyl acetate, 30 s). They were examined with a Philips 410 electron microscope at 80 kV (23). The adhesion of bacteria to intestinal tissue, cell monolayers, and human enterocytes was examined by thin-section electron microscopy. In brief, cells and tissue were fixed in 2.5% glutaraldehyde containing 0.05% ruthenium red. Samples were postfixed in 1% osmium tetroxide and then in 5% uranyl acetate, dehydrated through a graded series of ethanol and propylene oxide, and embedded in Epon (ProSciTech) at 60°C for 48 h. Thin sections were cut (Reichert ultramicrotome; Leica, Solms, Germany) and stained with 5% uranyl acetate (30 min) and 1% lead citrate (5 min).
SEM.
Scanning electron microscopy (SEM) (Philips 505 scanning electron microscope) at the Central Science Laboratory, University of Tasmania, Hobart, Tasmania, Australia, was used to visualize bacteria adhering to formalin-fixed rabbit intestinal tissue by the methods of Nakasone and Iwanaga (35) with the modification that PBS was used instead of Kreb’s Ringer Tris buffer.
FESEM.
Field-emission SEM (FESEM) was performed at the Centre for Electron Microscopy and Microstructure Analysis (CEMMSA), University of Adelaide, Adelaide, South Australia, Australia. Bacteria adhering to cell monolayers were fixed as for TEM. Samples were dehydrated through graded acetone solutions and dried to the critical point. Specimens were mounted on stubs, coated with gold, and examined with a Philips XL30 FESEM microscope at 15 kV.
Twitching motility agar and slide culture assays.
Twitching motility of Aeromonas type IV Bfp was assayed by two methods, a subsurface agar assay (1, 32) and a slide culture assay (42). For the first method, Aeromonas strains were grown and assayed for twitching motility under a variety of conditions (variations in temperature, pH, CO2, osmolarity, iron, and glucose levels) in addition to those developed for the standard Pseudomonas assay. P. aeruginosa PAO1, known to exhibit twitching motility, and a nontwitching mutant of this strain, strain PAO1pilA, served as positive and negative controls, respectively, and were included in each assay (standard and modified procedures). In brief, a thin (∼3-mm-thick) 1% Luria-Bertani agar (Oxoid) plate was inoculated with the bacteria being tested with a 27-gauge needle pushed through the agar until it touched the plastic. After incubation (37°C, 20 h), the agar was dehydrated by being blotted under weight and then was stained with 0.5% Coomassie blue R-250 (in 20% methanol–10% acetic acid) to visualize the zone of twitching at the plastic-agar interface. For the slide culture method, bacterial strains (A. veronii biovar sobria BC88 and P. aeruginosa control strains) were point inoculated onto triplicate sterile microscope slides that had been coated with GelGro medium (ICN). The inoculum was covered with a sterile coverslip, and the slide cultures were incubated for 4 h at 37°C in a humid environment to prevent drying of the medium. Slides were examined for twitching motility by use of an Olympus BX50 microscope with Nomarski optics (×400 magnification).
RESULTS
Piliation and adhesion to epithelial cells of Aeromonas species.
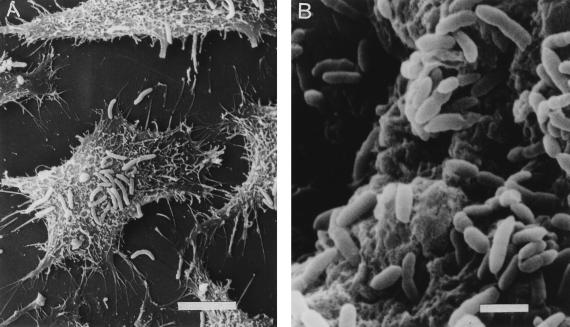
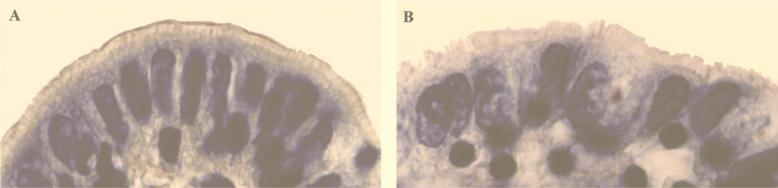
Our past studies have shown that fecal isolates of A. veronii biovar sobria and A. caviae generally adhere well (≥10 bacteria per cell) to cultured epithelial cell lines, such as HEp-2 cells, and to fixed rabbit intestinal tissue (7, 21, 22, 25). A. veronii biovar sobria BC88 exhibited such high-level adhesion (Fig. 1). In this study, we showed that strain BC88 is also able to adhere to a human intestinal cell line (Henle 407) and to freshly isolated human and rabbit intestinal tissues and human enterocytes (Fig. 2).
FIG. 1.
Scanning electron micrographs of A. veronii biovar sobria BC88 adhesion to a cultured HEp-2 cell (A) and formalin-fixed rabbit intestinal villi (B). Bars, 6 μm (A) and 2 μm (B).
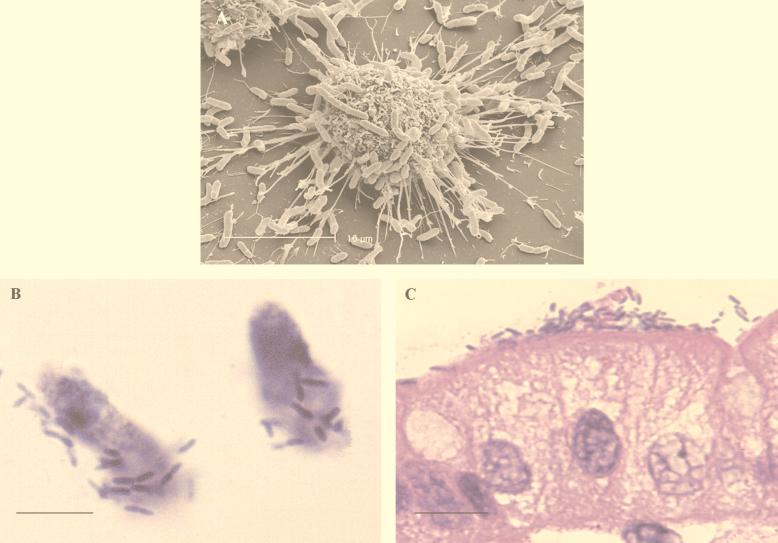
FIG. 2.
Scanning electron micrograph of A. veronii biovar sobria BC88 adhesion to a cultured small intestinal cell (Henle 407 cell) (A), a freshly isolated human enterocyte (bright-field microscopy) (B), and fresh human intestinal tissue (hematoxylin-eosin stain) (C). Bars, 10 μm (A), 10 μm (B), and 15 μm (C).
Bfp have been implicated in this adhesion process. These pili are the predominant pili on strain BC88 under the bacterial growth conditions used in adhesion experiments (pilus purification and immunogold electron microscopy) (26). Their removal by mechanical blending decreased the adhesion of this strain to HEp-2 and Caco-2 cells by up to 80% (22). In this study, adhesion to Henle 407 cells was similarly decreased (up to 80%) following the mechanical removal of pili. Adherence to fresh and fixed human intestinal tissue was also markedly decreased but was not quantitated.
Immunohistochemical studies with purified pili.
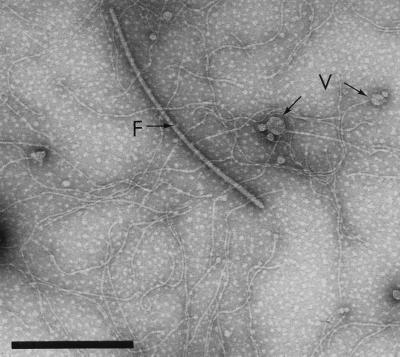
To establish that cell adhesion by A. veronii biovar sobria BC88 was indeed Bfp mediated, preparations of Bfp from this strain were purified and pooled for use in immunohistochemical pilus adhesion and bacterial adhesion inhibition experiments. TEM showed that this preparation (purified pili) contained predominantly long, flexible pili, although some contaminating polar flagellar fragments and outer membrane vesicles remained (Fig. 3). SDS-PAGE analysis of the purified pili revealed two major bands, corresponding to the Bfp pilin (21 kDa) and the polar flagellin (44 kDa). In Western blotting analysis, only the 21-kDa band reacted with the antipilus antibody at 1:1,000 to 1:10,000 dilutions.
FIG. 3.
Transmission electron micrograph of negatively stained crude pili (2% polyethylene glycol 6000-precipitated fraction) from A. veronii biovar sobria BC88 showing predominantly flexible pili, contaminating flagellar pieces (F), and outer membrane vesicles (V). Bar, 0.5 μm.
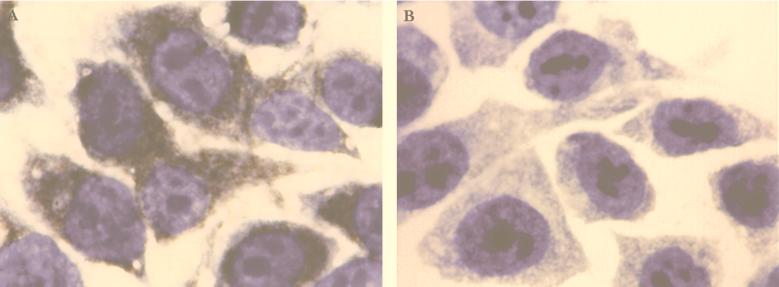
The ability of purified Bfp pili to bind to epithelial and intestinal cell lines (HEp-2 and Henle 407) (Fig. 4) and to fixed and fresh rabbit intestinal tissue (Fig. 5) was examined with anti-Bfp immune serum and an immunoperoxidase detection method. Cells and intestinal epithelium treated with purified pili developed a positive reaction, shown by brown color development on the cell or along the villous surface (Fig. 4A and 5A). Controls (no pili added or bovine serum albumin added instead of purified pili) included in each experiment showed a negative reaction (no color) (Fig. 4B and 5B).
FIG. 4.
Immunohistochemical detection of purified pili of A. veronii biovar sobria BC88 binding to Henle 407 cells. (A) Positive finding for adherent pili. (B) Control cells, to which no pili were added, showed no color reaction.
FIG. 5.
Immunohistochemical detection of purified pili of A. veronii biovar sobria binding to rabbit intestinal villi. (A) Enzymatic coloration of the villous surface showing pilus adhesion. (B) Control intestine, to which no pili were added, showed no color reaction. Sections were counterstained with hematoxylin-eosin.
Adhesion inhibition.
Purified pili blocked the adhesion of A. veronii biovar sobria BC88 to Henle 407 intestinal cells in a dose-dependent manner (Table 1). When tested at a single concentration (0.2 mg/ml), the purified pili also significantly decreased the adhesion of strain BC88 to HEp-2 cells from 12.3 ± 0.88 to 4.7 ± 0.7 bacteria per cell. Adhesion inhibition was specific. Pili from strain BC88 significantly inhibited the adhesion of this strain to Henle 407 and HEp-2 cells but had no significant effect on the adhesion of A. veronii biovar sobria BC96. Values for Henle 407 cells are shown in Table 1; for HEp-2 cells, adhesion values with strain BC96 were 10.2 ± 1.6 and 9.8 ± 1.1 bacteria per cell for no treatment and pretreatment with purified pili, respectively. Adhesion to Henle 407 cells was also significantly inhibited if bacteria were pretreated with nonagglutinating dilutions of antipilus antiserum or the Fab fraction of the antipilus antibody (Table 1). Normal rabbit serum contains anti-Bfp activity (26). Its Fab fraction also significantly inhibited adhesion, although not to the same extent as the IgG fraction of immune serum.
TABLE 1.
Adhesion inhibition test results
| Treatmenta | No. of the following bacteria per Henle 407 cellb:
|
|
|---|---|---|
| BC88 | BC96 | |
| None | 15.4 ± 3.2 | 11.3 ± 1.9 |
| Purified pili from strain BC88 (mg/ml) | ||
| 0.2 | 5.6 ± 0.5c | 9.4 ± 2.1 |
| 0.4 | 3.3 ± 0.2c | 8.9 ± 1.6 |
| 0.6 | 1.8 ± 0.1c | ND |
| BC88 anti-Bfp serum | ||
| 1:2,000 | 10.2 ± 0.1 | 11.1 ± 2.1 |
| 1:1,000 | 7.5 ± 0.2c | 10.9 ± 1.3 |
| Preimmune serum at 1:1,000 | 9.4 ± 0.3 | 10.2 ± 1.7 |
| Fab fraction (2 mg/ml) from: | ||
| BC88 anti-Bfp IgG | 4.6 ± 0.2c | ND |
| Normal rabbit serum IgG | 7.9 ± 0.1c | ND |
Henle 407 cells were pretreated with purified Bfp or bacteria were pretreated with BC88 anti-Bfp serum, preimmune serum, or the Fab fraction of anti-Bfp IgG or normal rabbit serum IgG.
Reported as mean ± standard deviation for triplicate coverslips from two experiments. ND, not done. Bacteria were inoculated onto cell monolayers and incubated for 90 min at 37°C in 5% CO2.
The P value was <0.05 in a comparison with no treatment (t test).
Ultrastructural analysis of cellular adhesion.
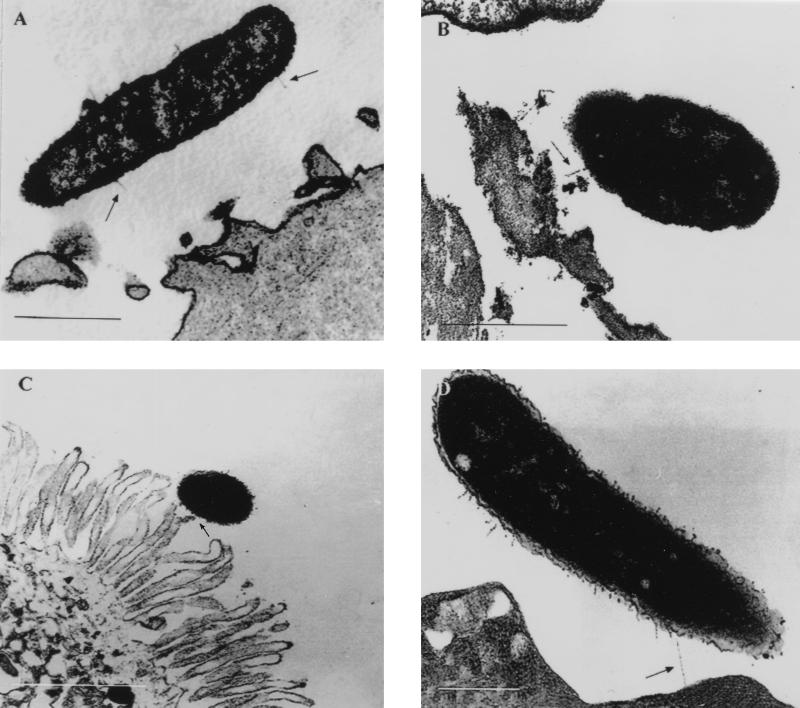
Adhesion of A. veronii biovar sobria BC88 to HEp-2 cells and isolated human enterocytes was examined by thin-section TEM. Preparations were stained with ruthenium red to enhance the visualization of pili. A gap was often seen between adherent bacteria and epithelial cells or enterocytes. Filamentous structures were occasionally visible bridging this gap or were seen in part in the plane of the section. Pili were seen interacting with the microvilli of the brush border of freshly isolated human enterocytes (Fig. 6).
FIG. 6.
Transmission electron micrographs (thin sections, ruthenium red stain) of A. veronii biovar sobria adhering to HEp-2 cells; (A and B) and freshly isolated human enterocytes (C and D). Note the gap between the organism and the cell membrane as well as pili (arrows) extending into space or interacting with the apical brush border. Bars, 0.5 μm (A), 0.5 μm (B), 1 μm (C), and 0.2 μm (D).
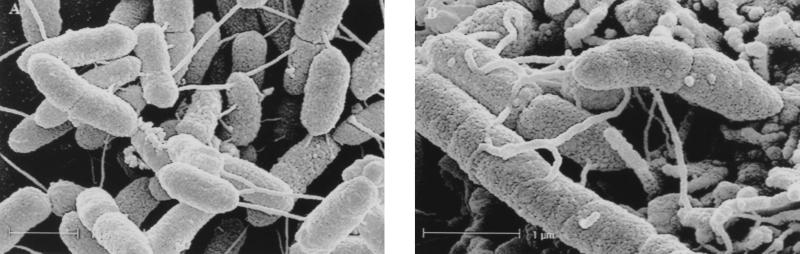
Adhesion of strain BC88 to Henle 407 cells was examined by FESEM. Filamentous structures appeared to link bacteria to each other as well as to the cell surface (Fig. 7).
FIG. 7.
Field-emission scanning electron micrographs of A. veronii biovar sobria BC88 adhering to Henle 407 cells. Filamentous structures (Bfp pili) link bacteria to the cells and also form bacterium-to-bacterium linkages. Bars, 1 μm.
Twitching motility function and Bfp.
Under standard assay conditions (see Materials and Methods), P. aeruginosa PAO1 yielded a mean spreading zone of 21.4 ± 1.2 mm (10 experiments) in the twitching motility subsurface agar assay for type IV pili. No spreading zone was seen with the P. aeruginosa mutant of this strain, PAO1pilA, which has an inactivated type IV pilus subunit gene. Aeromonas strains known to express type IV Bfp (A. veronii biovar sobria BC88 [26], A. caviae CA195 [27], and the Japanese Aeromonas strains Ae1, Ae6, Ae24, and Tap 13 [11–13, 16]) showed no twitching motility zones under standard assay conditions or after growth and assay under a variety of different conditions designed to approximate conditions that might operate in the intestine and/or stimulate type IV pilus expression. Five newly isolated clinical strains of Aeromonas (all from diarrheal feces and highly adhesive to HEp-2 cells) also showed no twitching motility zones under standard assay conditions. Similarly, in the slide culture assay, while the colony edge of the P. aeruginosa positive control strain was highly irregular, the colony edge of A. veronii biovar sobria BC88 was smooth and regular, demonstrating a lack of twitching motility.
DISCUSSION
This study has established that Bfp are likely to be important intestinal colonization factors for diarrheagenic Aeromonas species. Mechanical removal of Bfp from a diarrheal isolate of A. veronii biovar sobria (BC88) (26) significantly decreased the adhesion of this isolate to a variety of epithelial and intestinal cell lines. The adhesion was blocked by pretreatment of the cells with purified pili. Purified Bfp were shown to bind to epithelial cells and fresh and fixed rabbit and human intestinal tissues by immunohistochemistry. Pretreatment of bacteria with the Fab fraction of anti-Bfp serum also inhibited cell line adhesion (HEp-2 and Henle 407 cells). Bfp are antigenically diverse (11–14, 16, 26), and the pilus inhibition study showed that they can bind to different receptors on host cells.
To our knowledge, this is the first ultrastructural analysis of Aeromonas interactions with fresh human enterocytes and intestinal tissue. Ultrastructural studies support the conclusion that Bfp mediate binding to epithelial and intestinal cell lines and to freshly isolated human enterocytes. Pili were seen on adherent bacteria, bridging a gap at the cell surface, or interacting with enterocyte brush borders. TEM and FESEM analyses of strain BC88 adhering to Henle 407 cells also showed filamentous structures binding to cells. As Bfp are the predominant pili expressed on this strain, especially under the bacterial growth conditions used (22, 23), we conclude that the long, flexible pili seen mediating this binding are Bfp. This conclusion is supported by the adhesion inhibition studies and the fact that a mutation in the Tap pilin structural gene did not decrease the adhesion of this strain to intestinal cells (2a). FESEM analysis also revealed that filamentous structures appeared to mediate bacterium-to-bacterium interactions. We have previously observed that bundle formation by this pilus type is most often seen in transmission electron micrographs when bacteria are linked in this way (26). It is possible that these linkages facilitate colony formation at the mucosal surface and that this linkage formation is an important accessory function of Bfp.
Twitching motility was not observed for Bfp-positive Aeromonas species in the agar assay developed for P. aeruginosa for this type IV pilus function, even when bacteria were grown and tested under a wide variety of different conditions designed to mimic those encountered in vivo or known to induce Bfp expression. Moreover, twitching motility was not demonstrated by a Bfp-positive Aeromonas strain in a slide culture assay in which P. aeruginosa clearly exhibited twitching motility. These results suggest that twitching motility may not be an important role for Aeromonas Bfp.
It is likely that Bfp are just one type of a number of adhesins that can contribute to Aeromonas intestinal cell binding. In our study, the removal of Bfp never completely abolished cell adhesion. Other groups have implicated lipopolysaccharide as an adhesin in HEp-2 cell binding and intestinal colonization (5, 33, 34). Thornley and colleagues showed that patterns of adhesion to cell lines varied depending on the bacterial growth conditions, suggesting that Aeromonas bacteria are capable of expressing different adhesins, depending on their surrounding conditions (45). We propose that Bfp are important in the initial adhesion of Aeromonas bacteria to intestinal cells. Environmental strains of A. veronii biovar sobria are generally more heavily piliated that fecal isolates (23, 24). There is some evidence that Bfp promote binding to phagocytic cells, which may represent a selective pressure resulting in a shift in the degree of piliation in vivo (19).
As yet, there has been no published genetic analysis of Bfp. Evaluation of their role as critical intestinal colonization factors for diarrheagenic strains awaits the cloning and mutagenesis of Bfp pilin genes and the testing of wild-type and mutant strains in in vivo models.
ACKNOWLEDGMENTS
We thank Michael Piper for assistance in the preparation of the purified pili and Andrea Stimming, Andrew Boyd, and Annalese Semmler for contributions to the enterocyte and twitching motility investigations.
This work was supported by a grant from the Australian National Health and Medical Research Council.
REFERENCES
- 1.Alm R A, Mattick J S. Identification of a gene, pilV, required for type 4 fimbrial biogenesis in Pseudomonas aeruginosa whose product possesses a pre-pilin-like leader sequence. Mol Microbiol. 1995;16:485–496. doi: 10.1111/j.1365-2958.1995.tb02413.x. [DOI] [PubMed] [Google Scholar]
- 2.Barnett T C, Kirov S M, Strom M S, Sanderson K. Aeromonas spp. possess at least two distinct type IV pilus families. Microb Pathog. 1997;23:241–247. doi: 10.1006/mpat.1997.0152. [DOI] [PubMed] [Google Scholar]
- 2a.Barnett, T. C., S. M. Kirov, and M. S. Strom. Unpublished data.
- 3.Carnahan A M, Behram S, Joseph S W. Aerokey II: a flexible key for identifying clinical Aeromonas species. J Clin Microbiol. 1991;29:2843–2849. doi: 10.1128/jcm.29.12.2843-2849.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrello A, Silburn K A, Budden J R, Chang B J. Adhesion of clinical and environmental Aeromonas isolates to HEp-2 cells. J Med Microbiol. 1988;26:19–27. doi: 10.1099/00222615-26-1-19. [DOI] [PubMed] [Google Scholar]
- 5.Francki K T, Chang B J. Variable expression of O-antigen and the role of lipopolysaccharide as an adhesin in Aeromonas sobria. FEMS Microbiol Lett. 1994;122:97–102. doi: 10.1111/j.1574-6968.1994.tb07150.x. [DOI] [PubMed] [Google Scholar]
- 6.Gracey M, Burke V, Robinson J. Aeromonas-associated gastroenteritis. Lancet. 1982;ii:1304–1306. doi: 10.1016/s0140-6736(82)91510-0. [DOI] [PubMed] [Google Scholar]
- 7.Grey P A, Kirov S M. Adherence to HEp-2 cells and enteropathogenic potential of Aeromonas spp. Epidemiol Infect. 1993;110:279–287. doi: 10.1017/s0950268800068217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazlett L D, Moon M M, Singh A, Berk R S, Rudner X L. Analysis of adhesion, piliation, protease production and ocular infectivity of several P. aeruginosa strains. Curr Eye Res. 1991;10:351–362. doi: 10.3109/02713689108996341. [DOI] [PubMed] [Google Scholar]
- 9.Ho A S Y, Mietzner T A, Smith A J, Schoolnik G K. The pili of Aeromonas hydrophila: identification of an environmentally regulated “mini pilin.”. J Exp Med. 1990;172:795–806. doi: 10.1084/jem.172.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho A S Y, Sohel I, Schoolnik G K. Cloning and characterization of fxp, the flexible pilin gene of Aeromonas hydrophila. Mol Microbiol. 1992;6:2725–2732. doi: 10.1111/j.1365-2958.1992.tb01449.x. [DOI] [PubMed] [Google Scholar]
- 11.Hokama A, Honma Y, Nakasone N. Pili of an Aeromonas hydrophila strain as a possible colonization factor. Microbiol Immunol. 1990;34:901–915. doi: 10.1111/j.1348-0421.1990.tb01069.x. [DOI] [PubMed] [Google Scholar]
- 12.Hokama A, Iwanaga M. Purification and characterization of Aeromonas sobria pili, a possible colonization factor. Infect Immun. 1991;59:3478–3483. doi: 10.1128/iai.59.10.3478-3483.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hokama A, Iwanaga M. Purification and characterization of Aeromonas sobria Ae24 pili: a possible new colonization factor. Microb Pathog. 1992;13:325–334. doi: 10.1016/0882-4010(92)90042-m. [DOI] [PubMed] [Google Scholar]
- 14.Honma Y, Nakasone N. Pili of Aeromonas hydrophila: purification, characterization and biological role. Microbiol Immunol. 1990;34:83–98. doi: 10.1111/j.1348-0421.1990.tb00995.x. [DOI] [PubMed] [Google Scholar]
- 15.Institute of Medicine. Emerging infections: microbial threats to health in the United States. In: Lederberg J, Shope R E, Oaks S C Jr, editors. Committee on Emerging Microbial Threats to Health. Washington, D.C: National Academy Press; 1992. [PubMed] [Google Scholar]
- 16.Iwanaga M, Hokama A. Characterization of Aeromonas sobria TAP 13 pili: a possible new colonization factor. J Gen Microbiol. 1992;138:1913–1919. doi: 10.1099/00221287-138-9-1913. [DOI] [PubMed] [Google Scholar]
- 17.Janda J M. Recent advances in the study of the taxonomy, pathogenicity, and infectious syndromes associated with the genus Aeromonas. Clin Microbiol Rev. 1991;4:397–410. doi: 10.1128/cmr.4.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janda J M, Abbott S L. Evolving concepts regarding the genus Aeromonas: an expanding panorama of species, disease presentations and unanswered questions. Clin Infect Dis. 1998;27:332–344. doi: 10.1086/514652. [DOI] [PubMed] [Google Scholar]
- 19.Kamperman L, Kirov S M. Pili and the interaction of Aeromonas species with human peripheral blood polymorphonuclear cells. FEMS Immunol Med Microbiol. 1993;7:187–196. doi: 10.1111/j.1574-695X.1993.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 20.Kirov S M. Aeromonas and Plesiomonas. In: Doyle M P, Beuchat L R, Montville T J, editors. Food microbiology: fundamentals and frontiers. Washington, D.C: American Society for Microbiology; 1997. pp. 265–287. [Google Scholar]
- 21.Kirov S M. Adhesion and piliation of Aeromonas spp. Med Microbiol Lett. 1993;2:274–280. [Google Scholar]
- 22.Kirov S M, Hayward L J, Nerrie M A. Adhesion of Aeromonas sp. to cell lines used as models for intestinal colonization. Epidemiol Infect. 1995;115:465–473. doi: 10.1017/s0950268800058623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirov S M, Jacobs I, Hayward L J, Hapin R. Electron microscopic examination of factors influencing the expression of filamentous surface structures on clinical and environmental isolates of Aeromonas veronii biotype sobria. Microbiol Immunol. 1995;39:329–338. doi: 10.1111/j.1348-0421.1995.tb02209.x. [DOI] [PubMed] [Google Scholar]
- 24.Kirov S M, Rees B, Wellock R C, Goldsmid J M, Van Galen A D. Virulence characteristics of Aeromonas spp. in relation to source and biotype. J Clin Microbiol. 1986;24:827–834. doi: 10.1128/jcm.24.5.827-834.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirov S M, Sanderson K. Aeromonas cell line adhesion, surface structures and in vivo models of intestinal colonization. Med Microbiol Lett. 1995;4:305–315. [Google Scholar]
- 26.Kirov S M, Sanderson K. Characterization of a type IV bundle-forming pilus (SFP) from a gastroenteritis-associated strain of Aeromonas veronii biovar sobria. Microb Pathog. 1996;21:23–34. doi: 10.1006/mpat.1996.0039. [DOI] [PubMed] [Google Scholar]
- 27.Kirov S M, Sanderson K, Dickson T C. Characterisation of a type IV pilus produced by Aeromonas caviae. J Med Microbiol. 1998;47:527–531. doi: 10.1099/00222615-47-6-527. [DOI] [PubMed] [Google Scholar]
- 28.Knutton S, Lloyd D R, Candy D C A, McNeish A S. Ultrastructural study of adhesion of enterotoxigenic Escherichia coli to erythrocytes and human intestinal tissue. Infect Immun. 1984;44:519–527. doi: 10.1128/iai.44.2.519-527.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knutton S, Lloyd D R, Candy D C A, McNeish A S. Adhesion of enterotoxigenic Escherichia coli to human small intestinal enterocytes. Infect Immun. 1985;48:824–831. doi: 10.1128/iai.48.3.824-831.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 31.Martinetti Lucchini G, Altwegg M. rRNA gene restriction patterns as taxonomic tools for the genus Aeromonas. Int J Syst Bacteriol. 1992;42:384–389. doi: 10.1099/00207713-42-3-384. [DOI] [PubMed] [Google Scholar]
- 32.Mattick J S, Whitchurch C B, Alm R A. The molecular genetics of type-4 fimbriae in Pseudomonas aeruginosa—a review. Gene. 1996;179:147–155. doi: 10.1016/s0378-1119(96)00441-6. [DOI] [PubMed] [Google Scholar]
- 33.Merino S, Rubires X, Aguilar A, Tomás J M. The O:34-antigen lipopolysaccharide as an adhesin in Aeromonas hydrophila. FEMS Microbiol Lett. 1996;139:97–101. doi: 10.1111/j.1574-6968.1996.tb08186.x. [DOI] [PubMed] [Google Scholar]
- 34.Merino S, Rubires X, Aguilar A, Guillot J F, Tomás J M. The role of the O-antigen lipopolysaccharide on the colonization in vivo of the germfree chicken gut by Aeromonas hydrophila serogroup O:34. Microb Pathog. 1996;20:325–333. doi: 10.1006/mpat.1996.0031. [DOI] [PubMed] [Google Scholar]
- 35.Nakasone N, Iwanaga M. Quantitative evaluation of colonizing ability of Vibrio cholerae O1. Microbiol Immunol. 1987;31:753–761. doi: 10.1111/j.1348-0421.1987.tb03137.x. [DOI] [PubMed] [Google Scholar]
- 36.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pepe C M, Eklund M W, Strom M S. Cloning of an Aeromonas hydrophila type IV pilus biogenesis gene cluster: complementation of pilus assembly functions and characterization of a type IV leader peptidase/N-methyltransferase required for extracellular protein secretion. Mol Microbiol. 1996;19:857–869. doi: 10.1046/j.1365-2958.1996.431958.x. [DOI] [PubMed] [Google Scholar]
- 38.Picard B, Goullet P. Seasonal prevalence of nosocomial Aeromonas hydrophila infection related to aeromonas in hospital water. J Hosp Infect. 1987;10:152–155. doi: 10.1016/0195-6701(87)90141-1. [DOI] [PubMed] [Google Scholar]
- 39.Polotsky Y, Dragunsky E, Khavkin T. Morphologic evaluation of the pathogenesis of bacterial enteric infections. Crit Rev Microbiol. 1994;20:161–208. doi: 10.3109/10408419409114553. [DOI] [PubMed] [Google Scholar]
- 40.Popoff M. Genus III. Aeromonas Kluyver and Van Neil 1936, 398. In: Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 545–548. [Google Scholar]
- 41.Quinn D M, Atkinson H M, Bretag A H, Tester M, Trust T J, Wong C Y F, Flower R L P. Carbohydrate-reactive, pore-forming outer membrane proteins of Aeromonas hydrophila. Infect Immun. 1994;62:4054–4058. doi: 10.1128/iai.62.9.4054-4058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semmler, A. B. T., C. B. Whitchurch, and J. S. Mattick. A re-examination of twitching motility in Pseudomonas aeruginosa. Microbiology, in press. [DOI] [PubMed]
- 43.Tamamoto T, Nakashima K, Nakasone N, Honma Y, Higa N, Yamashiro T. Adhesive property of toxin-coregulated pilus of Vibrio cholerae O1. Microbiol Immunol. 1998;42:41–45. doi: 10.1111/j.1348-0421.1998.tb01967.x. [DOI] [PubMed] [Google Scholar]
- 44.Tennent J M, Mattick J S. Type IV fimbriae. In: Klemm P, editor. Fimbriae: adhesion, genetics, biogenesis, and vaccines. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 127–146. [Google Scholar]
- 45.Thornley J P, Shaw J G, Gryllos I A, Eley A. Adherence of Aeromonas caviae to human cell lines Hep-2 and Caco-2. J Med Microbiol. 1996;45:445–451. doi: 10.1099/00222615-45-6-445. [DOI] [PubMed] [Google Scholar]
- 46.Waldor K M, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]