Abstract
Background: The aim of our study was to search for predictive factors and to develop a model (index) for the risk of pneumonia following acute ischemic stroke. Material and methods: This study is an analysis of prospectively collected data from the neurology department of a district general hospital in Poland, comprising 1001 patients suffering from an acute ischemic stroke. Based on the medical data, the formula for the prediction of pneumonia was calculated. Results: Multivariate assessment for pneumonia occurrence was performed using the new PNEUMOINDEX. The study showed a significant increase in pneumonia risk with an increasing PNEUMOINDEX (OR non-adjusted = 2.738, p < 0.001). After accounting for age and comorbidities as confounders, the effect of the Index on pneumonia changed marginally (OR = 2.636, p < 0.001). Conclusions: This study presents factors that show a significant association with the occurrence of pneumonia in patients with acute ischemic stroke. The calculated PNEUMOINDEX consists of data obtained at admission, namely NYHA III and IV heart failure, COPD, generalized atherosclerosis, NIHHS score on admission, and CRP/Hgb ratio, and shows high prediction accuracy in predicting hospital-acquired pneumonia in ischemic stroke patients.
Keywords: acute ischemic stroke, pneumonia, COPD
1. Introduction
Hospital-acquired pneumonia is one of many serious complications that can occur following a stroke. Pneumonia considerably increases the risk of death (mainly in patients with acute ischemic stroke) and deteriorates patient outcomes, reducing the chances of a full recovery and frequently leading to disability [1,2]. While the incidence of pneumonia in stroke patients can range from 8% to 30% [3,4,5,6,7], it may reach even 44% in patients with chronic obstructive pulmonary disease (COPD) [7]. Therefore, it is important to provide an early prediction of the risk of hospital-acquired pneumonia in stroke patients.
Several risk factors have already been identified, such as age, male gender, comorbidities, and the severity of the stroke [8,9]. The occurrence of the stroke itself is associated with many well-studied risk factors, such as age, diabetes, smoking, dyslipidemia, and hypertension [10,11]. Recent studies suggest that anemia is also associated with high in-hospital mortality in stroke patients [12,13]. A study conducted in a hospital emergency department suggests a strong negative association between CRP and Hgb levels, increasing the incidence of hospitalization of the patients [14,15]. Therefore, in our study, we also decided to analyze the C-reactive protein-to-hemoglobin ratio.
Early recognition of patients with a high risk of developing pneumonia is essential, not only in the critical care unit, but also in every hospital ward admitting high-risk patients. Indeed, intensivists have created risk prediction models designed to predict pneumonia in stroke patients, demonstrating variable, but reasonable, degrees of sensitivity and specificity, including the A2DS2, ISAN, and PANTHERIS scores [16,17,18,19].
Investigating the relationships between medical history, symptoms on admission, and blood analyses is an important ongoing element throughout the treatment process [20]. Studies show that blood laboratory analyses influence 70% of medical decisions [21].
Our study aimed to search for predictive factors and to develop a model (index) for the risk of pneumonia following acute ischemic stroke.
2. Material and Methods
2.1. Study Population
This study is an analysis of prospectively collected data from the neurology department of a district general hospital in Poland between June, 2015, and March, 2018, comprising 1022 patients suffering from an acute ischemic stroke with symptom onset within 48 to 72 h. Exclusion criteria included incomplete laboratory results, no data regarding follow-up, and coexisting hematological disorders. Twenty-one subjects were excluded from the analysis; 1001 patients were further analyzed. The subjects were divided into two groups 774 patients without pneumonia and 227 patients with pneumonia.
2.2. Data Collection
From their medical records, information was collected regarding demographics (age, gender, BMI, smoking) and comorbidities (arterial hypertension, ischemic heart diseases, myocardial infarction, NYHA type III and IV, TIA, ischemic stroke, history of hemorrhagic stroke, acute chronic renal failure on admission, chronic dialysis, impaired insulin tolerance, diabetes, gout, extracardiac arteriopathy, COPD, atrial fibrillation, and carotid stenosis). Routine neurological examinations at admission and at discharge provided information regarding stroke severity, according to the National Institutes of Health Stroke Scale (NIHSS), and disability, according to the modified Rankin Scale. In addition, data regarding the results of routine laboratory tests (blood count, serum creatinine, C-reactive protein, troponin T, cholesterol, triglyceride, and liver function tests) were collected from their medical records.
Outcome data were obtained from medical records and by telephone at 30 and 90 days after the patient was discharged from the ward. Patient death was recorded as in-hospital mortality up to day 7. Information on subsequent death at the 1-year follow-up was obtained by telephone contact, and the mortality rates at 30 and 90 days and 1 year after stroke were calculated.
2.3. CRP and Hemoglobin
In our research, we decided to analyze the C-reactive protein-to-hemoglobin ratio [14]. From the medical records, we obtained information about the concentration of CRP and Hb in the blood on the day of admission of a patient with acute ischemic stroke. CRP/Hb was calculated based on the formula:
| CRP/Hgb = C-reactive Protein [mg/L]:Hemoglobin [g/dL]. | (1) |
2.4. Diagnosis of Pneumonia
The definition of pneumonia was based on the guidelines of the American Thoracic Society, where it is described as the presence of typical changes in chest radiography with at least two of the following: fever, leukocytosis (white blood cell WBC > 12 G/L) or leukopenia (WBC < 4 G/L), or expectoration of pus sputum [22].
2.5. Ethical Statement
Prospective data collection was performed, according to the guidelines of the Declaration of Helsinki. We obtained the approval of the Bioethical Committee of the Pomeranian Medical University in Szczecin, Poland, decision no. KBE-0012/84/03/19. The requirement to obtain informed consent was waived, as the study was performed as a routine clinical process of the diagnosis and treatment performed in every patient admitted to the hospital. Patient confidentiality was ensured with the use of anonymized data.
2.6. Statistical Analysis
Statistical analysis was performed using specialized software (StatSoft, Inc., Tulsa, OK, USA). Descriptive statistics, based on means, medians, standard deviations, counts, and percentages, were used to show the characteristics of the group. Continuous data was compared using Mann–Whitney U tests, and qualitative data using Chi-square or Chi-square with Yates’s correction tests. Regression analysis was performed to look for risk factors affecting the incidence of pneumonia. The formula for an optimal prediction of pneumonia was calculated and presented as PNEUMOINDEX. A receiver operating characteristic (ROC) analysis was performed to determine the diagnostic value of the PNEUMOINDEX and its components for predicting pneumonia. Multivariate logistic regression analysis, adjusted for age and comorbidities for the incidence of pneumonia, was performed. Differences were regarded as statistically significant at p < 0.05.
3. Results
Table 1 presents the baseline data collected on the day of hospital admission. In the group of stroke patients diagnosed with pneumonia, greater age (p < 0.001), higher RANKIN (p < 0.001) and higher NIHHS (p < 0.001) scores were observed. The patients with pneumonia on admission were more likely to have ischemic heart diseases (p < 0.001), myocardial infarction (p = 0.033), NYHA III, and IV heart failure (p < 0.001), history of ischemic stroke (p = 0.001), chronic renal failure (p < 0.001), impaired insulin tolerance (p = 0.031), diabetes on insulin (p = 0.002), extracardiac arteriopathy (p < 0.001), and atrial fibrillation (p < 0.001), COPD (p < 0.001).
Table 1.
Demographic data and comorbidities for the admitted ischemic stroke patients.
| No Pneumonia (n = 774) | Pneumonia (n = 227) | p | |
|---|---|---|---|
| Demographic data | |||
| Age [years], mean ± SD; Me | 70.55 ± 12.29; 70.0 | 77.09 ± 10.35; 79.0 | <0.001 |
| Gender [male], n (%) | 405 (52.33%) | 118 (51.98%) | 0.927 |
| BMI [kg/m2], mean ± SD; Me | 26.88 ± 4.74; 25.9 | 26.56 ± 4.58; 26.3 | 0.786 |
| Smoking, n (%) | 320 (41.34%) | 97 (42.73%) | 0.709 |
| Comorbidities | |||
| Arterial hypertension, n (%) | 664 (85.79%) | 205 (90.31%) | 0.077 |
| Ischemic heart diseases, n (%) | 167 (21.58%) | 91 (40.09%) | <0.001 |
| Myocardial infarction, n (%) | 74 (9.56%) | 33 (14.54%) | 0.033 |
| NYHA 3 & 4, n (%) | 16 (2.07%) | 26 (11.45%) | <0.001 |
| TIA, n (%) | 146 (18.86%) | 53 (23.35%) | 0.136 |
| History of ischemic stroke, n (%) | 154 (19.90%) | 68 (29.96%) | 0.001 |
| History of hemorrhagic stroke, n (%) | 19 (2.45%) | 5 (2.20%) | 0.827 |
| Acute renal failure on admission, n (%) | 5 (0.65%) | 4 (1.76%) | 0.117 |
| Chronic renal failure, n (%) | 90 (11.63%) | 53 (23.35%) | <0.001 |
| Chronic dialysis, n (%) | 1 (0.13%) | 1 (0.44%) | 0.354 |
| Impaired insulin tolerance, n (%) | 31 (4.01%) | 17 (7.49%) | 0.031 |
| Diabetes on oral medications, n (%) | 151 (19.51%) | 54 (23.79%) | 0.160 |
| Diabetes on insulin, n (%) | 86 (11.13%) | 43 (18.94%) | 0.002 |
| Gout, n (%) | 45 (5.81%) | 20 (8.81%) | 0.107 |
| Extracardiac arteriopathy, n (%) | 290 (37.47%) | 164 (72.25%) | <0.001 |
| COPD, n (%) | 53 (6.85%) | 42 (18.50%) | <0.001 |
| Atrial fibrillation, n (%) | 192 (24.84%) | 101 (44.49%) | <0.001 |
| Carotid artery stenosis, n (%) | 61 (36.53%) | 26 (52.00%) | 0.051 |
| Scale at admission | |||
| Rankin score, mean ± SD; Me | 9.20 ± 6.52; 7.0 | 18.74 ± 8.05; 20.0 | <0.001 |
| NIHSS, mean ± SD; Me | 2.73 ± 1.52; 2.0 | 4.43 ± 1.08; 5.0 | <0.001 |
Legend: n—number of patients, SD—standard deviation, Me-median, BMI—body mass index, NYHA—New York Heart Association, TIA—transient ischemic attack, COPD—chronic obstructive pulmonary disease, NIHSS—National Institutes of Health Stroke Scale.
Analysis of laboratory data performed on the day of admission showed higher levels of glycemia (p = 0.004), leukocytes (p < 0.001), neutrophils (p < 0.001), creatinine (p < 0.001), CRP (p < 0.001), and troponin T (p < 0.001) in the patients with pneumonia. and lower levels of lymphocytes (p < 0.001), hemoglobin (p < 0.001), cholesterol (p < 0.001), and triglyceride (p < 0.001). These results are presented in Table 2.
Table 2.
Laboratory data for the admitted ischemic stroke patients.
| Variable | No Pneumonia (n = 774) | Pneumonia (n = 227) | p | ||||
|---|---|---|---|---|---|---|---|
| Mean | Me | ±SD | Mean | Me | ±SD | ||
| Glycemia (mg/dl) | 141.56 | 123.00 | 61.85 | 147.99 | 135.00 | 56.94 | 0.004 |
| Leucocyte count (×109/L) | 9.45 | 8.89 | 3.37 | 11.02 | 9.71 | 5.38 | <0.001 |
| Neutrophil count (×109/L) | 6.61 | 5.82 | 4.35 | 8.26 | 7.18 | 4.93 | <0.001 |
| Lymphocyte count (×109/L) | 2.10 | 1.89 | 2.48 | 1.85 | 1.58 | 1.62 | <0.001 |
| Platelet count (×109/L) | 239.92 | 228.00 | 81.93 | 239.83 | 225.00 | 104.37 | 0.649 |
| Hemoglobin | 13.95 | 14.00 | 1.75 | 13.39 | 13.60 | 2.01 | <0.001 |
| Creatinine | 1.04 | 0.91 | 0.60 | 1.20 | 1.01 | 0.71 | <0.001 |
| C-reactive protein | 10.56 | 2.60 | 25.05 | 46.34 | 16.67 | 71.12 | <0.001 |
| Aspartate aminotransferase | 24.26 | 20.00 | 14.42 | 36.96 | 21.00 | 108.93 | 0.701 |
| Alanine aminotransferase | 22.70 | 18.00 | 14.01 | 29.21 | 17.00 | 72.94 | 0.133 |
| Cholesterol | 199.48 | 195.00 | 53.48 | 171.02 | 167.00 | 49.79 | <0.001 |
| Triglycerides | 146.26 | 122.00 | 91.21 | 121.54 | 105.00 | 63.92 | <0.001 |
| Troponin T | 27.52 | 10.00 | 89.47 | 58.09 | 21.98 | 137.19 | <0.001 |
| CRP/Hgb | 0.83 | 0.19 | 2.07 | 3.73 | 1.15 | 5.98 | <0.001 |
Legend: n—number of patients, SD—standard deviation, Me—median, CRP/HGB—C-reactive protein-to-hemoglobin ratio.
Table 3 presents the outcome data for the admitted ischemic stroke patients. Those with pneumonia during their hospitalization had a significantly higher risk of mortality at 7 days (p < 0.001), 30 days (p < 0.001), 90 days (p < 0.001), and 1 year (p < 0.001).
Table 3.
Outcome data for the admitted ischemic stroke patients.
| Variables | No Pneumonia (n = 774) |
Pneumonia (n = 227) |
p | ||||
|---|---|---|---|---|---|---|---|
| mean ± SD; Me/n (%) | mean ± SD; Me/n (%) | OR | CI − 95% | CI + 95% | |||
| Hospitalization time (days) | 9.34 ± 4.05; 8.0 | 15.43 ± 11.56; 13.0 | 1.168 | 1.131 | 1.206 | <0.001 | |
| NIHSS at discharge | 7.21 ± 10.02; 3.0 | 23.61 ± 13.75; 20.0 | 1.096 | 1.082 | 1.111 | <0.001 | |
| Rankin score at discharge | 1.99 ± 2.02; 1.0 | 4.79 ± 1.39; 5.0 | 2.090 | 1.884 | 2.317 | <0.001 | |
| Mortality until day 7 | 36 (4.65%) | 32 (14.10%) | 3.364 | 2.037 | 5.556 | <0.001 | |
| Mortality until day 30 | 63 (8.14%) | 104 (45.81%) | 9.542 | 6.612 | 13.771 | <0.001 | |
| Mortality until day 90 | 91 (11.76%) | 151 (66.52%) | 14.912 | 10.489 | 21.202 | <0.001 | |
| Mortality until year 1 | 135 (17.44%) | 169 (74.45%) | 13.792 | 9.706 | 19.598 | <0.001 | |
| Outcome | In-hospital death | 38 (4.91%) | 66 (29.07%) | 7.940 | 5.144 | 12.255 | <0.001 |
| Discharged home | 510 (65.89%) | 74 (32.60%) | 0.250 | 0.183 | 0.343 | <0.001 | |
| Nursing home | 44 (5.68%) | 48 (21.15%) | 4.449 | 2.864 | 6.912 | <0.001 | |
| Rehabilitation facility | 172 (22.22%) | 33 (14.54%) | 0.595 | 0.397 | 0.894 | 0.012 | |
| Another ward | 10 (1.29%) | 6 (2.64%) | 2.074 | 0.746 | 5.770 | 0.162 | |
Legend: n—number of patients, SD—standard deviation, Me—median, OR—odds ratio, CI—confidence interval, NIHSS—National Institutes of Health Stroke Scale.
A univariate logistic regression analysis was performed for pneumonia, as shown in Table 4. In stroke patients, the analysis showed an association between multiple factors at admission and pneumonia during the hospital stay (Table 4). This prompted the researchers to perform further analyses.
Table 4.
Regression analysis for pneumonia.
| Variables | p | OR | CI − 95% | CI + 95% |
|---|---|---|---|---|
| Demographic data | ||||
| Age [years] | <0.001 | 1.051 | 1.036 | 1.066 |
| Gender [male] | 0.927 | 0.986 | 0.734 | 1.326 |
| BMI [kg/m2] | 0.367 | 0.985 | 0.954 | 1.018 |
| Smoking | 0.709 | 1.059 | 0.785 | 1.428 |
| Co-morbidities | ||||
| Arterial hypertension | 0.079 | 1.544 | 0.952 | 2.504 |
| Ischemic heart diseases | <0.001 | 2.432 | 1.773 | 3.336 |
| Myocardial infarction | 0.034 | 1.609 | 1.036 | 2.498 |
| Heart failure III and IV NYHA | <0.001 | 6.128 | 3.225 | 11.644 |
| TIA | 0.137 | 1.310 | 0.917 | 1.871 |
| History of ischemic stroke | 0.001 | 1.722 | 1.232 | 2.405 |
| History of hemorrhagic stroke | 0.827 | 0.895 | 0.330 | 2.424 |
| Acute renal failure on admission | 0.133 | 2.759 | 0.735 | 10.361 |
| Chronic renal failure | <0.001 | 2.315 | 1.586 | 3.378 |
| Chronic dialysis | 0.384 | 3.436 | 0.214 | 55.147 |
| Impaired insulin tolerance | 0.033 | 1.940 | 1.053 | 3.575 |
| Diabetes on oral medications | 0.161 | 1.288 | 0.904 | 1.834 |
| Diabetes on insulin | 0.002 | 1.867 | 1.251 | 2.787 |
| Gout | 0.110 | 1.565 | 0.904 | 2.710 |
| Extracardiac arteriopathy | <0.001 | 4.345 | 3.139 | 6.013 |
| Atrial fibrillation | <0.001 | 2.426 | 1.782 | 3.302 |
| COPD | <0.001 | 3.088 | 1.997 | 4.776 |
| Carotid artery stenosis | 0.052 | 1.883 | 0.995 | 3.563 |
| Scale at admission | ||||
| Rankin score | <0.001 | 1.175 | 1.148 | 1.203 |
| NIHSS | <0.001 | 2.436 | 2.112 | 2.809 |
| Laboratory data | ||||
| Glycemia 0 (mg/dl) | 0.164 | 1.002 | 0.999 | 1.004 |
| Leucocyte count (×109/L) | <0.001 | 1.094 | 1.055 | 1.135 |
| Neutrophil count (×109/L) | <0.001 | 1.088 | 1.047 | 1.131 |
| Lymphocyte count (×109/L) | 0.064 | 0.854 | 0.724 | 1.009 |
| Platelet count (×109/L) | 0.990 | 1.000 | 0.998 | 1.002 |
| Hemoglobin | <0.001 | 0.848 | 0.783 | 0.919 |
| Creatinine | 0.003 | 1.444 | 1.134 | 1.838 |
| C-reactive protein | <0.001 | 1.020 | 1.015 | 1.025 |
| Aspartate aminotransferase | 0.295 | 1.004 | 0.996 | 1.013 |
| Alanine aminotransferase | 0.281 | 1.004 | 0.997 | 1.011 |
| Cholesterol | <0.001 | 0.989 | 0.985 | 0.992 |
| Triglycerides | <0.001 | 0.996 | 0.993 | 0.998 |
| Troponin T | 0.002 | 1.002 | 1.001 | 1.004 |
| CRP/HGB | <0.001 | 1.266 | 1.198 | 1.339 |
Legend: n—number of patients, SD standard deviation, Me—median, OR—odds ratio, CI—confidence interval, BMI—body mass index, NYHA—New York Heart Association, TIA—transient ischemic attack, COPD—chronic obstructive pulmonary disease, NIHSS—National Institutes of Health Stroke Scale, CRP/HGB—C-reactive protein-to-hemoglobin ratio.
In the search for factors determining the occurrence of pneumonia during the hospital stay of ischemic stroke patients, multiple multivariate regression models were performed, clustering all statistically significant parameters from the univariate analysis. These analyses in the study group resulted in a model with factors that had the highest influence on the occurrence of pneumonia. The model is presented in Table 5. The analysis showed an association between pneumonia and heart failure on NYHA III and IV scale (OR = 4.56, p < 0.001), COPD (OR = 2.66, p < 0.001), extracardiac arteriopathy (OR = 3.06, p < 0.001), NIHHS scale at discharge (OR = 1.15, p < 0.001), and CRP/HGB ratio (OR = 1.17, p < 0.001).
Table 5.
Regression model for patients with pneumonia.
| p | OR | CI − 95% | CI + 95% | |
|---|---|---|---|---|
| NYHA III & IV | <0.001 | 4.555 | 1.979 | 10.487 |
| COPD | <0.001 | 2.659 | 1.540 | 4.593 |
| Extracardiac atherosclerosis | <0.001 | 3.055 | 2.060 | 4.530 |
| NIHHS score (points) | <0.001 | 1.152 | 1.122 | 1.181 |
| CRP/HGB | <0.001 | 1.166 | 1.101 | 1.234 |
Legend: OR—odds ratio, CI—confidence interval, NYHA—New York Heart Association, COPD—chronic obstructive pulmonary disease, NIHSS—National Institutes of Health Stroke Scale, CRP/HGB—C-reactive protein-to-hemoglobin ratio.
Based on the obtained factors in Table 5, with the highest association with pneumonia, a predictive index for pneumonia was created. The PNEUMOINDEX is predictive of the occurrence of pneumonia during the hospital stay, and was calculated using the formula:
| PNEUMOINDEX = (1.516 × NYHA III & IV) + (0.978 × COPD) + (1.117 × extracardiac arteriopathy) + (0.141 × NIHHS on the day of admission) + (0.153 × CRP/HGB) | (2) |
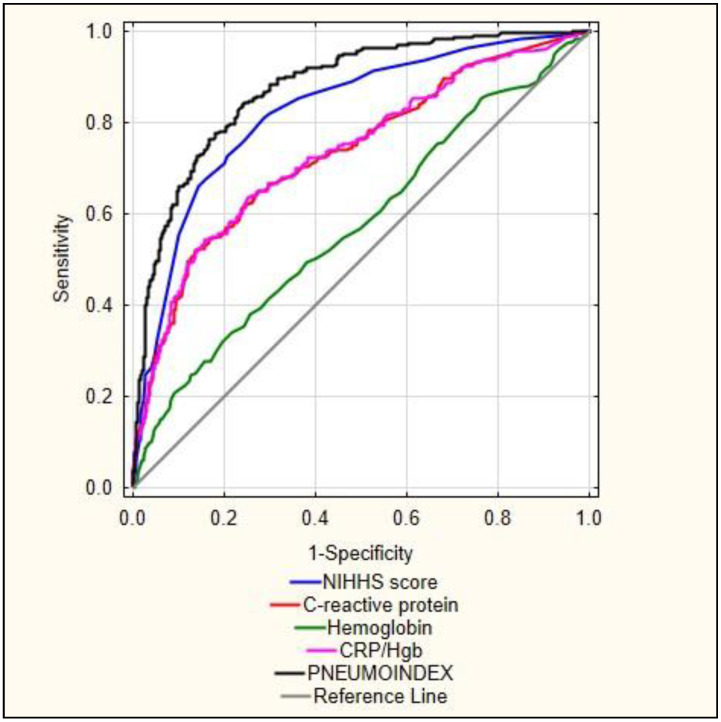
ROC curve analysis for the quantitative data: NIHHS score on the day of admission, CRP and Hb values every day, CRP/Hb and calculated PNEUMOINDEX value is presented in Figure 1. Table 6 presents the results of the ROC curve. This analysis showed a very high area under the curve for PNEUMOINDEX (AUC = 0.876, p < 0.001). PNEUMOINDEX obtained a significantly higher predictive value than each parameter separately.
Figure 1.
ROC analysis curves for pneumonia. Legend: NIHSS—National Institutes of Health Stroke Scale, CRP/HGB—C-reactive protein-to-hemoglobin ratio.
Table 6.
Details of the receiver operating characteristics (ROC) analysis for pneumonia.
| AUC | AUC − 95% | AUC + 95% | p | |
|---|---|---|---|---|
| PNEUMOINDEX | 0.876 | 0.851 | 0.902 | <0.001 |
| NIHHS score | 0.825 | 0.794 | 0.856 | <0.001 |
| CRP/Hgb | 0.735 | 0.696 | 0.775 | <0.001 |
| C-reactive protein | 0.734 | 0.695 | 0.773 | <0.001 |
| Haemoglobin | 0.577 | 0.534 | 0.621 | <0.001 |
Legend: AUC—Area under the ROC Curve, OR—odds ratio, CI-confidence interval, NIHSS—National Institutes of Health Stroke Scale, CRP/HGB—C-reactive protein-to-hemoglobin ratio.
Multivariate assessment for pneumonia occurrence was performed using the newly calculated PNEUMOINDEX. The study showed a significant increase in pneumonia risk with an increasing PNEUMOINDEX (OR non-adjusted = 2.738, p < 0.001). After accounting for age and comorbidities as confounders, the effect of the Index on pneumonia changed marginally (OR = 2.636, p < 0.001) Table 7.
Table 7.
Multivariate logistic regression for pneumonia using PNUEMOINDEX.
| p | OR | CI − 95% | CI + 95% | |
|---|---|---|---|---|
| PNEUMOINDEX | <0.001 | 2.738 | 2.381 | 3.149 |
| PNEUMOINDEX * | <0.001 | 2.639 | 2.292 | 3.037 |
| PNEUMOINDEX ** | <0.001 | 2.636 | 2.281 | 3.047 |
Legend: OR—odds ratio, CI—confidence interval. Notes: * adjusted by age, ** adjusted by age and comorbidities not included in PNEUMOINDEX.
4. Discussion
In current clinical practice, assessing the risk of pneumonia in patients with acute ischemic stroke remains a major challenge. Therefore, in this study we attempted to create a simple universal index that would be helpful for clinicians to quickly detect/identify patients with acute ischemic stroke having the highest risk of pneumonia.
Indeed, our study reports a relatively high risk of pneumonia in stroke patients. The research indicated that the incidence of pneumonia in stroke patients ranged from 7.1% to over 30%. Our research showed that over 20% of patients developed pneumonia, which fell within the ranges reported by other authors. A study by Finlayson et al., reported that 587 out of 8251 (7.1%) patients developed stroke-associated pneumonia [6]. In a more recent study by Liu et al., the authors were able to propose a care bundle that included interventions aimed at reducing the incidence of pneumonia after stroke, such as the utilization of the following tools: for SAP risk screening, dysphagia screening, and adequate rehabilitation; for feeding modification, oral care, airway management, and position management. They also proposed utilizing the nursing techniques of traditional Chinese medicine. The authors reported an initial incidence of 37.2% in the pre-implementation period and 14% in the post-implementation group (p = 0.025) [23].
Our study showed that an index meeting the above assumptions could be provided by the pneumonia index we developed. This index included the following variables: NYHA III and IV heart failure, COPD, generalized atherosclerosis, NIHHS scale, and CRP/hemoglobin ratio. Patients with CHF (chronic heart failure) are thought to have twice the risk of pneumonia compared with age- and sex-matched subjects in the general population, while survival from pneumonia is lower in patients with coexisting CHF than in those without CHF. Conversely, pneumonia increases the risk of worsening CHF and is often considered a factor in cardiopulmonary decompensation leading to hospitalization or death. An analysis by Shen et al. revealed that 6.3% of patients participating in the PARADIGM-HF trial, and 10.6% of patients participating in the PARAGON-HF trial, developed pneumonia. In addition, the researchers also found that patients with heart failure with preserved ejection fraction (HFpEF) were at the highest risk of developing pneumonia [24,25,26].
COPD is one of the most common medical conditions, and is also a risk factor for developing pneumonia. Numerous clinical studies of pneumonia, that included outpatient, inpatient, and ICU cohorts, indicated that COPD is a frequently reported comorbid condition [27,28,29,30,31]. Patients with COPD had more severe pneumonia that led to unavoidable hospitalization and had a poorer prognosis [32,33,34] than those without. In the first year after COPD diagnosis, patients were 16 times more likely to develop pneumonia compared to patients without COPD. The incidence of out-of-hospital pneumonia was 22.4 events per 1000 person-years within 10 years of COPD diagnosis and was 50% higher in those classified as having severe COPD [35,36]. Hospitalized patients with COPD and coexisting pneumonia had significantly higher 30- and 90-day mortality rates than patients without COPD [33].
Increased disability, impaired consciousness, impaired awareness, or the presence of pseudobulbar syndrome generate higher NIHSS scores and are associated with a propensity for aspiration and hypostatic pneumonia. The results of other studies also support our observations that high NIHSS scale scores increase the risk of pneumonia in patients with acute ischemic stroke [37,38,39].
To the best of our knowledge, the relationship between serum CRP and Hb concentration has not been previously determined for stroke unit patients. The results of this study indicated that blood Hb and CRP concentrations were strongly associated with each other. The mean Hb concentration was significantly lower in patients with high CRP. In the study by Santos-Silva et al., a significant association between Hb and CRP was observed, especially in patients with respiratory diagnoses [15]. The CRP/Hb ratio included in the PNEUMOINDEX significantly increased the predictive value of this Index.
Comparing different models to predict pneumonia in a meta-analysis, Zhang et al. demonstrated that the AIS–SAP model showed the highest clinical value for assessing the prediction of pneumonia after AIS. The authors described that, for predicting in-hospital pneumonia after AIS, the area under the ROC curve for AIS–APS was 0.79, for A2DS2 score AUC was 0.74, and for the ISAN score AUC was 0.76 to [40]. In our study, the area under the PNEUMOINDEX curve was 0.87, making it an important, easily obtainable tool for the prediction of pneumonia after acute ischemic stroke that may be useful in everyday clinical practice.
5. Limitations
This study is not without limitations. It is a single-center observational series, so it would be useful to extend the research into a multi-center study. Further prospective studies with a larger sample size are needed.
6. Conclusions
This study presents factors that show a significant association with the occurrence of pneumonia in patients with acute ischemic stroke. The calculated PNEUMOINDEX consists of data obtained at admission, namely NYHA III & IV heart failure, COPD, generalized atherosclerosis, NIHHS score on admission, and CRP/Hgb ratio, and shows high accuracy in predicting hospital-acquired pneumonia in ischemic stroke patients.
Author Contributions
Conceptualization, A.S., M.B.-O., I.R. and K.K.; methodology, A.S. and M.B.-O.; software, A.S.; validation, A.S., M.B.-O., I.R. and K.K.; formal analysis, A.S.; investigation, A.S., M.B.-O., P.W., D.K., I.R. and K.K.; resources, M.B.-O. and D.K.; data curation, M.B.-O. and D.K.; visualization, A.S.; supervision, I.R. and K.K.; project administration, A.S. and M.B.-O.; writing—original draft preparation, A.S., M.B.-O., P.W., D.K., I.R. and K.K.; writing—review and editing, A.S., M.B.-O., P.W., D.K., I.R. and K.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Bioethical Committee of Pomeranian Medical University in Szczecin, Poland (KBE-0012/84/03/19).
Informed Consent Statement
The requirement to obtain informed consent was waived, as the study was performed as a routine clinical process of the diagnosis and treatment performed in every patient admitted to the hospital. Patient confidentiality was ensured with the use of anonymized data.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray B.D., Smith C.J., Cloud G.C., Enderby P., James M., Paley L., Tyrrell P.J., Wolfe C.D.A., Rudd A.G., SSNAP Collaboration The association between delays in screening for and assessing dysphagia after acute stroke, and the risk of stroke-associated pneumonia. J. Neurol. Neurosurg. Psychiatry. 2017;88:25–30. doi: 10.1136/jnnp-2016-313356. [DOI] [PubMed] [Google Scholar]
- 2.Al-Khaled M., Matthis C., Binder A., Mudter J., Schattschneider J., Pulkowski U., Strohmaier T., Niehoff T., Zybur R., Eggers J., et al. Dysphagia in patients with acute ischemic stroke: Early dysphagia screening may reduce stroke-related Pneumonia and Improve Stroke Outcomes. Cerebrovasc. Dis. 2016;42:81–89. doi: 10.1159/000445299. [DOI] [PubMed] [Google Scholar]
- 3.Cugy E., Sibon I. Stroke-associated pneumonia risk score: Validity in a French stroke unit. J. Stroke Cerebrovasc. Dis. 2017;26:225–229. doi: 10.1016/j.jstrokecerebrovasdis.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Kishore A.K., Vail A., Chamorro A., Garau J., Hopkins S.J., di Napoli M., Kalra L., Langhorne P., Montaner J., Roffe C., et al. How is pneumonia diagnosed in clinical stroke research? A systematic review and meta-analysis. Stroke. 2015;46:1202–1209. doi: 10.1161/STROKEAHA.114.007843. [DOI] [PubMed] [Google Scholar]
- 5.Szylińska A., Kotfis K., Bott-Olejnik M., Wańkowicz P., Rotter I. Post-Stroke Outcomes of Patients with Chronic Obstructive Pulmonary Disease. Brain Sci. 2022;12:106. doi: 10.3390/brainsci12010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finlayson O., Kapral M., Hall R., Asllani E., Selchen D., Saposnik G. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. 2011;77:1338–1345. doi: 10.1212/WNL.0b013e31823152b1. [DOI] [PubMed] [Google Scholar]
- 7.Westendorp W.F., Nederkoorn P.J., Vermeij J.-D., Dijkgraaf M.G., Beek D. Post-stroke infection: A systematic review and meta-analysis. BMC Neurol. 2011;11:110. doi: 10.1186/1471-2377-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon H.M., Jeong S.W., Lee S.H., Yoon B.W. The pneumonia score: A simple grading scale for prediction of pneumonia after acute stroke. Am. J. Infect. Control. 2006;34:64–68. doi: 10.1016/j.ajic.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Ovbiagele B., Hills N.K., Saver J.L., Johnston S.C. Frequency and determinants of pneumonia and urinary tract infection during stroke hospitalization. J. Stroke Cerebrovasc. Dis. 2006;15:209–213. doi: 10.1016/j.jstrokecerebrovasdis.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bang O.Y., Ovbiagele B., Kim J.S. Nontraditional Risk Factors for Ischemic Stroke: An Update. Stroke. 2015;46:3571–3578. doi: 10.1161/STROKEAHA.115.010954. [DOI] [PubMed] [Google Scholar]
- 11.Li L., Yiin G.S., Geraghty O.C., Schulz U.G., Kuker W., Mehta Z. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: A population-based study. Lancet Neurol. 2015;14:903–913. doi: 10.1016/S1474-4422(15)00132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabjan T.H., Penko M., Hojs R. Anemia on admission and long-term mortality risk in patients with acute ischemic stroke. Adv. Clin. Exp. Med. 2019;28:1419–1424. doi: 10.17219/acem/104540. [DOI] [PubMed] [Google Scholar]
- 13.Li Z., Zhou T., Li Y., Chen P., Chen L. Anemia increases the mortality risk in patients with stroke: A meta-analysis of cohort studies. Sci. Rep. 2016;23:26636. doi: 10.1038/srep26636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziv-Baran T., Wasserman A., Goldiner I., Stark M., Shenhar-Tsarfaty S., Shapira I., Zeltser D., Mailis I., Berliner S., Rogowski O. The association between C-reactive protein and common blood tests in apparently healthy individuals undergoing a routine health examination. Clin. Chim. Acta. 2020;501:33–41. doi: 10.1016/j.cca.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Santos-Silva M.A., Sousa N., Sousa J.C. Correlation Analysis between Hemoglobin and C-Reactive Protein in Patients Admitted to an Emergency Unit. J. Clin. Med. 2021;10:5411. doi: 10.3390/jcm10225411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji R., Shen H., Pan Y., Wang P., Liu G., Wang Y., Li H., Wang Y. Novel risk score to predict pneumonia after acute ischemic stroke. Stroke. 2013;44:1303–1309. doi: 10.1161/STROKEAHA.111.000598. [DOI] [PubMed] [Google Scholar]
- 17.Smith C.J., Bray B.D., Hoffman A., Meisel A., Heuschmann P.U., Wolfe C.D.A., Tyrrell P.J., Rudd A.G., the Intercollegiate Stroke Working Party Group Can a novel clinical risk score improve pneumonia prediction in acute stroke care? A UK multicenter cohort study. J. Am. Heart Assoc. 2015;4:e001307. doi: 10.1161/JAHA.114.001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harms H., Grittner U., Dröge H., Meisel A. Predicting post-stroke pneumonia: The PANTHERIS score. Acta Neurol. Scand. 2013;128:178–184. doi: 10.1111/ane.12095. [DOI] [PubMed] [Google Scholar]
- 19.Huang G.-Q., Lin Y.-T., Wu Y.-M., Cheng Q.-Q., Cheng H.-R., Wang Z. Individualized prediction of stroke associated pneumonia for patients with acute ischemic stroke. Clin. Interv. Aging. 2019;14:1951–1962. doi: 10.2147/CIA.S225039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badrick T. Evidence-Based Laboratory Medicine. Clin. Biochem. Rev. 2013;34:43–46. [PMC free article] [PubMed] [Google Scholar]
- 21.Hallworth M.J. The ‘70% claim’: What is the evidence base? Ann. Clin. Biochem. 2011;48:487–488. doi: 10.1258/acb.2011.011177. [DOI] [PubMed] [Google Scholar]
- 22.Kalil A.C., Metersky M.L., Klompas M., Muscedere J., Sweeney D.A., Palmer L.B., Napolitano L.M., O’Grady N.P., Bartlett J.G., Carratala J., et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z.Y., Wei L., Ye R.C., Chen J., Nie D., Zhang G., Zhang X.P. Reducing the incidence of stroke-associated pneumonia: An evidence-based practice. BMC Neurol. 2022;22:297. doi: 10.1186/s12883-022-02826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alon D., Stein G.Y., Korenfeld R., Fuchs S. Predictors and outcomes of infection-related hospital admissions of heart failure patients. PLoS ONE. 2013;8:e72476. doi: 10.1371/journal.pone.0072476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jobs A., Simon R., de Waha S., Rogacev K., Katalinic A., Babaev V., Thiele H. Pneumonia and inflammation in acute decompensated heart failure: A registry-based analysis of 1939 patients. Eur. Heart J. Acute Cardiovasc. Care. 2018;7:362–370. doi: 10.1177/2048872617700874. [DOI] [PubMed] [Google Scholar]
- 26.Shen L., Jhund P.S., Anand I.S., Bhatt A.S., Desai A.S., Maggioni A.P., Martinez F.A., Pfeffer M.A., Rizkala A.R., Rouleau J.L., et al. Incidence and Outcomes of Pneumonia in Patients With Heart Failure. J. Am. Coll. Cardiol. 2021;77:1961–1973. doi: 10.1016/j.jacc.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Almirall J., Bolíbar I., Balanzó X., González C.A. Risk factors for community-acquired pneumonia in adults: A population-based case-control study. Eur. Respir. J. 1999;13:349–355. doi: 10.1183/09031936.99.13234999. [DOI] [PubMed] [Google Scholar]
- 28.García-Ordóñez M.A., García-Jiménez J.M., Páez F., Álvarez F., Poyato B., Franquelo M., Colmenero J.D., Juárez C. Clinical aspects and prognostic factors in elderly patients hospitalised for community-acquired pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 2001;20:14–19. doi: 10.1007/s100960000413. [DOI] [PubMed] [Google Scholar]
- 29.Lim W.S., Lewis S., Macfarlane J.T. Severity prediction rules in community acquired pneumonia: A validation study. Thorax. 2000;55:219–223. doi: 10.1136/thorax.55.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim W.S., van der Eerden M.M., Laing R., Boersma W.G., Karalus N., I Town G., A Lewis S., Macfarlane J.T. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramirez J.A., Wiemken T.L., Peyrani P., Arnold F.W., Kelley R., A Mattingly W., Nakamatsu R., Pena S., E Guinn B., Furmanek S.P., et al. Adults Hospitalized with Pneumonia in the United States: Incidence, Epidemiology, and Mortality. Clin. Infect. Dis. 2017;65:1806–1812. doi: 10.1093/cid/cix647. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y., Stewart P., Dales R., Johansen H., Bryan S., Taylor G. In a retrospective study of chronic obstructive pulmonary disease inpatients, respiratory comorbidities were significantly associated with prognosis. J. Clin. Epidemiol. 2005;58:1199–1205. doi: 10.1016/j.jclinepi.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Restrepo M.I., Mortensen E.M., Pugh J.A., Anzueto A. COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur. Respir. J. 2006;28:346–351. doi: 10.1183/09031936.06.00131905. [DOI] [PubMed] [Google Scholar]
- 34.Rello J., Rodriguez A., Torres A., Roig J., Sole-Violan J., Garnacho-Montero J., De La Torree M.V., Sirvent J.M., Bodí M. Implications of COPD in patients admitted to the intensive care unit by community-acquired pneumonia. Eur. Respir. J. 2006;27:1210–1216. doi: 10.1183/09031936.06.00139305. [DOI] [PubMed] [Google Scholar]
- 35.Soriano J.B., Visick G.T., Muellerova H., Payvandi N., Hansell A.L. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. 2005;128:2099–2107. doi: 10.1378/chest.128.4.2099. [DOI] [PubMed] [Google Scholar]
- 36.Müllerova H., Chigbo C., Hagan G.W., Woodhead M.A., Miravitlles M., Davis K.J., Wedzicha J.A. The natural history of community-acquired pneumonia in COPD patients: A population database analysis. Respir. Med. 2012;106:1124–1133. doi: 10.1016/j.rmed.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Chumbler N.R., Williams L.S., Wells C.K., Lo A.C., Nadeau S., Peixoto A.J., Gorman M., Boice J.L., Concato J., Bravata D.M. Derivation and validation of a clinical system for predicting pneumonia in acute stroke. Neuroepidemiology. 2010;34:193–199. doi: 10.1159/000289350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann S., Malzahn U., Harms H., Koennecke H., Berger K., Kalic M., Walter G., Meisel A., Heuschmann P.U., Berlin Stroke Register and the Stroke Register of Northwest Germany Development of a clinical score (A2DS2) to predict pneumonia in acute ischemic stroke. Stroke. 2012;43:2617–2623. doi: 10.1161/STROKEAHA.112.653055. [DOI] [PubMed] [Google Scholar]
- 39.Kumar S., Marchina S., Massaro J., Feng W., Lahoti S., Selim M., Herzig S.J. ACDD4score: A simple tool for assessing risk of pneumonia after stroke. J. Neurol. Sci. 2017;372:399–402. doi: 10.1016/j.jns.2016.10.050. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X., Xiao L., Niu L., Tian Y., Chen K. Comparison of six risk scores for stroke-associated pneumonia in patients with acute ischemic stroke: A systematic review and Bayesian network meta-analysis. Front. Med. 2022;9:964616. doi: 10.3389/fmed.2022.964616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable.