Abstract
Tumor necrosis factor (TNF) is a critical mediator in the immune response to mycobacteria, particularly in the formation and maintenance of granulomas. Treatment of Mycobacterium bovis BCG-infected mice with TNF and a TNF-mimetic peptide (TNF70–80) altered the number and cellular composition of granulomas. This change was associated with a moderate decrease in the bacterial burden.
The hallmark of infection with Mycobacterium tuberculosis and Mycobacterium bovis BCG is the formation of granulomas (18). Granulomas function both to limit the spread of infection and to provide an environment of activated macrophages which, through autocrine and paracrine stimulation, kill the mycobacteria. The formation of granulomas, although critical to the resolution and control of infection, is also the primary cause of the tissue destruction and pathology seen in mycobacterial infections (7).
Granulomas are composed of activated mononuclear phagocytic cells and T lymphocytes (20); however, the evolution of the cellular composition during infection is less well defined. The formation of granulomas is dependent on cytokines, notably gamma interferon (IFN-γ) and tumor necrosis factor (TNF) (2, 13). Numerous in vitro studies have shown that TNF and IFN-γ act in synergy to activate bactericidal mechanisms in murine macrophages, in particular the induction of nitric oxide through the up-regulation of inducible nitric oxide synthase (4, 10). In vivo studies with both ligand- and receptor-deficient mice and monoclonal antibody neutralization have revealed that deficiency of TNF and IFN-γ causes increased susceptibility to infection, with manifestations including retarded granuloma formation and increased bacterial loads (2, 6, 11, 12, 14). The importance of TNF is further highlighted by studies in which treatment with TNF increased host resistance to M. tuberculosis (8) and Listeria monocytogenes (9) infection in mice.
Recently a short peptide which mimics some of the actions of TNF has been described (19). This 11-mer mimetic peptide, TNF70–80, corresponds to residues 70 to 80 of human TNF and differs from that sequence only by the substitution of isoleucine for leucine at position 76. This substitution confers increased stability without affecting peptide binding to the 55- and 75-kDa TNF receptors (15, 19). TNF70–80 enhanced human polymorphonuclear cell-mediated killing of Plasmodium falciparum in vitro by stimulating and priming the polymorphonuclear cells for increased respiratory burst and granule release (15). Treatment of both Plasmodium chabaudi-infected mice (15) and Pseudomonas aeruginosa-infected mice (19a) with TNF70–80 reduced the parasite or bacterial burden, as well as decreasing the systemic effects of P. aeruginosa infection.
In this study, the cellular components of the granulomas that form during the normal course of M. bovis BCG infection were analyzed. Based on this analysis we selected the height of infection to compare the effects of treatments with TNF and TNF70–80 on the immunopathology of M. bovis BCG infection. Both treatments altered the number and cellular composition of the granulomas compared to those in infected untreated mice. The change in pathology was also associated with a moderate decrease in bacterial burden in the spleen.
M. bovis BCG (CSL) was obtained from CSL Biosciences (Melbourne, Australia) and prepared as previously described (3). Specific-pathogen-free female C57B1/6 mice from Little Bay Animal Facility (University of New South Wales, Sydney, Australia), at 6 to 10 weeks of age, were infected intravenously with 106 viable BCG organisms. The course of bacterial infection was determined by plating serial dilutions of whole spleen homogenates on nutrient oleic acid-albumin-dextrose-catalase (OADC)-enriched 7H11 agar and counting bacterial colonies formed after 21 days’ incubation (37°C, 5% CO2). Formaldehyde-fixed, hematoxylin and eosin (H&E)-stained liver sections were used to quantify granulomas. A granuloma was defined as a cluster of 8 to 10 macrophages and lymphocytes, and the average number in 10 randomly selected high-power (×400) fields was determined. The cellular phenotypes of the granulomas were analyzed by quantitative image analysis. Liver sections were immunohistochemically stained, and the area of positively stained tissue was determined in 10 randomly selected fields of view by using the automated Chromatic color image analysis software package version 2.2 (Leading Edge). Following image capture, the primary color levels of individual pixels were compared to predetermined values, based on the color of the enzymatic product. Pixels which met these criteria were classed as positive and were then used to calculate the areas of positive staining. The antibodies used were as follows: rat anti-murine major histocompatibility complex (MHC) class II (hybridoma line P7/7), rat anti-murine CD4 (GK1.5), rat anti-murine CD8 (53.6.7), biotinylated hamster anti-mouse γδ T-cell receptor (TCR) (GL3-1A), and biotinylated mouse anti-NK1.1 (PK136), followed by biotinylated rabbit anti-rat immunoglobulin G (Dako) and/or streptavidin-conjugated alkaline phosphatase (Amersham). Bound antibodies were visualized by color development with New Fuchsin. The area of positive staining reflects the number of cells of that phenotype in that field of view.
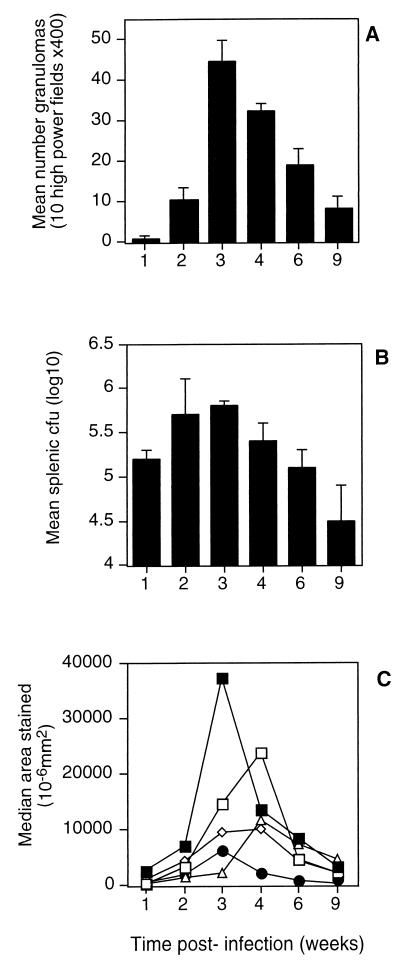
The primary sites of infection following intravenous BCG exposure are the liver and spleen (5). The time at which the maximum number of granulomas were observed was 3 weeks postinfection (Fig. 1A), while the bacterial burden peaked at week 2 of infection (Fig. 1B). Quantitative image analysis determined the areas of positive tissue staining for MHC class II, CD4, CD8, γδ TCR, and NK1.1, which were indicative of the numbers of cells of each phenotype in the tissue. The phenotypic profile of the cellular composition varied over time. Early in infection granulomas contained large numbers of MHC class II+ cells and CD4+ T cells and a smaller number of NK1.1+ cells. At 4 weeks the granulomas were predominated by CD4+, CD8+, and γδ TCR+ T cells, MHC class II+ cells were reduced in number, and NK1.1+ cells were absent (Fig. 1C). Uninfected liver tissue was negative for all cell types tested, including MHC class II (data not shown).
FIG. 1.
Time course of intravenous M. bovis BCG infection. Mice were infected with 106 BCG cells intravenously, and the course of infection was monitored over 9 weeks, with six mice being examined at each time point. (A) Mean number of granulomas in 10 high-power fields of H&E-stained liver sections (magnification, ×400). (B) Mean CFU numbers recoverable from spleens. (C) Phenotypes of cells constituting granulomas evaluated by measuring the areas of immunohistochemically stained liver tissues. Closed squares, MHC class II+; open squares, CD4+; open diamonds, γδ TCR+; open triangles, CD8+; closed circles, NK1.1+. The results are representative of two experiments.
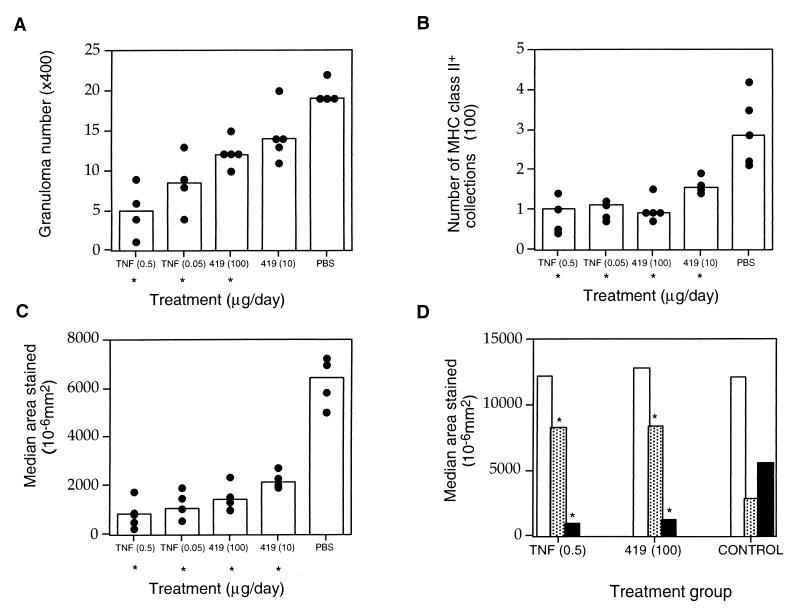
In a second set of experiments the effects of treatments, prior to the peak of infection, with TNF and TNF70–80 were examined. Recombinant human TNF (3.4 × 104 U/μg) was obtained from Peptide Technology Ltd. (Sydney, Australia). The sequence and synthesis of TNF70–80 have been previously described (3), and 1 μg of TNF70–80 had activity equivalent to approximately 200 U of TNF. Mice were infected with 106 BCG organisms intravenously and rested for 7 days. Mice were then treated intraperitoneally, daily for 10 days, with 0.5 μg of TNF, 0.05 μg of TNF, 100 μg of TNF70–80, or 10 μg of TNF70–80, all in phosphate-buffered saline (PBS), or with PBS alone. Control mice received daily saline injections. Analysis 24 h after the last injection revealed significantly less pathology in the livers of TNF- or TNF70–80-treated mice than in those of the PBS-treated control mice, as revealed by decreased numbers of granulomas and reduction in the number of foci and the total area of MHC class II+ tissue (P < 0.05) (Fig. 2A, B, and C and Fig. 3). Treatment with TNF or TNF70–80 also altered the cellular composition of the granulomas. In addition to a reduction in MHC class II+ cells, mice had a two- to threefold increase in the area of CD8+-stained tissue and a reduction in the area of NK1.1+ tissue staining compared with saline-treated or untreated BCG-infected control mice (Fig. 1C and 2D). There were no significant differences in the median area of staining for CD4+ (Fig. 2D) or γδ TcR+ tissue (data not shown) in the livers of TNF- or TNF70–80-treated mice. The phenotype of the cellular components of the granulomas in the TNF- and TNF70–80-treated mice at day 17 was similar to that occurring later in the normal course of infection, that is, there were high numbers of CD8+ T cells, low numbers of NK1.1+ cells, and reduced numbers of MHC class II+ cells (Fig. 1C). Furthermore, in mice treated with exogenous TNF and TNF70–80, the numbers of viable BCG organisms in the spleens were reduced by 66 and 41%, respectively (P < 0.05) (Table 1).
FIG. 2.
Effect of treatment with TNF or TNF-mimetic peptide on M. bovis BCG infection. Mice were infected with 106 BCG cells intravenously and treated intraperitoneally from days 7 to 16 with the doses indicated on the figure. Each data point represents a value for an individual mouse, and each column indicates the median value of the group (n = 4). (A) Number of granulomas visible in 10 high-power fields of H&E-stained liver sections (magnification, ×400). (B) Number of MHC class II+ collections in 10 image analysis fields (magnification, ×100). (C) Total area of MHC class II+ tissue staining in 10 fields of view. (D) Total areas stained for CD4+ (open columns), CD8+ (shaded columns), and NK1.1+ (closed columns). Asterisks denote significant differences compared to PBS-treated mice (P < 0.05, Mann-Whitney U test). The results are representative of three experiments. 419, TNF70–80.
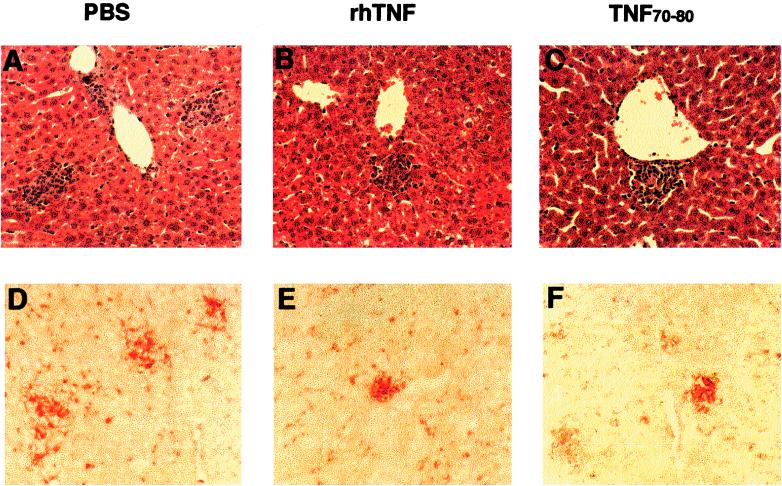
FIG. 3.
Effect of treatment on pathology at 17 days after M. bovis BCG infection. (A, B, and C) Liver sections stained with H&E. (D, E, and F) Liver tissues stained with anti-MHC class II antibody. Panels A and D show tissues from PBS-treated mice, panels B and E show those from TNF-treated mice, and panels C and F show those from TNF70–80-treated mice (magnification, ×200). Tissues are representative of five mice in each of three separate experiments.
TABLE 1.
The anti-mycobacterial effect of in vivo treatment with TNF and TNF70–80 on M. bovis BCG-infected micea
| Treatment (per day) | 103 CFU per spleenb | % Decreasec |
|---|---|---|
| 0.5 μg of TNF | 124d (58) | 60 |
| 0.05 μg of TNF | 160d (59) | 48 |
| 100 μg of TNF70–80 | 183d (19) | 40 |
| 10 μg of TNF70–80 | 253 (71) | 18 |
| PBS | 307 (18) |
Mice were infected, rested for 7 days, and then treated daily for 10 days. Mice were sacrificed 24 h after the last treatment.
Splenic bacterial load (mean ± standard deviation [shown in parentheses] for five mice). Spleens were homogenized and serial-dilution plated onto 7H11 agar plates.
Percent decreases were calculated as a percentage of the value for the control (PBS-treated) group.
Significant difference from value for PBS-treated group by Mann-Whitney U test (P < 0.05).
TNF is required to control acute mycobacterial infections and to prevent reactivation during the chronic stages of infection (1, 2, 12) through the formation and maintenance of granulomas (2, 12) and macrophage activation leading to mycobacterial killing (3). Treatment with TNF or TNF70–80, over the period in which the granulomatous response develops, leads to decreased numbers of granulomas and reduced bacterial load. Moreover, the cellular constituents of granulomas resembled those observed late in the normal course of infection. This suggests that treatment with TNF or TNF70–80 induced early macrophage activation leading to increased clearance of BCG and therefore fewer granulomas. Both TNF and TNF70–80 synergize with IFN-γ to induce macrophage production of reactive nitrogen intermediates (RNI) (4). The production of RNI is associated with killing of mycobacteria (3, 10). During infection TNF is localized in granulomas, at high concentration relative to that in the surrounding tissues (14). Exogenous TNF or TNF70–80 peptide may act synergistically with IFN-γ to produce RNI prior to granuloma formation and thus facilitate killing without the presence of a granuloma. Reducing the bacterial burden early in infection may in turn lead to the more rapid maturation of the granulomas. Treatment with TNF70–80 has recently been shown to increase the clearance of other bacterial and fungal infections (16, 17).
In summary, treatment with TNF70–80 or TNF early in BCG infection reduced the granulomatous response, modified the cellular infiltrate, and reduced the bacterial burden. This activity of TNF70–80 is consistent with its in vitro effects on macrophage activation and inducible nitric oxide synthase induction.
Acknowledgments
This study was supported by grants from the Community Health and Anti-Tuberculosis Association of New South Wales and the National Health and Medical Research Council of Australia.
We thank Danielle Avery for technical assistance and Philip Mack for provision of TNF70–80.
REFERENCES
- 1.Adams L B, Mason C M, Kolls J K, Scollard D, Krahenbuhl J L, Nelson S. Exacerbation of acute and chronic murine tuberculosis by administration of a tumour necrosis factor receptor-expressing adenovirus. J Infect Dis. 1995;171:400–405. doi: 10.1093/infdis/171.2.400. [DOI] [PubMed] [Google Scholar]
- 2.Bean A G D, Roach D R, Briscoe H, France M P, Korner H, Sedgwick J D, Britton W J. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection which is not compensated by lymphotoxin. J Immunol. 1999;162:3504–3511. [PubMed] [Google Scholar]
- 3.Britton W J, Meadows N, Rathjen D A, Roach D R, Briscoe H. A tumor necrosis factor mimetic peptide activates a murine macrophage cell line to inhibit mycobacterial growth in a nitric oxide-dependent fashion. Infect Immun. 1998;66:2122–2127. doi: 10.1128/iai.66.5.2122-2127.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan J, Xing Y, Magliozzo R S, Bloom B R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins F M, Montalbine V. Distribution of in vivo grown mycobacteria in the organs of intravenously infected mice. Am Rev Respir Dis. 1976;113:281–286. doi: 10.1164/arrd.1976.113.3.281. [DOI] [PubMed] [Google Scholar]
- 6.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon-γ gene disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dannenberg A M. Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunol Today. 1991;12:228–233. doi: 10.1016/0167-5699(91)90035-R. [DOI] [PubMed] [Google Scholar]
- 8.Denis M. Involvement of cytokines in determining resistance and acquired immunity in murine tuberculosis. J Leukoc Biol. 1991;50:495–501. doi: 10.1002/jlb.50.5.495. [DOI] [PubMed] [Google Scholar]
- 9.Desiderio J V, Kiener P A, Lin P-F, Warr G A. Protection of mice against Listeria monocytogenes infection by recombinant tumor necrosis factor alpha. Infect Immun. 1989;57:1615–1617. doi: 10.1128/iai.57.5.1615-1617.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flesch I E A, Kaufmann S H E. Activation of tuberculostatic macrophage functions by gamma interferon, interleukin-4, and tumor necrosis factor. Infect Immun. 1990;58:2675–2677. doi: 10.1128/iai.58.8.2675-2677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon-γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Shreiber R, Mak T W, Bloom B R. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann S H E. Immunity to intracellular mycobacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 14.Kindler V, Sappino A P, Grau G E, Piguet P-F, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 15.Kumaratilake L M, Rathjen D A, Mack P, Widmer F, Prasertsiriroj V, Ferrante A. A synthetic tumor necrosis factor-α agonist peptide enhances human polymorphonuclear leukocyte-mediated killing of Plasmodium falciparum in vitro and suppresses Plasmodium chabaudi infection in mice. J Clin Investig. 1995;95:2315–2323. doi: 10.1172/JCI117923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laichalk L L, Bucknell K A, Huffnagle G B, Wilkowski J M, Moore T A, Romanelli R J, Standiford J J. Intrapulmonary delivery of tumor necrosis factor agonist peptide augments host defense in murine gram-negative bacterial pneumonia. Infect Immun. 1998;66:2822–2826. doi: 10.1128/iai.66.6.2822-2826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehrad B, Strieter R M, Stadiford T J. Role of TNF-alpha in pulmonary host defense in murine invasive aspergillosis. J Immunol. 1999;136:1633–1640. [PubMed] [Google Scholar]
- 18.Orme I M. The immunopathogenesis of tuberculosis: a new working hypothesis. Trends Microbiol. 1996;6:94–97. doi: 10.1016/s0966-842x(98)01209-8. [DOI] [PubMed] [Google Scholar]
- 19.Rathjen D A, Ferrante A, Aston R. Differential effects of small tumour necrosis factor α peptides on tumour cytotoxicity, neutrophil activation and endothelial cell procoagulant. Immunology. 1993;80:293–299. [PMC free article] [PubMed] [Google Scholar]
- 19a.Rathjen, D. A. Unpublished observations.
- 20.Sheffield E A. The granulomatous inflammatory response. J Pathol. 1990;160:1–2. doi: 10.1002/path.1711600102. [DOI] [PubMed] [Google Scholar]