Abstract
Structures similar to the melanin “ghosts” of melanized cryptococcal cells were isolated from pigeon excreta contaminated with Cryptococcus neoformans, and their growth in pigeon excreta supported melanization. The results suggest that environmental C. neoformans cells are melanized and imply that initial infection may involve exposure to melanized cells.
Cryptococcus neoformans has a laccase that catalyzes the synthesis of melanin in the presence of phenolic compounds (9, 17). Melanin synthesis by C. neoformans is associated with virulence (11, 14). Melanin can protect C. neoformans against antifungal compounds, oxidants, UV light, macrophages, and extremes in temperature (reviewed in reference 2). Since melanization confers reduced susceptibility to many insults, this trait may have originally been selected for environmental survival.
Infections with C. neoformans are thought to occur by inhalation of aerosolized organisms from environmental sources, and the sizes of cryptococcal cells collected from pigeon excreta are compatible with aveolar deposition (10). Although pigeon excreta have not formally been considered to be a source of human infections, four independent DNA-typing studies have identified the same cryptococcal strains in collected pigeon excreta and clinical isolates (3–5, 18). Furthermore, there is considerable anecdotal evidence suggesting that humans were infected after exposure to avian excreta (reviewed in reference 2). In this study we present evidence that cryptococcal cells are melanized in pigeon excreta.
(The data in this paper are from a thesis to be submitted by Angel Luis Rosas in partial fulfillment of the requirements for the degree of doctor of philosophy from the Sue Golding Graduate Division of Medical Sciences, Albert Einstein College of Medicine, Yeshiva University, Bronx, N.Y.)
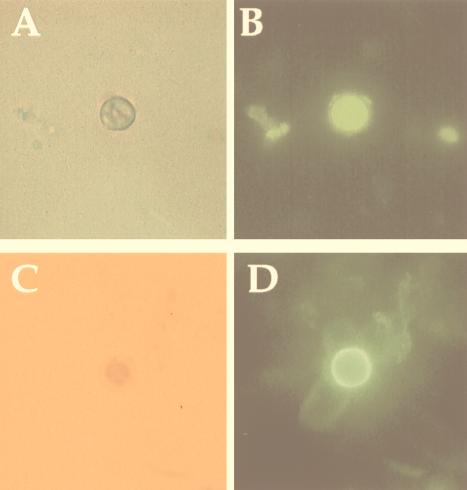
Samples of pigeon excreta were collected from areas adjacent to our institution. The presence of C. neoformans in the feces was initially investigated by a C. neoformans cell capture enzyme-linked immunosorbent assay (ELISA) and immunofluorescence (IF) to screen pigeon excreta slurries. The ELISA captures and immobilizes yeast cells and was performed as described previously (1). For IF, 50 μl of pigeon excreta supernatant was dried on poly-l-lysine-coated slides (Sigma Chemical Corp., St. Louis, Mo.). Blocking for nonspecific binding was done with 0.05% Tween 20 in 2% bovine serum albumin (BSA) for 1 h at 37°C. Fifty-microliter samples of the polysaccharide-binding immunoglobulin M (IgM) monoclonal antibody (MAb) 2D10 (1) or antimelanin IgM MAb 11B11 (13) (1:100 [vol/vol]) in 1% BSA were applied to the slides, which were incubated in a moisture chamber for 1 h at 37°C. After excess MAb was washed off, each slide was covered with 50 μl of a 1:100 dilution of fluorescein isothiocyanate-conjugated goat anti-mouse IgM (Southern Biotech, Birmingham, Ala.) in 1% BSA for 1 h at room temperature. The slide was washed, a coverslip was placed over it with a mounting solution (50% glycerol, 50% phosphate-buffered saline [PBS]–0.1 M N-propyl gallate), and the slide was examined with an Olympus (Melville, N.Y.) AX70 microscope. Negative controls included slides with nonmelanized cells (ATCC 24067), NSO ascites fluid, or no primary MAb. IF with the MAb 2D10 and whole-cell capture ELISA demonstrated yeast cells that stained for cryptococcal polysaccharide in pigeon feces (Fig. 1A and B). IF with the melanin-binding MAb also stained yeast cells in the pigeon excreta supernatants (Fig. 1C and D).
FIG. 1.
Light and IF images of C. neoformans in pigeon excreta suspensions (magnification, ×1,000). (A and B) Images depict the binding of MAb 2D10 to a yeast cell. (C and D) Images depict the staining of the melanin-binding MAb 11B11 to a yeast cell.
Excreta that demonstrated C. neoformans by capture ELISA and/or IF were plated with 50-μl aliquots on isolation agar (1 mM l-dopa, 29.4 mM KH2PO4, 10 mM MgSO4, 13 mM glycine, 15 mM glucose, and 6 μM thiamine [Sigma] with 0.1% biphenyl [Eastman Fine Chemicals, Eastman Kodak Company, Rochester, N.Y.], 0.025% vancomycin [Fugisawa USA, Inc., Deerfield, Ill.], 0.025% imipenem–cilastin [Merck and Co.], and 1.5% agar [Difco Laboratories, Detroit, Mich.]) (3) and incubated at 30°C. After a minimum of 6 days, darkly pigmented colonies were selected for further analysis. India ink suspensions of cells from colonies or after growth in Sabouraud dextrose medium (Difco) at 30°C were viewed with an Olympus AX70 microscope. Isolates from pigeon excreta melanized when they were grown in a defined chemical medium with l-dopa (15 mM glucose, 10 mM MgSO4, 29.4 mM KH2PO4, 13 mM glycine, 3 μM thiamine, 1 mM l-dopa [Sigma]) at 30 or 37°C. Urease activity was measured with BBL urea agar slants (Becton Dickinson, Cockeysville, Md.). Selected isolates that were India ink positive, stained with MAb 2D10, melanized in l-dopa medium, grew at 37°C, and utilized urea were further identified as C. neoformans with the API 20C Clinical Yeast System (BioMerieux Vitek, Inc., Hazelwood, Mo.).
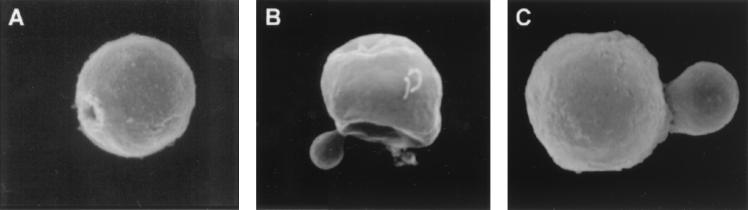
Pigeon excreta that tested positive for C. neoformans were treated with enzymes, denaturant, and hot acid and examined by scanning electron microscopy for particles that resembled melanin “ghosts” (16). Briefly, dried pigeon excreta (250 ml) were dissolved in 1 M sorbitol–0.1 M sodium citrate (pH 5.0) and then treated with 10 mg of Novozym 234 (Calbiochem, La Jolla, Calif.) per ml for 1 h at 30°C. The debris was collected by centrifugation, suspended in 4 M guanidinium isothiocyanate for 2 h at room temperature, and then incubated in 6 M HCl for 1 h at 100°C. The denaturant- and acid-resistant material was dialyzed exhaustively against distilled water. Samples of the isolated material were prepared as previously described (7) and viewed with a model JSM-6400 electron microscope (JEOL, Tokyo, Japan). Spherical structures very similar to the melanin ghosts isolated from melanized cells (16) were observed in pigeon excreta that had been treated with enzyme, denaturant, and boiling acid. These particles contained buds and bud scars, consistent with an origin from melanized yeast (Fig. 2A and B). One particle had a hole in the surface, suggestive of a budding site where the daughter cell was sheared off during the melanin isolation.
FIG. 2.
Scanning electron micrographs of particles isolated following treatment with enzyme, detergent, and hot acid of pigeon excreta (A and B) or cryptococcal cells grown on excreta agar (C) (magnification, ×15,000). The structures are remarkably similar to cryptococcal melanin ghosts (8, 16) and demonstrate what appear to be bud scars (A) and budding (B and C).
To examine whether pigeon excreta had precursors for melanization, C. neoformans was grown on pigeon excreta agar plates. Pigeon feces were suspended in a minimal amount of PBS and autoclaved. Agar plates were prepared with a defined chemical medium (15 mM glucose, 10 mM MgSO4, 29.4 mM KH2PO4, 13 mM glycine, and 3 μM thiamine [Sigma] with 2% Bacto-Agar [Difco]) with 5, 10, or 20% excreta. Positive control plates were made with 1 mM l-dopa, and negative control plates lacked a substrate. C. neoformans ATCC 24067 or Candida albicans SC5314 (gift of M. Ghannoun) was grown overnight in Sabouraud dextrose medium at 30°C with shaking and then collected and washed three times in PBS. Ten-day-old C. neoformans cells growing on 10% excreta plates were collected, subjected to the melanin ghost isolation protocol (16), and prepared for scanning electron microscopy. C. neoformans grown in medium without phenolic substrate completely solubilize after treatment with enzyme, detergent, and hot HCl (16). Colonies of C. neoformans grown on the defined agar plates with or without 10% excreta were selected and suspended in PBS, and the cells were observed in India ink suspensions. Measurements of total cell diameter were done with an Olympus AX70 microscope (magnification, ×1,000) with a grid (number of segments, 20) with resolutions to 0.1 μm. Colonies of strain 24067 on 5, 10, or 20% excreta plates turned brown by day 5 and became progressively darker over time. Colonies on the defined agar without phenolic substrate were white, whereas those on plates with l-dopa were black. C. albicans grown on the excreta plates did not become pigmented, suggesting that colony pigmentation was not due simply to the uptake of some type of pigment from the excreta agar. Treatment of C. neoformans cells from excreta agar by the melanin isolation protocol yielded particles that were very similar to melanin ghosts (Fig. 2C). The diameters of the ghost-like structures isolated from C. neoformans grown on 10% excreta agar were similar to those of ghosts prepared from cells grown on the same agar (3.2 ± 0.77 μm versus 2.88 ± 0.92 μm; n = 20; P > 0.05 by Student’s t test). Similarly, the sizes of the particles visualized in pigeon feces were 2.91 ± 1.07 μm (n = 20; P > 0.05 by Student’s t test).
We conclude that C. neoformans in pigeon excreta is melanized from the following evidence: (i) yeast cells in pigeon excreta stain with MAbs to C. neoformans polysaccharide and melanin; (ii) structures remarkably similar in size and appearance to melanin ghosts can be isolated both from pigeon feces contaminated with C. neoformans and from cryptococcal cells grown on excreta agar after treatment with enzyme, denaturant, and hot acid; and (iii) C. neoformans colonies on pigeon excreta agar plates are darkly pigmented. Melanization of C. neoformans in the environment has important implications for our understanding of cryptococcal ecology and pathogenesis. Melanin synthesis in pigeon excreta may potentially protect C. neoformans cells against environmental stresses such as UV light (15) and extremes in temperature (12). Human C. neoformans infection is acquired from the environment, and there is extensive circumstantial evidence implicating pigeon excreta as a potential reservoir for infection (reviewed in reference 2). Our finding suggests that the initial infection may involve melanized C. neoformans cells. Since there is evidence that the immune responses to melanized and nonmelanized cells are different (6), our results suggest that it is important to study the effect of melanization in initial infection.
Acknowledgments
This work was supported by grants from the NIH (grants RO1-AI22774, AI13342, and HL59842 [A.C.]; grant K08-AI01489 [J.D.N.]; and training grant 5T32GM07491 [A.L.R.]), the Burroughs Wellcome Trust (A.C.), and the Infectious Diseases Society of America (J.D.N.).
REFERENCES
- 1.Casadevall A, Cleare W, Feldmesser M, Glatman-Freedman A, Goldman D L, Kozel T R, Lendvai N, Mukherjee J, Pirofski L-A, Rivera J, Rosas A L, Scharff M D, Valadon P, Westin K, Zhong Z. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother. 1998;42:1437–1446. doi: 10.1128/aac.42.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casadevall A, Perfect J R. Cryptococcus neoformans. Washington, D.C: American Society for Microbiology; 1998. [Google Scholar]
- 3.Currie B P, Freundlich L F, Casadevall A. Restriction fragment length polymorphism analysis of Cryptococcus neoformans isolates from environmental (pigeon excreta) and clinical sources in New York City. J Clin Microbiol. 1994;32:1188–1192. doi: 10.1128/jcm.32.5.1188-1192.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franzot S P, Hamdan J S, Currie B P, Casadevall A. Molecular epidemiology of Cryptococcus neoformans in Brazil and the United States: evidence for both local genetic differences and a global clonal population structure. J Clin Microbiol. 1997;35:2243–2251. doi: 10.1128/jcm.35.9.2243-2251.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Hermoso D, Mathoulin-Pelissier S, Couprie B, Ronin O, Dupont B, Dromer F. DNA typing suggests pigeon droppings as a source of pathogenic Cryptococcus neoformans serotype D. J Clin Microbiol. 1997;35:2683–2685. doi: 10.1128/jcm.35.10.2683-2685.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huffnagle G B, Chen G-H, Curtis J L, McDonald R A, Strieter R M, Toews G B. Down-regulation of the afferent phase of T cell-mediated pulmonary inflammation and immunity by a high melanin-producing strain of Cryptococcus neoformans. J Immunol. 1995;155:3507–3516. [PubMed] [Google Scholar]
- 7.Nosanchuk J D, Rosas A L, Casadevall A. The antibody response to fungal melanin in mice. J Immunol. 1998;160:6026–6031. [PubMed] [Google Scholar]
- 8.Nosanchuk J D, Valadon P, Feldmesser M, Casadevall A. Melanization of Cryptococcus neoformans in murine infection. Mol Cell Biol. 1999;19:745–750. doi: 10.1128/mcb.19.1.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polacheck I, Kwon-Chung K J. Melanogenesis in Cryptococcus neoformans. J Gen Microbiol. 1988;134:1034–1041. doi: 10.1099/00221287-134-4-1037. [DOI] [PubMed] [Google Scholar]
- 10.Powell K E, Dahl B A, Weeks R J, Tosh F E. Airborne Cryptococcus neoformans: particles from pigeon excreta compatible with alveolar deposition. J Infect Dis. 1972;125:412–415. doi: 10.1093/infdis/125.4.412. [DOI] [PubMed] [Google Scholar]
- 11.Rhodes J C, Polacheck I, Kwon-Chung K J. Phenoloxidase activity and virulence in isogenic strains of Cryptococcus neoformans. Infect Immun. 1982;36:1175–1184. doi: 10.1128/iai.36.3.1175-1184.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosas A L, Casadevall A. Melanization affects susceptibility of Cryptococcus neoformans to heat and cold. FEMS Microbiol Lett. 1997;153:265–272. doi: 10.1111/j.1574-6968.1997.tb12584.x. [DOI] [PubMed] [Google Scholar]
- 13.Rosas A L, Nosanchuk J D, Casadevall A. Abstracts of the General Meeting of the American Society for Microbiology 1999. Washington, D.C: American Society for Microbiology; 1999. Melanin-binding monoclonal antibodies for the study of melanogenesis of Cryptococcus neoformans in vivo. [Google Scholar]
- 14.Salas S D, Bennett J E, Kwong-Chung K J, Perfect J R, Williamson P R. Effect of the laccase gene, CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med. 1996;184:377–386. doi: 10.1084/jem.184.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Casadevall A. Decreased susceptibility of melanized Cryptococcus neoformans to UV light. Appl Environ Microbiol. 1994;60:3864–3866. doi: 10.1128/aem.60.10.3864-3866.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Casadevall A. Melanin, melanin “ghosts,” and melanin composition in Cryptococcus neoformans. Infect Immun. 1996;64:2420–2424. doi: 10.1128/iai.64.7.2420-2424.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williamson P R. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: identification as a laccase. J Bacteriol. 1994;176:656–664. doi: 10.1128/jb.176.3.656-664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto Y, Kohno S, Koga H, Kakeya H, Tomono K, Kaku M, Yamazaki T, Arisawa M, Hara K. Random amplified polymorphic DNA analysis of clinically and environmentally isolated Cryptococcus neoformans in Nagasaki. J Clin Microbiol. 1995;33:3328–3332. doi: 10.1128/jcm.33.12.3328-3332.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]