Abstract
Circadian rhythms control almost all aspects of physiology and behavior, allowing temporal synchrony of these processes between each other, as well as with the external environment. In the immune system, daily rhythms of leukocyte functions can determine the strength of the immune response, thereby regulating the efficiency of defense mechanisms to cope with infections or tissue injury. The natural light/dark cycle is the prominent synchronizing agent perceived by the circadian clock, but this role of light is highly compromised by irregular working schedules and unintentional exposure to artificial light at night (ALAN). The primary concern is disrupted circadian control of important physiological processes, underlying potential links to adverse health effects. Here, we first discuss the immune consequences of genetic circadian disruption induced by mutation or deletion of specific clock genes. Next, we evaluate experimental research into the effects of disruptive light/dark regimes, particularly light-phase shifts, dim ALAN, and constant light on the innate immune mechanisms under steady state and acute inflammation, and in the pathogenesis of common lifestyle diseases. We suggest that a better understanding of the mechanisms by which circadian disruption influences immune status can be of importance in the search for strategies to minimize the negative consequences of chronodisruption on health.
Keywords: circadian rhythms, chronodisruption, inflammation, innate immunity, light at night, phase shifts
1. Introduction
Circadian rhythms (circa = about; dies = day) represent endogenous oscillations with a period of approximately 24 h. In most species, circadian rhythms are effectively entrained by external factors, primarily by a light/dark (LD) cycle, allowing the anticipation of daily periodic changes in the environment [1,2]. Mammalian circadian rhythms are governed by a master clock located in the suprachiasmatic nuclei (SCN) of the hypothalamus. The SCN receives photic input from the environment and transmits the information to peripheral oscillators to coordinate the optimal timing of physiological and behavioral processes [3].
Life on Earth has evolved under relatively stable conditions of bright days and dark nights. The sun is the primary light source for the majority of organisms, with daylight illumination varying from 50,000 to 100,000 lx, and low illuminance levels during the night, reaching up to 0.3 lx at the full moon [4,5]. Nowadays, light exposure is no longer limited by the natural LD cycle in the industrialized world. Recent studies show that more than 80% of the world’s population lives in light-polluted areas [6] and increasing exposure to artificial light at night (ALAN) represents a novel challenge for both humans and wildlife [7,8]. The straightforward impact of compromised LD cycles is linked with circadian disruption, which can be manifested at multiple levels, depending on the nature of mistimed light information. Such situations are a common part of modern society and include especially various shift work schedules, time-zone transitions, or unintentional ALAN exposure. Here, circadian disruption refers to transient or chronic misalignment between the external LD cycle and endogenous circadian clocks, which can further lead to internal misalignment (impaired phase relationships) or desynchronization (changes in period) among individual endogenous rhythms, diminished peak-trough differences in these rhythms (changes in amplitude) or complete arrhythmicity [9]. The main result is attenuated or abolished circadian control of important physiological processes, underlying potential links to adverse health effects [10,11]. Many epidemiological studies examining the risk of common lifestyle diseases among shift workers or due to ALAN found a positive correlation with the incidence of sleep disorders [12,13], cancer [14,15], metabolic and cardiovascular diseases [16,17,18]. A common feature of most lifestyle and chronic diseases is low-grade inflammation, which can further potentiate disease progression [19]. Therefore, a better understanding of the mechanisms by which circadian disruption influences the status of the immune system and inflammatory responses can be of importance in the search for strategies to minimize the negative consequences of environmentally induced circadian disruption on health.
In the current review, we document the effects of circadian disruption resulting from compromised LD information on fundamental aspects of the innate immune defense under homeostatic conditions, as well as in response to acute inflammation and in the pathogenesis of diseases. We focus on data obtained from experimental studies in rodents and first compare the immune consequences in transgenic animal models with genetic mutation or deletion of specific clock genes. In the following sections, we evaluate the impact of different disruptive LD regimes, particularly light-phase shifts, dim ALAN, and constant light (LL) on innate immune cells and their effector functions.
The literature search was performed in the PubMed and Google Scholar databases based on the following keywords: artificial light at night, circadian disruption, constant light, dim light at night, innate immunity, inflammation, jet lag, macrophages, monocytes, neutrophils, NK cells, shift work. Relevant papers were evaluated by title and abstract, followed by a full-text overview.
2. Mammalian Circadian System
In mammals, circadian timekeeping is organized into a multi-oscillator system operating in a hierarchical manner, with the SCN as a master oscillator [20]. The SCN neurons are located alongside the third ventricle above the optic chiasm and form a unified circadian network [21]. Light information is perceived by the intrinsically photosensitive retinal ganglion cells, containing the photopigment melanopsin, and conveyed via the retinohypothalamic tract into the SCN [22]. Subsequently, the SCN communicates timing information to individual peripheral oscillators via neural and humoral pathways [23].
At the molecular level, circadian rhythms are generated through transcriptional-translational feedback loops of clock genes and their protein products, forming a basis of the self-sustained and cell-autonomous molecular clocks [24]. The core feedback loop consists of positive and negative regulators. The CLOCK and BMAL1 proteins heterodimerize to form the CLOCK/BMAL1 complex, which activates transcription via binding to E-box enhancer elements in the promoters of clock genes, Period (Per1, Per2, and Per3) and Cryptochrome (Cry1 and Cry2). The PER and CRY proteins represent a negative limb of the loop, as they form the repressive PER/CRY complex, which enters the nucleus, combines with CLOCK/BMAL1, and inhibits the transcription of E-box-controlled genes [25]. The availability and stability of PER and CRY proteins are regulated by protein kinases and phosphatases [26].
Additionally, the core loop is stabilized by accessory feedback loops, consisting of transcriptional activators and repressors, which regulate target genes either through ROR response elements (RORE) or D-boxes [27]. In this way, nuclear receptors REV-ERBs (α/β) repress and retinoic acid-related orphan receptors (RORα/β/γ) activate the transcription of Bmal1, which contains RORE in its promoter. On the other hand, the CLOCK/BMAL1 complex can activate the transcription of genes encoding REV-ERBs [28]. The next feedback loop is formed by nuclear factor interleukin-3 (NFIL3, also known as E4BP4) and D-box binding protein (DBP), which competitively repress or activate the transcription of D-box regulated genes, such as those encoding the circadian proteins PER, REV-ERBs, and RORs [24]. Importantly, circadian regulatory elements have also been identified in the promoters of numerous immune genes, underlying direct crosstalk between the components of the molecular clockwork and the immune system [29,30].
3. Circadian Rhythms in Innate Immunity
Innate immune mechanisms represent the first line of defense against invading pathogens. Circulating and tissue-specific innate immune cells recognize pathogens or cell injury via pattern recognition receptors [31]. Subsequently, initiated signaling pathways induce the release of specific immune mediators, such as cytokines, chemokines, and antimicrobial peptides, which are involved in numerous effector functions [32]. Effective host defense against infection is based on tightly regulated immune processes. Inflammation is an essential part of the innate immunity in response to infection or tissue injury. However, deregulated inflammatory responses or disbalance between favoring and limiting factors can lead to chronic inflammation and tissue damage [19].
Immune functions, including innate immune mechanisms, are under circadian control. Leukocyte trafficking, inflammatory responses and susceptibility to pathogens exhibit their peaks and troughs at specific times of the day [33,34]. In steady state, circulating immune cell numbers reach a peak during the day in mice and rats [35,36] and during the night in humans [37]. High and low leukocyte numbers in the blood over 24 h mirror their mobilization from the bone marrow in the passive phase (light phase for rats) and their recruitment to tissues at the onset of the active phase (dark phase for rats) [38]. Leukocyte oscillations persist in an absence of external entraining cues, such as the LD cycle, thereby indicating their endogenous nature [39,40]. Rhythmic leukocyte trafficking is complementary controlled by extrinsic factors, including neural and humoral outputs of the central oscillator, immune cell-autonomous clocks, and tissue-specific microenvironment [35,41,42]. For example, reported data show that β3-adrenergic signaling in the mouse bone marrow down-regulates C-X-C motif chemokine ligand 12 (Cxcl12) expression during the light phase, controlling the rhythmic release of hematopoietic progenitors from the bone marrow into the circulation [43]. Additionally, low corticosterone levels at the onset of the light phase allow proliferation of hematopoietic cells and contribute to their egress into the circulation [44].
The exit of leukocytes from the circulation to the tissues is facilitated by coordinated interactions between adhesion molecules on the endothelium and the surface of leukocytes [45]. In general, rhythmic expression of adhesion molecules, such as intercellular adhesion molecule 1 (ICAM1), vascular cell adhesion molecule 1 (VCAM1), and selectins on endothelial cells promotes time-of-day-dependent leukocyte transmigration into the lymphoid and non-lymphoid tissues [35].
Susceptibility of the immune system to bacterial, viral, and parasitic infections varies across 24 h [46]. One of the first evidence was provided by the experiment, in which mice were administrated a lethal dose of lipopolysaccharide (LPS). An immune challenge given at the end of the rest period led to a mortality rate of 80%, whereas the same LPS dose given in the middle of the active period resulted in a mortality rate of only about 20% [47]. A subsequent study demonstrated that this time-of-day-dependent mortality rate following LPS administration correlates with the increased cytokine response at the end of the light phase (ZT11; ZT—Zeitgeber time) compared to the dark period (ZT19) [48]. Daily variation in susceptibility to inflammatory challenge has also been shown to correlate with nuclear factor kappa B (NF-κB) activation, as mice administrated with a toll-like receptor (TLR) 5 ligand in the middle of their passive phase (ZT6) displayed higher NF-κB activation compared to mice injected in their active phase (ZT18) [49].
Macrophages represent one of the main sources of pro-inflammatory cytokines, and their inflammatory response is controlled by the circadian clock [50]. Mouse peritoneal macrophages show higher LPS-induced expression of inflammatory cytokines, mainly interleukins Il-6, Il-12b, and chemokines Cxcl1 and C-C motif chemokine ligand 2 (Ccl2), when isolated at the end than at the beginning of the subjective passive phase [51]. Moreover, the rhythm of inflammatory monocytes Ly6Chigh in the blood corresponds with the time-of-day-dependent immune response to Listeria monocytogenes infection, reflected by higher levels of CCL2 in the serum and peritoneal fluid upon the induction of infection at ZT8 compared to ZT0 [52].
Neutrophil infiltration into the skeletal muscle was increased upon tumor necrosis factor-alpha (TNFα) challenge at the beginning of the active phase (ZT13) compared to the passive phase (ZT5), and positively correlated with greater Icam1 expression on the muscle endothelial cells [53]. On the other hand, in a mouse model of acute lung inflammation, the recruitment of neutrophils was promoted by the rhythmic release of chemokine CXCL5 from bronchiolar epithelial cells with higher levels upon LPS administration at the beginning of the resting phase compared to the active phase [41].
4. Effects of Circadian Disruption on Innate Immunity
Disruption of the circadian timing system can directly impact daily rhythms in the immune parameters, bearing potential negative consequences on the host’s ability to effectively cope with pathogens or tissue injury. Other complications can include a disturbed balance between anti- and pro-inflammatory mechanisms that can lead to either immunosuppression or promote a pro-inflammatory microenvironment favorable for chronic inflammatory diseases.
The following sections will evaluate the abovementioned ways, in which innate immune cells can respond to circadian disruption induced by the targeted deletion of individual clock genes or by exposure to disruptive LD regimes, including light-phase shifts, dim ALAN, and LL.
4.1. Genetic Circadian Disruption
Many important functional interactions between components of the molecular clock and the immune system have been revealed using animal models with the deletion of clock genes at the systemic or cell-specific levels [29,30]. These studies show that individual clock proteins can differ in their pro-inflammatory and anti-inflammatory properties. Typical immune phenotypes associated with deficiency of the main circadian genes, including Bmal1, Clock, Per1/2, Cry1/2, Rev-erbα, Rorα, and Nfil3, are presented in Table 1.
Table 1.
Animal models with genetic disruption in specific clock components and their effects on processes related to innate immune functions and inflammation.
| Genotype | Immune Challenge | Effects | Refs. |
|---|---|---|---|
| Bmal1−/− mice (global KO) | Lost daily rhythms in the circulating numbers of white blood cells and their progenitors | [43,54] | |
| Bmal1−/− mice (global KO) | KLA (in vitro) | Disturbed transcriptome response to TLR4 activation in BMDMs (enhanced and prolonged response of Il-1β, iNos and Hif1α) | [55] |
| Bmal1−/− mice (global KO) | ↑ severity of DSS-induced colitis | [56] | |
|
ArntlLoxP/LoxPLyz2Cre mice (myeloid-specific Bmal1 KO) |
Lost daily variability in Ly6Chigh monocyte counts in the blood, spleen, and bone marrow | [52] | |
|
ArntlLoxP/LoxPLyz2Cre mice (myeloid-specific Bmal1 KO) |
TG-induced peritoneal inflammation |
↑ peritoneal recruitment of Ly6Chigh monocytes and amplified CCL2, CCL8, IL-1β, and IL-6 response | [52] |
|
ArntlLoxP/LoxPLyz2Cre mice (myeloid-specific Bmal1 KO) |
Listeria monocytogenes infection |
↓ survival and ↑ serum levels of IL-1β, IL-6, IFNγ, and CCL2 | [52] |
|
Bmal1−/−Lys-MCre mice (myeloid-specific Bmal1 KO) |
LPS 25 mg/kg (i.p.) | Lost protection to LPS-induced lethality at ZT0 compared to ZT12 | [57] |
|
Bmal1−/−Lys-MCre mice (myeloid-specific Bmal1 KO) |
LPS 100 ng/mL (in vitro) | ↑ LPS-induced production of IL-6, TNFα, CXCL1 and CCL2 and ↓ levels of IL-10 in BMDMs ↑ pro-inflammatory microRNA miR-155 induction upon LPS in BMDMs |
[57] |
|
Bmal1LoxP/LoxPLyz2Cre mice (myeloid-specific Bmal1 KO) |
LPS 100 ng/mL (in vitro) or LPS 5 mg/kg (i.p.) |
↓ NRF2 response in LPS stimulated BMDMs ↑ basal and LPS stimulated ROS levels, ↑ LPS stimulated IL-1β and HIF1α levels in BMDMs ↑ serum IL-1β response to in vivo LPS stimulation |
[58] |
|
Bmal1FloxP/FloxP;LysMCre mice (myeloid-specific Bmal1 KO) |
LPS 10 or 100 ng/mL (in vitro) | Lost daily variability in IL-12p40-producing cells in LPS-stimulated peritoneal macrophages | [59] |
|
LysM-Bmal1−/− mice (myeloid-specific Bmal1 KO) |
Streptococcus pneumoniae or Staphylococcus aureus infection | Protection against pneumococcal infection ↑ phagocytic activity in peritoneal and alveolar macrophages |
[60] |
|
LysM-Bmal1−/− mice (myeloid-specific Bmal1 KO) |
LPS 1 mg/kg (i.p.) | Lost daily variability in IL-6 response to LPS in peritoneal macrophages | [51] |
|
BmallLoxP/LoxPLyz2Cre mice (ApoE−/− background) (myeloid-specific Bmal1 KO) |
↑ size of atherosclerotic lesions ↑ recruitment of Ly6Chigh monocytes and accumulation of pro-inflammatory M1 macrophages in atherosclerotic lesions |
[61] | |
|
Bmal1ΔN mice (neutrophil-specific deletion of Bmal1) |
Lost daily variability in neutrophil proteome, granule content and NET formation | [62] | |
| Clock−/− mice (global KO) | TNFα 2 ng/mL or CBLB502 100 ng/mL (in vitro) |
↓ NF-κB activation upon TNFα treatment in MEFs and upon bacterial flagellin (CBLB502) treatment in hepatocytes | [49] |
| Clock mutant mice | LPS 1 µg/mL or S. Typhimurium (in vitro) |
↓ expression of pro-inflammatory genes Il-6, Il-1β, Tnfα, Cxcl1, Ifnβ, and Ccl2 and ↓ TNFα and IL-6 response in BMDMs | [63] |
| Clock mutant mice | Salmonella infection (in vivo) | Impaired rhythmicity in bacterial colonization in the gut and reduced pro-inflammatory gene expression | [63] |
| Clock mutant mice | LPS 1 µg/mL (in vitro) | ↓ LPS-induced expression of Il-6, Il-1β and Cxcl1 in MEFs ↑ RELB and p100/52 protein levels in MEFs independent of LPS |
[64] |
| Per1tm1Drw mutant mice | Modified circadian rhythms of perforin, granzyme B and IFNγ in the splenic NK cells | [65] | |
| mPer2Brdml mutant mice | Lost daily IFNγ rhythms (splenic mRNA and protein expression, and serum levels) in the spleen | [66] | |
| mPer2Brdml mutant mice | LPS 25 mg/kg (i.p.) | ↑ survival upon lethal dose of LPS and suppressed daily rhythm in susceptibility to endotoxic shock ↓ serum IFNγ and IL-1β levels and ↓ IFNγ production by splenic NK cells in response to LPS |
[67] |
| mPer2Brdml mutant mice | TLR9 ligand (in vitro) | ↓ TNFα and IL-12 production in challenged peritoneal macrophages and ↓ Tlr9 expression | [68] |
| Cry1−/−Cry2−/− mice and fibroblasts (double KO) | Constitutive activation NF-κB via PKA signaling in fibroblasts ↑ constitutive expression of pro-inflammatory molecules in the hypothalamus and fibroblasts (Il-6, Tnfα and iNos), and in the BMDMs (Il-6, Cxcl1 and iNos) ↑ inflammatory response of BMDMs to LPS (TNFα and IL-6) |
[69] | |
| Rev-erbα−/− mice (global KO) | LPS 1 mg/kg (i.p.) LPS 1 µg/mL (in vitro) |
Lost circadian response of IL-6 to LPS challenge in vivo and in vitro using isolated PECs | [51] |
| Rev-erbα−/− mice (global KO) | aerosolized LPS 2 mg/mL | ↑ neutrophil numbers and CXCL1, CXCL2 and CXCL5 levels in BAL fluid | [70] |
| Rev-erbα−/− mice (global KO) | LPS 100 ng/mL (ex vivo) | ↑ cytokine and chemokine response to LPS (Il-6, Ccl2 and Ccl5 expression) in alveolar macrophages | [70] |
| Rev-erbα−/− mice (global KO) | LPS 1 µg/mL (in vitro) | ↑ basal and LPS-stimulated Ccl2 gene expression in peritoneal macrophages | [71] |
| Rev-erbα−/− mice (global KO) | ↑ basal NF-κB signaling and pro-inflammatory microglial activation in the hippocampus ↑ LPS-induced neuroinflammation |
[72] | |
| Rev-erbα−/− mice (global KO) | ↑ complement transcripts (C4b and C3) in the hippocampus | [73] | |
| Rev-erbα−/− mice (global KO) | DSS-induced colitis | ↑ severity of DSS-induced colitis ↑ colonic levels of NLRP3, IL-1β, and IL-18 in DSS-induced colitis suppressed daily rhythm of Nlrp3 in the colon |
[56] |
| Rev-erbα−/− mice (global KO) | LPS 100 ng/mL (in vitro) | ↑ LPS-induced protein levels of NLRP3 and IL-1β in peritoneal macrophages | [56] |
| staggerer (RORαsg/sg) mice | intra-tracheal LPS 2 µg/50 µL | ↑ susceptibility to LPS-induced airway inflammation ↑ neutrophil counts and cytokine levels IL-1β, IL-6, and MIP-2 in BAL fluid |
[74] |
| staggerer (RORαsg/sg) mice | LPS 5 µg/mL (in vitro) | ↑ Il-1β, Il-1α, and Tnfα expression in LPS-stimulated splenocytes | [75] |
| Nfil3−/− mice (global KO) | Lack of CD8α+ cDC population in the lymphoid organs | [76] | |
| Nfil3−/− mice (global KO) | Lack of NK cells and impaired NK-cell mediated cytotoxicity | [77] | |
| Nfil3−/− mice (global KO) | Clostridium difficile infection | ↓ numbers of innate lymphoid cells in the intestinal mucosa ↓ immune defence against acute intestinal bacterial infection with Clostridium difficile |
[78] |
| Nfil3−/− mice (global KO) | LPS 10 ng/mL (in vitro) | ↑ LPS-induced Il-12b expression and IL-12p40 release from BMDMs Spontaneous expression of Il-12b in colonic CD11b+ LPMCs |
[79] |
| Nfil3−/− mice (global KO) | LPS 10 ng/mL (in vitro) | ↑ proportion of IL-12p40 producing macrophages in response to LPS and ↑ expression of Ccr2 in unstimulated BMDMs | [59] |
APOE—apolipoprotein E; BAL-bronchoalveolar lavage; BMDMs—bone marrow-derived macrophages; CCL2/5/8—CC motif chemokine ligand 2/5/8; cDC—conventional dendritic cells; CXCL1/2/5—CXC motif chemokine ligand 1/2/5; DSS—dextran sulphate sodium; HIF1α—hypoxia-inducible factor 1α; IFNγ—interferon gamma; IL—interleukin; iNOS—inducible nitric oxide synthase; KLA—Kdo2-lipid A (TLR4 ligand); KO—knock-out; LPMCs—lamina propria mononuclear cells; LPS—lipopolysaccharide; MEFs—mouse embryonic fibroblasts; MIP-2—macrophage inflammatory protein 2; NET—neutrophil extracellular trap; NF-κB—nuclear factor kappa B; NLRP3—NOD-like receptor family pyrin domain containing 3; NRF2—nuclear factor-like 2; PECs—peritoneal exudate cells; PKA—protein kinase A; RELB—subunit of nuclear factor kappa B; ROS—reactive oxygen species; TG—thioglycolate; TLR—toll-like receptor; TNFα—tumor necrosis factor alpha; ZT—zeitgeber time.
BMAL1 is a central component of the mammalian molecular clock and plays a central role in circadian–immune interactions. Systemic deletion of Bmal1 eliminated circadian rhythmicity in the central pacemaker and periphery, resulting in a complete behavioral arrhythmicity [80,81]. Bmal1−/− mice also lost rhythmicity in the numbers of leukocytes and immature hematopoietic cells in the peripheral blood [43,54]. However, particularly models with targeted Bmal1 deletion in myeloid cell lineages have revealed an essential role of BMAL1 in the control of the time-of-day-dependent effector functions of monocytes and macrophages. Mice with deletion of Bmal1 in myeloid cells lost daily variability in circulating inflammatory Ly6Chigh monocytes, showing higher susceptibility to Listeria monocytogenes infection [52]. In another study, myeloid Bmal1-deficient mice on the Apoe−/− background showed increased recruitment of Ly6Chigh monocytes to atherosclerotic lesions with polarization to pro-inflammatory M1 macrophages [61]. In vitro experiments using bone marrow-derived macrophages (BMDMs) demonstrated that Bmal1 deficiency amplified acute inflammatory response to LPS, as was manifested by enhanced production of pro-inflammatory cytokines, suppressed antioxidant pathways. and increased reactive oxygen species levels [57,58]. Surprisingly, myeloid Bmal1 deficiency was also found to confer protection against pneumococcal infection that was attributed to increased motility and phagocytic activity of Bmal1 deficient macrophages [60]. In neutrophils, specific deletion of Bmal1 eliminated daily variability in granule content and neutrophil extracellular traps formation [62]. In general, the above-mentioned studies demonstrated the anti-inflammatory effects of BMAL1, which are probably mediated by CLOCK/BMAL1-dependent transcriptional regulation of genes containing E-box. For example, circadian monocyte trafficking is driven by time-of-day-dependent expression of chemokines (such as Ccl2), which are under the repressive transcriptional control of BMAL1 through recruitment of the polycomb repressive complex 2 [52].
In contrast to BMAL1, CLOCK protein has been shown to enhance NF-κB-mediated transcription and production of pro-inflammatory cytokines, and these effects were independent of the transactivation capacity of the CLOCK/BMAL1 complex on E-box containing promoters [49]. CLOCK was found in protein complexes with the p65 subunit of NF-κB and CLOCK overexpression was associated with enhanced NF-κB activation [49]. These findings were supported by reduced activation of NF-κB in response to immune challenge in mouse embryonic fibroblasts (MEFs), as well as hepatocytes of Clock-deficient mice compared to wild-type controls [49]. Similarly, reduced induction of pro-inflammatory cytokines upon LPS challenge has been observed in MEFs and BMDMs from Clock-mutant mice [63,64]. Moreover, day/night differences in inflammatory response to Salmonella infection were eliminated in the gut of Clock mutants [63].
Models with genetic disruption of clock genes Per and Cry have revealed distinct roles of these clock components in the regulation of immune functions. A study in Per1 mutant mice showed that they maintained circadian expression of perforin, granzyme B, and interferon-gamma (IFNγ) in splenic NK cells, though these rhythms were either attenuated or phase-shifted [65]. On the other hand, in Per2 mutant mice, serum IFNγ concentrations as well as mRNA and protein levels in the spleen completely lost daily rhythmicity [66]. These eliminated IFNγ rhythms can be translated to impaired IFNγ production by the splenic NK cells upon LPS challenge in Per2 mutant mice, and suppressed response in serum IFNγ and IL-1β levels [67]. Moreover, this study found an increased survival rate of Per2 mutants following a lethal dose of LPS compared to controls [67]. Mutation of Per2, disrupting the ability of PER2 to interact with other clock proteins, can also significantly affect TLR9-mediated immune responses, as peritoneal macrophages from Per2 mutants showed reduced expression of Tlr9 and decreased TLR9 ligand-induced production of TNFα and IL-12 [68]. In contrast to Per2 defects, the absence of Cry genes leads to a pro-inflammatory phenotype. In Cry1 and Cry2 double knockout fibroblasts, enhanced constitutive expression of pro-inflammatory factors was observed, and this was mediated by the constitutive activation of NF-κB and protein kinase A (PKA) signaling [69]. The proposed mechanism shows that CRY1 can inhibit PKA-mediated phosphorylation of p65 through binding to adenylyl cyclase and suppression of cyclic adenosine monophosphate levels [69]. In this study, Cry1−/−Cry2−/− mice exhibited not only enhanced basal expression of Il-6, Cxcl1, and inducible nitric oxide synthase (iNos) in the BMDMs but also elevated cytokine responses to LPS compared to wild-type animals [69].
The nuclear receptors REV-ERBα and RORα represent important regulatory components linking the circadian and immune systems and exert mostly anti-inflammatory effects. Peritoneal macrophages isolated from global REV-ERBα knockout mice (Rev-erbα−/−) displayed augmented pro-inflammatory response to LPS [51,56,71]. Simultaneously, the absence of circadian rhythmicity in LPS-induced IL-6 response was demonstrated in the cultured Rev-erbα−/− macrophages and in vivo upon endotoxin challenge in Rev-erbα−/− mice [51]. Furthermore, these studies showed that REV-ERBα is a direct transcriptional repressor of several pro-inflammatory genes, including Ccl2 and NOD-like receptor family pyrin domain containing 3 (Nlrp3), which contain RORE binding sites in their promoter regions [56,71]. Moreover, Rev-erbα−/− mice have been found to display exaggerated LPS-induced pulmonary inflammation [70], increased severity of dextran sulphate sodium (DSS)-induced colitis [56], as well as a neuroinflammatory phenotype with basal activation of microglia in the hippocampus [72]. Likewise, in mice with deficient Rorα expression (RORαsg/sg, staggerer mutants), several immune defects were described besides typical cerebellar neurodegeneration. For example, splenocytes isolated from these mice were more sensitive to LPS challenge, showing increased expression of pro-inflammatory cytokines compared to wild-type controls [75]. Moreover, similarly to Rev-erbα deficient mice, also RORαsg/sg mice showed increased susceptibility to LPS-induced lung inflammation, higher neutrophil numbers, and increased levels of pro-inflammatory cytokines (IL-1β, IL-6, and macrophage inflammatory protein 2) in the bronchoalveolar lavage compared to wild-type mice [74]. An anti-inflammatory action of RORα can occur through RORE-mediated up-regulation of inhibitor of NF-κB (IκBα) and reduced p65 nuclear translocation [82].
REV-ERBα can transcriptionally regulate and repress another circadian repressor NFIL3 [83], which is also implicated in numerous immune processes. Studies in NFIL3-deficient (Nfil3−/−) mice have shown a critical role of NFIL3 in the development of several types of immune cells, including CD8+ conventional dendritic cells [76], NK cells, as well as all other innate lymphoid cell lineages [77,78]. Later, NFIL3 was identified as an important regulator of macrophage responses via transcriptional repression of Il-12b [79]. Interestingly, the inflammatory response of macrophages has been shown to depend on the phase of circadian oscillations of NFIL3 and DBP, which competitively bind at the Il-12b enhancer [59]. Therefore, desynchronization of the molecular clock in the macrophage population can contribute to the heterogeneity of the inflammatory response [59].
Together, accumulating evidence indicates a complexity of circadian-immune crosstalk, highlighting diverse immunomodulatory effects of individual clock components, which are determined by transcription-dependent mechanisms, direct protein–protein interactions, or the phase of circadian oscillations.
4.2. Light-Phase Shifts
Shift work and jet lag represent frequent circadian challenges associated with a modern lifestyle that lead to desynchronization of the SCN and downstream oscillators with the external environment [84]. Shift work refers to work outside the regular daytime hours and involves non-standard work schedules, such as night shifts, early morning shifts, or rotating shifts, which are also associated with alterations in the sleep/wake cycle [85]. Reduced amplitude or disturbance of the key circadian rhythms, such as melatonin, cortisol, and body temperature, has been observed among shift workers [86]. Misalignment between endogenous circadian rhythms and the LD cycle in shift workers can also predispose to an increased risk of negative health outcomes, such as cancer, and metabolic and cardiovascular diseases [87,88]. Moreover, shift workers are at a higher risk of common respiratory infections, including cold, flu, or COVID-19 [89,90,91].
Epidemiological studies revealed increased numbers of total leukocytes, neutrophils, monocytes, and lymphocytes [92,93,94], and reduced activity of NK cells in the circulation of shift workers compared to daytime workers [95]. Moreover, shift workers had significantly elevated markers of systemic inflammation, including C-reactive protein and the cytokines TNFα, IL-6, IL-1β, and IL-10 than daytime workers [94]. One limitation of most observational studies is that they measure these parameters only during the daytime and do not consider their 24 h variability, emphasizing the importance of an interventional approach. In healthy volunteers under laboratory conditions, simulated night shift work protocol with a 10 h delayed sleep period resulted in the reduced amplitude of rhythmic transcripts in peripheral blood mononuclear cells [96] and caused a misalignment of the rhythmic secretion of cytokines IL-6, IL-1β, and TNFα following ex vivo immune stimulation [97].
In animal models, jet lag is induced by single (acute models) or repeated (chronic models) phase delays or advances in the LD cycle [98], while shift work experimental schedules use exposure to contrasting signals, such as light during the dark phase and forced activity or food consumption during the resting period [99]. Chronic jet lag (CJL) protocols have been shown to induce circadian desynchronization in the locomotor activity pattern, which is documented by the appearance of two components of activity rhythms, one with a free-running period and the other with the mean period of a specific shift-lag schedule [100]. Furthermore, CJL modified acrophases of the main clock gene rhythms in the central oscillator, as well as in the peripheral tissues [101].
Experimental studies in mice and rats exploring how circadian disruption induced by light-phase shifts impacts innate immunity and inflammatory responses are summarized in Table 2.
Table 2.
The effects of different light-phase shift paradigms on the immune parameters and functions under the steady state and challenged conditions in rodents.
| Species | Shift Paradigm | Immune Challenge | Effects | Ref. |
|---|---|---|---|---|
| Humanized NSG mice | 8 h PA/2 days for 10 days | Eliminated circadian rhythm of mouse and human blood leukocytes | [102] | |
| Lyzs-Cre mice | LD reverse/5 days for 3 weeks | Eliminated circadian rhythm of neutrophil hepatic infiltration and ↑ triacylglycerol levels in the liver | [103] | |
| C57BL/6J mice | 6 h PA/7 days for 4 weeks and 1 week of re-synchronization |
LPS 50 μg/mL (ex vivo) or 1 µg/mL (in vitro) |
↑ LPS-induced IL-6 response of the whole blood and incubated PECs, preserving a daily rhythm in this immune response | [104] [105] |
| C57BL/6J Per2Luc mice | 6 h PA/7 days for 4 weeks and 1 week of re-synchronization |
LPS 10 µg/mL (in vitro) | ↑ LPS-induced IL-6 response of incubated PECs | [106] |
| C57BL/6J Per2Luc mice | 6 h PA/7 days for 4 weeks and 1 week of re-synchronization |
LPS 12.5 mg/kg (i.p.) | Persistent hypothermia, ↑ mortality rate, ↑ response of pro-inflammatory cytokines (IL-1β, GM-CSF, IL-12, IL-13) to LPS | [106] |
| C57BL/6J mice | 6 h PA/2 days for 3 weeks | LPS 20 mg/kg (i.p.) | 80% mortality rate independent of time of LPS administration, ↑ hypothermic and serum TNFα response | [107] |
| C57BL/6J Per2Luc mice on HFD | LD reverse/5 days for 10 weeks | ↑ adipose tissue macrophage infiltration and pro-inflammatory M1 polarization associated with amplified expression of Il-1β, Il-6 and Tnfα
↑ pro-inflammatory activation of BMDMs with ↑ LPS-induced expression of Il-1β, Il-6, and Tnfα |
[108] | |
| APOE*3-Leiden.CETP mice on HFD | LD reverse/7 days for 10 and 15 weeks | ↑ atherosclerosis development in the aortic root ↑ lesion macrophage content and ↑ vascular expression of markers for inflammation (Nfκb1, Tnfα, iNos), oxidative stress (Sod1, Gpx1, Hif1α, Nox2), and leukocyte recruitment (Icam1, Ccr2, CCL2) at ZT0 Phase-shifted rhythms of circulating leukocytes |
[109] | |
| C57BL/6J mice | 8 h PA/2–3 days for 8 weeks | ↑ severity of DSS-induced colitis | [56] | |
| C57BL/6J mice | 6 h PA/2 days for 3 weeks | B16F0 nonmetastatic melanoma cells (s.c.) | ↑ mortality rate, ↑ tumor growth rate, lost daily variability and M1/M2 macrophage ratio in melanoma tumors | [110] |
| C57BL/6J mice | LD reverse/4 days for 12 weeks | ↓ NK cell numbers in the spleen and lungs ↓ expression of CD107a and IFNγ in non-stimulated and activated splenic NK cells ↑ lung metastasis of B16 melanoma |
[111] | |
| Fischer rats | 6 h PA/2 days for 3 weeks and 1 week in DD |
↓ rhythm and cytotoxicity of splenic NK cells ↓ or shifted circadian rhythms of perforin, granzyme B and IFNγ in NK cells |
[112] | |
| Fischer rats | 6 h PA/2 days for 3 weeks and 1 week in DD |
MADB106 tumor cells (i.v.) |
↑ lung tumor frequency (after 6–8 weeks in LD) and ↑ cytolytic activity of NK cells (24 h post stimulation) | [112] |
BMDMs—bone marrow-derived macrophages; CCL2—CC motif chemokine ligand 2; CCR2—CC chemokine receptor 2; DD—constant darkness; DSS—dextran sulphate sodium; GM-CSF—granulocyte-macrophage colony-stimulating factor; GPX1—glutathione peroxidase 1; HFD—high fat diet; HIF1α—hypoxia-inducible factor 1α; ICAM1—intercellular adhesion molecule 1; IFNγ—interferon gamma; IL—interleukin; iNOS—inducible nitric oxide synthase; LD—light/dark; LPS—lipopolysaccharide; NF-κB1—nuclear factor kappa B; NOX2—NADPH oxidase 2; PA—phase advance; PECs—peritoneal exudate cells; SOD1—superoxide dismutase type 1; TNFα—tumor necrosis factor alpha; ZT0—beginning of the light phase.
In mice, 24 h following acute jet lag (12 h phase advance), an arrhythmic pattern of circulating blood progenitors [43] and abolished daily variability of leukocyte recruitment to the skeletal muscle were found under both steady state and inflammatory conditions [53]. Similar results were obtained using a CJL protocol. Specifically, jet lag eliminated circadian rhythms in mouse and human blood leukocytes in humanized mice [102] and abolished the rhythm in neutrophil infiltration into the liver, which correlated with increased hepatic accumulation of lipids [103]. The consequences of chronic jet lag on inflammatory responses have been demonstrated in mice using a protocol with 6 h phase advances of the LD cycle every 7 days for 4 weeks followed by 1 week of re-synchronization [104,105,106]. The CJL mice challenged in vivo with a lethal dose of LPS exhibited a higher mortality rate, persistent hypothermia, and amplified serum response of pro-inflammatory cytokines [106]. Corresponding data showing an exaggerated IL-6 response to LPS were also found after ex vivo stimulation of the whole blood or in vitro stimulation of isolated peritoneal macrophages harvested from CJL mice [104,105,106]. Interestingly, LPS-induced IL-6 responses showed a rhythmic pattern in CJL mice, indicating adaptation to a new LD cycle during 1 week of re-synchronization [104]. On the other hand, increased frequency of shifts over one week eliminated time-of-day-dependent mortality rate upon the lethal dose of LPS and enhanced hypothermic and serum TNFα responses in mice [107].
Circadian disruption in shift workers is associated with an increased incidence of metabolic diseases. Macrophages represent key mediators of obesity-induced inflammation in mice and humans [113,114]. In mice on a high-fat diet (HFD), chronic exposure to shifts of the LD cycle amplified adipose tissue macrophage infiltration and pro-inflammatory M1 polarization, together with enhanced expression of pro-inflammatory cytokines [108]. Similarly, in hyperlipidemic APOE*3-Leiden.CETP mice on an HFD, circadian disruption induced by weekly LD reversals over several weeks accelerated the development of atherosclerosis, increased the macrophage content in atherosclerotic lesions, and promoted a pro-inflammatory state in the vessel wall [109].
Circadian clocks have been studied as an important player in many aspects of cancer-immune cell interactions [115]. Experimental research has shown that circadian disruption induced by different jet lag models can accelerate tumor growth and the incidence of metastasis as compared to a normal lighting regime [110,111,112,116]. Innate lymphoid NK cells are an integral part of anti-tumor immunity and provide effective immune surveillance by destroying tumor cells [117]. This ability is ensured by a stable count of NK cells and their production of various cytolytic factors and cytokines, mainly perforin, granzyme B, and IFNγ [118,119]. In mice, chronic shifts in the LD cycle reduced the numbers of NK cells in the spleen and lungs [111] and attenuated their cytolytic activity through suppressed expression of CD107a, a sensitive indicator of NK cell cytotoxicity and degranulation [120]. Another study in rats showed that repeated phase advances of the LD cycle suppressed rhythmic cytotoxicity of splenic NK cells, and modified circadian expression of granzyme B, perforin, and IFNγ in NK cells [112]. In addition to NK cells, tumor progression is controlled by the tumor microenvironment, which contains a variety of immune cells with a tumor-promoting or tumor-suppressing phenotype [121]. In a melanoma mouse model, circadian disruption induced by CJL abolished daily variability and decreased the M1 (pro-inflammatory)/M2 (anti-inflammatory) macrophage ratio in the tumor, promoting immunosuppression of the tumor microenvironment [110]. These effects accelerated tumor growth, and were also associated with increased mortality [110]. Other studies found reduced survival in aged mice exposed to chronic phase-advances for 8 weeks [122] or even as a result of long-term exposure (for 85 weeks) to phase-advances in 4-day intervals [123]. Additionally, epigenetic changes are known to participate in carcinogenesis, and they can also have the potential to mediate deregulation of immune mechanisms induced by circadian disruption. For example, rats, experienced chronic circadian disruption, exhibited aberrant changes in the expression of several cancer-related microRNAs in mammary tissues and, these changes were associated with increased protein levels of pro-inflammatory transcription factors, phosphorylated NF-κB and STAT3 [124].
4.3. Dim ALAN
The advancement of lighting technologies, including the implementation of light-emitting diode (LED) technology, goes in parallel with increasing levels of light pollution [125]. Moreover, evening use of devices with light-emitting screens as well as the use of night lamps, especially for small children while sleeping, considerably contribute to unintentional exposure to ALAN [126].
Evidence provided by experimental studies has demonstrated that dim ALAN (≤5 lx) can compromise circadian coordination in laboratory rodents. The rhythmic profile of locomotor activity was preserved in rats exposed to dim ALAN for 2 weeks, but mean night-time levels were reduced, and daytime activity was increased compared to controls [127]. Another study in rats reported that dim ALAN diminished the power of 24 h activity rhythm and induced a second approximately 25 h free-running rhythm, indicating internal desynchronization of locomotor activity [128]. In the SCN, dim ALAN exposure clearly suppressed the daily rhythms of clock genes in both rats [129,130] and mice [131,132]. In peripheral tissues, clock gene rhythms appeared to be less affected by ALAN than in the master oscillator, though they showed lowered amplitude or shifts in acrophase [127,129,131]. The daily plasma melatonin rhythm was eliminated in rats after 2 weeks of dim ALAN (2 lx) exposure due to suppressed nocturnal melatonin levels [129], which were also reported in other studies, not only in rats [133,134] but also in diurnal birds [135,136] and humans [137]. Moreover, circadian disruption induced by dim ALAN has been observed in other hormonal rhythms, e.g., suppressed and phase-advanced corticosterone rhythm, and abolished daily rhythmicity in plasma testosterone and vasopressin levels in rats [129].
Several experimental studies have demonstrated that ALAN can affect innate immune mechanisms, including inflammatory response (Table 3). However, in most of these studies, the immune status was evaluated only at one time point, neglecting consequences on circadian rhythms in the immune system. Indeed, a recent study showed that rats exposed to dim ALAN (2 lx) for 5 weeks exhibited impaired daily variation of the main leukocyte subsets in the blood, especially monocytes and T cells [138]. Moreover, ALAN reduced blood monocyte counts and altered gene expression of macrophage marker Cd68 and chemokine Ccl2 in the kidney, indicating that weakened circadian control of circulating leukocyte numbers was associated with disturbed renal immune homeostasis [138].
Table 3.
Summary of the effects of dim artificial light at night (ALAN) on the immune parameters under steady state and challenged conditions in rodents.
| Species | ALAN Intensity and Duration |
Immune Challenge | Effects | Ref. |
|---|---|---|---|---|
| Wistar rats | L: 150 lx; dimL: 2 lx for 2 and 5 weeks |
Impaired daily variation in the numbers of circulating monocytes and T cells ↓ numbers of blood monocytes ↑ expression of macrophage marker Cd68 and ↓ Ccl2 expression in the kidney |
[138] | |
| Swiss Webster mice | L: 150 lx; dimL: 5 lx for 4 weeks |
↑ expression of Mac-1 and Tnfα in WAT Exacerbated peripheral inflammation associated with HFD |
[139] | |
| CFW mice | L: 125 lx; dimL: 5 lx for 4 weeks |
↑ Il-6 expression in the medulla associated with cold hyperalgesia and mechanical allodynia | [140] | |
| Swiss Webster mice | L: 150 lx; dimL: 5 lx for 4 weeks |
LPS 0.5 mg/kg (i.p.) | Exaggerated changes in body temperature and prolonged sickness responses to LPS ↑ LPS-induced expression of Tnfα and Il-6 in microglia |
[141] |
| Swiss Webster mice | L: 150 lx; dimL: 5 lx for 1 week |
Model of global cerebral ischemia | ↑ mortality rate 7 days following injury ↑ neuroinflammation 24 h following injury (amplified Tnfα mRNA levels in the hippocampus) |
[142] |
| C3H mice | L: 150 lx; dimL: 5 lx for 3 weeks |
FM3A mammary carcinoma cells | ↓ latency to tumor onset and ↑ tumor volume | [143] |
| Nude rats | L: 345 lx; dimL: 0.2 lx for 6 weeks |
MCF-7 human breast cancer xenografts | ↑ tumor growth rate | [144] |
| Siberian hamsters | L: 150 lx; dimL: 5 lx for 4 weeks |
↑ Tnfα and ↓ Bdnf expression in the hippocampus ↓ hippocampal dendritic spine density |
[145] | |
| Siberian hamsters | L: 150 lx; dimL: 5 lx for 4 weeks |
LPS 0.4 mg/kg (i.p.) or DNFB treatment |
↓ plasma bactericidal capacity following LPS ↓ delayed-type hypersensitivity response to DNFB |
[146] |
| Nile grass rats | L: 150 lx; dimL: 5 lx for 3 weeks |
↑ basal plasma bactericidal capacity ↑ delayed-type hypersensitivity response to DNFB |
[147] |
BDNF—brain-derived neurotrophic factor; CCL2—CC motif chemokine ligand 2; dimL—dim light phase; DNFB—2,4-dinitro-1-fluorobenzene; HFD—high fat diet; IL—interleukin; L—light phase; LPS—lipopolysaccharide; MAC-1—macrophage-1 antigen; TNFα—tumor necrosis factor-alpha; WAT—white adipose tissue.
Immune disbalance caused by ALAN is considered one of the key mechanisms that can promote a pro-inflammatory state or accelerate various pathologies. For example, mice exposed to either ALAN (5 lx) or an HFD for 4 weeks showed up-regulated expression of inflammatory markers Tnfα and macrophage-1 antigen (Mac-1) in white adipose tissue, while ALAN further potentiated HFD-induced inflammation [139]. In cancer research, dim ALAN has been shown to favor tumor growth, especially in models of mammary cancer [143,144]. C3H mice exposed to ALAN (5 lx) for 3 weeks and then injected with FM3A mammary carcinoma cells displayed earlier tumor onset and increased terminal tumor volume compared to tumor-bearing mice housed in the LD regime [143]. In another study in nude rats, chronic ALAN even with a very low light intensity of 0.2 lx accelerated mammary tumor growth [144].
Another process that can drive the impact of ALAN on the progression of diseases is the ability of ALAN to promote neuroinflammation. Exposure to ALAN for 4 weeks increased hippocampal Tnfα and Il-6 expression simultaneously with depression-like behavior in female Siberian hamsters (Phodopus sungorus) [145], and up-regulated Il-6 mRNA levels in the medulla of mice that concomitantly exhibited cold hyperalgesia and mechanical allodynia [140]. Moreover, mice that underwent global cerebral ischemia and were subsequently exposed to ALAN showed decreased survival associated with increased neuronal damage that was preceded by amplified neuroinflammation, compared to animals in the control regime [142].
Till now, the effects of ALAN on inflammatory response were examined only in a limited number of studies. In mice challenged with LPS following 4 weeks of dim ALAN (5 lx), exaggerated changes in body temperature, prolonged sickness responses, and elevated pro-inflammatory cytokine expression (Tnfα and Il-6) in microglia were found compared to controls [141]. Additionally, diminished bactericidal capacity of blood upon LPS challenge and reduced delayed-type hypersensitivity response was observed in Siberian hamsters exposed to dim ALAN compared to animals in the standard LD regime [146]. Interestingly, the opposite effects of ALAN were obtained in a diurnal rodent model, Nile grass rats (Arvicanthis niloticus), which exhibited enhanced delayed-type hypersensitivity response and elevated basal bactericidal capacity when exposed to ALAN for 3 weeks [148]. Thus, the currently available data demonstrate that ALAN affects the responsiveness of the immune system to challenges, but clearly more studies are needed to reveal potentially differential responses between diurnal and nocturnal mammals, and to evaluate whether immune responses are impacted by ALAN in a time-of-day-dependent manner. Moreover, surprisingly limited data are available on the effects of ALAN on innate immunity and inflammation in humans.
4.4. Constant Light
Exposure to LL and low-intensity ALAN are often considered interchangeable conditions. However, circadian disruption caused by LL differs from that induced by low-intensity ALAN in several ways [149]. In general, LL leads to the complete loss of locomotor activity rhythms [150], and this behavioral arrhythmicity develops as soon as one month after changed lighting conditions in rats [151,152]. In the master clock, LL causes desynchronization of SCN neurons [150] and reduces the amplitude of SCN neuronal activity rhythm [153], which is further attenuated by long-term LL exposure [154]. Suppressed nocturnal melatonin levels have been found under both LL and dim ALAN regimes [155,156] but corticosterone is arrhythmic in LL [133,157], and preserves its rhythmicity with decreased amplitude in the dim ALAN regime [129].
The effects of LL exposure on immune functions have been reported by several studies, which are summarized in Table 4. Circadian disruption induced by LL was shown to facilitate a pro-inflammatory state even under unchallenged conditions. Specifically, in rats, 4-week LL exposure up-regulated the expression of the pro-inflammatory markers Stat3, Il-17ra, and Il-1α in the colonic mucosa [151]. In another study, rats exposed to LL for 5 weeks displayed amplified plasma TNFα response and sickness symptoms, such as febrile reaction and food intake reduction, following LPS administration [152]. Interestingly, in rats, 24 h leukocyte rhythms in the circulation persisted 8 weeks after LL exposure, despite suppressed circadian rhythms in body temperature and locomotor activity [158]. Nevertheless, in the same study, prolonged LL exposure (for 11 and 16 weeks) did eliminate the circadian rhythm in blood leukocytes that was not restored even 16 weeks after re-synchronization in the LD regime [158]. In mice exposed to LL for 8 weeks, increased numbers of blood neutrophils and reduced numbers of lymphocytes were found together with an enhanced response of pro-inflammatory cytokines to LPS challenge [154]. Interestingly, these effects were transient, as no further changes were observed in mice after 24 weeks of the LL regime [154]. However, the study did not monitor the whole 24 h profile in white blood cells.
Table 4.
Summary of the effects of constant light (LL) on the immune parameters under steady state and challenged conditions in rodents.
| Species | LL Intensity and Duration |
Immune Challenge | Effects | Ref. |
|---|---|---|---|---|
| Sprague-Dawley rats | 300 lx for 17 weeks and 16 weeks of re-synchronization |
Lost 24 h rhythm in blood leukocytes after 11/16 weeks in LL, (the rhythm was not restored after 16 weeks of re-synchronization in LD regime) ↓ NK cell counts in the blood |
[158] | |
| Wistar rats | 150 lx for 4 weeks | Activated pro-inflammatory state (↑ expression of Stat3, Il-1α and Il-17ra) in the colonic mucosa | [151] | |
| Wistar rats | 200–250 lx for 5 weeks | LPS 2 µg/kg (i.v.) | ↑ plasma TNFα response and sickness symptoms upon LPS | [152] |
| Wistar rats | 200–250 lx for 5 weeks | C6 tumor cells (s.c.) | ↑ tumor growth ↑ tumor infiltration of monocytes/macrophages |
[152] |
| Sprague-Dawley rats | 200 lx for 7 days | Endotoxemia model (daily i.p. LPS injection for 7 days) |
↑ hypothalamic expression of Il-1β and Tnfα | [159] |
| C56BL/6J mice | 105 lx for 24 weeks | LPS 50 µg/kg (i.v.) | Transient ↑ of neutrophil and ↓ of lymphocyte numbers in the blood was associated with enhanced inflammatory response to LPS (↑ IL-1β, TNFα, IL-6, and ↓ IL-10 plasma levels) after 8 weeks of LL No immune changes after 24 weeks of LL |
[154] |
| CD-1 mice | 750 lx for 4 weeks | Complete Freund’s adjuvant (100 µL) | ↑ proportion of myeloid-derived suppressor cells in the spleen under LL was potentiated by chronic inflammation ↑ plasma TGF-β1 levels and ↑ chronic inflammation induced elevation of IL-6 levels |
[160] |
IL—interleukin; LD—light/dark; LPS—lipopolysaccharide; STAT3—signal transducer and activator of transcription 3; TGF-β1—transforming growth factor beta 1; TNFα—tumor necrosis factor-alpha.
The disruption of circadian rhythms due to LL has also been demonstrated to promote tumorigenesis and adversely affect chronic inflammatory processes [152,159,160]. Rats implanted with C6 tumor cells and exposed to LL for 5 weeks exhibited faster tumor growth and increased tumor infiltration with macrophages compared to controls in the LD regime [152]. The mechanisms behind these effects are not clear, but may involve reduced NK cell counts, also found in rats exposed to LL [158]. In addition, recent evidence suggests that myeloid-derived suppressor cells are associated with a poor prognosis in cancer [161]. In the mouse model of chronic inflammation, 4-week LL potentiated the accumulation of myeloid-derived suppressor cells (granulocytic CD11b+Ly6Ghigh and monocytic CD11b+CD49d+ cell subsets) in the spleen and elevated IL-6 levels in the circulation [160].
5. Conclusions
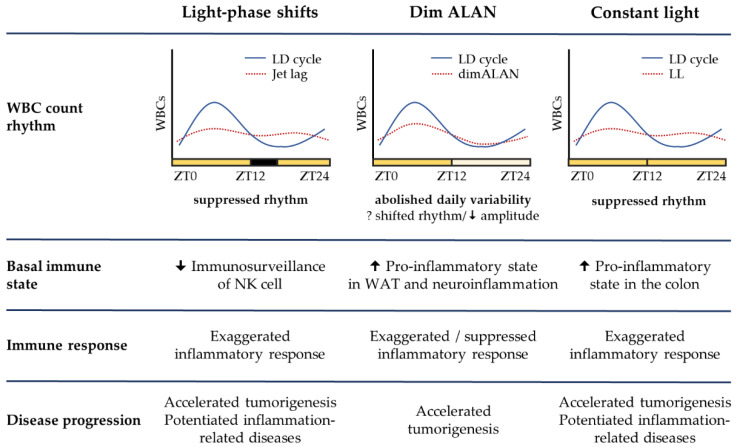
Chronodisruptive risk factors, such as mistimed light information due to shift work or ALAN exposure, are not associated with acute pain, increasing the chance that their negative health consequences will be overlooked. Innate immune cells represent the first line of defense against pathogenic stimuli. The effector functions of innate immune cells are profoundly controlled by cell-intrinsic molecular clocks, which can coordinate immune cell trafficking and the production of immune-regulatory molecules in a time-of-day-dependent manner. Accumulating evidence from experimental studies in mice and rats demonstrates that disruptive LD regimes can compromise these surveillance mechanisms and shift the immune balance to a pro-inflammatory state. Indeed, the obvious effects, observed under light-phase shifts, dim ALAN, and LL, are represented by exaggerated acute inflammatory response upon LPS challenge, promoted tumorigenesis, and amplified symptoms associated with chronic inflammation (Figure 1).
Figure 1.
Comparison of three disruptive light/dark regimes and their effects on the immune measures based on the current knowledge in laboratory animals. ALAN—artificial light at night; LD—standard light/dark cycle of 12/12 h; LL—constant light; WAT—white adipose tissue; WBCs—white blood cells; ZT—Zeitgeber time.
These effects can result from disrupted immune rhythms and disrupted circadian gating of immune responses, though there is still a lack of experimental data, particularly under dim ALAN exposure. Moreover, existing studies rarely specify other details regarding the quality of light than light intensity, and this can be critical for the evaluation of adverse health consequences.
Collectively, a better understanding of the mechanisms by which circadian disruption influences the immune status can be of importance in the search for strategies to prevent or limit the negative consequences of chronodisruption on health.
Author Contributions
Conceptualization, writing—original draft preparation, writing—review and editing, V.J., M.Z. and M.O.; visualization, V.J. and M.O.; supervision, project administration, funding acquisition, M.Z. and M.O. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The study was supported by the Slovak Research and Development Agency APVV-17-0178 and the Scientific Grant Agency of the Ministry of Education of the Slovak Republic VEGA 1/0565/22.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Albrecht U. Timing to perfection: The biology of central and peripheral circadian clocks. Neuron. 2012;74:246–260. doi: 10.1016/j.neuron.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Golombek D.A., Rosenstein R.E. Physiology of circadian entrainment. Physiol. Rev. 2010;90:1063–1102. doi: 10.1152/physrev.00009.2009. [DOI] [PubMed] [Google Scholar]
- 3.Pilorz V., Astiz M., Heinen K.O., Rawashdeh O., Oster H. The concept of coupling in the mammalian circadian clock network. J. Mol. Biol. 2020;432:3618–3638. doi: 10.1016/j.jmb.2019.12.037. [DOI] [PubMed] [Google Scholar]
- 4.Blume C., Garbazza C., Spitschan M. Effects of light on human circadian rhythms, sleep and mood. Somnologie. 2019;23:147–156. doi: 10.1007/s11818-019-00215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaston K.J., Bennie J., Davies T.W., Hopkins J. The ecological impacts of nighttime light pollution: A mechanistic appraisal. Biol. Rev. 2013;88:912–927. doi: 10.1111/brv.12036. [DOI] [PubMed] [Google Scholar]
- 6.Falchi F., Cinzano P., Duriscoe D., Kyba C.C., Elvidge C.D., Baugh K., Portnov B.A., Rybnikova N.A., Furgoni R. The new world atlas of artificial night sky brightness. Sci. Adv. 2016;2:e1600377. doi: 10.1126/sciadv.1600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kernbach M.E., Miller C., Alaasam V., Ferguson S., Francis C.D. Introduction to the symposium: Effects of light pollution across diverse natural systems. Integr. Comp. Biol. 2021;61:1089–1097. doi: 10.1093/icb/icab157. [DOI] [PubMed] [Google Scholar]
- 8.Davies T.W., Smyth T. Why artificial light at night should be a focus for global change research in the 21st century. Glob. Chang. Biol. 2018;24:872–882. doi: 10.1111/gcb.13927. [DOI] [PubMed] [Google Scholar]
- 9.Vetter C. Circadian disruption: What do we actually mean? Eur. J. Neurosci. 2020;51:531–550. doi: 10.1111/ejn.14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lunn R.M., Blask D.E., Coogan A.N., Figueiro M.G., Gorman M.R., Hall J.E., Hansen J., Nelson R.J., Panda S., Smolensky M.H., et al. Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci. Total Environ. 2017;607:1073–1084. doi: 10.1016/j.scitotenv.2017.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens R.G., Brainard G.C., Blask D.E., Lockley S.W., Motta M.E. Adverse health effects of nighttime lighting: Comments on American Medical Association policy statement. Am. J. Prev. Med. 2013;45:343–346. doi: 10.1016/j.amepre.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Yazdi Z., Sadeghniiat-Haghighi K., Loukzadeh Z., Elmizadeh K., Abbasi M. Prevalence of sleep disorders and their impacts on occupational performance: A comparison between shift workers and nonshift workers. Sleep Disord. 2014;2014:870320. doi: 10.1155/2014/870320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohayon M.M., Milesi C. Artificial outdoor nighttime lights associate with altered sleep behavior in the American general population. Sleep. 2016;39:1311–1320. doi: 10.5665/sleep.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Saenz A., Sánchez de Miguel A., Espinosa A., Valentin A., Aragonés N., Llorca J., Amiano P., Martín Sánchez V., Guevara M., Capelo R., et al. Evaluating the association between artificial light-at-night exposure and breast and prostate cancer risk in Spain (MCC-Spain study) Environ. Health Perspect. 2018;126:047011. doi: 10.1289/EHP1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X., Ji A., Zhu Y., Liang Z., Wu J., Li S.Q., Meng S., Zheng X.Y., Xie L.P. A meta-analysis including dose-response relationship between night shift work and the risk of colorectal cancer. Oncotarget. 2015;6:25046–25060. doi: 10.18632/oncotarget.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun S., Cao W., Ge Y., Ran J., Sun F., Zeng Q., Guo M., Huang J., Lee R.S.-Y., Tian L., et al. Outdoor light at night and risk of coronary heart disease among older adults: A prospective cohort study. Eur. Heart J. 2021;42:822–830. doi: 10.1093/eurheartj/ehaa846. [DOI] [PubMed] [Google Scholar]
- 17.Vetter C., Devore E.E., Wegrzyn L.R., Massa J., Speizer F.E., Kawachi I., Rosner B., Stampfer M.J., Schernhammer E.S. Association between rotating night shift work and risk of coronary heart disease among women. JAMA-J. Am. Med. Assoc. 2016;315:1726–1734. doi: 10.1001/jama.2016.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koo Y.S., Song J.-Y., Joo E.-Y., Lee H.-J., Lee E., Lee S.-K., Jung K.-Y. Outdoor artificial light at night, obesity, and sleep health: Cross-sectional analysis in the KoGES study. Chronobiol. Int. 2016;33:301–314. doi: 10.3109/07420528.2016.1143480. [DOI] [PubMed] [Google Scholar]
- 19.Furman D., Campisi J., Verdin E., Carrera-Bastos P., Targ S., Franceschi C., Ferrucci L., Gilroy D.W., Fasano A., Miller G.W., et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honma S. The mammalian circadian system: A hierarchical multi-oscillator structure for generating circadian rhythm. J. Physiol. Sci. 2018;68:207–219. doi: 10.1007/s12576-018-0597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welsh D.K., Takahashi J.S., Kay S.A. Suprachiasmatic nucleus: Cell autonomy and network properties. Annu. Rev. Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucas R.J., Peirson S.N., Berson D.M., Brown T.M., Cooper H.M., Czeisler C.A., Figueiro M.G., Gamlin P.D., Lockley S.W., O’Hagan J.B., et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014;37:1–9. doi: 10.1016/j.tins.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buijs F.N., León-Mercado L., Guzmán-Ruiz M., Guerrero-Vargas N.N., Romo-Nava F., Buijs R.M. The circadian system: A regulatory feedback network of periphery and brain. Physiology. 2016;31:170–181. doi: 10.1152/physiol.00037.2015. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017;18:164–179. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reppert S.M., Weaver D.R. Molecular analysis of mammalian circadian rhythms. Annu. Rev. Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- 26.Alessandro M.S., Golombek D.A., Chiesa J.J. Protein kinases in the photic signaling of the mammalian circadian clock. Yale J. Biol. Med. 2019;92:241–250. [PMC free article] [PubMed] [Google Scholar]
- 27.Ueda H.R., Hayashi S., Chen W., Sano M., Machida M., Shigeyoshi Y., Iino M., Hashimoto S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat. Genet. 2005;37:187–192. doi: 10.1038/ng1504. [DOI] [PubMed] [Google Scholar]
- 28.Preitner N., Damiola F., Luis Lopez M., Zakany J., Duboule D., Albrecht U., Schibler U. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/S0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 29.Curtis A.M., Bellet M.M., Sassone-Corsi P., O’Neill L.A. Circadian clock proteins and immunity. Immunity. 2014;40:178–186. doi: 10.1016/j.immuni.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Hergenhan S., Holtkamp S., Scheiermann C. Molecular interactions between components of the circadian clock and the immune system. J. Mol. Biol. 2020;432:3700–3713. doi: 10.1016/j.jmb.2019.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li D., Wu M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021;6:291. doi: 10.1038/s41392-021-00687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newton K., Dixit V.M. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect. Biol. 2012;4:a006049. doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abele S.H., Meadows K.E., Medeiros D., Silver A.C. Time is on the immune system’s side, Yes it is. Yale J. Biol. Med. 2019;92:225–231. [PMC free article] [PubMed] [Google Scholar]
- 34.Waggoner S.N. Circadian rhythms in immunity. Curr. Allergy Asthma Rep. 2020;20:2. doi: 10.1007/s11882-020-0896-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He W., Holtkamp S., Hergenhan S.M., Kraus K., de Juan A., Weber J., Bradfield P., Grenier J.M.P., Pelletier J., Druzd D., et al. Circadian expression of migratory factors establishes lineage-specific signatures that guide the homing of leukocyte subsets to tissues. Immunity. 2018;49:1175–1190.e7. doi: 10.1016/j.immuni.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pelegrí C., Vilaplana J., Castellote C., Rabanal M., Franch À., Castell M. Circadian rhythms in surface molecules of rat blood lymphocytes. Am. J. Physiol. Cell Physiol. 2003;284:C67–C76. doi: 10.1152/ajpcell.00084.2002. [DOI] [PubMed] [Google Scholar]
- 37.Haus E., Smolensky M.H. Biologic rhythms in the immune system. Chronobiol. Int. 1999;16:581–622. doi: 10.3109/07420529908998730. [DOI] [PubMed] [Google Scholar]
- 38.Pick R., He W., Chen C.-S., Scheiermann C. Time-of-day-dependent trafficking and function of leukocyte subsets. Trends Immunol. 2019;40:524–537. doi: 10.1016/j.it.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Casanova-Acebes M., Pitaval C., Weiss L.A., Nombela-Arrieta C., Chèvre R., A-González N., Kunisaki Y., Zhang D., van Rooijen N., Silberstein L.E., et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153:1025–1035. doi: 10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Druzd D., Matveeva O., Ince L., Harrison U., He W., Schmal C., Herzel H., Tsang A.H., Kawakami N., Leliavski A., et al. Lymphocyte circadian clocks control lymph node trafficking and adaptive immune responses. Immunity. 2017;46:120–132. doi: 10.1016/j.immuni.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibbs J., Ince L., Matthews L., Mei J., Bell T., Yang N., Saer B., Begley N., Poolman T., Pariollaud M., et al. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat. Med. 2014;20:919–926. doi: 10.1038/nm.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palomino-Segura M., Hidalgo A. Circadian immune circuits. J. Exp. Med. 2020;218:e20200798. doi: 10.1084/jem.20200798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Méndez-Ferrer S., Lucas D., Battista M., Frenette P.S. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 44.Kollet O., Vagima Y., D’Uva G., Golan K., Canaani J., Itkin T., Gur-Cohen S., Kalinkovich A., Caglio G., Medaglia C., et al. Physiologic corticosterone oscillations regulate murine hematopoietic stem/progenitor cell proliferation and CXCL12 expression by bone marrow stromal progenitors. Leukemia. 2013;27:2006–2015. doi: 10.1038/leu.2013.154. [DOI] [PubMed] [Google Scholar]
- 45.Yadav R., Larbi K.Y., Young R.E., Nourshargh S. Migration of leukocytes through the vessel wall and beyond. Thromb. Haemost. 2003;90:598–606. doi: 10.1160/TH03-04-0220. [DOI] [PubMed] [Google Scholar]
- 46.Nobis C.C., Labrecque N., Cermakian N. From immune homeostasis to inflammation, a question of rhythms. Curr. Opin. Physiol. 2018;5:90–98. doi: 10.1016/j.cophys.2018.09.001. [DOI] [Google Scholar]
- 47.Halberg F., Johnson E.A., Brown B.W., Bittner J.J. Susceptibility rhythm to E. coli endotoxin and bioassay. Proc. Soc. Exp. Biol. Med. 1960;103:142–144. doi: 10.3181/00379727-103-25439. [DOI] [PubMed] [Google Scholar]
- 48.Marpegan L., Leone M.J., Katz M.E., Sobrero P.M., Bekinstein T.A., Golombek D.A. Diurnal variation in endotoxin-induced mortality in mice: Correlation with proinflammatory factors. Chronobiol. Int. 2009;26:1430–1442. doi: 10.3109/07420520903408358. [DOI] [PubMed] [Google Scholar]
- 49.Spengler M.L., Kuropatwinski K.K., Comas M., Gasparian A.V., Fedtsova N., Gleiberman A.S., Gitlin I.I., Artemicheva N.M., Deluca K.A., Gudkov A.V., et al. Core circadian protein CLOCK is a positive regulator of NF-κB–mediated transcription. Proc. Natl. Acad. Sci. USA. 2012;109:E2457–E2465. doi: 10.1073/pnas.1206274109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Timmons G.A., O’Siorain J.R., Kennedy O.D., Curtis A.M., Early J.O. Innate rhythms: Clocks at the center of monocyte and macrophage function. Front. Immunol. 2020;11:1743. doi: 10.3389/fimmu.2020.01743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gibbs J.E., Blaikley J., Beesley S., Matthews L., Simpson K.D., Boyce S.H., Farrow S.N., Else K.J., Singh D., Ray D.W., et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Natl. Acad. Sci. USA. 2012;109:582. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen K.D., Fentress S.J., Qiu Y., Yun K., Cox J.S., Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6Chi inflammatory monocytes. Science. 2013;341:1483–1488. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scheiermann C., Kunisaki Y., Lucas D., Chow A., Jang J.E., Zhang D., Hashimoto D., Merad M., Frenette P.S. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012;37:290–301. doi: 10.1016/j.immuni.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stenzinger M., Karpova D., Unterrainer C., Harenkamp S., Wiercinska E., Hoerster K., Pfeffer M., Maronde E., Bonig H. Hematopoietic-extrinsic cues dictate circadian redistribution of mature and immature hematopoietic cells in blood and spleen. Cells. 2019;8:1033. doi: 10.3390/cells8091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oishi Y., Hayashi S., Isagawa T., Oshima M., Iwama A., Shimba S., Okamura H., Manabe I. Bmal1 regulates inflammatory responses in macrophages by modulating enhancer RNA transcription. Sci. Rep. 2017;7:7086. doi: 10.1038/s41598-017-07100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang S., Lin Y., Yuan X., Li F., Guo L., Wu B. REV-ERBα integrates colon clock with experimental colitis through regulation of NF-κB/NLRP3 axis. Nat. Commun. 2018;9:4246. doi: 10.1038/s41467-018-06568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Curtis A.M., Fagundes C.T., Yang G., Palsson-McDermott E.M., Wochal P., McGettrick A.F., Foley N.H., Early J.O., Chen L., Zhang H., et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc. Natl. Acad. Sci. USA. 2015;112:7231–7236. doi: 10.1073/pnas.1501327112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Early J.O., Menon D., Wyse C.A., Cervantes-Silva M.P., Zaslona Z., Carroll R.G., Palsson-McDermott E.M., Angiari S., Ryan D.G., Corcoran S.E., et al. Circadian clock protein BMAL1 regulates IL-1β in macrophages via NRF2. Proc. Natl. Acad. Sci. USA. 2018;115:E8460–E8468. doi: 10.1073/pnas.1800431115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allen N.C., Philip N.H., Hui L., Zhou X., Franklin R.A., Kong Y., Medzhitov R. Desynchronization of the molecular clock contributes to the heterogeneity of the inflammatory response. Sci. Signal. 2019;12:eaau1851. doi: 10.1126/scisignal.aau1851. [DOI] [PubMed] [Google Scholar]
- 60.Kitchen G.B., Cunningham P.S., Poolman T.M., Iqbal M., Maidstone R., Baxter M., Bagnall J., Begley N., Saer B., Hussell T., et al. The clock gene Bmal1 inhibits macrophage motility, phagocytosis, and impairs defense against pneumonia. Proc. Natl. Acad. Sci. USA. 2020;117:1543–1551. doi: 10.1073/pnas.1915932117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huo M., Huang Y., Qu D., Zhang H., Wong W.T., Chawla A., Huang Y., Tian X.Y. Myeloid Bmal1 deletion increases monocyte recruitment and worsens atherosclerosis. FASEB J. 2017;31:1097–1106. doi: 10.1096/fj.201601030R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adrover J.M., Aroca-Crevillén A., Crainiciuc G., Ostos F., Rojas-Vega Y., Rubio-Ponce A., Cilloniz C., Bonzón-Kulichenko E., Calvo E., Rico D., et al. Programmed ‘disarming’ of the neutrophil proteome reduces the magnitude of inflammation. Nat. Immunol. 2020;21:135–144. doi: 10.1038/s41590-019-0571-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bellet M.M., Deriu E., Liu J.Z., Grimaldi B., Blaschitz C., Zeller M., Edwards R.A., Sahar S., Dandekar S., Baldi P., et al. Circadian clock regulates the host response to Salmonella. Proc. Natl. Acad. Sci. USA. 2013;110:9897–9902. doi: 10.1073/pnas.1120636110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bellet M.M., Zocchi L., Sassone-Corsi P. The RelB subunit of NFκB acts as a negative regulator of circadian gene expression. Cell Cycle. 2012;11:3304–3311. doi: 10.4161/cc.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Logan R.W., Wynne O., Levitt D., Price D., Sarkar D.K. Altered circadian expression of cytokines and cytolytic factors in splenic natural killer cells of Per1−/− mutant mice. J. Interferon Cytokine Res. 2013;33:108–114. doi: 10.1089/jir.2012.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arjona A., Sarkar D.K. The circadian gene mPer2 regulates the daily rhythm of IFN-gamma. J. Interferon Cytokine Res. 2006;26:645–649. doi: 10.1089/jir.2006.26.645. [DOI] [PubMed] [Google Scholar]
- 67.Liu J.G., Mankani G., Shi X.Y., Meyer M., Cunningham-Runddles S., Ma X.J., Sun Z.S. The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect. Immun. 2006;74:4750–4756. doi: 10.1128/IAI.00287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silver A.C., Arjona A., Walker W.E., Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. 2012;36:251–261. doi: 10.1016/j.immuni.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Narasimamurthy R., Hatori M., Nayak S.K., Liu F., Panda S., Verma I.M. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc. Natl. Acad. Sci. USA. 2012;109:12662–12667. doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pariollaud M., Gibbs J.E., Hopwood T.W., Brown S., Begley N., Vonslow R., Poolman T., Guo B., Saer B., Jones D.H., et al. Circadian clock component REV-ERBα controls homeostatic regulation of pulmonary inflammation. J. Clin. Investig. 2018;128:2281–2296. doi: 10.1172/JCI93910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sato S., Sakurai T., Ogasawara J., Takahashi M., Izawa T., Imaizumi K., Taniguchi N., Ohno H., Kizaki T. A circadian clock gene, Rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J. Immunol. 2014;192:407–417. doi: 10.4049/jimmunol.1301982. [DOI] [PubMed] [Google Scholar]
- 72.Griffin P., Dimitry J.M., Sheehan P.W., Lananna B.V., Guo C., Robinette M.L., Hayes M.E., Cedeño M.R., Nadarajah C.J., Ezerskiy L.A., et al. Circadian clock protein Rev-erbα regulates neuroinflammation. Proc. Natl. Acad. Sci. USA. 2019;116:5102–5107. doi: 10.1073/pnas.1812405116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Griffin P., Sheehan P.W., Dimitry J.M., Guo C., Kanan M.F., Lee J., Zhang J., Musiek E.S. REV-ERBα mediates complement expression and diurnal regulation of microglial synaptic phagocytosis. eLife. 2020;9:e58765. doi: 10.7554/eLife.58765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stapleton C.M., Jaradat M., Dixon D., Kang H.S., Kim S.-C., Liao G., Carey M.A., Cristiano J., Moorman M.P., Jetten A.M. Enhanced susceptibility of staggerer (RORαsg/sg) mice to lipopolysaccharide-induced lung inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L144–L152. doi: 10.1152/ajplung.00348.2004. [DOI] [PubMed] [Google Scholar]
- 75.Kopmels B., Mariani J., Delhaye-Bouchaud N., Audibert F., Fradelizi D., Wollman E.E. Evidence for a hyperexcitability state of staggerer mutant mice macrophages. J. Neurochem. 1992;58:192–199. doi: 10.1111/j.1471-4159.1992.tb09295.x. [DOI] [PubMed] [Google Scholar]
- 76.Kashiwada M., Pham N.-L.L., Pewe L.L., Harty J.T., Rothman P.B. NFIL3/E4BP4 is a key transcription factor for CD8α+ dendritic cell development. Blood. 2011;117:6193–6197. doi: 10.1182/blood-2010-07-295873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gascoyne D.M., Long E., Veiga-Fernandes H., de Boer J., Williams O., Seddon B., Coles M., Kioussis D., Brady H.J.M. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat. Immunol. 2009;10:1118–1124. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]
- 78.Geiger T.L., Abt M.C., Gasteiger G., Firth M.A., O’Connor M.H., Geary C.D., O’Sullivan T.E., van den Brink M.R., Pamer E.G., Hanash A.M., et al. Nfil3 is crucial for development of innate lymphoid cells and host protection against intestinal pathogens. J. Exp. Med. 2014;211:1723–1731. doi: 10.1084/jem.20140212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kobayashi T., Matsuoka K., Sheikh S.Z., Elloumi H.Z., Kamada N., Hisamatsu T., Hansen J.J., Doty K.R., Pope S.D., Smale S.T., et al. NFIL3 is a regulator of IL-12 p40 in macrophages and mucosal immunity. J. Immunol. 2011;186:4649. doi: 10.4049/jimmunol.1003888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bunger M.K., Wilsbacher L.D., Moran S.M., Clendenin C., Radcliffe L.A., Hogenesch J.B., Simon M.C., Takahashi J.S., Bradfield C.A. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/S0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baggs J.E., Price T.S., DiTacchio L., Panda S., FitzGerald G.A., Hogenesch J.B. Network features of the mammalian circadian clock. PLoS Biol. 2009;7:e1000052. doi: 10.1371/journal.pbio.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Delerive P., Monté D., Dubois G., Trottein F., Fruchart-Najib J., Mariani J., Fruchart J.-C., Staels B. The orphan nuclear receptor RORα is a negative regulator of the inflammatory response. EMBO Rep. 2001;2:42–48. doi: 10.1093/embo-reports/kve007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Duez H., van der Veen J.N., Duhem C., Pourcet B., Touvier T., Fontaine C., Derudas B., Baugé E., Havinga R., Bloks V.W., et al. Regulation of bile acid synthesis by the nuclear receptor Rev-erbα. Gastroenterology. 2008;135:689–698. doi: 10.1053/j.gastro.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 84.Vosko A.M., Colwell C.S., Avidan A.Y. Jet lag syndrome: Circadian organization, pathophysiology, and management strategies. Nat. Sci. Sleep. 2010;2:187–198. doi: 10.2147/NSS.S6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wickwire E.M., Geiger-Brown J., Scharf S.M., Drake C.L. Shift work and shift work sleep disorder: Clinical and organizational perspectives. Chest. 2017;151:1156–1172. doi: 10.1016/j.chest.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boivin D.B., Boudreau P., Kosmadopoulos A. Disturbance of the circadian system in shift work and its health impact. J. Biol. Rhythm. 2021;37:3–28. doi: 10.1177/07487304211064218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hemmer A., Mareschal J., Dibner C., Pralong J.A., Dorribo V., Perrig S., Genton L., Pichard C., Collet T.-H. The effects of shift work on cardio-metabolic diseases and eating patterns. Nutrients. 2021;13:4178. doi: 10.3390/nu13114178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X.S., Armstrong M.E.G., Cairns B.J., Key T.J., Travis R.C. Shift work and chronic disease: The epidemiological evidence. Occup. Med. 2011;61:78–89. doi: 10.1093/occmed/kqr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Loef B., van Baarle D., van der Beek A.J., Sanders E.A.M., Bruijning-Verhagen P., Proper K.I. Shift work and respiratory infections in health-care workers. Am. J. Epidemiol. 2019;188:509–517. doi: 10.1093/aje/kwy258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohren D.C.L., Jansen N.W.H., Kant I., Galama J.M.D., van den Brandt P.A., Swaen G.M.H. Prevalence of common infections among employees in different work schedules. J. Occup. Environ. Med. 2002;44:1003–1011. doi: 10.1097/00043764-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 91.Fatima Y., Bucks R.S., Mamun A.A., Skinner I., Rosenzweig I., Leschziner G., Skinner T.C. Shift work is associated with increased risk of COVID-19: Findings from the UK Biobank cohort. J. Sleep Res. 2021;30:e13326. doi: 10.1111/jsr.13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu L.-F., Wang C.-P., Tsai I.T., Hung W.-C., Yu T.-H., Wu C.-C., Hsu C.-C., Lu Y.-C., Chung F.-M., Jean M.-C.Y. Relationship between shift work and peripheral total and differential leukocyte counts in Chinese steel workers. J. Occup. Health. 2016;58:81–88. doi: 10.1539/joh.15-0137-OA. [DOI] [PubMed] [Google Scholar]
- 93.Wirth M.D., Andrew M.E., Burchfiel C.M., Burch J.B., Fekedulegn D., Hartley T.A., Charles L.E., Violanti J.M. Association of shiftwork and immune cells among police officers from the Buffalo Cardio-Metabolic Occupational Police Stress study. Chronobiol. Int. 2017;34:721–731. doi: 10.1080/07420528.2017.1316732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Atwater A.Q., Immergluck L.C., Davidson A.J., Castanon-Cervantes O. Shift work predicts increases in lipopolysaccharide-binding protein, interleukin-10, and leukocyte counts in a cross-sectional study of healthy volunteers carrying low-grade systemic inflammation. Int. J. Environ. Res. Public Health. 2021;18:13158. doi: 10.3390/ijerph182413158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Okamoto H., Tsunoda T., Teruya K., Takeda N., Uemura T., Matsui T., Fukazawa S., Ichikawa K., Takemae R., Tsuchida K., et al. An occupational health study of emergency physicians in Japan: Health assessment by immune variables (CD4, CD8, CD56, and NK cell activity) at the beginning of work. J. Occup. Health. 2008;50:136–146. doi: 10.1539/joh.L6084. [DOI] [PubMed] [Google Scholar]
- 96.Kervezee L., Cuesta M., Cermakian N., Boivin D.B. Simulated night shift work induces circadian misalignment of the human peripheral blood mononuclear cell transcriptome. Proc. Natl. Acad. Sci. USA. 2018;115:5540–5545. doi: 10.1073/pnas.1720719115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cuesta M., Boudreau P., Dubeau-Laramée G., Cermakian N., Boivin D.B. Simulated night shift disrupts circadian rhythms of immune functions in humans. J. Immunol. 2016;196:2466. doi: 10.4049/jimmunol.1502422. [DOI] [PubMed] [Google Scholar]
- 98.Arble D.M., Ramsey K.M., Bass J., Turek F.W. Circadian disruption and metabolic disease: Findings from animal models. Best Pract. Res. Clin. Endocrinol. Metab. 2010;24:785–800. doi: 10.1016/j.beem.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Opperhuizen A.L., van Kerkhof L.W.M., Proper K.I., Rodenburg W., Kalsbeek A. Rodent models to study the metabolic effects of shiftwork in humans. Front. Pharmacol. 2015;6:50. doi: 10.3389/fphar.2015.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Casiraghi L.P., Oda G.A., Chiesa J.J., Friesen W.O., Golombek D.A. Forced desynchronization of activity rhythms in a model of chronic jet lag in mice. J. Biol. Rhythm. 2012;27:59–69. doi: 10.1177/0748730411429447. [DOI] [PubMed] [Google Scholar]
- 101.Iwamoto A., Kawai M., Furuse M., Yasuo S. Effects of chronic jet lag on the central and peripheral circadian clocks in CBA/N mice. Chronobiol. Int. 2014;31:189–198. doi: 10.3109/07420528.2013.837478. [DOI] [PubMed] [Google Scholar]
- 102.Zhao Y., Liu M., Chan X.Y., Tan S.Y., Subramaniam S., Fan Y., Loh E., Chang K.T.E., Tan T.C., Chen Q. Uncovering the mystery of opposite circadian rhythms between mouse and human leukocytes in humanized mice. Blood. 2017;130:1995–2005. doi: 10.1182/blood-2017-04-778779. [DOI] [PubMed] [Google Scholar]
- 103.Crespo M., Gonzalez-Teran B., Nikolic I., Mora A., Folgueira C., Rodríguez E., Leiva-Vega L., Pintor-Chocano A., Fernández-Chacón M., Ruiz-Garrido I., et al. Neutrophil infiltration regulates clock-gene expression to organize daily hepatic metabolism. eLife. 2020;9:e59258. doi: 10.7554/eLife.59258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Adams K.L., Castanon-Cervantes O., Evans J.A., Davidson A.J. Environmental circadian disruption elevates the IL-6 response to lipopolysaccharide in blood. J. Biol. Rhythm. 2013;28:272–277. doi: 10.1177/0748730413494561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brager A.J., Ehlen J.C., Castanon-Cervantes O., Natarajan D., Delisser P., Davidson A.J., Paul K.N. Sleep loss and the inflammatory response in mice under chronic environmental circadian disruption. PLoS ONE. 2013;8:e63752. doi: 10.1371/journal.pone.0063752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Castanon-Cervantes O., Wu M.W., Ehlen J.C., Paul K., Gamble K.L., Johnson R.L., Besing R.C., Menaker M., Gewirtz A.T., Davidson A.J. Dysregulation of inflammatory responses by chronic circadian disruption. J. Immunol. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mul Fedele M.L., Aiello I., Caldart C.S., Golombek D.A., Marpegan L., Paladino N. Differential thermoregulatory and inflammatory patterns in the circadian response to LPS-induced septic shock. Front. Cell. Infect. Microbiol. 2020;10:100. doi: 10.3389/fcimb.2020.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim S.-M., Neuendorff N., Alaniz R.C., Sun Y., Chapkin R.S., Earnest D.J. Shift work cycle-induced alterations of circadian rhythms potentiate the effects of high-fat diet on inflammation and metabolism. FASEB J. 2018;32:3085–3095. doi: 10.1096/fj.201700784R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schilperoort M., van den Berg R., Bosmans L.A., van Os B.W., Dollé M.E.T., Smits N.A.M., Guichelaar T., van Baarle D., Koemans L., Berbée J.F.P., et al. Disruption of circadian rhythm by alternating light-dark cycles aggravates atherosclerosis development in APOE*3-Leiden.CETP mice. J. Pineal Res. 2020;68:e12614. doi: 10.1111/jpi.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aiello I., Fedele M.L.M., Román F., Marpegan L., Caldart C., Chiesa J.J., Golombek D.A., Finkielstein C.V., Paladino N. Circadian disruption promotes tumor-immune microenvironment remodeling favoring tumor cell proliferation. Sci. Adv. 2020;6:eaaz4530. doi: 10.1126/sciadv.aaz4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zeng X., Liang C., Yao J. Chronic shift-lag promotes NK cell ageing and impairs immunosurveillance in mice by decreasing the expression of CD122. J. Cell. Mol. Med. 2020;24:14583–14595. doi: 10.1111/jcmm.16088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Logan R.W., Zhang C., Murugan S., O’Connell S., Levitt D., Rosenwasser A.M., Sarkar D.K. Chronic shift-lag alters the circadian clock of NK cells and promotes lung cancer growth in rats. J. Immunol. 2012;188:2583–2591. doi: 10.4049/jimmunol.1102715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Olefsky J.M., Glass C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 114.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003;112:1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Z., Zeng P., Gao W., Zhou Q., Feng T., Tian X. Circadian clock: A regulator of the immunity in cancer. Cell Commun. Signal. 2021;19:37. doi: 10.1186/s12964-021-00721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Filipski E., Delaunay F., King V.M., Wu M.-W., Claustrat B., Gréchez-Cassiau A., Guettier C., Hastings M.H., Francis L.V. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004;64:7879–7885. doi: 10.1158/0008-5472.CAN-04-0674. [DOI] [PubMed] [Google Scholar]
- 117.Pierce S., Geanes E.S., Bradley T. Targeting natural killer cells for improved immunity and control of the adaptive immune response. Front. Cell. Infect. Microbiol. 2020;10:231. doi: 10.3389/fcimb.2020.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim S., Iizuka K., Aguila H.L., Weissman I.L., Yokoyama W.M. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc. Natl. Acad. Sci. USA. 2000;97:2731–2736. doi: 10.1073/pnas.050588297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paul S., Lal G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front. Immunol. 2017;8:1124. doi: 10.3389/fimmu.2017.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aktas E., Kucuksezer U.C., Bilgic S., Erten G., Deniz G. Relationship between CD107a expression and cytotoxic activity. Cell. Immunol. 2009;254:149–154. doi: 10.1016/j.cellimm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 121.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Davidson A.J., Sellix M.T., Daniel J., Yamazaki S., Menaker M., Block G.D. Chronic jet-lag increases mortality in aged mice. Curr. Biol. 2006;16:R914–R916. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Inokawa H., Umemura Y., Shimba A., Kawakami E., Koike N., Tsuchiya Y., Ohashi M., Minami Y., Cui G., Asahi T., et al. Chronic circadian misalignment accelerates immune senescence and abbreviates lifespan in mice. Sci. Rep. 2020;10:2569. doi: 10.1038/s41598-020-59541-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kochan D.Z., Ilnytskyy Y., Golubov A., Deibel S.H., McDonald R.J., Kovalchuk O. Circadian disruption-induced microRNAome deregulation in rat mammary gland tissues. Oncoscience. 2015;2:428–442. doi: 10.18632/oncoscience.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sánchez de Miguel A., Bennie J., Rosenfeld E., Dzurjak S., Gaston K.J. First estimation of global trends in nocturnal power emissions reveals acceleration of light pollution. Remote Sens. 2021;13:3311. doi: 10.3390/rs13163311. [DOI] [Google Scholar]
- 126.Cajochen C., Frey S., Anders D., Späti J., Bues M., Pross A., Mager R., Wirz-Justice A., Stefani O. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J. Appl. Physiol. 2011;110:1432–1438. doi: 10.1152/japplphysiol.00165.2011. [DOI] [PubMed] [Google Scholar]
- 127.Rumanova V.S., Okuliarova M., Foppen E., Kalsbeek A., Zeman M. Exposure to dim light at night alters daily rhythms of glucose and lipid metabolism in rats. Front. Physiol. 2022;13:973461. doi: 10.3389/fphys.2022.973461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stenvers D.J., van Dorp R., Foppen E., Mendoza J., Opperhuizen A.L., Fliers E., Bisschop P.H., Meijer J.H., Kalsbeek A., Deboer T. Dim light at night disturbs the daily sleep-wake cycle in the rat. Sci. Rep. 2016;6:35662. doi: 10.1038/srep35662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Okuliarova M., Dzirbikova Z., Rumanova V.S., Foppen E., Kalsbeek A., Zeman M. Disrupted circadian control of hormonal rhythms and anticipatory thirst by dim light at night. Neuroendocrinology. 2022;112:1116–1128. doi: 10.1159/000524235. [DOI] [PubMed] [Google Scholar]
- 130.Stenvers D.J., Scheer F., Schrauwen P., la Fleur S.E., Kalsbeek A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 2019;15:75–89. doi: 10.1038/s41574-018-0122-1. [DOI] [PubMed] [Google Scholar]
- 131.Fonken L.K., Aubrecht T.G., Meléndez-Fernández O.H., Weil Z.M., Nelson R.J. Dim light at night disrupts molecular circadian rhythms and increases body weight. J. Biol. Rhythm. 2013;28:262–271. doi: 10.1177/0748730413493862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shuboni D., Yan L. Nighttime dim light exposure alters the responses of the circadian system. Neuroscience. 2010;170:1172–1178. doi: 10.1016/j.neuroscience.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 133.Dauchy R.T., Dauchy E.M., Tirrell R.P., Hill C.R., Davidson L.K., Greene M.W., Tirrell P.C., Wu J., Sauer L.A., Blask D.E. Dark-phase light contamination disrupts circadian rhythms in plasma measures of endocrine physiology and metabolism in rats. Comp. Med. 2010;60:348–356. [PMC free article] [PubMed] [Google Scholar]
- 134.Molcan L., Sutovska H., Okuliarova M., Senko T., Krskova L., Zeman M. Dim light at night attenuates circadian rhythms in the cardiovascular system and suppresses melatonin in rats. Life Sci. 2019;231:116568. doi: 10.1016/j.lfs.2019.116568. [DOI] [PubMed] [Google Scholar]
- 135.Moaraf S., Heiblum R., Okuliarová M., Hefetz A., Scharf I., Zeman M., Barnea A. Evidence that artificial light at night induces structure-specific changes in brain plasticity in a diurnal bird. Biomolecules. 2021;11:1069. doi: 10.3390/biom11081069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moaraf S., Vistoropsky Y., Pozner T., Heiblum R., Okuliarová M., Zeman M., Barnea A. Artificial light at night affects brain plasticity and melatonin in birds. Neurosci. Lett. 2020;716:134639. doi: 10.1016/j.neulet.2019.134639. [DOI] [PubMed] [Google Scholar]
- 137.Grubisic M., Haim A., Bhusal P., Dominoni D.M., Gabriel K.M.A., Jechow A., Kupprat F., Lerner A., Marchant P., Riley W., et al. Light pollution, circadian photoreception, and melatonin in vertebrates. Sustainability. 2019;11:6400. doi: 10.3390/su11226400. [DOI] [Google Scholar]
- 138.Okuliarova M., Mazgutova N., Majzunova M., Rumanova V.S., Zeman M. Dim light at night impairs daily variation of circulating immune cells and renal immune homeostasis. Front. Immunol. 2021;11:614960. doi: 10.3389/fimmu.2020.614960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fonken L.K., Lieberman R.A., Weil Z.M., Nelson R.J. Dim light at night exaggerates weight gain and inflammation associated with a high-fat diet in male mice. Endocrinology. 2013;154:3817–3825. doi: 10.1210/en.2013-1121. [DOI] [PubMed] [Google Scholar]
- 140.Bumgarner J.R., Walker W.H., Liu J.A., Walton J.C., Nelson R.J. Dim light at night exposure induces cold hyperalgesia and mechanical allodynia in male mice. Neuroscience. 2020;434:111–119. doi: 10.1016/j.neuroscience.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fonken L.K., Weil Z.M., Nelson R.J. Mice exposed to dim light at night exaggerate inflammatory responses to lipopolysaccharide. Brain Behav. Immun. 2013;34:159–163. doi: 10.1016/j.bbi.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 142.Fonken L.K., Bedrosian T.A., Zhang N., Weil Z.M., DeVries A.C., Nelson R.J. Dim light at night impairs recovery from global cerebral ischemia. Exp. Neurol. 2019;317:100–109. doi: 10.1016/j.expneurol.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Walker W.H., Kvadas R.M., May L.E., Liu J.A., Bumgarner J.R., Walton J.C., DeVries A.C., Dauchy R.T., Blask D.E., Nelson R.J. Artificial light at night reduces anxiety-like behavior in female mice with exacerbated mammary tumor growth. Cancers. 2021;13:4860. doi: 10.3390/cancers13194860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Blask D.E., Dauchy R.T., Dauchy E.M., Mao L., Hill S.M., Greene M.W., Belancio V.P., Sauer L.A., Davidson L. Light exposure at night disrupts host/cancer circadian regulatory dynamics: Impact on the Warburg effect, lipid signaling and tumor growth prevention. PLoS ONE. 2014;9:e102776. doi: 10.1371/journal.pone.0102776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bedrosian T.A., Weil Z.M., Nelson R.J. Chronic dim light at night provokes reversible depression-like phenotype: Possible role for TNF. Mol. Psychiatry. 2013;18:930–936. doi: 10.1038/mp.2012.96. [DOI] [PubMed] [Google Scholar]
- 146.Bedrosian T.A., Fonken L.K., Walton J.C., Nelson R.J. Chronic exposure to dim light at night suppresses immune responses in Siberian hamsters. Biol. Lett. 2011;7:468–471. doi: 10.1098/rsbl.2010.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fonken L.K., Haim A., Nelson R.J. Dim light at night increases immune function in nile grass rats, a diurnal rodent. Chronobiol. Int. 2012;29:26–34. doi: 10.3109/07420528.2011.635831. [DOI] [PubMed] [Google Scholar]
- 148.Fonken L.K., Kitsmiller E., Smale L., Nelson R.J. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J. Biol. Rhythm. 2012;27:319–327. doi: 10.1177/0748730412448324. [DOI] [PubMed] [Google Scholar]
- 149.Rumanova V.S., Okuliarova M., Zeman M. Differential effects of constant light and dim light at night on the circadian control of metabolism and behavior. Int. J. Mol. Sci. 2020;21:5478. doi: 10.3390/ijms21155478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ohta H., Yamazaki S., McMahon D.G. Constant light desynchronizes mammalian clock neurons. Nat. Neurosci. 2005;8:267–269. doi: 10.1038/nn1395. [DOI] [PubMed] [Google Scholar]
- 151.Polidarová L., Houdek P., Sumová A. Chronic disruptions of circadian sleep regulation induce specific proinflammatory responses in the rat colon. Chronobiol. Int. 2017;34:1273–1287. doi: 10.1080/07420528.2017.1361436. [DOI] [PubMed] [Google Scholar]
- 152.Guerrero-Vargas N.N., Navarro-Espíndola R., Guzmán-Ruíz M.A., Basualdo M.D.C., Espitia-Bautista E., López-Bago A., Lascurain R., Córdoba-Manilla C., Buijs R.M., Escobar C. Circadian disruption promotes tumor growth by anabolic host metabolism; experimental evidence in a rat model. BMC Cancer. 2017;17:625. doi: 10.1186/s12885-017-3636-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Coomans C.P., van den Berg S.A.A., Houben T., van Klinken J.-B., van den Berg R., Pronk A.C.M., Havekes L.M., Romijn J.A., van Dijk K.W., Biermasz N.R., et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2013;27:1721–1732. doi: 10.1096/fj.12-210898. [DOI] [PubMed] [Google Scholar]
- 154.Lucassen E.A., Coomans C.P., van Putten M., de Kreij S.R., van Genugten J.H.L.T., Sutorius R.P.M., de Rooij K.E., van der Velde M., Verhoeve S.L., Smit J.W.A., et al. Environmental 24-hr cycles are essential for health. Curr. Biol. 2016;26:1843–1853. doi: 10.1016/j.cub.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 155.Báez-Ruiz A., Guerrero-Vargas N.N., Cázarez-Márquez F., Sabath E., Basualdo M.d.C., Salgado-Delgado R., Escobar C., Buijs R.M. Food in synchrony with melatonin and corticosterone relieves constant light disturbed metabolism. J. Endocrinol. 2017;235:167–178. doi: 10.1530/JOE-17-0370. [DOI] [PubMed] [Google Scholar]
- 156.Gale J.E., Cox H.I., Qian J.Y., Block G.D., Colwell C.S., Matveyenko A.V. Disruption of circadian rhythms accelerates development of diabetes through pancreatic beta-cell loss and dysfunction. J. Biol. Rhythm. 2011;26:423–433. doi: 10.1177/0748730411416341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Claustrat B., Valatx J.L., Harthé C., Brun J. Effect of constant light on prolactin and corticosterone rhythms evaluated using a noninvasive urine sampling protocol in the rat. Horm. Metab. Res. 2008;40:398–403. doi: 10.1055/s-2008-1065330. [DOI] [PubMed] [Google Scholar]
- 158.Deprés-Brummer P., Bourin P., Pages N., Metzger G., Lévi F. Persistent T lymphocyte rhythms despite suppressed circadian clock outputs in rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1997;273:R1891–R1899. doi: 10.1152/ajpregu.1997.273.6.R1891. [DOI] [PubMed] [Google Scholar]
- 159.Chen Y., Cheng M., Su T., Gao T., Yu W. Constant light exposure aggravates POMC-mediated muscle wasting associated with hypothalamic alteration of circadian clock and SIRT1 in endotoxemia rats. Biochem. Biophys. Res. Commun. 2019;508:811–817. doi: 10.1016/j.bbrc.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 160.Perfilyeva Y.V., Abdolla N., Ostapchuk Y.O., Tleulieva R., Krasnoshtanov V.C., Belyaev N.N. Expansion of CD11b+Ly6Ghigh and CD11b+CD49d+ myeloid cells with suppressive potential in mice with chronic inflammation and light-at-night-induced circadian disruption. Inflamm. Res. 2017;66:711–724. doi: 10.1007/s00011-017-1052-4. [DOI] [PubMed] [Google Scholar]
- 161.Katoh H., Watanabe M. Myeloid-derived suppressor cells and therapeutic strategies in cancer. Mediat. Inflamm. 2015;2015:159269. doi: 10.1155/2015/159269. [DOI] [PMC free article] [PubMed] [Google Scholar]