Abstract
The CDC1551 strain of Mycobacterium tuberculosis was compared with the H37Rv strain of M. tuberculosis and the Ravenel strain of Mycobacterium bovis for virulence in mice. Although all three strains gave rise to the same level of stationary infection in major organs, mice infected with the Ravenel strain died much earlier from lung disease.
A recently isolated strain of Mycobacterium tuberculosis (CDC1551) responsible for a relatively large number cases of active tuberculosis in a small geographical area near the Kentucky-Tennessee border was shown to have a greatly faster doubling time in the lungs of mice than the long established Erdman strain (14). It was reasoned, on the basis of this finding, that strain CDC1551 has a very high virulence for mice and that this could indicate that it has a high virulence for humans, which would explain, in turn, its unusually high transmissibility. This seems a reasonable interpretation, given that the communicability of tuberculosis depends on the ability of M. tuberculosis to cause lung pathology. On the other hand, the communicability of a pathogen can depend on properties other than virulence, such as an ability to resist desiccation. It seemed important to determine, therefore, whether the reported superior ability of CDC1551 to multiply in the lungs of mice over a relatively short period of observation is associated with a superior ability to cause disease.
To investigate this, 10-week-old male C57BL/6 mice (Jackson Laboratories, Bar Harbor, Maine) were infected via the respiratory route in an aerosol infection apparatus, as previously described (6), with approximately 102 CFU of M. tuberculosis H37Rv, Mycobacterium bovis Ravenel (TMC 102 and TMC 401; originally obtained from the Trudeau Mycobacterial Culture Collection and currently available from American Type Culture Collection), or M. tuberculosis CDC1551 (kindly provided by Barry Kriesworth, Public Health Research Institute, New York, N.Y.). All three organisms were grown in suspension culture in Proskauer and Beck medium containing 0.01% Tween 80 and were harvested while in log-phase growth. The cultures were subjected to two 5-s bursts of ultrasound to break up clumps and passed through a 5-μm-pore-size filter (Gelman Sciences, Ann Arbor, Mich.) to ensure that differences in virulence could not be attributed to differences in the number of bacilli per CFU. At 1, 10, 20, 50, and 120 days, five mice infected with each organism were sacrificed and their lungs, livers, and spleens were removed and homogenized in phosphate-buffered saline–Tween. The homogenates were subjected to 10-fold serial dilution, the dilutions were plated on 7H11 agar, and the plates were incubated for 2 to 3 weeks. Colonies were counted with the aid of a dissecting microscope.
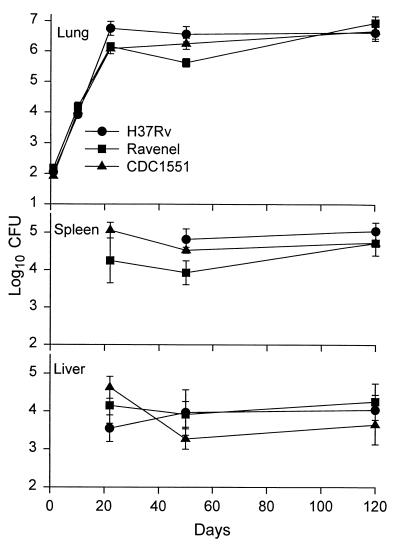
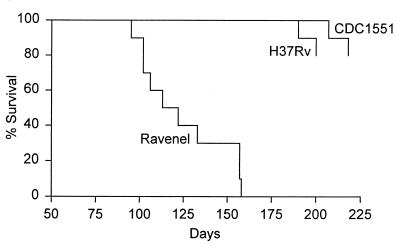
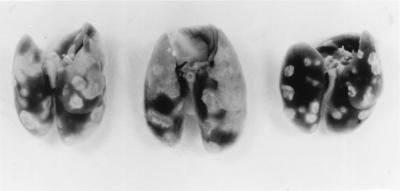
It can be seen in Fig. 1 that strains CDC1551, H37Rv, and Ravenel multiplied at essentially the same rates in the lungs of mice over the first 20 days of infection, after which infection in each case was controlled and caused to plateau at a level of about 6.5 logs for the duration of the experiment. It can also be seen that these strains caused similar levels of infection in the liver and spleen. In all cases, infection was not detected in these organs until day 20 and remained approximately stationary in both organs from day 20 on. However, in spite of the fact that all three pathogens gave rise to the same level of infection, Ravenel proved much more virulent than the other two strains, according to host survival. The survival curves (Fig. 2) for 10 mice infected as described above with each of the three strains shows that Ravenel caused mice to die, with a median survival time of 115 days. Moreover, all mice infected with this pathogen died before any mice infected with H37Rv or CDC1551 succumbed. The basis for the differences in survival times can be deduced from the macroscopic appearance (Fig. 3) of lungs harvested on day 120 of infection, when more than 50% of mice infected with Ravenel were dead. It can be seen that lung disease in Ravenel-infected mice was much more extensive than that in H37Rv-infected mice, which appeared more extensive, in turn, than lung disease in CDC1551-infected mice. Because there was no macroscopic pathology evident in other major organs of mice infected with any one of the pathogens, it seems reasonable to suggest that all three pathogens caused lung disease but that Ravenel caused lung disease to develop most rapidly. The possibility that Ravenel is more pathogenic because it induces the macrophages in which it resides to secrete more proinflammatory cytokines needs consideration.
FIG. 1.
Time course of infection with CDC1551, H37Rv, and Ravenel in C57BL/6 mice infected via the respiratory route with approximately 102 CFU. The strains were almost identical in their ability to grow in the organs of mice. Means for five mice per group per time point ± standard deviations are shown.
FIG. 2.
Survival of times of 10 mice infected by aerosol with 102 CFU of CDC1551, H37Rv, or Ravenel. Mice infected with Ravenel died with a median survival time of 115 days. The experiment was terminated before mice infected with the other strains had all died.
FIG. 3.
Appearance of the lungs of mice infected by aerosol with approximately 102 CFU of CDC1551 (right), H37Rv (left), or Ravenel (center) on day 120 of infection. Ravenel caused the most lung pathology, followed in turn by H37Rv and CDC1551.
The ability of mice to cause M. tuberculosis infection to become stationary in their lungs and other organs after the onset of expression of immunity has been stressed in earlier publications from this laboratory (6, 8). Guinea pigs also acquire the ability to stabilize infection in their lungs after several weeks of progressive M. tuberculosis growth (1). It has been suggested that the peculiar susceptibility of the lungs of mice to infection-induced disease makes mouse tuberculosis similar to tuberculosis in humans, because in over 85% of infected humans the disease is confined to the lungs (4). The confinement of disease to the lungs in humans, and in mice, undoubtedly is the result of the acquisition of a state of systemic immunity that is capable of inhibiting disease progression in all organs except the lungs. This is evident from the knowledge that loss of immune competence in mice (3) and humans (2, 5) can result in systemic disease involving multiple organs. It is apparent in the case of mice that although immunity can stabilize infection in the lungs and cause infection to become stationary, the pathogen is able to take advantage of something peculiar to the lungs to induce chronic inflammation and organ consolidation. It is known (3, 11) that in the mouse, chronic lung infection is associated with massive interstitial thickening and fibrosis and that fibrosis is the key manifestation of human pulmonary tuberculosis (16).
We have not attempted to distinguish between the terms pathogenicity and virulence, although a distinction has been made by some (12, 15) and not by others (9, 13). According to one group (10), the extent to which a given M. tuberculosis strain is able to cause pathology in a given period of time is the most useful measure of its virulence. Based on this measure, strain CDC1551 is not a particularly virulent strain of M. tuberculosis, in spite of its highly infectious nature. At this time there seems to be no scientific basis for believing that recently isolated strains are more virulent than long-established reference strains such as H37Rv and Ravenel, which were isolated nearly 100 years ago. It should be pointed out that the C57BL/6 mouse strain employed in this study is considered to be an M. tuberculosis-resistant strain (7).
ADDENDUM
After our paper was submitted, an elegant paper by Manca et al. (5a) showing that strain CDC1551 is not more virulent than two other strains of M. tuberculosis, based on mouse survival data, was published.
Acknowledgments
This work was supported by grants AI-37844 and AI-40071 from the U.S. Public Health Service and a grant from the G. Harold and Leila Y. Mathers Charitable Foundation.
REFERENCES
- 1.Alsaadi A-I, Smith D W. The fate of virulent and attenuated mycobacteria in guinea pigs infected by the respiratory route. Am Rev Respir Dis. 1975;107:1041–1046. doi: 10.1164/arrd.1973.107.6.1041. [DOI] [PubMed] [Google Scholar]
- 2.Barnes P, Block A B, Davidson P T, Snider D E. Tuberculosis in patients with immunodeficiency virus infection. N Engl J Med. 1991;324:1644–1650. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- 3.Dunn P L, North R J. Virulence ranking of some Mycobacterium tuberculosis and Mycobacterium bovis strains according to their ability to multiply in the lungs, induce lung pathology and cause mortality in mice. Infect Immun. 1995;63:3428–3437. doi: 10.1128/iai.63.9.3428-3437.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farer L S. Extrapulmonary tuberculosis in the United States. Am J Epidemiol. 1997;109:205–211. doi: 10.1093/oxfordjournals.aje.a112675. [DOI] [PubMed] [Google Scholar]
- 5.Hill R A, Pemkuma S, Brustein S, Vaidya K, Powell S, Li P-W, Suster B. Disseminated tuberculosis in the acquired immunodeficiency syndrome era. Am Rev Respir Dis. 1991;144:1164–1170. doi: 10.1164/ajrccm/144.5.1164. [DOI] [PubMed] [Google Scholar]
- 5a.Manca C, Tsenova L, Barry III C E, Bergtold A, Freeman S, Haslett P A, Musser J M, Freedman V H, Kaplan G. Mycobacterium tuberculosis CDC1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J Immunol. 1999;162:6740–6746. [PubMed] [Google Scholar]
- 6.Medina E, North R J. Evidence inconsistent with a role for the Bcg gene (Nramp1) in resistance to infection with Mycobacterium tuberculosis. J Exp Med. 1996;183:1045–1051. doi: 10.1084/jem.183.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medina E, North R J. Resistance ranking of some common inbred mouse strains to Mycobacterium tuberculosis and relationship to major histocompatibility haplotype and Nramp1 genotype. Immunology. 1998;83:270–274. doi: 10.1046/j.1365-2567.1998.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medina E, North R J. Genetically susceptible mice remain proportionally more susceptible to tuberculosis after vaccination. Immunology. 1999;96:16–21. doi: 10.1046/j.1365-2567.1999.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mims C A. The pathogenesis of infectious disease. 2nd ed. New York, N.Y: Academic Press, Inc.; 1982. pp. 238–241. [Google Scholar]
- 10.Mitchison D A, Bhatia L A, Radhakrishna S, Selkon J B, Subaiah T V, Wallace J G. The virulence in the guinea pig of tubercle bacilli isolated before treatment from south Indian patients with pulmonary tuberculosis. 1. Homogeneity of the investigation and a critique of the virulence test. Bull W H O. 1961;25:285–312. [PMC free article] [PubMed] [Google Scholar]
- 11.Rhoades E R, Frank A A, Orme I M. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuberc Lung Dis. 1997;78:57–66. doi: 10.1016/s0962-8479(97)90016-2. [DOI] [PubMed] [Google Scholar]
- 12.Rich A R. Pathogenesis of tuberculosis. Springfield, Ill: Charles C. Thomas, Publisher; 1944. pp. 80–101. [Google Scholar]
- 13.Salyers A A, Whitt D D. Bacterial pathogenesis: a molecular approach. Washington, D.C: ASM Press; 1994. pp. 31–32. [Google Scholar]
- 14.Valway S E, Sanchez M P C, Shinnick T F, Orme I, Agerton T, Hoy D, Jones J S, Westmoreland H, Onorato I M. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. N Engl J Med. 1998;338:633–639. doi: 10.1056/NEJM199803053381001. [DOI] [PubMed] [Google Scholar]
- 15.Wilson G S, Miles A. Topley and Wilson’s principles of bacteriology, virology and immunity. 6th ed. Vol. 2. London, United Kingdom: Edward Arnold Ltd.; 1975. pp. 275–279. [Google Scholar]
- 16.Yeager H, Azumi N, Underhill C B. Fibrosis: the formation of the granuloma matrix. In: Rom W N, Garay S, editors. Tuberculosis. New York, N.Y: Little, Brown & Co.; 1996. pp. 363–370. [Google Scholar]