Abstract
Thyroid hormones (THs) and TH receptor-beta (TRβ) reduce hepatic triglycerides, indicating a therapeutic potential for TH analogs in liver steatosis. To avoid adverse extrahepatic, especially TRα-mediated effects such as tachycardia and bone loss, TH analogs with combined TRβ and hepatocyte specificity are desired. MGL-3196 is a new TH analog that supposedly meets these criteria. Here, we characterize the thyromimetic potential of MGL-3196 in cell-based assays and address its cellular uptake requirements. We studied the contribution of liver-specific organic anion transporters (OATP)1B1 and 1B3 to MGL-3196 action. The TR isoform-specific efficacy of MGL-3196 compared with 3,5,3′-triiodothyronine (T3) was determined with luciferase assays and gene expression analysis in OATP1B1 and OATP1B3 and TRα- or TRβ-expressing cells and in primary murine hepatocytes (PMHs) from wild-type and TRβ knockout mice. We measured the oxygen consumption rate to compare the effects of MGL-3196 and T3 on mitochondrial respiration. We identified OATP1B1 as the primary transporter for MGL-3196. MGL-3196 had a high efficacy (90% that of T3) in activating TRβ, while the activation of TRα was only 25%. The treatment of PMHs with T3 and MGL-3196 at EC50 resulted in a similar induction of Dio1 and repression of Serpina7. In HEK293 cells stably expressing OATP1B1, MGL-3196 had comparable effects on mitochondrial respiration as T3. These data indicate that MGL-3196’s hepatic thyromimetic action, the basis for its therapeutic use, results from a combination of hepatocyte-specific transport by OATP1B1 and the selective activation of TRβ over TRα.
Keywords: MGL-3196, Resmetirom, thyromimetic, thyroid hormone action, thyroid hormone analog, thyroid hormone transport, OATP1B1
1. Introduction
Thyroid hormone (TH; T4, 3,5,3′,5′-tetraiodothyroxine, thyroxine, and its active form T3, 3,5,3′-triiodothyronine, thyronine) is essential for development and physiology, including hepatic triglyceride metabolism [1,2]. The uptake of T4 and T3 into target cells is mediated by plasma membrane transporter proteins. TH transporters are members of the monocarboxylate transporter family (e.g., MCT8 and MCT10), organic anion transporting polypeptides (OATPs), e.g., OATP1C1, and L-type amino acid transporters (e.g., LAT1 and LAT2) [3]. T3 acts by binding to the TH receptors (TRs) α and β that are expressed in virtually all cells. The TR isoform expression differs between cell types [4,5]. In hepatocytes, TRβ is the predominant TR isoform, while TRα is mainly expressed in cardiomyocytes and osteoblasts [4,6]. Therefore, local, cell-type-specific TH action is determined by TH transport into these cells and their TR isoform expression.
In the liver, local TRβ action lowers lipid content, thus offering a therapeutic potential of TH action in the treatment of non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). However, the systemic supra-physiological doses of TH lead to thyrotoxicosis with adverse effects on the cardiovascular system and the bone, mainly mediated by the activation of TRα. Therefore, TRβ agonists have been developed, e.g., GC-1 (Sobetirome), GC-24, KB-141, and KB-2115 (Eprotirome). Eprotirome was highly successful in reducing LDL cholesterol and triglycerides in patients with familial hypercholesterolemia in a phase 3 clinical trial. However, a significant increase in transaminases in patients and deleterious effects on the cartilage in dogs led to the termination of the clinical trial. In a phase 1 trial, Sobetirome treatment resulted in a reduction in LDL cholesterol by up to 41% but was terminated due to concerns over a class effect in light of the adverse effects of Eprotirome [7,8]. For these reasons, to date, no TRβ agonist obtained NIH approval despite its demonstrated beneficial effects on serum and hepatic lipid concentration [9,10]. The recent development of TH analogs aimed at better focusing TH action by combining hepatocyte specificity and TRβ selectivity, which would preserve the beneficial hepatic effects while reducing the extrahepatic adverse effects.
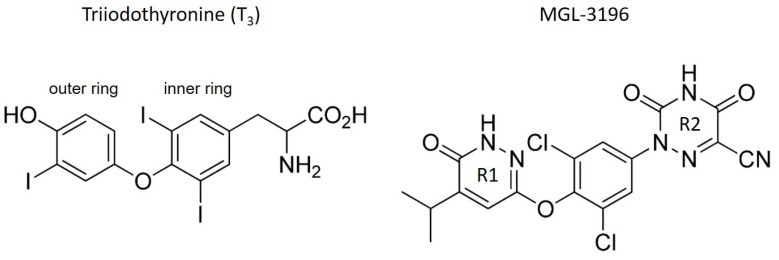
Presumably, MGL-3196 (also called VIA-3196 or Resmetirom) meets these criteria [11]. MGL-3196 is a pyridazinone-based TH analog with an azauracil group containing a cyano substitution that increases TRβ selectivity (Figure 1). In preclinical studies, the oral application of MGL-3196 reduced serum cholesterol without affecting bone mineral density in mice or cardiac α-MHC expression in thyroidectomized rats [11]. Recently, results from a phase 2 trial of MGL-3196 in NASH patients and from a 36-week active treatment open-label extension study have been reported [12,13]. Relative to the baseline, 74 patients treated with MGL-3196 for 36 weeks in the main study showed a 37.3% reduction in liver fat compared with 8.9% of the 34 placebo-treated patients, determined by MRI, and reduced liver fibrosis, as determined by liver biopsy. This was accompanied by a significant reduction in serum LDL cholesterol and triglyceride concentration. No adverse effects such as the suppression of thyroid stimulating hormone (TSH) concentration, reduction in bone mineral density, or increased heart rate were reported. These promising results from the phase 2 study led to a phase 3 study (MAESTRO-NASH; NCT03900429).
Figure 1.
Structures of triiodothyronine (T3) and MGL-3196. In comparison to the structure of T3, the phenolic outer ring is replaced by a pyridazinone ring (R1) in MGL-3196, resulting in a heterocyclic structure. Instead of the α-amino acid group, MGL-3196 possesses an azauracil residue with a cyano group (R2) ([11]).
MGL-3196 appears to have a favorable overall pharmacological profile with significant lipid-lowering efficacy in the absence of adverse effects. Presumably, this is due to a combination of TRβ and hepatocyte specificity, reducing extrahepatic, especially TRα-mediated, adverse effects.
In a cell-free co-factor binding assay, MGL-3196 selectivity for TRβ was determined to be 28-fold with an EC50 of 0.21 µM compared with 3.74 µM for TRα [11]. Another study found a 12.5-fold selectivity for TRβ over TRα using HEK293T cells overexpressing retinoic X receptor-α (RXRα) in combination with either TRα or TRβ [14]. This study also revealed high variations in EC50 values between different cell lines, possibly explained by differences in MGL-3196 cellular uptake. The transport of TH and TH derivatives is facilitated by OATPs. Interestingly, the expression of OATP1B1 and OATP1B3 is restricted to the liver [3,15,16]. It was, therefore, hypothesized that MGL-3196 could be a substrate for OATP1B1 and OATP1B3, which would explain MGL-3196’s hepatocyte-specific action [14]. However, the requirement of OATP1B1 and/or OATP1B3 for the transport of MGL-3196 has not been studied to date. Studies on MGL-3196 action were mostly carried out either in cell-free systems or in HEK293 cells that do not express OATP1B1 or OATP1B3. We aimed to study the contribution of hepatocyte-specific OATP1B1 and OATP1B3 to MGL-3196 action and its TR isoform selectivity in a human cell model and primary murine hepatocytes.
2. Results and Discussion
2.1. Expression of TH Transporters and TRs in HEK293 Cells
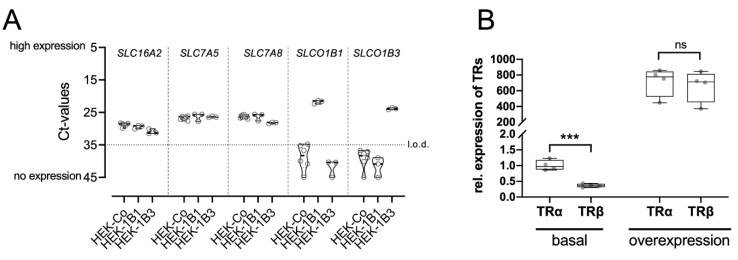
To determine the role of hepatocyte-specific transporters in MGL-3196 action, we studied wild-type HEK293 cells in comparison to HEK293 cells stably expressing the hepatocyte-specific transporters OATP1B1 (HEK-1B1) and OATP1B3 (HEK-1B3). First, we measured the endogenous expression of OATP1B1, OTAP1B3, and TH transporters MCT8, LAT1, and LAT2 [17,18,19]. MCT8, LAT1, and LAT2 were all expressed in all three cell lines. OATP1B1 and B3 were not expressed in the wild-type HEK cells and were detected only in the respective stably transfected cell line (Figure 2A).
Figure 2.
Expression of TH transporters and TRs in HEK293 cell lines: (A) RT-PCR analysis of genes encoding for TH transporters MCT8 (SLC16A2), LAT1 (SLC7A5), and LAT2 (SLC7A8) in wild-type HEK293 cells (HEK-Co) as well as in HEK-1B1 and HEK-1B3 cells stably expressing OATP1B1 (SLCO1B1) or OATP1B3 (SLCO1B3), respectively. The dotted line marks the limit of detection (l.o.d.) at Ct ≥ 35. Ct = cycle threshold; LAT = l-type amino acid transporter; MCT = monocarboxylate transporter; (B) expression of TRα and TRβ in HEK293 cells. Transient transfection led to similar expressions of both receptors. Student’s t-test: ns = not significant; *** p < 0.001.
Next, we determined the expression of TRα and TRβ in HEK293 cells and found a threefold higher expression of TRα compared with TRβ (Figure 2B). This TRα:TRβ ratio is comparable to that found in human and mouse kidneys [4,5]. To overcome this difference, we transiently transfected the three cell lines with plasmids encoding for TRα and TRβ. This significantly increased the expression levels of both receptors and equalized their expression (Figure 2B).
2.2. MGL-3196 Transport Is Mediated by OATP1B1
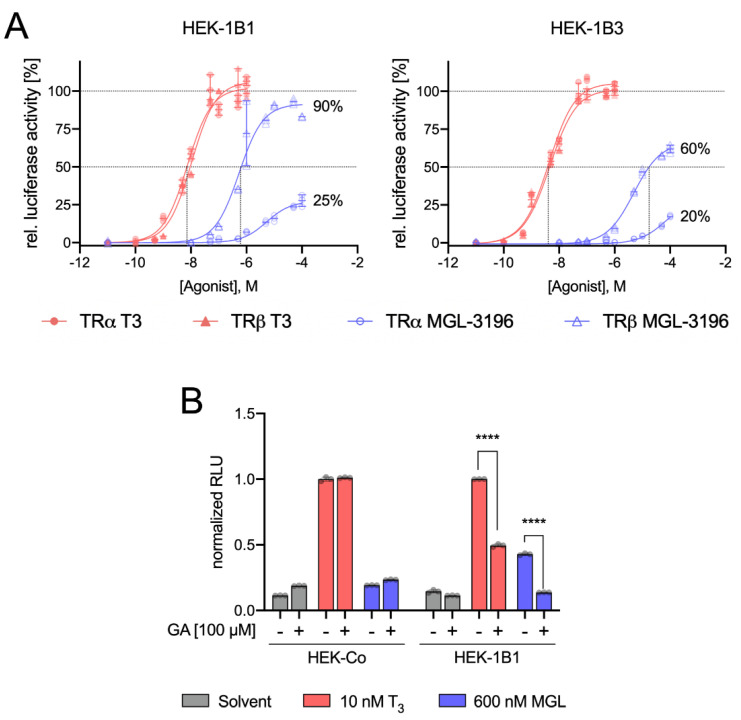
We used a DR4 luciferase reporter assay to assess MGL-3196 action in HEK-1B1 and HEK-1B3 cells either overexpressing TRα or TRβ in comparison to T3 [20]. For T3, we used concentrations ranging from 0.1 to 1000 nM and MGL-3196 concentrations from 10 to 10,000 nM. Luciferase activity was increased by T3 in all cell lines. While there was no difference in the T3-mediated increase in the luciferase action of OATP1B1- or OATP1B3-expressing cells, treatment with MGL-3196 resulted in a stronger increase in luciferase activity in HEK-1B1 cells than in HEK-1B3 cells (Figure 3A), indicating that OATP1B1 is the preferred transporter for MGL-3196.
Figure 3.
Dose-response curve and luciferase activity: (A) EC50 values were determined by DR4 luciferase reporter assay in cells stably expressing OATP1B1 or OATP1B3 (HEK-1B1 and HEK-1B3, respectively). Additionally, the cells were transfected with plasmids either encoding for TRα (circles) or TRβ (triangles). Cells were cultured in TH-depleted serum and treated with T3 (0.1–1000 nM; red-filled triangles or circles) or MGL-3196 (10–10,000 nM; blue open triangles or circles) for 48 h; (B) inhibition of OATP1B1-dependent uptake of MGL-3196 was determined in a DR4 luciferase reporter assay with HEK-Co (control) cells and HEK-1B1 cells (stably expressing OATP1B1) and 100 µM GA (glycyrrhizinic acid) as OATP1B1 inhibitor. RLU in T3-treated cells without GA = 1.00 in each cell line. Two-way ANOVA with Tukey’s post hoc test: **** p < 0.0001.
To confirm the relevance of OATP1B1 for MGL-3196 transport, we used the OATP1B1 inhibitor glycyrrhizinic acid (GA) [21,22]. Pretreatment with 100 µM GA had no effect on the luciferase signal in T3-treated wild-type HEK cells that did not express OAPT1B1, demonstrating that GA does not inhibit T3 uptake in the absence of OATP1B that is mediated by MCT8, LAT1, and LAT2. However, in HEK-1B1 cells, the T3 effect was significantly reduced by GA pretreatment (Figure 3B), demonstrating that OATP1B1 contributes to T3 transport, which is in accordance with previous reports [3,16,23]. Importantly, MGL-3196 induced a luciferase signal only in HEK-1B1 cells but not in wild-type cells. This induction was completely abolished by GA pretreatment (Figure 3B).
From these data, we conclude that MGL-3196 is predominantly transported by OATP1B1, while OATP1B3 and other TH transporters (e.g., MCT8, LAT1, and LAT2) contribute little at the studied doses. OATP1B1 differs from other members of the OATP family in its exclusive expression in hepatocytes. Therefore, the requirement of OATP1B1 for the cellular action of MGL-3196 possibly explains the high liver specificity of MGL-3196 [24]. These results cannot exclude a possible transport of MGL-3196 by other transporters, e.g., OATP1C1 and SLC17A4, into other cells outside the liver. However, no extrahepatic adverse effects of MGL-3196, e.g., on TSH serum concentrations in probands or patients [11,25] or the heart and kidney weight in mice have been reported so far [26].
2.3. MGL-3196 Predominantly Acts via TRβ
The activation of TRα and TRβ by T3 resulted in almost superimposable dose–response curves for both receptors, demonstrating that T3 is not a selective TR agonist, whereas MGL-3196 induced a stronger response with TRβ (Figure 3A). Compared with T3, the activation of TRβ reached 90% in HEK-1B1 and 60% in HEK-1B3 cells, respectively, compared with 25% and 20% for TRα (Figure 3A). Based on the T3-mediated receptor activation, we calculated the EC50 values of MGL-3196 in OATP1B1- and OATP1B3-expressing cells [27]. In HEK-1B1 cells, the EC50 of MGL-3196 for TRβ was 0.601 µM, compared with 0.007 µM for T3 (Table 1), which illustrates that in those cells expressing OATP1B1, MGL-3196 is about 100-fold less potent than T3. Interestingly, in OATP1B3-expressing cells, the EC50 value of MGL-3196 for TRβ was 17,660 µM, which is about 30 times higher than in OATP1B1-expressing cells, further demonstrating that OATP1B1 and not OATB1B3 is the predominant transporter for MGL-3196. Remarkably, in this cell-based luciferase reporter assay, MGL-3196 failed to reach the 50% activation threshold in TRα-overexpressing cells, precluding the calculation of EC50 values for MGL-3196 and TRα, which further demonstrates the much higher affinity of MGL-3196 to TRβ over TRα.
Table 1.
EC50 values for T3 and MGL-3196.
| TR Isoform | Transporter | log(EC50) ± SD | EC50 [µM] | |
|---|---|---|---|---|
| T3 | TRα | OATP1B1 | −8.091 ± 0.043 | 0.007 |
| OATP1B3 | −8.441 ± 0.030 | 0.004 | ||
| TRβ | OATP1B1 | −8.059 ± 0.031 | 0.009 | |
| OATP1B3 | −8.356 ± 0.022 | 0.004 | ||
| MGL-3196 | TRα | OATP1B1 | - | - |
| OATP1B3 | - | - | ||
| TRβ | OATP1B1 | −6.221 ± 0.046 | 0.601 | |
| OATP1B3 | −4.753 ± 0.013 | 17.660 |
The EC50 value of MGL-3196 in HEK-1B1 cells was about three to nine times higher than previously reported in two independent studies (601 nM vs. 210 nM [11] and 73.1 nM [14]). In these studies, a cell-free FRET assay was used to determine the EC50 values. The probable explanation for this difference is the additional requirement of the cellular import of MGL-3196 in our cell-based luciferase reporter assay, which more closely resembles the physiological situation. Luong et al. also determined the EC50 values for MGL-3196 with luciferase assays in wild-type HEK293T cells transiently expressing TRβ and found a fourfold higher EC50 (2365.8 nM) than that found in our experiments [14]. This difference reflects the absence of the transporter OATP1B1 and demonstrates that transport needs to be considered for the action of TH analogs such as MGL-3196. Furthermore, in Huh-7 cells (established from a human well-differentiated hepatocyte-derived carcinoma cell line), the EC50 value was much lower at 303.1 ± 50.9 nM and closer to the EC50 values determined in HEK-1B1 cells. Interestingly, Huh-7 cells express OATP1B1 [28], which again underscores the importance of transport for MGL-3196 action [11,14].
2.4. Equivalent Induction of TH Target Genes by MGL-3196 and T3
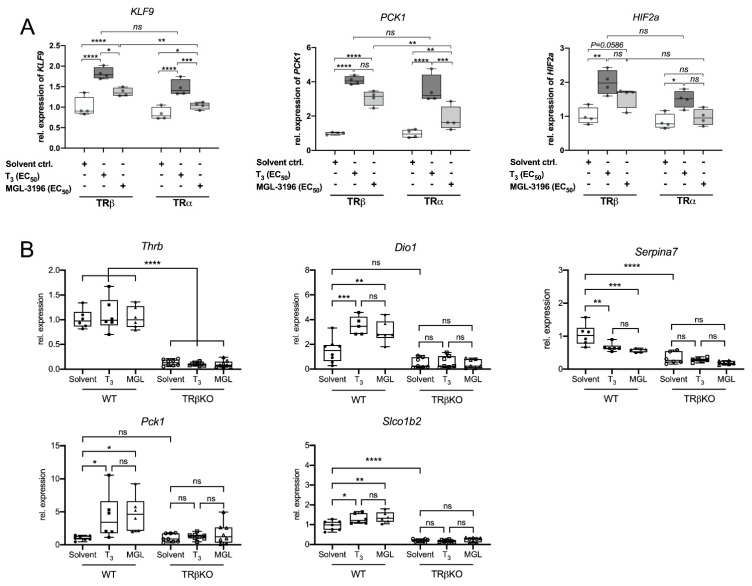
Next, we compared the MGL-3196- and T3-mediated induction of endogenous TH target genes in HEK-1B1 cells overexpressing either TRβ or TRα. The cells were treated with the EC50 values of T3 (10 nM) and MGL-3196 (600 nM) for 24 h. The expression of krüppel-like factor 9 (KLF9) and phosphoenolpyruvate carboxykinase 1 (PCK1) was similarly increased by T3 in TRβ- and TRα-overexpressing cells (Figure 4A). MGL-3196 treatment led to the significantly stronger induction of KLF9 and PCK1 in the cells overexpressing TRβ than in those overexpressing TRα, which demonstrates its TRβ selectivity [11].
Figure 4.
Gene expression analysis in HEK-1B1 cells and primary murine hepatocytes: (A) HEK-1B1 cells either expressing TRβ or TRα were treated with 10 nM T3 (dark grey) or 600 nM MGL-3196 (light grey) for 48 h and expression of TH target genes KLF9 (krüppel-like factor 9), PCK1 (pyruvate carboxylase kinase 1) and HIF2a (hypoxia-inducible factor 2a) was measured (n = 3); (B) primary murine hepatocytes of WT and TRβKO mice were treated with EC50 of T3 or MGL-3196 for 24 h and expression of hepatic TH target genes was analyzed by using RT-PCR. MGL-3196 and T3 induced similar changes in gene expression of Dio1, Serpina7, and Pck1 (n = 6). Both T3 and MGL-3196 led to an increased expression of Slco1b2, the murine orthologue of the human SLCO1B1 and SLCO1B3. One-way ANOVA with Tukey’s post hoc test: ns = not significant; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
To verify these results in a more physiological model, we used the primary murine hepatocytes (PMHs) from wild-type C57BL6/J mice and TRβ−/− mice from the same genetic background. The thyromimetic action of MGL-3196 was compared with that of T3 in PMHs treated for 24 h with the EC50 of T3 and MGL-3196. T3 and MGL-3196 led to an equivalent induction of Dio1 and Pck1 as well as the repression of the negatively regulated TH target gene Serpina7 (Figure 4B). Neither T3 nor MGL-3196 led to a transcriptional regulation in PMH of TRβ−/− mice.
Notably, human cells express two OAPT1B isoforms (1B1 and 1B3), whereas in mice, only one isoform exists. The common murine orthologue of OATP1B1 and OATP1B3 is Oatp1b2, encoded by Slco1b2 [24]. Interestingly, the expression of Slco1b2 was induced by T3 and by MGL-3196 in a TRβ-dependent manner (Figure 4B). Moreover, in PMHs from TRβ−/− cells, the expression of Slco1b2 was significantly decreased, independent of the treatment regime, suggesting a strong TRβ-mediated influence on Slco1b2 expression. Therefore, testing for residual TRα-mediated off-target effects of MGL-3196 in TRβ−/− cells is not possible, as a lack of Oatp1b2 expression prevents proper MGL-3196 uptake. However, the induction of Slco1b2 expression by T3 and MGL-3196 suggests the possibility of a positive feedback loop of TH and MGL-3196 uptake into hepatocytes, which would further increase MGL-3196 accumulation in hepatocytes and, consequently, enhance its hepatocyte specificity.
2.5. MGL-3196 Improves Mitochondrial Function
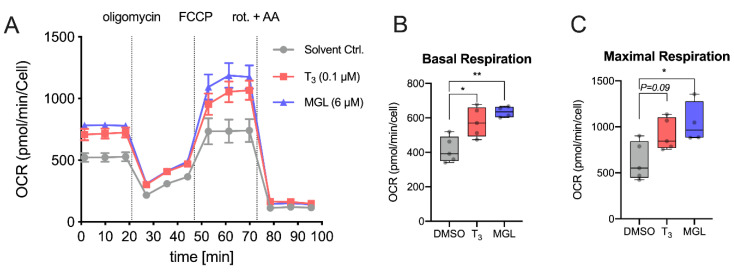
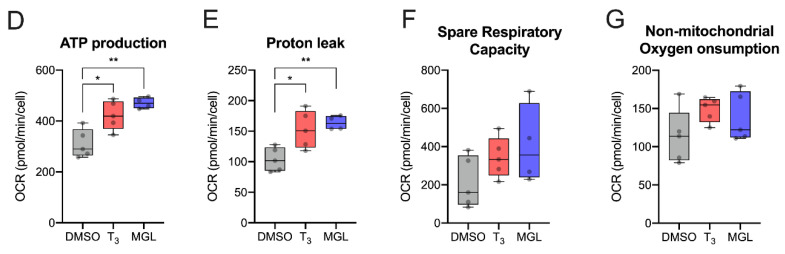
T3 increases mitochondrial function in HepG2 cells [29]. As HepG2 cells do not express OATP1B1 [28], we used the HEK-1B1 cell line to study whether MGL-3196 activates mitochondrial function as T3. HEK-1B1 cells were treated with a receptor-saturating dose of 100 nM T3 and 6 µM MGL-3196 for 48 h, and the oxygen consumption rate (OCR) was measured (Figure 5A). Both T3 and MGL-3196 increased basal respiration, maximal respiration, ATP production, and proton leak (Figure 5B–E). The increased basal respiration accounts for a higher energy demand of the cells that is accompanied by an increase in ATP production. The increased maximum rate of respiration in T3- and MGL-3196-treated cells reflects a higher oxidation rate of substrates and thus an increased metabolic rate. The increased proton leak could indicate mitochondrial damage. The spare respiration capacity and non-mitochondrial oxygen consumption rate were not significantly increased (Figure 5F,G). This analysis of oxygen consumption rate and respiration demonstrates that MGL-3196 functionally mimics T3.
Figure 5.
MGL-3196 increases mitochondrial activity in OATP1B1-expressing cells: (A) measurement of oxygen consumption rate (OCR) in HEK-1B1 cells treated for 48 h with T3 (100 nM) or MGL-3196 (6 µM) (n = 5 biological replicates per group); (B) basal OCR ([last rate measurement before oligomycin injection] − [non-mitochondrial respiration rate]); (C) maximal respiration ([maximum rate measured after FCCP injection] − [non-mitochondrial respiration rate]); (D) ATP production ([last rate measurement before oligomycin injection] − [minimum rate measured after oligomycin injection]); (E) proton leak ([minimum rate measured after oligomycin injection] − [non-mitochondrial respiration rate]); (F) spare respiratory capacity ([maximal respiration] − [basal respiration]); (G) non-mitochondrial oxygen consumption (minimum rate measured after rotenone/antimycin A injection). One-way ANOVA with Tukey’s post hoc test: ns = not significant; * p < 0.05, ** p < 0.01. FCCP, carbonyl cyanide-p-trifluoromethoxyphenylhydrazone; rot., rotenone; AA, antimycin A.
3. Conclusions
THs harbor therapeutic potential for liver diseases such as NAFLD or NASH. Yet, as THs act in virtually all organs and cells, the challenge is to harness the desired beneficial TRβ-mediated effects on liver lipid metabolism while avoiding adverse effects on the cardiovascular system and bone turnover, mostly mediated by TRα [10,30,31]. This could be achieved with a hepatocyte-specific TRβ agonist. The present data show that MGL-3196 action requires the expression of OAT1B1, a hepatocyte-specific OATP. Furthermore, the activation of TRβ by MGL-3196 reached 90% of that of T3 compared with only 25% with TRα, demonstrating MGL-3196’s TR isoform-specific efficacy. MGL-3196 elicits the same effects as T3 on gene expression and cell metabolism, stimulating respiration and ATP production. Therefore, the present results demonstrate that the local, hepatic thyromimetic action of MGL-3196 is a result of hepatocyte-specific transport and high TRβ specificity. These features serve to explain the efficacy of reducing hepatic lipid content in patients and the absence of extrahepatic adverse results in clinical studies. MGL-3196 is an example of how local control of TH action, here achieved through transport and receptor specificity, allows for the therapeutic use of TH action.
4. Material and Methods
4.1. Chemicals
T3 (3,3′,5-triiodo-l-thyronine sodium salt; Merk, Darmstadt, Germany) and MGL-3196 (Cayman Chemical, Ann Arbor, MI, USA) were both dissolved in dimethyl sulfoxide (DMSO; Sigma Aldrich, Darmstadt, Germany) at a concentration of 10 mM. Glycyrrhizinic acid (GA; Sigma Aldrich, Darmstadt, Germany) was dissolved in DMSO at a concentration of 50 mM.
4.2. Cell Culture and Transfection
Human embryonic kidney cells (HEK293) stably overexpressing human OATP1B1 (HEK-1B1) and OATP1B3 (HEK-1B3) were contributed by Dr. Jörg König, and their generation has been described previously [32]. The cells were maintained in a DMEM containing 4.5 g/L glucose, 1% pyruvate (DMEM+ GlutaMAX, Gibco™, Thermo Fisher Scientific, Schwerte, Germany), 1% ZellShield (Minerva Biolabs GmbH, Berlin, Germany), and 10% FCS (Fetal Calf Serum, Gibco™, Thermo Fisher Scientific, Schwerte, Germany) supplemented with 800 µg/mL G418 (Capricorn Scientific, Ebsdorfergrund, Germany) [33]. Only those cells in a passage number below 7 were used for the experiments. Transient transfection with expression vectors for TRα and TRβ was performed as previously described [20], with few modifications. Briefly, polyethyleneimine (Pei, Polyscience, Hirschberg an der Bergstraße, Germany) was used as a transfection reagent in a Pei/DNA ratio of 3:1 using a total amount of 0.5 µg DNA/24-well and 2 µg DNA/6-well.
4.3. Luciferase Assay
HEK293 cells were grown to 60–80% confluence on a 24-well plate. The transient transfections of DR4-Luc reporter plasmid, RL-TK control plasmid, and plasmids encoding for TRα, TRβ, or empty vector pcDNA3 were carried out as previously described [20]. After transfection, cells were maintained under hypothyroid conditions for another 24 h using 5% TH-depleted FCS. TH-depleted FCS was generated through treatment with anion-exchange resin, as previously described [34,35]. The cells were stimulated with 0.1 Nm–1 µM T3 or 10 nM–100 µM MGL-3196 for 24 h, respectively.
The highest concentration of DMSO was used as a solvent control. The cells were harvested, and the activities of firefly and renilla luciferases were determined (Dual-Glo Luciferase Assay System; Promega, Mannheim, Germany) in a Sirius luminometer (Berthold Detection Systems GmbH, Pforzheim, Germany). Firefly luciferase luminescence was normalized to renilla luciferase luminescence from the same transfection sample to control for differences in transfection efficiency.
OATP1B1-mediated transport was inhibited with the competitive inhibitor glycyrrhizinic acid (GA). HEK-Co and HEK-1B1 cell lines were transiently transfected with a plasmid encoding for TRβ and cultured under TH-free conditions. The cells were treated with 100 µM GA 30 min prior to treatment with T3 (10 nM) or MGL-3196 (600 nM). Luciferase expression was determined after 24 h as described above.
4.4. Isolation and Cultivation of Primary Murine Hepatocytes
Primary murine hepatocytes (PMHs) were isolated from WT and TRβ knockout (TRβKO) mice through a 2-step perfusion procedure of the livers of 8–10-week-old male mice via the portal vein [36]. After perfusion with 37 °C prewarmed HBSS without Ca2+ and Mg2+ (Gibco™, Thermo Fisher Scientific, Schwerte Germany) containing 0.5 mM EGTA (Sigma Aldrich, Darmstadt, Germany), the livers were digested with 2 mg/mL collagenase II (Worthington Biochemical, Lakewood, NJ, USA) in a DMEM (low-glucose) (Thermo Fisher Scientific Gibco, Schwerte, Germany) supplemented with 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 15 mM HEPES (Sigma Aldrich, Darmstadt, Germany). After the excision of the liver and mechanical disruption, the liver cell suspension was filtered through a 70 µm cell strainer and centrifuged for 2 min at 50× g. Viability was determined using a trypan blue exclusion test. Hepatocytes were seeded on collagen-coated 6-well plates at a density of 3.5 × 105 cells/mL in a 1:1 mixture of William’s E Medium (Thermo Fisher Scientific Gibco, Schwerte, Germany) and low-glucose DMEM supplemented with penicillin–streptomycin, 1 µM insulin (Sigma Aldrich, Darmstadt, Germany), 1 µM dexamethasone (Sigma Aldrich, Darmstadt, Germany), and 10% of TH-depleted FCS. PMHs were treated with the respective doses of T3 and MGL-3196 for 24 h.
4.5. Gene Expression Analysis
Briefly, 8 × 105 HEK-1B1 cells were seeded in a 6-well dish and grown to 60–80% confluence overnight. Transfection with the plasmids encoding for TRα or TRβ was carried out the next day as described above. After 24 h in the TH-depleted medium, HEK-1B1 cells were treated with the EC50 of T3 and MGL-3196 for another 24 h. PMHs were treated with the EC50 of T3 and MGL-3196 directly after 4 h of attachment for 24 h. The total RNA was isolated from HEK-1B1 and PMH using a QIAGEN RNeasy Mini Kit (QIAGEN, Hilden, Germany). The concentration and purity of the RNA samples were measured using a NanoDrop2000 device (Thermo Fisher Scientific, Schwerte, Germany). cDNA synthesis real-time PCR was performed on a LightCycler480 (Roche, Mannheim, Germany) [20]. For the normalization of gene expression, 18S, Ppia, β-Actin, and Rpl13a were used as reference genes. Primer sequences are provided in Table 2.
Table 2.
qRT-PCR primer sequences.
| Gene | Forward Primer 5′-->3′ | Reverse Primer 5′-->3′ | Accession No. | |
|---|---|---|---|---|
| human | 18S | CGG CTA CCA CAT CCA AGG AA | GCT GGA ATT ACC GCG GCT | NR_145820.1 |
| ACTB | AGA GCT ACG AGC TGC CTG AC | AGC ACT GTG TTG GCG TAC AG | NM_001101.5 | |
| PPIA | AAC GTG GTA TAA AAG GGG CGG | CTG CAA ACA GCT CAA AGG AGA C | NM_021130.5 | |
| SLCO1B1 | CAC TTG CAC TGG GTT TCC AC | AAG CCC AAG TAG ACC CTT GAA A | NM_006446.5 | |
| SLCO1B3 | TTT TTG GAA GGG TCT ACT TGG G | TCA TTG TCC GAT GCC TTG GTA | NM_019844.4 | |
| PCK-1 | GGA GAA GGA GGT GGAAGA C | GAA CAC TTG CCC TCT CTT GC | NM_002591.4 | |
| KLF9 | CTC CCA TCT CAA AGC CCA TTA C | TGA GCG GGA GAA CTT TTT AAG G | NM_001206.2 | |
| SLC16A2 | AGC TCC TTC ACC AGC TCC CTA AGC | TCC TCC ACA TAC TTC ATC AGG TG | NM_006517.5 | |
| SLC7A5 | GGG AAG GGT GAT GTG TCC AAT CTA | CAA GTA ATT CCA TCC TCC | NM_003486.7 | |
| SLC7A8 | GTG CTA TCA TCG TAG GGA AGA TC | CAC AGC CTC AGG AAC CCA G | NM_012244.4 | |
| murine | 18s | CGG CTA CCA CAT CCA AGG AA | GCT GGA ATT ACC GCG GCT | NR_003278.3 |
| Ppia | CTT GGG CCG CGT CTC CTT CG | GCG TGT AAA GTC ACC ACC CTG GC | NM_008907.2 | |
| Actb | GGC CCA GAG CAA GAG AGG TA | CTG GAT GGC TAC GTA CAT GGC | NM_007393.5 | |
| Rpl13a | GGG CAG GTT CTG GTA TTG GA | GGG GTT GGT ATT CAT CCG CT | NM_009438.5 | |
| Slco1b2 | ATC GGA CCA ATC CTT GGC TTT | TTA TGC GGA CAC TTC TCA GGT | NM_020495.2 | |
| Pck-1 | ATC TTT GGT GGC CGT AGA CC | ATC TTG CCC TTG TGT TCT GC | NM_011044.3 | |
| Dio1 | GGG CAG GAT CTG CTA CAA GG | CGT GTC TAG GTG GAG TGC AA | NM_007860.4 | |
| Serpina7 | TGG GCA TGT GCT ATC ATC TTC A | GAG TGG CAT TTT GTT GGG GC | NM_177920.5 | |
| Thrb | GGA CAA GCA CCC ATC GTG AA | ACA TGG CAG CTC ACA AAA CAT | NM_001113417.1 |
4.6. Seahorse XF-24 Mito Stress Test
The oxygen consumption rate (OCR) was measured with an XF24 extracellular analyzer (Seahorse Bioscience, North Billerica, MA, USA). Briefly, 7.5 × 103 HEK-1B1 cells were seeded in poly-l lysine-coated XF24 culture plates and cultured in a DMEM with 10% TH-depleted serum for 4 days. The cells were treated with T3 (100 nM) or MGL-3196 (6 µM) 48 h before OCR measurement. Reagent concentration was optimized using a Mito Stress Test kit from Seahorse Bioscience (103015-100) according to the protocol and the XF24 program’s algorithm. Oligomycin, FCCP, antimycin A, and rotenone were all used at a concentration of 1 µM. On the day of measurement, the TH-depleted medium was replaced by 500 µL of the assay medium, and the plate was incubated at 37 °C for 1 h in a non-CO2 incubator.
Basal respiration was calculated as [baseline O2 consumption] − [OCR after rotenone and antimycin A]. ATP production corresponds to the OCR used for mitochondrial ATP synthesis via ATP synthetase, which is inhibited by oligomycin. The expression [OCR after FCCP] − [OCR after rotenone and antimycin A] determined the maximal respiration (respiratory capacity), and spare respiratory capacity was calculated as [OCR after FCCP] − [Basal OCR].
4.7. Statistics
We used one-way ANOVA with Tukey’s post hoc test for the statistical analysis of normally distributed datasets unless otherwise noted. Differences were considered significant when p < 0.05. For gene expression data, statistical significance was calculated using log-transformed data (to obtain normal distribution) as recommended by the MIQE guidelines [37]. Data were analyzed and plotted with GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA).
Acknowledgments
We thank Andrea Jaeger, Stefanie Rehn, Julius Göbel, and Andrea Odersky for their dedicated technical assistance.
Author Contributions
Conceptualization, G.S.H. and L.C.M.; methodology, G.S.H., L.C.M., and J.K.; validation, L.C.M., G.S.H., R.G.S., and C.H.; formal analysis, G.S.H.; investigation, G.S.H., R.G.S., and C.H.; resources, J.K., L.C.M., and D.F.; data curation, G.S.H., R.G.S., and C.H.; writing—original draft preparation, G.S.H., R.G.S., and L.C.M.; writing—review and editing, L.C.M., G.S.H., J.K., C.H., and D.F.; visualization, G.S.H.; supervision, L.C.M.; project administration, L.C.M. and D.F.; funding acquisition, L.C.M. and D.F. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
L.C.M. and D.F. are funded by Deutsche Forschungsgemeinschaft (DFG)—Project-ID 424957847-TRR 296. L.C.M. was also funded by the Faculty of Medicine, University of Duisburg-Essen (IFORES).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sinha R.A., Singh B.K., Yen P.M. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat. Reviews. Endocrinol. 2018;14:259–269. doi: 10.1038/nrendo.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araki O., Ying H., Zhu X.G., Willingham M.C., Cheng S.Y. Distinct dysregulation of lipid metabolism by unliganded thyroid hormone receptor isoforms. Mol. Endocrinol. 2009;23:308–315. doi: 10.1210/me.2008-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groeneweg S., van Geest F.S., Peeters R.P., Heuer H., Visser W.E. Thyroid Hormone Transporters. Endocr. Rev. 2019;41:146–201. doi: 10.1210/endrev/bnz008. [DOI] [PubMed] [Google Scholar]
- 4.Bookout A.L., Jeong Y., Downes M., Yu R.T., Evans R.M., Mangelsdorf D.J. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minakhina S., Bansal S., Zhang A., Brotherton M., Janodia R., De Oliveira V., Tadepalli S., Wondisford F.E. A Direct Comparison of Thyroid Hormone Receptor Protein Levels in Mice Provides Unexpected Insights into Thyroid Hormone Action. Thyroid. 2020;30:1193–1204. doi: 10.1089/thy.2019.0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvey C.B., O’Shea P.J., Scott A.J., Robson H., Siebler T., Shalet S.M., Samarut J., Chassande O., Williams G.R. Molecular mechanisms of thyroid hormone effects on bone growth and function. Mol. Genet. Metab. 2002;75:17–30. doi: 10.1006/mgme.2001.3268. [DOI] [PubMed] [Google Scholar]
- 7.Elbers L.P., Kastelein J.J., Sjouke B. Thyroid Hormone Mimetics: The Past, Current Status and Future Challenges. Curr. Atheroscler. Rep. 2016;18:14. doi: 10.1007/s11883-016-0564-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scanlan T.S. Sobetirome: A case history of bench-to-clinic drug discovery and development. Heart Fail. Rev. 2010;15:177–182. doi: 10.1007/s10741-008-9122-x. [DOI] [PubMed] [Google Scholar]
- 9.Vatner D.F., Weismann D., Beddow S.A., Kumashiro N., Erion D.M., Liao X.H., Grover G.J., Webb P., Phillips K.J., Weiss R.E., et al. Thyroid hormone receptor-beta agonists prevent hepatic steatosis in fat-fed rats but impair insulin sensitivity via discrete pathways. Am. J. Physiology. Endocrinol. Metab. 2013;305:E89–E100. doi: 10.1152/ajpendo.00573.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kowalik M.A., Columbano A., Perra A. Thyroid Hormones, Thyromimetics and Their Metabolites in the Treatment of Liver Disease. Front. Endocrinol. 2018;9:382. doi: 10.3389/fendo.2018.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly M.J., Pietranico-Cole S., Larigan J.D., Haynes N.E., Reynolds C.H., Scott N., Vermeulen J., Dvorozniak M., Conde-Knape K., Huang K.S., et al. Discovery of 2-[3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl]-3,5-dio xo-2,3,4,5-tetrahydro[1,2,4]triazine-6-carbonitrile (MGL-3196), a Highly Selective Thyroid Hormone Receptor beta agonist in clinical trials for the treatment of dyslipidemia. J. Med. Chem. 2014;57:3912–3923. doi: 10.1021/jm4019299. [DOI] [PubMed] [Google Scholar]
- 12.Harrison S.A., Bashir M.R., Guy C.D., Zhou R., Moylan C.A., Frias J.P., Alkhouri N., Bansal M.B., Baum S., Neuschwander-Tetri B.A., et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394:2012–2024. doi: 10.1016/S0140-6736(19)32517-6. [DOI] [PubMed] [Google Scholar]
- 13.Harrison S.A., Bashir M., Moussa S.E., McCarty K., Pablo Frias J., Taub R., Alkhouri N. Effects of Resmetirom on Noninvasive Endpoints in a 36-Week Phase 2 Active Treatment Extension Study in Patients With NASH. Hepatol. Commun. 2021;5:573–588. doi: 10.1002/hep4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luong X.G., Stevens S.K., Jekle A., Lin T.I., Gupta K., Misner D., Chanda S., Mukherjee S., Williams C., Stoycheva A., et al. Regulation of gene transcription by thyroid hormone receptor β agonists in clinical development for the treatment of non-alcoholic steatohepatitis (NASH) PLoS ONE. 2020;15:e0240338. doi: 10.1371/journal.pone.0240338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obaidat A., Roth M., Hagenbuch B. The expression and function of organic anion transporting polypeptides in normal tissues and in cancer. Annu. Rev. Pharmacol. Toxicol. 2012;52:135–151. doi: 10.1146/annurev-pharmtox-010510-100556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abe T., Kakyo M., Tokui T., Nakagomi R., Nishio T., Nakai D., Nomura H., Unno M., Suzuki M., Naitoh T., et al. Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1. J. Biol. Chem. 1999;274:17159–17163. doi: 10.1074/jbc.274.24.17159. [DOI] [PubMed] [Google Scholar]
- 17.Kinne A., Wittner M., Wirth E.K., Hinz K.M., Schulein R., Kohrle J., Krause G. Involvement of the L-Type Amino Acid Transporter Lat2 in the Transport of 3,3’-Diiodothyronine across the Plasma Membrane. Eur. Thyroid J. 2015;4:42–50. doi: 10.1159/000381542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trajkovic M., Visser T.J., Mittag J., Horn S., Lukas J., Darras V.M., Raivich G., Bauer K., Heuer H. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J. Clin. Invest. 2007;117:627–635. doi: 10.1172/JCI28253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Visser W.E., Friesema E.C., Visser T.J. Minireview: Thyroid hormone transporters: The knowns and the unknowns. Mol. Endocrinol. 2011;25:1–14. doi: 10.1210/me.2010-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hones G.S., Rakov H., Logan J., Liao X.H., Werbenko E., Pollard A.S., Praestholm S.M., Siersbaek M.S., Rijntjes E., Gassen J., et al. Noncanonical thyroid hormone signaling mediates cardiometabolic effects in vivo. Proc. Natl. Acad. Sci. USA. 2017;114:E11323–E11332. doi: 10.1073/pnas.1706801115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong J., Olaleye O.E., Jiang R., Li J., Lu C., Du F., Xu F., Yang J., Wang F., Jia W., et al. Glycyrrhizin has a high likelihood to be a victim of drug-drug interactions mediated by hepatic organic anion-transporting polypeptide 1B1/1B3. Br. J. Pharmacol. 2018;175:3486–3503. doi: 10.1111/bph.14393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishida K., Ullah M., Tóth B., Juhasz V., Unadkat J.D. Transport Kinetics, Selective Inhibition, and Successful Prediction of In Vivo Inhibition of Rat Hepatic Organic Anion Transporting Polypeptides. Drug Metab. Dispos. Biol. Fate Chem. 2018;46:1251–1258. doi: 10.1124/dmd.118.080770. [DOI] [PubMed] [Google Scholar]
- 23.van der Deure W.M., Friesema E.C., de Jong F.J., de Rijke Y.B., de Jong F.H., Uitterlinden A.G., Breteler M.M., Peeters R.P., Visser T.J. Organic anion transporter 1B1: An important factor in hepatic thyroid hormone and estrogen transport and metabolism. Endocrinology. 2008;149:4695–4701. doi: 10.1210/en.2008-0169. [DOI] [PubMed] [Google Scholar]
- 24.Roth M., Obaidat A., Hagenbuch B. OATPs, OATs and OCTs: The organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br. J. Pharmacol. 2012;165:1260–1287. doi: 10.1111/j.1476-5381.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taub R., Chiang E., Chabot-Blanchet M., Kelly M.J., Reeves R.A., Guertin M.C., Tardif J.C. Lipid lowering in healthy volunteers treated with multiple doses of MGL-3196, a liver-targeted thyroid hormone receptor-beta agonist. Atherosclerosis. 2013;230:373–380. doi: 10.1016/j.atherosclerosis.2013.07.056. [DOI] [PubMed] [Google Scholar]
- 26.Caddeo A., Kowalik M.A., Serra M., Runfola M., Bacci A., Rapposelli S., Columbano A., Perra A. TG68, a Novel Thyroid Hormone Receptor-β Agonist for the Treatment of NAFLD. Int. J. Mol. Sci. 2021;22:13105. doi: 10.3390/ijms222313105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Black J.W., Leff P. Operational models of pharmacological agonism. Proc. R. Soc. Lond. B Biol. Sci. 1983;220:141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- 28.Louisa M., Suyatna F.D., Wanandi S.I., Asih P.B., Syafruddin D. Differential expression of several drug transporter genes in HepG2 and Huh-7 cell lines. Adv. Biomed. Res. 2016;5:104. doi: 10.4103/2277-9175.183664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinha R.A., Singh B.K., Zhou J., Wu Y., Farah B.L., Ohba K., Lesmana R., Gooding J., Bay B.-H., Yen P.M. Thyroid hormone induction of mitochondrial activity is coupled to mitophagy via ROS-AMPK-ULK1 signaling. Autophagy. 2015;11:1341–1357. doi: 10.1080/15548627.2015.1061849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein I., Ojamaa K. Thyroid hormone and the cardiovascular system. N. Engl. J. Med. 2001;344:501–509. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 31.Bassett J.H., Nordstrom K., Boyde A., Howell P.G., Kelly S., Vennstrom B., Williams G.R. Thyroid status during skeletal development determines adult bone structure and mineralization. Mol. Endocrinol. 2007;21:1893–1904. doi: 10.1210/me.2007-0157. [DOI] [PubMed] [Google Scholar]
- 32.Seithel A., Eberl S., Singer K., Auge D., Heinkele G., Wolf N.B., Dörje F., Fromm M.F., König J. The influence of macrolide antibiotics on the uptake of organic anions and drugs mediated by OATP1B1 and OATP1B3. Drug Metab. Dispos. Biol. Fate Chem. 2007;35:779–786. doi: 10.1124/dmd.106.014407. [DOI] [PubMed] [Google Scholar]
- 33.Konig J., Klatt S., Dilger K., Fromm M.F. Characterization of ursodeoxycholic and norursodeoxycholic acid as substrates of the hepatic uptake transporters OATP1B1, OATP1B3, OATP2B1 and NTCP. Basic Clin. Pharmacol. Toxicol. 2012;111:81–86. doi: 10.1111/j.1742-7843.2012.00865.x. [DOI] [PubMed] [Google Scholar]
- 34.Moeller L.C., Wardrip C., Niekrasz M., Refetoff S., Weiss R.E. Comparison of thyroidectomized calf serum and stripped serum for the study of thyroid hormone action in human skin fibroblasts in vitro. Thyroid. 2009;19:639–644. doi: 10.1089/thy.2008.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samuels H.H., Stanley F., Casanova J. Depletion of L-3,5,3′-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology. 1979;105:80–85. doi: 10.1210/endo-105-1-80. [DOI] [PubMed] [Google Scholar]
- 36.Echtermeyer F., Eberhardt M., Risser L., Herzog C., Gueler F., Khalil M., Engel M., Vondran F., Leffler A. Acetaminophen-induced liver injury is mediated by the ion channel TRPV4. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019;33:10257–10268. doi: 10.1096/fj.201802233R. [DOI] [PubMed] [Google Scholar]
- 37.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.