Abstract
The basic surface protein, BspA, has been used as a fusion partner to direct peptide antigens from the human immunodeficiency virus gp41 protein and the Chlamydia psittaci OmpA protein to the cell surface of Lactobacillus fermentum BR11. BspA has potential utility in the construction of live vaccines and diagnostic reagents.
Display of heterologous polypeptides on the surface of gram-positive bacteria has many potential applications in the vaccine, biotechnology, and diagnostic fields (20). Anchoring of the heterologous polypeptide to gram-positive bacterial cell envelope components may be either covalent or noncovalent. Covalent attachment to the cell wall peptidoglycan is directed by a cell wall sorting signal found in a large number of gram-positive bacterial surface polypeptides, including Staphylococcus aureus protein A and Streptococcus pyogenes M protein (19). A variety of heterologous polypeptides have been covalently anchored to the gram-positive bacterial cell wall, including antigens (5, 15), single-chain antibody fragments (4), multisubunit polypeptides (7), and enzymes (21). Noncovalent attachment of heterologous polypeptides to the gram-positive bacterial cell envelope has only been investigated more recently. Specific anchoring domains of noncovalently anchored surface polypeptides, such as those from S-layer proteins (9), muralytic enzymes (1–3), and the Listeria monocytogenes internalin B protein (3), have been used to surface display several antigens and enzymes on gram-positive bacteria.
There is current interest in developing strains of the nonpathogenic bacterial genus Lactobacillus as hosts for surface expression of heterologous polypeptides (14, 26). The ability of lactobacilli to colonize mucosal surfaces indicates that they have potential in mucosal vaccination strategies as live recombinant antigen delivery vehicles (14). Thus far, surface expression systems developed for lactobacilli have been limited to covalent anchoring of the heterologous polypeptide to the cell wall peptidoglycan by using cell wall sorting signals of the Lactobacillus casei proteinase PrtP (14) and the S. pyogenes M protein (12, 13).
The guinea pig vaginal tract isolate Lactobacillus fermentum BR11 (17) has been used previously to express and secrete antigens (16). In this report, we investigate the potential of the noncovalently anchored surface protein, BspA (23), as a fusion partner for expression of heterologous antigens on the surface of L. fermentum BR11. BspA is a member of family III of the solute binding proteins as defined by Tam and Saier (22, 23). It has recently been shown to be part of an l-cystine uptake system in L. fermentum BR11 (24). BspA can be selectively extracted from whole L. fermentum BR11 cells by a single 5 M LiCl wash and is presumed to be electrostatically anchored to negatively charged cell wall components (23). To test BspA as a surface presentation vector, we have selected antigens expressed by two important pathogens (human immunodeficiency virus [HIV] and Chlamydia) for which there is currently no vaccine.
Construction of strains expressing BspA fusion proteins.
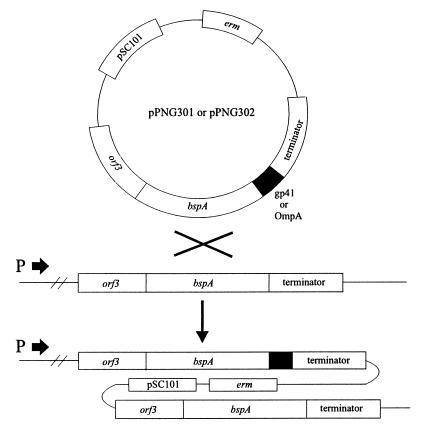
The strategy to obtain expression of bspA gene fusions from the bspA locus of the L. fermentum BR11 chromosome is shown in Fig. 1. We chose to fuse the heterologous antigens to the carboxyl terminus of BspA, because it has been shown that another family III solute binding protein member has an exposed carboxyl terminus (6). The major outer membrane protein (OmpA) of Chlamydia has been extensively investigated as a target for vaccine development. Therefore, the first antigen tested in this system, termed OmpA(293–346), is 54 amino acids from the Chlamydia psittaci guinea pig inclusion conjunctivitis (GPIC) OmpA protein (amino acids 293 to 346), which encompasses the variable domain 4 region (27). Regions of the HIV Env polyprotein (e.g., the gp41 protein) have been targets for HIV vaccine development as well as for the detection of anti-HIV antibodies in diagnostic assays (8). The second antigen used in this system, termed gp41(556–590), is 34 amino acids from the HIV-1 Env polyprotein (amino acids 556 to 590), which includes part of the gp41 protein (10). To maximize the possibility of the heterologous sequences being exposed on the fusion molecule, hydrophilic linker peptides were inserted immediately downstream of the BspA carboxyl terminus [GSGIP for BspA-OmpA(293–346) and GSGI for BspA-gp41(556–590)]. All PCR experiments were performed with the High Fidelity system (Boehringer Mannheim) according to the manufacturer’s instructions. The bspA gene and part of orf3 were amplified by PCR from pMFT3 (23) by using oligonucleotides A (5′-CGTTTCTAGAACTTGTTAGTAATGCCGG-3′) and B (5′-GCGAATTCCTGAACCTTCTGTAATATCCGCACCAA-3′). The putative bspA transcription terminator was amplified from pMFT3 with oligonucleotides C (5′-AAGGATCCTTTTGCAGTTCATTCGTTAG-3′) and D (5′-AAAGGAAGCTTTGGTAATGGGGATTGCC-3′) DNA encoding OmpA(293–346) was amplified from a plasmid clone by using the oligonucleotides E (5′-AAGAATTCCAACATTTGATGCTGACTCTA-3′) and F (5′-CTGGATCCTTATTTGTTGATTTGAAGCGAAG-3′). DNA encoding gp41(556–590) was amplified from a plasmid clone by using oligonucleotides G (5′-AAGAATTCGTATCCTGGCCGTCGAAC-3′) and H (5′-TTGGATCCTTAAGACGCATTCCACGGGACC-3′). The OmpA(293–346) and gp41(556–590) antigen-encoding PCR fragments were digested with EcoRI and BamHI and cloned into similarly digested pBluescript SK+II (Stratagene). Ligation reactions were prepared with the following DNA molecules: EcoRI-digested bspA PCR fragment, BamHI-digested bspA terminator PCR fragment, and either OmpA(293–346)- or gp41(556–590)-encoding DNA fragments digested with EcoRI and BamHI. PCRs were performed with the products of both ligation reactions with oligonucleotides A and D. The amplified 2.1-kb products consisted of the entire BspA fusion protein expression cassettes. These fragments were digested with XbaI and HindIII and cloned into similarly digested pBluescript SK+II. DNA sequencing confirmed that no misincorporations had occurred in the bspA gene fusion during PCR amplification. Both of the 2.1-kb fragments were ligated to pJRS233 (11), and the pJRS233 derivative containing the OmpA(293–346)-encoding DNA was named pPNG301, while the pJRS233 derivative containing the gp41(556–590)-encoding DNA was named pPNG302. pPNG301 and pPNG302 were introduced into L. fermentum BR11 by electroporation, and clones which contained integrated plasmids were isolated as previously described (24) and named PNG301 and PNG302, respectively. Escherichia coli, L. fermentum BR11, and recombinant strains were cultivated as described previously (24).
FIG. 1.
Proposed mechanism for integration of the pPNG301 and pPNG302 plasmids into the L. fermentum BR11 chromosome, downstream of the bspA promoter, via single-crossover homologous recombination. The bspA promoter is indicated as P ➞. The DNAs encoding the gp41(556–590) and OmpA(293–346) antigens are shown as gp41 and OmpA, respectively.
Analysis of expressed BspA fusion proteins.
Overnight-grown L. fermentum BR11, PNG301, or PNG302 cells (10 ml) were extracted with 5 M LiCl as described previously (24). Following addition of an equal volume of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (18), the proteins were separated by SDS-PAGE and then either stained with Coomassie brilliant blue or electroblotted onto a nitrocellulose membrane (Schleicher & Schuell) as previously described (25). The fusion proteins were detected by standard Western blotting procedures (18) with a horseradish peroxidase chemiluminescent detection kit (Boehringer Mannheim) according to the manufacturer’s instructions.
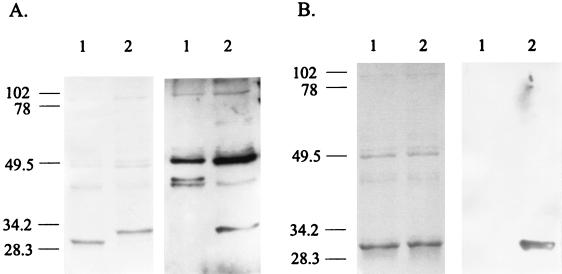
Analysis of 5 M LiCl extracts of 13 erythromycin-resistant pPNG301 putative integrants revealed that 6 expressed BspA-OmpA(293–346). This fusion protein was approximately 2.5 kDa larger than BspA, as judged by SDS-PAGE analysis, and reacted with guinea pig anti-C. psittaci GPIC serum (Fig. 2A). Analysis of 5 M LiCl extracts of 10 erythromycin-resistant pPNG302 putative integrants revealed that 8 expressed BspA-gp41(556–590). There was no significant difference in the electrophoretic mobility of BspA and BspA-gp41(556–590), but the latter reacted strongly with the mouse monoclonal immunoglobulin M (IgM) antibody MAb-2A6, which is specific for the gp41(556–590) antigen (Fig. 2B). Comparison of band intensities in Coomassie brilliant blue-stained gels showed that the fusions were expressed at approximately the same level as BspA (Fig. 2A and B).
FIG. 2.
Analysis of 5 M LiCl extracts from L. fermentum BR11, PNG301, and PNG302 cells. (A) On the left is Coomassie brilliant blue-stained SDS-PAGE of 5 M LiCl extracts from L. fermentum BR11 (lane 1) and PNG301 (lane 2). On the right is a Western blot of 5 M LiCl extracts from L. fermentum BR11 (lane 1) and PNG301 (lane 2) reacted with anti-C. psittaci GPIC serum. (B) On the left is Coomassie brilliant blue-stained SDS-PAGE of 5 M LiCl extracts from L. fermentum BR11 (lane 1) and PNG302 (lane 2). On the right is a Western blot of 5 M LiCl extracts from L. fermentum BR11 (lane 1) and PNG302 (lane 2) reacted with MAb-2A6. The numbers on the left are in kilodaltons.
The amount of BspA-gp41(556–590) extracted with 5 M LiCl was estimated by comparing the intensity of this protein band in Coomassie brilliant blue-stained SDS-PAGE to dilutions of known amounts of lysozyme (Boehringer Mannheim). Approximately 0.75 mg of BspA-gp41(556–590) per liter of PNG302 stationary-phase culture could be removed selectively by a single 5 M LiCl wash. Quantitation of the total amount of BspA-gp41(556–590) expressed by PNG302 was estimated by scanning Western blots and comparing the signal of the BspA-gp41(556–590) band in the whole-cell lysate to that from the 5 M LiCl extract. Whole-cell lysates were prepared by first washing stationary-phase cells with phosphate-buffered saline (PBS) (pH 7.4) and then resuspending them in 1 ml of 5 M LiCl, followed by homogenization on ice for 5 × 2 min at 20,000 rpm with a Polytron homogenizer (Kinematica AG, Lucerne, Switzerland). This extraction procedure yielded approximately 3 mg of processed BspA-gp41(556–590) per liter of stationary-phase culture. This equates to approximately 7 × 103 BspA-gp41(556–590) molecules per cell. The proportion of these molecules which are surface exposed, however, is unknown. Plasmid-based expression of the S. pyogenes M6 protein on the surface of Lactococcus lactis has been reported at levels of around 4 mg per liter of culture (culture corrected to an optical density at 600 nm [OD600] of 1) (12). By comparison, an equivalent culture of PNG302 cells (OD600 = 1) expresses 1.3 mg of extractable BspA-gp41(556–590) per liter.
Surface display of BspA-gp41(556–590).
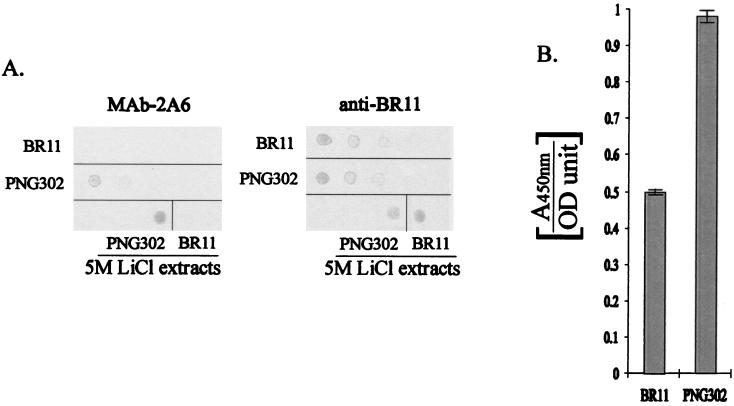
The accessibility of expressed BspA-gp41(556–590) in whole cells was assessed by using two different enzyme-linked immunosorbent assays. The first involved the immobilization of cells on a nitrocellulose membrane, followed by detection of the gp41(556–590) antigen with Mab-2A6. Exponential-phase-grown cells (L. fermentum BR11 or PNG302) were washed with PBS (pH 7) and then resuspended to an OD600 of ≈1.1 in PBS. Two microliters of dilutions of these cell suspensions was spotted onto a nitrocellulose membrane. Two microliters of 5 M LiCl extracts from L. fermentum BR11 and PNG302 was also spotted onto the membrane. The membrane then was blocked with Boehringer-Mannheim blocking reagent and probed with MAb-2A6 at a 1:3,000 dilution for 2 h and then washed with PBS and probed with horseradish peroxidase-conjugated anti-mouse IgM (Sigma) at a 1:500 dilution. Following three more PBS wash steps, antibody binding was detected with chloro-1-naphthol and H2O2. To control for the amount of cells spotted onto the membrane, an identically spotted membrane was probed with rabbit anti-L. fermentum BR11 serum (23) at a 1:100 dilution, washed, and then probed with horseradish peroxidase-conjugated anti-rabbit IgG (Dako) at a 1:1,000 dilution. The membrane was then washed three times, and antibody binding was detected by using chloro-1-naphthol and H2O2. The results show that PNG302 cells bound MAb-2A6, whereas L. fermentum BR11 cells did not (Fig. 3A). As expected, the 5 M LiCl extract of PNG302 bound MAb-2A6 more strongly than the 5 M LiCl extract of L. fermentum BR11 (Fig. 3A). There was no visible difference in the ability of these strains to bind anti-L. fermentum BR11 antibodies (Fig. 3A).
FIG. 3.
Colorimetric assays for the detection of cell surface-displayed gp41(556–590). (A) Dilutions of L. fermentum BR11 (BR11) and PNG302 cell suspensions were spotted onto duplicate nitrocellulose membranes (from left to right: undiluted, 1:2 diluted, and 1:4 diluted). Also, 5 M LiCl extracts from L. fermentum BR11 and PNG302 were spotted onto the bottom right of the membranes. The membranes were either reacted with MAb-2A6 or with anti-L. fermentum BR11 serum. (B) L. fermentum BR11 (BR11) and PNG302 cells were incubated with MAb-2A6, allowing it to bind to surface-displayed gp41(556–590) antigen. This binding was quantified by using a horseradish peroxidase-conjugated secondary antibody and a chromogenic substrate. The results are displayed as the means of the A450 per OD600 unit of the cells, and the standard deviations are shown by error bars.
The second surface accessibility protocol is based on a procedure previously reported (7) and was designed to ensure that any binding observed was not due to fusion protein released from lysed cells. First, exponential-phase-grown cells (L. fermentum BR11 or PNG302) were washed with PBS and then resuspended to an OD600 of ≈1.1 in PBS-Tween (0.05% Tween 20). Three aliquots (900 μl each) of each strain were then incubated for 30 min with 100 μl of MAb-2A6 diluted 1:300. Following two washes with PBS-Tween, the cells were incubated for 15 min with 1 ml of horseradish peroxidase-conjugated antimouse IgM diluted 1:1,000. After being washed twice in PBS-Tween, the cells were resuspended in 1.5 ml of Tris-buffered saline. Four aliquots (50 μl each) of each of the cell suspensions were loaded into wells of a microtiter plate, and 50 μl of 3,3′,5,5′-tetramethylbenzidine (ELISA Systems, Graphic Scientific Pty. Ltd., Brisbane, Australia) was added. Following color development, the reaction was stopped by the addition of 50 μl of 3 M HCl, and the increase in A450 was measured by an automated plate reader. A total of 12 absorbance readings of each of the strains were taken into account when calculating the means and standard deviations. Although some background MAb-2A6 binding to L. fermentum BR11 cells was observed with this assay, a significant increase in binding was observed for PNG302 cells (Fig. 3B), thus confirming that BspA-gp41(556–590) is exposed on the surface of PNG302. Due to the strong cross-reactivity of the anti-C. psittaci GPIC serum with L. fermentum BR11 proteins, surface display of BspA-OmpA(293–346) was not tested. The reason for the significant binding of unmodified L. fermentum BR11 to Mab-2A6 in the centrifugation-based assay, but not in the membrane-based assay, is unknown, but it may be because a blocking procedure was carried out immediately prior to the application of the antibody in the membrane-based assay, but not in the centrifugation-based assay.
BspA is involved in l-cystine uptake and therefore must be able to directly contact the cytoplasmic membrane in order to interact with the membrane-located translocation complex (24). Since BspA-gp41(556–590) in whole L. fermentum cells can bind MAb-2A6, it is likely that at least some BspA is also located near the external side of the cell wall peptidoglycan of L. fermentum BR11. Alternatively, MAb-2A6 may be able to penetrate the cell wall peptidoglycan; however since this antibody is of the IgM class and is therefore a large pentameric molecule, this seems unlikely. Due to the proposed nonspecific nature of anchoring of BspA to the surface of L. fermentum BR11 cells, BspA may be able to be used to anchor heterologous polypeptides to the surface of other Lactobacillus strains.
The BspA surface expression system developed in this study has potential for the development of live vaccine candidates or diagnostic reagents. Other biotechnological applications for this system include the immobilization of enzymatic molecules or single-chain antibodies on gram-positive bacterial cell surfaces. The fast and simple 5 M LiCl extraction procedure for purifying BspA fusion proteins from whole cells indicates that this system may also be useful simply for the expression and purification of recombinant proteins. We are currently investigating several of these applications.
Acknowledgments
We thank Louise Hafner and Peter Timms for critically reviewing the manuscript. Our thanks also go to Andrea McCracken for supplying E. coli strains containing plasmids pCUTV4 and PCUTG4, which contain DNA encoding OmpA(293–346) and gp41(556–590), respectively; Peter Hudson (CSIRO) for supplying pGC1201, which contains DNA encoding gp41(556–590); Kym Volp and Dean Moss (Agen Biomedical) for supplying us with guinea pig anti-C. psittaci GPIC serum and MAb-2A6, respectively; Cynthia Cooper for expert DNA sequencing assistance; and June Scott for supplying pJRS233.
This work was supported by a QUT Meritorious grant to P.M.G. and NHMRC Project grant 941114. M.S.T. is the recipient of an Australian postgraduate award and a vice-chancellors scholarship initiative.
REFERENCES
- 1.Baba T, Schneewind O. Target cell specificity of a bacteriocin molecule: a C-terminal signal directs lysostaphin to the cell wall of Staphylococcus aureus. EMBO J. 1996;15:4789–4797. [PMC free article] [PubMed] [Google Scholar]
- 2.Baba T, Schneewind O. Targeting of muralytic enzymes to the cell division site of gram-positive bacteria: repeat domains direct autolysin to the equatorial surface ring of Staphylococcus aureus. EMBO J. 1998;17:4639–4646. doi: 10.1093/emboj/17.16.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun L, Dramsi S, Dehoux P, Bierne H, Lindahl G, Cossart P. InlB: an invasion protein of Listeria monocytogenes with a novel type of surface association. Mol Microbiol. 1997;25:285–294. doi: 10.1046/j.1365-2958.1997.4621825.x. [DOI] [PubMed] [Google Scholar]
- 4.Gunneriusson E, Samuelson P, Uhlén M, Nygren P-Å, Ståhl S. Surface display of a functional single-chain Fv antibody on staphylococci. J Bacteriol. 1996;178:1341–1346. doi: 10.1128/jb.178.5.1341-1346.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansson M, Ståahl S, Nguyen T N, Bächi T, Robert A, Binz H, Sjölander A, Uhlén M. Expression of recombinant proteins on the surface of the coagulase-negative bacterium Staphylococcus xylosus. J Bacteriol. 1992;174:4239–4245. doi: 10.1128/jb.174.13.4239-4245.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsiao C D, Sun Y J, Rose J, Wang B C. The crystal structure of glutamine-binding protein from Escherichia coli. J Mol Biol. 1996;262:225–242. doi: 10.1006/jmbi.1996.0509. [DOI] [PubMed] [Google Scholar]
- 7.Liljeqvist S, Samuelson P, Hansson M, Nguyen T N, Binz H, Ståhl S. Surface display of the cholera toxin B subunit on Staphylococcus xylosus and Staphylococcus carnosus. Appl Environ Microbiol. 1997;63:2481–2488. doi: 10.1128/aem.63.7.2481-2488.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lilley G G, Dolezal O, Hillyard C J, Bernard C, Hudson P J. Recombinant single-chain antibody peptide conjugates expressed in Escherichia coli for the rapid diagnosis of HIV. J Immunol Methods. 1994;171:211–226. doi: 10.1016/0022-1759(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 9.Mesnage S, Tosi-Couture E, Fouet A. Production and cell surface anchoring of functional fusions between the SLH motifs of the Bacillus anthracis S-layer proteins and the Bacillus subtilis levansucrase. Mol Microbiol. 1999;31:927–936. doi: 10.1046/j.1365-2958.1999.01232.x. [DOI] [PubMed] [Google Scholar]
- 10.Muesing M A, Smith D H, Cabradilla C D, Benton C V, Lasky L A, Capon D J. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retrovirus. Nature. 1985;313:450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Casal J, Price J A, Maguin E, Scott J R. An M protein with a single C repeat prevents phagocytosis of Streptococcus pyogenes: use of a temperature-sensitive shuttle vector to deliver homologous sequences to the chromosome of S. pyogenes. Mol Microbiol. 1993;8:809–819. doi: 10.1111/j.1365-2958.1993.tb01628.x. [DOI] [PubMed] [Google Scholar]
- 12.Piard J-C, Hautefort I, Fischetti V A, Ehrlich S D, Fons M, Gruss A. Cell wall anchoring of the Streptococcus pyogenes M6 protein in various lactic acid bacteria. J Bacteriol. 1997;179:3068–3072. doi: 10.1128/jb.179.9.3068-3072.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piard J-C, Jimenez-Diaz R, Fischetti V A, Ehrlich S D, Gruss A. The M6 protein of Streptococcus pyogenes and its potential as a tool to anchor biologically active molecules at the surface of lactic acid bacteria. Adv Exp Med Biol. 1997;418:545–550. doi: 10.1007/978-1-4899-1825-3_126. [DOI] [PubMed] [Google Scholar]
- 14.Pouwels P H, Leer R J, Shaw M, Heijne den Bak-Glashouwer M-J, Tielen F D, Smit E, Martinez B, Jore J, Conway P L. Lactic acid bacteria as antigen delivery vehicles for oral immunization purposes. Int J Food Microbiol. 1998;41:155–167. doi: 10.1016/s0168-1605(98)00048-8. [DOI] [PubMed] [Google Scholar]
- 15.Pozzi G, Contorni M, Oggioni M R, Manganelli R, Tommasino M, Cavalieri F, Fischetti V A. Delivery and expression of a heterologous antigen on the surface of streptococci. Infect Immun. 1992;60:1902–1907. doi: 10.1128/iai.60.5.1902-1907.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rush C, Hafner L, Timms P. Protein A as a fusion partner for the expression of heterologous proteins in Lactobacillus. Appl Microbiol Biotechnol. 1997;47:537–542. doi: 10.1007/s002530050969. [DOI] [PubMed] [Google Scholar]
- 17.Rush C M, Hafner L M, Timms P. Genetic modification of a vaginal strain of Lactobacillus fermentum and its maintenance within the reproductive tract after intravaginal administration. J Med Microbiol. 1994;41:272–278. doi: 10.1099/00222615-41-4-272. [DOI] [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 19.Schneewind O, Mihaylova-Petkov D, Model P. Cell wall sorting signals in surface proteins of gram-positive bacteria. EMBO J. 1993;12:4803–4811. doi: 10.1002/j.1460-2075.1993.tb06169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ståhl S, Uhlén M. Bacterial surface display: trends and progress. Trends Biotechnol. 1997;15:185–192. doi: 10.1016/S0167-7799(97)01034-2. [DOI] [PubMed] [Google Scholar]
- 21.Strauss A, Götz F. In vivo immobilization of enzymatically active polypeptides on the cell surface of Staphylococcus carnosus. Mol Microbiol. 1996;21:491–500. doi: 10.1111/j.1365-2958.1996.tb02558.x. [DOI] [PubMed] [Google Scholar]
- 22.Tam R, Saier M H., Jr Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol Rev. 1993;57:320–346. doi: 10.1128/mr.57.2.320-346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner M S, Timms P, Hafner L M, Giffard P M. Identification and characterization of a basic cell surface-located protein from Lactobacillus fermentum BR11. J Bacteriol. 1997;179:3310–3316. doi: 10.1128/jb.179.10.3310-3316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner M S, Woodberry T, Hafner L M, Giffard P M. The bspA locus of Lactobacillus fermentum BR11 encodes an l-cystine uptake system. J Bacteriol. 1999;181:2192–2198. doi: 10.1128/jb.181.7.2192-2198.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wanitchakorn R, Harding R M, Dale J L. Banana bunchy top virus DNA-3 encodes the viral coat protein. Arch Virol. 1997;142:1673–1680. doi: 10.1007/s007050050188. [DOI] [PubMed] [Google Scholar]
- 26.Wells J M, Robinson K, Chamberlain L M, Schofield K M, LePage R W F. Lactic acid bacteria as vaccine delivery vehicles. Antonie Leeuwenhoek. 1996;70:317–330. doi: 10.1007/BF00395939. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y-X, Morrison S G, Caldwell H D, Baehr W. Cloning and sequence analysis of the major outer membrane protein genes of two Chlamydia psittaci strains. Infect Immun. 1989;57:1621–1625. doi: 10.1128/iai.57.5.1621-1625.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]