Abstract
Opa proteins of Neisseria gonorrhoeae bind to CD66 receptors on human phagocytes, thereby inducing efficient uptake of the bacteria in the absence of opsonins. The interaction of Opa proteins and CD66 receptors leads to activation of Src family tyrosine kinases, a process that is of critical importance for the efficient, CD66-mediated internalization. Here we show that during Opa-mediated stimulation of CD66 the activity of the host cell tyrosine phosphatase SHP-1 is strongly downregulated, concomitant with increases in the tyrosine phosphorylation of several cellular proteins. Since the SHP-1 tyrosine phosphorylation level itself is influenced by Opa-induced events, this phosphatase comprises an important regulatory checkpoint of the pathogen-triggered signaling cascade in human phagocytes.
Pathogens and their host cells engage in intimate cross talk (9). This is reflected on the molecular level by the activation of cellular signal transduction pathways upon binding of specific bacterial adhesins to plasma membrane receptors (3). In many examples, the pathogens trigger tyrosine phosphorylation-based signaling cascades that lead to their uptake by eukaryotic cells (2, 10).
The human-specific, gram-negative pathogen Neisseria gonorrhoeae is known to interact with different cell types by means of a given set of phase-variable outer membrane proteins, the opacity-associated (Opa) proteins (24). These bacterial adhesins bind to CD66 molecules that comprise a family of immunoglobulin-like glycoproteins expressed on a variety of human cells (11, 36). On human phagocytes, Opa52-expressing gonococci recognize CD66a (BGP), CD66c (NCA90), and CD66d (CGM1) (12). In contrast to CD66c, a glycosylphosphatidylinositol (GPI)-anchored membrane protein, both CD66a and CD66d contain intracellular domains encompassing tyrosine residues which can be phosphorylated in response to extracellular stimuli (25, 31). In the case of CD66a, phosphorylation of tyrosine residue Tyr-488 has been reported elsewhere (26). The phosphorylation of this residue in turn leads to the recruitment of SH2 domain-containing signal transduction molecules. In particular, Src family kinases have been found to interact with the cytoplasmic tails of CD66 proteins (8, 30). In line with these results, stimulation of CD66 on human phagocytes by Opa52-expressing bacteria triggers an intracellular signaling cascade involving the Src family kinases Hck and Fgr (14). Interestingly, treatment of cells with the general phosphatase inhibitor vanadate results in phosphorylation of CD66a at Tyr-488 (21). This observation led us to speculate that tyrosine phosphatase(s) might play an important regulatory role in the early signaling events following CD66 receptor occupation by bacterial adhesins.
In this paper, we demonstrate that the activity of the cytoplasmic tyrosine phosphatase SHP-1 is downregulated in response to CD66 stimulation by Opa-expressing gonococci. The strong decrease in phosphatase activity is observed in an in vitro-differentiated myelomonocytic cell line as well as in primary neutrophils and is accompanied by decreased tyrosine phosphorylation of SHP-1. Downregulation of SHP-1 phosphatase activity parallels the reported increases in Hck and Fgr tyrosine kinase activity and therefore seems to be an important prerequisite for the observed accumulation of tyrosine-phosphorylated proteins upon bacterial infection.
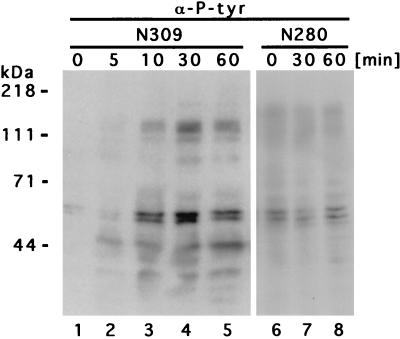
To demonstrate the significance of tyrosine phosphorylation in the interaction between phagocytes and pathogenic gonococci, we analyzed the tyrosine-phosphorylation events following infection of human phagocytes with Opa-expressing gonococci. Therefore, human myelomonocytic JOSK-M cells, cultured in RPMI 1640 containing 5% fetal calf serum, were differentiated for 4 to 6 days in RPMI 1640 with 5% heat-inactivated fetal calf serum containing bufalin (10 nM; Sigma, Deisenhofen, Germany) and retinoic acid (100 nM; Sigma) as described elsewhere (13). The gonococcal strains N280, a piliated variant exhibiting the transparent phenotype (Opa− P+), and N309, an isogenic nonpiliated (P−) variant constitutively expressing a CD66-specific Opa protein (Opa52) (14, 19), were grown on GC agar (Life Science Technologies, Paisley, United Kingdom) supplemented with vitamins and corresponding antibiotics at 37°C in 5% CO2. To investigate cellular tyrosine phosphorylation events, differentiated JOSK-M cells (4 × 106 cells) were infected at a multiplicity of infection (MOI) of 50 bacteria/cell for the indicated times, at which the cells were lysed at 4°C with radioimmunoprecipitation assay (RIPA) buffer (25 mM HEPES [pH 7.4]; 0.1% sodium dodecyl sulfate [SDS]; 0.5% sodium deoxycholate; 1% Triton X-100; 125 mM NaCl; 10 mM [each] NaF, Na3VO4, and sodium pyrophosphate; and 10 μg each of aprotinin and leupeptin per ml). After centrifugation at 20,000 × g for 15 min, 5× sample buffer was added to the supernatant, and the whole-cell lysates were separated by SDS-polyacrylamide gel electrophoresis (PAGE), transferred to polyvinylidene difluoride membranes (Bio-Rad, Munich, Germany), and incubated overnight at 4°C with a monoclonal antiphosphotyrosine antibody (clone 4G10; Upstate Biotechnology Inc., Lake Placid, N.Y.). Immunoblots were developed by incubation with horseradish peroxidase-conjugated protein G (Bio-Rad) and use of the ECL chemiluminescent substrate kit (Amersham, Braunschweig, Germany). As shown in Fig. 1, infection with Opa52-expressing gonococci (N309) did lead to strong increases in the tyrosine phosphorylation of multiple phagocyte proteins. Most notably, tyrosine phosphorylation was augmented in proteins with estimated molecular masses of ∼32, 43, 55 to 65, 95, and 115 to 120 kDa and was maximal between 30 and 60 min (Fig. 1, lanes 1 to 5). In contrast, N. gonorrhoeae N280 (Opa− P+) did not elicit comparable effects, and cellular tyrosine phosphorylation in infected cells was comparable to background levels in uninfected cells (Fig. 1, lanes 6 to 8; note that lanes 6 to 8 in Fig. 1 show a longer exposure than that in lanes 1 to 5). In addition, only Opa-mediated binding to CD66 has been shown to result in effective internalization of the bacteria (14), though gonococci are capable of interacting with human neutrophils in the absence of opsonins via both Opa52 and pili (17).
FIG. 1.
Stimulation of tyrosine phosphorylation in JOSK-M cells upon phagocytosis of Opa52-expressing N. gonorrhoeae. Within 10 min after infection with N309 (Opa52), increased tyrosine phosphorylation of several cellular proteins was detectable and peaked between 30 and 60 min. In contrast, N280 (Opa− P+) did not lead to alterations in tyrosine phosphorylation levels. The cells were infected at an MOI of 50, and infection was terminated at the indicated time points. Whole-cell lysates were analyzed by SDS-PAGE and Western blotting with monoclonal antiphosphotyrosine antibodies (α-P-tyr). The blots were developed with enhanced chemiluminescence reagents and exposed for 20 s (lanes 1 to 5) or 1 min (lanes 6 to 8). The background phosphorylation of unstimulated cells is reflected by the zero time points.
Interestingly, the increases in tyrosine phosphorylation paralleled the increase in Src family tyrosine kinase activities observed with JOSK-M cells and primary neutrophils during non-opsonin-mediated uptake of Opa52-expressing gonococci (14). It is now well established that the activity of Src family tyrosine kinases can be directly regulated by the action of tyrosine phosphatases (33). In particular, dephosphorylation of a tyrosine residue close to the C terminus of these kinases can lead to activation due to the release of an intramolecular autoinhibition involving this residue and the SH2 domain (38), whereas dephosphorylation of a tyrosine residue in the kinase domain downregulates kinase activity (4, 34). Therefore, activation of Src kinases can be accomplished by increased phosphatase activity directed to the C-terminal tyrosine and decreased phosphatase activity directed toward the tyrosine residues in the kinase domain. Since the cytosolic, SH2 domain-containing tyrosine phosphatases SHP-1 (PTP1C, HCP) and SHP-2 (PTP1D, Syp) have been implicated in controlling the activity of Src family tyrosine kinases (22, 23), we wondered whether these enzymes could be involved in the nonopsonic uptake of gonococci. Whereas myelomonocytic JOSK-M cells expressed SHP-1, SHP-2 protein expression was not detectable in differentiated and undifferentiated JOSK-M cells by Western blotting (data not shown).
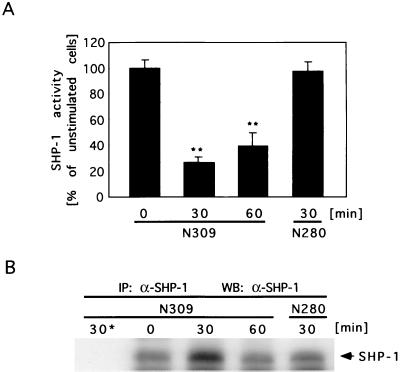
To test whether SHP-1 activity was affected by infection of phagocytes with gonococci, differentiated JOSK-M cells (107 cells) were infected with Opa52-expressing N309 or N280 (Opa− P+) for the indicated times and lysed in RIPA buffer. SHP-1 was immunoprecipitated from the lysates with monoclonal anti-SHP-1 antibodies (3 μg/sample; clone 52 [immunoglobulin G1]; Transduction Laboratories, Lexington, Ky.) for 4 h at 4°C followed by Protein A/G Plus-Sepharose beads (Santa Cruz Biotechnology, Santa Cruz, Calif.) for 1 h at 4°C. Control immunoprecipitations from cells infected with N309 for 30 min were performed with isotype-matched control antibodies (3 μg/sample; clone EH7a [immunoglobulin G1]; Developmental Studies Hybridoma Bank, Iowa City, Iowa). SHP-1 phosphatase activity in the immunoprecipitates was measured according to the procedures described in references 29 and 32. Briefly, the samples were washed three times at 4°C with RIPA buffer and four times with phosphatase buffer (40 mM morpholineethanesulfonic acid [pH 5.5], 50 mM NaCl, 10 mM dithiothreitol, 5 mM EDTA) and incubated at 23°C in 150 μl of phosphatase buffer containing 15 mM p-nitrophenyl phosphate (NPP; Sigma) for 30 min. The reaction was stopped by addition of 200 μl of 0.5 M NaOH, and the absorbance of the samples at 405 nm was determined in 96-well plates with an enzyme-linked immunosorbent assay reader (Digiscan; ASYS Hitech, Eugendorf, Austria). Control immunoprecipitates were also employed in phosphatase assays and subtracted as background values from all samples. Interestingly, SHP-1 activity decreased markedly in JOSK-M cells upon infection with Opa52-expressing N. gonorrhoeae (N309) (Fig. 2A). Within 30 min postinfection, phosphatase activity decreased to 43% of the initial activity. In contrast, phagocytes infected with N280 (Opa− P+) showed no significant change in SHP-1 activity over the observed time course. Western blotting of aliquots of each sample demonstrated similar amounts of SHP-1 in the immunoprecipitates used for phosphatase assays (Fig. 2B).
FIG. 2.
SHP-1 activity is downregulated in phagocytes infected with Opa52-expressing N. gonorrhoeae. (A) The phosphatase activity of SHP-1 was significantly reduced in JOSK-M cells upon phagocytosis of Opa52-expressing N. gonorrhoeae (N309) within 30 to 60 min, whereas infection with Opa− P+ N. gonorrhoeae (N280) had no significant effect on SHP-1 activity. Cells were infected at an MOI of 50 and lysed at the indicated time points. Following SHP-1 immunoprecipitation, phosphatase activity was measured with NPP as substrate. Values are given as amounts relative to SHP-1 activity in unstimulated cells. Bars represent mean values of a representative experiment with triplicate samples. Overall significance was determined by one-way analysis of variance, and significance between samples was determined by the Tukey-Kramer multiple-comparison t test. Double asterisks indicate samples that are significantly (P < 0.01) different from the control. (B) Aliquots of SHP-1 immunoprecipitates (IP: α-SHP-1) used for in vitro phosphatase assays were separated by SDS-PAGE and employed in a Western blot with anti-SHP-1 antibodies (WB: α-SHP-1) to demonstrate equal amounts of precipitated enzyme in the samples. Control immunoprecipitates from cells infected with N309 for 30 min were obtained with isotype-matched control antibodies (30*).
To further substantiate these findings, we tested whether SHP-1 activity follows a similar change in primary human neutrophils upon infection with gonococci expressing the CD66-binding Opa52 protein. Therefore, neutrophils were isolated from human blood by the method of Brandt et al. (6). Neutrophils (107) in RPMI 1640–5% heat-inactivated fetal calf serum were seeded in 60-mm-diameter cell culture dishes and infected with N309 and N280 at an MOI of 50. At the indicated times, cells were lysed in RIPA buffer, SHP-1 was immunoprecipitated, and immunocomplex phosphatase assays were performed as described above. Again, infection with Opa52-expressing N. gonorrhoeae led to a reduction in SHP-1 activity, whereas nonopaque, piliated gonococci (N280) did not cause any alteration in the activity of this tyrosine phosphatase (Fig. 3A). The kinetics of SHP-1 inactivation in primary neutrophils paralleled the time course observed with JOSK-M cells with a minimum of phosphatase activity at 30 to 60 min following infection. Interestingly, the decrease in SHP-1 activity induced by N309 was even more pronounced in primary neutrophils than in JOSK-M cells. Within 30 min after the start of the infection, approximately 26% of the initial SHP-1 activity could be detected in cells infected with the Opa52-expressing strain. Western blotting of aliquots of each sample demonstrated that the observed decrease in SHP-1 activity was not due to lower amounts of immunoprecipitated SHP-1 in these samples (Fig. 3B).
FIG. 3.
SHP-1 activity is downregulated in neutrophils upon infection with Opa52-expressing N. gonorrhoeae. (A) SHP-1 phosphatase activity was strongly reduced in primary human neutrophils following infection with Opa52-expressing N. gonorrhoeae (N309). In contrast, Opa− P+ gonococci (N280) did not lead to changes in SHP-1 activity in primary phagocytes. Cells were infected at an MOI of 50 and lysed at the indicated time points. SHP-1 was immunoprecipitated, and its phosphatase activity was measured by using NPP as substrate. Values are given as amounts relative to SHP-1 activity in unstimulated cells. Bars represent mean values of a representative experiment with triplicate samples. Overall significance was determined by one-way analysis of variance, and significance between samples was determined by the Tukey-Kramer multiple-comparison t test. Double asterisks indicate samples that are significantly (P < 0.01) different from the control. (B) Samples of SHP-1 immunoprecipitates (IP: α-SHP-1) used for in vitro phosphatase assays were separated by SDS-PAGE and probed with anti-SHP-1 antibodies (WB: α-SHP-1) to show the amounts of precipitated enzyme. Control immunoprecipitates from cells infected with N309 for 30 min were obtained with isotype-matched control antibodies (30*).
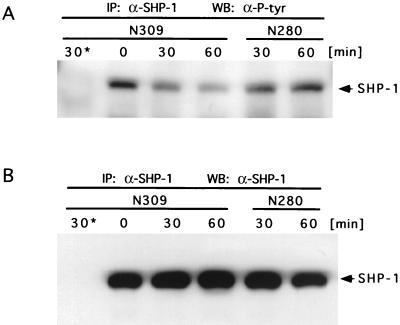
Since the activities of SHP-1 and SHP-2 can be influenced by tyrosine phosphorylation (5, 20, 23, 35, 37), we tested whether there is a change in tyrosine phosphorylation of SHP-1 upon encounter of phagocytes with Opa52-expressing N. gonorrhoeae (N309). Immunoprecipitates of SHP-1 from infected JOSK-M cells (1.5 × 107 cells) were analyzed by using monoclonal antiphosphotyrosine antibodies (clone 4G10; Upstate Biotechnology Inc.). Upon infection with N309, tyrosine phosphorylation of SHP-1 decreased within 30 to 60 min (Fig. 4A). This reduction paralleled the time course observed for SHP-1 activity in JOSK-M cells and primary neutrophils. Significantly, tyrosine phosphorylation of SHP-1 did not markedly change in JOSK-M cells infected with nonopaque, piliated gonococci (N280). Samples of the SHP-1 immunoprecipitates were separated by SDS-PAGE and probed with anti-SHP-1 antibodies to demonstrate equal amounts of precipitated enzyme (Fig. 4B).
FIG. 4.
Tyrosine phosphorylation of SHP-1 in JOSK-M cells is reduced upon infection with Opa52-expressing N. gonorrhoeae. (A) JOSK-M cells were infected with Opa52-expressing N. gonorrhoeae (N309) or Opa− P+ gonococci (N280) at an MOI of 50, and infection was stopped at the indicated time points. SHP-1 was immunoprecipitated (IP: α-SHP-1), and samples were analyzed by SDS-PAGE and Western blotting with monoclonal antiphosphotyrosine antibodies (WB: α-P-tyr). Isotype-matched control antibodies were used to obtain control immunoprecipitates from cells infected with N309 for 30 min (30*). (B) Aliquots of the SHP-1 immunoprecipitates (IP: α-SHP-1) were separated by SDS-PAGE and probed with anti-SHP-1 antibodies (WB: α-SHP-1) to demonstrate equal amounts of precipitated enzyme in the samples.
The observation that SHP-1 tyrosine phosphorylation is reduced synchronously with its phosphatase activity in response to CD66 stimulation is in agreement with previous reports (35, 37). Nevertheless, it is remarkable with regard to the overall increase in tyrosine phosphorylation (Fig. 1). This emphasizes the complexity of signaling cascades, where the counteracting processes of tyrosine phosphorylation as well as dephosphorylation have to be integrated (16). Since activation of Src family kinases requires a tyrosine phosphatase activity directed toward the C-terminal tyrosine residue, it is tempting to speculate that the same enzyme might act on SHP-1. Thereby, such a regulatory molecule could coordinately stimulate Hck and Fgr and downregulate SHP-1 activity. Interestingly, upon vanadate treatment of mouse colon carcinoma cells overexpressing CD66a, an association between SHP-1 or SHP-2 and CD66a has been reported elsewhere (1, 15). Therefore, the simultaneous recruitment of Src family tyrosine kinases and SHP-1 to the phosphorylated CD66 cytoplasmic tail could place these signaling molecules in proximity to a common upstream regulator.
Our results show for the first time the physiological consequences of CD66 receptor activation for the function of the tyrosine phosphatase SHP-1. In immortalized as well as primary cells that do not overexpress the receptor or the phosphatase, SHP-1 activity is downregulated following CD66 engagement. Therefore, the results of this study imply that SHP-1 constitutes a negative regulator of CD66-triggered tyrosine phosphorylation by Src family kinases. This conclusion is in line with results from SHP-1-deficient (moth-eaten) mice, where decreased SHP-1 activity leads to hyperactive Fyn and Lyn kinases in T cells (22, 27). In addition, stimulation of human neutrophils with phorbol myristate acetate or zymosan results in decreased SHP-1 activity, and this event is accompanied by increased tyrosine phosphorylation of cellular proteins (7).
Since CD66a is downregulated in colorectal carcinomas and has the potential to function as a tumor suppressor molecule in colon epithelial cells (18, 28), CD66-mediated signaling events are of major importance. Based on our findings, we would predict that constitutively activated versions of SHP-1 inhibit a CD66-initiated signaling cascade in human phagocytes and possibly prevent the uptake of Opa52-expressing bacteria. The further use of Opa52-expressing gonococci as a specific multivalent and spatially confined stimulus for CD66 molecules will help in elucidating the signaling mechanisms triggered by these receptors.
Acknowledgments
We thank F. Bohmer, M. Coggeshall, H. Keilhack, and B. Neel for valuable discussions. We thank Carolin Müller for excellent technical assistance.
C.R.H. acknowledges the generous support of the Tübinger Stipendienstiftung and the Stifterverband für die deutsche Wissenschaft. The study was supported by DFG grant Gu 335/2-2 to E.G. and by the Fonds der Chemischen Industrie grant to T.F.M.
C. R. Hauck and E. Gulbins contributed equally to the work.
REFERENCES
- 1.Beauchemin N, Kunath T, Robitaille J, Chow B, Turbide C, Daniels E, Veillette A. Association of biliary glycoprotein with protein tyrosine phosphatase SHP-1 in malignant colon epithelial cells. Oncogene. 1997;14:783–790. doi: 10.1038/sj.onc.1200888. [DOI] [PubMed] [Google Scholar]
- 2.Bliska J, Falkow S. The role of host tyrosine phosphorylation in bacterial pathogenesis. Trends Genet. 1993;9:85–89. doi: 10.1016/0168-9525(93)90229-b. [DOI] [PubMed] [Google Scholar]
- 3.Bliska J B, Galan J E, Falkow S. Signal transduction in the mammalian cell during bacterial attachment and entry. Cell. 1993;73:903–920. doi: 10.1016/0092-8674(93)90270-z. [DOI] [PubMed] [Google Scholar]
- 4.Bolen J B, Rowley R B, Spana C, Tsygankov A Y. The Src family of tyrosine protein kinases in hemopoietic signal transduction. FASEB J. 1992;6:3403–3409. doi: 10.1096/fasebj.6.15.1281458. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard P, Zhao Z, Banville D, Dumas F, Fischer E H, Shen S H. Phosphorylation and identification of a major tyrosine phosphorylation site in protein tyrosine phosphatase 1C. J Biol Chem. 1994;269:19585–19589. [PubMed] [Google Scholar]
- 6.Brandt E, Van Damme J, Flad H-D. Neutrophils can generate their activator neutrophil-activating peptide 2 by proteolytic cleavage of platelet-derived connective tissue-activating peptide III. Cytokine. 1991;3:311–321. doi: 10.1016/1043-4666(91)90499-4. [DOI] [PubMed] [Google Scholar]
- 7.Brumell J H, Chan C K, Butler J, Borregaard N, Siminovitch K A, Grinstein S, Downey G P. Regulation of Src homology 2-containing tyrosine phosphatase 1 during activation of human neutrophils. Role of protein kinase C. J Biol Chem. 1997;272:875–882. doi: 10.1074/jbc.272.2.875. [DOI] [PubMed] [Google Scholar]
- 8.Brummer J, Neumaier M, Gopfert C, Wagener C. Association of pp60c-src with biliary glycoprotein (CD66a), an adhesion molecule of the carcinoembryonic antigen family downregulated in colorectal carcinomas. Oncogene. 1995;11:1649–1655. [PubMed] [Google Scholar]
- 9.Finlay B B, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 10.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray-Owen S D, Dehio C, Haude A, Grunert F, Meyer T F. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J. 1997;16:3435–3445. doi: 10.1093/emboj/16.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray-Owen S D, Lorenzen D R, Haude A, Meyer T F, Dehio C. Differential opa specificities for CD66 receptors influence tissue interactions and cellular response to Neisseria gonorrhoeae. Mol Microbiol. 1997;26:971–980. doi: 10.1046/j.1365-2958.1997.6342006.x. [DOI] [PubMed] [Google Scholar]
- 13.Hauck C R, Lorenzen D, Saas J, Meyer T F. An in vitro-differentiated human cell line as a model system to study the interaction of Neisseria gonorrhoeae with phagocytic cells. Infect Immun. 1997;65:1863–1869. doi: 10.1128/iai.65.5.1863-1869.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hauck C R, Meyer T F, Lang F, Gulbins E. CD66-mediated phagocytosis of Opa52Neisseria gonorrhoeae requires a Src-like tyrosine kinase- and Rac1-dependent signalling pathway. EMBO J. 1998;17:443–454. doi: 10.1093/emboj/17.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huber M, Izzi L, Grondin P, Houde C, Kunath T, Veillette A, Beauchemin N. The carboxyl-terminal region of biliary glycoprotein controls its tyrosine phosphorylation and association with protein-tyrosine phosphatases SHP-1 and SHP-2 in epithelial cells. J Biol Chem. 1999;274:335–344. doi: 10.1074/jbc.274.1.335. [DOI] [PubMed] [Google Scholar]
- 16.Hunter T. Protein kinases and phosphatases: the Yin and Yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 17.Knepper B, Heuer I, Meyer T F, van Putten J P M. Differential response of human monocytes to Neisseria gonorrhoeae variants expressing pilus and opacity protein. Infect Immun. 1997;65:4122–4129. doi: 10.1128/iai.65.10.4122-4129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunath T, Ordonez-Garcia C, Turbide C, Beauchemin N. Inhibition of colonic tumor cell growth by biliary glycoprotein. Oncogene. 1995;11:2375–2382. [PubMed] [Google Scholar]
- 19.Kupsch E M, Knepper B, Kuroki T, Heuer I, Meyer T F. Variable opacity (Opa) outer membrane proteins account for the cell tropisms displayed by Neisseria gonorrhoeae for human leukocytes and epithelial cells. EMBO J. 1993;12:641–650. doi: 10.1002/j.1460-2075.1993.tb05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li R Y, Gaits F, Ragab A, Ragab-Thomas J M, Chap H. Tyrosine phosphorylation of an SH2-containing protein tyrosine phosphatase is coupled to platelet thrombin receptor via a pertussis toxin-sensitive heterotrimeric G-protein. EMBO J. 1995;14:2519–2526. doi: 10.1002/j.1460-2075.1995.tb07249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin S H, Luo W, Earley K, Cheung P, Hixson D C. Structure and function of C-CAM1: effects of the cytoplasmic domain on cell aggregation. Biochem J. 1995;311:239–245. doi: 10.1042/bj3110239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorenz U, Ravichandran K S, Burakoff S J, Neel B G. Lack of SHPTP1 results in src-family kinase hyperactivation and thymocyte hyperresponsiveness. Proc Natl Acad Sci USA. 1996;93:9624–9629. doi: 10.1073/pnas.93.18.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorenz U, Ravichandran K S, Pei D, Walsh C T, Burakoff S J, Neel B G. Lck-dependent tyrosyl phosphorylation of the phosphotyrosine phosphatase SH-PTP1 in murine T cells. Mol Cell Biol. 1994;14:1824–1834. doi: 10.1128/mcb.14.3.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer T F, Pohlner J, van Putten J P. Biology of the pathogenic Neisseriae. Curr Top Microbiol Immunol. 1994;192:283–317. doi: 10.1007/978-3-642-78624-2_13. [DOI] [PubMed] [Google Scholar]
- 25.Najjar S M, Accili D, Philippe N, Jernberg J, Margolis R, Taylor S I. pp120/ecto-ATPase, an endogenous substrate of the insulin receptor tyrosine kinase, is expressed as two variably spliced isoforms. J Biol Chem. 1993;268:1201–1206. [PubMed] [Google Scholar]
- 26.Najjar S M, Philippe N, Suzuki Y, Ignacio G A, Formisano P, Accili D, Taylor S I. Insulin-stimulated phosphorylation of recombinant pp120/HA4, an endogenous substrate of the insulin receptor tyrosine kinase. Biochemistry. 1995;34:9341–9349. doi: 10.1021/bi00029a009. [DOI] [PubMed] [Google Scholar]
- 27.Neel B G, Tonks N K. Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- 28.Neumaier M, Paululat S, Chan A, Matthaes P, Wagener C. Biliary glycoprotein, a potential human cell adhesion molecule, is down-regulated in colorectal carcinomas. Proc Natl Acad Sci USA. 1993;90:10744–10748. doi: 10.1073/pnas.90.22.10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pei D, Wang J, Walsh C T. Differential functions of the two Src homology 2 domains in protein tyrosine phosphatase SH-PTP1. Proc Natl Acad Sci USA. 1996;93:1141–1145. doi: 10.1073/pnas.93.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skubitz K M, Campbell K D, Ahmed K, Skubitz A P. CD66 family members are associated with tyrosine kinase activity in human neutrophils. J Immunol. 1995;155:5382–5390. [PubMed] [Google Scholar]
- 31.Skubitz K M, Ducker T P, Goueli S A. CD66 monoclonal antibodies recognize a phosphotyrosine-containing protein bearing a carcinoembryonic antigen cross-reacting antigen on the surface of human neutrophils. J Immunol. 1992;148:852–860. [PubMed] [Google Scholar]
- 32.Sugimoto S, Lechleider R J, Shoelson S E, Neel B G, Walsh C T. Expression, purification, and characterization of SH2-containing protein tyrosine phosphatase, SH-PTP2. J Biol Chem. 1993;268:22771–22776. [PubMed] [Google Scholar]
- 33.Superti-Furga G. Regulation of the Src protein tyrosin kinase. FEBS Lett. 1995;369:62–66. doi: 10.1016/0014-5793(95)00636-n. [DOI] [PubMed] [Google Scholar]
- 34.Thomas S M, Brugge J. Cellular functions regulated by src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 35.Uchida T, Matozaki T, Noguchi T, Yamao T, Horita K, Suzuki T, Fujioka Y, Sakamoto C, Kasuga M. Insulin stimulates the phosphorylation of Tyr538 and the catalytic activity of PTP1C, a protein tyrosine phosphatase with Src homology-2 domains. J Biol Chem. 1994;269:12220–12228. [PubMed] [Google Scholar]
- 36.Virji M, Makepeace K, Ferguson D J P, Watt S M. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol Microbiol. 1996;22:941–950. doi: 10.1046/j.1365-2958.1996.01551.x. [DOI] [PubMed] [Google Scholar]
- 37.Vogel W, Lammers R, Huang J, Ullrich A. Activation of a phosphotyrosine phosphatase by tyrosine phosphorylation. Science. 1993;259:1611–1614. doi: 10.1126/science.7681217. [DOI] [PubMed] [Google Scholar]
- 38.Xu W, Harrison S C, Eck M J. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–601. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]