Abstract
Background
The effect of extended thromboprophylaxis in improving the prognosis of adult patients with coronavirus disease 2019 (COVID-19) after discharge remains debatable. This meta-analysis was aimed to determine the advantages and disadvantages of extended thromboprophylaxis in these patients.
Methods
Different databases such as PubMed, Embase, Web of Science, and Cochrane Library were systematically searched for studies that evaluated the effects of extended thromboprophylaxis in post-discharge patients with COVID-19 until 13 June 2022. The primary efficacy outcome was defined by the composite outcome of thromboembolism and all-cause mortality, and the safety outcome was defined by bleeding events. The odds ratios (ORs) and 95 % confidence intervals (CIs) of efficacy and safety outcomes were calculated using fixed- or random-effects model. Interaction analysis was performed to assess and compare observational studies and randomised controlled trials (RCTs). A sensitivity analysis was performed after excluding studies of poor quality.
Results
Eight studies involving 10,148 patients were included. The results confirmed that extended thromboprophylaxis, primarily prophylactic use of anticoagulants for <35 days, was significantly associated with reduced composite outcome in high-risk post-discharge patients with COVID-19 (OR: 0.52; 95 % CI: 0.41–0.67, P = 0.000). Interaction analysis revealed that the effect estimates were consistent between the RCT and observational studies (Pinteraction = 0.310). Furthermore, extended thromboprophylaxis did not increase the risk of major bleeding events (OR: 1.64; 95 % CI: 0.95–2.82, P = 0.075).
Conclusion
In post-discharge patients with COVID-19 at high risk of thromboembolism, extended thromboprophylaxis, primarily prophylactic use of anticoagulants for <35 days, can significantly reduce the risk of thrombosis and all-cause mortality without increasing the risk of major bleeding events.
Registration
PROSPERO CRD42022339399.
Keywords: COVID-19, Extended thromboprophylaxis, Thromboembolism, Major bleeding events, meta-analysis
1. Introduction
Coronavirus disease 2019 (COVID-19) has spread throughout the world [1], [2]. Recent research has shown that despite adequate thromboprophylaxis during hospitalization, the risk of thromboembolism and the incidence of venous thromboembolism (VTE) (1.5–2.5 %) in post-discharge patients with COVID-19 remain high [3], [4], [5]. Previous studies have revealed that thrombotic events are caused by interactions among the immune system, endothelial injury, and vascular inflammation [6], [7], [8], which complicate COVID-19 [4], [9] and may contribute to poor prognosis.
Extended thromboprophylaxis may exert a protective effect in these patients [4]. However, previous meta-analyses of randomised controlled trials (RCTs) demonstrated that extended thromboprophylaxis can significantly reduce the incidence of thromboembolism after discharge but increase major bleeding in high-risk patients without COVID-19 [10], [11], [12]. However, the risk of bleeding and net benefit of extended thromboprophylaxis in patients with COVID-19 remains unclear. Guidelines are conflicting because of the limited data available regarding the effects of extended thromboprophylaxis [13], [14], [15].
Hitherto, no meta-analysis has specifically revealed the positive and negative effects of extended thromboprophylaxis in post-discharge patients with COVID-19 [16]. The protective effects of different types of anticoagulants, such as low-molecular-weight heparin (LMWH) and direct oral anticoagulants (DOACs), in post-discharge patients with COVID-19 remain controversial. Furthermore, the optimal intensity and duration of extended thromboprophylaxis remain unclear. Therefore, the present meta-analysis was aimed to comprehensively and meticulously investigate the above issues.
2. Materials and methods
This study was performed in accordance with a protocol prospectively registered in PROSPERO (CRD42022339399) and the PRISMA statement.
2.1. Data sources and search strategy
Different databases such as PubMed, Embase, Web of Science, and Cochrane Library were searched to identify studies that evaluated the effects of extended thromboprophylaxis from inception till 13 June 2022 without language restriction. Briefly, the search strategy included three key concepts according to the PICOS format: (1) post-discharge patients with COVID-19, (2) extended thromboprophylaxis, and (3) human studies (Supplementary Table 1). Medical Subject Headings and relevant keywords were used to identify eligible articles. The search strategy is presented in Supplementary Table 2. The references of relevant articles were screened to further identify potential articles.
2.2. Study selection and outcomes
Studies that met the following predetermined criteria were included in this systematic review: (1) RCTs, cohort studies, or case-control studies; (2) adult patients (aged ≥18 years); (3) patients who were hospitalized with COVID-19 at discharge; (4) patients who received extended thromboprophylaxis post discharge; and (5) incidence of thromboembolism or bleeding events reported as outcomes. Studies that met the following criteria were excluded: (1) reviews, conference abstracts, and case reports; (2) no comparison group; (3) outpatients or inpatients instead of post-discharge patients with COVID-19; and (4) patients suspected or confirmed to have a thrombotic event.
Guidelines for prescribing extended thromboprophylaxis have evolved over the course of the pandemic, resulting in inconsistent thromboprophylaxis indications at different times. The prescription and duration of thromboprophylaxis after discharge were at the discretion of the professional medical team, based on prevailing guidelines. However, the included studies met the following criteria: extended thromboprophylaxis was performed in patients with an increased risk of thromboembolism with active cancer, immobilisation, respiratory failure, or personal/family history of VTE as the underlying risk factors, or an International Medical Prevention Registry on Venous Thromboembolism (IMPROVE VTE) score of ≥4 or a score of 2–3 with D-dimer level of >500 ng/mL, or indications for anticoagulation at hospital discharge.
The primary efficacy outcome was a composite outcome consisting of thromboembolic events and all-cause mortality, and only thromboembolic events were analysed as the secondary efficacy outcome. Bleeding was the primary safety outcome as per the criteria of the International Society on Thrombosis and Haemostasis (ISTH). Two investigators (MFD and WXX) independently performed literature search and reviewed the content to determine eligible articles. Another investigator (LF) arbitrated any discrepancies.
2.3. Data extraction
The following information was collected independently by the two investigators using a pre-specified form: study design, sample size (number of patients in each group), incidence of intensive care unit (ICU) stay, drugs for thromboprophylaxis, drug doses, duration of thromboprophylaxis, follow-up duration, and outcomes. Adjusted effect estimates were preferred to unadjusted estimates and extracted to reduce confounding factors [17]. Unadjusted estimates were extracted when adjusted estimates were not provided. All the extracted information was cross-checked for accuracy by the third author (LF).
2.4. Quality assessment
The methodological quality of the RCTs was identified in accordance with the Cochrane Collaboration Risk of Bias Tool [18]. The Newcastle–Ottawa Scale (NOS) score was used to evaluate methodological quality of observational studies while assessing the selection, comparability, and ascertainment of either the exposure or outcome of interest for case-control and cohort studies, respectively [19]. The NOS scores ranged from 0 to 9, with scores <5 indicating a high risk of bias [19].
2.5. Statistical analysis
The odds ratios (ORs) and 95 % confidence intervals (CIs) of the efficacy and safety outcomes were calculated and pooled in the random- or fixed-effects model depending on between-study heterogeneity. An I 2-test >50 % indicated considerable heterogeneity [20]. The fixed-effects model was used based on the generic inverse variance method unless considerable heterogeneity was present [20]. Interaction analysis (P interaction) was conducted to evaluate comparability between RCTs and observational studies. Sensitivity analyses involving sequential removal of each study were performed to determine robustness of the results. Furthermore, the estimates were calculated after excluding studies of poor quality to minimise the risk of bias. In addition, a qualitative analysis was conducted to provide recommendations regarding the anticoagulant type, intensity, and duration of extended thromboprophylaxis. The potential publication bias was assessed through funnel plots and Egger's test [21], [22]. Meta-analyses were performed using the STATA 15.1 (Stata, College Station, TX, USA).
3. Results
3.1. Study selection and characteristics
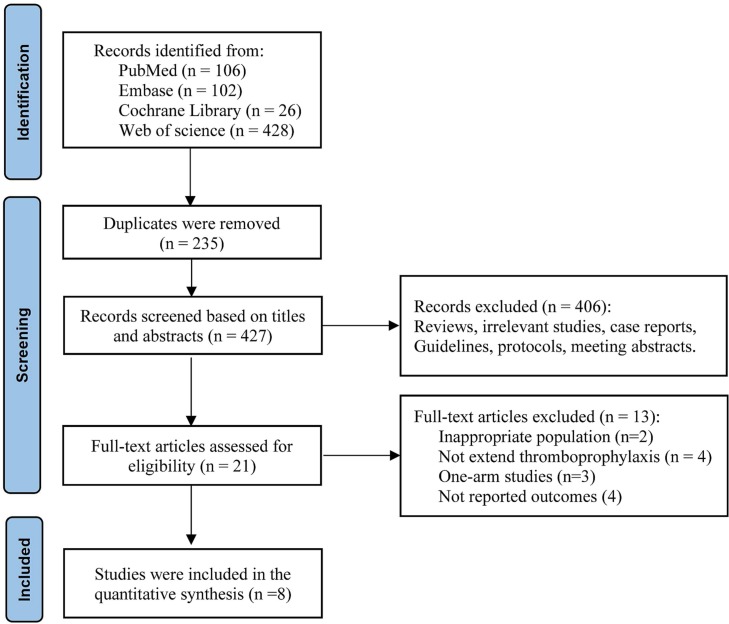
The initial search using electronic databases identified 662 studies. After screening the title, abstract, and full text, eight studies [4], [23], [24], [25], [26], [27], [28], [29] involving 10,148 post-discharge patients with COVID-19 at high risk of thromboembolism were selected. The study selection process is illustrated in Fig. 1 . Supplementary Table 3 shows patient selection for extended thromboprophylaxis from each study.
Fig. 1.
Flow diagram representing study selection process.
The eight studies include seven observational studies and one RCT. Four studies were conducted in the USA, and the other four were conducted in Belgium, Israel, Brazil, and England. Detailed information on extended thromboprophylaxis, including the type of drug, dosage, and duration, in these studies is summarised in Table 1 . More than 80 % of patients in the thromboprophylaxis group received DOACs, and >70 % of patients received prophylactic dose. Four studies have reported the duration of extended thromboprophylaxis, which was <35 days after discharge in all studies.
Table 1.
Characteristics of the included studies.
| Authors | Study design | Country | Sample size (TP/non-TP) | ICU stay (%) | Drugs for thromboprophylaxis | Drug dosage | Duration of thromboprophylaxis | Follow-up duration | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Courtney et al. (2022) | Observational study | USA | 1171 (132/1039) | NA | Rivaroxaban (86.3 %), enoxaparin (12.9 %), and apixaban (0.8 %) | Prophylactic doses (100 %) | 28 days | 35 days | 8 |
| Engelen et al. (2021) | Observational study | Belgium | 146 (41/105) | 39 | Enoxaparin (100 %) | Prophylactic doses (100 %) | 14 days | 42 days | 7 |
| Eswaran et al. (2020) | Observational study | USA | 447 (190/257) | 39.4 | DOACs (90 %) | Prophylactic doses (100 %) | NA | 30 days | 5 |
| Giannis et al. (2021) | Observational study | USA | 4906 (612/4294) | 11.8 | Enoxaparin (12.3 %), apixaban (29.4 %), and rivaroxaban (54.9 %) | Prophylactic doses (94.9 %) and therapeutic doses (5.1 %) | NA | 90 days | 8 |
| Li et al. (2021) | Observational study | USA | 2832 (682/2150) | 15.2 | NA | Prophylactic doses (27.6 %) and therapeutic doses (72.4 %) | NA | 90 days | 8 |
| Patell et al. (2020) | Observational study | Israel | 176 (13/163) | NA | LWMH (76.9 %) and DOACs (15.4 %) | Prophylactic doses (100 %) | NA | 30 days | 5 |
| Ramacciotti et al. (2021) | RCT | Brazil | 318 (159/159) | 52 | Rivaroxaban (100 %) | Prophylactic doses (100 %) | 35 days | 35 days | Low risk |
| Salisbury et al. (2020) | Observational study | England | 152 (5/147) | 22 | LWMH (100 %) | Prophylactic doses (100 %) | 7 days | 42 days | 5 |
RCT, randomised controlled trial; TP, thromboprophylaxis; ICU, intensive care unit; DOACs, direct oral anticoagulants; LWMH, low-molecular-weight heparin; NA, not available.
The methodological quality of the observational studies and RCT was evaluated. Three studies were of low quality, primarily because of comparability issues, whereas the other five studies were fair and of good quality (Table 1).
3.2. Association of extended thromboprophylaxis with thromboembolism and all-cause mortality
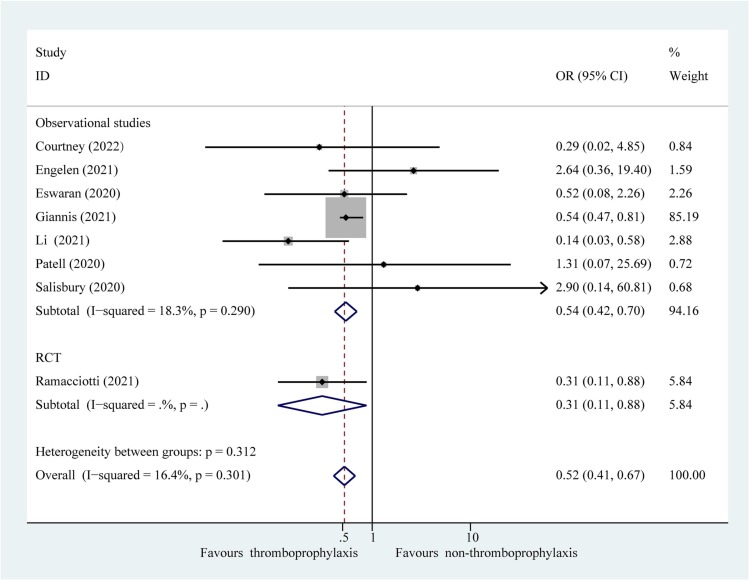
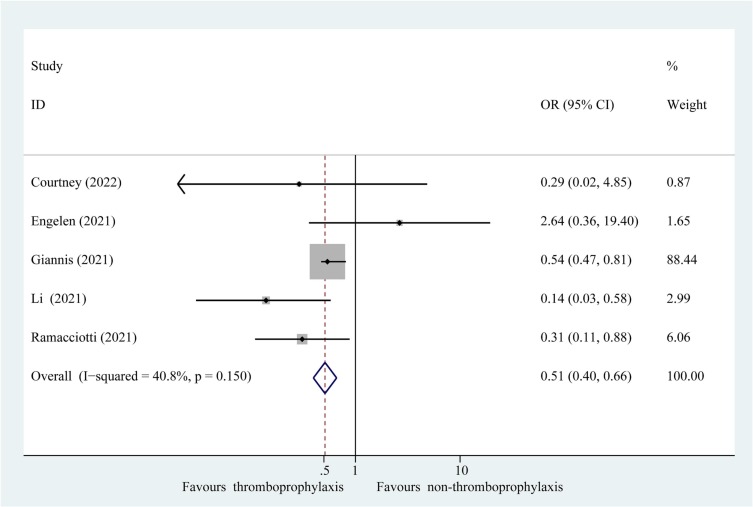
Composite outcomes consisting of thromboembolism and all-cause mortality were represented in the eight studies. Composite outcomes occurred in 83 of 1834 patients (4.53 %) receiving extended thromboprophylaxis and in 459 of 8314 patients (5.52 %) who did not receive this treatment. The results of the meta-analysis revealed that extended thromboprophylaxis was significantly associated with a reduced composite outcome of thrombosis and all-cause mortality in post-discharge patients with COVID-19 (Fig. 2 ; OR: 0.52; 95 % CI: 0.41–0.67, P = 0.000; I 2 = 16.4 %). Interaction analysis revealed no significant difference in the effect estimates between the RCT and observational studies (P interaction = 0.310). Furthermore, sensitivity analysis after excluding poor-quality studies confirmed the results of the primary analyses (Fig. 3 ; OR: 0.51; 95 % CI: 0.40–0.66, P = 0.000; I 2 = 40.8 %). Sequential elimination of each study failed to identify those having a significant influence on the results (Supplementary Table 4). In addition, for the secondary efficacy outcome, patients receiving extended thromboprophylaxis had a significantly lower risk of post-discharge thrombosis than those without this treatment (Supplementary Fig. 1; OR: 0.62; 95 % CI: 0.42–0.94, P = 0.023; I 2 = 30.2 %).
Fig. 2.
Fixed effects model showing association of extended thromboprophylaxis with composite outcome of thrombosis and all-cause mortality in post-discharge patients with COVID-19. P, heterogeneity test.
Fig. 3.
Fixed effects model of sensitivity analysis after excluding studies with a high risk of bias showing association of extended thromboprophylaxis with composite outcome of thrombosis and all-cause mortality in post-discharge patients with COVID-19. P, heterogeneity test.
3.3. Association of extended thromboprophylaxis with bleeding events
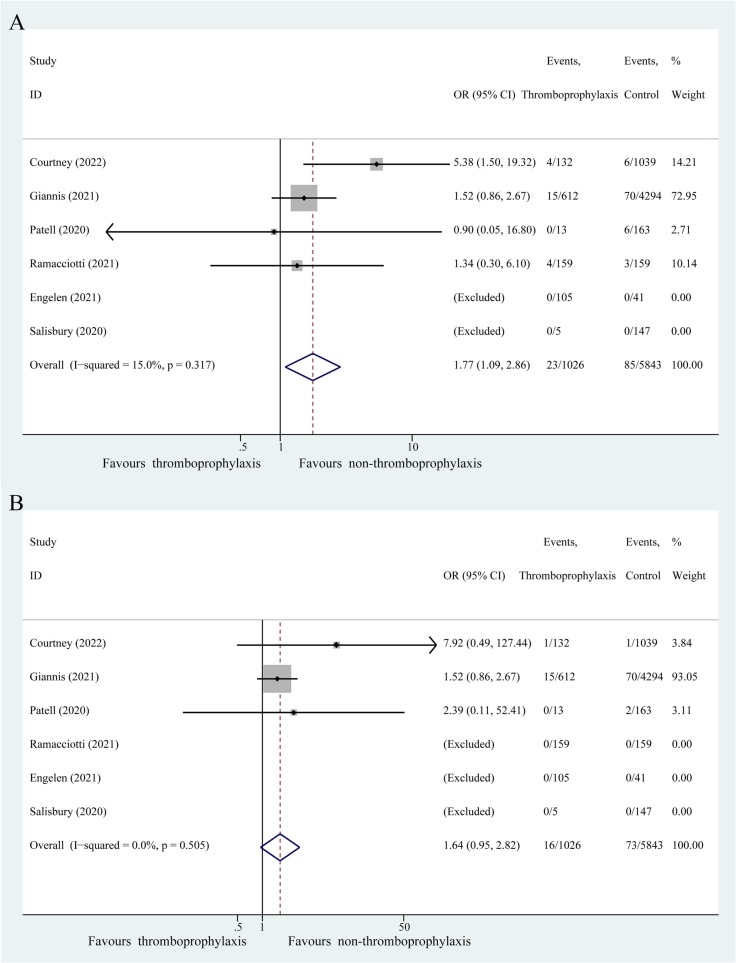
Six studies reported primary safety outcomes, including all and major bleeding events. All bleeding events occurred in 23 of 1026 patients (2.24 %) receiving extended thromboprophylaxis and in 85 of 5843 patients (1.45 %) without this treatment (Fig. 4A). Patients receiving extended thromboprophylaxis were associated with a significantly higher risk of all bleeding events than those without this treatment (OR: 1.77; 95 % CI: 1.09–2.86, P = 0.021; I 2 = 15.0 %). Major bleeding events occurred in 16 of 1026 patients (1.56 %) receiving extended thromboprophylaxis and in 73 of 5843 patients (1.25 %) without this treatment (Fig. 4B). Moreover, extended thromboprophylaxis was not associated with a significant increase in major bleeding events (OR: 1.64; 95 % CI: 0.95–2.82, P = 0.075; I 2 = 0.0 %).
Fig. 4.
Fixed effects model showing association of extended thromboprophylaxis with bleeding events in post-discharge patients with COVID-19. (A) All bleeding events. (B) Major bleeding events. P, heterogeneity test.
3.4. Publication bias
Visual inspection of funnel plots showed qualitative symmetry (Supplementary Fig. 2). Egger's test (P = 0.875) failed to detect significant publication bias, consistent with the results of funnel plots.
4. Discussion
This study is the first, to the best of our knowledge, to provide a comprehensive investigation of the effectiveness and safety of extended thromboprophylaxis in post-discharge patients with COVID-19 based on evidences from RCT and observational studies simultaneously. The results suggested that extended thromboprophylaxis significantly reduced the incidence of composite outcome of thrombosis and all-cause mortality in post-discharge patients with COVID-19 without increasing the risk of major bleeding events. No significant difference in effectiveness estimates was observed between the RCT and observational studies. Although the analysis of safety suggested a higher risk of all bleeding events in post-discharge patients with COVID-19 receiving extended thromboprophylaxis than in those without this treatment, no significant difference in major bleeding events was detected. Together, these results indicate that extended thromboprophylaxis can improve the clinical outcomes in post-discharge patients with COVID-19 at high risk of thromboembolism.
Post-discharge patients with COVID-19 have a higher incidence of thrombosis than those with other infectious diseases [4], [30]. Extended thromboprophylaxis has been proposed to minimise the risk of thrombosis. Based on evidence from the eight studies involving 10,148 post-discharge patients with COVID-19, this meta-analysis is the first to investigate the effectiveness and safety of extended thromboprophylaxis. The results demonstrate that extended thromboprophylaxis can reduce the risk of post-discharge thrombosis and all-cause mortality without increasing the risk of major bleeding events. However, a meta-analysis by Rungjirajittranon et al. [3] reported no association between extended thromboprophylaxis and thrombosis in post-discharge patients with COVID-19 (OR: 0.84; 95 % CI: 0.26–2.70; P = 0.49), which was inconsistent with the results of the present study. However, this previous meta-analysis [3] included only four observational studies (of which two were of poor quality) and did not include RCTs, thereby providing inadequate preliminary conclusions. The present study is a comprehensive review of one RCT and seven observational studies to provide high-quality evidence. In particular, the pooled results of the primary outcome from the RCT (OR: 0.31; 95 % CI: 0.11–0.88) were consistent with those from observational studies (OR: 0.54; 95 % CI: 0.42–0.70), with a P interaction of 0.31. The results of the RCT were replicated and validated in observational studies. Observational studies in real-world clinical practice could provide critical clinical evidence of the benefits and risks of interventions when RCTs are lacking [31] and are complementary to RCTs. The results of the meta-analysis of RCTs were confirmed by observational studies, which supported extrapolation of the results to clinical practice. In addition, the effect estimates after excluding studies of poor quality were consistent with the results of the primary analysis, which provided robust evidence for the efficacy and safety of the intervention.
Based on data from the MICHELLE trial [4], patients with COVID-19 and increased risk of thromboembolism, defined by an IMPROVE VTE score [32] of 2–3 with D-dimer level >500 ng/mL or a score ≥4 independent of the D-dimer level at hospital discharge, should be considered for extended thromboprophylaxis at hospital discharge to improve clinical outcomes, including reducing major and fatal thromboembolic events, without increase in major bleeding events [4]. The results of this meta-analysis supported the recommendation from the MICHELLE trial [4] that extended thromboprophylaxis is an attractive strategy to improve prognosis in patients with an increased risk of thromboembolism.
No previous systematic review has investigated which between DOACs and LMWH should be recommended as a priority anticoagulant for post-discharge patients with COVID-19. Furthermore, the optimal intensity and duration of extended thromboprophylaxis remain unclear. Qualitative analysis can provide critical clinical evidence for decision making when quantitative data are lacking. A comprehensive qualitative analysis was conducted to provide preliminary recommendations on these issues. First, >80 % of patients in the thromboprophylaxis group received DOACs, and pooled estimates showed that thromboprophylaxis could improve clinical outcomes. Furthermore, oral administration of DOACs has advantages of convenience of use and economic significance over injection administration of LMWH. Therefore, it is reasonable to recommend DOACs for extended thromboprophylaxis. Regarding the optimal intensity of thromboprophylaxis, >70 % of patients in the thromboprophylaxis group received a prophylactic dose, and previous studies have shown that prophylactic doses were associated with a lower risk of major bleeding events than that of therapeutic doses [33]. Therefore, the results of this systematic review support the use of prophylactic doses for extended thromboprophylaxis. Furthermore, regarding the duration of thromboprophylaxis after discharge, extended thromboprophylaxis for <35 days was appropriate and supported by the results of the MICHELLE trial [4]; however, additional RCTs are required to draw more definitive conclusions on the optimal duration of thromboprophylaxis.
However, these results should be interpreted with caution for two reasons. First, patients included in this study had a high risk of thromboembolism at discharge. Second, although extended thromboprophylaxis did not increase the risk of major bleeding events, it was associated with a significant increase in all bleeding events. Therefore, extended thromboprophylaxis after discharge should not be generalised to all patients with COVID-19. However, patients at high risk of thromboembolism should adhere to extended thromboprophylaxis, which is consistent with the recommendations of the American Society of Hematology (ASH) living guidelines [34]. A comprehensive assessment of thrombotic and bleeding risks should be performed before decision making, which is the cornerstone of extended thromboprophylaxis.
Several ongoing RCTs have evaluated the effectiveness and safety of extended thromboprophylaxis in patients with COVID-19 [3], [35], [36]; high-quality evidence from RCTs is required to substantiate this finding.
5. Limitations
The results of the present study should be interpreted with caution because of the following limitations: First, seven of the eight included studies were observational studies and one was an open-label RCT. The meta-analysis of data from observational studies had some limitations, including selection bias and clinical event ascertainment. Second, the absence of clarity and consistency in defining high risk of thromboembolism might introduce selection bias for patients included in the studies; moreover, differences in diagnostic methods and collection of thromboembolism-related data across trials might introduce certain biases. Nevertheless, patient selection and outcome data collection were managed by a professional medical team, and bias was considered acceptable. Finally, as most studies lacked information on drug type and dosage, drug type and dosage subgroups were not included in this study. Nonetheless, qualitative analysis in this study provided important evidence that can be used to select drugs and dosages; however, additional RCTs are needed to support these recommendations.
6. Conclusion
The current systematic review and meta-analysis revealed that extended thromboprophylaxis administered for <35 days could significantly reduce the risk of post-discharge thrombosis in patients with COVID-19, without increasing the risk of major bleeding events. Additional RCTs are warranted to verify the findings of the present study.
Funding
This study was funded by the Zhejiang Pharmaceutical Association (2022ZYJ18) and Medical Health Science and Technology Project of Zhejiang Province (2022KY105).
Declaration of competing interest
The author reports no conflicts of interest in this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.thromres.2022.11.019.
Appendix A. Supplementary data
Supplementary material
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.John Hopkins University John Hopkins University covid-19. https://coronavirus.jhu.edu/map.html
- 3.Rungjirajittranon T., Owattanapanich W., Leelakanok N., Sasijareonrat N., Suwanawiboon B., Chinthammitr Y., Ruchutrakool T. Thrombotic and hemorrhagic incidences in patients after discharge from COVID-19 infection: a systematic review and meta-analysis. Clin. Appl. Thromb. Hemost. 2021;27 doi: 10.1177/10760296211069082. 10760296211069082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramacciotti E., Agati L.Barile, Calderaro D., Aguiar V.C.R., Spyropoulos A.C., de Oliveira C.C.C., Santos J.Lins Dos, Volpiani G.G., Sobreira M.L., Joviliano E.E., Júnior M.S.Bohatch, da Fonseca B.A.L., Ribeiro M.S., Dusilek C., Itinose K., Sanches S.M.V., de Almeida Araujo Ramos K., de Moraes N.F., Tierno P., de Oliveira A., Tachibana A., Chate R.C., Santos M.V.B., de Menezes Cavalcante B.B., Moreira R.C.R., Chang C., Tafur A., Fareed J., Lopes R.D. Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. Lancet (London, England) 2022;399(10319):50–59. doi: 10.1016/S0140-6736(21)02392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuin M., Engelen M.M., Barco S., Spyropoulos A.C., Vanassche T., Hunt B.J., Vandenbriele C., Verhamme P., Kucher N., Rashidi F., Zuliani G., Konstantinides S.V., Roncon L. Incidence of venous thromboembolic events in COVID-19 patients after hospital discharge: a systematic review and meta-analysis. Thromb. Res. 2022;209:94–98. doi: 10.1016/j.thromres.2021.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet (London, England) 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belen-Apak F.B., Sarıalioğlu F. Pulmonary intravascular coagulation in COVID-19: possible pathogenesis and recommendations on anticoagulant/thrombolytic therapy. J. Thromb. Thrombolysis. 2020;50(2):278–280. doi: 10.1007/s11239-020-02129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Fagot Gandet F., Fafi-Kremer S., Castelain V., Schneider F., Grunebaum L., Anglés-Cano E., Sattler L., Mertes P.M., Meziani F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zayed Y., Kheiri B., Barbarawi M., Banifadel M., Abdalla A., Chahine A., Obeid M., Haykal T., Yelangi A., Malapati S., Bachuwa G., Seedahmed E. Extended duration of thromboprophylaxis for medically ill patients: a systematic review and meta-analysis of randomised controlled trials. Intern. Med. J. 2020;50(2):192–199. doi: 10.1111/imj.14417. [DOI] [PubMed] [Google Scholar]
- 11.Spyropoulos A.C., Ageno W., Albers G.W., Elliott C.G., Halperin J.L., Hiatt W.R., Maynard G.A., Steg P.G., Weitz J.I., Lu W., Spiro T.E., Barnathan E.S., Raskob G.E. Post-discharge prophylaxis with rivaroxaban reduces fatal and major thromboembolic events in medically ill patients. J. Am. Coll. Cardiol. 2020;75(25):3140–3147. doi: 10.1016/j.jacc.2020.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen A.T., Harrington R.A., Goldhaber S.Z., Hull R.D., Wiens B.L., Gold A., Hernandez A.F., Gibson C.M. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N. Engl. J. Med. 2016;375(6):534–544. doi: 10.1056/NEJMoa1601747. [DOI] [PubMed] [Google Scholar]
- 13.Spyropoulos A.C., Levy J.H., Ageno W., Connors J.M., Hunt B.J., Iba T., Levi M., Samama C.M., Thachil J., Giannis D., Douketis J.D. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020;18(8):1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moores L.K., Tritschler T., Brosnahan S., Carrier M., Collen J.F., Doerschug K., Holley A.B., Jimenez D., Le Gal G., Rali P., Wells P. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158(3):1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Society of Hematology ASH guidelines on use of anticoagulation in patients with COVID-19. https://www.hematology.org/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines/ash-guidelines-on-use-of-anticoagulation-in-patients-with-covid-19#rec3
- 16.Tunjungputri R.N., Tetrasiwi E.N., Mulansari N.A., Harimurti K., Nelwan E.J. Parenteral and oral anticoagulant treatment for hospitalized and post-discharge COVID-19 patients: a systematic review and meta-analysis. Acta Med. Indones. 2022;54(2):190–209. [PubMed] [Google Scholar]
- 17.Kamel A.M., Sobhy M., Magdy N., Sabry N., Farid S. Anticoagulation outcomes in hospitalized Covid-19 patients: a systematic review and meta-analysis of case-control and cohort studies. Rev. Med. Virol. 2021;31(3) doi: 10.1002/rmv.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed.) 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells G., Shea B., O'Connell D., Peterson J., Welch V., Losos M., Tugwell P. Ottawa Hospital Research Institute Research Programs; 2021. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 20.Cumpston M., Li T., Page M.J., Chandler J., Welch V.A., Higgins J.P., Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019;10 doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 22.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Courtney L.A., Trujillo T.C., Saseen J.J., Wright G., Palkimas S. Evaluation of the clinical impact of thromboprophylaxis in patients with COVID-19 following hospital discharge. Ann. Pharmacother. 2022;56(9):981–987. doi: 10.1177/10600280211064306. 10.1177/10600280211064306. [DOI] [PubMed] [Google Scholar]
- 24.Engelen M.M., Vandenbriele C., Balthazar T., Claeys E., Gunst J., Guler I., Jacquemin M., Janssens S., Lorent N., Liesenborghs L., Peerlinck K., Pieters G., Rex S., Sinonquel P., Van der Linden L., Van Laer C., Vos R., Wauters J., Wilmer A., Verhamme P., Vanassche T. Venous thromboembolism in patients discharged after COVID-19 hospitalization. Semin. Thromb. Hemost. 2021;47(4):362–371. doi: 10.1055/s-0041-1727284. [DOI] [PubMed] [Google Scholar]
- 25.Eswaran H., Jarmul J.A., Shaheen A.W., Meaux D., Long T., Saccoccio D., Moll S. Vascular thromboembolic events following COVID-19 hospital discharge: incidence and risk factors. Res. Pract. Thromb. Haemost. 2021;5(2):292–295. doi: 10.1002/rth2.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giannis D., Allen S.L., Tsang J., Flint S., Pinhasov T., Williams S., Tan G., Thakur R., Leung C., Snyder M., Bhatia C., Garrett D., Cotte C., Isaacs S., Gugerty E., Davidson A., Marder G.S., Schnitzer A., Goldberg B., McGinn T., Davidson K.W., Barish M.A., Qiu M., Zhang M., Goldin M., Matsagkas M., Arnaoutoglou E., Spyropoulos A.C. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood. 2021;137(20):2838–2847. doi: 10.1182/blood.2020010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li P., Zhao W., Kaatz S., Latack K., Schultz L., Poisson L. Factors associated with risk of postdischarge thrombosis in patients with COVID-19. JAMA Netw. Open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.35397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patell R., Bogue T., Koshy A., Bindal P., Merrill M., Aird W.C., Bauer K.A., Zwicker J.I. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136(11):1342–1346. doi: 10.1182/blood.2020007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salisbury R., Iotchkova V., Jaafar S., Morton J., Sangha G., Shah A., Untiveros P., Curry N., Shapiro S. Incidence of symptomatic, image-confirmed venous thromboembolism following hospitalization for COVID-19 with 90-day follow-up. Blood Adv. 2020;4(24):6230–6239. doi: 10.1182/bloodadvances.2020003349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Samkari H., Karp Leaf R.S., Dzik W.H., Carlson J.C.T., Fogerty A.E., Waheed A., Goodarzi K., Bendapudi P.K., Bornikova L., Gupta S., Leaf D.E., Kuter D.J., Rosovsky R.P. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei A.H., Gu Z.C., Zhang C., Ding Y.F., Liu D., Li J., Liu X.Y., Lin H.W., Pu J. Increased risk of myocardial infarction with dabigatran etexilate: fact or fiction? A critical meta-analysis of over 580,000 patients from integrating randomized controlled trials and real-world studies. Int. J. Cardiol. 2018;267:1–7. doi: 10.1016/j.ijcard.2018.05.048. [DOI] [PubMed] [Google Scholar]
- 32.Spyropoulos A.C., Anderson F.A., Jr., FitzGerald G., Decousus H., Pini M., Chong B.H., Zotz R.B., Bergmann J.F., Tapson V., Froehlich J.B., Monreal M., Merli G.J., Pavanello R., Turpie A.G.G., Nakamura M., Piovella F., Kakkar A.K., Spencer F.A. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140(3):706–714. doi: 10.1378/chest.10-1944. [DOI] [PubMed] [Google Scholar]
- 33.Zhang S., Li Y., Liu G., Su B. Intermediate-to-therapeutic versus prophylactic anticoagulation for coagulopathy in hospitalized COVID-19 patients: a systemic review and meta-analysis. Thromb. J. 2021;19(1):91. doi: 10.1186/s12959-021-00343-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuker A., Tseng E.K., Nieuwlaat R., Angchaisuksiri P., Blair C., Dane K., Davila J., DeSancho M.T., Diuguid D., Griffin D.O., Kahn S.R., Klok F.A., Lee A.I., Neumann I., Pai A., Righini M., Sanfilippo K.M., Siegal D., Skara M., Terrell D.R., Touri K., Akl E.A., Jabiri R.N.Al, Jabiri Y.N.Al, Barbara A.M., Bognanni A., Akl I.Bou, Boulos M., Brignardello-Petersen R., Charide R., Chan M., Colunga-Lozano L.E., Dearness K., Darzi A.J., Hussein H., Karam S.G., Kolb P., Mansour R., Morgano G.P., Morsi R.Z., Muti-Schünemann G., Nadim M.K., Noori A., Philip B.A., Piggott T., Qiu Y., Benitez Y.R., Schünemann F., Stevens A., Solo K., Wiercioch W., Mustafa R.A., Schünemann H.J. American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19: July 2021 update on postdischarge thromboprophylaxis. Blood Adv. 2022;6(2):664–671. doi: 10.1182/bloodadvances.2021005945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.XACT Factor Xa inhibitor versus standard of care heparin in hospitalized patients with COVID-19 (XACT) November 23, 2020. https://clinicaltrials.gov/ct2/show/NCT04640181
- 36.ACTIV-4c COVID-19 thrombosis prevention trials: post-hospital thromboprophylaxis. December 2, 2020. https://clinicaltrials.gov/ct2/show/study/NCT04650087
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material