Abstract
Background
Prolonged cognitive deficits (“brain fog”) following COVID19 infection (long-COVID) are common and debilitating, yet there are currently no approved treatments. Cognitive impairment particularly targets the working memory and executive functions of the prefrontal cortex (PFC). The PFC has unusual neurotransmission and neuromodulation that render it vulnerable to stressors, and basic research has identified mechanisms that protect PFC connections. Based on the basic neuroscience data, we tried a combined open label treatment to bolster prefrontal function: the α2A-adrenoceptor agonist, guanfacine, which strengthens prefrontal connectivity, and the anti-oxidant, N- acetylcysteine (NAC), which protects mitochondria and reduces kynurenic acid blockade of NMDA receptors.
Case report
Twelve patients with “brain fog” including difficulties in executive functions were treated with guanfacine (1mg, PO bedtime for the first month, increased to 2mg after 1 month, if well-tolerated) and 600 mg NAC daily. Guanfacine+NAC improved cognitive abilities in eight of the twelve patients; four patients discontinued therapy, two for unspecified reasons and two due to hypotension and/or dizziness, common side effects of guanfacine. Those who stayed on guanfacine+NAC reported improved working memory, concentration, and executive functions, including a resumption of normal workloads. One patient briefly stopped taking guanfacine due to a hypotensive episode and reported a return of cognitive deficits that abated with resumed guanfacine treatment.
Conclusion
Although placebo-controlled trials will be needed to more rigorously demonstrate efficacy, as these agents have established safety, they may be immediately helpful in treating the large number of patients suffering from prolonged cognitive deficits following COVID19 infection.
Keywords: Intuniv, SARS-CoV-2, Cognition, Brain fog, Guanfacine, NAC, PFC
Background
A common consequence of post-COVID-19 infection is residual cognitive impairment (“brain fog”), which interferes with work and daily living. The most consistent deficits are in working memory and the executive functions e.g. (Vanderlind et al., 2021), cognitive functions that are generated by the prefrontal cortex (PFC) (Arnsten et al., 2021). There are no approved treatments for these symptoms, despite urgent need.
Neurobiological data help explain the preferential vulnerability of the PFC, and offer clues to treatment. The PFC has extensive recurrent excitatory connections on spines to keep information “in mind” (Arnsten et al., 2021). These synapses are particularly vulnerable due to two unusual features (Fig. 1 ): 1) they heavily rely on NMDA receptor (NMDAR) rather than AMPA receptor (AMPAR) neurotransmission (Arnsten et al., 2021); and 2) they express feedforward calcium-cAMP-PKA signaling, which opens potassium (K+) channels to weaken connectivity, e.g. during stress. With chronic stress, calcium overload of mitochondria signals microglia to remove spines (Arnsten et al., 2021).
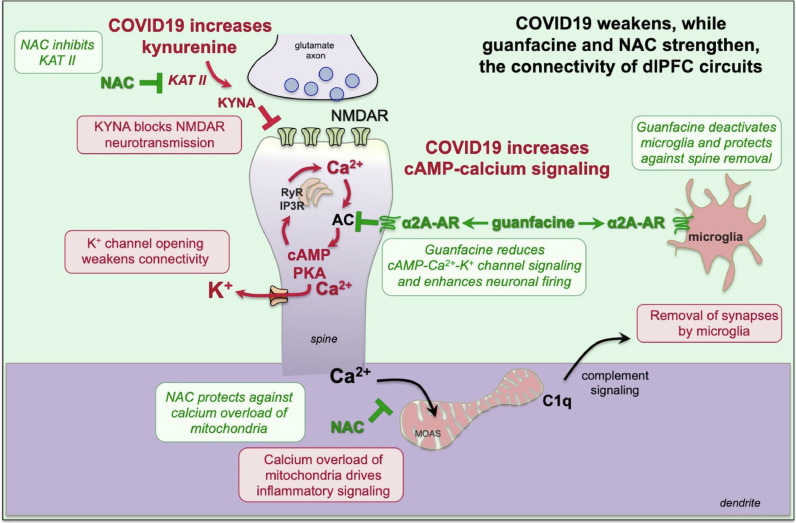
Fig. 1.
Schematic illustration of how the α2A-AR agonist, guanfacine, and the anti-oxidant NAC, can have therapeutic effects in treating the symptoms of TBI and long-COVID by strengthening PFC neurotransmission and synaptic connections and reducing neuroinflammation. Layer III dlPFC microcircuits depend on NMDAR synapses on dendritic spines to produce the persistent neuronal firing needed for higher cognition. Inflammation drives the production of kynurenic acid, generated from kynurenine by KAT II, which blocks NMDAR and markedly reduces dlPFC neuronal firing. These synapses are also weakened by the opening of nearby potassium (K+) channels by high levels of cAMP-calcium signaling (Arnsten et al., 2021). Stressors increase catecholamine release in the PFC, which stimulates α1-AR and D1R to drive intracellular calcium-cAMP-PKA signaling (Arnsten et al., 2021). This in turn opens K+ channels to weaken synaptic efficacy, reduce neuronal firing, and impair working memory and executive functioning. With sustained stress, calcium overloads mitochondria driving a MOAS phenotype, and the initiation of complement C1q signaling, which communicates to microglia to remove spines and dendrites (phagocytosis), leading to loss of PFC gray matter. Guanfacine and NAC can reduce many of these harmful actions: NAC can inhibit KAT II to reduce kynurenic acid production, restoring NMDR neurotransmission, while the α2A-AR agonist, guanfacine, inhibits cAMP-PKA signaling in spines to reduce calcium dysregulation and close K+ channels, strengthening network connectivity. These agents can also reduce neuroinflammatory actions: NAC reduces calcium overload of mitochondria, and guanfacine deactivates microglia to reduce phagocytosis. Note that α2A-AR have high affinity for NE and are thus engaged under nonstress conditions with modest levels of NE release, while α1-AR have low affinity for NE and thus are engaged under conditions of stress when there are high levels of NE release (Arnsten et al., 2021). NMDAR=NMDA glutamate receptors; MOAS=mitochondria-on-a-string which are associated with calcium overload; KAT II=kynurenine aminotransferase.
COVID19 may impair PFC function by blocking NMDAR neurotransmission and reducing calcium regulation (Fig. 1). COVID19 increases tryptophan metabolism to kynurenine, which is metabolized by kynurenine aminotransferase (KAT II) to kynurenic acid, which blocks NMDAR (Pullan and Cler, 1989) (Figs. 1 and 2 ). Kynurenic acid is increased in brains of COVID19 patients, and there are also increases in GCPII (glutamate carboxypeptidase II) and cAMP-PKA-calcium signaling (Reiken et al., 2022). GCPII disinhibits cAMP-calcium-K+ signaling and weakens PFC connectivity (Arnsten et al., 2021) (Fig. 1). Thus, an agent that regulates cAMP-calcium signaling could be helpful in restoring PFC function.
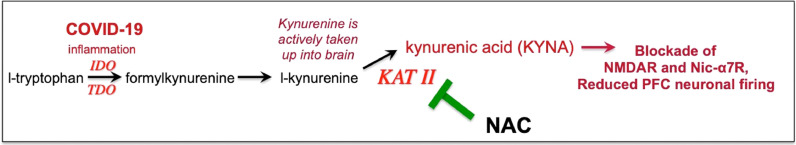
Fig. 2.
Inflammation increases tryptophan metabolism to kynurenine, which in turn can be metabolized to kynurenic acid, which blocks NMDAR and nic- α7R, receptors needed for layer III dlPFC neurotransmission.
The α2A-adrenoceptor agonist, guanfacine: 1) inhibits cAMP-PKA-K+ channel signaling in dlPFC, strengthening network connectivity (Arnsten et al., 2021); and 2) is anti-inflammatory, deactivating microglia}, and macrophages (Arnsten et al., 2021). Guanfacine protects PFC from hypoxia and psychological stress, and improves cognition in patients with encephalomyelitis (reviewed in (Arnsten et al., 2021)), providing compelling rationale to test guanfacine in patients with cognitive deficits from COVID-19.
N-acetylcysteine (NAC) is an anti-oxidant that protects mitochondria by increasing levels of the antioxidant, glutathione, and reduces calcium overload (Fig. 1). NAC also reduces inflammation by inhibiting KAT II (Figs. 1 and 2), the enzyme that converts kynurenine into KYNA (Blanco-Ayala et al., 2020). Taken together, these data suggest that NAC may help restore NMDAR neurotransmission, while guanfacine would strengthen that signal by closing K+ channels to strengthen PFC connections. The two agents may also reduce neuroinflammation, by protecting mitochondria and deactivating microglia. As these agents are typically well-tolerated, they were given to patients with cognitive deficits from COVID19.
Case report series- treatment of patients with long-COVID
We have followed 12 patients (11 women, 1 man; ages 21-73 years, 11 Caucasian, 1 African American) who were prescribed extended release guanfacine and NAC for long-COVID cognitive complaints starting in December 2020 and continuing to the present. The range of time post-COVID infection ranged from 3-14 months. The patients described “brain fog”, with significant difficulties in in executive functions such as multi-tasking, focus and concentration. Patients described feeling overwhelmed when under stress, e.g. when faced with multiple different salient stimuli. A case example involves a nurse on a medical floor, who after contracting COVID, felt overwhelmed at work and had to markedly decrease her caseload.
Guanfacine was initiated as a 1 mg tablet PO at night for the first month, and the dose was often increased to 2mg after 1 month, if well-tolerated. Guanfacine was usually given in its extended release (ER) form, although in older patients guanfacine's long half-life is compatible with the immediate release formulation. In addition to guanfacine, the patients were also started on NAC 600 mg tab PO daily. The nurse described above was additionally treated with 100 mg sertraline and behavioral therapy for her anxiety.
Four patients discontinued therapy, two for unspecified reasons and two due to hypotension and/or dizziness. There was no relationship between discontinuation of guanfacine and sex, age or time since COVID infection. The eight remain on medication with reported benefit. Some patients reported that their brain fog had decreased or gone away completely. One patient reported having less difficulty with word-finding difficulties. Many reported improved working memory, concentration, and executive functions, e.g. multi-tasking. Two patients described feeling more like themselves again.
The experience of the nurse is particularly illuminating, as it may be helpful in dealing with the shortage of medical personnel due to the pandemic. The nurse experienced significant benefits in her working memory, executive functioning and cognitive processing speed. She reported being able to fluidly manage her tasks at work and return to a normal caseload. She also reported significantly diminished situational anxiety symptoms, which she attributed to the combination of her guanfacine, sertraline, as well as weekly therapy sessions. Due to her significant prior COVID infection, she had also suffered cardiopulmonary complications. She reported guanfacine to be the “most effective medication” that she has been on. Her cardiologist had briefly stopped the guanfacine due to an acute transient episode of hypotension, and while stopping the guanfacine she experienced a significant worsening of her cognitive functioning and concentration. She decided to go back on the guanfacine regimen, without recurrence of the hypotensive episode. The patient continues to be on this regimen after one year of starting this medication, and continues to tolerate it well.
Summary
The current report demonstrates beneficial neurocognitive benefits of combined guanfacine and NAC in long-COVID19. Future, placebo-controlled trials are warranted for more rigorous understanding of these drug actions. The finding that one patient's cognitive abilities worsened when guanfacine treatment was suspended, and improved with guanfacine reinstatement, supports a therapeutic role for this compound.
Neurobiological data help to explain why this regimen is helpful. NAC and guanfacine may be mutually beneficial, restoring upstream NMDAR neurotransmission, and strengthening that signal through downstream closure of K+ channels on spines, respectively. Their inhibition of neuroinflammation may also restore connectivity, helping patients return to more normal lives.
Declaration of Competing Interest
Arman Fesharaki-Zadeh and Naomi Lowe have no conflicts of interest to declare.
Amy F.T. Arnsten and Yale University received royalties from the USA sales of Intuniv (extended release guanfacine) from Takeda/Shire Pharmaceuticals. They do not receive royalties from international sales nor from generic Intuniv nor immediate release guanfacine.
References
- Arnsten A.F.T., Datta D., Wang M. The Genie in the Bottle- Magnified calcium signaling in dorsolateral prefrontal cortex. Molecul. Psychiatry. 2021;26:3684–3700. doi: 10.1038/s41380-020-00973-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Ayala T., Sathyasaikumar K.V., Uys J.D., Pérez-de-la-Cruz V., Pidugu L.S., Schwarcz R. N-acetylcysteine inhibits kynurenine aminotransferase II. Neuroscience. 2020;444:160–169. doi: 10.1016/j.neuroscience.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullan L.M., Cler J.A. Schild plot analysis of glycine and kynurenic acid at the N-methyl-D-aspartate excitatory amino acid receptor. Brain Res. 1989;497:59–63. doi: 10.1016/0006-8993(89)90969-4. [DOI] [PubMed] [Google Scholar]
- Reiken S., Sittenfeld L., Dridi H., Liu Y., Liu X., Marks A.R. Alzheimer's-like signaling in brains of COVID-19 patients. Alzheimers Dement. Epub. ahead of print Feb. 2022;3 doi: 10.1002/alz.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderlind W.M., Rabinovitz B.B., Miao I.Y., Oberlin L.E., Bueno-Castellano C., Fridman C., Jaywant A., Kanellopoulos D. A systematic review of neuropsychological and psychiatric sequalae of COVID-19: implications for treatment. Curr. Opin. Psychiatry. 2021;4:420–433. doi: 10.1097/YCO.0000000000000713. [DOI] [PMC free article] [PubMed] [Google Scholar]