Abstract
Background
In patients with pneumonia or acute respiratory distress syndrome who survived hospitalization, one-year mortality can affect up to one third of discharged patients. Therefore, significant long-term mortality after COVID-19 respiratory failure could be expected. The primary outcome of the present study was one-year all-cause mortality in hospitalized COVID-19 patients.
Methods
Observational study of COVID-19 patients hospitalized at Papa Giovanni XXIII Hospital (Bergamo, Italy), during the first pandemic wave.
Results
A total of 1326 COVID-19 patients were hospitalized. Overall one-year mortality was 33.6% (N 446/1326), with the majority of deaths occurring during hospitalization (N=412, 92.4%). Thirty-four patients amongst the 914 discharged (3.7%) subsequentely died within one year. A third of these patients died for advanced cancer, while death without a cause other than COVID-19 was uncommon (8.8% of the overall post-discharge mortality). In-hospital late mortality (i.e. after 28 days of admission) interested a population with a lower age, and fewer comorbidities, more frequentely admitted in ICU. Independent predictors of post-discharge mortality were age over 65 years (HR 3.19; 95% CI 1.28-7.96, p-value=0.013), presence of chronic obstructive pulmonary disease (COPD) (HR 2.52; 95% CI 1.09-5.83, p-value=0.031) or proxy of cardiovascular disease (HR 4.93; 95% CI 1.45-16.75, p-value=0.010), and presence of active cancer (HR 3.64; 95% CI 1.50-8.84, p-value=0.004), but not pneumonia severity.
Conclusions
One-year post-discharge mortality depends on underlying patients’ comorbidities rather than COVID-19 pneumonia severity per se. Awareness among physicians of predictors of post-discharge mortality might be helpful in structuring a follow-up program for discharged patients.
Keywords: COVID-19, Pneumonia, Respiratory failure, Mortality, Comorbidities
1. Introduction
Patients with community-acquired pneumonia (CAP) are at higher risk of long-term morbidity and mortality compared with the general population who have never had CAP [1,2]. Indeed, when considering hospital survivors of CAP, studies have reported 1-year mortality rates of 17 to 33.6% [3,4]. Furthermore, in the more severe forms of acute respiratory failure (i.e., Acute Respiratory Distress Syndrome - ARDS), studies on long-term outcomes found a one-year mortality ranging between 11% and 22%, with significant residual muscle wasting and weakness in those who survived [5,6].
Nowadays, two years from the beginning of the Coronavirus disease 2019 (COVID-19) pandemic, it has been widely described that in-hospital mortality due to COVID-19 can be relevant, interesting up to one third of critically ill hospitalized patients [7,8]. It is also well known that ARDS is a frequent complication of COVID-19, affecting approximately one-third of the hospitalized population [9]. Increasing age, male gender, and various chronic comorbidities, especially cardiometabolic ones, were identified as major risk factors for the more severe forms of illness [10,11].
Given these premises, it would seem reasonable to expect a significant long-term mortality after COVID-19 respiratory failure and hospitalization; however little is known about post-discharge mortality and scientific evidence is still limited [12].
The aims of the present study were to evaluate the overall one-year mortality of patients initially admitted for COVID-19 and to assess the features of those who died within the first year since index hospitalization.
2. Material and methods
2.1. Study design and participants
This was a retrospective monocentric observational study, approved by the local Ethics Committee (Comitato Etico di Bergamo, Italy. N°37/2020). Verbal consent was obtained when feasible, according to local protocol. Written consent was not collected during the first Italian pandemic peak to avoid paper contamination, in accordance with hospital dispositions at that time. We included all consecutive adult patients with laboratory confirmed SARS-CoV-2 infection, hospitalized at Papa Giovanni XXIII Hospital (Bergamo, Italy) and its affiliate Hospital, San Giovanni Bianco, between February 23rd and April 7th, 2020. Patients already hospitalized for other conditions, who became positive during hospitalization, were not considered in the study. Data were derived from electronic medical records. For discharged or transferred patients, survival status, as well as the date of death, was obtained from the Regional Healthcare Informatic System (SISS, Lombardy Region, Italy), at least one year after index hospital admission. The cause of death after discharge was evaluated by a multidisciplinary team (FDM, FLL, MS), who considered all the available medical information (e.g., information regarding new admissions to our hospital, reports of outpatient visits, information from general practitioners, etc.). Covid-19 sequelae are referred to consequences related to severe forms of respiratory failure (e.g. tracheostomy with multiple complications, deconditioning and critical illness polyneuropathy with severe overall deterioration). Presence of respiratory failure at admission was assessed by SpO2 evaluation in room air (i.e., SpO2 < 90%) or by an arterial blood gas analysis (i.e., PaO2 < 60 mmHg). In accordance with regional dispositions, patients were considered suitable for discharge at home if apyretic for at least 48 h, with SpO2 > 92% in room air for at least 48 h, hemodynamically stable and self-sufficient in daily activities. Those who did not meet the criteria, if clinically stable, were referred to health care facilities with a lower intensity of care and considered discharged.
2.2. Outcomes
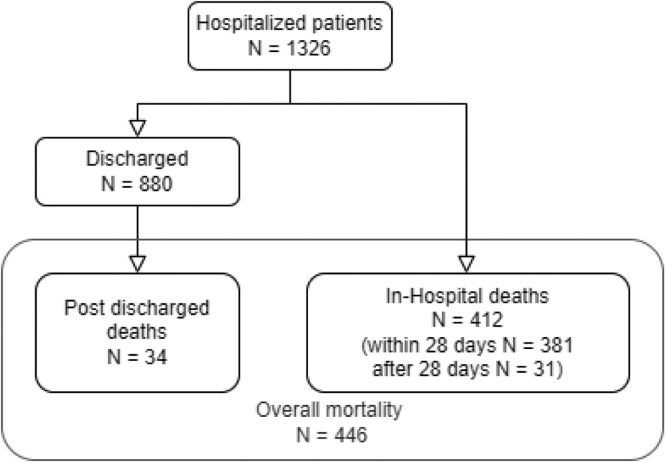
The primary outcome was one-year all-cause mortality, occurring either during hospital stay, or after discharge. Secondary outcomes were focused on (1) in-hospital mortality, evaluating early and late mortality (i.e., death within or after 28 days, respectively); (2) post-discharge mortality, comparing those who survived versus the deceased (Fig. 1 ).
Fig. 1.
Flow-chart about overall 1-year mortality divided into in-hospital and post-discharge deaths.
2.3. Statistical analysis
We summarized COVID-19 patient characteristics at baseline using descriptive statistics. Continuous variables were expressed as medians and interquartile ranges (IQRs) and categorical ones as frequencies and percentages. Study population characteristics were stratified for overall one-year death, in-hospital death (within or after 28 days) and post-discharge death. Differences between groups were tested using the Mann-Whitney test for continuous variables and the chi-square test (or Fisher's exact test when appropriate) for categorical variables.
The survival after discharge was estimated using the Kaplan Meier method. We calculated time to death as the time, expressed in months, between discharge and the death date and we censored the time for survivors at the last date of follow-up. Since forty-six alive patients had no information on discharge date, their follow-up period was estimated as the median time of follow-up of alive patients. Post-discharge survival was also estimated in strata of age, chronic obstructive pulmonary disease (COPD), proxy of cardiovascular disease (i.e., at least one of the following conditions or medications: coronary artery disease, atrial fibrillation, diabetes, use of angiotensin-converting-enzyme inhibitors, angiotensin receptor blockers, loop diuretics, mineralocorticoid receptor antagonists, beta-blockers, oral anticoagulants, antiplatelets), and cancer.
The effect of potential predictors on post-discharge mortality was estimated by Cox proportional hazards models and expressed as hazard ratios (HRs) and corresponding 95% confidence intervals (CIs). The multivariable model included terms for demographic characteristics and covariates that resulted significantly different between patients who die and those who did not in the univariate analysis at a p-value level of 0.05, or covariates clinically relevant. Thus, the Cox model included terms for age, sex, COPD, proxy of cardiovascular diseases, cancer and pneumonia severity during hospitalization (i.e., having at least one of the following conditions: at least one continuous positive airway pressure (CPAP) intervention during hospitalization, at least one non-invasive ventilation (NIV) intervention during hospitalization, PaO2/FIO2 ratio <200).
For all tested hypotheses, two-sided p-values of 0.05 or less were considered significant. Statistical analysis was performed using STATA Software, release 16.1 (StataCorp LP, College Station TX, USA) and was carried out at the biostatistical laboratory of the Foundation for Research (FROM) at Papa Giovanni XXIII Hospital in Bergamo.
3. Results
A total of 1326 COVID-19 patients were hospitalized at Papa Giovanni XXIII Hospital and its affiliate Hospital, San Giovanni Bianco (Bergamo, Italy), with laboratory confirmed SARS-CoV-2 infection, between February 23rd and April 7th, 2020. Overall one-year mortality was 33.6% (N 446/1326): 412 deaths occurred in-hospital (92.4% of the overall one-year mortality, N 412/446), while 34 patients died after discharge (7.6% of the overall one-year mortality, N 34/446, Fig. 2 ). When considering in-hospital mortality, 31 patients have died after 28 days (7.5% of the overall in-hospital mortality, N 31/412).
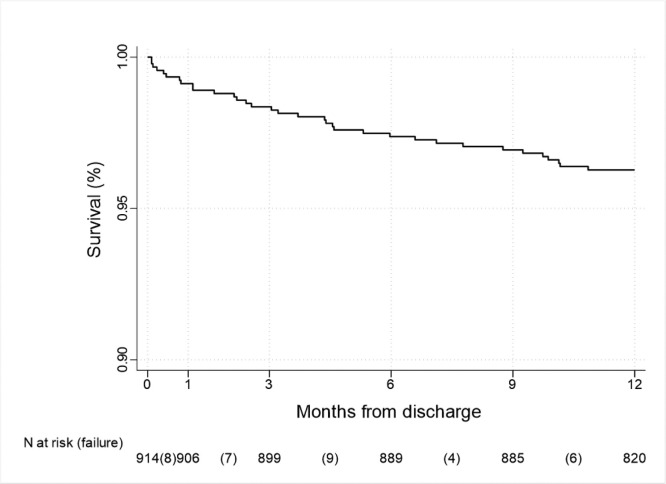
Fig. 2.
Kaplan-Meier survival curve of post-discharge mortality. 34 (3.7%) patients subsequently died after discharge within 1 year (N = 914).
3.1. General features of study population and overall one-year mortality
Demographic and clinical characteristics of 1326 COVID-19 patients are shown in Table 1 . Patients were mainly male (over 70%), with a median age of 68 years [58–76 years] and generally overweight (median BMI of 26.5 Kg/m2 [24.5–29.7 Kg/m2]). About 25% were current or former smokers. Univariate analysis described an higher mortality in the elderly, but no differences in terms of gender, BMI or smoking habits. In our sample, chronic comorbidities were frequent, as indicated by a median Charlson Comorbidity Index of 3 [2], [3], [4], [5], especially cardiometabolic conditions were represented.In particular, systemic hypertension, diabetes and coronary artery disease resulted highly prevalent. Overall one-year mortality was significantly higher in the presence of almost all comorbidities.
Table 1.
Anamnestic characteristics of patients and overall mortality, in hospital and post-discharge mortality.
| All patients (N = 1326) | Overall mortality |
In-hospital death |
Post-discharge death |
|||||
|---|---|---|---|---|---|---|---|---|
| Alive (N = 880) | Dead (N = 446) | No (N = 914) | Yes, whithin 28 days (N = 381) | Yes, after 28 days (N = 31) | No (N = 880) | Yes (N = 34) | ||
| Male gender, N (%) | 948 (71.5) | 616 (70.0) | 332 (74.4) | 634 (69.4) | 292 (76.6) | 22 (71.0) | 616 (70.0) | 18 (52.9) |
| Age, median [IQR] | 68 (58-76) | 63 (54-73) | 75 (69-81) | 63 (54-73) | 75 (70-81) | 69 (58-75) | 63 (54-73) | 77.5 (71-82) |
| ≤59, N (%) | 388 (29.3) | 351 (39.9) | 37 (8.3) | 352 (38.5) | 28 (7.3) | 8 (25.8) | 351 (39.9) | 1 (2.9) |
| 60-69, N (%) | 331 (25.0) | 249 (28.3) | 82 (18.4) | 256 (28.0) | 67 (17.6) | 8 (25.8) | 249 (28.3) | 7 (20.6) |
| 70-79, N (%) | 383 (28.9) | 201 (22.8) | 182 (40.8) | 213 (23.3) | 158 (41.5) | 12 (38.7) | 201 (22.8) | 12 (35.3) |
| ≥80, N (%) | 224 (16.9) | 79 (9.0) | 145 (32.5) | 93 (10.2) | 128 (33.6) | 3 (9.7) | 79 (9.0) | 14 (41.2) |
| BMI, median [IQR] | 26.5[24.5-29.7] | 26.5[24.4-29.4] | 26.7[24.6-30.2] | 26.4[24.4-29.4] | 26.8[24.5-30.1] | 27.3[24.6-31.8] | 26.5[24.4-29.4] | 25.4[23.1-30.6] |
| <30, N (%) | 715 (76.0) | 536 (76.9) | 179 (73.4) | 553 (76.8) | 150 (73.9) | 12 (66.7) | 536 (76.9) | 17 (73.9) |
| ≥30, N (%) | 226 (24.0) | 161 (23.1) | 65 (26.6) | 167 (23.2) | 53 (26.1) | 6 (33.3) | 161 (23.1) | 6 (26.1) |
| Obesity, N (%) | 232 (24.3) | 162 (23.0) | 70 (27.9) | 168 (23.1) | 58 (27.6) | 6 (33.3) | 162 (23.0) | 6 (26.1) |
| Smoking history, N (%) | ||||||||
| Current smoker | 47 (4.0) | 35 (4.3) | 12 (3.3) | 36 (4.3) | 11 (3.6) | 0 (0.0) | 35 (4.3) | 1 (3.2) |
| Former smoker | 248 (21.2) | 165 (20.5) | 83 (22.7) | 172 (20.5) | 72 (23.5) | 4 (14.3) | 165 (20.5) | 7 (22.6) |
| Never smoker | 877 (74.8) | 606 (75.2) | 271 (74.0) | 629 (75.1) | 224 (73.0) | 24 (85.7) | 606 (75.2) | 23 (74.2) |
| Comorbidity, N (%) | ||||||||
| Hypertension | 683 (52.1) | 393 (44.8) | 290 (67.0) | 411 (45.1) | 254 (69.0) | 18 (58.1) | 393 (44.8) | 18 (52.9) |
| Diabetes mellitus | 251 (19.2) | 135 (15.4) | 116 (26.8) | 141 (15.5) | 105 (28.5) | 5 (16.1) | 135 (15.4) | 6 (17.6) |
| Chronic kidney failure | 99 (7.6) | 49 (5.6) | 50 (11.6) | 53 (5.8) | 42 (11.5) | 4 (12.9) | 49 (5.6) | 4 (11.8) |
| COPD | 82 (6.3) | 44 (5.0) | 38 (8.8) | 51 (5.6) | 30 (8.2) | 1 (3.2) | 44 (5.0) | 7 (20.6) |
| Active solid cancer | 47 (3.6) | 23 (2.6) | 24 (5.6) | 26 (2.9) | 20 (5.5) | 1 (3.2) | 23 (2.6) | 3 (8.8) |
| Active hematologicalMalignancy | 48 (3.7) | 23 (2.6) | 25 (5.8) | 26 (2.9) | 19 (5.2) | 3 (9.7) | 23 (2.6) | 3 (8.8) |
| Cancer history | 92 (7.0) | 44 (5.0) | 48 (11.1) | 50 (5.5) | 38 (10.4) | 4 (12.9) | 44 (5.0) | 6 (17.6) |
| Cerebrovascular disease | 69 (5.3) | 40 (4.6) | 29 (6.7) | 43 (4.7) | 25 (6.8) | 1 (3.2) | 40 (4.6) | 3 (8.8) |
| Previous myocardialInfarction | 117 (9.0) | 53 (6.1) | 64 (14.9) | 58 (6.4) | 56 (15.3) | 3 (9.7) | 53 (6.1) | 5 (14.7) |
| Coronary artery disease | 137 (10.7) | 64 (7.5) | 73 (17.3) | 70 (7.8) | 61 (17.1) | 6 (19.4) | 64 (7.5) | 6 (17.6) |
| Atrial fibrillation | 122 (9.5) | 63 (7.3) | 59 (13.9) | 76 (8.5) | 43 (12.0) | 3 (9.7) | 63 (7.3) | 13 (38.2) |
| Chronic heart failure | 55 (4.2) | 26 (3.0) | 29 (6.7) | 31 (3.4) | 24 (6.6) | 0 (0.0) | 26 (3.0) | 5 (14.7) |
| Vasculopathy | 111 (8.5) | 54 (6.2) | 57 (13.2) | 56 (6.1) | 52 (14.2) | 3 (9.7) | 54 (6.2) | 2 (5.9) |
| Rheumatic pathology | 68 (5.2) | 41 (4.7) | 27 (6.3) | 45 (5.0) | 20 (5.5) | 3 (9.7) | 41 (4.7) | 4 (12.1) |
| Peptic ulcer | 16 (1.2) | 12 (1.4) | 4 (0.9) | 14 (1.5) | 2 (0.5) | 0 (0.0) | 12 (1.4) | 2 (5.9) |
| Immunodepression | 71 (5.4) | 38 (4.3) | 33 (7.7) | 41 (4.5) | 25 (6.8) | 5 (16.1) | 38 (4.3) | 3 (8.8) |
| Cirrhosis | 20 (1.5) | 7 (0.8) | 13 (3.0) | 9 (1.0) | 10 (2.7) | 1 (3.2) | 7 (0.8) | 2 (5.9) |
| CCI score, median [IQR] | 3 [2–5] | 2 [1-4] | 4 [3–6] | 3 [1–4] | 4 [3–6] | 3 [2–4] | 2 [1–4] | 4 [4–6] |
| CCI=0, N (%) | 127 (9.8) | 121 (14.0) | 6 (1.4) | 122 (13.6) | 3 (0.8) | 2 (6.5) | 121 (14.0) | 1 (3.0) |
| CCI=1-2, N (%) | 368 (28.4) | 323 (37.4) | 45 (10.4) | 325 (36.3) | 36 (9.8) | 7 (22.6) | 323 (37.4) | 2 (6.1) |
| CCI=3+, N (%) | 799 (61.7) | 419 (48.6) | 380 (88.2) | 449 (50.1) | 328 (89.4) | 22 (71.0) | 419 (48.6) | 30 (90.9) |
Age in years, Body mass index (BMI) was calculated if weight and height data were available, Obesity refers to medical history records, Chronic obstructive pulmonary disease (COPD), Cancer history refers to a previously treated disease, currently in follow-up, Charlson Comorbidity Index (CCI). In bold statistically significant differences at a 0.05 or less p-value level. For in-hospital analysis, differences were tested between deaths within and after 28 days. Interquartile range in square brackets [IRQ].
Information about respiratory failure severity, heart and respiratory rate, gas exchange and ventilatory support are shown in Table 2 . During the first 24 h since hospital admission, 83% (N = 1101) of the patients required oxygen or ventilatory support. Continuous positive airway pressure (CPAP) or noninvasive mechanical ventilation (NIV) were needed in almost a third of the cases, while only 16 patients underwent endotracheal intubation (ETI) during the first 24 h. Of note, during the overall hospitalization, the cumulative number of patients who required CPAP or NIV was higher, representing the 46.6% of the cohort and ICU admission was needed in 14.5% of the patients. Median PaO2/FIO2 ratio at admission resulted 194 [108–271]. Overall one-year mortality was greater in those with a more severe respiratory impairment at presentation, as indicated by a higher respiratory rate, a lower PaO2/FIO2 ratio, a bilateral chest X-ray involvement and a more intense level of treatment (i.e., use of CPAP, NIV or ETI). Supplemental information about medication history, symptoms at onset, intervals between significant events, and laboratory test can be found in Appendix Tables A.1 and A.2.
Table 2.
Clinical and radiological characteristics of patients and overall mortality, in hospital and post-discharge mortality.
| All patients (N = 1326) | Overall mortality |
In-hospital death |
Post-discharge death |
|||||
|---|---|---|---|---|---|---|---|---|
| Alive (N = 880) | Dead (N = 446) | No (N = 914) | Yes, whithin 28 days (N = 381) | Yes, after 28 days (N=31) | No (N = 880) | Yes (N = 34) | ||
| In the first 24h | ||||||||
| Oxygen and ventilatory support, N (%) | ||||||||
| None | 94 (7.9) | 75 (9.4) | 19 (4.8) | 79 (9.5) | 14 (4.2) | 1 (3.7) | 75 (9.4) | 4 (13.3) |
| Nasal cannula | 314 (26.3) | 266 (33.2) | 48 (12.2) | 281 (33.8) | 28 (8.3) | 5 (18.5) | 266 (33.2) | 15 (50.0) |
| Venturi mask/Reservoir | 415 (34.7) | 279 (34.8) | 136 (34.6) | 287 (34.5) | 121 (36.0) | 7 (25.9) | 279 (34.8) | 8 (26.7) |
| CPAP/NIV | 356 (29.8) | 173 (21.6) | 183 (46.6) | 175 (21.0) | 170 (50.6) | 11 (40.7) | 173 (21.6) | 2 (6.7) |
| ETI | 16 (1.3) | 9 (1.1) | 7 (1.8) | 10 (1.2) | 3 (0.9) | 3 (11.1) | 9 (1.1) | 1 (3.3) |
| FIO2, median [IQR] | 60 [35-70] | 50 [33-70] | 70 [60-70] | 50 [33-70] | 70 [60-70] | 70 [60-70] | 50 [33-70] | 36 [30-50] |
| HR bpm [IQR] | 85 [75-95] | 85 [76-95] | 82 [73-94] | 85 [76-95] | 82 [73-94] | 85 [75-89] | 85 [76-95] | 80 [72-90] |
| RR acts/min [IQR] | 24 [19-28] | 23 [18-28] | 25 [20-30] | 22 [18-28] | 25.5 [20-30] | 20 [18-28.5] | 23 [18-28] | 19 [15.5-27] |
| SBP mmHg [IQR] | 127 [115-140] | 126 [113-140] | 128 [115-140] | 126 [113-140] | 130 [115-142] | 122 [113-145] | 126 [113-140] | 123 [115-136] |
| DBP mmHg [IQR] | 73 [65-80] | 74 [67-80] | 70 [60-80] | 74 [66-80] | 72 [62-80] | 69 [53-78] | 74 [67-80] | 70 [56-78] |
| ABG at presentation, median [IQR] | ||||||||
| pH | 7.5 [7.5-7.5] | 7.5 [7.5-7.5] | 7.5 [7.4-7.5] | 7.5 [7.5-7.5] | 7.5 [7.4-7.5] | 7.5 [7.5-7.5] | 7.5 [7.5-7.5] | 7.5 [7.4-7.5] |
| PaO2, mmHg | 64 [54.9-81.5] | 65 [56-81] | 62 [52-84] | 65 [56-83] | 61 [51.5-79] | 67 [51-81.5] | 65 [56-81] | 86 [60-102] |
| PaCO2, mmHg | 32 [29-35] | 32 [29-35] | 31.6 [29-35] | 32 [29-35] | 31.7 [28.2-35] | 30.1 [29-33] | 32 [29-35] | 32 [30-35] |
| HCO3−, mmol/L | 24 [22-26] | 24 [22-26] | 23 [21-25] | 24 [22-26] | 23 [21-25] | 21 [20-25] | 24 [22-26] | 21 [18-24] |
| Lac, mmol/L | 1.4 [1.1-1.8] | 1.3 [1.0-1.6] | 1.5 [1.2-2.1] | 1.3 [1.0-1.7] | 1.6 [1.2-2.2] | 1.2 [0.9-1.6] | 1.3 [1.0-1.6] | 1.6 [1.3-2.0] |
| FIO2 | 0.4 [0.2-0.7] | 0.3 [0.2-0.7] | 0.6 [0.2-0.7] | 0.3 [0.2-0.7] | 0.6 [0.3-0.7] | 0.6 [0.3-0.7] | 0.3 [0.2-0.7] | 0.3 [0.2-0.5] |
| PaO2/FIO2 ratio | 194 [108-271] | 214 [124-282] | 151 [89-238] | 217 [126-286] | 137 [87-224] | 150 [96-210] | 214 [124-282] | 259 [207-333] |
| SatO2 | 92 [89-95] | 92 [90-95] | 91 [86-94.5] | 92 [90-95] | 91 [86-94] | 94 [90-95] | 92 [90-95] | 93 [89-100] |
| Chest X-ray abnormalities, N (%) | ||||||||
| Bilateral | 1017 (78.7) | 665 (76.7) | 352 (82.6) | 685 (76.0) | 304 (84.2) | 28 (90.3) | 665 (76.7) | 20 (58.8) |
| Monolateral | 159 (12.3) | 120 (13.8) | 39 (9.2) | 127 (14.1) | 29 (8.0) | 3 (9.7) | 120 (13.8) | 7 (20.6) |
| Absent | 117 (9.0) | 82 (9.5) | 35 (8.2) | 89 (9.9) | 28 (7.8) | 0 (0.0) | 82 (9.5) | 7 (20.6) |
| During hospitalization(need of/admission to) | ||||||||
| CPAP | 513 (38.7) | 282 (32.0) | 231 (51.8) | 286 (31.3) | 212 (55.6) | 15 (48.4) | 282 (32.0) | 4 (11.8) |
| NIV | 105 (7.9) | 60 (6.8) | 45 (10.1) | 61 (6.7) | 39 (10.2) | 5 (16.1) | 60 (6.8) | 1 (2.9) |
| ETI | 202 (15.2) | 115 (13.1) | 87 (19.5) | 117 (12.8) | 68 (17.8) | 17 (54.8) | 115 (13.1) | 2 (5.9) |
| ECMO | 8 (0.6) | 5 (0.6) | 3 (0.7) | 5 (0.5) | 0 (0.0) | 3 (9.7) | 5 (0.6) | 0 (0.0) |
| Semi-ICU | 89 (6.7) | 62 (7.0) | 27 (6.1) | 62 (6.8) | 23 (6.0) | 4 (12.9) | 62 (7.0) | 0 (0.0) |
| ICU | 192 (14.5) | 116 (13.2) | 76 (17.0) | 117 (12.8) | 57 (15.0) | 18 (58.1) | 116 (13.2) | 1 (2.9) |
| Severe Pneumonia* | 696 (52.5) | 407 (46.3) | 289 (64.8) | 415 (45.4) | 261 (68.5) | 20 (64.5) | 407 (46.3) | 8 (23.5) |
Continuous positive airway pressure (CPAP), Noninvasive ventilation (NIV), Fraction of inspired oxygen (FIO2), Heart rate (HR), Respiratory rate (RR), Systolic and diastolic blood pressure (SBP and DBP), Arterial blood gases (ABG), Arterial pressure of Oxygen (PaO2), Arterial pressure of carbon dioxide (PaCO2), lactate (Lac), Arterial oxygen saturation (SaO2), Endotracheal tube (ETI), Extracorporeal membrane oxygenation (ECMO), High dependency od semi-intensive care unit (Semi-ICU), Intensive care unit (ICU). *Pneumonia severity indicates at least one of the following conditions: at least one CPAP intervention during hospitalization, at least one NIV intervention during hospitalization, PaO2/FIO2 ratio <200. In bold statistically significant differences at a 0.05 or less p-value level. For in-hospital analysis, differences were tested between deaths within and after 28 days. Interquartile range in square brackets [IRQ].
3.2. In-hospital mortality
In-hospital late mortality (i.e., after 28 days) consists of 31 cases (7.5% of overall in-hospital mortality, and 2.3% of the overall population), while 381 patients have died within the 28th day (92.5% of overall in-hospital mortality, and 28.7% of the overall population). Comparison of several characteristics between patients who died during hospitalization, before and after 28-day, is provided in Tables 1 and 2. As indicated in bold, in-hospital late mortality interested a population with a lower age, and fewer comorbidities (69 vs 75 years and Charlson Comorbidity Index 3 vs 4, p-value<0.001 and p-value=0.002, respectively). They more frequentely underwent ETI, needed ICU admission and eventually extracorporeal membrane oxygenation (ECMO) (54.8% vs 17.8%, 58.1% vs 15.0% and 9.7% vs 0%, respectively, all p-values<0.001). Patients with late in-hospital mortality, had also a longer interval between hospitalization and ICU referral, compared to those who died within the 28th day (6 vs 2 days, p-value=0.021; Appendix Table 1). Univariate analysis did not show significant differences in terms of gender, BMI, smoking history, and specific comorbidities. Previous medication history, symptoms at onset, laboratory test evaluation and arterial blood gas (ABG) at admission are reported in Appendix Tables 1, and 2.
3.3. Post-discharge mortality
Thirty-four patients amongst the 914 discharged (3.7%), subsequentely died within 1 year since index hospital admission (Fig. 2). Individuals who died after discharge were more frequentely female (47.1% vs 30.0%, p-value=0.034), and older (median age around 78 vs. 63, p-value<0.001, Table 1). When considering comorbidities, extra-hospital deceased population more commonly had active cancer and were affected by chronic cardiovascular and respiratory comorbidities. Furthermore, these patients had a remarkable medication history, with use of angiotensin receptor blockers, diuretics, anticoagulant or antiplatelets therapies and proton pump inhibitor therapy (PPI) (Appendix Table 1). Patients who died, less frequentely had severe pneumonia (i.e., at least one CPAP or NIV intervention during hospitalization or PaO2/FIO2 ratio <200 at admission). In general, they required lower FIO2 (36 vs 50, p-value=0.022), had better gas exchange parameters and fewer chest X-ray abnormalities (PaO2/FIO2 ratio 259 vs 214, p-value=0.018 and bilateral infiltrates 58.8% vs 76.7%, p-value=0.038), and less frequently needed a CPAP support during hospitalization (11.8 vs 32.0%, p-value=0.013). Blood test analysis are reported in Appendix Table 2.
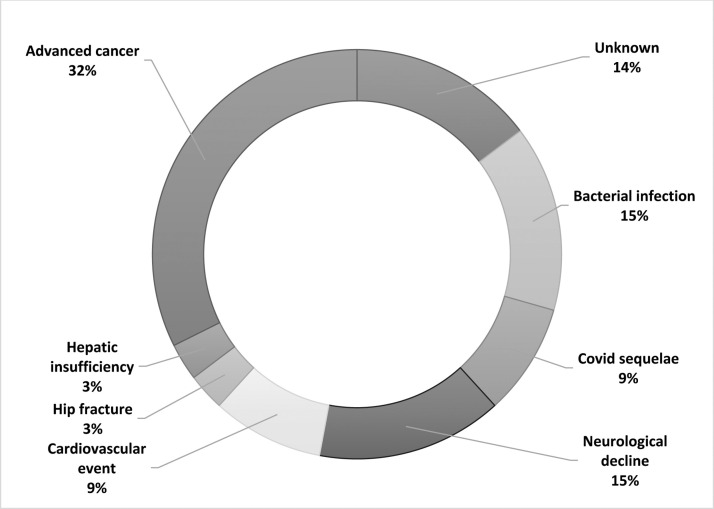
The post-discharge causes of death are reported in Fig. 3 . A third of the patients died because of direct consequences of advanced cancer (32%), representing the most common scenario. In addition, cardiovascular events, neurological decline and bacterial infectious complications accounted for nearly forty percent of the cases. In a minority of the patients a specific fatal event was identifiable (i.e., hip fracture and hepatic insufficiency), while in five cases information about death were unavailable. Finally, death in the absence of causes other than COVID-19 was unconventional, representing an event that occurred in three patients (8.8%).
Fig. 3.
Causes of post-discharge mortality evaluated by multidisciplinary team (N = 34).
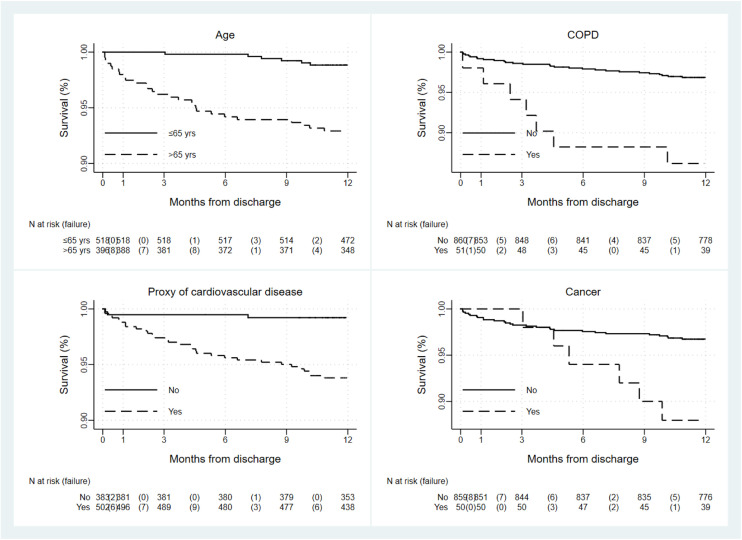
Multivariate analysis of selected variables was conducted to identify predictors of mortality after discharge (Table 3 , Fig. 4 ). Independent association was observed with age over 65 years (HR 3.18; 95% CI 1.28-7.95, p-value=0.013), presence of COPD (HR 2.59; 95% CI 1.12–6.00, p-value =0.027) or proxy of cardiovascular disease (HR 4.98; 95% CI 1.47-16.91, p-value=0.010), and presence of active cancer (HR 3.64; 95% CI 1.50-8.84, p-value=0.004). Conversely, gender and severity of respiratory disease during hospitalization did not result associated with post-discharge mortality.
Table 3.
Hazard ratios https://www.clinicalkey.com/tbl3fna(HRs) and corresponding 95% confidence intervals (CIs) for post-discharge mortality according to selected characteristics. Multivariate analysis.
| HR (95% CI) | p-value | |
|---|---|---|
| Age | ||
| ≤65 years | 1 (ref) | 0.013 |
| >65 years | 3.18 (1.28-7.95) | |
| Sex | ||
| Women | 1 (ref) | 0.088 |
| Men | 0.56 (0.28-1.09) | |
| COPD | ||
| No | 1 (ref) | 0.027 |
| Yes | 2.59 (1.12-6.00) | |
| Proxy of cardiovascular diseasea | ||
| No | 1 (ref) | 0.010 |
| Yes | 4.98 (1.47-16.91) | |
| Cancerb | ||
| No | 1 (ref) | 0.004 |
| Yes | 3.64 (1.50-8.84) | |
| Severe Pneumoniac | ||
| No | 1 (ref) | 0.075 |
| Yes | 0.49 (0.22-1.08) |
Chronic obstructive pulmonary disease (COPD).
At least one of the following conditions or medications: Coronary artery disease, Atrial fibrillation, diabetes, Angiotensin-converting enzyme (ACE) inhibitors, Sartans, Loop diuretics, Mineralocorticoid antagonist (MRAs), Beta-blocker, Oral anticoagulant therapy (OAT) with direct oral anticoagulants (DOACs), antiplatelets.
at least one of the following conditions: active hematological or solid cancer.
At least one of the following conditions: at least one CPAP intervention during hospitalization, at least one NIV intervention during hospitalization, and PaO2/FIO2 ratio <200.
Fig. 4.
Kaplan-Meier survival curves of post-discharge mortality by age (N = 914), COPD (N = 911), proxy of cardiovascular disease (N = 885) and cancer (N = 909).
4. Discussion
Our study provides information on long-term mortality in COVID-19 patients who required hospitalization, specifically focusing on post-discharge mortality. Results can be summarized as follows: (1) overall one-year mortality is significant, accounting for 33.6% of the study population; (2) patients who died in-hospital after 28 days, are typically younger and less comorbid subjects, who received a prolonged and more intense level of care; (3) One-year post-discharge mortality is 3.7%; (4) a third of these patients dies because of direct consequences of advanced cancer, while death in the absence of cause other than COVID-19 is rare; (5) independent predictors of post-discharge mortality are age over 65 years, presence of COPD or proxy of cardiovascular disease, and presence of active cancer, but not pneumonia severity during hospitalization.
Considerations about the population evaluated are needed. We investigated consecutively hospitalized patients in one of the hardest hit hospital during the most intense peak of the Italian COVID-19 outbreak [13,14]. As demonstrated by the severity of respiratory compromise upon admission (i.e., median PaO2/FIO2 ratio below 200) and during hospitalization (nearly 60% of study population need CPAP, NIV or ETI), our cohort is characterized by a greater severity than those described in early report from China [15], and more similar to other population in the UK or in the USA [16,17]. Therefore, data should be interpreted in the light of this background.
In-hospital overall mortality was 31.1%. This finding is consistent with other large studies describing an in-hospital mortality between 20.5% and 53.4% during the first pandemic wave [7,[16], [17], [18]]. Death usually occurred within few weeks since hospitalization (i.e., <28 days), except for a subgroup of younger and less comorbid patients (7.5% of in-hospital deaths). This minority of patients received a higher and prolonged intensity of care, which has probably influenced a longer hospital stay before death. It is less clear why these subjects had a longer interval between hospitalization and ICU referral. Probably, because of having a greater functional reserve, a non-invasive management was attempted for longer. Interestingly, in a previous research we described a comparable mortality irrespectively of time intervals between hospitalization and ETI or between CPAP/NIV initiation and ETI [13].
We found a one-year post-discharge mortality of 3.7%. Similarly, a recent systematic review and meta-analysis by Ramzi, specifically focused on hospital readmission and post-discharge all-cause mortality in COVID-19, describes an overall one-year mortality of 7.5%, falling to 4.0% when considering only low bias studies, comparable to that observed in our series [12]. As already anticipated, literature on long-term mortality after hospitalization for ARDS provides considerably worse outcomes. Herridge et al., conducted a multicentre longitudinal study on ARDS survivors, describing a one-year mortality of 11%. Of note, three quarter of post-discharge deaths were related to pre-existing medical problems [5]. Interestingly, other longitudinal studies on ARDS survivors, identify older age and pre-ICU comorbidities as independent predictors of mortality, rather than severity of illness or ICU factors [6,19]. It must be considered that the aforementioned cohorts consisted of ICU patients, who were younger than our study population. Guillon et al., in a cross-sectional retrospective study reported a significant increase in 6-month mortality in COVID-19 invasive ventilated patients ≥80-year-old patients admitted to ICU as compared with the younger age [20]. Noteworthy is the results of a multicentre prospective analysis of 3210 COVID-19 patients who completed 1-year follow-up after having required ICU admission. In this study, Ceccato et al. found that 1-year mortality was 35% and only 1% of patients discharged died within the first year of follow-up [21]. This work does not mention other details relating to the age or comorbidities of the patients. When evaluating literature on long-term outcomes in CAP survivors, one-year mortality is higher than in COVID-19 pneumonia, ranging between 17% and 33.6% [3,4]. The aforementioned work by Ceccato et al. agrees that long-term mortality is lower when compared with other causes of pneumonia [21]. Similarly, predictors of post-discharge mortality in CAP survivors are the pre-existing comorbidities, especially malignancy, COPD and presence of cardiovascular disease, while the role of age is not always described as a risk factor [1], [2], [3].
Our study suggests that COVID-19 mortality could be high during the acute phase, but the long-term impact is lower than that described for ARDS and CAP. To investigate differences in pathophysiological mechanisms is beyond the scope of this research, but this aspect is clearly worthy of further investigations to better define the role of viral infection on long-term outcomes. Having said that, the predictors of long-term mortality are similar between COVID-19 and ARDS or CAP, as well as the absence of association with the severity of the acute event for ARDS [19,[22], [23], [24]]. The deceased subjects had in most cases an a priori unfavourable prognosis determined by their clinical condition [24,25]. We further investigated extra-hospital deaths in the eleven oncological patients, collecting data about cancer diagnosis and stage at the time of the last specialistic evaluation available. We found that nine of them were known for cancer and all had already been treated with multiple lines of chemo or radiotherapy, whereas only two of them received a de-novo diagnosis. Malignancies were all in advanced stage although being heterogeneous in type and site. Of note, only two patients had lung cancer while three patients had brain cancer, two had haematological malignancies and the remaining ones had ovarian, gastric, renal and hepatic cancers. Taking into account the role of active cancer in COVID-19, scientific literature identifies the oncologic population at a higher risk of death [26,27]. Thus, in this subgroup of patients, it seems reasonable to consider COVID-19 as a precipitating factor rather than the main cause of death.
The reason for a milder respiratory disease in the post-discharge deceased group remains uncertain. We interpreted this data as a further confirmation that mortality depended on the underlying fragilities and comorbidities, while COVID-19 do not represent per se the cause of death. Two main considerations support this interpretation. First, the fact of having examined a severely ill population reinforces the negative association between post-discharge mortality and disease severity, even if prospective studies are needed to clarify this aspect. Second, post-discharge actual causes of death were mainly characterized by oncological, cardiovascular aetiologies or infectious complications and not by COVID-19.
This study has a number of limitations. First, because of the retrospective design involving patients hospitalized in a single centre during a pandemic peak, the accuracy and completeness of the data can be reasonably questioned. However, the study cohort is relatively large, and specifically focused on an emergency setting with severely ill COVID-19 patients. Second, one-year mortality was assessed by using the Regional Healthcare Informatic System, which only provides information about the date of the death. Therefore, the cause of death after discharge was sought case by case, and it was not always possible to trace it. Nevertheless, our study is aimed to identifying predictors of post-discharge mortality and the multivariate analysis is not affected by the actual cause of death. Third, we did not investigate the readmission rate and did not compare post-discharge mortality of COVID-19 patients with a control group because it was beyond the scope of this study, however it may be useful in further characterizing this population. Finally, for those patients who were transferred in other hospital, we still have reliable mortality data obtained by investigating the regional system, but no information on their clinical course, therefore the need for CPAP, NIV, ETI or ECMO may be underestimated.
5. Conclusions
Post-discharge mortality interests a minority of the hospitalized COVID-19 patients, and it does not depend on pneumonia severity that resulted in index hospitalization. Older patients, those with active malignancy, COPD or cardiovascular comorbidities have a greater risk of dying within one-year after index hospitalization. Therefore, awareness among physicians of predictors of post-discharge mortality might be helpful in structuring a follow-up program for discharged patients, focusing on their comorbidities and not just on the severity of the index episode.
Authors’ contributions
Luca Novelli, Federico Raimondi and Fabiano Di Marco conceived the idea and designed the research. Luca Novelli, Federico Raimondi, Simone Pappacena, Roberta Biza, Roberta Trapasso, Marisa Anelli, Mariangela Amoroso, Chiara Allegri, Luca Malandrino, Gianluca Imeri, Carterina Conti and Marta Beretta collected clinical records data. The cause of death after discharge was evaluated by a multidisciplinary team (Fabiano Di Marco, Ferdinando Luca Lorini and Michele Senni). Greta Carioli and Alessandra Carobbio analysed study data and developed statistical models and design of methodology. Fabiano Di Marco and Stefano Fagiuoli were the responsible for the research activity, management and coordination. Luca Novelli, Federico Raimondi, Fabiano Di Marco, and Arianna Ghirardi created and wrote the initial draft. All the authors critically analysed data and revised the draft. Luca Novelli, Federico Raimondi, Simone Pappacena, Luca Malandrino and Fabiano Di Marco prepared the final version of manuscript. All authors read and approved the final version of the manuscript.
Primary funding source
This work was supported by Fondazione Cariplo.
Funding
This work was supported by Fondazione Cariplo [Grant for improving research in COVID-19, 2021].
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations of Competing Interest
The authors have no conflict of interest in relationship with the present manuscript.
Acknowledgements
We would like to acknowledge all the members of HPG23 Covid-19 Study Group listed in Appendix B.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.resmer.2022.100976.
Appendix. Supplementary materials
References
- 1.D.T. Eurich, T.J. Marrie, J.K. Minhas-Sandhu, S.R. Majumdar Ten-year mortality after community-acquired pneumonia a prospective cohort 2015 doi:10.1164/rccm.201501-0140OC. [DOI] [PubMed]
- 2.Mortensen E.M., Kapoor W.N., Chang C.C.H., Fine M.J. Assessment of mortality after long-term follow-up of patients with community-acquired pneumonia. Clin Infect Dis. 2003;37:1617–1624. doi: 10.1086/379712. [DOI] [PubMed] [Google Scholar]
- 3.Bruns A.H.W., Oosterheert J.J., Cucciolillo M.C., El Moussaoui R., Groenwold R.H.H., Prins J.M., et al. Cause-specific long-term mortality rates in patients recovered from community-acquired pneumonia as compared with the general Dutch population. Clin Microbiol Infect. 2011;17:763–768. doi: 10.1111/j.1469-0691.2010.03296.x. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan V., Clermont G., Griffin M.F., Kasal J., Watson R.S., Linde-Zwirble W.T., et al. Pneumonia: still the old man's friend? Arch Intern Med. 2003;163:317–323. doi: 10.1001/archinte.163.3.317. [DOI] [PubMed] [Google Scholar]
- 5.Herridge M.S., Cheung A.M., Tansey C.M., Matte-Martyn A., Diaz-Granados N., Al-Saidi F., et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 6.Wang C.Y., Calfee C.S., Paul D.W., Janz D.R., May A.K., Zhuo H., et al. One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome. Intensive Care Med. 2014;40:388–396. doi: 10.1007/s00134-013-3186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt M., et al. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2020;471:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzotzos S.J., Fischer B., Fischer H., Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care. 2020;24:1–4. doi: 10.1186/s13054-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semenzato L., et al. Chronic diseases, health conditions and risk of COVID-19-related hospitalization and in-hospital mortality during the first wave of the epidemic in France: a cohort study of 66 million people. Lancet Reg Heal Eur. 2021;8 doi: 10.1016/j.lanepe.2021.100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ioannou G.N., Locke E., Green P., Berry K., O'Hare A.M., Shah J.A., et al. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 infection. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramzi ZS. Hospital readmissions and post-discharge all-cause mortality in COVID-19 recovered patients; a systematic review and meta-analysis. Am J Emerg Med. 2021;51:267–279. doi: 10.1016/j.ajem.2021.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novelli L., Raimondi F., Ghirardi A., Pellegrini D., Capodanno D., Sotgiu G., et al. At the peak of covid-19 age and disease severity but not comorbidities are predictors of mortality: Covid-19 burden in Bergamo, Italy. Panminerva Med. 2021;63:51–61. doi: 10.23736/S0031-0808.20.04063-X. [DOI] [PubMed] [Google Scholar]
- 14.Fagiuoli S., Lorini F.L., Remuzzi G. Adaptations and lessons in the province of Bergamo. N Engl J Med. 2020;382:e71. doi: 10.1056/NEJMc2011599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 16.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O'Donnell L., Chernyak Y., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020 doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murthy S., Archambault P.A., Atique A., Carrier F.M., Cheng M.P., Codan C., et al. Characteristics and outcomes of patients with COVID-19 admitted to hospital and intensive care in the first phase of the pandemic in Canada: a national cohort study. CMAJ Open. 2021;9:E181–E188. doi: 10.9778/cmajo.20200250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfoh E.R., Wozniak A.W., Colantuoni E., Dinglas V.D., Mendez-Tellez P.A., Shanholtz C., et al. Physical declines occurring after hospital discharge in ARDS survivors: a 5-year longitudinal study. Intensive Care Med. 2016;42:1557–1566. doi: 10.1007/s00134-016-4530-1. [DOI] [PubMed] [Google Scholar]
- 20.GuillonA L.E., Godillon L., Kimmoun A., Grammatico-Guillon L. Long-term mortality of elderly patients after intensive care unit admission for COVID-19. Intensive Care Med. 2021;47:710–712. doi: 10.1007/s00134-021-06399-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceccato A., Pérez-Arnal R., Motos A., Barbé F., Torres A. One-year mortality after ICU admission due to COVID-19 infection. Intensive Care Med. 2022;48:366–368. doi: 10.1007/s00134-021-06611-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Günster C., Busse R., Spoden M., Rombey T., Schillinger G., Hoffmann W., et al. 6-month mortality and readmissions of hospitalized COVID-19 patients: a nationwide cohort study of 8,679 patients in Germany. PLoS One. 2021;16 doi: 10.1371/journal.pone.0255427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donnelly J.P., Wang X.Q., Iwashyna T.J., Prescott H.C. Readmission and death after initial hospital discharge among patients with COVID-19 in a large multihospital system. JAMA. 2021;325:304–306. doi: 10.1001/jama.2020.21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowles K.H., McDonald M., Barrón Y., Kennedy E., O'Connor M., Mikkelsen M. Surviving COVID-19 after hospital discharge: symptom, functional, and adverse outcomes of home health recipients. Ann Intern Med. 2021;174:316–325. doi: 10.7326/M20-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zettersten E., Engerström L., Bell M., Jäderling G., Mårtensson J., Block L., et al. Long-term outcome after intensive care for COVID-19: differences between men and women—a nationwide cohort study. Crit Care. 2021;25(1):86. doi: 10.1186/s13054-021-03511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertuzzi A.F., Ciccarelli M., Marrari A., Gennaro N., Dipasquale A., Giordano L., et al. Impact of active cancer on COVID-19 survival: a matched-analysis on 557 consecutive patients at an academic hospital in Lombardy, Italy. Brit J Cancer. 2021;125:358–365. doi: 10.1038/s41416-021-01396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu C., Stoeckle J.H., Masri L., Pandey A., Cao M., Littman D., et al. COVID-19 outcomes in hospitalized patients with active cancer: experiences from a major New York City health care system. Cancer. 2021;127:3466–3475. doi: 10.1002/cncr.33657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.