Abstract
The recent COVID-19 infection outbreak has raised the demand for rapid, highly sensitive POC biosensing technology for intelligent health and wellness. In this direction, efforts are being made to explore high-performance nano-systems for developing novel sensing technologies capable of functioning at point-of-care (POC) applications for quick diagnosis, data acquisition, and disease management. A combination of nanostructures [i.e., 0D (nanoparticles & quantum dots), 1D (nanorods, nanofibers, nanopillars, & nanowires), 2D (nanosheets, nanoplates, nanopores) & 3D nanomaterials (nanocomposites and complex hierarchical structures)], biosensing prototype, and micro-electronics makes biosensing suitable for early diagnosis, detection & prevention of life-threatening diseases. However, a knowledge gap associated with the potential of 0D, 1D, 2D, and 3D nanostructures for the design and development of efficient POC sensing is yet to be explored carefully and critically. With this focus, this review highlights the latest engineered 0D, 1D, 2D, and 3D nanomaterials for developing next-generation miniaturized, portable POC biosensors development to achieve high sensitivity with potential integration with the internet of medical things (IoMT, for miniaturization and data collection, security, and sharing), artificial intelligence (AI, for desired analytics), etc. for better diagnosis and disease management at the personalized level.
Keywords: Biosensors, 0D to 3D nanomaterials, Point-of-care testing, Efficient diagnostics, Wearable, Personalized health management
1. Introduction
In recent years, the medical field has garnered astounding advancements concerning the diagnosis & cure of certain deadly diseases. Still, a large percentage of the population worldwide continues to succumb to diseases such as cancer, diabetes, ischemic heart disease, tuberculosis, acquired immunodeficiency syndrome (AIDS), Coronavirus disease (COVID-19), Alzheimer's & Parkinson's disease, malaria, dengue, and several other common respiratory, viral & bacterial infections. The lack of availability of simple, quick, easy & portable detection methods as well as the time lag between a patient's sample collection & its analysis by an expert can be considered as some of the prominent reasons contributing to this state of healthcare & diagnostics especially, in the developing countries. Point-of-care (POC) devices having low cost, illustrating features like robustness, selectivity, sensitivity, and offering accurate quick diagnostics near the patient's bedside can offer a potential solution to improve the current healthcare scenario (Quesada-González and Merkoçi, 2018). Moreover, the Internet of Medical Things (IoMT) based on various nanomaterials also becoming popular in intelligent healthcare (Kaushik et al., 2021).
In the last decade, nanoengineering & nanotechnology have emerged as a research methodology entailing the combined study of various sciences, monitoring the changes occurring in the material properties at the nanoscale level & which have led to increased use of nanostructures in the biosensors (Welch et al., 2021). Nanomaterials are materials synthesized using nanotechnology principles, depicting macroscopic solids and atomic system's intermediate properties. The particles with internal structure dimensions or external dimensions, in the range of 1 nm–100 nm are considered nanomaterials. Nanomaterials such as gold nanoparticles (AuNPs)(Mahari et al., 2022; Shahdeo et al., 2021), semiconductor quantum dots (QDs), polymeric nanoparticles, carbon nanotubes (CNTs), nanodiamonds & graphene are considered to be promising candidates for improving the sensitivities & limit of detection (LOD) down to individual molecules. Generally, all nanomaterials confer an advantage of a high specific surface, thereby allowing for the immobilization of bioreceptor units in a greater amount. However, the immobilization technique chosen for carrying out this conjugation of the bio-specific entity onto such nanomaterials comes with its challenges & is thus a defining step in developing a reliable biosensor (Holzinger et al., 2014).
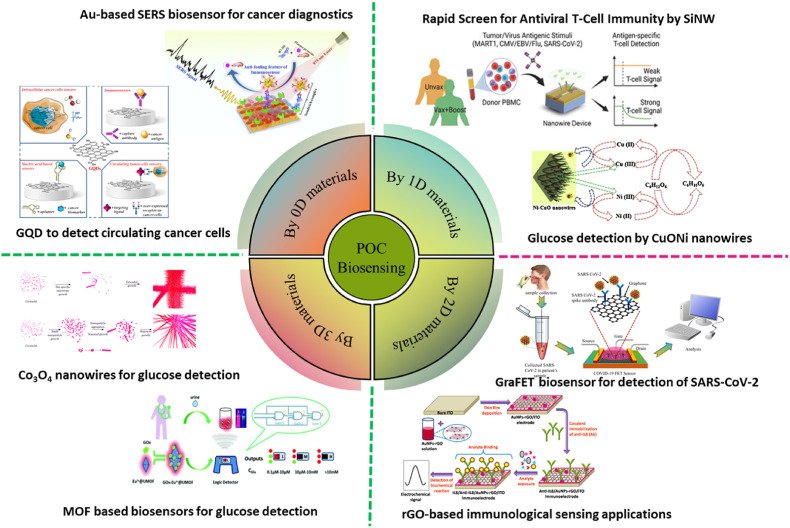
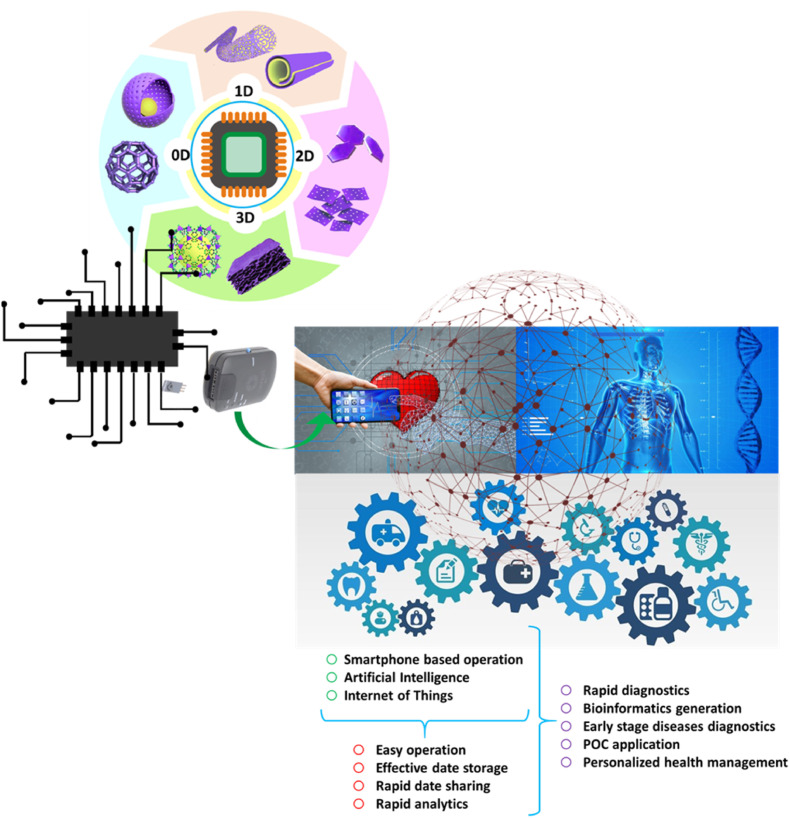
Nanomaterials are categorized as zero-dimensional (0D) (for example, nanoparticles), one-dimensional (1D) (for example, nanotubes & nanorods), two-dimensional (2D) (for example, graphene), and three-dimensional (3D) (for example, nanoprisms & nanoflowers)(Singh Chouhan et al., 2021). The incorporation of nanomaterials in biosensing systems as the active elements has paved the way for a significant breakthrough in the field, resulting in enhanced detection of signals in small sample volumes, stable sensing probes, miniaturized tools, and systems for multiplex detection (Yüce and Kurt, 2017). Fig. 1 illustrates the different nanomaterial-based biosensors. In this review, we emphasized the various 0D, 1D, 2D, and 3D nanostructures-based POC systems that have been used extensively for biosensing purposes along with the benefits & drawbacks associated with their application in diagnostics.
Fig. 1.
Illustration of 0D, 1D, 2D, and 3D nanomaterial-based POC biosensing devices (adopted from Iannazzo et al., 2021; Panikar et al., 2020; Nami et al., 2022; Bai et al., 2017; Qiao et al., 2018; Zhang and Yan., 2019; Kannan et al., 2017).
2. Emergence of POC biosensing
Identification of infection-causing pathogens by their culture characteristic feature has been the conventional method. But this method has a major limitation. This method is not suitable for on-site detection in developing countries which usually don't have well-equipped laboratories or diagnostic centers (Kaittanis et al., 2010). POC is defined extensively in various scientific journals. The mobile devices which can be hand-held or transported, readily available for the patients, in-charge physicians, etc, to conduct on-site diagnostic tests could be considered POC testing (Lamb and B, 1995; Urdea et al., 2006). In the proximity of the patient, when testing is done, it's termed POC (Kiechle et al., 1990). Another crucial feature of POC testing is the short assaying time. Though in some cases the reduction in assay time doesn't hold much clinical significance, it aids in alleviating the patient's as well as the close relative's anxiety (Holland and Kiechle, 2005).
In general terms, POC tests are categorized into 4 main categories (Holland and Kiechle, 2005; Hu et al., 2013):
-
1)
Diagnostic tests which require immediate results for patients in critical conditions (example: Group B Streptococcus [GBS])
-
2)
Diagnostic tests that are needed to initiate the treatment (for example, resistance testing)
-
3)
Diagnostic tests that need the results for a quick decision in case of organism contaminants (for example, Mycobacterium tuberculosis, methicillin-resistant Staphylococcus aureus [MRSA])
-
4)
Diagnostic tests are carried out for patients that don't usually return for follow-up treatment (example: STD i.e., sexually transmitted diseases clinics)
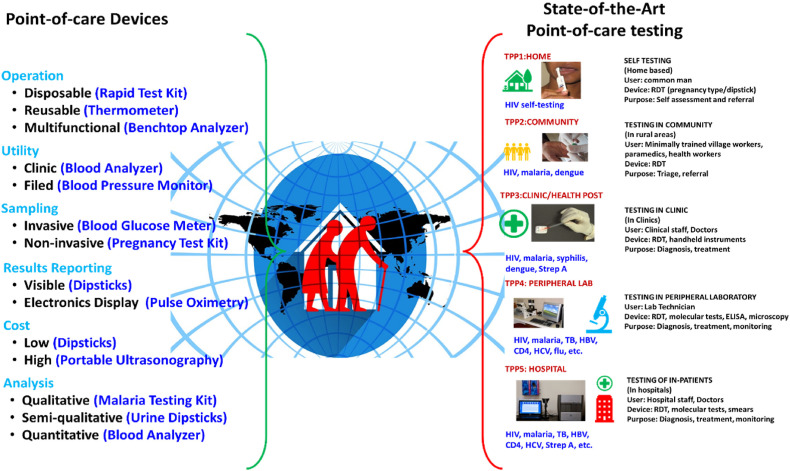
Thus, POC devices are considered for rapid on-site detection of infections. The POC devices are fabricated to meet the specific requirements i.e., either qualitatively or quantitatively assessing the specific biomarkers such as nucleic acids, antigens, etc. (Thien et al., 2016). Further classification of POC based on the aspects associated with it and examples of POC is given in Fig. 2 .
Fig. 2.
Left - different types of POC devices with examples (left), and right - different target product profiles (TPP), users, and settings/levels within the spectrum of POC testing (Reprinted from Pai et al., 2012).
The POC tests are proven to be advantageous over conventional laboratory diagnostic tests in the following ways: ease of use, little need for specialized equipment, the minimal need for requirements for the analysis, and cost-effective and rapid accurate results (Manocha and Bhargava, 2019; Syedmoradi et al., 2017). POC devices are developed usually with the aim of it being a chip-based miniaturized version of the actual assay, and a self-containing portable system for performing an assay of various analytes in the samples (Syedmoradi et al., 2017). There are challenges associated with POC test devices, for example, non-orientation to the variable factors affecting the test results in preanalytical, analytical, and post-analytical phases of testing by the clinical as well as non-laboratory trained operators. The non-clinical operators who are not laboratory trained are inclined to increase errors while performing tests as they are not trained in quality control of the tests (Manocha and Bhargava, 2019).
Pai et al., have deduced that POC testing can be considered as a combination of a spectrum of technologies, people using it, and the site where it's used. The spectrum of technologies ranges from simple dipsticks to sophisticated molecule sensors. Users comprise the common man with no technical knowledge of the principle of working the device to scientists/doctors. The site of its use also plays an important role as the surrounding conditions such as temperature, and clean environment/household environment may hinder the results. This diversity of the target product profiles within the POC testing has been illustrated in Fig. 2. The POC testing can be done in 5 diverse settings/levels, i.e., in the home, community, clinic, peripheral laboratory, and hospital. There may be distinct barriers associated with each level. The importance of these levels may vary from region to region, for example in developing countries, due to insufficient funds, laboratories, and lack of expertise, the importance of cost-effective, simple to use, and on-site detection POC test devices are much of a necessity when compared to metro cities of the world. The author has also stated that the type of device doesn't necessarily define a POC test. From Fig. 2 we can notice that the same lateral flow assay can be used almost at all sites ranging from ill-equipped rural areas to well-equipped urban areas. But some POC tests such as ELISA and florescent biosensors cannot be used at all sites of use, they will require expertise and equipment to handle the POC test. The POC testing also depends on the end user and the actual setting. POC tests may not be directly used to prescribe medicines, while some results can be used for treatments, as the POC tests that are performed just for qualitative analysis (present/absent type of tests) will have different accuracy, low specificity, nonuniformity when compared to the POC tests whose results will be used further for treatment (Gift et al., 1999).
Further, the POC wearable biosensors are gathering more attention in recent times due to their ability in providing non-invasive, real-time, and continuous biomarkers monitoring. Wearables have been developed using nanomaterials for the detection of biomolecules in tears, sweat, interstitial fluid, and saliva. The electrochemical, and optical biosensors are in the trend due to their non-invasive detection of biomarkers such as microorganisms, hormones, metabolites, etc. The amalgamation of microfluidic sampling in smaller volumes, biosensing, transport systems integration, and miniaturized flexible substrates have made the wearables easy to wear and operate. But there's still a lot of scope in research and development in improving the monitoring and analysis of physiological information. With further advancements in nanotechnology, POC biosensing can be improved with the integration of novel highly sensitive nanomaterials/composites incorporation in POC devices (Kim et al., 2019).
3. Emerging nanomaterials for POC biosensing application
The novel opportunities offered by nanotechnology in the next generation advancement have made their way to the limelight. In the diagnostic field, using modified nanomaterials for the selective detection of biomolecules has given thrust to this field (Thien et al., 2016). In the Diagnostic field, nanomaterials have made a major imprint, especially the nanoengineering technology which has been extensively used in biosensors (Welch et al., 2021). Nanomaterials are classically categorized according to the number of dimensions that fall within the nanoscale. Hence, dimension, morphology, size, shape, structure & phase of matter are considered some of the defining parameters for the classification of nanomaterials (Miernicki et al., 2019).
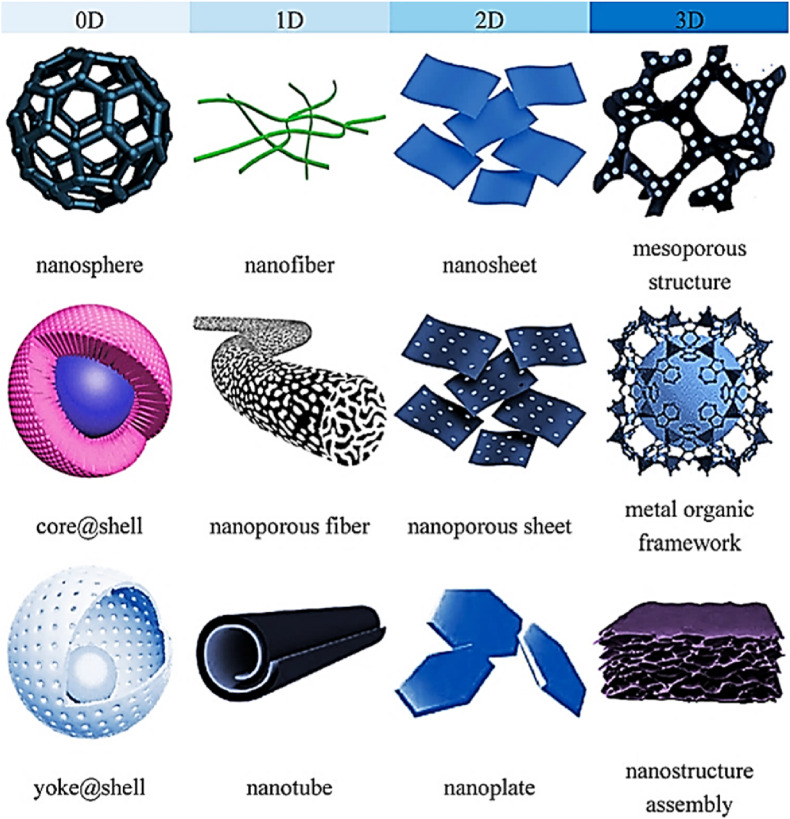
Fig. 3 shows the classification of nanomaterials based on dimension. For instance, nanoparticles are nanomaterials having all three dimensions on the nanoscale with no substantial difference between their longest & shortest axes (Jeevanandam et al., 2018). Nanofibres, on the other hand, have one of their dimensions on the nanoscale (Tian et al., 2018) & furthermore, may be solid (nanorods) or hollow (nanotubes) (Fan et al., 2007) in structure. Nanoplates and nanoribbons are also having one dimension on the nanoscale. Consolidated materials & nano-dispersions (Joshy et al., 2020) are two broad types of nanomaterials based on their morphology. Examples of 1D nanodispersive systems include nanopowders & nanoparticles. The latter is sub-divided into nanoclusters, nanocrystals, nanotubes, supermolecules, etc. (Rashtbari et al., 2020). Nanocomposites are solids having one chemically or physically unique region & minimum of a single region with nanoscale dimensions. Nanofoams are comprised of a liquid or solid matrix filled with a gaseous phase, one of which has nanoscale dimensions. Nanoporous are solids with cavities having nanoscale dimensions & nanocrystalline are some other nanostructured materials that have crystal grains (Qureshi et al., 2020) in the nanoscale. Some examples of nanomaterials categorized under 0D, 1D, 2D, and 3D nanomaterials are shown. Table 1 shown below depicts the various nanomaterials used to date for the POC application. It shows the big picture of recently developed various biosensors using 0D, 1D, 2D, and 3D nanomaterials for the detection of various diseases biomarkers/related analytes. From table 1, it can be inferred that gold nanomaterials and graphene nanomaterials are the most common choice of researchers for POC biosensing devices due to their enhanced optical properties, electrical properties, etc.
Fig. 3.
Classification of 0D, 1D, 2D, and 3D nanostructures (Goh et al., 2020).
Table 1.
Different nanomaterials of various dimensions for POC biosensing for health and wellness.
| Dimensionality | Name of nanomaterial | POC detection of | Limit of detection | Ref. |
|---|---|---|---|---|
| 0D (Nanoparticle) | Au@SCX8-RGO-TB | SARS-CoV-2 | 200 RNA copies/mL | Zhao et al. (2021) |
| 0D (Nanoparticle) | Colloidal gold | Human papilloma virus (HPV) | 1000 copies/μL | Ma et al. (2019) |
| 0D (Quantum dot) | Quantum dot | High-sensitivity cardiac troponin (hs-cTn) | 0.04 ng/mL (critical value) | Zhou et al. (2019) |
| 0D (Nanoparticle) | Fe2O3 Nanoparticles | Ovarian cancer ctRNA | 1 fM | Gorgannezhad et al. (2018) |
| 0D (Nanoparticle) | Gold Nanoparticle | Glucose | 0.1 mM | Xuan et al. (2018) |
| 0D (Quantum dot) | Graphene quantum dots (GQD) | Cephalexin | 0.53 fM | Kolhe et al. (2023) |
| 0D (Nanoparticle) | Gold Nanoparticle | Aflatoxin M1(carcinogen) | 3 pM | Kasoju et al. (2020a) |
| 0D (Nanoparticle) | Gold Nanoparticle | Aflatoxin B1(carcinogen) | 10 nM | Kasoju et al. (2020b) |
| 0D (Nanoparticle) | Gold Nanoparticle | Aflatoxin B1(carcinogen) | 33 fg/mL | Li et al. (2021) |
| 1D (Nanowire) | Gold Nanowire | Alzheimer's disease (miRNA-137) | 1.7 fM | Azimzadeh et al. (2017) |
| 1D (Nanowire) | Gold Nanowire | Parkinson's disease (miRNA-195) | 2.9 fM | Aghili et al. (2017) |
| 1D (Nanorod) | Gold Nanorod | Glucose | 0.8 mM | Tao et al. (2021) |
| 1D (Nanorod) | Gold Nanorod | SARS-CoV-2 RBD antigen | 0.73 fM | Shahdeo et al. (2022) |
| 1D (Nanorod) | Gold Nanorod | Japanese Encephalitis Virus (JEV) | 0.36 nM | Roberts et al. (2022b) |
| 2D (Nanosheet) | Graphene | Zika virus (NS1 antigen) | 450 pM | Afsahi et al. (2018) |
| 2D (Nanosheet) | Graphene oxide | Cardiac biomarker BNP (brain natriuretic peptide) | 100 fM | Lei et al. (2017) |
| 2D (Nanosheet) | Reduced graphene oxide | Patulin (PAT) | 0.66 nM | Shukla et al. (2020) |
| 2D (Nanosheet) | Graphene oxide | 4T1 breast cancer cells | 100 cells/mL | Zhang et al. (2016) |
| 2D (Nanosheet) | Graphene oxide | Lung cancer miRNA | 0.87 fM | Khoothiam et al. (2019) |
| 2D (Nanosheet) | Reduced graphene oxide | Prostate specific antigen (PSA) | 10 pg/mL | Wei et al. (2018) |
| 2D (Nanosheet) | Graphene | DNA mutations for Duchenne muscular dystrophy | 1.7 fM | Hajian et al. (2019) |
| 2D (Nanosheet) | Graphene | Folic acid protein (FAP) | 5 fM | He et al. (2017) |
| 2D (Metal organic framework) | Cobalt metal-organic framework | Glucose | 16.3 μM | (al Lawati and Hassanzadeh, 2020) |
| 2D (Metal organic framework) | Metal organic framework | carcinoembryonic antigen (CEA) | 0.742 pg/mL | Zeng et al. (2022) |
| 2D (Nanosheet) | Nickel metal-organic framework 2D nanosheet | Peroxidase | 8 nM | Chen et al. (2018) |
| 3D (Metal organic framework) | zeolitic imidazole framework-90 (ZIF-90) | Adenosine triphosphate | 233 nM | Cao et al. (2022) |
| 3D (Metal organic framework) | lanthanide functionalized metal organic framework | Glucose | 0.2 μM | Zhang and Yan (2019) |
| 3D (Metal organic framework) | Copper-Metal organic framework (Cu-MOF) | Glucose | 0.11 μM | Lu et al. (2020) |
| 3D (Metal organic framework) | Copper-Metal organic framework (Ni-MOF) | Glucose | 0.25 μM | Xiao et al. (2017) |
| 3D (Metal organic framework) | Cobalt-Metal organic framework (Co-MOF) | Glucose | 1.6 μM | Zhang et al. (2020) |
The diverse nanomaterials of different dimensions for biosensing applications can be synthesized through two approaches i.e., bottom-up approach or top-down approach. In the bottom-up approach, the dimensions of the starting material/precursors used are smaller when compared to synthesized nanomaterials. It's vice-versa in the top-down approach. Another category of classification of the nanomaterial synthesis approaches reported by (Kolahalam et al., 2019) was biological, chemical, and physical methods. The biological method was reported to be simple, eco-friendly, and usually a single-step process. In biological different bacteria, algae, fungi, plant parts, sap, etc can be used for nanomaterial synthesis (Sarkar et al., 2022).
For developing a qualitative assay, to evaluate the presence or absence of analyte/biomarker, 0D nanostructures i.e gold nanoparticles, magnetic nanoparticles, etc., are used. For example, Lateral flow assay, which usually uses gold nanoparticles for optical detection of the presence/absence of biomarkers. For the fabrication of sensitive quantitative detection of biomarkers, 1D, 2D, and 3D nanomaterials such as carbon nanotubes, graphene nanosheets, MXenes, etc., can be used for the development of biosensors. For fluorescence-based detection, the use of 0D nanomaterials such as QD nanostructures of CdSe, CdTe, graphene, etc., is much preferred. For colorimetric biosensors, the researchers have various options for using 0D nanomaterials such as gold nanoparticles, silver nanoparticles, magnetic nanoparticles, etc. Generally, for electrochemical biosensor development, 1D, and 2D nanomaterials such as carbon nanotubes, gold nanowires, graphene, and MXenes are preferred.
Looking at the trend in the development of POCT using nanomaterials, the 0D nanomaterials, nanoparticles, especially of gold are among the widely used nanomaterials from time immemorial and are still trending. In the case of 1D nanomaterials, carbon nanotubes, are the choice of material for POCT development till today. 2D nanosheets of graphene have been used widely because of their electrical conducting properties (Shahdeo et al., 2020), but recently, MXenes, transition metal dichalcogenides, etc., are trending in usage as it offers better advantages when compared to graphene conducting properties in the development of sensitive biosensors. In the case of 3D nanomaterials for the development of POCT, the Metal-organic framework has been extensively researched.
4. 0D nanomaterials for POC biosensing
Also referred to as spherical nanomaterials, 0D nanomaterials do not have any of their dimensions greater than 100 nm, are thus ultrasmall in size, display an effect termed quantum confinement, show good biocompatibility, possess excellent physical & chemical properties, and are therefore amongst the most widely used nanomaterials for biosensing purposes (Wang et al., 2020). Nanoparticles & QDs are two well-known examples of this category. Attachment of a 0D nanomaterial to a biomarker causes the formation of a molecule that will react only under well-optimized pathological conditions and the resultant modified nanosphere will act as a biological label, which will detect the analyte presence. 0D nanomaterials are discussed in detail as mentioned below.
4.1. 0D Nanoparticles
Nanomaterials are used in both optical (Kasoju et al., 2020b) & electrochemical biosensors as these aid in improving the ultrasensitive detection of biomarkers occurring in biofluidic samples (Roberts and Gandhi, 2020). For example, Hussein et al. have utilized gold nanoparticles (AuNPs) to develop a quick and simple POC lateral flow immunoassay (LFA) for the detection of human IgM & IgG antibodies against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in the blood (Hussein et al., 2020). Further, another study has reported the use of LFA in which mesoporous silica encapsulated up-conversion nanoparticles (UCNPs@mSiO2) were used for the detection of SARS-CoV-2, additionally, the sensor was IoMT enabled, and was shown to be accessible to edge hardware devices such as 5G smartphones, computers, etc. via Bluetooth (Guo et al., 2021). Similarly, another group has focussed on the use of noble nanoparticles i.e., AuNPs & silver nanoparticles (AgNPs) for the generation of nanoparticles-based biosensors as a detection strategy against emerging pathogenic RNA viruses. They have depicted that, with the increase in size, the color of nans suspensions changes from red to purple/blue & vice-versa, and the way the electrons are transferred from AuNPs to the electrode surface, thereby allowing electrochemical response measurement (Ibrahim et al., 2021).
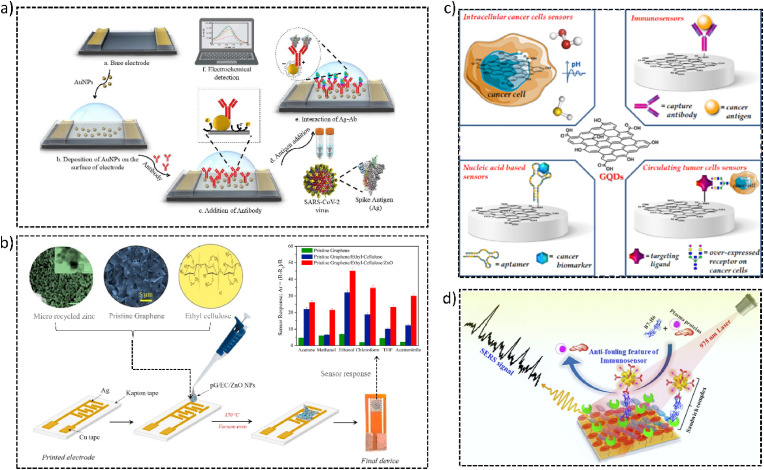
Furthermore, Roberts et al. fabricated an electrochemical biosensor, using AuNPs, for quick, convenient, and time-saving detection of COVID-19 in clinical samples as shown in Fig. 4 a. They coated AuNPs onto the fluorine-doped tin oxide (FTO) electrode, followed by attaching in-house generated antibody against SARS-CoV-2 via physisorption & then tested electrochemical changes due to binding on the addition of antigen (Roberts et al., 2021b). Another study has identified a potential in magnetic nanoparticles (MNPs) to be used as multifunctional agents for the diagnosis & therapy of diseases (Chouhan et al., 2021). Further, a group has utilized MNPs for the labeling of breast cancer cells. They have also analyzed the sensitivity of electrical bioimpedance patterns (González-Díaz and Golberg, 2020). Gold-based hybrid nanomaterials have also been popularly used by researchers for the detection of diseases such as the use of AuNPs conjugated with graphene for monitoring Parkinson's disease (Aminabad et al., 2022), as they offer multi-functionalities in high specificity and sensitivity molecular detection (Kim et al., 2016). To perform biosensing of human immunodeficiency virus (HIV) (Islam et al., 2019a, Islam et al., 2019b; Nandi et al., 2020), a group first treated glass substrates with silane layer evenly, then added HIV pseudovirus which was followed by adding anti-HIV gp41 antibodies. These antibodies were fused with selenium nanoparticles (SeNPs) and gold nanoclusters (AuNCs) (Manoto et al., 2021). Various microscopic & surface morphology characterizations were executed & ultimately Raman spectroscopy was used to confirm the presence of biomarkers related to HIV. This novel biosensing system thus depicted a potential for developing a POC HIV biosensor.
Fig. 4.
(a) Schematic representation of AuNP-based electrochemical biosensor for COVID detection (Reprinted from (Roberts et al., 2021b) with copyright permission for figure obtained from Elsevier). (b) Schematic representation of paper-based immunological sensor for Prostate-specific antigen of zinc oxide nanoparticles (ZnO NP) used for VOC detection using ZnO sensor (Reprinted from Hassan et al., 2021 with copyright permission for figure obtained from Elsevier). (c) Use of GQDs in various detection systems including intracellular cancer cells sensors, immunosensors, nucleic acid-based sensors, and circulating tumor cells sensors (Reprinted from Iannazzo et al., 2021). (d) Schematic representation of anti-fouling SERS-based immunosensor for POC detection of the B7-H6 tumor biomarker in cervical cancer patient serum (Reprinted from Panikar et al., 2020 with copyright permission for figure obtained from Elsevier).
Hassan et al. have reported a Zinc Oxide nanoparticle (ZnO NPs) based sensor for Volatile Organic Compounds (VOC) detection which has the potential to be developed for POC testing. A study states that human exhales more than 3500 components which contain a wide range of VOC. These VOCs could be considered biomarkers to diagnose a patient's health status non-intrusively (Pleil et al., 2013). Non-intrusive biomonitoring, (e.g., breath testing), is an evolving diagnostic field to support POC diagnostics advancement. Hassan et al. have developed the ZnO NP from discarded Zn-C batteries (Hassan et al., 2021). The sensor was fabricated by direct drop-casting of microrecycled ZnO NPs based nanocomposite material (pG/EC/ZnO NPs), where pG/EC is pristine graphene/ethyl-cellulose, onto the printed inter-digitated electrodes (IDE) on Kapton film (Hassan et al., 2021). The schematic of the biosensor is shown in Fig. 4b. The fabricated sensor was cost-effective and displayed increased sensing performance, better sensitivity, and selectivity towards chloroform and ethanol VOCs at room temperature (Hassan et al., 2021). As the awareness of climate change and pollution is constantly increasing, this research has proven to be a step toward climate-smart practices.
4.2. 0D QDs nanostructures
Owning to the diverse properties depicted by QDs (Farzin and Abdoos, 2021), such as exclusive photochemical stability, photobleaching resistance, superior brightness offered, their narrow emission spectrum, large absorption coefficient, allowing size-tunable light emission, etc., QDs or semiconductor nanocrystals have widely been employed for developing a range of sensors with fluorescence, bioluminescence & chemiluminescence biosensing and bioimaging (Martynenko et al., 2017), pathogen detection & for therapeutic purposes (Liang et al., 2014). Common examples of QDs include Cadmium telluride (CdTe), Cadmium selenide (CdSe), and Indium phosphide (InP). But, since these are heavy metal based, others such as silicon-based QDs (SiQDs) synthesized in an aqueous solution are considered less toxic, have better biocompatibility & thus offer a better option in some cases (Liu et al., 2016; Atkins et al., 2011).
Campuzano et al. have demonstrated how Graphene QDs (GQDs) & carbon dots (CDs) are advantageous in the development of nanomaterial-based biosensors. GQDs are 0D materials, belong to the carbon family, and have properties of graphene as well as CDs, hence these are considered good electron transporters, and offer increased contact area with the target analyte, thereby improving the active electrochemical response area. CDs, on the other hand, are quasi-spherical NPs depicting enhanced solubility, more biocompatibility & less toxicity (Campuzano et al., 2019). Another study has reported the production & functionalization of GQDs with various agents for early cancer diagnosis. These GQDs thus developed were able to selectively recognize & convert specific cancer biomarkers such as enzymes, proteins, antigens, hormones, other cancer cell-related by-products, biomolecules present on the cancer cell's surface, pH change, etc. into a detectable signal, help in cancer therapy & also aid in treatment evaluation as shown in Fig. 4c (Iannazzo et al., 2021).
4.3. 0D Fullerene
Fullerene has been reported to have good electrical conductivity, low manufacturing cost, high mechanical strength, high surface area, and good biocompatibility. In recent years fullerene-C60 has gathered attention among researchers because of its electrochemical properties. It has been found that in an aqueous solution partially reduced fullerene-C60 films exhibit increased electrochemical behaviors. It's being used in the modification of electrodes (Goyal et al., 2007, Goyal et al., 2008, Rather and de Wael, 2013). Suresh et al., have demonstrated the development of immunosensing device with immobilized fullerene-C60, hydroquinone (HQ), and copper nanoparticles (CuNPs) composite film on glassy carbon electrode (HQ@CuNPs-reduced-fullerene-C60/GCE). The immunosensor detects PSA selectively, quickly, and in trace amounts. The collective effect of CuNPs and fullerene-C60 nanocomposite film revealed exceptional catalytic activity towards H2O2 reduction for significantly amplified immunological sensing signals. A distinct redox peak and accelerated electrochemical reduction of hydrogen peroxide were observed. There was no intrusion of dissolved O2 and neither false-positive result in phosphate buffer solution at pH 7.0 exhibited by the HQ@CuNPs-fullerene- C60/GCE. The developed immunosensor showed a wide range of linearity in 0.005 ng/mL and 20 ng/mL with a limit of detection of 0.002 ng/mL (Suresh et al., 2020).
4.4. 0D Nanospike
Panikar et al. (2020), have reported an immunosensor with sandwich type Surface Enhanced Raman Spectroscopy (SERS) modified to be zwitterionic for B7-H6 biomarker spotting in cervical cancer patient blood serum. To combat the serum fouling, the immunosensor's thin gold film substrate was integrated with a self-assembled monolayer of zwitterionic L-cysteine. Further, to capture the B7-H6 biomarker, it was cojoined with NKp30 receptor protein. To make sure that the SERS signal was dependable in a complex mixture of media, the ATP reporter molecule was functionalized with the spiky gold nanoparticles (AuNPs) based SERS nanoprobe. It was later combined with the anti-B7-H6 antibody to form the SERS nanoprobe, which is complex anti-B7-H6@ATP@AuNPs. Fig. 4d shows the schematic representation of the immunosensor working. It has been demonstrated by many research groups that the sandwich of AuNps and gold film enhanced the Raman enhancement factor due to the formation of many “hotspots” (Pérez-Mayen et al., 2015; Rodríguez-Lorenzo et al., 2009). The stated immunosensor exhibited high reproducibility for detection of B7–H6 between 10−10 M to 10−14 M, and LOD of 10−14 M or 10.8 fg/mL. It's also stated that the LOD exceeded hundred-fold from the LOD of extensively used commercial ELISA kits. The lower LOD was claimed to be partly the result of the zwitterionic modification done. This modification reduced serum fouling by fifty-five percent in comparison to the conventionally utilized control which is BSA capture substrates.
The 0D nanostructures have served as efficient nanomaterials to develop various point-of-care diagnostics for health and wellness. QDs have also served as fluorescent probes in cancer diagnostics. Further, other material dots such as carbon dots, when doped with other molecules such as nitrogen can be used as fluorescent probes in biosensors (Zhang et al., 2015). However, nanoparticles have more scope for further improvement with respect to the sensitivity of the biosensor. 0D nanomaterials as stated, have shown excellent SERS-based applications, and more sensitive biosensors can be developed based on these properties of 0D nanomaterials.
5. 1D nanomaterials for POC biosensing
Nanomaterials having two of their dimensions ranging from 1 to 100 nm, offering high aspect ratios, providing improved compatibility with biological structures, and depicting numerous morphologies are categorized under 1D nanostructures. Metal oxides based 1D nanomaterials (Hahm, 2016) are not only cheaper but also present good electrochemical properties suitable for the creation of biosensing systems. Zhang et al. have devised 1D nanostructure arrays which are self-assembled, apt for high sensitivity & specificity, quick SARS-CoV-2 detection, perform clinical sample testing in 10 min & offer LOD of 100 pfu/mL (pfu: plaque forming unit) (Z. Zhang et al., 2022, Zhang et al., 2022). Various types of 1D nanostructures are described in the following section.
5.1. 1D Nanorods
Nanorods designed using metal oxides, in addition to depicting improved electrocatalytic properties, also offer a large surface area to volume ratio. These are widely utilized as an alternative to traditional methods of sensing glucose in diabetes patients. Normally glucose oxidase enzyme is required to be used & it becomes difficult to bring stable results with repeatability. Hence, Chakraborty et al. fabricated a copper oxide (CuO) based electrode which was of low cost, porous, followed the non-enzymatic approach of glucose biosensing, showed high sensitivity of nearly 2299 μA mM−1 cm−2 in human saliva samples, and was a comparatively quicker method of checking glucose level without any pain of pricking needles into the patient's finger (Chakraborty et al., 2020).
5.2. 1D Nanofibers
A group of researchers applied the method of electrospinning to deposit cellulose nanofibers (CNs) onto paper which was at first deacetylated in an alkaline solution followed by trimethyl chitosan (TMC) treatment. They further carried out its characterization via various microscopy techniques to confirm deposition had taken place properly & also fabricated screen-printed electrodes (SPEs) via sputtering of a thick gold layer onto TMC/CNs substrate. Later, the reduced graphene oxide was used in the surface modification of the working electrode (Dey et al., 2022; Mahari and Gandhi, 2022; Roberts et al., 2022a). Electrochemical analysis revealed the biosensor was indeed very useful in glucose enzymatic detection in whole blood, exhibited LOD of 0.1 mM with quite high sensitivity around 9.9 × 10 −4 KΩ −1 · mM −1 & depicted excellent selectivity & results in reproducibility as well (Ahmadi et al., 2021). For detecting ferulic acid in cosmetics, Alexandra et al. have devised a carbon nanofibers-based novel electrochemical biosensor that also utilized AuNPs & enzyme tyrosinase in the system for the modification of carbon. The result validation was done spectrophotometrically and the LOD offered by this novel biosensor was observed to be 2.89 × 10−9 mol L−1 (Bounegru and Apetrei, 2020).
5.3. 1D Nanopillars
Being compact in structure, nanopillars are considered to be reliable materials for mass-scale production as they have unique optical properties, provide high surface area and the cost of their fabrication is also low as compared to other nanomaterials (Pradana et al., 2021). Lee et al. used the principle of biomimetics to develop a biosensor using nanopillars, for label-free influenza virus detection. They made use of polymerized dopamine and carried out the immobilization of a peptide onto the nanopillar surface, which mimics sialic acid so that it would bind to hemagglutinin present on the viral surface. The sensor thus generated as an alternative to conventional methods of diagnosis, allowed them to detect viral particles in the range of 103 to 105 pfu (Lee et al., 2021).
5.4. 1D Nanowires
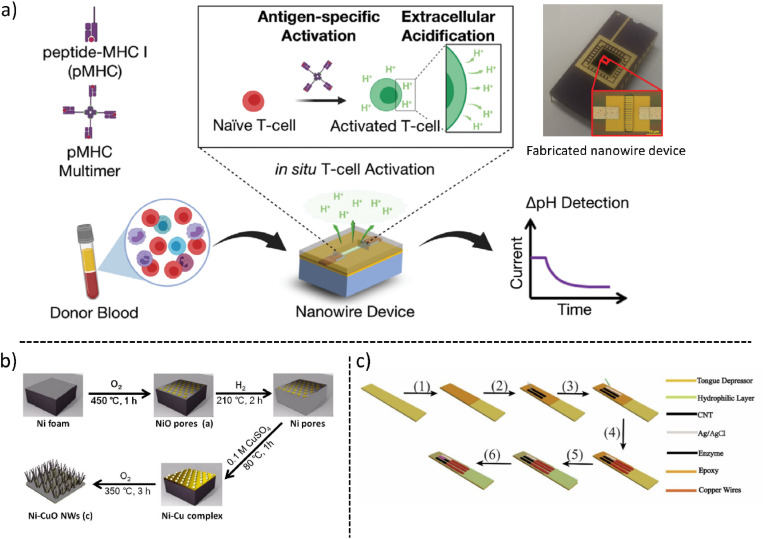
Serge et al. fabricated a field-effect transistor (FET) biosensor based on silicon nanowires. Vascular endothelial growth factor (VEGF) was chosen as the biomarker target for the diagnosis of cancer cells. They chose an approach to modify the nanowire assembly, observed changes in physical properties via scanning electron microscopy and atomic force microscopy, made DNA aptamers against VEGF, functionalized them onto the surface of nanowires & measured their conductance (Zida et al., 2020). Another group has also exploited multichannel silicon nanowire-based FET biosensors for human T-cell immune response analysis. Conventional methods for measuring pathogen-specific T-cell immune response are laborious, time-consuming and resource intensive whereas this novel approach is applicable for T-cell immune analysis. In this, they have observed a weak electrochemical signal in unvaccinated individuals and a strong signal in vaccinated individuals against a variety of common viruses, seasonal viruses & emergent pandemic viruses such as SARS-CoV-2 (Nami et al., 2022). In Fig. 5 a the schematic representation of the device principle is shown. To avoid cumbersome routes for reliable and enzymeless glucose detection, an electrochemical sensor using nickel-copper oxide nanowires. The fabrication of CuONi nanowire is shown in Fig. 5b. It showed greater sensitivity (5610.6 μA mM−1 cm−2), lower limit of detection (0.07 μM), and better selectivity (Bai et al., 2017).
Fig. 5.
(a) Illustration of silicon nanowire-based biosensor for the Rapid Screen for Antiviral T-Cell Immunity (Reprinted from Nami et al., 2022 with copyright permission for figure obtained from Wiley) (b) Synthesized CuONi showing the hierarchical chemical reaction for the glucose detection schematic (Reprinted from Bai et al., 2017 with copyright permission for figure obtained from Elsevier). (c) The tongue-depressor biosensor fabrication and schematic representation of the device (Reprinted from Luo et al., 2019). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
5.5. 1D Nanotubes
Luo et al., have fabricated a smart tongue-depressor based biosensor using carbon nanotube, for salivary glucose detection. The tongue-depressor is a medical device widely used for the detection of biomolecules by inserting the device into the mouth, in close contact with the saliva(Leeming et al., 1996; Paydas et al., 2003). Saliva is reported to be a storehouse of biomarkers related to health status and diseases. Another advantage is that saliva can be procured non-invasively, thus making it a suitable medium for the detection of biomarkers (Dodds et al., 2005; Humphrey and Williamson, 2001; Kaufman and Lamster, 2002; Ngamchuea et al., 2018). One of the biomarkers that can be detected in the saliva is glucose. The author has claimed (as of 2019) that the device reported and tested in phosphate buffer as well as in real human saliva, is the first of its kind. The sensor is fabricated by printing three electrodes, working and counter electrodes from carbon nanotubes (CNTs), and silver/silver chloride for reference electrodes in the electrochemical cell. CNTs are a widely used electronic material and studies have shown that they have very good electrical properties (Mishra et al., 2018; Yasun et al., 2020), hence can be used for amperometric biosensor fabrication (Fujisawa et al., 2018; Li et al., 2018). The schematic representation of the fabrication and the device developed is shown in Fig. 5c. Glucose oxidase was immobilized on the working electrode. The LOD range of the reported biosensor was from 7.3 μM to 6 mM and the time taken for glucose detection was reported to be about 3 min (Luo et al., 2019).
Literature survey has shown extensive use of nanotubes and nanowires-based biosensors, but nanopillars and nanofibres based-biosensors are less reported. Hence more research can be carried out in these areas. Nanowires and nanotubes are proven to be excellent 1D conductive nanomaterials for building efficient electrochemical biosensors. Miniaturization of the device is possible with 1D nanomaterials, for example, FET-based devices. Although 1D nanomaterial-based FET biosensors are sensitive, there is still scope for further developing more sensitive biosensors by tuning their conductive properties.
6. 2D materials for POC biosensing
As mentioned earlier, 2D nanomaterials are the materials in which any two dimensions are outside the nanoscale, for example, MXenes (Khunger et al., 2021), Phosphorenes, Transition Metal Dichalcogenides (TMD), Metal oxide Nanosheets, silicenes, Boron Nitride Nanosheets (BNNSs), Borophene(Kumar Sharma et al., 2022), etc. In recent years, numerous 2D materials have been used for POC biosensing purposes. Some of these are mentioned below.
6.1. 2D Nanosheets
Graphene a carbon allotrope and other forms of graphene exhibit a unique combination of optical, electronic, electrochemical (Prattis et al., 2021), and biomolecular surface adsorption properties (Narlawar and Gandhi, 2021) which make them a suitable candidate for the POC biosensing systems.
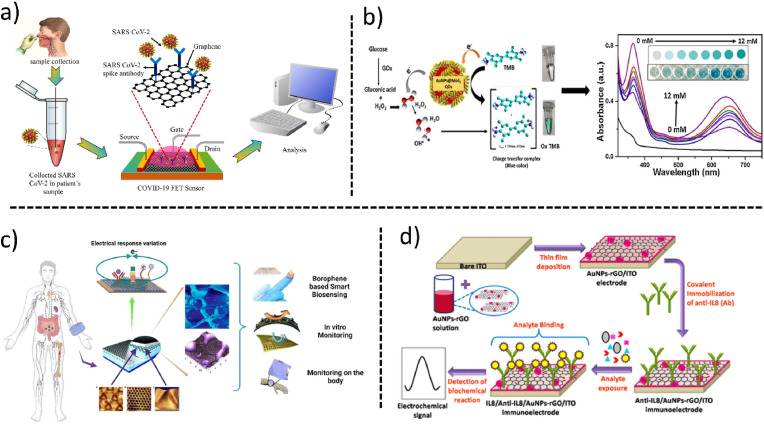
Graphene when combined with Field Effect Transistors (Islam et al., 2019a, Islam et al., 2019b), forms an integrated system, known as GraFET (Roberts et al., 2020) or GFET. Recently GFET has been used to detect SARS-CoV-2 during the COVID-19 pandemic (Roberts et al., 2021a). The first efforts to fabricate GFET for the detection of SARS-CoV-2 were taken by Seo et al. They fabricated a GFET decorated with the anti-spike antibody of SARS-CoV-2. First, they transferred the graphene sheets to SiO2/Si substrate by conventional wet transfer method and then attached anti-spike antibodies to the surface of graphene using 1-pyrenebutyric acid N-hydroxy succinimide ester, which was an interfacing molecule for the graphene surface and antibody. The fabricated biosensor is shown in Fig. 6 a. The limit of detection of SARS-CoV-2 with the fabricated biosensor was 1.6 × 101 pfu/mL which was the pioneer to develop the POC biosensors during the COVID-19 pandemic (Seo et al., 2020).
Fig. 6.
(a) Schematics and working of fabricated graphene-based GraFET biosensor for detection of SARS-CoV-2. (b) Glucose detection via synthesized AuNP@MoS2-QDs nanocomposites (Reprinted from Vinita et al., 2018 with copyright permission for Fig. obtained from Elsevier). (c) Borophene-based wearable POC biosensor (Reprinted from Kumar Sharma et al., 2022 with copyright permission for figure obtained from Elsevier) (d) Schematic representation of the gold nanoparticles and reduced graphene oxide-based immuno-electrode for immunological sensing applications (Reprinted from Verma et al., 2017). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Apart from sensing the virus, Graphene and graphene-related materials have also been reportedly used for POC detection of Ovarian cancer biomolecules (Wei et al., 2018), Lung cancer microRNA (Khoothiam et al., 2019), Glucose (Xuan et al., 2018a, Xuan et al., 2018b) and Duchenne muscular dystrophy (Hajian et al., 2019). Furthermore, graphene nanosheets have been used to detect urokinase-type plasminogen activator receptor, which is also a cancer biomarker. The experimentation was done using the FTO electrode. The immunosensor generated as a result of this had LOD of 4.8 fM and offered a linear range between 1 fM to 1 μM (Roberts et al., 2019).
Another 2D nanomaterial is MoS2 (Molybdenum disulfide). It has attracted attention among scientists worldwide due to its graphene-like properties. It is a 2D layered nanomaterial (Huang et al., 2013). The semiconducting MoS2 nanomaterial and depending on its thickness has an indirect to direct bandgap transition (Eda et al., 2011; Radisavljevic et al., 2011). This property offers a solution for overcoming the limitations associated with graphene. Due to this advantage over graphene, MoS2 is showing increasing potential use in developing sensors combined with surface-enhanced Raman scattering techniques, electrochemistry, and fluorescence (Fu et al., 2017; Saleh et al., 2017, 2018; Zhang et al., 2017). MoS2 possesses increased efficiency of fluorescence quenching, it's reportedly made use of as a nanoprobe in biomarkers homogeneous detection. MoS2 nanosheets have also reported the ability to adsorb single-stranded DNA through the van der Waals force (Ge et al., 2014a; Zhu et al., 2013). In previous works of literature, MoS2 nanosheets have also been reported as efficient dye quenchers, with potential application in developing fluorescent sensors for DNA detection with the help of recognition units such as aptamers.
Recently, Vinita et al. developed a biosensor to detect the glucose in serum, saliva, and tears. They have synthesized AuNP@MoS2-QDs composite in which MoS2-QDs were synthesized using one-step hydrothermal procedure. Then the developed composite was able to detect glucose by peroxidase mimicking property of AuNP@MoS2-QDs as shown in Fig. 6b. The developed biosensor has a good wide range of 1–400 μM with the LOD of 0.068 μΜ in Phosphate buffered saline. The fabricated biosensor was sensitive enough to detect low levels of glucose in fluids such as saliva, serum, and tear (Vinita et al., 2018). Apart from this MoS2 has also been used in the biosensing of Prostate-specific antigen (PSA) (Kong et al., 2014), Important enzymes such as DNA methyl transferases (Deng et al., 2015) and T4 polynucleotide kinases (Ge et al., 2014b).
Qiao et al., have reported developing a unique aptamer-based electrochemical sensor. The authors have fabricated the sensor based on layer structured MoS2 nanosheets affixed onto the glassy carbon electrode, conjugated with aptamer for Cardiac troponin I (cTnI) detection. The cTnI presence combines with aptamer immobilized on MoS2. It adopts a definite and rigid tertiary structure causing a feeble affinity with MoS2. Hence, the aptamer coupled cTnI will be freed from the surface of the nanosheet, in turn decreasing the resistance. For the aptamer sensor, the detection range of cTnI was from 10 pM to 1.0 μM with a LOD of 0.95 pM. This sensor had been successfully tested for cTnI presence in human blood serums (Qiao et al., 2018). It has shown the potential to be developed for POC detection.
Kumar Sharma et al., have reported another 2D material borophene, a 2D allotrope of boron with a potential application in biosensing. It has emerged in prominence because of its highly electroactive surface, controlled optical properties, anisotropic behavior, the feasibility of deposition in thin films, high electron transport, and it is also possible to create surface functionalities. Borophene due to its similar flexibility to graphene can be deposited on flexible substrates. It can also be functionalized for immobilizing bio-actives. In addition to this, its surface functionalities can be controlled for fabricating a sensitive biosensor that can be operated by a smartphone for early diagnosis using POC testing as shown in Fig. 6c.
6.2. 2D Nanocomposite
Graphene oxide is making its way into the field of biosensors as a potential matrix material because of its 2D structure, biocompatibility, mechanical stability, and ease of electronic properties tunability (Tiwari et al., 2016). In addition to these properties, simple covalent binding of biomolecules on the graphene oxide surface containing oxygen functional groups, especially -COOH, is possible without linker molecules. Metallic nanoparticles can be embodied in graphene oxide for composite synthesis. This composite will aid the electronic, electrochemical properties modulation which in turn helps in signal amplification, and time measurement for the optimization of biosensors (Gutés et al., 2012).
Verma et al. have demonstrated a highly stable electrode immunosensor made up of gold nanoparticle-reduced graphene oxide (AuNPs-rGO) composite for noninvasive, label-free detection of interleukin – 8 (IL8), a salivary oral cancer biomarker. The sensor was fabricated using Indium tin oxide glazed glass, and on top of which a thin film of AuNPs-rGO nanocomposites was layered. The receptor anti-IL8 antibodies were immobilized on the film via a covalent bond between the amine group of the receptor and the carboxylic group of reduced graphene oxide on the electrode's surface. The schematic of the same is shown in Fig. 6d below. The coaction of electrochemical and electronic properties of AuNPs as well as rGO in the composite increases the rate of electron transfer. This resulted in quick efficient detection of interleukin – 8 within 9 min. The LOD exhibited by the immunosensor 72.73 ± 0.18 pg/mL was below the clinical salivary expression level of IL8 i.e. 720 pg/mL in oral cancer patients. This biosensor also exhibited exceptional performance of 94.15% average recovery of IL8 in spiked saliva. That stability, elevated precision, and specificity made the biosensor a potential platform for the detection of initial-stage oral cancer (Verma et al., 2017). This biosensor can be further modified for the detection of other cancer biomarkers in saliva, blood serum samples, urine samples, etc.
2D nanomaterial composites have also shown promising results in wearable biosensor technology. One such wearable monitoring system is developed by Zahed et al. The authors have fabricated hybrid epidermal biosensing (bi-HEB) patch. The patch has a unique nanoporous carbon and MXene layer which acts as a transducer. The patch was coupled with a miniaturized monitoring system. This patch houses glucose, pH, and temperature biosensors. The patch within the physiological levels of 0.003–1.5 mM, demonstrated exceptional sensitivity of 100.85 μAmM−1 cm−2. In this way, the nanocomposite played a crucial role in accurately monitoring glucose in sweat and also ECG signals. This patch can also be used by human subjects in homes, gyms, etc (Zahed et al., 2022).
2D nanomaterials are one of the most widely explored and used nanomaterials for developing biosensors. Graphene nanosheet is one of the trending 2D nanomaterials used in various industries, including the healthcare sector to build biosensors. Further, exfoliated semiconductor 2D nanosheets such as Tungsten diselenide (WSe2), being photovoltaic and transparent in nanoscale can be explored more for the development of wearable biosensors with LED properties.
7. 3D nanomaterials for POC diagnosis
While 0D, 1D & 2D nanomaterials have comparatively simpler morphology, 3D nanostructures are usually an amalgamation of different nanoscale features in one single structure. Since these nanomaterials offer superior electroactive surface area, hence, they are often considered to be better at targeting analytes of interest, signal amplification & perform efficient biosensing. Complex hierarchical structures and nanocomposites are mainly categorized under 3D materials. A study reported that photonic crystals (PCs) & inverse opals (IOs) having an ordered 3D nanostructure are perfectly suitable candidates for biomarker detection & development of novel biosensors. For instance, a group fabricated ZnO IOs & ZnO PCs using the sol-gel technique & spin –coating method. The structures ultimately obtained had hexagonal compact arrangements & depicted better photoluminescence peaks. Furthermore, PCs based on TiO2 IOs & colloidal SiO2-based PCs have also been synthesized (Fathi et al., 2021). Another study was shown of the application of barcode technology for biosensing by Xu et al. This barcode was synthesized using PEG i.e., polyethylene glycol hydrogel conjugated with inverse opal particles.
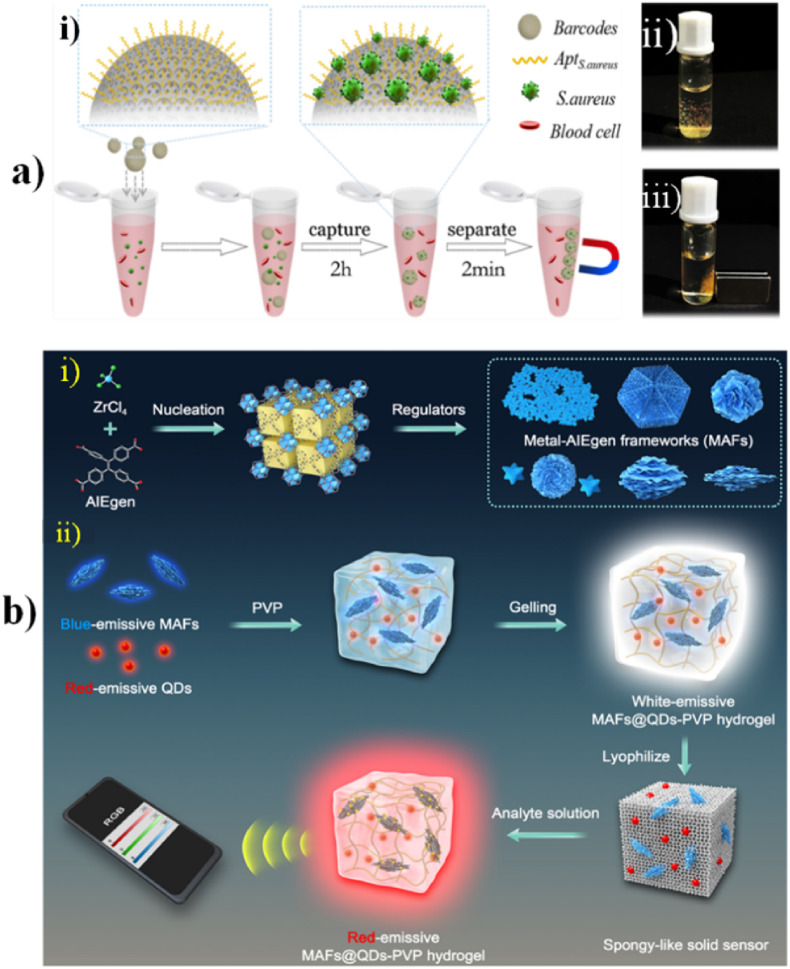
Fig. 7a illustrates aptamer probes immobilized on the IO-structured magnetic hydrogel barcodes and the real images of the barcodes which can be mobilized with the magnet. The entire barcode was reported to be less than 500 nm in size. These barcodes had specific reflection peak codes which were reported to be stable during the process of bacteria capturing on the barcode surface. The spherical surface of barcodes had ordered porous nanostructure which made it suitable for providing greater surface area for immobilization of probe and also a platform that is nanopatterned for carrying out highly efficient bioreactions. The conjugated aptamers on the barcode surface increased the sensitivity, specificity, and reliability of bacteria detection and capturing. In addition to this, the magnetic nanoparticles were tagged in the hydrogel scaffold which enabled magnetic field-based controlled movement for simplification of bioassays. The barcodes could capture bacteria with concentrations as low as 100 CFU/mL within 2.5 h which is shorter when compared to the ‘gold standard’ adopted in clinics. All the features stated above made the barcodes having IO a potential 3D nanomaterial for POC hematological infection diagnostics (Xu et al., 2018).
Fig. 7.
(a) Illustration of aptamer probes immobilized on the IO structured magnetic hydrogel barcodes. The real images of the barcode spheres were mobilized with the magnet. (Reprinted from Xu et al., 2018 with copyright permission for figure obtained from Elsevier). (b) Different MAFs synthesis, development of MAFs@QDs-PVP hydrogel complex, HDS coupled with the technology of digital sensing (Reprinted from J. Zhang et al., 2022).
In another attempt to perform glucose sensing without the use of any enzymes, as the enzymatic approach is time-consuming, there's a constant fear of enzymes getting denatured or digested by proteases, their costly purification, non-consistent results, etc., 3D cobalt oxide (Co3O4) nanowires (Fig. 7a) were fabricated onto carbon fiber paper substrates, former being extremely active & latter being quite flexible. The novel sensor thus developed showed potential in glucose monitoring in both food samples as well as clinical blood samples (Kannan et al., 2017). Fang et al. utilized the synergistic effect of zinc oxide (ZnO) providing a large surface area & unique property contributed by AuNPs to aid in the creation of a 3D functionalized hierarchical nanostructure for glucose sensing (Fang et al., 2016). The sensor showed satisfactory performance with LOD 0.02 mM & the linear range was considered acceptable having values between 1 and 20 mM. Manganese oxide (MnO2) based multiwalled CNTs nanocomposites for amperometric glucose detection have also been developed. These had a wide linear range between 5 and 200 μM and 0.2–1 mM whereas LOD was 2 μM (Hao et al., 2020). Similarly, multi-walled CNTs have also been used for the biosensing of myoglobin (Mani et al., 2014).
In addition to the above 3D nanomaterials, in recent times, Metal-Organic Frameworks (MOFs) are gaining more attention from scholars for enzyme immobilization. It's due to the MOF's intriguing properties such as large surface areas, tuneable porosity, structural flexibility, and diverse post-synthetic modifications (Fig. 7b). Lanthanide has intrinsic 4f–4f transitions. Hence, the lanthanide metal-organic frameworks (L-MOF) showed excellent luminescence having potential application in sensors for sensing metal anions and cations, biomarkers, and molecules (Hao and Yan, 2017; Lian and Yan, 2016; Xu and Yan, 2016). L-MOF is also accompanied by pros for logic detectors due to outstanding fluorescence properties and high sensitivity toward analytes (Xu and Yan, 2018).
Y. Zhang et al., have reportedly developed for the first time (as of 2019), an L-MOF-enzyme composite. To fabricate the L-MOF-enzyme composite, glucose oxidase (GOx) was incorporated in an H2O2-sensitive L-MOF. The enzyme cascade catalysis process produces H2O2. Through this process, the GOx-Eu3+@UMOF showed good sensitivity and selectivity towards glucose under neutral conditions and room temperature. The LOD of serum and urine was reported to be 0.23 μM and 0.25 μM. The was sensor constructed having three INHIBIT logic gates. The logic detector displayed three different outputs of low, medium, and high, equivalent to three inputs i.e., 0.1 μM–10 μM, 10 μM–10 mM, >10 mM. The testing results being distinguishable via the naked eye on the screen, by the three lights and fluorescence, makes it suitable for self-diagnosis of Glucose, and on-site detection with ease (Zhang and Yan, 2019). Another MOF used for ultrasensitive biosensing was reported by J. Zhang et al. The aggregation-induced emission luminogen (AIEgen) acted as the ligand, hence they named it as metal-AIEgen frameworks (MAFs). Fig. 7b shows different MAFs synthesis and also the development of MAFs@QDs-PVP hydrogel complex for HDS coupled with the technology of digital sensing. MAFs were reported to process a luminescent mechanism related to the structural rigidity-enhanced emission. This had proven to give a high quantum yield (∼99.9%). The MAFs were optimized to display enhanced sensitivity of 102- to 103-fold, for the lateral flow immunoassays (LFIA) and hydrogel-based POC digital sensor. Since MAFs showed greater affinity for direct protein adsorption, they can be used for robust serum detection for POC testing (J. Zhang et al., 2022).
3D nanocomposite assembly is difficult and hence not as popular as other dimensional nanomaterials for developing biosensors. Since 3D nanostructures have a larger electroactive surface area so more sensitive biosensors can be developed. 3D nanostructures assembly can be made simpler in order to easily fabricate biosensors. 3D nanomaterials especially nanocomposites can be developed with different semiconducting materials with enhanced electro-chemical properties that can have synergistic effects and can prove to be advantageous over a single material.
8. Challenges and alternative approaches
Point of care (POC) diagnostics, as the name suggests should be available for diagnosis on-site at various locations such as hospitals, homes, gyms, etc. To achieve this, the developed biosensor/detection system must be portable with ease of diagnosis. Portability being the most significant feature of POC testing, miniaturization is the biggest challenge encountered in the field of POC biosensing. Due to the revolution in technology, for example in microelectronics, the circuit dimensions have been shirked making it possible to fabricate miniaturized POC devices, but with time and extensive advancement in technology, there is a lot of scope for improvement in designs that can offer better functionality.
In addition to the portable miniaturized feature of POC devices, another important feature is the ease of operation of the device, such as the paper-based microfluidic devices and lateral flow assays utilizing gold nanoparticles (0D material) as a colorimetric agent. But there are challenges associated with the operation of some POC devices, e.g., the non-orientation to the variable factors affecting the test results in preanalytical, analytical, and post-analytical phases of testing by the clinical as well as non-laboratory trained operators. The non-clinical operators who are not laboratory trained are inclined to increase errors while performing assays as they are not trained in quality control of the tests (Manocha and Bhargava, 2019). Hence, with the aid of digital media awareness can be spread regarding the use of the readily available POC devices in use for users in homes, social media can also add an advantage to this. Adequate training via set SOPs should be made compulsory for clinical workers, especially in rural areas.
Further, when looking into the biomarkers used to develop the POC detection kits, there is always a challenge associated with the stability of the biological components. Numerous PoC biosensors have been reported to use antibodies as a biorecognition element. Antibodies are proteins and proteins are heat labile. So, there is a need to improve this storage stability. To address the issue, an alternative i.e., aptamers can be used. But there is also a challenge in the generation of aptamers. It is a tedious and complicated process, but improvement in the SELEX (aptamer generation process) can resolve this issue. As nanomaterials are the heart of many sensors, their monodisperse synthesis poses a challenge. Rigorous and thorough optimization of those protocols considering various parameters involved can fix the inconsistency issue.
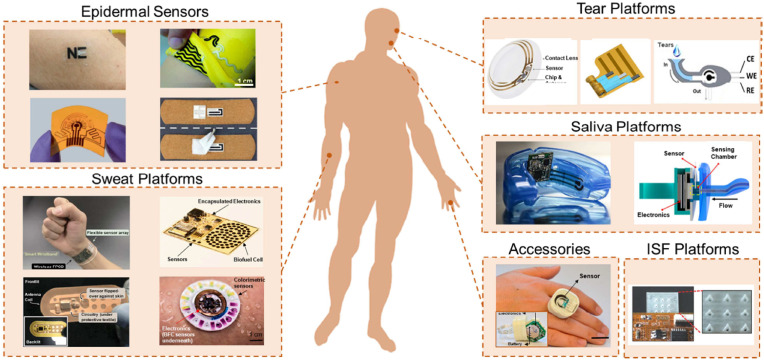
Even if all the above-mentioned approaches are inculcated, proper diagnosis will still be an issue if the bio-analyte is in trace amounts in the sample being tested. Hence the POC system sensitivity is a very important factor for POC testing. The disease-associated biomarkers are secreted in trace amounts hence, the colorimetric detection using plasmonic nanoparticles is not efficient to display proper results. In such cases graphene (2D material) based or carbon nanotube (1D material) based electrochemical biosensors are useful. But many of these are not portable (only miniaturized for lab setup). Miniaturizing them for homes and hospitals are necessary area of improvement. To address this issue, the advancement of nanotechnology has directed the development of miniaturization of POC devices with better sensing performance at the point of care, integration, interfacing, etc (Mujawar et al., 2020). Advanced 2D nanomaterials such as MXenes, and borophene have emerged as alternative nanomaterials incorporated to develop next-gen POC testing devices. These nanomaterials are portable, wearable, biocompatible, smart, and intelligent when amalgamated with 5G technologies, AI, ML, and IoT. Wearable biosensors or in other terms wearable electronic devices such as smartwatches and smart bands are trending due to various health-tracking functionality. These devices are operated via a computer algorithm and detected via embedded sensors. Conventionally, these devices don't use nanomaterials for their functionality. But researchers around the world are making an effort to develop nanomaterials-based wearable biosensors for health and wellness. In the last three years, wearable electrochemical sensors research and development for the detection of biomarkers is trending. In addition to this, the innovation in the field of science and technology in nano/microfabrication methods, bioelectronics, material chemistry, wireless sensor networks, and digital communication technology, the wearable chemical sensors has witnessed breakthroughs over the last decade (Min et al., 2021). Fig. 8 shows the recently developed advanced wearables and platforms. The epidermal sensors were developed on a stretchable/flexible platform by patterning electrodes. Epidermal sensors (Ciui et al., 2018) conjugated with wearable sweat platforms result in a POC device that should acquire, process, and transmit data from the electrochemical sweat (Bandodkar et al., 2019; Gao et al., 2016) to a user interface. Tear monitoring wearable platforms have also been developed for biomolecules such as google lens for glucose monitoring. In addition to this, for continuous monitoring of saliva analytes, saliva-based platforms (García-Carmona et al., 2019) are developed. To enhance wearable performance, accessories are added with sensing capabilities. For in-situ monitoring of analytes in the interstitial fluid, wearable devices containing hollow microneedles with biosensors have also been developed. Wearable nanomaterial-based POC biosensors for the detection of common electrolytes, metabolites, and biomarkers such as proteins, DNA, etc is the emerging area of research that will soon prove its potential in the field of POC biosensing for health and wellness (Min et al., 2021).
Fig. 8.
Recently developed advanced wearables and platforms (adapted from Bandodkar et al., 2019; Ciui et al., 2018; Gao et al., 2016; García-Carmona et al., 2019; Jia et al., 2013; Kagie et al., 2008; Kim et al., 2015; Lv et al., 2018; Min et al., 2021; Pal et al., 2018; Rose et al., 2014; Sempionatto et al., 2020, 2017; Yang et al., 2019; Yu et al., 2020).
These devices can be further transformed into futuristic POC detection kits by surface functionalization and hybridization of the 2D nanocomposite materials, rapid data analysis and acquisition strategies, and the use of advanced machine learning algorithms (Chaudhary et al., 2022).
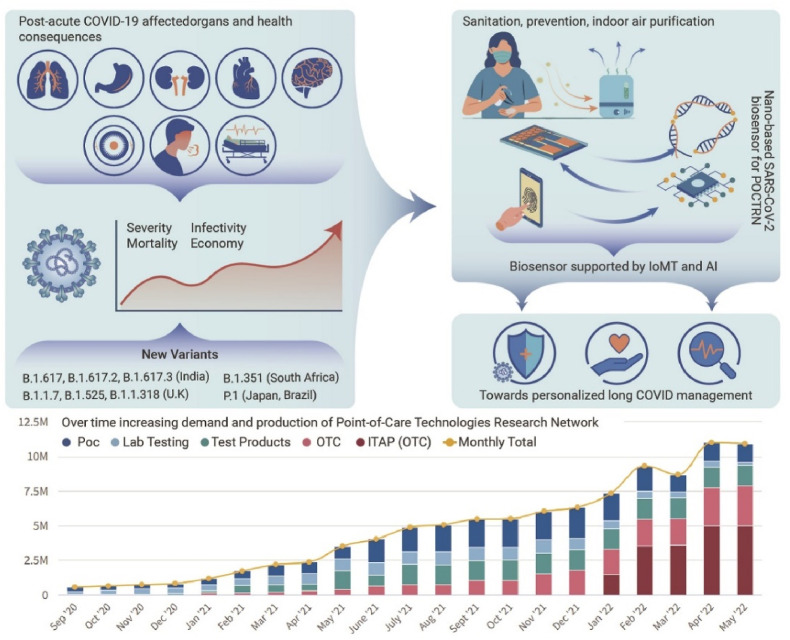
Recently, another promising technology has emerged which has proven to be a potential tool for the development of POC biosensing and disease management, i.e., Internet of Medical Things (IoMT)-assisted miniaturized biomedical electronics (Kaushik et al., 2020). This played a major role in the recent COVID-19 outbreak. The COVID-19 status and recommendations for the better development of COVID sensing platforms have been shown in Fig. 9 (Kaushik and Mostafavi, 2022). For better diagnosis and disease management, the optimized combination and collective approach of various technologies such as biosensing by advanced nanomaterial, POC testing, IoMT interfaced testing, predictive analysis via AI, rapid testing system, Bioinformatics sharing, and timely therapy decisions, emerged as a powerful tool for efficient diagnosis and for personal level disease management (Jain et al., 2021; Manickam et al., 2022). This is more elaborated in Fig. 10 , which shows the schematic of the nano-enabled biosensing prototype for efficient diagnosis (Kaushik et al., 2021). With the help of IoMT, the data collection from the biosensor is possible, thus, the diagnosis data can be analyzed. With the help of AI, and ML this data can be further processed to evaluate the performance of various POC devices in the field. This analysis can be used for the development of enhanced POC biosensors, similar to the feedback loop process.
Fig. 9.
Schematic representation of COVID-19 status and recommendations for the better development of COVID sensing platforms (Reprinted from Kaushik and Mostafavi, 2022).
Fig. 10.
Schematic of the nano-enabled biosensing prototype for efficient diagnosis (Reprinted from Kaushik et al., 2021a).
Overall, the cost-effective scale-up technological set-up has remained as one of the biggest challenges. Many POC diagnostics survive in their prototype phase but failed in scale-up scenarios. But in due time, with the advancement of technology and with the aid of funding from various institutions and government support, the research in the POC testing arena will further progress and will show promising outcomes to aid humanity.
9. Conclusion and future perspective
The development of novel methods to aid in the early diagnosis & prediction of many diseases continues to remain the topmost priority for all researchers around the world to mitigate the occurrence of deadly outcomes that may follow later. Conventional methods for medical diagnosis & therapeutics used are laborious and time-consuming. In addition, the fear of denaturation of enzymes in the enzymatic approach is associated with improper handling, a time gap in sample collection and analysis, and is less accurate. Hence, in recent times, point-of-care sensing has emerged as a leading alternative, offering portable detection devices, which can be conveniently used even at the patient's bedside. For example, Lateral flow assay POC tests, wearable patches, etc., have made POC biosensing of biomarkers easy for the common man. Wearable POC biosensors have shown potential in the non-invasive, real-time, continuous monitoring of biomolecules. Integration of nanomaterials/nanocomposites in the wearables has made biosensors highly sensitive and further reduces the limit of detection. Nanomaterials conferring unique properties in different dimensions such as 0D, 1D, 2D & 3D have made a mark for themselves by making things simpler by providing enhanced surface area for immobilization of biomarkers against the target to be detected, improved electrochemical & optical properties, change in which can be inferred by a change in their conductance values. The existence of nanomaterials in a variety of shapes, structures, morphologies, dimensions, phases of matter, etc., has made them suitable candidates for use in POC-based biosensing time and time again.
As discussed in this review, nanostructures-based POC detection of certain life-threatening diseases such as various cancers, diabetes, Parkinson's, influenza, Duchenne muscular dystrophy, COVID-19, etc., along with biomarkers involved in their detection has already been achieved with increased specificity, enhanced sensitivity with low LOD values, good selectivity & overall better biocompatibility. 0D Nanoparticles such as AuNPs, AgNPs & QDs have proven to be one of the most utilized nanomaterials for biosensing purposes. Also, these are often conjugated with other dimensional materials such as graphene, MXenes to create a modified & much more efficient detection system showing synergistic effects of both kinds of nanomaterials as mentioned in the literature of this review.
Since the COVID-19 pandemic, the world has realized the importance of highly mutating viruses, it has become extremely necessary to diagnose the potential threat causing viruses. The post-COVID times have also made it necessary for continuous monitoring of complications associated with post-infections like weakening of lungs, pulmonary diseases, cardiac disorders, etc., in these cases, POC diagnostics have established their importance. With the aid of IoMT, AI, etc POC biosensing is proven to show potential in the development of highly sensitive POC testing devices which will aid in personal-level disease management. To conclude, nanostructures-based POC biosensing & diagnostics is a field that has proven its mettle over time & continues to evolve for the betterment of the healthcare sector & prevention of potentially harmful diseases. We believe that this review opens new ideas for further future improvement in POC detection using 0D, 1D, 2D & 3D nanomaterials for health and wellness.
CRediT authorship contribution statement
Manisha Byakodi: Writing – original draft. Narlawar Sagar Shrikrishna: Writing – original draft. Riya Sharma: Writing – original draft. Shekhar Bhansali: Supervision, Writing – review & editing. Yogendra Mishra: Supervision, Writing – review & editing. Ajeet Kaushik: Supervision, Writing – review & editing. Sonu Gandhi: Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank for their kind support and the Indian Council of Medical Research (ICMR), New Delhi, (Grant number BT/AAQ/01/NIAB-Flagship/2019) and are grateful to Science and Engineering Research Board (SERB), New Delhi (Grant Number-WEA/2020/000036 & CRG/2020/003014). The authors are grateful for the research endowment under the Intensification of Research in High Priority Area (IRHPA) program from the Science and Engineering Research Board (SERB), New Delhi (Grant Number IPA/2020/000069). The authors are grateful for funding provided by the Department of Biotechnology (DBT), New Delhi (Grant number BT/PR34216/AAQ/1/765/2019).
Data availability
No data was used for the research described in the article.
References
- Afsahi S., Lerner M.B., Goldstein J.M., Lee J., Tang X., Bagarozzi D.A., Pan D., Locascio L., Walker A., Barron F., Goldsmith B.R. Biosens. Bioelectron. 2018;100:85–88. doi: 10.1016/j.bios.2017.08.051. [DOI] [PubMed] [Google Scholar]
- Aghili Z., Nasirizadeh N., Divsalar A., Shoeibi S., Yaghmaei P. Biosens. Bioelectron. 2017;95:72–80. doi: 10.1016/j.bios.2017.02.054. [DOI] [PubMed] [Google Scholar]
- Ahmadi A., Khoshfetrat S.M., Kabiri S., Fotouhi L., Dorraji P.S., Omidfar K. IEEE Sensor. 2021;21:9210–9217. [Google Scholar]
- al Lawati H.A.J., Hassanzadeh J. Anal. Chim. Acta. 2020;1139:15–26. doi: 10.1016/j.aca.2020.09.026. [DOI] [PubMed] [Google Scholar]
- Aminabad E.D., Mobed A., Hasanzadeh M., Hosseinpour Feizi M.A., Safaralizadeh R., Seidi F. RSC Adv. 2022;12:4346–4357. doi: 10.1039/d1ra06437a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins T.M., Thibert A., Larsen D.S., Dey S., Browning N.D., Kauzlarich S.M. J. Am. Chem. Soc. 2011;133:20664–20667. doi: 10.1021/ja207344u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimzadeh M., Nasirizadeh N., Rahaie M., Naderi-Manesh H. RSC Adv. 2017;7:55709–55719. [Google Scholar]
- Bai X., Chen W., Song Y., Zhang J., Ge R., Wei W., Jiao Z., Sun Y. Appl. Surf. Sci. 2017;420:927–934. [Google Scholar]
- Bandodkar A.J., Gutruf P., Choi J., Lee K.H., Sekine Y., Reeder J.T., Jeang W.J., Aranyosi A.J., Lee S.P., Model J.B., Ghaffari R., Su C.J., Leshock J.P., Ray T., Verrillo A., Thomas K., Krishnamurthi V., Han S., Kim J., Krishnan S., Hang T., Rogers J.A. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aav3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bounegru A.V., Apetrei C. Sensors. 2020;20:6724. doi: 10.3390/s20236724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campuzano S., Yáñez-Sedeño P., Pingarrón J.M. Nanomaterials. 2019;9:634. doi: 10.3390/nano9040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Mo F., Liu Yahua, Liu Yu, Li G., Yu W., Liu X. Biosens. Bioelectron. 2022;198 doi: 10.1016/j.bios.2021.113819. [DOI] [PubMed] [Google Scholar]
- Chakraborty P., Dhar S., Deka N., Debnath K., Mondal S.P. Sensor. Actuator. B Chem. 2020;302 [Google Scholar]
- Chaudhary V., Kaushik A., Furukawa H., Khosla A. ECS Sensors Plus. 2022;1 [Google Scholar]
- Chen J., Shu Y., Li H., Xu Q., Hu X. Talanta. 2018;189:254–261. doi: 10.1016/j.talanta.2018.06.075. [DOI] [PubMed] [Google Scholar]
- Chouhan R.S., Horvat M., Ahmed J., Alhokbany N., Alshehri S.M., Gandhi S. Cancers. 2021;13:2213. doi: 10.3390/cancers13092213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciui B., Martin A., Mishra R.K., Brunetti B., Nakagawa T., Dawkins T.J., Lyu M., Cristea C., Sandulescu R., Wang J. Adv. Healthc. Mater. 2018;7 doi: 10.1002/adhm.201701264. [DOI] [PubMed] [Google Scholar]
- Deng H., Yang X., Gao Z. Analyst. 2015;140:3210–3215. doi: 10.1039/c4an02133a. [DOI] [PubMed] [Google Scholar]
- Dey J., Roberts A., Mahari S., Gandhi S., Tripathi P.P. Front. Bioeng. Biotechnol. 2022:873811. doi: 10.3389/fbioe.2022.873811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds M.W.J., Johnson D.A., Yeh C.K. J. Dent. 2005;33:223–233. doi: 10.1016/j.jdent.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Eda G., Yamaguchi H., Voiry D., Fujita T., Chen M., Chhowalla M. Nano Lett. 2011;11:5111–5116. doi: 10.1021/nl201874w. [DOI] [PubMed] [Google Scholar]
- Fan H.J., Gösele U., Zacharias M. Small. 2007;3:1660–1671. doi: 10.1002/smll.200700382. [DOI] [PubMed] [Google Scholar]
- Fang L., Liu B., Liu L., Li Y., Huang K., Zhang Q. Sensor. Actuator. B Chem. 2016;222:1096–1102. [Google Scholar]
- Farzin M.A., Abdoos H. Talanta. 2021;224 doi: 10.1016/j.talanta.2020.121828. [DOI] [PubMed] [Google Scholar]
- Fathi F., Rashidi M.R., Pakchin P.S., Ahmadi-Kandjani S., Nikniazi A. Talanta. 2021;221 doi: 10.1016/j.talanta.2020.121615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Sheng Q., Zheng J. Anal. Methods. 2017;9:2812–2820. [Google Scholar]
- Fujisawa K., Hayashi T., Endo M., Terrones M., Kim J.H., Kim Y.A. Nanoscale. 2018;10:12723–12733. doi: 10.1039/c8nr02323a. [DOI] [PubMed] [Google Scholar]
- Gao W., Emaminejad S., Nyein H.Y.Y., Challa S., Chen K., Peck A., Fahad H.M., Ota H., Shiraki H., Kiriya D., Lien D.H., Brooks G.A., Davis R.W., Javey A. Nature. 2016;529:509–514. doi: 10.1038/nature16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Carmona L., Martín A., Sempionatto J.R., Moreto J.R., González M.C., Wang J., Escarpa A. Anal. Chem. 2019;91:13883–13891. doi: 10.1021/acs.analchem.9b03379. [DOI] [PubMed] [Google Scholar]
- Ge J., Ou E.C., Yu R.Q., Chu X. J. Mater. Chem. B. 2014;2:625–628. doi: 10.1039/c3tb21570a. [DOI] [PubMed] [Google Scholar]
- Ge J., Tang L.J., Xi Q., Li X.P., Yu R.Q., Jiang J.H., Chu X. Nanoscale. 2014;6:6866–6872. doi: 10.1039/c4nr00944d. [DOI] [PubMed] [Google Scholar]
- Gift T.L., Pate M.S., Hook E.W., Kassler W.J. Sex. Transm. Dis. 1999;26:232–240. doi: 10.1097/00007435-199904000-00010. [DOI] [PubMed] [Google Scholar]
- Goh P.S., Wong K.C., Ismail A.F. Membranes. 2020;10:1–29. doi: 10.3390/membranes10100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Díaz C.A., Golberg A. Physiol. Meas. 2020;41 doi: 10.1088/1361-6579/ab9377. [DOI] [PubMed] [Google Scholar]
- Gorgannezhad L., Umer M., Kamal Masud M., Hossain M.S.A., Tanaka S., Yamauchi Y., Salomon C., Kline R., Nguyen N.T., Shiddiky M.J.A. Electroanalysis. 2018;30:2293–2301. [Google Scholar]
- Goyal R.N., Gupta V.K., Bachheti N., Sharma R.A. Electroanalysis. 2008;20:757–764. [Google Scholar]
- Goyal R.N., Gupta V.K., Oyama M., Bachheti N. Talanta. 2007;71:1110–1117. doi: 10.1016/j.talanta.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Guo Jiuchuan, Chen S., Tian S., Liu K., Ni J., Zhao M., Kang Y., Ma X., Guo Jinhong. Biosens. Bioelectron. 2021;181 doi: 10.1016/j.bios.2021.113160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutés A., Hsia B., Sussman A., Mickelson W., Zettl A., Carraro C., Maboudian R. Nanoscale. 2012;4:438–440. doi: 10.1039/c1nr11537e. [DOI] [PubMed] [Google Scholar]
- Hahm J.I. Annu. Rev. Phys. Chem. 2016;67:691. doi: 10.1146/annurev-physchem-031215-010949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajian R., Balderston S., Tran T., deBoer T., Etienne J., Sandhu M., Wauford N.A., Chung J.Y., Nokes J., Athaiya M., Paredes J., Peytavi R., Goldsmith B., Murthy N., Conboy I.M., Aran K. Nat. Biomed. Eng. 2019;3:427–437. doi: 10.1038/s41551-019-0371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J.N., Yan B. Adv. Funct. Mater. 2017;27 doi: 10.1002/adfm.201604281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L., Li S.S., Wang J., Tan Y., Bai L., Liu A. J. Electroanal. Chem. 2020;878 [Google Scholar]
- Hassan K., Hossain R., Farzana R., Sahajwalla V. Anal. Chim. Acta. 2021;1165:338563. doi: 10.1016/j.aca.2021.338563. [DOI] [PubMed] [Google Scholar]
- He L., Pagneux Q., Larroulet I., Serrano A.Y., Pesquera A., Zurutuza A., Mandler D., Boukherroub R., Szunerits S. Biosens. Bioelectron. 2017;89:606–611. doi: 10.1016/j.bios.2016.01.076. [DOI] [PubMed] [Google Scholar]
- Holland C.A., Kiechle F.L. Curr. Opin. Microbiol. 2005;8:504–509. doi: 10.1016/j.mib.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Holzinger M., Goff A. le, Cosnier S. Front. Chem. 2014;2:63. doi: 10.3389/fchem.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Wang L., Li F., Han Y.L., Lin M., Lu T.J., Xu F. Lab Chip. 2013;13:4352–4357. doi: 10.1039/c3lc50672j. [DOI] [PubMed] [Google Scholar]
- Huang X., Zeng Z., Zhang H. Chem. Soc. Rev. 2013;42:1934–1946. doi: 10.1039/c2cs35387c. [DOI] [PubMed] [Google Scholar]
- Humphrey S.P., Williamson R.T. J. Prosthet. Dent. 2001;85:162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- Hussein H.A., Hassan R.Y.A., Chino M., Febbraio F. Sensors. 2020;20:4289. doi: 10.3390/s20154289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannazzo D., Espro C., Celesti C., Ferlazzo A., Neri G. Cancers. 2021;13:3194. doi: 10.3390/cancers13133194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim N., Jamaluddin N.D., Tan L.L., Yusof N.Y.M. Sensors. 2021;21:5114. doi: 10.3390/s21155114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam S., Shukla S., Bajpai V.K., Han Y.K., Huh Y.S., Ghosh A., Gandhi S. Sci. Rep. 2019;9:276. doi: 10.1038/s41598-018-36746-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam S., Shukla S., Bajpai V.K., Han Y.K., Huh Y.S., Kumar A., Ghosh A., Gandhi S. Biosens. Bioelectron. 2019;126:792–799. doi: 10.1016/j.bios.2018.11.041. [DOI] [PubMed] [Google Scholar]
- Jain S., Nehra M., Kumar R., Dilbaghi N., Hu T.Y., Kumar S., Kaushik A., Li C. zhong. Biosens. Bioelectron. 2021;179 doi: 10.1016/j.bios.2021.113074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeevanandam J., Barhoum A., Chan Y.S., Dufresne A., Danquah M.K. Beilstein J. Nanotechnol. 2018;9:1050–1074. doi: 10.3762/bjnano.9.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W., Bandodkar A.J., Valdés-Ramírez G., Windmiller J.R., Yang Z., Ramírez J., Chan G., Wang J. Anal. Chem. 2013;85:6553–6560. doi: 10.1021/ac401573r. [DOI] [PubMed] [Google Scholar]
- Joshy K.S., Augustine R., Li T., Snigdha S., Hasan A., Komalan C., Kalarikkal N., Thomas S. Int. J. Biol. Macromol. 2020;157:350–358. doi: 10.1016/j.ijbiomac.2020.04.175. [DOI] [PubMed] [Google Scholar]
- Kagie A., Bishop D.K., Burdick J., la Belle J.T., Dymond R., Felder R., Wanga J. Electroanalysis. 2008;20:1610–1614. [Google Scholar]
- Kaittanis C., Santra S., Perez J.M. Adv. Drug Deliv. Rev. 2010;62:408–423. doi: 10.1016/j.addr.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan P., Maiyalagan T., Marsili E., Ghosh S., Guo L., Huang Y., Rather J.A., Thiruppathi D., Niedziolka-Jönsson J., Jönsson-Niedziolka M. Analyst. 2017;142:4299–4307. doi: 10.1039/c7an01084b. [DOI] [PubMed] [Google Scholar]
- Kasoju A., Shahdeo D., Khan A.A., Shrikrishna N.S., Mahari S., Alanazi A.M., Bhat M.A., Giri J., Gandhi S. Scientific Reports. 2020;10:4627. doi: 10.1038/s41598-020-60926-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasoju A., Shrikrishna N.S., Shahdeo D., Khan A.A., Alanazi A.M., Gandhi S. RSC Adv. 2020;10:11843–11850. doi: 10.1039/d0ra00062k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman E., Lamster I.B. Crit. Rev. Oral Biol. Med. 2002;13:197–212. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- Kaushik A., Mostafavi E. Innovation. 2022;3 doi: 10.1016/j.xinn.2022.100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik A.K., Dhau J.S., Gohel H., Mishra Y.K., Kateb B., Kim N.Y., Goswami D.Y. ACS Appl. Bio Mater. 2020;11:7306–7325. doi: 10.1021/acsabm.0c01004. [DOI] [PubMed] [Google Scholar]
- Kaushik A., Khan R., Solanki P., Gandhi S., Gohel H., Mishra Y.K. Biosensors. 2021;11:359. doi: 10.3390/bios11100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoothiam K., Treerattrakoon K., Iempridee T., Luksirikul P., Dharakul T., Japrung D. Analyst. 2019;144:4180–4187. doi: 10.1039/c9an00517j. [DOI] [PubMed] [Google Scholar]
- Khunger A., Kaur N., Mishra Y.K., Ram Chaudhary G., Kaushik A. Mater. Lett. 2021;304 [Google Scholar]
- Kiechle F.L., Malinski H., Dandurand D.M., McGill J.B. Mol. Cell. Biochem. 1990;93:195–206. doi: 10.1007/BF00226192. [DOI] [PubMed] [Google Scholar]
- Kim J., Imani S., de Araujo W.R., Warchall J., Valdés-Ramírez G., Paixão T.R.L.C., Mercier P.P., Wang J. Biosens. Bioelectron. 2015;74:1061–1068. doi: 10.1016/j.bios.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.E., Choi J.H., Colas M., Kim D.H., Lee H. Biosens. Bioelectron. 2016;80:543–549. doi: 10.1016/j.bios.2016.02.015. [DOI] [PubMed] [Google Scholar]
- Kim J., Campbell A.S., de Ávila B.E.F., Wang J. Nat. Biotechnol. 2019;37:389–406. doi: 10.1038/s41587-019-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolahalam L.A., Kasi Viswanath I.v., Diwakar B.S., Govindh B., Reddy V., Murthy Y.L.N. Mater. Today Proc. 2019;18:2182–2190. [Google Scholar]
- Kolhe P., Roberts A., Gandhi S. Food Chem. 2023;398:133846. doi: 10.1016/j.foodchem.2022.133846. [DOI] [PubMed] [Google Scholar]
- Kong R.M., Ding L., Wang Z., You J., Qu F. Anal. Bioanal. Chem. 2014;407:369–377. doi: 10.1007/s00216-014-8267-9. [DOI] [PubMed] [Google Scholar]
- Kumar Sharma P., Ruotolo A., Khan R., Mishra Y.K., Kumar Kaushik N., Kim N.Y., Kumar Kaushik A. Mater. Lett. 2022;308 [Google Scholar]
- Lamb L.S., Jr., Parish R.S., Goran S.F., Biel M.H. Current Nursing Practice of Point-Of-Care Laboratory Diagnostic Testing in Critical Care Units. Am. J. Crit. Care. 1995 https://pubmed.ncbi.nlm.nih.gov/8556083/ [PubMed] [Google Scholar]
- Lee W.S., Ahn J., Jung S., Lee J., Kang T., Jeong J. Biochip J. 2021;15:260–267. doi: 10.1007/s13206-021-00027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeming J.G., Moss H.A., Elliott T.S.J. Lancet. 1996;348:889. doi: 10.1016/s0140-6736(05)64758-7. [DOI] [PubMed] [Google Scholar]
- Lei Y.M., Xiao M.M., Li Y.T., Xu L., Zhang H., Zhang Z.Y., Zhang G.J. Biosens. Bioelectron. 2017;91:1–7. doi: 10.1016/j.bios.2016.12.018. [DOI] [PubMed] [Google Scholar]
- Li X., Cheng Y., Zhao L., Zhang Q., Wang M.S. Carbon N Y. 2018;133:186–192. [Google Scholar]
- Li B., Zhang Y., Ren X., Ma H., Wu D., Wei Q. Talanta. 2021;235 doi: 10.1016/j.talanta.2021.122772. [DOI] [PubMed] [Google Scholar]
- Lian X., Yan B. Dalton Trans. 2016;45:18668–18675. doi: 10.1039/c6dt02693a. [DOI] [PubMed] [Google Scholar]
- Liang R., Yan D., Tian R., Yu X., Shi W., Li C., Wei M., Evans D.G., Duan X. Chem. Mater. 2014;26:2595–2600. [Google Scholar]
- Liu X., Zhang Y., Yu T., Qiao X., Gresback R., Pi X., Yang D. Part. Part. Syst. Char. 2016;33:44–52. [Google Scholar]
- Lu S.M., Peng Y.Y., Ying Y.L., Long Y.T. Anal. Chem. 2020;92:5621–5644. doi: 10.1021/acs.analchem.0c00931. [DOI] [PubMed] [Google Scholar]
- Luo X., Shi W., Liu Y., Sha P., Chu Y., Cui Y. Sensors. 2019;19:3864. doi: 10.3390/s19183864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J., Jeerapan I., Tehrani F., Yin L., Silva-Lopez C.A., Jang J.H., Joshuia D., Shah R., Liang Y., Xie L., Soto F., Chen C., Karshalev E., Kong C., Yang Z., Wang J. Energy Environ. Sci. 2018;11:3431–3442. [Google Scholar]
- Ma B., Fang J., Lin W., Yu X., Sun C., Zhang M. Anal. Bioanal. Chem. 2019;411:7451–7460. doi: 10.1007/s00216-019-02113-5. [DOI] [PubMed] [Google Scholar]
- Mahari S., Gandhi S. Bioelectrochemistry. 2022;144:108036. doi: 10.1016/j.bioelechem.2021.108036. [DOI] [PubMed] [Google Scholar]
- Mahari S., Roberts A., Gandhi S. Food Chem. 2022;390 doi: 10.1016/j.foodchem.2022.133219. [DOI] [PubMed] [Google Scholar]
- Mani V., Dinesh B., Chen S.M., Saraswathi R. Biosens. Bioelectron. 2014;53:420–427. doi: 10.1016/j.bios.2013.09.075. [DOI] [PubMed] [Google Scholar]
- Manickam P., Mariappan S.A., Murugesan S.M., Hansda S., Kaushik A., Shinde R., Thipperudraswamy S.P. Biosensors. 2022;12:562. doi: 10.3390/bios12080562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manocha A., Bhargava S. Curr. Med. Res. Pract. 2019;9:227–230. [Google Scholar]
- Manoto S.L., El-Hussein A., Malabi R., Thobakgale L., Ombinda-Lemboumba S., Attia Y.A., Kasem M.A., Mthunzi-Kufa P. Saudi J. Biol. Sci. 2021;28:78–89. doi: 10.1016/j.sjbs.2020.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martynenko I.V., Litvin A.P., Purcell-Milton F., Baranov A.V., Fedorov A.V., Gun’Ko Y.K. J. Mater. Chem. B. 2017;5:6701–6727. doi: 10.1039/c7tb01425b. [DOI] [PubMed] [Google Scholar]
- Miernicki M., Hofmann T., Eisenberger I., von der Kammer F., Praetorius A. Nat. Nanotechnol. 2019;14:208–216. doi: 10.1038/s41565-019-0396-z. [DOI] [PubMed] [Google Scholar]
- Min J., Sempionatto J.R., Teymourian H., Wang J., Gao W. Biosens. Bioelectron. 2021;172 doi: 10.1016/j.bios.2020.112750. [DOI] [PubMed] [Google Scholar]
- Mishra P., Banga I., Tyagi R., Munjal T., Goel A., Capalash N., Sharma P., Suri C.R., Gandhi S. RSC Adv. 2018;8:23163–23170. doi: 10.1039/c8ra02018c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujawar M.A., Gohel H., Bhardwaj S.K., Srinivasan S., Hickman N., Kaushik A. Mater. Today Chem. 2020;17 doi: 10.1016/j.mtchem.2020.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nami M., Han P., Hanlon D., Tatsuno K., Wei B., Sobolev O., Pitruzzello M., Vassall A., Yosinski S., Edelson R., Reed M. Adv. Mater. 2022 doi: 10.1002/adma.202109661. [DOI] [PubMed] [Google Scholar]
- Nandi S., Mondal A., Roberts A., Gandhi S. Adv. Clin. Chem. 2020;98:1–34. doi: 10.1016/bs.acc.2020.02.001. [DOI] [PubMed] [Google Scholar]
- Narlawar S.S., Gandhi S. Mater Today Adv. 2021;12 [Google Scholar]
- Ngamchuea K., Chaisiwamongkhol K., Batchelor-Mcauley C., Compton R.G. pubs.rsc.org. 2018;143:777–783. doi: 10.1039/c7an90101a. [DOI] [PubMed] [Google Scholar]
- Pai N.P., Vadnais C., Denkinger C., Engel N., Pai M. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A., Goswami D., Cuellar H.E., Castro B., Kuang S., Martinez R.v. Biosens. Bioelectron. 2018;117:696–705. doi: 10.1016/j.bios.2018.06.060. [DOI] [PubMed] [Google Scholar]
- Panikar S.S., Banu N., Haramati J., Gutierrez-Silerio G.Y., Bastidas-Ramirez B.E., Tellez-Bañuelos M.C., Camacho-Villegas T.A., del Toro-Arreola S., de la Rosa E. Anal. Chim. Acta. 2020;1138:110–122. doi: 10.1016/j.aca.2020.09.019. [DOI] [PubMed] [Google Scholar]
- Paydas S., Yavuz S., Disel U., Yildirim M., Besen A., Sahin B., Gumurdulu D., Yuce E. Am. J. Med. 2003;114:618–620. doi: 10.1016/s0002-9343(03)00092-5. [DOI] [PubMed] [Google Scholar]
- Pérez-Mayen L., Oliva J., Torres-Castro A., de La Rosa E. Nanoscale. 2015;7:10249–10258. doi: 10.1039/c5nr02004b. [DOI] [PubMed] [Google Scholar]
- Pleil J.D., Stiegel M.A., Risby T.H. J. Breath Res. 2013;7 doi: 10.1088/1752-7155/7/1/017107. [DOI] [PubMed] [Google Scholar]
- Pradana A., Septiani N.L.W., Dipojono H.K., Suyatman, Yuliarto B. J. Electrochem. Soc. 2021;168 [Google Scholar]
- Prattis I., Hui E., Gubeljak P., Kaminski Schierle G.S., Lombardo A., Occhipinti L.G. Trends Biotechnol. 2021;39:1065–1077. doi: 10.1016/j.tibtech.2021.01.005. [DOI] [PubMed] [Google Scholar]
- Qiao X., Li K., Xu J., Cheng N., Sheng Q., Cao W., Yue T., Zheng J. Biosens. Bioelectron. 2018;113:142–147. doi: 10.1016/j.bios.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Quesada-González D., Merkoçi A. Chem. Soc. Rev. 2018;47:4697–4709. doi: 10.1039/c7cs00837f. [DOI] [PubMed] [Google Scholar]
- Qureshi A., Tufani A., Corapcioglu G., Niazi J.H. Sensor. Actuator. B Chem. 2020;321 [Google Scholar]
- Radisavljevic B., Radenovic A., Brivio J., Giacometti V., Kis A. Nat. Nanotechnol. 2011;6:147–150. doi: 10.1038/nnano.2010.279. [DOI] [PubMed] [Google Scholar]
- Rashtbari S., Dehghan G., Amini M. Anal. Chim. Acta. 2020;1110:98–108. doi: 10.1016/j.aca.2020.03.021. [DOI] [PubMed] [Google Scholar]
- Roberts A., Gandhi S. Front. Biosci. - Landmark. 2020;25:1875–1893. doi: 10.2741/4882. [DOI] [PubMed] [Google Scholar]
- Roberts A., Tripathi P.P., Gandhi S. Biosens. Bioelectron. 2019;141:111398. doi: 10.1016/j.bios.2019.111398. [DOI] [PubMed] [Google Scholar]
- Rather J.A., de Wael K. Sens. Actuators B Chem. 2013;176:110–117. [Google Scholar]
- Roberts A., Chauhan N., Islam S., Mahari S., Ghawri B., Gandham R.K., Majumdar S.S., Ghosh A., Gandhi S. Sci. Rep. 2020;10:14546. doi: 10.1038/s41598-020-71591-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Chauhan RS., Shahdeo D., Shrikrishna SN., Kesarwani V., Horvat M., Gandhi G. Front Immunol. 2021;12:732756. doi: 10.3389/fimmu.2021.732756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Mahari S., Shahdeo D., Gandhi S. Anal Chim Acta. 2021;1188:339207. doi: 10.1016/j.aca.2021.339207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Kesarwani V., Gupta R., Gandhi S. Biosens Bioelectron. 2022;198:113837. doi: 10.1016/j.bios.2021.113837. [DOI] [PubMed] [Google Scholar]
- Roberts A., Mahari S., Gandhi S. J. Electroanal. Chem. 2022;919 [Google Scholar]
- Rodríguez-Lorenzo L., Álvarez-Puebla R.A., Pastoriza-Santos I., Mazzucco S., Stéphan O., Kociak M., Liz-Marzán L.M., de Abajo F.J.G. J. Am. Chem. Soc. 2009;131:4616–4618. doi: 10.1021/ja809418t. [DOI] [PubMed] [Google Scholar]
- Rose D.P., Ratterman M., Griffin D.K., Hou L., Kelley-Loughnane N., Naik R.K., Hagen J.A., Papautsky I., Heikenfeld J. IEEE Engineering in Medicine and Biology Society EMBC; 2014. pp. 4038–4041. EMBC 2014. [DOI] [PubMed] [Google Scholar]
- Saleh T.A., Al-Shalalfeh M.M., Al-Saadi A.A. Mater. Res. Bull. 2017;91:173–178. [Google Scholar]
- Saleh T.A., Al-Shalalfeh M.M., Al-Saadi A.A. Sensor. Actuator. B Chem. 2018;254:1110–1117. [Google Scholar]
- Sarkar B., Mahanty A., Gupta S.K., Choudhury A.R., Daware A., Bhattacharjee S. Aquaculture. 2022;546 [Google Scholar]
- Sempionatto J.R., Jeerapan I., Krishnan S., Wang J. Anal. Chem. 2020;92:378–396. doi: 10.1021/acs.analchem.9b04668. [DOI] [PubMed] [Google Scholar]
- Sempionatto J.R., Mishra R.K., Martín A., Tang G., Nakagawa T., Lu X., Campbell A.S., Lyu K.M., Wang J. ACS Sens. 2017;2:1531–1538. doi: 10.1021/acssensors.7b00603. [DOI] [PubMed] [Google Scholar]
- Seo G., Lee G., Kim M.J., Baek S.H., Choi M., Ku K.B., Lee C.S., Jun S., Park D., Kim H.G., Kim S.J., Lee J.O., Kim B.T., Park E.C., Kim S. Il. ACS Nano. 2020;14:5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- Shahdeo D., Roberts A., Abbineni N., Gandhi S. Compr. Anal. Chem. 2020;91:175–199. doi: 10.1016/bs.coac.2020.08.007. [DOI] [Google Scholar]
- Shahdeo D., Kesarwani V., Suhag D., Ahmed J., Alshehri S.M., Gandhi S. Carbohydr. Polym. 2021;266 doi: 10.1016/j.carbpol.2021.118138. [DOI] [PubMed] [Google Scholar]
- Shahdeo D., Roberts A., Archana G.J., Shrikrishna N.S., Mahari S., Nagamani K., Gandhi S. Biosens. Bioelectron. 2022;212 doi: 10.1016/j.bios.2022.114406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla, S., Haldorai, Y., Khan, I., Kang, S.M., Kwak, C.H., Gandhi, S., Bajpai, V.K., Huh, Y.S. and Han, Y.K., 2020. Mater. Sci. Eng. C 113,110916. [DOI] [PubMed]
- Singh Chouhan R., Jerman I., Heath D., Bohm S., Gandhi S., Sadhu V., Baker S., Horvat M., Raghuraj Singh Chouhan C. Nano Select. 2021;2:712–743. [Google Scholar]
- Suresh L., Bondili J.S., Brahman P.K. Mater. Today Chem. 2020;16 [Google Scholar]
- Syedmoradi L., Daneshpour M., Alvandipour M., Gomez F.A., Hajghassem H., Omidfar K. Biosens. Bioelectron. 2017;87:373–387. doi: 10.1016/j.bios.2016.08.084. [DOI] [PubMed] [Google Scholar]
- Tao Y., Luo F., Lin Y., Dong N., Li C., Lin Z. Talanta. 2021;230 doi: 10.1016/j.talanta.2021.122364. [DOI] [PubMed] [Google Scholar]
- Thien, D., Tram, N., Wang, H., Sugiarto, S., Li, T., Ang, H., Lee, C., Pastorin, G., 2016. [DOI] [PMC free article] [PubMed]
- Tian, D., Li, X.X., He, J.H., 2018. 10.1177/0263617418813826%2037, 367–388. [DOI]
- Tiwari J.N., Vij V., Kemp K.C., Kim K.S. ACS Nano. 2016;10:46–80. doi: 10.1021/acsnano.5b05690. [DOI] [PubMed] [Google Scholar]
- Urdea M., Penny L.A., Olmsted S.S., Giovanni M.Y., Kaspar P., Shepherd A., Wilson P., Dahl C.A., Buchsbaum S., Moeller G., Hay Burgess D.C. Nature. 2006;444:73–79. doi: 10.1038/nature05448. [DOI] [PubMed] [Google Scholar]
- Verma S., Singh A., Shukla A., Kaswan J., Arora K., Ramirez-Vick J., Singh P., Singh S.P. ACS Appl. Mater. Interfaces. 2017;9:27462–27474. doi: 10.1021/acsami.7b06839. [DOI] [PubMed] [Google Scholar]
- Vinita, Nirala N.R., Prakash R. Sensor. Actuator. B Chem. 2018;263:109–119. [Google Scholar]
- Wang Z., Hu T., Liang R., Wei M. Front. Chem. 2020;8:320. doi: 10.3389/fchem.2020.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B., Mao K., Liu N., Zhang M., Yang Z. Biosens. Bioelectron. 2018;121:41–46. doi: 10.1016/j.bios.2018.08.067. [DOI] [PubMed] [Google Scholar]
- Welch E.C., Powell J.M., Clevinger T.B., Fairman A.E., Shukla A. Adv. Funct. Mater. 2021;31 [Google Scholar]
- Xiao X., Zheng S., Li X., Zhang G., Guo X., Xue H., Pang H. J. Mater. Chem. B. 2017;5:5234–5239. doi: 10.1039/c7tb00180k. [DOI] [PubMed] [Google Scholar]
- Xu X.Y., Yan B. Sensor. Actuator. B Chem. 2016;230:463–469. [Google Scholar]
- Xu X.Y., Yan B. J. Mater. Chem. C Mater. 2018;6:1863–1869. [Google Scholar]
- Xu Y., Wang H., Luan C., Liu Y., Chen B., Zhao Y. Biosens. Bioelectron. 2018;100:404–410. doi: 10.1016/j.bios.2017.09.032. [DOI] [PubMed] [Google Scholar]
- Xuan, X., Yoon, H., Bioelectronics, J.P.-B. and, 2018a, undefined, n.d. Elsevier.
- Xuan X., Yoon H.S., Park J.Y. Biosens. Bioelectron. 2018;109:75–82. doi: 10.1016/j.bios.2018.02.054. [DOI] [PubMed] [Google Scholar]
- Yang Y., Song Y., Bo X., Min J., Pak O.S., Zhu L., Wang M., Tu J., Kogan A., Zhang H., Hsiai T.K., Li Z., Gao W. Nat. Biotechnol. 2019;38:217–224. doi: 10.1038/s41587-019-0321-x. [DOI] [PubMed] [Google Scholar]
- Yasun E., Gandhi S., Choudhury S., Mohammadinejad R., Benyettou F., Gozubenli N., Arami H. J. Drug Deliv. Sci. Technol. 2020;60 doi: 10.1016/j.jddst.2020.102094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Nassar J., Xu C., Min J., Yang Y., Dai A., Doshi R., Huang A., Song Y., Gehlhar R., Ames A.D., Gao W. Sci Robot. 2020;5:aaz7946. doi: 10.1126/scirobotics.aaz7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yüce M., Kurt H. RSC Adv. 2017;7:49386–49403. [Google Scholar]
- Zahed M.A., Sharifuzzaman M., Yoon H., Asaduzzaman M., Kim D.K., Jeong S., Pradhan G.B., Shin Y. do, Yoon S.H., Sharma S., Zhang S., Park J.Y. Adv. Funct. Mater. 2022 [Google Scholar]
- Zeng Y., Wang M., Sun Z., Sha L., Yang J., Li G. J. Mater. Chem. B. 2022;10:450–455. doi: 10.1039/d1tb02192c. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yan B. Nanoscale. 2019;11:22946–22953. doi: 10.1039/c9nr06475c. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Zhang C., Li Z., Ge J., Li C., Dong C., Shuang S. RSC Adv. 2015;5:95054–95060. [Google Scholar]
- Zhang H., Zhang Z., Wang Y., Wu C., Li Q., Tang B. ACS Appl. Mater. Interfaces. 2016;8:29933–29938. doi: 10.1021/acsami.6b09490. [DOI] [PubMed] [Google Scholar]
- Zhang S., Fu Y., Sheng Q., Zheng J. New J. Chem. 2017;41:13076–13084. [Google Scholar]
- Zhang L., Wang N., Cao P., Lin M., Xu L., Ma H. Microchem. J. 2020;159 [Google Scholar]
- Zhang J., Li Y., Chai F., Li Q., Wang D., Liu L., Tang B.Z., Jiang X. Sci. Adv. 2022;8 doi: 10.1126/sciadv.abo1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Zhao M., Su M., Sun Y., Ponkratova E., Tan S.-J., Pan Q., Chen B., Li Z., Cai Z., Wang H., Wu D., Shi L., Song Y. Matter. 2022 [Google Scholar]
- Zhao H., Liu F., Xie W., Zhou T.C., OuYang J., Jin L., Li H., Zhao C.Y., Zhang L., Wei J., Zhang Y.P., Li C.P. Sensor. Actuator. B Chem. 2021;327 doi: 10.1016/j.snb.2020.128899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Liu H., Gong L., Tang B., Shi Y., Yang C., Han Z. J. Thorac. Dis. 2019;11:1506–1513. doi: 10.21037/jtd.2019.03.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Zeng Z., Li H., Li F., Fan C., Zhang H. J. Am. Chem. Soc. 2013;135:5998–6001. doi: 10.1021/ja4019572. [DOI] [PubMed] [Google Scholar]
- Zida S.I., Yang C.C., Khung Y.L., Lin Y. Der. J. Med. Biol. Eng. 2020;40:601–609. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.