Abstract
Neurodegenerative diseases and neurodevelopmental disorders have become increasingly prevalent; however, the development of new pharmaceuticals to treat these diseases has lagged. Animal models have been extensively utilized to identify underlying mechanisms and to validate drug efficacies, but they possess inherent limitations including genetic heterogeneity with humans. To overcome these limitations, human cell-based in vitro brain models including brain-on-a-chip and brain organoids have been developed. Each technique has distinct advantages and disadvantages in terms of the mimicry of structure and microenvironment, but each technique could not fully mimic the structure and functional aspects of the brain tissue. Recently, a brain organoid-on-a-chip (BOoC) platform has emerged, which merges brain-on-a-chip and brain organoids. BOoC can potentially reflect the detailed structure of the brain tissue, vascular structure, and circulation of fluid. Hence, we summarize recent advances in BOoC as a human brain avatar and discuss future perspectives. BOoC platform can pave the way for mechanistic studies and the development of pharmaceuticals to treat brain diseases in future.
I. INTRODUCTION
As society ages, various geriatric disorders have emerged as social problems.1 Among them, the most common diseases are neurodegenerative diseases.2–4 Annually, the number of patients suffering from neurodegenerative diseases such as Alzheimer's, Parkinson's, and Huntington's diseases are on the rise. Dementia is one of the representative cases of neurodegenerative disease.5,6 According to the World Health Organization (WHO), 55 million people are affected by dementia, and the number is expected to grow exponentially to 139 million by 2050.7 Also, the global cost of dementia is estimated to be USD 1.3 trillion and is expected to reach approximately USD 1.7–2.8 trillion by 2030.8 Thus, neurodegenerative diseases have become a major issue associated with significant economic and social costs. The hallmarks of neurodegenerative disease are progressive deterioration in structural and functional properties of the nervous system as a result of neuronal death.9–11 However, the underlying mechanism of these diseases is not entirely understood because of the complex structure and function of the brain.12
In addition to neurodegenerative diseases, neurodevelopmental disorders, including autism spectrum disorders and intellectual disability, are also a significant problem, as the environmental toxicity issues of particulate matter and microplastics are emerging. Approximately, 120 million people worldwide suffer from neurodevelopmental disorders.13,14 It is estimated that approximately 1% of children worldwide have autism, with rates increasing over the past few decades.15
Although many pharmaceutical companies have developed new drugs to treat neurodegenerative diseases, only six of them have been approved by the Food and Drug Administration (FDA), beginning in 1993 with Tacrine.16–18 A new drug called aducanumab was recently approved by the FDA about 20 years after memantine was approved in 2003; however, its efficacy remains controversial.19–22 Moreover, the current treatment for neurodevelopmental disorder consists of a combination of behavior therapy and some medications to alleviate comorbid symptoms such as anxiety and depression.23 Currently, there are only two FDA-approved drugs that are used to treat autism disorders: riprazole, which is a partial dopamine agonist, and risperidone, which is an antagonist of serotonin and dopamine.24 However, their use is extremely limited. Since the underlying mechanism of neurodevelopmental disorders has not yet been identified, there is still no fundamental medication for the patient, and the core symptoms remain unsolved. As such, developing new drugs for neurodegenerative diseases and neurodevelopmental disorders is challenging because of the poor understanding of pathogenesis and the absence of an appropriate experimental model.
In the preclinical stages of drug development, conventional in vivo animal models play an important role; however, they have drawbacks, including ethical concerns, genetic heterogeneity with humans, and high costs.25–27 As an alternative to in vivo animal models, a variety of in vitro two-dimensional (2D) and three-dimensional (3D) cell culture models that are prepared using human-derived cells have been proposed. 2D-based cell cultures are the most widely used in vitro models. 2D-based cell cultures have numerous advantages, including simplicity, reproducibility, low cost, and versatility of analysis tools.28,29 However, they also have limitations in that they do not reflect the real microenvironment of human tissue, such as mechanical properties, cell–extracellular matrix (ECM) interactions, cell–cell interactions, fluidic conditions, and dynamic organ-level motion, leading to low physiological relevance.30,31
Cells in the human body exist in a 3D environment and spontaneously interact with other cells. Two techniques have been extensively studied: brain-on-a-chip (BoC) and brain organoids. BoC is fabricated using microfabrication techniques and is capable of constructing sophisticated and complex microstructures for 3D cell cultures.32–37 The BoC allows the co-culture of heterogeneous cell types, as well as spatial-temporal dynamic stimulation of soluble factors.38–40 However, the BoC cannot replicate the complex features of brain tissue, such as the cortical layer structure formation and the rich diversity of cell types appropriate for the human brain, both of which are characteristic of the brain organoid model.41,42 Although BoCs can recapitulate the layer structure by arranging several microchannels in parallel, the thickness of the layers differs from the thickness of individual layers in the cortical layer structure. Moreover, although there have been attempts to co-culture various types of cells on BoCs, the number of co-cultured cell types has not yet reached the diversity of the cell types found in brain organoids. Second, brain organoids are based on mimicry of the spontaneous developmental process of brain tissue. It has great potential for modeling the complex structures of brain tissue. However, brain organoid technology has limitations in mimicking brain-specific microenvironments, such as interstitial flow, brain microvasculature, and the brain immune system. In this regard, a hybrid technology called the brain organoid-on-a-chip (BOoC) has emerged. The BOoC can replicate the structure of the in vivo brain and microenvironmental factors, such as the microvasculature, flow, and diffusion-mediated molecular transport.43–45 Remarkably, brain organoids are advantageous in the modeling of neurodevelopmental disorders.46,47 Brain organoids are composed of newly differentiated cells from stem cells, and are therefore too young to reflect a degenerative process that occurs primarily in the elderly. However, the cells used for BoC are relatively matured, highly differentiated cells, which are ideal for generating an aging model. It would be potentially beneficial if these models were integrated to recapitulate various aspects of neurological diseases.
From this perspective, we summarize the recent advances in BOoC, with a particular focus on the mimicry of structural and functional aspects of in vivo brain tissue. To this end, we first briefly describe the key features of BoCs and brain organoids and their applications in brain disease modeling. Recent advances in BOoC technology are presented. Finally, the remaining issues with the BOoC are described. In the future, it is envisioned that a BOoC that closely mimics human brain tissue will contribute to the understanding of the underlying mechanisms of brain diseases and accelerate the development of new drugs.
II. BOC
A. Characteristics of the BoC
Microfluidics is a technology that can precisely control fluid in microchannels with a micrometer-scale size.48 The organ-on-a-chip (OoC) utilizes microfluidic technology to accurately model the structure and physiology of the human organs as well as the microenvironment.49,50 The BoC is a part of OoC, and it is mainly developed in the field of engineering. To construct the BoC, the cells are seeded in a chip incorporating (i) 2D surfaces of microchannels (2D BoC), (ii) on porous membranes, (iii) within the hydrogels, and (iv) in neural spheroid formation. Likewise, neural spheroids are cultured in microchannels. It is crucial to note that an instrumental factor in the 2D BoC type, which involves attaching cells to the two-dimensional surfaces of microchannels for culture, is the height of the microchannel. The microchannels in the 2D BoC are divided into two types: the height of the microchannel being higher than the size of the neuron, and the height being lower than that of the neuron, to allow only the axons to pass through. Using this height difference, inducing the growth of only axons in specific microchannels without soma is possible.51 On the other hand, the pores in the porous membrane-based BoC have a smaller size than the cells, causing the cells to attach to the surface of the porous membrane, while the substances secreted by the cells can penetrate the porous membrane. Owing to their characteristics, porous membrane-based BoCs are primarily used to recapitulate the blood–brain barrier (BBB).52,53 In the hydrogel-based BoC, cell-laden hydrogels are patterned and cultured within microchannels to achieve three-dimensional cell culture.54,55 In addition, the BoC, containing the neural spheroids in microchannels, achieves three-dimensional cell culture by forming and culturing scaffold-free neural spheroids in microchannels.56 The number of cell types and cell densities were controlled during fabrication [Fig. 1(a)]. It is noted that microenvironmental factors such as the concentration gradient of molecules, the existence of fluid flow, and matrix stiffness can be controlled in a spatiotemporal manner, enabling the mimicry of brain physiology.57–61 One of the greatest advantages of BoC is its ability to decouple various control factors such as the addition or removal of certain cell types, the presence or existence of flow, and the concentration of soluble factors.62,63 Depending on the cultured cell types, current BoCs can be classified largely into the neural circuit and the BBB In the following section, the representative cases of the two BoCs are described.
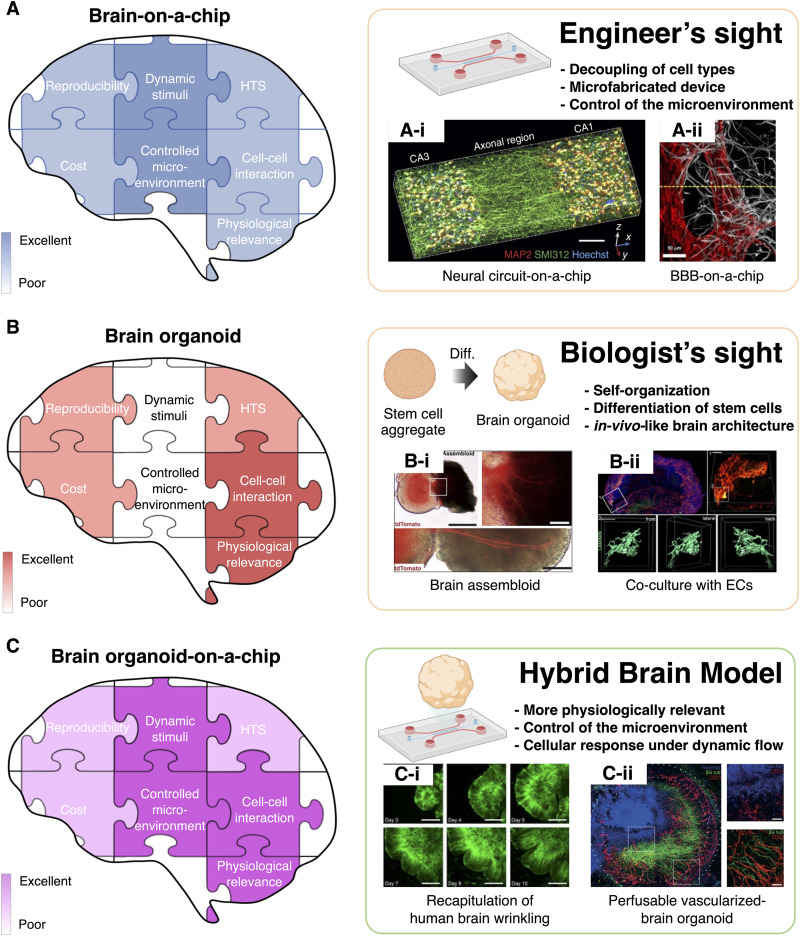
FIG. 1.
Comparison of human brain avatars and representative examples. (a) The feature of brain-on-a-chip. (a)-(i) A neural circuit-on-a-chip able to three-dimensional neuron patterning and neurite alignment was developed through the alignment of microfibrils in the hydrogel. Reproduced with permission from Kim et al., Nat. Commun. 8, 1 (2017). Copyright 2017 Springer Nature. (a)-(ii) The perfusable blood–brain barrier-on-a-chip recapitulated the low permeability of brain microvasculature to brain tissue. Reproduced with permission from Bang et al., Sci. Rep. 7, 1 (2017). Copyright 2017 Springer Nature. (b) The feature of brain organoid. (b)-(i) Brain assembloid created by attaching two brain organoids formed neural circuits through the extension of neurites. Reproduced with permission from Fligor et al., Stem Cell Rep. 16, 9 (2021). Copyright 2021 Cell press. (b)-(ii) In brain organoids co-cultured with endothelial cells, lumen structures were formed that are not perfusable. (c) The feature of brain organoid-on-ah-chip. (c)-(i) Brain organoids cultured in microchannels exhibited wrinkles, which are a structural characteristic of the brain. Reproduced from Karzbrun et al., Nat. Phys. 14, 5 (2018) Copyright 2018 Pub Med Central. (c)-(ii) Perfusable vascularized brain organoids were created by culturing brain organoids on a perfusable microvasculature-on-a-chip. Reproduced with permission from Shi et al., PLoS Biol. 18, 5 (2020) Copyright 2020 Public Library of Science.109
B. Featured BoC models: Neural circuits and BBB
Representative BoCs include neural circuits on a chip and BBBs on a chip. Neural circuit-on-a-chip aims to mimic the structure and function of the neural circuit, thus enabling real-time monitoring and functional analysis that is largely hampered in animal models.64–66 The first neural circuit-on-a-chip was a micro-ladder-type microfluidic device that could only pass the axon but not the soma.51–67 In this case, neurons were attached on the two-dimensional surface of the microchannel and cultured throughout the experiment. Later, a three-dimensional neural circuit-on-a-chips were developed with the introduction of hydrogel. The neural circuit-on-a-chip with three-dimensional hydrogel patterning differs significantly from the conventional hydrogel-mixed culture model in terms of (i) the formation of aligned neurite structure by the guidance of directional growth and (ii) the spatial separation of soma and neurite with the help of localized cell patterning54,55 [Fig. 1(a)-(i)]. As well, there is a BoC model for neural tube development by culturing stem cells on the hydrogel surface and generating a gradient of neural differentiation inducers through microchannels on the hydrogel surface.59 Therefore, the neural circuit-on-a-chip enables the modeling of propagation of signals and structural collapse in various pathologies of neurodegenerative diseases, including neuronal propagation of pTau in Alzheimer's disease and neuronal propagation of alpha-synuclein in Parkinson's disease (PD).68–70 An alternative method for three-dimensional neuronal cell culture is one that involves loading a neural spheroid into a microchannel rather than culturing a mixture of hydrogel and neurons.56 A microchannel is utilized to apply interstitial flow around the neural spheroids in this case, and the neural spheroids that have been exposed to the interstitial flow show more mature characteristics, such as extended neurites and enhanced synapse formation, compared to those without.
On the other hand, the BBB-on-a-chip aims to mimic the functional aspects of the brain microvasculature and its surroundings that show selective molecular transport characteristics. Unlike capillaries in other organs, brain capillaries are structurally surrounded by pericytes and astrocytic endfeets, which usually selectively control the transport of molecules and ions across the blood vessel wall, except for water, oxygen, and metabolically important substances.71 These brain capillaries, called the BBB, play an important role in controlling the influx and efflux of biological substances that are essential for the metabolic activity of the brain as well as the function of its neurons.72 In this regard, the BBB is crucial for the precise control of the central nervous system (CNS) and homeostasis of the brain microenvironment. The molecular transport occurs via a passive and active mechanism. While most lipophilic drugs are susceptible to the efflux functions of the BBB, all hydrophilic drugs face severe penetration barriers as a result of the tight cellular junctions of the BBB.73 Small, lipophilic molecules of less than 500 Da in size are typically transported through the endothelial cells by passive transport. Passive transport is regulated by physicochemical properties, including molecular weight, electrical charge, and lipophilicity.74,75 In contrast, active transport mechanisms are required for nutrients and proteins that are larger or hydrophilic. This active transport mechanism includes receptor-mediated, adsorption-mediated, and carrier-mediated transport.76–78 Such limited transport of drugs across the BBB is one of the biggest obstacles in the development of drugs for neuropathologies.79 Thus, the BBB-on-a-chip is an effective tool for evaluating the efficiency of drug delivery through the BBB, in vitro. Another advantage of the BBB-on-a-chip is that the tissue can be readily accessed. Electrodes are often integrated with BBB-on-a-chip to measure the transendothelial electrical resistance (TEER) to determine the integrity of tight junction dynamics in the BBB.80,81 BBB-on-a-chip can be classified into two types. One is a two-dimensional method of culturing endothelial cells on the surface of a porous membrane,52–82 and the other is a three-dimensional method of culturing endothelial cells on the surface or inside a hydrogel83–85 [Fig. 1(a)-(ii)]. It is noted that the pore size of the porous membrane-based BBB-on-a-chip is smaller than the size of the cells. Consequently, cells are cultured only on the surface of the membrane, and only astrocyte endfeet or substances can pass through the pores. Hence, the cell culture environment within the porous membrane-based BBB-on-a-chip is two-dimensional. In a hydrogel-based BBB-on-a-chip, cells are cultured on a three-dimensional scaffold, while being surrounded by hydrogel. Further, endothelial cells within the hydrogel form a vascular network with three-dimensional lumens.45,86 However, given the complex morphology of the vascular network and differences between sample to sample, a hydrogel-based BBB-on-a-chip is more difficult to analyze than a porous membrane-based BBB-on-a-chip.
III. BRAIN ORGANOID
A. Characteristics of the brain organoid
Cell spheroids usually refer to an aggregated form of readily differentiated cells, while an organoid refers to a spontaneously differentiated form of the stem cell that aggregates into a specific organ-like structure.87 The growth and differentiation are organized by stem cells themselves, but also partially controlled by the addition of signaling factors, following the developmental process of brain tissue. Therefore, studies of brain organoids have mainly been conducted in biology. Brain organoid formation is largely divided into two stages: the first stage involves induction into neuroectodermal lineages, and the second stage involves differentiation into subsequent region-specific lineages.88 Since stem cells self-organize their morphological structure during differentiation, the structure of the brain organoid closely resembles the architecture and function of the in vivo brain.89 Based on signaling factors, stem cell aggregates can differentiate into various brain regions such as cerebral organoids, midbrain organoids, and cerebellum organoids.90–92 For instance, a midbrain organoid contains dopaminergic neurons and exhibits characteristics of the midbrain, including the accumulation of neuromelanin.93 Furthermore, brain organoids are capable of modeling neurological diseases beyond simply modeling brain regions. Brain organoids have been used to model degenerative brain diseases such as Parkinson's disease (PD) and Huntington's disease (HD). For instance, Parkinson's disease is known to be caused by mutations in the leucine-rich repeat kinase 2 (LRRK2) gene.94 Smits et al. developed midbrain organoids derived from PD patients with the LRRK2-G2019S mutation.95 In this model, the number of dopaminergic neurons that produce and secrete dopamine in the PD midbrain organoid was reduced compared to that of the normal midbrain organoid. Based on these results, brain organoids could be used to recapitulate disease-relevant phenotypes. Likewise, mutations in the glucocerebrosidase (Gcase) gene and duplication of the α-synuclein gene are known to cause PD. Jo et al. developed a midbrain organoid using an embryonic stem that knocked out one of either the Gcase or GBA1, and overexpressed α-synuclein.96 Consequently, the Lewy bodies, characteristic of Parkinson's disease patients' brains, were reproduced in the midbrain organoids. In addition, disrupted cytoarchitectural characteristics of HD have been reproduced in brain cortical organoids derived from induced pluripotent stem cells (iPSCs) of HD patients.97
Moreover, brain organoids can be used to model neurodevelopmental disorders, besides neurodegenerative diseases. Qian et al. exposed the brain cortical organoid to Zika virus (ZIKV) at various incubation periods that corresponded to different pregnancy stages.98 The proliferation of neural progenitor cells in the organoid infected with ZIKV was suppressed and the thickness of the ventricular zone was greatly reduced. This phenomenon is a representative characteristic of microcephaly and appears more dramatically in the early stages of development of cortical organoids infected with the Zika virus than in the later stages.
B. Featured brain organoid models
Brain organoids have the potential to mimic the ultrastructure of human brain tissues, such as multilayer structures and region-specific cellular phenotypes.70,99,100 As organoid technology advances, brain organoids can reflect the more complex structure of human brain tissues by forming assembloids or vascularized brain organoids.101,102
An assembloid refers to the physical connection between multiple organoids, forming a route for cellular migration and molecular transport. The formation of assembloids enables axonal projection from one organoid to another, forming a neural circuit103,104 [Fig. 1(b)-(i)]. Moreover, recent studies have suggested that an assembloid can be formed from a single organoid rather than by attaching two organoids. An example is the neuromuscular system in which two types of cells aggregate in opposite directions within a sphere, one of which becomes a nerve and the other a muscle.105 The assembloids, however, cannot mimic long-range neural circuits because they are in direct contact with organoids. This limitation can be overcome by placing brain organoids at both ends of the microstructure where axons grow.106,107
It is necessary to develop vascularized brain organoids as a prerequisite for mature brain organoid production. Non-vascularized brain organoids are unable to provide oxygen and nutrients to the deep core, resulting in limited growth and inherent apoptosis in the core.108 Vascularized brain organoids can be formed by co-culturing of brain organoid and endothelial cells109 or by promoting the differentiation of certain portions of stem cells within the stem cell aggregates into brain endothelial cells.110 However, the vascularized brain organoid does not allow perfusion through the blood vessel, despite the lumen structures in the vascular structures. Nevertheless, co-culture with endothelial cells, even without perfusion, appears to facilitate the maturation of neurons and the formation of the BBB, a characteristic of the microvasculature of the brain109 [Fig. 1(b)-(ii)].
IV. BOoC, MERGING OF BOC AND BRAIN ORGANOIDS
Although brain organoids have great potential in mimicking the ultrastructures of brain tissue, the BoC is good at reconstructing brain microenvironmental characteristics in an engineered platform. However, each technology also has limitations in the recapitulation of microenvironmental features and structural aspects, implying the need for more in vivo-relevant brain models.111,112 In this regard, the hybrid platform of brain organoids and BoCs has emerged, termed BOoC, and this model is believed to serve as a next-generation “human brain avatar.” BOoC can be formed by incorporating matured brain organoids into the BoC with hydrogels.
Pioneering studies have indicated that the BOoC can present more human brain-relevant physiological and pathological features than those presented by brain organoids and BoCs [Fig. 1(c)]. Even though the brain organoid is expected to significantly contribute to the research on the brain, limitations such as insufficient maturation, heterogeneous differentiation, apoptosis in the core, and insufficient differentiation are observed in cerebral organoids.90,113–115 These limitations can be improved by integrating brain organoids with the BoC. An important advantage of the integration of BoC is its ability to adapt to fluid flows. A continuous flow of fluid supplied by a microchannel to transport nutrients and oxygen and eliminate metabolites from the brain organoid, consequently reducing apoptosis in the core of the brain organoid and improved differentiation.56,116 For example, Cho et al. demonstrated that human pluripotent stem cell (hPSC)-derived brain organoids could be cultured in a microfluidic device having a decellularized brain matrix. It has been demonstrated that gravity-driven flow, which mimics the fluid flow in the cerebrospinal and interstitial spaces in the body, can facilitate the delivery of nutrients and oxygen and eliminate waste, thus reducing cell death across organoids.117 They also confirmed the increased volume of organoids and improved neurogenesis in cortical development and electrophysiological function. Recently, Spitz et al. also introduced a multi-sensor integrated organoid-on-a-chip to monitor electrochemical signals in the midbrain.118 By culturing brain organoids under the interstitial fluid flow, they improved brain organogenesis and reduced necrotic core formation. Furthermore, they confirmed functional network maturity by monitoring a time-dependent increase in dopamine signals with significantly higher catecholamine levels.
BOoC can similarly generate a variety of stimuli using fluid flow, which allows the evaluation of the effects of various stimuli on the development of a given organoid. For instance, Cui et al. validated the effect of exosomes derived from breast cancer on human-iPSC-derived brain organoids using a microfluidic device with a micropillar array.119 Brain organoids exposed to breast cancer cell-derived exosomes were shown to exhibit impaired neurodevelopment, indicating that women with breast cancer may be at risk for impaired neurodevelopmental disorders after the birth of their fetus. Furthermore, Wang et al. examined the effects of nicotine on early fetal brain development.120 Under continuous fluid flow containing nutrients and nicotine, abnormal neuronal differentiation and migration were observed in BoC-incorporated brain organoids. Additionally, Ao et al. introduced a microfluidic device to validate the effect of prenatal cannabis exposure (PCE) on early human brain development.121 They confirmed that fluid flow with PCE resulted in reduced neuronal maturation, downregulation of cannabinoid type 1 receptors, and impaired neurite outgrowth. Using the microfluidic device, they demonstrated sequential processes including embryonic body formation, neuroectoderm induction, ECM embedding, and brain organoid maturation within a single device. Additionally, they used a microfluidic device to create air-liquid interface culture conditions that minimized the heterogeneity of brain organoids and hypoxic core formation. Under static conditions without continuous fluid flow containing PCE, these results cannot be obtained.
Meanwhile, BOoC is also useful for studying the underlying mechanisms related to the development of organoids. Karzbrun et al., for the first time, demonstrated the physics of human brain surface wrinkles using BOoC.122 They revealed that the contraction force at the center of the organoid and cell cycle-dependent nuclear expansion force at the perimeter of the organoids drive the brain wrinkles [Fig. 1(c)-(i)].
Furthermore, a brain organoid capable of perfusion was recently proposed by combining vasculature-on-a-chip with a brain organoid. Salmon et al. developed integrated neurovascular organoids using a 3D-printed microfluidic device.123 They co-cultured human pluripotent stem cell (hPSC)-derived cerebral organoids, hPSC-derived pericytes, and endothelial cells on a chip to recapitulate the characteristics of human embryonic development, especially organoid vascularization under spatially and temporally controlled conditions [Fig. 1(c)-(ii)]. They also demonstrated the perfusion and permeability of the vascular networks. FITC-dextran of 40 kDa diffused out of the neurovascular network, whereas microbeads with a 1-mm diameter remained within the microvasculature.
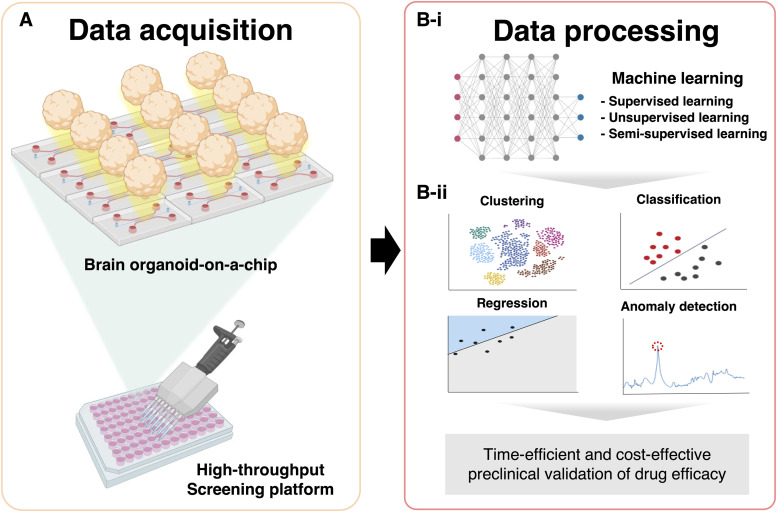
FIG. 2.
High-throughput screening of human brain avatar and machine learning techniques for new drug discovery. (a) Large size of data are required to apply machine learning techniques for biological data analysis. The injection-molded microfluidic chip allows the high-throughput screening of brain-organoids-on-a-chip. (b)-(i) From supervised learning to unsupervised learning, a variety of machine learning algorithms can be applied. (b)-(ii) Representative data that can be achieved from the application of machine learning techniques are displayed for the time-efficient and cost-effective preclinical validation of drug efficacy. The figures were created using BioRender (https://biorender.com/).
Based on their research, they concluded that vascularized organoids can be used in several applications, including the delivery of small membrane-diffusible molecules to brain organoids via blood vessels.
As shown above, the merging of two independently developed technologies opens a new avenue for studying the mechanisms underlying brain diseases and development.
V. FUTURE PERSPECTIVES
Compared with conventional 2D-based culture models, BOoC has heterogeneous 3D structures within a single organoid and large unit sizes that is difficult to image in high magnification. Therefore, imaging should be performed in series to visualize the height-dependent structures, which is essential for high-content screening (HCS). Furthermore, for high-throughput screening (HTS), multiple organoids should be imaged with an automated imaging system. In both cases, the number of images is too many to identify the readouts in a labor-intensive manner. Therefore, a more robust and scalable analysis technique is required [Fig. 2(a)]. Therefore, the adaptation of machine learning (ML) techniques for data analysis is required for both HCS and HTS. Therefore, a more robust and scalable analysis technique is required [Fig. 2(a)]. Therefore, the adaptation of ML techniques for data analysis is required for both HCS and HTS.
In recent years, artificial intelligence has achieved remarkable success, leading to tremendous interest in applying deep-learning technology to analyze biological data.124–127 Various ML algorithms have been proposed, ranging from supervised learning techniques such as multilayer perception, convolutional neural networks (CNN), and recurrent neural networks to unsupervised learning techniques such as deep generative models128,129 [Fig. 2(b)-(i)]. The types of ML models depend on the types of input data and purpose. Data used in supervised ML are labeled, whereas data used in unsupervised ML are unlabeled.130–132 These algorithms are capable of clustering, classification, regression, and anomaly detection.128,129,133,134 Therefore, it is necessary to choose the most appropriate model according to the type of data and purpose [Fig. 2(b)-(ii)].
In particular, as many deep-learning models have been developed using image-based data, such as variational autoencoder and a generative adversarial network, they show outstanding performance when applied to image-based data analysis.135 Therefore, the analysis of biological data using deep-learning technology appears to be more attractive, as a large portion of biological experiment data is image-based.
Even though the emerging technology of brain organoids and BoC has emerged as a promising technique, the amount of data that can be acquired at a time in the hybrid BooC platform is still insufficient for HTS. In general, the amount of data obtained from a single BoC is usually less than 10. Moreover, the fabrication time for a single BoC is 1–2 h, excluding 4 h for baking.136 Considering the photolithography process for master fabrication, an additional 3–4 h is required. Consequently, the device preparation process for BoCs is labor-intensive and time-consuming. The properties derived from these BoC manufacturing processes make it difficult to introduce high-throughput analysis or ML.
Hence, to train the model, approximately 1000 datasets per class are required.137,138 Therefore, enormous efforts have been made to overcome this gap by increasing the throughput of the experiments. Recently, an injection-molded plastic chip for 3D culture was proposed as a breakthrough for providing a sufficient amount of data.139–142 The plastic-based microfluidic devices were fabricated using an injection-molding technique. The master mold was usually obtained by milling, and the melted polymer was injected into the mold.143 Injection molding requires only a few seconds after the mold is made. As the device is ready-made at the factory, it can be immediately used. Scalability is another advantage of injection molding.144 In addition to being easy to use, a large number of plastic-based microfluidic devices in a short period of time at a low cost can be produced. Therefore, injection-molded microfluidic chips are capable of simultaneously testing dozens of experimental sets, greatly reducing the device preparation time and cost. There are also several commercial chips available for 3D culture.145–147 These chips are mostly ready to use, allowing people to easily move from a 2D culture based on a Petri-dish to a 3D culture using hydrogel.
Recently, Yu et al. introduced a 384 format 28-well injection-molded plastic array for the generation of angiogenic sprouts and vasculogenic networks with high throughput.148 The device was used to identify the optimal conditions for angiogenesis and vasculogenesis because of its ability to process 28 samples at a time without preparation time. As it requires minimal preparation time and labor for devices, a relatively large number of datasets at a time can be provided; obtaining 1000 datasets per condition would be expected to be easier, which is the minimum amount of data required for ML. Moreover, Tung et al. also developed 384 hanging drop array plates that can be used for 3D spheroid culture of a variety of cell types.149 It should be noted that the 384-format microfluidic devices mentioned above are compatible with existing liquid dispensers and plate readers. Compatibility with existing laboratory equipment can accelerate the automation of experiments and allow for the rapid and easy acquisition of large amounts of data. Moreover, Paek et al. developed a wellplate-based high-throughput bone-on-a-chip and analyzed the effect of drugs on osteoporosis using a deep-learning algorithm based on a CNN.150 This platform has overcome the limitations of previous HTS systems, enabling the possibility of obtaining a sufficient amount of data per class for training the model.
There are several additional examples of the application of deep learning in OoC if the organ is not limited to the brain.151 Considering that brain science research still largely relies on time-consuming in vivo experiments, this new experimental method can not only provide a large number of useful datasets in a simple and robust manner but also allows for a time-efficient analysis of drug efficacy using state-of-the-art ML techniques.
ACKNOWLEDGMENTS
This study was supported by a National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MSIT) (Nos. 2021R1A2B5B02086828 and 2022M3A9B6082678).
AUTHOR DECLARATIONS
Conflict of Interest
The authors declare that they have no competing interests.
Author Contributions
J.S. and S.B. conceived of the project. J.S. and S.B. conceptualize the ideas and wrote the manuscript. N.C. and H.N.K. supervised the study and revised the manuscript.
Jiyoung Song: Conceptualization (equal); Data curation (lead); Investigation (lead); Writing – original draft (equal). Seokyoung Bang: Conceptualization (equal); Formal analysis (equal); Investigation (equal); Writing – original draft (equal). Nakwon Choi: Funding acquisition (equal); Supervision (equal); Writing – review & editing (equal). Hong Nam Kim: Conceptualization (equal); Funding acquisition (equal); Project administration (equal); Supervision (equal); Writing – review & editing (equal).
DATA AVAILABILITY
The data supporting the findings of this study are available from the corresponding authors upon reasonable request.
REFERENCES
- 1.Arai H., Ouchi Y., Yokode M., Ito H., Uematsu H., Eto F., Oshima S., Ota K., Saito Y., and Sasaki H., Geriatr. Gerontol. Int. 12(1), 16–22 (2012). 10.1111/j.1447-0594.2011.00776.x [DOI] [PubMed] [Google Scholar]
- 2.Forman M. S., Trojanowski J. Q., and Lee V. M., Nat. Med. 10(10), 1055–1063 (2004). 10.1038/nm1113 [DOI] [PubMed] [Google Scholar]
- 3.Johnson I. P., “Age-related neurodegenerative disease research needs aging models,” Front.Aging Neurosci. (published online 2022). 10.3389/fnagi.2015.00168 [DOI] [PMC free article] [PubMed]
- 4.Wyss-Coray T., Nature 539(7628), 180–186 (2016). 10.1038/nature20411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fratiglioni L. and Qiu C., Exp. Gerontol. 44(1–2), 46–50 (2009). 10.1016/j.exger.2008.06.006 [DOI] [PubMed] [Google Scholar]
- 6.Baquero M. and Martín N., World J. Clin. Cases 3(8), 682 (2015). 10.12998/wjcc.v3.i8.682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuehn B. M., JAMA 326(15), 1471 (2021). 10.3389/fnagi.2021.662474 [DOI] [PubMed] [Google Scholar]
- 8.W. H. Organization, Global Status Report on the Public Health Response to Dementia (World Health Organization, 2021); available at https://www.who.int/publications/i/item/9789240033245.
- 9.Chen W. W., Zhang X., and Huang W. J., Mol. Med. Rep. 13(4), 3391–3396 (2016). 10.3892/mmr.2016.4948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ou G.-y., Lin W.-w., and Zhao W.-j., Front. Aging Neurosci. 13, 170 (2021). 10.3389/fnagi.2021.662474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt M. F., Gan Z. Y., Komander D., and Dewson G., Cell Death Differ. 28(2), 570–590 (2021). 10.1038/s41418-020-00706-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stolp H. and Dziegielewska K., Neuropathol. Appl. Neurobiol. 35(2), 132–146 (2009). 10.1111/j.1365-2990.2008.01005.x [DOI] [PubMed] [Google Scholar]
- 13.James S. L., Abate D., Abate K. H., Abay S. M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., and Abdelalim A., Lancet 392(10159), 1789–1858 (2018). 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maulik P. K., Mascarenhas M. N., Mathers C. D., Dua T., and Saxena S., Res. Dev. Disabil. 32(2), 419–436 (2011). 10.1016/j.ridd.2010.12.018 [DOI] [PubMed] [Google Scholar]
- 15.Zarafshan H., Salmanian M., Aghamohammadi S., Mohammadi M. R., and Mostafavi S.-A., Basic Clin. Neurosci. 8(2), 95 (2017). 10.18869/nirp.bcn.8.2.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummings J. L., Morstorf T., and Zhong K., Alzheimer's Res. Ther. 6(4), 37 (2014). 10.1186/alzrt269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crismon M. L., Ann. Pharmacother. 28(6), 744–751 (1994). 10.1177/106002809402800612 [DOI] [PubMed] [Google Scholar]
- 18.Athar T., Al Balushi K., and Khan S. A., Mol. Biol. Rep. 48(7), 5629–5645 (2021). 10.1007/s11033-021-06512-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tampi R. R., Forester B. P., and Agronin M., Drugs Context 10, 1 (2021). 10.7573/dic.2021-7-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fillit H. and Green A., Nat. Rev. Neurol. 17(3), 129–130 (2021). 10.1038/s41582-020-00454-9 [DOI] [PubMed] [Google Scholar]
- 21.Rubin R., JAMA 326(6), 469–472 (2021). 10.1001/jama.2021.11558 [DOI] [PubMed] [Google Scholar]
- 22.Tagliavini F., Tiraboschi P., and Federico A., “Alzheimer's disease: The controversial approval of aducanumab,” Neurol. Sci. (published online 2021). 10.1007/s10072-021-05497-4 [DOI] [PubMed] [Google Scholar]
- 23.Tărlungeanu D. C. and Novarino G., Exp. Mol. Med. 50(8), 1–7 (2018). 10.1038/s12276-018-0129-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashmawi N. S. and Hammoda M. A., Cureus 14(3), e23465 (2022). 10.7759/cureus.23465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell S. J., Scheibye-Knudsen M., Longo D. L., and de Cabo R., Annu. Rev. Anim. Biosci. 3(1), 283–303 (2015). 10.1146/annurev-animal-022114-110829 [DOI] [PubMed] [Google Scholar]
- 26.Kim J., Koo B.-K., and Knoblich J. A., Nat. Rev. Mol. Cell Biol. 21(10), 571–584 (2020). 10.1038/s41580-020-0259-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamas-Paz A., Hao F., Nelson L. J., Vázquez M. T., Canals S., Del Moral M. G., Martínez-Naves E., Nevzorova Y. A., and Cubero F. J., World J. Gastroenterol. 24(45), 5063 (2018). 10.3748/wjg.v24.i45.5063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoarau-Véchot J., Rafii A., Touboul C., and Pasquier J., Int. J. Mol. Sci. 19(1), 181 (2018). 10.3390/ijms19010181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinrich M. A., Mostafa A. M., Morton J. P., Hawinkels L. J., and Prakash J., Adv. Drug Delivery Rev. 174, 265–293 (2021). 10.1016/j.addr.2021.04.018 [DOI] [PubMed] [Google Scholar]
- 30.Kapałczyńska M., Kolenda T., Przybyła W., Zajączkowska M., Teresiak A., Filas V., Ibbs M., Bliźniak R., Łuczewski Ł, and Lamperska K., Arch. Med. Sci. 14(4), 910–919 (2018). 10.5114/aoms.2016.63743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frühauf M., Zeitschel U., Höfling C., Ullm F., Rabiger F. V., Alber G., Pompe T., Müller U., and Roßner S., Eur. J. Neurosci. 53(12), 4034–4050 (2021). 10.1111/ejn.14978 [DOI] [PubMed] [Google Scholar]
- 32.Huh D., Hamilton G. A., and Ingber D. E., Trends Cell Biol. 21(12), 745–754 (2011). 10.1016/j.tcb.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhuang P., Sun A. X., An J., Chua C. K., and Chew S. Y., Biomaterials 154, 113–133 (2018). 10.1016/j.biomaterials.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 34.Dong R., Liu Y., Mou L., Deng J., and Jiang X., Adv. Mater. 31(45), 1805033 (2019). 10.1002/adma.201805033 [DOI] [PubMed] [Google Scholar]
- 35.Rothbauer M., Zirath H., and Ertl P., Lab Chip 18(2), 249–270 (2018). 10.1039/C7LC00815E [DOI] [PubMed] [Google Scholar]
- 36.Atat O. E., Farzaneh Z., Pourhamzeh M., Taki F., Abi-Habib R., Vosough M., and El-Sibai M., Human Cell 35 1–14 (2021). 10.1007/s13577-021-00642-9 [DOI] [PubMed] [Google Scholar]
- 37.Ryu H., Chung M., Song J., Lee S. S., Pertz O., and Jeon N. L., Sci. Rep. 8(1), 1–7 (2018). 10.1038/s41598-018-28873-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kook Y.-M., Jeong Y., Lee K., and Koh W.-G., J. Tissue Eng. 8, 2041731417724640 (2017). 10.1177/2041731417724640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song J., Choi H., Koh S. K., Park D., Yu J., Kang H., Kim Y., Cho D., and Jeon N. L., Front. Immunol. 12 3848 (2021). 10.3389/fimmu.2021.733317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H., Brown P. C., Chow E. C., Ewart L., Ferguson S. S., Fitzpatrick S., Freedman B. S., Guo G. L., Hedrich W., and Heyward S., Clin. Transl. Sci. 14(5), 1659–1680 (2021). 10.1111/cts.13066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qian X., Su Y., Adam C. D., Deutschmann A. U., Pather S. R., Goldberg E. M., Su K., Li S., Lu L., and Jacob F., Cell Stem Cell 26(5), 766–781.e9 (2020). 10.1016/j.stem.2020.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Velasco S., Kedaigle A. J., Simmons S. K., Nash A., Rocha M., Quadrato G., Paulsen B., Nguyen L., Adiconis X., and Regev A., Nature 570 (7762), 523–527 (2019). 10.1038/s41586-019-1289-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jang M., Choi N., and Kim H. N., “Hyperglycemic neurovasculature-on-a-chip to study the effect of SIRT1-targeted therapy for the Type 3 Diabetes “Alzheimer's Disease”,” Adv. Sci. (published online 2022). 10.1002/advs.202201882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hajal C., Offeddu G. S., Shin Y., Zhang S., Morozova O., Hickman D., Knutson C. G., and Kamm R. D., Nat. Protoc. 17(1), 95–128 (2022). 10.1038/s41596-021-00635-w [DOI] [PubMed] [Google Scholar]
- 45.Shin Y., Choi S. H., Kim E., Bylykbashi E., Kim J. A., Chung S., Kim D. Y., Kamm R. D., and Tanzi R. E., Adv. Sci. 6(20), 1900962 (2019). 10.1002/advs.201900962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adams J. W., Cugola F. R., and Muotri A. R., Physiology 34(5), 365–375 (2019). 10.1152/physiol.00005.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu X., Yang J., and Xiang Y., Cell Regen. 11(1), 1–13 (2022). 10.1186/s13619-021-00103-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sia S. K. and Whitesides G. M., Electrophoresis 24(21), 3563–3576 (2003). 10.1002/elps.200305584 [DOI] [PubMed] [Google Scholar]
- 49.Wikswo J. P., Curtis E. L., Eagleton Z. E., Evans B. C., Kole A., Hofmeister L. H., and Matloff W. J., Lab Chip 13(18), 3496–3511 (2013). 10.1039/c3lc50243k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perestrelo A. R., Águas A. C., Rainer A., and Forte G., Sensors 15(12), 31142–31170 (2015). 10.3390/s151229848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor A. M., Blurton-Jones M., Rhee S. W., Cribbs D. H., Cotman C. W., and Jeon N. L., Nat. Methods 2(8), 599–605 (2005). 10.1038/nmeth777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahn S. I., Sei Y. J., Park H.-J., Kim J., Ryu Y., Choi J. J., Sung H.-J., MacDonald T. J., Levey A. I., and Kim Y., Nat. Commun. 11(1), 1–12 (2020). 10.1038/s41467-019-13896-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park T.-E., Mustafaoglu N., Herland A., Hasselkus R., Mannix R., FitzGerald E. A., Prantil-Baun R., Watters A., Henry O., and Benz M., Nat. Commun. 10(1), 1–12 (2019). 10.1038/s41467-019-10588-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bang S., Na S., Jang J. M., Kim J., and Jeon N. L., Adv. Healthcare Mater. 5(1), 159–166 (2016). 10.1002/adhm.201500397 [DOI] [PubMed] [Google Scholar]
- 55.Kim S. H., Im S.-K., Oh S.-J., Jeong S., Yoon E.-S., Lee C. J., Choi N., and Hur E.-M., Nat. Commun. 8(1), 1–16 (2017). 10.1038/ncomms14346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park J., Lee B. K., Jeong G. S., Hyun J. K., Lee C. J., and Lee S.-H., Lab Chip 15(1), 141–150 (2015). 10.1039/C4LC00962B [DOI] [PubMed] [Google Scholar]
- 57.Huang C. P., Lu J., Seon H., Lee A. P., Flanagan L. A., Kim H.-Y., Putnam A. J., and Jeon N. L., Lab Chip 9(12), 1740–1748 (2009). 10.1039/b818401a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bang S., Lee B.-J., Lee S.-R., Na S., Jang J. M., Kang M., Kim S.-Y., Min D.-H., Song J. M., and Ho W.-K., Biofabrication 11(1), 015008 (2018). 10.1088/1758-5090/aaeb66 [DOI] [PubMed] [Google Scholar]
- 59.Rifes P., Isaksson M., Rathore G. S., Aldrin-Kirk P., Møller O. K., Barzaghi G., Lee J., Egerod K. L., Rausch D. M., and Parmar M., Nat. Biotechnol. 38(11), 1265–1273 (2020). 10.1038/s41587-020-0525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee D., Golden K., Rahman M. M., Moran A., Gonzalez B., and Ryu S., Exp. Mech. 59(9), 1249–1259 (2019). 10.1007/s11340-018-0416-1 [DOI] [Google Scholar]
- 61.Wang W., Li L., Ding M., Luo G., and Liang Q., BioChip J. 12(2), 93–101 (2018). 10.1007/s13206-017-2202-z [DOI] [Google Scholar]
- 62.Tavakol D. N., Fleischer S., and Vunjak-Novakovic G., Cell Stem Cell 28(6), 993–1015 (2021). 10.1016/j.stem.2021.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim H. N. and Choi N., BioChip J. 13(1), 8–19 (2019). 10.1007/s13206-018-3101-7 [DOI] [Google Scholar]
- 64.Bang S., Hwang K. S., Jeong S., Cho I.-J., Choi N., Kim J., and Kim H. N., Acta Biomater. 132, 379–400 (2021). 10.1016/j.actbio.2021.06.024 [DOI] [PubMed] [Google Scholar]
- 65.Zhao Y., Demirci U., Chen Y., and Chen P., Lab Chip 20(9), 1531–1543 (2020). 10.1039/C9LC01010F [DOI] [PubMed] [Google Scholar]
- 66.Herreros P., Ballesteros-Esteban L. M., Laguna M. F., Leyva I., Sendiña-Nadal I., and Holgado M., Biotechnol. J. 16(7), 2000355 (2021). 10.1002/biot.202000355 [DOI] [PubMed] [Google Scholar]
- 67.Park J., Koito H., Li J., and Han A., Biomed. Microdevices 11(6), 1145–1153 (2009). 10.1007/s10544-009-9331-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takeda S., Wegmann S., Cho H., DeVos S. L., Commins C., Roe A. D., Nicholls S. B., Carlson G. A., Pitstick R., and Nobuhara C. K., Nat. Commun. 6(1), 1–15 (2015). 10.1038/ncomms9490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gribaudo S., Tixador P., Bousset L., Fenyi A., Lino P., Melki R., Peyrin J.-M., and Perrier A. L., Stem Cell Rep. 12(2), 230–244 (2019). 10.1016/j.stemcr.2018.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bang S., Lee S., Choi N., and Kim H. N., Adv. Healthcare Mater. 10(12), 2002119 (2021). 10.1002/adhm.202002119 [DOI] [PubMed] [Google Scholar]
- 71.Hajal C., Le Roi B., Kamm R. D., and Maoz B. M., Annu. Rev. Biomed. Eng. 23, 359–384 (2021). 10.1146/annurev-bioeng-082120-042814 [DOI] [PubMed] [Google Scholar]
- 72.Kadry H., Noorani B., and Cucullo L., Fluids Barriers CNS 17(1), 1–24 (2020). 10.1186/s12987-020-00230-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barar J., Rafi M. A., Pourseif M. M., and Omidi Y., BioImpacts 6(4), 225 (2016). 10.15171/bi.2016.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pardridge W. M., J. Cereb. Blood Flow Metab. 32(11), 1959–1972 (2012). 10.1038/jcbfm.2012.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pardridge W. M., NeuroRx 2(1), 3–14 (2005). 10.1602/neurorx.2.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pulgar V. M., Front. Neurosci. 12, 1019 (2019). 10.3389/fnins.2018.01019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aleynik A., Gernavage K. M., Mourad Y. S., Sherman L. S., Liu K., Gubenko Y. A., and Rameshwar P., Clin. Transl. Med. 3(1), 1–10 (2014). 10.1186/2001-1326-3-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Curley S. M. and Cady N. C., Sci. Prog. 101(3), 273–292 (2018). 10.3184/003685018X15306123582346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Terstappen G. C., Meyer A. H., Bell R. D., and Zhang W., Nat. Rev. Drug Discovery 20(5), 362–383 (2021). 10.1038/s41573-021-00139-y [DOI] [PubMed] [Google Scholar]
- 80.Wang Y. I., Abaci H. E., and Shuler M. L., Biotechnol. Bioeng. 114(1), 184–194 (2017). 10.1002/bit.26045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cucullo L., McAllister M. S., Kight K., Krizanac-Bengez L., Marroni M., Mayberg M. R., Stanness K. A., and Janigro D., Brain Res. 951(2), 243–254 (2002). 10.1016/S0006-8993(02)03167-0 [DOI] [PubMed] [Google Scholar]
- 82.Pediaditakis I., Kodella K. R., Manatakis D. V., Le C. Y., Hinojosa C. D., Tien-Street W., Manolakos E. S., Vekrellis K., Hamilton G. A., and Ewart L., Nat. Commun. 12(1), 1–17 (2021). 10.1038/s41467-021-26066-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bang S., Lee S.-R., Ko J., Son K., Tahk D., Ahn J., Im C., and Jeon N. L., Sci. Rep. 7(1), 1–10 (2017). 10.1038/s41598-017-07416-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seo S., Nah S. Y., Lee K., Choi N., and Kim H. N., Adv. Funct. Mater. 32(10), 2106860 (2022). 10.1002/adfm.202106860 [DOI] [Google Scholar]
- 85.Campisi M., Shin Y., Osaki T., Hajal C., Chiono V., and Kamm R. D., Biomaterials 180, 117–129 (2018). 10.1016/j.biomaterials.2018.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim H. N., J.: Organoid 1, e10 (2021). 10.51335/organoid.2021.1.e10 [DOI] [Google Scholar]
- 87.Sakalem M. E., De Sibio M. T., da Costa F. A. d. S., and de Oliveira M., Biotechnol. J. 16(5), 2000463 (2021). 10.1002/biot.202000463 [DOI] [PubMed] [Google Scholar]
- 88.Quadrato G. and Arlotta P., Curr. Opin. Cell Biol. 49, 47–52 (2017). 10.1016/j.ceb.2017.11.010 [DOI] [PubMed] [Google Scholar]
- 89.Di Lullo E. and Kriegstein A. R., Nat. Rev. Neurosci. 18(10), 573–584 (2017). 10.1038/nrn.2017.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lancaster M. A., Renner M., Martin C.-A., Wenzel D., Bicknell L. S., Hurles M. E., Homfray T., Penninger J. M., Jackson A. P., and Knoblich J. A., Nature 501(7467), 373–379 (2013). 10.1038/nature12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nickels S. L., Modamio J., Mendes-Pinheiro B., Monzel A. S., Betsou F., and Schwamborn J. C., Stem Cell Res. 46, 101870 (2020). 10.1016/j.scr.2020.101870 [DOI] [PubMed] [Google Scholar]
- 92.Nayler S., Agarwal D., Curion F., Bowden R., and Becker E. B., Sci. Rep. 11(1), 1–17 (2021). 10.1038/s41598-021-91846-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jo J., Xiao Y., Sun A. X., Cukuroglu E., Tran H.-D., Göke J., Tan Z. Y., Saw T. Y., Tan C.-P., and Lokman H., Cell Stem Cell 19(2), 248–257 (2016). 10.1016/j.stem.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rui Q., Ni H., Li D., Gao R., and Chen G., Curr. Neuropharmacol. 16(9), 1348–1357 (2018). 10.2174/1570159X16666180222165418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smits L. M., Reinhardt L., Reinhardt P., Glatza M., Monzel A. S., Stanslowsky N., Rosato-Siri M. D., Zanon A., Antony P. M., and Bellmann J., NPJ Parkinson's Dis. 5(1), 1–8 (2019). 10.1038/s41531-019-0078-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jo J., Yang L., Tran H. D., Yu W., Sun A. X., Chang Y. Y., Jung B. C., Lee S. J., Saw T. Y., and Xiao B., Ann. Neurol. 90(3), 490–505 (2021). 10.1002/ana.26166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Conforti P., Besusso D., Bocchi V., Faedo A., Cesana E., Rossetti G., Ranzani V., Svendsen C., Thompson L., and Toselli M., Proc. Natl. Acad. Sci. U.S.A. 115(4), E762–E771 (2018). 10.1073/pnas.1715865115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qian X., Nguyen H. N., Song M. M., Hadiono C., Ogden S. C., Hammack C., Yao B., Hamersky G. R., Jacob F., and Zhong C., Cell 165(5), 1238–1254 (2016). 10.1016/j.cell.2016.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim S., Cho A. N., Min S., Kim S., and Cho S. W., Adv. Ther. 2(1), 1800087 (2019). 10.1002/adtp.201800087 [DOI] [Google Scholar]
- 100.Del Dosso A., Urenda J.-P., Nguyen T., and Quadrato G., Neuron 107(6), 1014–1028 (2020). 10.1016/j.neuron.2020.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tambalo M. and Lodato S., Brain Res. 1746, 147028 (2020). 10.1016/j.brainres.2020.147028 [DOI] [PubMed] [Google Scholar]
- 102.Matsui T. K., Tsuru Y., Hasegawa K., and Kuwako K.-i., Stem Cells 39(8), 1017–1024 (2021). 10.1002/stem.3368 [DOI] [PubMed] [Google Scholar]
- 103.Fligor C. M., Lavekar S. S., Harkin J., Shields P. K., VanderWall K. B., Huang K.-C., Gomes C., and Meyer J. S., Stem Cell Rep. 16(9), 2228–2241 (2021). 10.1016/j.stemcr.2021.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miura Y., Li M.-Y., Birey F., Ikeda K., Revah O., Thete M. V., Park J.-Y., Puno A., Lee S. H., and Porteus M. H., Nat. Biotechnol. 38(12), 1421–1430 (2020). 10.1038/s41587-020-00763-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Faustino Martins J.-M., Fischer C., Urzi A., Vidal R., Kunz S., Ruffault P.-L., Kabuss L., Hube I., Gazzerro E., and Birchmeier C., Cell Stem Cell 26(2), 172–186.e6 (2020). 10.1016/j.stem.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 106.Cullen D. K., Gordián-Vélez W. J., Struzyna L. A., Jgamadze D., Lim J., Wofford K. L., Browne K. D., and Chen H. I., Iscience 21, 57–67 (2019). 10.1016/j.isci.2019.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Robles D. A., Boreland A. J., Pang Z. P., and Zahn J. D., Micromachines 12(12), 1574 (2021). 10.3390/mi12121574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shi Y., Wu Q., and Wang X., Curr. Opin. Neurobiol. 66, 103–115 (2021). 10.1016/j.conb.2020.09.006 [DOI] [PubMed] [Google Scholar]
- 109.Shi Y., Sun L., Wang M., Liu J., Zhong S., Li R., Li P., Guo L., Fang A., and Chen R., PLoS Biol. 18(5), e3000705 (2020). 10.1371/journal.pbio.3000705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cakir B., Xiang Y., Tanaka Y., Kural M. H., Parent M., Kang Y.-J., Chapeton K., Patterson B., Yuan Y., and He C.-S., Nat. Methods 16(11), 1169–1175 (2019). 10.1038/s41592-019-0586-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Song J., Ryu H., Chung M., Kim Y., Blum Y., Lee S. S., Pertz O., and Jeon N. L., Biosens. Bioelectron. 104, 58–64 (2018). 10.1016/j.bios.2017.12.038 [DOI] [PubMed] [Google Scholar]
- 112.Lee S. S., Vizcarra I. A., Huberts D. H., Lee L. P., and Heinemann M., Proc. Natl. Acad. Sci. U.S.A. 109(13), 4916–4920 (2012). 10.1073/pnas.1113505109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Choi S. H., Kim Y. H., Quinti L., Tanzi R. E., and Kim D. Y., Mol. Neurodegener. 11(1), 1–11 (2016). 10.1186/s13024-016-0139-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jalink P. and Caiazzo M., Biology 10(08), 740 (2021). 10.3390/biology10080740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hernández D., Rooney L. A., Daniszewski M., Gulluyan L., Liang H. H., Cook A. L., Hewitt A. W., and Pébay A., Stem Cell Rev. Rep. 18 1–14 (2021). 10.1007/s12015-021-10147-5 [DOI] [PubMed] [Google Scholar]
- 116.Berger E., Magliaro C., Paczia N., Monzel A. S., Antony P., Linster C. L., Bolognin S., Ahluwalia A., and Schwamborn J. C., Lab Chip 18(20), 3172–3183 (2018). 10.1039/C8LC00206A [DOI] [PubMed] [Google Scholar]
- 117.Cho A.-N., Jin Y., An Y., Kim J., Choi Y. S., Lee J. S., Kim J., Choi W.-Y., Koo D.-J., and Yu W., Nat. Commun. 12(1), 1–23 (2021). 10.1038/s41467-021-24775-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Spitz S., Bolognin S., Brandauer K., Fuessl J., Schuller P., Schobesberger S., Jordan C., Schaedl B., Grillari J., and Wanzenboeck H. D., bioRxiv (2022).
- 119.Cui K., Chen W., Cao R., Xie Y., Wang P., Wu Y., Wang Y., and Qin J., Cell Regen. 11(1), 1–12 (2022). 10.1186/s13619-021-00102-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Y., Wang L., Zhu Y., and Qin J., Lab Chip 18(6), 851–860 (2018). 10.1039/C7LC01084B [DOI] [PubMed] [Google Scholar]
- 121.Ao Z., Cai H., Havert D. J., Wu Z., Gong Z., Beggs J. M., Mackie K., and Guo F., Anal. Chem. 92(6), 4630–4638 (2020). 10.1021/acs.analchem.0c00205 [DOI] [PubMed] [Google Scholar]
- 122.Karzbrun E., Kshirsagar A., Cohen S. R., Hanna J. H., and Reiner O., Nat. Phys. 14(5), 515–522 (2018). 10.1038/s41567-018-0046-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Salmon I., Grebenyuk S., Abdel Fattah A. R., Rustandi G., Pilkington T., Verfaillie C., and Ranga A., Lab Chip 22(8), 1615–1629 (2022). 10.1039/D1LC00535A [DOI] [PubMed] [Google Scholar]
- 124.Min S., Lee B., and Yoon S., Briefings Bioinf. 18(5), 851–869 (2017). 10.1093/bib/bbw068b [DOI] [PubMed] [Google Scholar]
- 125.Vatansever S., Schlessinger A., Wacker D., Kaniskan HÜ, Jin J., Zhou M. M., and Zhang B., Med. Res. Rev. 41(3), 1427–1473 (2021). 10.1002/med.21764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dlamini Z., Francies F. Z., Hull R., and Marima R., Comput. Struct. Biotechnol. J. 18, 2300–2311 (2020). 10.1016/j.csbj.2020.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barragán-Montero A., Javaid U., Valdés G., Nguyen D., Desbordes P., Macq B., Willems S., Vandewinckele L., Holmström M., and Löfman F., Phys. Med. 83, 242–256 (2021). 10.1016/j.ejmp.2021.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sarker I. H., SN Comput. Sci. 2(3), 1–21 (2021). 10.1007/s42979-021-00592-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sarker I. H., SN Comput. Sci. 2(6), 1–20 (2021). 10.1007/s42979-021-00815-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Libbrecht M. W. and Noble W. S., Nat. Rev. Genet. 16(6), 321–332 (2015). 10.1038/nrg3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Najafabadi M. M., Villanustre F., Khoshgoftaar T. M., Seliya N., Wald R., and Muharemagic E., J. Big Data 2(1), 1–21 (2015). 10.1186/s40537-014-0007-7 [DOI] [Google Scholar]
- 132.Kourou K., Exarchos T. P., Exarchos K. P., Karamouzis M. V., and Fotiadis D. I., Comput. Struct. Biotechnol. J. 13, 8–17 (2015). 10.1016/j.csbj.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Patcha A. and Park J.-M., Comput. Netw. 51(12), 3448–3470 (2007). 10.1016/j.comnet.2007.02.001 [DOI] [Google Scholar]
- 134.Pang G., Shen C., Cao L., and Hengel A. V. D., ACM Comput. Surv. (CSUR) 54(2), 1–38 (2021). 10.1145/3439950 [DOI] [Google Scholar]
- 135.Song J., Lee Y. C., and Lee J., “Deep generative model with time series-image encoding for manufacturing fault detection in die casting process,” J. Intell. Manuf. (published online 2022). 10.1007/s10845-022-01981-6 [DOI] [Google Scholar]
- 136.Hoelzle D., Lake M., Narciso C., Cowdrick K., Storey T., Zhang S., and Zartman J., “Microfluidic device design, fabrication, and testing protocols,” Protocol Exchange (published online 2015). 10.1038/protex.2015.069 [DOI]
- 137.Olsen A., Konovalov D. A., Philippa B., Ridd P., Wood J. C., Johns J., Banks W., Girgenti B., Kenny O., and Whinney J., Sci. Rep. 9(1), 1–12 (2019). 10.1038/s41598-018-38343-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ravuri S. and Vinyals O., Adv. Neural. Inf. Process Syst., arXiv.1905.10887 [Google Scholar]
- 139.Kim Y., Song J., Lee Y., Cho S., Kim S., Lee S.-R., Park S., Shin Y., and Jeon N. L., Lab Chip 21(16), 3150–3158 (2021). 10.1039/D0LC01245A [DOI] [PubMed] [Google Scholar]
- 140.Lee S., Lim J., Yu J., Ahn J., Lee Y., and Jeon N. L., Lab Chip 19(12), 2071–2080 (2019). 10.1039/C9LC00148D [DOI] [PubMed] [Google Scholar]
- 141.Jung S., Lee J., Lim J., Suh J., Kim T., Ahn J., Kim W. J., and Kim Y., Adv. Healthcare Mater. 9(22), 2001633 (2020). 10.1002/adhm.202001633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Malandrino A., Trepat X., Kamm R. D., and Mak M., PLoS Comput. Biol. 15(4), e1006684 (2019). 10.1371/journal.pcbi.1006684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee K. G., Lee T. J., Jeong S. W., Choi H. W., Heo N. S., Park J. Y., Park T. J., and Lee S. J., Sensors 12(8), 10810–10819 (2012). 10.3390/s120810810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sharifi E., Chaudhuri A., Waehrens B. V., Staal L. G., and Davoudabadi Farahani S., Sustainability 13(3), 1313 (2021). 10.3390/su13031313 [DOI] [Google Scholar]
- 145.Singh A. V., Romeo A., Scott K., Wagener S., Leibrock L., Laux P., Luch A., Kerkar P., Balakrishnan S., and Dakua S. P., Adv. Healthcare Mater. 10(18), 2100633 (2021). 10.1002/adhm.202100633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xiao Y., Kim D., Dura B., Zhang K., Yan R., Li H., Han E., Ip J., Zou P., and Liu J., Adv. Sci. 6(8), 1801531 (2019). 10.1002/advs.201801531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mavrakis E. and Pergantis S. A., Anal. Chim. Acta 1179, 338830 (2021). 10.1016/j.aca.2021.338830 [DOI] [PubMed] [Google Scholar]
- 148.Yu J., Lee S., Song J., Lee S.-R., Kim S., Choi H., Kang H., Hwang Y., Hong Y.-K., and Jeon N. L., Nano Converg. 9(1), 1–11 (2022). 10.1186/s40580-022-00306-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hsiao A. Y., Tung Y. C., Qu X., Patel L. R., Pienta K. J., and Takayama S., Biotechnol. Bioeng. 109(5), 1293–1304 (2012). 10.1002/bit.24399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Paek K., Kim S., Tak S., Kim M. K., Park J., Chung S., Park T. H., and Kim J. A., “A high-throughput biomimetic bone-on-a-chip platform with artificial intelligence-assisted image analysis for osteoporosis drug testing,” Bioeng. Trans. Med. (published online 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jena B. P., Gatti D. L., Arslanturk S., Pernal S., and Taatjes D. J., Micron 117, 55–59 (2019). 10.1016/j.micron.2018.11.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding authors upon reasonable request.