Abstract
The effect of growth phase on expression of virulence-associated factors was studied by Northern hybridization in an M1T1 clinical isolate of Streptococcus pyogenes. Expression of M protein, C5a peptidase, and capsule was maximal in the exponential phase of growth, while streptococcal pyrogenic exotoxins A and B and mitogenic factor were maximally expressed in later phases of growth.
Streptococcus pyogenes is an aggressive human pathogen responsible for a variety of serious illnesses, ranging from local infections such as pharyngitis to severe invasive infections such as scarlet fever, necrotizing fasciitis, and streptococcal toxic shock syndrome (25). The incidence of severe invasive group A streptococcal infections, in particular, those due to serotype M1 streptococci, has increased over the past decade (7, 9).
S. pyogenes possesses numerous cell surface-associated and secreted factors that are believed to contribute to its virulence. The hyaluronic acid capsule is responsible for resistance to complement-mediated phagocytic killing (27). M protein and C5a peptidase, which are transcriptionally controlled by mga (which encodes multiple gene activator), are both important virulence factors of S. pyogenes with roles in antiphagocytic activity and complement inactivation, respectively (1, 11). Among the extracellular secreted proteins, streptococcal pyrogenic exotoxin A (SPEA), streptococcal pyrogenic exotoxin B (SPEB), and mitogenic factor (MF) have been well studied. The gene encoding the superantigenic toxin SPEA is present in 85% of strains which cause streptococcal toxic shock syndrome (8). MF, though not a proven virulence factor, is a DNase with superantigenic properties (10, 18). SPEB is a cysteine protease known to activate and process a variety of important host proteins (18).
Environmental conditions, cell density, and growth phase are all believed to influence the expression of virulence factors by a pathogen (14). In Staphylococcus aureus the global regulator, agr, controls many important genes in a growth-dependent manner (19). Expression of toxins in Yersinia enterocolitica and Clostridium difficile is reported to be growth-phase specific (6, 17). As S. pyogenes is a pathogen able to survive in a variety of host locations, it is likely to have an environmentally sensitive circuit to regulate expression of virulence factors.
Growth-phase-dependent regulation of the mga locus of a serotype M6 S. pyogenes strain has been reported previously (15). In this study, we examined the expression of virulence-associated factors of an M1T1 clinical isolate of S. pyogenes at the transcriptional level, focusing on expression of a range of cell wall-associated and secreted factors that are thought to be important in virulence.
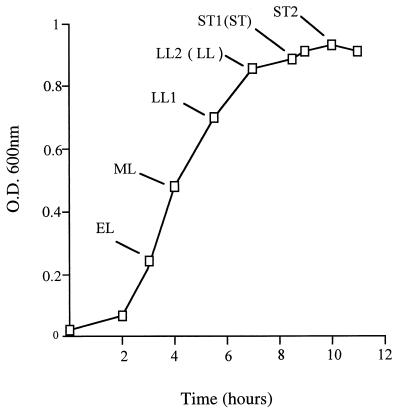
A scarlet fever-associated M1T1 S. pyogenes isolate (H305), confirmed to be speA+ speB+ mf+ and ssa mutant and speC by PCR, was used in this study. Strains were cultured in Todd-Hewitt broth supplemented with 0.2% yeast extract (THY) (Oxoid, Basingstoke, United Kingdom) at 37°C. Overnight culture of H305 (1 ml) was used to inoculate 10 ml of fresh THY, and growth was monitored by measuring optical density at 600 nm (OD600) by using a Pharmacia Ultrospec III spectrophotometer. RNA was extracted as described by Podbielski et al. (20) and quantitated by measuring OD260. The phases of growth at which total RNAs were extracted are shown in Fig. 1. RNA (20 μg) was run on a denaturing 1.5% agarose gel and transferred to a Hybond N membrane (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom) and cross-linked in a UV Stratalinker 1800 (Stratagene, Cambridge, United Kingdom). Uniformity of loading was confirmed by ethidium bromide gel staining and measurement of OD260. Blots were hybridized to digoxigenin (DIG)-UTP (Boehringer Mannheim, Lewes, United Kingdom)-labelled DNA probes at 50°C overnight and visualized by using CSPD, a chemiluminescent substrate (Boehringer Mannheim). Results of all hybridizations were replicated in at least two further experiments. Densitometric studies were done to compare intensities of bands on Northern blots by using the Scion Image program. Probes were generated from PCR products obtained by amplifying H305 genomic DNA (21). The primer pairs used in this study are shown in Table 1.
FIG. 1.
Growth curve for S. pyogenes H305 in Todd-Hewitt broth. Different points of growth at which total RNA was extracted are indicated. EL, early log phase; ML, mid-log phase; LL, late log phase; and ST, stationary phase.
TABLE 1.
Oligonucleotides used to generate PCR probes used in this study
| Target gene | Name of the primer | Sequence of the primer | Source or reference |
|---|---|---|---|
| recA | recAF | GCGTTCAGGAAGTCTAGCTC | 15 |
| recAR | CTGATGCTACTGCCATAGCAG | 15 | |
| emm1 | M1 | GAATCCACTATTCGCTTAGA | 4 |
| M2 | GAATTCAGTTCTTCAGCTTGT | 4 | |
| scpA | scp1 | GGCGAGTGGGTCAATGATAA | This study |
| scp2 | ACCGTCTTTTCGACTGATAAAG | 15 | |
| hasA | has1 | GAAAACGCCATGCTCAAGCG | This study |
| has2 | GATTGGTAGACAGTGCGTCC | This study | |
| speA | spea1 | GGCGGATCCGCCAACAAGACCCCGTA | This lab (22) |
| spea2 | GCGGATCCGCAGTAGGTAAGGTTGCA | This lab (22) | |
| speB | speb1 | GATAACCATACGATTCAGCT | 15 |
| speb2 | TCTGTGTCTGATGGATAGC | 15 | |
| mf | mf1 | GCGAATTCGGTATAGCGCATGCC | This study |
| mf2 | CCGAATTCCAAACACAGGTCTCA | This study | |
| 16S rRNA | rRNA1 | CGGTAACTAACCAGAAAGGG | This study |
| rRNA2 | CGTTGTACCAACCATTGTAGC | This study |
Expression of cell wall-associated virulence genes.
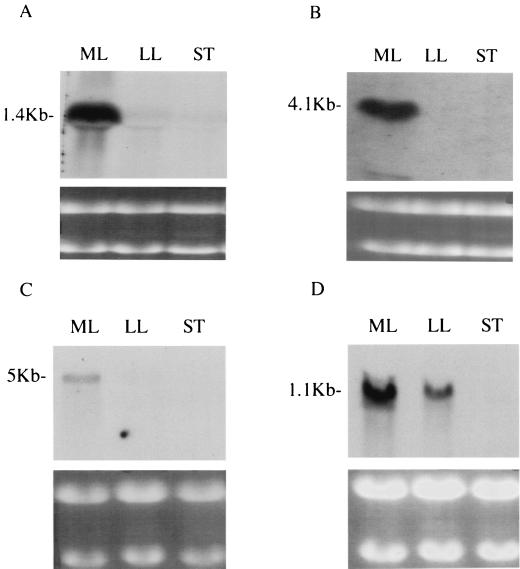
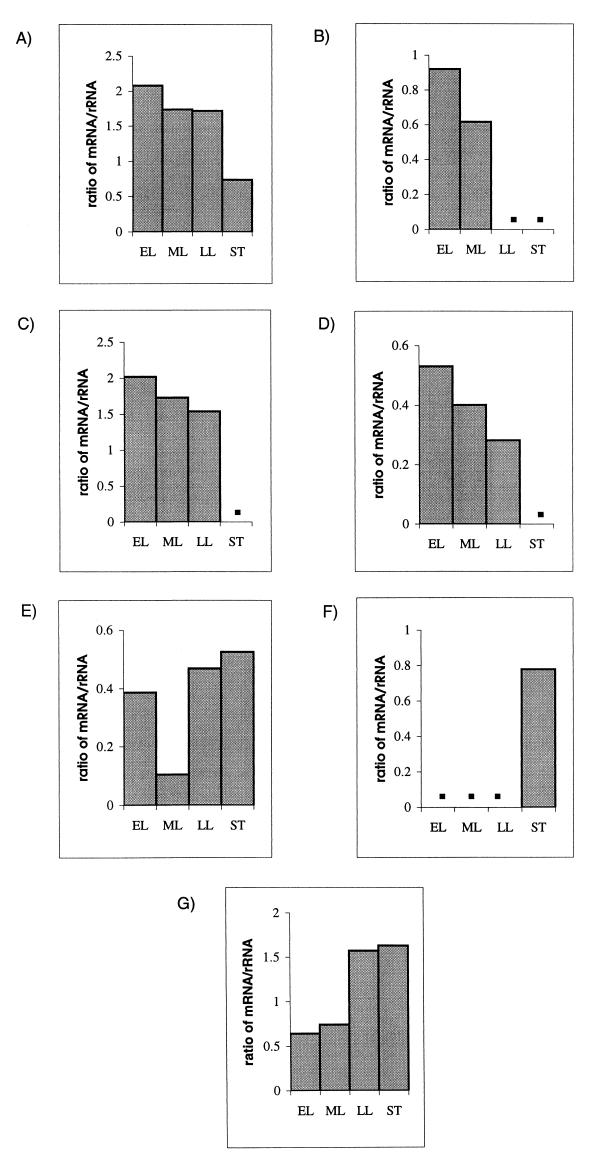
Expression of the emm, scpA, and hasA genes, which encode M protein, C5a peptidase, and hyaluronic acid capsule, respectively, was studied at different phases of growth of S. pyogenes. In experiments spanning three time points, transcripts of all three genes were detectable by Northern hybridization for cells in exponential growth phase but not for those in stationary phase (Fig. 2A, B, and C). As an internal control for the amount of RNA used, replica RNA blots were probed with recA, which is thought to be a housekeeping gene. However, we found that recA transcript levels decreased as the organism entered later phases of growth (Fig. 2D). In separate experiments spanning four time points, blots were stripped and reprobed with a 16S rRNA probe and densitometry was performed by comparing mRNA and rRNA band intensities at different stages of growth (Fig. 3A, through D).
FIG. 2.
Expression of the genes emm1, scpA, hasA, and recA. RNA (20 μg), extracted from H305 at the mid-log (ML), late-log (LL), and stationary (ST) phases of growth, was hybridized to DIG-labelled PCR probes specific for emm1 (A), hasA (B), scpA (C), and recA (D) mRNAs. Photographs of ethidium bromide-stained gels are shown below the corresponding Northern blots.
FIG. 3.
Densitometric analysis of expression of genes at early and late growth phases. Ratios of band intensities of mRNA to band intensities of the corresponding 16S rRNA from cells collected at different stages of growth are shown. EL, early log phase; ML, mid-log phase; LL, late log phase; and ST, stationary phase. Data for emm1 (A), hasA (B), scpA (C), recA (D), speA (E), speB (F), and mf (G) are shown. Densitometry was not performed for the lanes with no bands, and this is indicated by solid squares in the graphs.
Growth-phase-regulated expression of genes is thought to be a mechanism adopted by bacteria to save energy, especially under conditions of low nutrient supply. McIver and Scott studied an M6 strain using RNA slot blot techniques and showed that S. pyogenes expression of mga and the mga-regulated genes emm and scpA was maximal in the exponential phase (15). hasA expression has also been reported to be maximal in the early exponential phase of growth (5). Production of cell surface proteins in the early exponential phase of growth is seen in other bacteria, such as S. aureus (19). Previously recA was shown to be constitutively expressed in an M6 strain of S. pyogenes by slot blot analysis (15). However, we found that dot blot hybridizations with some of the DIG-labelled probes was very nonspecific. Growth-phase-dependent expression of recA appears to be a general feature of S. pyogenes, as we found a similar pattern of expression of recA in three other clinical streptococcal strains (an M1 isolate associated with bacteremia, an M3 isolate associated with toxic shock, and an M89 isolate producing necrotizing fasciitis; data not shown). As recA is involved in regulation of homologous recombination and chromosomal partitioning, it is perhaps not surprising that recA expression is maximal during the exponential growth phase (29). RecA is believed to have a role in the virulence of Salmonella typhimurium and S. aureus (2, 16). Furthermore, it is known to be important in coordination of virulence factor expression in Shigella flexneri and Neisseria gonorrhoeae (12, 28).
Expression of genes coding for secreted proteins.
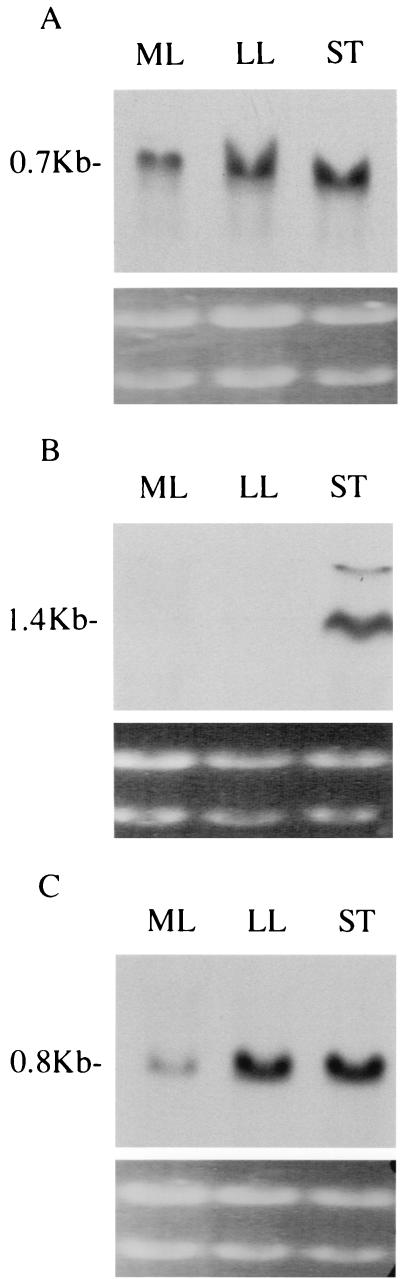
Production of SPEA, SPEB, and MF was studied by monitoring the corresponding transcript levels through different phases of growth. spea and mf transcripts were maximum in the late exponential and stationary phases of growth. speb transcripts were detected only in the stationary phase (Fig. 4A, B, and C). Densitometry was performed by comparing the band intensities of mRNAs from cells collected at different growth phases to the intensities of the corresponding 16S rRNAs, in separate experiments (Fig. 3E, F, and G). Probes for the cell wall genes hybridized strongly to the mid-exponential phase RNA, showing that mRNA at this phase was suitable for hybridization.
FIG. 4.
Expression of speA, speB, and mf. RNA (20 μg), extracted from H305 at the mid-log (ML), late log (LL), and stationary (ST) phases of growth, was hybridized to DIG-labelled PCR probes for speA (A), speB (B), and mf mRNAs. Photographs of ethidium bromide-stained gels are shown below the corresponding Northern blots.
Stationary-phase-specific expression of proteins has also been observed in many other bacteria. For example, in C. difficile, toxin genes are turned on only when the bacterium enters the stationary phase (6). Delayed expression of toxin genes may allow survival of the pathogen under conditions of stress or nutrient starvation. Production of SPEA by this strain was found to occur in the late log phase of growth in broth (23). It has been reported that SPEB production is maximal under conditions of nutrient starvation (3). Secreted levels of SPEA and SPEB mirror the patterns of transcription seen for speA and speB. Though this indicates transcriptional regulation of these genes, the possibility of translational regulation cannot be excluded. SPEA is a phage-encoded toxin, and the mechanisms involved in regulation of SPEA expression are still unclear (26). It is of interest that the kinetics of SPEA transcription are similar to those of a chromosomally encoded protein like MF, although in this strain SPEA transcription can be easily detected in early phases of growth; the reasons for this are unclear.
Instability in expression of speb.
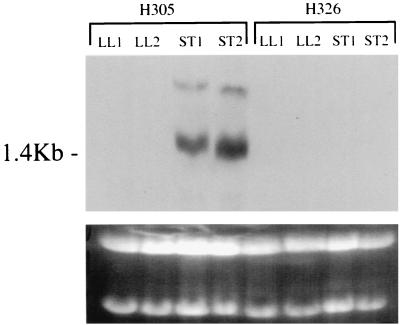
Expression of speB was also studied in H326, a kanamycin-resistant isogenic mutant of H305 with a disruption in the spea gene (24). In contrast to H305, the speB transcript was undetectable in H326 when the cells were cultured in antibiotic-free medium and examined by Northern hybridization. To ensure that speB expression was not being missed, RNA was prepared from cells collected at each of two time points in the late log phase and two points in the stationary phase (Fig. 5). The expression of speB was also undetectable by Northern hybridization in a strain of H305 transformed with the plasmid pDL413, a derivative of pVA380-1 which confers kanamycin resistance (13). However, we could detect low-level expression of SPEB by reverse transcription-PCR and Western blotting (data not shown). The effect on speB transcription was not specific to the SPEA-negative mutant, as speB transcripts were markedly reduced in plasmid-transformed H305 strains without chromosomal mutation. Electroporation per se was not found to affect speB expression (data not shown). Observations of the regulation of genes such as speB in S. pyogenes must therefore be interpreted with caution as, at least in our strain, reduced speB expression was a nonspecific effect associated with transformation and kanamycin selection.
FIG. 5.
Instability of speB expression. RNAs extracted from H305 and H326 (speA) at two time points in the late log phase (LL1 and LL2) and two time points in the stationary phase (ST1 and ST2) were hybridized by using DIG-labelled probes for speB.
Growth-phase-dependent regulation of genes could indicate the presence of a global regulatory circuit in this pathogen, similar to the agr regulatory system of Staphylococcus spp. Stationary-phase sigma factors, as found in other gram-positive bacteria, could also play a role in the control of gene expression in S. pyogenes.
Acknowledgments
This work was supported by the Medical Research Council through a Clinician Scientist Award to S.S.
REFERENCES
- 1.Ashbaugh C D, Warren H B, Carey V J, Wessels M R. Molecular analysis of streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft-tissue infection. J Clin Investig. 1998;102:550–560. doi: 10.1172/JCI3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchmeier N A, Lipps C J, So M Y, Heffron F. Recombination-deficient mutants of Salmonella typhimurium are avirulent and sensitive to the oxidative burst of macrophages. Mol Microbiol. 1993;7:933–936. doi: 10.1111/j.1365-2958.1993.tb01184.x. [DOI] [PubMed] [Google Scholar]
- 3.Chausee M S, Phillips E R, Ferretti J J. Temporal production of streptococcal erythrogenic toxin B (streptococcal cysteine proteinase) in response to nutrient depletion. Infect Immun. 1997;65:1956–1959. doi: 10.1128/iai.65.5.1956-1959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cleary P P, McLandsborough L, Ikeda L, Cue D, Lam H. High-frequency intracellular infection and erythrogenic toxin A expression undergo phase variation in M1 group A streptococci. Mol Microbiol. 1998;28:157–167. doi: 10.1046/j.1365-2958.1998.00786.x. [DOI] [PubMed] [Google Scholar]
- 5.Crater D L, Van de Rijn I. Hyaluronic acid synthesis operon (has) expression in group A streptococci. J Biol Chem. 1994;270:18452–18458. doi: 10.1074/jbc.270.31.18452. [DOI] [PubMed] [Google Scholar]
- 6.Dupuy B, Sonenshein A L. Regulated transcription of Clostridium difficile toxin genes. Mol Microbiol. 1998;27:107–120. doi: 10.1046/j.1365-2958.1998.00663.x. [DOI] [PubMed] [Google Scholar]
- 7.Erikson B K G, Andersson J, Holm S E, Norgren M. Epidemiological and clinical aspects of invasive group A streptococcal infections and the streptococcal toxic shock syndrome. Clin Infect Dis. 1998;27:1428–1436. doi: 10.1086/515012. [DOI] [PubMed] [Google Scholar]
- 8.Hauser A R, Stevens D L, Kaplan E L, Schlievert P M. Molecular analysis of pyrogenic exotoxins from Streptococcus pyogenes isolates associated with toxic shock-like syndrome. J Clin Microbiol. 1991;29:1562–1567. doi: 10.1128/jcm.29.8.1562-1567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoge C W, Schwartz B, Talkington D F, Breiman R F, MacNeill E M, Englender S J. The changing epidemiology of invasive group A streptococcal infections and the emergence of streptococcal toxic shock-like syndrome. JAMA. 1993;269:384–389. [PubMed] [Google Scholar]
- 10.Iwasaki M, Igarashi H, Yutsudo T. Mitogenic factor secreted by Streptococcus pyogenes is a heat stable nuclease requiring His122 for activity. Microbiology. 1997;143:2449–2455. doi: 10.1099/00221287-143-7-2449. [DOI] [PubMed] [Google Scholar]
- 11.Ji Y, McLandsborough L, Kondagunta A, Cleary P P. C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect Immun. 1996;64:503–510. doi: 10.1128/iai.64.2.503-510.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koomy J M, Falkow S. Cloning of the recA gene of Neisseria gonorrhoeae and construction of gonococcal recA mutants. J Bacteriol. 1987;169:790–795. doi: 10.1128/jb.169.2.790-795.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeBlanc D J, Inamine J M, Lee L N. Broad geographical distribution of homologous erythromycin, kanamycin, and streptomycin resistance determinants among group D streptococci of human and animal origin. Antimicrob Agents Chemother. 1986;29:549–555. doi: 10.1128/aac.29.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McIver K S, Heath A S, Scott J R. Regulation of virulence by environmental signals in group A streptococci: influence of osmolarity, temperature, gas exchange, and iron limitation on emm transcription. Infect Immun. 1995;63:4540–4542. doi: 10.1128/iai.63.11.4540-4542.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McIver K S, Scott J R. Role of mga in growth phase regulation of virulence genes of the group A streptococcus. J Bacteriol. 1997;179:5178–5187. doi: 10.1128/jb.179.16.5178-5187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mei J, Nourbakhsh F, Ford C W, Holden D. Identification of Staphylococcus aureus virulence genes in a murine model of bacteremia using signature tagged mutagenesis. Mol Microbiol. 1997;26:399–407. doi: 10.1046/j.1365-2958.1997.5911966.x. [DOI] [PubMed] [Google Scholar]
- 17.Mikulskis A V, Delor I, Thi V H, Cornelis G R. Regulation of the Yersinia enterocolitica enterotoxin Yst gene: influence of growth phase, temperature, osmolarity, pH and bacterial host factors. Mol Microbiol. 1994;14:905–915. doi: 10.1111/j.1365-2958.1994.tb01326.x. [DOI] [PubMed] [Google Scholar]
- 18.Musser J M. Streptococcal superantigen, mitogenic factor and pyrogenic exotoxin B expressed by Streptococcus pyogenes. Prep Biochem Biotechnol. 1997;27:143–172. doi: 10.1080/10826069708000074. [DOI] [PubMed] [Google Scholar]
- 19.Novick R P, Projan S J, Kornblum J, Ross H F, Ji G, Kreiswirth B, Vandenesch F, Moghazeh S. The agr P2 operon: an autocatalytic sensory transduction system in Staphylococcus aureus. Mol Gen Genet. 1995;248:446–458. doi: 10.1007/BF02191645. [DOI] [PubMed] [Google Scholar]
- 20.Podbielski A, Flosdorff A, Weber-Heynemann J. Group A streptococcal vir49 gene controls expression of four structural vir regulon genes. Infect Immun. 1995;63:9–20. doi: 10.1128/iai.63.1.9-20.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pospiech A, Neumann B. A versatile quick-prep of genomic DNA from Gram-positive bacteria. Trends Genet. 1995;11:217–218. doi: 10.1016/s0168-9525(00)89052-6. [DOI] [PubMed] [Google Scholar]
- 22.Sriskandan S, Moyes D, Buttery L, Krautz T, Evans T J, Polak J, Cohen J. Streptococcal pyrogenic exotoxin A release, distribution, and role in a murine model of fasciitis and multiorgan failure due to Streptococcus pyogenes. J Infect Dis. 1996;173:1399–1407. doi: 10.1093/infdis/173.6.1399. [DOI] [PubMed] [Google Scholar]
- 23.Sriskandan S, McKee A, Hall L, Cohen J. Comparative effects of clindamycin and ampicillin on superantigenic activity of Streptococcus pyogenes. J Antimicrob Chemother. 1997;40:1–3. doi: 10.1093/jac/40.2.275. [DOI] [PubMed] [Google Scholar]
- 24.Sriskandan S, Unnikrishnan M, Krausz T, Cohen J. Molecular analysis of the role of streptococcal pyrogenic exotoxin A (SPEA) in invasive soft-tissie infection resulting in Streptococcus pyogenes. Mol Microbiol. 1999;33:778–790. doi: 10.1046/j.1365-2958.1999.01525.x. [DOI] [PubMed] [Google Scholar]
- 25.Stevens D. Invasive group A streptococcal infections. Clin Infect Dis. 1992;14:2–13. doi: 10.1093/clinids/14.1.2. [DOI] [PubMed] [Google Scholar]
- 26.Weeks C R, Ferretti J J. Nucleotide sequence of the type A streptococcal exotoxin (erythrogenic toxin) gene from Streptococcus pyogenes bacteriophage T12. Infect Immun. 1986;52:144–150. doi: 10.1128/iai.52.1.144-150.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wessels M R, Moses A E, Goldberg J B, Di Cesare T J. Hyaluronic acid is a virulence factor for mucoid group A streptococci. Proc Natl Acad Sci USA. 1991;88:8317–8321. doi: 10.1073/pnas.88.19.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zagaglia C, Casalino M, Colonna B, Conti C, Calconi A, Nicoletti M. Virulence plasmids of enteroinvasive Escherichia coli and Shigella flexneri integrate into a specific site on the host chromosome: integration greatly reduces expression of plasmid-carried virulence genes. Infect Immun. 1991;59:792–799. doi: 10.1128/iai.59.3.792-799.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zyskind J W, Svitil A L, Stine W B, Biery M C, Smith D W. RecA protein of Escherichia coli and chromosome partitioning. Mol Microbiol. 1992;6:2525–2537. doi: 10.1111/j.1365-2958.1992.tb01429.x. [DOI] [PubMed] [Google Scholar]