Abstract
Three different Yersinia enterocolitica serotype O8 strains harboring mutations in virulence-associated genes coding for Yersinia adhesin A (YadA), Mn-cofactored superoxide dismutase (SodA), and high-molecular-weight protein 1 were analyzed for their ability to colonize and persist in tissues after orogastric immunization of C57BL/6 mice. We demonstrated that all three Yersinia mutant strains were markedly impaired in their ability to disseminate into the spleens and livers of immunized mice but were able to colonize the Peyer’s patches for at least 12 days, resulting in the induction of significant antibody titers against Yersinia outer proteins (Yops) and in the priming of Yersinia antigen-specific CD4+ Th1 cells isolated from spleens. The high level of attenuation did not diminish the immunogenic properties of the mutant strains. In fact, mice immunized with a single oral dose of any of the mutant strains were protected against a lethal oral-challenge infection with wild-type Y. enterocolitica. Moreover, adoptive transfer of Yersinia-specific antibodies from sera of mice immunized with the mutant WAP-314 sodA revealed that this protection could be mediated by Yersinia-specific immunoglobulins.
Live replicating bacteria are being considered as attractive antigen delivery vectors. A variety of attenuated Salmonella typhimurium, Yersinia enterocolitica, Mycobacterium tuberculosis, and Listeria monocytogenes mutant strains have been evaluated as potential carrier vaccines to present heterologous antigens to the immune systems of vaccinated mice (1, 12, 14, 25, 33).
Despite the progress in the development of new bacterial live carrier vaccines, it has become increasingly clear that new strategies are needed. For example, instead of knocking out genes that result in auxotrophic mutations (e.g., ΔaroA or ΔaroCD) (9, 23, 48) or interference in global gene expression and regulation (e.g., ΔphoP or ΔphoQ) (16, 22), an attractive alternative might be to mutate genes that code for virulence-associated factors of bacteria, leading to newly designed vector strains with tissue tropism and restriction.
Y. enterocolitica causes enteritis and lymphadenitis in humans and rodents (17). In mice, yersiniae preferentially bind to M cells, thereby promoting bacterial uptake and transepithelial transport to the Peyer’s patches. Both dissemination into the spleen and liver and further proliferation within these organs mark the initiation of a symptomatic infection. The virulence is controlled by chromosomally encoded (Inv, Ail, and the siderophore yersiniabactin) and plasmid-encoded (Yersinia outer proteins and Yersinia adhesin A) determinants (11). These virulence factors and the pathogenesis of Y. enterocolitica have been extensively studied (5, 19, 24, 38–40).
Y. enterocolitica has evolved a strategy to survive and multiply within the lymphoid tissue, predominantly extracellularly (27, 29, 44). This strategy might be an advantageous feature for a carrier vaccine strain. The extracellular location may help the host’s immune system to eliminate the recombinant strain after a decent time interval post-oral immunization and thus prevent a chronic colonization.
In our laboratory, we have previously described three Y. enterocolitica O8 mutant strains (34, 35, 37): (i) the yadA-2 mutant, obtained by substituting tyrosine residues for two histidine residues in the YadA protein, which is a plasmid-encoded surface protein that mediates binding to extracellular-matrix proteins, adherence to host cells, and resistance to complement lysis and is essential for virulence of yersiniae; (ii) the Mn-cofactored superoxide dismutase (sodA) mutant, which is deficient in resistance to exogenous oxygen radicals produced by phagocytes; and (iii) the irp-1 mutant, lacking the 384.6-kDa high-molecular-weight protein 1, which is part of the siderophore yersiniabactin biosynthesis apparatus. The aim of this study was to assess the capacity of these three isogenic Y. enterocolitica O8 strains carrying mutations in virulence-associated genes to act as potential live oral vaccine candidates in mice.
The Yersinia strains used in this study and their construction were described previously (34, 35, 37). Strain WA-314 is a clinical isolate of Y. enterocolitica serotype O8 and bears the virulence plasmid pYVO8. This isolate was used as the parental strain for the construction of WA(pYVO8-A-2) and WA-314 sodA. Strain WA(pYVO8-A-2) was constructed by site-directed mutagenesis, resulting in the substitution of tyrosine residues for histidine-156 and histidine-159 of the YadA protein. In strain WA-314 sodA, the wild-type sodA gene has been replaced by sodA::Km. To construct WA irp1, the previously described cointegrant pRK290B8-5::pO8 was mobilized into the virulence plasmidless mutant WA-CS irp1::Kanr (20).
The significance of the differences among control and experimental groups in all experiments was determined by the Student t test. P values of <0.05 were considered statistically significant.
Determination of the course of colonization and persistence in mouse tissues.
The virulence of the Y. enterocolitica mutant strains was tested in the orogastric mouse infection model as described previously (37). Prior to infection of 6- to 8-week-old C57BL/6 mice, Yersinia stock suspensions were thawed and washed twice in sterile phosphate-buffered saline (PBS; pH 7.4). After appropriate dilution, bacteria were fed to groups of eight C57BL/6 mice by the use of a microliter pipette. The actual number of bacteria administered was determined by plating serial dilutions on Mueller-Hinton agar and counting CFU after incubation for 36 h at 27°C. Control mice were given an equal volume of sterile PBS. At various days postinfection (p.i.), mice were sacrificed. After aseptic removal of the organs, the Peyer’s patches, spleen, and liver of each mouse were homogenized in 1, 5, and 5 ml, respectively, of sterile PBS containing 0.1% Tergitol TMN 10 (Fluka, Buchs, Switzerland) and 0.1% bovine serum albumin (E. Merck AG, Darmstadt, Germany) by the use of tissue homogenizers, whereas the small intestine was washed with 10 ml of ice-cold PBS.
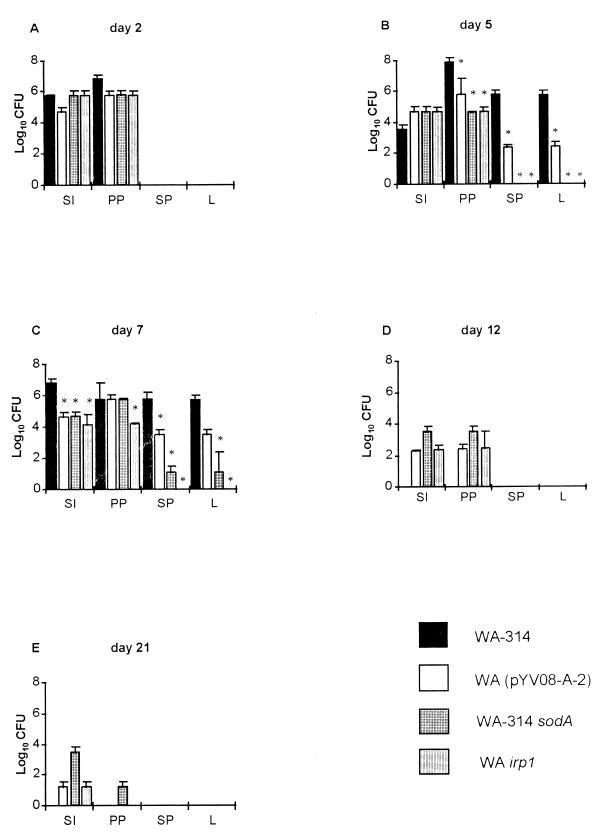
The course of immunization was determined by counting the numbers of surviving bacteria, as CFU, in the lumen of the small intestine, the Peyer’s patches, the spleen, and the liver on days 2, 5, 7, 12, and 21 postimmunization. The results are summarized in Fig. 1. Two days after orogastric immunization, the mutant strains and the wild-type strain colonized the small intestine and the Peyer’s patches (Fig. 1A). The course of infection with WA-314 was progressive, with dissemination of the bacteria into the spleen (mean ± standard deviation, 5.7 × 105 ± 5.5 × 105 CFU) and the liver (5.0 × 105 ± 5.1 × 105 CFU) by day 5 (Fig. 1B). At this time point, only the mutant strain WA(pYVO8-A-2) was detected in the spleen (2.2 × 102 ± 0.9 × 102 CFU) and the liver (2.4 × 102 ± 2.5 × 102 CFU), whereas no dissemination of WA-314 sodA and WA irp1 into these organs was observed. In contrast, all three mutant strains profoundly colonized the gut and the Peyer’s patches (Fig. 1B). On day 7 p.i., half of the mice infected with the wild-type Y. enterocolitica strain died due to the high bacterial load in the spleen and liver leading to a septic course of infection (Fig. 1C). In contrast, bacterial counts of WA(pYVO8-A-2) in the spleen (3.1 × 103 ± 3.3 × 103 CFU) and liver (1.8 × 103 ± 2.1 × 103 CFU) were more than 100 times lower than those of the wild-type strain. The mutant strain WA-314 sodA colonized both organs in smaller numbers (10 to 200 CFU per organ), whereas WA irp1 could not be reisolated from the spleen or liver throughout the investigated period of time, although this strain was able to colonize the Peyer’s patches (1.7 × 104 ± 0.5 × 104 CFU on day 7). While all mice infected with wild-type strain WA-314 died between days 6 and 10 p.i., all mice immunized with mutant strains showed markedly reduced signs of illness and survived. By day 12 p.i., WA(pYVO8-A-2) and WA-314 sodA were eliminated from the spleens and livers of immunized mice (Fig. 1D). The latter strain was reisolated from Peyer’s patches (3.5 × 103 ± 3.5 × 103 CFU) and the small intestine (3.4 × 103 ± 3.6 × 103 CFU) in numbers that were 10 times higher than those for WA(pYVO8-A-2) or WA irp1. Twenty-one days after oral inoculation of the mutant strains, only WA-314 sodA was still able to colonize the Peyer’s patches, although it did so in small numbers (∼40 CFU per organ). In addition, this mutant strain was reisolated from the small intestine at a 100-fold-higher concentration (3.2 × 103 ± 3.3 × 103 CFU) than WA(pYVO8-A-2) or WA irp1. Thus, all three mutant strains were able to colonize the Peyer’s patches of immunized C57BL/6 mice, to different degrees, for at least 2 weeks but were markedly impaired in their ability to disseminate into the spleen and liver compared to the fully virulent parental strain.
FIG. 1.
Time course of colonization and persistence of Y. enterocolitica in the liver (L), spleen (SP), Peyer’s patches (PP), and small intestine (SI). C57BL/6 mice were orally immunized with 108 Y. enterocolitica O8 mutant or isogenic wild-type organisms. Two, 5, 7, 12, and 21 days later, the mice were killed and the numbers of bacteria (CFU) present in the different mouse tissues were determined. Four of the eight mice immunized with Yersinia wild-type strain WA-314 succumbed on day 7 p.i., whereas the rest of the group died between days 8 and 10. Values are means for eight animals, with standard errors of the means indicated by error bars. ∗, value differs from that of mice infected with the Yersinia wild-type strain (P < 0.05).
Antibody responses against Yersinia outer proteins.
In the next set of experiments, it was investigated whether the Yersinia mutant strains were able to elicit humoral immune responses against Yersinia outer proteins, for which the acronym Yop is used. Yersinia-specific anti-Yop antibodies in sera of immunized mice were detected by a Yersinia-specific enzyme-linked immunosorbent assay (ELISA) as described previously (21, 28, 42). Yersinia outer proteins, at a concentration of 10 μg/ml in PBS, were used to coat 96-well microtiter plates (Greiner, Frickenhausen, Germany). Serial dilutions of sera from each of eight mice per group were carried out in PBS containing 0.5% Tween 20 (Merck, Darmstadt, Germany) and 2% bovine serum albumin BSA. Alkaline phosphatase-conjugated anti-mouse immunoglobulin G (IgG), IgA, and IgM (Sigma, Deisenhofen, Germany) were diluted 1:1,000 with PBS containing 0.5% Tween 20 and used as secondary antibodies. Disodium p-nitrophenylphosphate (Sigma) was used as the substrate. Optical densities were measured with an ELISA reader (Flow Laboratories, Meckenheim, Germany) at a wavelength of 405 nm. Five duplicates of sera from nonimmunized control mice were tested as negative controls to obtain cutoff values. The cutoff value in this study was defined as the mean absorbance of the negative controls plus 2 standard deviations.
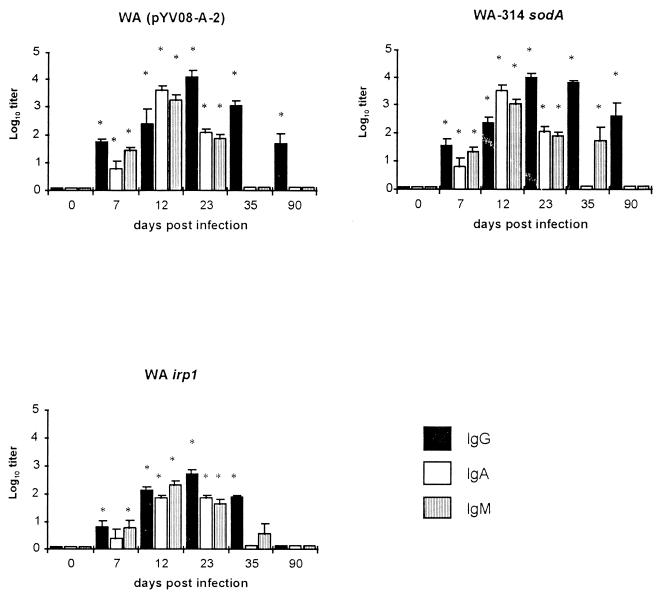
Groups of five mice were immunized with a single oral dose of 108 organisms of one of the various strains, and blood samples were collected on days 7, 12, 23, 35, and 90 after immunization. The results are shown in Fig. 2. All mice immunized with the mutant strains showed the highest serum IgA and IgM antibody titers on day 12 p.i. and the highest serum IgG antibody titers on day 23 p.i. Thereafter, a continuous decline of the titers was observed. Over the course of 90 days, the mutant strains differed in the magnitude of Yersinia-specific IgG, IgA, and IgM responses elicited in sera of mice. On day 23, WA(pYVO8-A-2) elicited a 23-fold-higher titer (1:14,000) and WA-314 sodA elicited a 17-fold-higher titer (1:10,000) of Yersinia-specific serum IgG antibody than WA irp1 (1:600) (P < 0.05) (Fig. 2). Mice immunized with WA-314 sodA elicited a 10-fold-higher titer (1:600) of serum IgG antibody than those given WA(pYVO8-A-2) (1:60) 90 days after the immunization (P < 0.05). At this time point, significant Yersinia-specific IgG titers were no longer detectable in sera from mice immunized with WA irp1.
FIG. 2.
Serum IgG, IgA, and IgM antibody responses of C57BL/6 mice prior to immunization (day 0) and 7, 12, 23, 35, and 90 days after oral immunization with 108 organisms of the indicated Yersinia mutant strains, as determined by using a Yersinia outer protein-specific ELISA. Columns represent means and standard deviations of results (log10 titer) obtained from eight mice. ∗, value differs from that of a control serum obtained prior to oral immunization (P < 0.05).
Induction of Yersinia-specific splenic T cells.
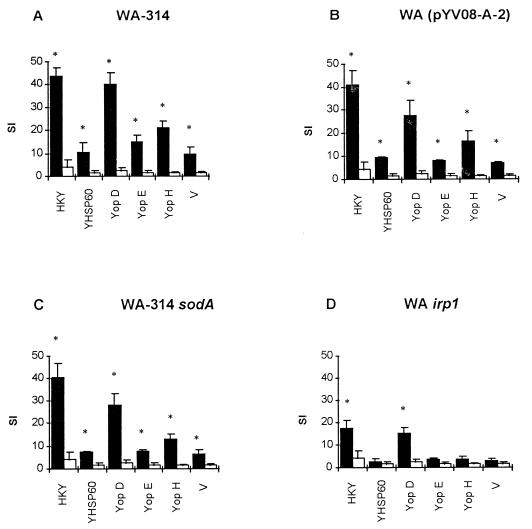
To investigate the abilities of the three mutant strains to elicit Yersinia-specific T-cell responses, mice were orally immunized with 108 yersiniae. Eight days after immunization, spleens were removed and single-cell suspensions were prepared as described previously (4). Purified T cells (26) were stained with a fluorescein isothiocyanate-coupled anti-CD3ɛ (145 2C11) (Becton Dickinson, Heidelberg, Germany) monoclonal antibody (MAb) and analyzed by flow cytometry (Epics XL-MCL; Coulter Electronics, Krefeld, Germany) as described previously (31). For proliferation assays, 2 × 105 purified T cells, 2 × 105 irradiated (3,000 rads) syngeneic splenic cells, as antigen-presenting cells, and 10 μg of antigen/ml were incubated in 96-well microtiter plates (Nunc, Wiesbaden, Germany) with 200 μl of culture medium per well (Click-RPMI 1640 (Biochrom, Berlin, Germany) supplemented with 2 mM l-glutamine, HEPES, 5 × 10−5 M 2-mercaptoethanol, 10 μg of streptomycin/ml 100 U of penicillin/ml and 10% heat-inactivated fetal calf serum). The following antigens were used at a concentration of 10 μg/ml of culture medium: heat-killed whole bacterial cells of Y. enterocolitica O8 (4), recombinant purified Yersinia heat shock protein 60 (HSP60) (31), and recombinant purified Yersinia outer proteins (YopD, -E, -H, and V antigen) (36). After incubation for 3 days, the cultures were pulsed with [3H]thymidine and the uptake of [3H]thymidine was determined with a liquid scintillation counter (Pharmacia) (31). Proliferative responses were expressed as stimulation indices (SI), which were calculated as follows: SI = [3H]thymidine uptake (in counts per minute) in the presence of the indicated antigen/[3H]thymidine uptake in the absence of that antigen. All experiments were repeated at least three times for verification.
The proliferation of T cells depended on the presence of antigen-presenting cells (APC) and antigen. Heat-killed Y. enterocolitica, Yersinia heat shock protein (HSP60), and recombinant Yersinia outer proteins (YopD, YopE, YopH, and V antigen) induced significant proliferative responses, as summarized in Fig. 3. Upon antigenic stimulation, T cells isolated from mice immunized with WA(pYVO8-A-2) or WA-314 sodA showed proliferative responses almost equivalent to those of T cells from mice infected with Yersinia wild-type strain WA-314. In contrast, T cells obtained from mice immunized with WA irp1 exhibited significantly weaker proliferative responses to the different antigens (P < 0.05) than T cells isolated from mice inoculated with either of the two other Yersinia mutant strains investigated in this study. Comparisons of proliferative responses to different Yersinia antigens revealed distinct patterns. T cells showed only a weak proliferative response to HSP60, YopE, and the V antigen (SI < 10), whereas stimulation with YopH induced a moderate proliferation (SI = 10 to 20). A strong T-cell proliferative response was observed upon stimulation with heat-killed Y. enterocolitica or YopD (SI = 25 to 45).
FIG. 3.
Proliferative T-cell responses to various Yersinia-specific antigens after oral immunization with wild-type or mutant Yersinia strains. Splenic T cells were stimulated with 10 μg of heat-killed Y. enterocolitica (HKY), Yersinia heat shock protein (YHSP60), and recombinant Yersinia outer proteins (YopD, YopE, YopH, and the V antigen) or without Yersinia antigen. Proliferative responses are expressed as SI. Solid bars represent SI values of T cells stimulated with the indicated antigen, whereas open bars represent SI values of T cells exhibiting non-antigen-stimulated (spontaneous) proliferation. Values are the means of triplicate cultures, with standard deviations indicated by error bars. ∗, value differs from that of the control (P < 0.05).
Cytokine production by Yersinia-specific T cells.
For determination of cytokine production, the supernatants of T cells stimulated with heat-killed Yersinia organisms were collected and used in cytokine assays. Gamma interferon (IFN-γ) levels were determined by capture ELISA as described previously (2, 4). Briefly, ELISA microtiter plates were coated with anti-IFN-γ MAb (AN-18.17.24). Biotin-labeled anti-IFN-γ MAb (R4-6A2) and avidin-biotin-alkaline phosphatase complex (Vectastain ABC-AP kit; Camon Wiesbaden, Germany) were used, and the optical densities at wavelengths of 405 and 490 nm were determined with an ELISA reader. In parallel, an interleukin-4 (IL-4)-specific ELISA, including the anti-IL4 MAbs 11B11 (biotin labeled) and BVD6 24G2, was carried out as described for IFN-γ.
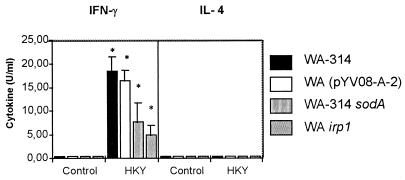
Determination of IFN-γ levels revealed that restimulated T cells from mice immunized with the mutant strains produced significant quantities of IFN-γ by day 8 after oral immunization compared to the control group of nonrestimulated T cells from immunized mice (P < 0.05) (Fig. 4). However, the amounts of IFN-γ produced by T cells from mice immunized with the mutant strains were lower than those of mice immunized with the wild-type Y. enterocolitica strain. In contrast, no significant quantities of IL-4 were detected after immunization with the wild-type or mutant strains (Fig. 4).
FIG. 4.
IFN-γ and IL-4 production by splenic T cells of mice after oral immunization with wild-type or mutant Y. enterocolitica strains. T cells were stimulated with 10 μg of heat-killed Y. enterocolitica (HKY) per ml. Supernatants were used in an IFN-γ- and IL-4-specific ELISA. The optical density values revealed in the ELISA are expressed as units of IFN-γ and IL-4 per milliliter according to the linear portion of the standard curve. Results are the means ± standard deviations (error bars) of values for five animals. ∗, value differs from that of the control (nonstimulated T cells) (P < 0.05).
Protective immunity.
Groups of eight mice were orally immunized with a single dose of 108 attenuated Yersinia mutant organisms. Ten weeks after this immunization, mice were challenged with 5 × 108 wild-type Y. enterocolitica WA-314 organisms (10 times the 50% lethal dose [LD50]) by the oral route. To determine the extent of protection, the bacterial loads in the lumen of the small intestines, the Peyer’s patches, the spleens, and the livers of infected animals were determined as described above.
In comparison to the control group of nonimmunized mice, no signs of illness were observed in mice immunized with the attenuated Yersinia strains. Five days after the oral challenge, mice were sacrificed and the colonization and persistence of wild-type Y. enterocolitica WA-314 in vivo was investigated (Table 1). Three of the eight mice in the nonimmunized group died due to the challenge on day 5. In the remaining five mice, wild-type yersiniae were present in large numbers in the lumen of the small intestine (3.4 × 105 ± 1.7 × 105 CFU), the Peyer’s patches (1.8 × 108 ± 0.9 × 106 CFU), the spleen (4.1 × 105 ± 0.8 × 105 CFU), and the liver (5.9 × 104 ± 1.2 × 104 CFU). In contrast, the wild-type Y. enterocolitica strain was not isolated from the spleen or the liver of any immunized mouse. In addition, mice immunized with WA(pYVO8-A-2) or WA-314 sodA had 1,000- to 10,000-fold-lower bacterial counts in the small intestine and the Peyer’s patches than nonimmunized mice (P < 0.05). In mice immunized with WA irp1, 100- to 1,000-fold-lower numbers of wild-type Yersinia were detected in the latter organs (P < 0.05).
TABLE 1.
Protection against wild-type Y. enterocolitica infection
| Immunizing strain | No. of bacteria in tissues of mice 5 days after challengea
|
|||
|---|---|---|---|---|
| Small intestine | Peyer’s patches | Spleen | Liver | |
| WA(pYVO8-A-2) | (4.4 ± 0.2) × 102* | (3.0 ± 1.4) × 102* | 0 | 0 |
| WA-314 sodA | (1.9 ± 0.6) × 102* | (6.3 ± 1.5) × 102* | 0 | 0 |
| WA irp1 | (8.9 ± 1.2) × 103* | (8.3 ± 0.8) × 103* | 0 | 0 |
| Controlb | (3.4 ± 1.7) × 105 | (1.8 ± 0.9) × 106 | (4.1 ± 0.8) × 105 | (5.9 ± 1.2) × 104 |
Groups of eight mice were orally immunized with 108 organisms of the indicated Yersinia mutant strain. Ten weeks after immunization, mice were orally challenged with 5 × 108 (10 times the LD50) Yersinia wild-type strain (WA-314) organisms. Five days after the challenge, the bacterial loads in organs of the mice were determined. Values are means ± standard deviations of data for eight animals. Values that differ from the control values at P < 0.05 are marked with asterisks.
Nonimmunized mice.
Adoptive transfer of Yersinia-specific antibodies.
To determine whether Yersinia antiserum from mice orally immunized with the Yersinia WA-314 sodA mutant strain can mediate protection against an intravenous challenge with a lethal dose of wild-type Y. enterocolitica, adoptive-transfer experiments were carried out. Four weeks after oral inoculation of 108 WA-314 sodA organisms, hyperimmune sera from mice were collected. After ammonium sulfate precipitation, the immunoglobulins were dialyzed against PBS overnight at 4°C and the protein content was determined as described previously (49). C57BL/6 mice (eight per group) were each treated intravenously with 400 μg of a mouse immunoglobulin-rich fraction 1 day prior to an intravenous (i.v.) challenge with 10 times the LD50 of Y. enterocolitica O8 wild-type strain WA-314. Five and 11 days after the challenge, bacterial counts in spleens of mice were determined.
As demonstrated in Table 2, the group of mice treated with the WA-314 sodA antiserum showed a 100-fold-lower bacterial load in their spleens than the group of mice treated with the purified serum from naive mice (P < 0.05) 5 days after the challenge. Moreover, in the former group, all mice survived the Yersinia infection and no bacteria were isolated from spleens on day 11 after the challenge, whereas all mice of the latter group died between days 6 and 9 p.i.
TABLE 2.
Adoptive transfer of Yersinia-specific antibodies
| Source of seruma | No. of bacteria in the spleenb
|
|
|---|---|---|
| Day 5 | Day 11 | |
| WA-314 sodA mice | (6.4 ± 0.2) × 105* | 0 |
| Naive mice | (4.0 ± 0.2) × 107 | —c |
C57BL/6 mice were each treated intravenously with 400 μg of purified serum from mice orally immunized with WA-314 sodA or from nonimmunized mice 1 day prior to intravenous challenge with 105 Y. enterocolitica O8 wild-type strain organisms (200 times the LD50).
Five and 11 days after the challenge, bacterial counts per spleen were determined. Values are means ± standard deviations of data for eight mice per group. Values that differ from the control group (naive mice) values at P < 0.05 are marked with asterisks.
All C57BL/6 mice which were given serum from naive mice died between days 6 and 9 after the challenge.
Y. enterocolitica has been recognized as a potential bacterial live carrier by several groups. Sory et al. made use of Y. enterocolitica strains to induce mucosal and serum antibody responses against the cholera toxin B subunit and the cytoplasmic CRA protein of Trypanosoma cruzi (46, 47). However, in those studies, the authors did not carry out the experiments with attenuated Yersinia strains but rather used Y. enterocolitica serotype O9 strains (biotype II), which are less virulent in mice (low-pathogenicity group) than those of serotype O8 (biotype I B; high-pathogenicity group). In contrast, Bowe et al. (7) and O’Gaora et al. (32, 33) constructed a highly attenuated Y. enterocolitica O8 aroA mutant strain which was found to persist in the Peyer’s patches, mesenteric lymph nodes, spleen, and liver for only 3 days after oral infection. Consequently, mice immunized orally with a single dose of this mutant strain were not protected against a lethal wild-type infection. More recently, Dorrell et al. described a Y. enterocolitica O8 ompR mutant strain which did not cause a lethal course of infection in orally immunized BALB/c mice (13). Spleens and livers of infected mice were colonized with the mutant strain for 21 days. Mice orally immunized with a single dose of the O8 ompR mutant strain were partially protected against an oral challenge with the virulent Y. enterocolitica parent strain.
The Yersinia mutant strains investigated in this study were capable of translocating from the intestinal lumen to the Peyer’s patches, where they persisted for at least 12 days. All three mutant strains were markedly impaired in their ability to disseminate from Peyer’s patch tissue into the spleen and liver, resulting in survival of all infected mice. The yadA-2 mutant strain WA(pYVO8-A-2) was reisolated from these organs on days 5 and 7 p.i. 100 times less than the isogenic wild-type strain, whereas the mutant strain WA-314 sodA was detected in the spleen and liver only on day 7 p.i. even 1,000 times less than the wild-type Yersinia strain. Surprisingly, the mutant strain WA irp1 did not colonize the latter organs at any time during the course of immunization.
A yadA null mutant of Y. enterocolitica is characterized by an impaired ability to colonize the intestinal mucosa (27). Previously, we showed that the substitution of tyrosine residues for two histidine residues in YadA resulted in abrogation of binding to various extracellular-matrix proteins (42, 43) and of adherence to HEp-2 cells, whereas autoagglutination (45) and serum complement resistance (10) remained unaffected. However, the collagen-binding function appeared to not be required for translocation of WA(pYVO8-A-2) from the gut lumen to the Peyer’s patches.
The attenuation of Y. enterocolitica resulting from the mutation of the sodA gene is due to the additive effects of the reduced detoxification abilities of metabolically produced bacterial superoxide and of the increased susceptibility to killing by polymorphonuclear leukocytes (35). The sodA gene was found to be upregulated under conditions of aerobiosis and iron starvation in Escherichia coli (15). Such conditions are encountered by extracellular yersiniae in the spleen and liver. In contrast, sodA is known to be downregulated under anaerobic or microaerophilic conditions, such as in the gut or in abscesses of Peyer’s patches during Yersinia infection (3, 17, 30). In fact, WA-314 sodA colonized the small intestine and the Peyer’s patches for at least 3 weeks after the orogastric immunization, indicating that the sodA gene is not required for intraluminal growth.
Highly pathogenic Y. enterocolitica strains possess a chromosomal cluster of iron-regulated genes located in the high-pathogenicity island. This gene cluster carries genes for the biosynthesis and uptake of the Yersinia siderophore yersiniabactin (irp1-9 and fyuA), which is a high-affinity ferric iron uptake system that significantly contributes to the virulence of yersiniae (8, 18). WA irp1 was able to translocate from the intestinal lumen to the Peyer’s patches, but its abilities to cause a systemic infection and to colonize the spleen and liver were totally impaired. As mentioned above, the evasion strategy of wild-type Y. enterocolitica in the host eventually results in extracellular survival and multiplication in the spleen and liver. Evidently, the siderophore yersiniabactin is essential for the ability of yersiniae to survive and multiply within these organs, whereas presumed accessory iron uptake systems in these organisms are sufficient to mediate survival during the first stage of colonization of the host.
The investigation of humoral and cellular immune responses to Yersinia-specific antigens elicited by the Yersinia mutant strains revealed that WA(pYVO8-A-2) and WA-314 sodA were able to induce significantly higher IgG, IgA, and IgM antibody titers against Yersinia outer proteins than WA irp1. It is conceivable that the prolonged colonization of the spleen and liver by the former strains provoked a stronger humoral immune response. On the other hand, it appears that the higher bacterial load of WA-314 sodA in the Peyer’s patches and the small intestine at 21 days p.i. contributed to the 10-fold-higher IgG antibody titer compared to WA(pYVO8-A-2) at 90 days p.i.
The mutant strains WA(pYVO8-A-2) and WA-314 sodA elicited stronger cellular immune responses against a variety of Yersinia-specific antigens than WA irp1. These data suggest that the transient and weak colonization of the spleens and livers of infected mice by attenuated Yersinia strains enhances Yersinia-specific antibody and T-cell responses. However, this is not an essential prerequisite of an effective Yersinia live oral carrier vaccine, because WA irp1 was also able to induce significant humoral and cellular immune responses against Yersinia-specific antigens. On the other hand, we cannot exclude the possibility that much larger numbers of yersiniae eventually reached the spleen and liver but were rapidly killed and thus did not appear in the CFU counting assay.
It has been previously shown that T cells from C57BL/6 mice immunized with a sublethal dose of wild-type Yersinia produced significant levels of IFN-γ upon exposure to antigen (heat-killed Y. enterocolitica), while they did not produce IL-4 (2). T cells from mice immunized with the Yersinia mutant strains showed the same pattern of cytokine production. Thus, like wild-type Y. enterocolitica, the mutant strains induced pronounced Th1 responses. IFN-γ-producing Th1 cells are known to provide help for cell-mediated immune responses which are crucial for the defense from intracellular pathogens (6).
A single oral immunization of a mouse with any of the mutant strains resulted in full protection against a lethal wild-type Yersinia infection. Moreover, in experiments involving adoptive transfer of Yersinia-specific antibodies from sera of mice immunized with WA-314 sodA, we were able to demonstrate that this protection could be mediated by Yersinia-specific immunoglobulins. Thus, the high level of attenuation did not diminish the immunogenic properties of the mutant strains.
The mutant Yersinia strains investigated in this study elicited pronounced humoral and cellular immune responses against Yersinia outer proteins, which are effector proteins of Yersinia’s type III secretion system. Recently, Rüssmann et al. showed that the delivery of viral epitopes through the S. typhimurium type III secretion system resulted in efficient stimulation of MHC class I-restricted protective antiviral immune responses in vaccinated mice (41). The use of Yersinia outer proteins as carriers for heterologous antigens in attenuated Yersinia strains may be an attractive strategy to stimulate both humoral and cellular immune responses.
We have shown, based on designed mutations of virulence-associated genes, that rationally attenuated Y. enterocolitica O8 strains have a great potential to serve as safe and effective live oral carrier vaccines for the delivery of heterologous antigens in future studies.
Acknowledgments
E.I.I. and H.R. contributed equally to this work.
We thank Jeannette Sauer for expert technical assistance.
This work was supported by the Bayerische Forschungsförderung (FORGEN). H.R. was supported by the AIDS-Stipendienprogramm of the Bundesministerium für Forschung und Technologie/Germany.
REFERENCES
- 1.Aldovini A, Young R A. Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature. 1991;351:479–482. doi: 10.1038/351479a0. [DOI] [PubMed] [Google Scholar]
- 2.Autenrieth I B, Beer M, Bohn E, Kaufmann S H E, Heesemann J. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect Immun. 1994;62:2590–2599. doi: 10.1128/iai.62.6.2590-2599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autenrieth I B, Firsching R. Penetration of M cells and destruction of Peyer’s patches by Yersinia enterocolitica: an ultrastructural and histological study. J Med Microbiol. 1996;44:285–294. doi: 10.1099/00222615-44-4-285. [DOI] [PubMed] [Google Scholar]
- 4.Autenrieth I B, Tingle A, Reske-Kunz A, Heesemann J. T lymphocytes mediate protection against Yersinia enterocolitica in mice: characterization of murine T-cell clones specific for Y. enterocolitica. Infect Immun. 1992;60:1140–1149. doi: 10.1128/iai.60.3.1140-1149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bliska J B, Guan K L, Dixon J E, Falkow S. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc Natl Acad Sci USA. 1991;88:1187–1191. doi: 10.1073/pnas.88.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988;9:268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- 7.Bowe F, O’Gaora P, Maskell D, Cafferkey M, Dougan G. Virulence, persistence, and immunogenicity of Yersinia enterocolitica O:8 aroA mutants. Infect Immun. 1989;57:3234–3236. doi: 10.1128/iai.57.10.3234-3236.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carniel E, Mazigh D, Mollaret H H. Expression of iron-regulated proteins in Yersinia species and their relation to virulence. Infect Immun. 1987;55:277–280. doi: 10.1128/iai.55.1.277-280.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatfield S N, Strahan K, Pickard D, Charles I G, Hormaeche C E, Dougan G. Evaluation of Salmonella typhimurium strains harbouring defined mutations in htrA and aroA in the murine salmonellosis model. Microb Pathog. 1992;12:145–151. doi: 10.1016/0882-4010(92)90117-7. [DOI] [PubMed] [Google Scholar]
- 10.China B, Sory M-P, N’Guyen B T, De Bruyere M, Cornelis G R. Role of the YadA protein in prevention of opsonization of Yersinia enterocolitica by C3b molecules. Infect Immun. 1993;61:3129–3136. doi: 10.1128/iai.61.8.3129-3136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornelis G R. Yersinia pathogenicity factors. Curr Top Microbiol Immunol. 1994;192:243–263. doi: 10.1007/978-3-642-78624-2_11. [DOI] [PubMed] [Google Scholar]
- 12.Dietrich G, Bubert A, Gentschev I, Sokolovic Z, Simm A, Catic A, Kaufmann S H, Hess J, Szalay A A, Goebel W. Delivery of antigen-encoding plasmid DNA into the cytosol of macrophages by attenuated suicide Listeria monocytogenes. Nat Biotechnol. 1998;16:181–185. doi: 10.1038/nbt0298-181. [DOI] [PubMed] [Google Scholar]
- 13.Dorrell N, Li S R, Everest P H, Dougan G, Wren B W. Construction and characterisation of a Yersinia enterocolitica O:8 ompR mutant. FEMS Microbiol Lett. 1998;165:145–151. doi: 10.1111/j.1574-6968.1998.tb13139.x. [DOI] [PubMed] [Google Scholar]
- 14.Everest P, Griffiths P, Dougan G. Live Salmonella vaccines as a route towards oral immunisation. Biologicals. 1995;23:119–124. doi: 10.1006/biol.1995.0022. [DOI] [PubMed] [Google Scholar]
- 15.Fee J A. Regulation of sod genes in Escherichia coli: relevance to superoxide dismutase function. Mol Microbiol. 1991;5:2599–2610. doi: 10.1111/j.1365-2958.1991.tb01968.x. [DOI] [PubMed] [Google Scholar]
- 16.Galan J E, Curtiss R., III Virulence and vaccine potential of phoP mutants of Salmonella typhimurium. Microb Pathog. 1989;6:433–443. doi: 10.1016/0882-4010(89)90085-5. [DOI] [PubMed] [Google Scholar]
- 17.Hanski C, Kutschka U, Schmoranzer H P, Naumann M, Stallmach A, Hahn H, Menge H, Riecken E O. Immunohistochemical and electron microscopic study of interaction of Yersinia enterocolitica serotype O8 with intestinal mucosa during experimental enteritis. Infect Immun. 1989;57:673–678. doi: 10.1128/iai.57.3.673-678.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heesemann J. Chromosomal encoded siderophores are required for mouse virulence of enteropathogenic Yersinia species. FEMS Microbiol Lett. 1989;48:229–233. [Google Scholar]
- 19.Heesemann J, Grüter L. Genetic evidence that the outer membrane protein YOP1 of Yersinia enterocolitica mediates adherence and phagocytosis resistance to human epithelial cells. FEMS Microbiol Lett. 1987;40:37–41. [Google Scholar]
- 20.Heesemann J, Laufs R. Construction of a mobilizable Yersinia enterocolitica virulence plasmid. J Bacteriol. 1983;155:761–767. doi: 10.1128/jb.155.2.761-767.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heesemann J, Schroder J, Ulrich M. Analysis of the class-specific immune response to Yersinia enterocolitica virulence-associated antigens in orogastrically infected rabbits. Microb Pathog. 1988;5:437–447. doi: 10.1016/0882-4010(88)90005-8. [DOI] [PubMed] [Google Scholar]
- 22.Hohmann E L, Oletta C A, Killeen K P, Miller S I. phoP/phoQ-deleted Salmonella typhi (Ty800) is a safe and immunogenic single-dose typhoid fever vaccine in volunteers. J Infect Dis. 1996;173:1408–1414. doi: 10.1093/infdis/173.6.1408. [DOI] [PubMed] [Google Scholar]
- 23.Hoiseth S K, Stocker B A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 24.Iriarte M, Sory M-P, Boland A, Boyd A P, Mills S D, Lambermont I, Cornelis G R. TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 1998;17:1907–1918. doi: 10.1093/emboj/17.7.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobs W R, Jr, Snapper S B, Lugosi L, Bloom B R. Development of BCG as a recombinant vaccine vehicle. Curr Top Microbiol Immunol. 1990;155:153–160. doi: 10.1007/978-3-642-74983-4_11. [DOI] [PubMed] [Google Scholar]
- 26.Julius M H, Simpson E, Herzenberg L A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973;3:645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 27.Kapperud G, Namork E, Skurnik M, Nesbakken T. Plasmid-mediated surface fibrillae of Yersinia pseudotuberculosis and Yersinia enterocolitica: relationship to the outer membrane protein YOP1 and possible importance for pathogenesis. Infect Immun. 1987;55:2247–2254. doi: 10.1128/iai.55.9.2247-2254.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kono D H, Ogasawara M, Yu D T. An animal model to study serum immunoglobulin response to infection by Yersinia enterocolitica. Clin Exp Rheumatol. 1985;3:117–121. [PubMed] [Google Scholar]
- 29.Lian C-J, Hwang W S, Pai C H. Plasmid-mediated resistance to phagocytosis in Yersinia enterocolitica. Infect Immun. 1987;55:1176–1183. doi: 10.1128/iai.55.5.1176-1183.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller R A, Britigan B E. Role of oxidants in microbial pathophysiology. Clin Microbiol Rev. 1997;10:1–18. doi: 10.1128/cmr.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noll A, Autenrieth I B. Immunity against Yersinia enterocolitica by vaccination with Yersinia HSP60 immunostimulating complexes or Yersinia HSP60 plus interleukin-12. Infect Immun. 1996;64:2955–2961. doi: 10.1128/iai.64.8.2955-2961.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Gaora P, Maskel D, Coleman D, Cafferkey M, Dougan G. Cloning and characterisation of the serC and aroA genes of Yersinia enterocolitica, and construction of an aroA mutant. Gene. 1989;84:23–30. doi: 10.1016/0378-1119(89)90135-2. [DOI] [PubMed] [Google Scholar]
- 33.O’Gaora P, Roberts M, Bowe F, Hormaeche C, Demarco de Hormaeche R, Cafferkey M, Tite J, Dougan G. Yersinia enterocolitica aroA mutants as carriers of the B subunit of the Escherichia coli heat-labile enterotoxin to the murine immune system. Microb Pathog. 1990;9:105–116. doi: 10.1016/0882-4010(90)90084-4. [DOI] [PubMed] [Google Scholar]
- 34.Pelludat C, Rakin A, Jacobi C A, Schubert S, Heesemann J. The yersiniabactin biosynthetic gene cluster of Yersinia enterocolitica: organization and siderophore-dependent regulation. J Bacteriol. 1998;180:538–546. doi: 10.1128/jb.180.3.538-546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roggenkamp A, Bittner T, Leitritz L, Sing A, Heesemann J. Contribution of the Mn-cofactored superoxide dismutase (SodA) to the virulence of Yersinia enterocolitica serotype O8. Infect Immun. 1997;65:4705–4710. doi: 10.1128/iai.65.11.4705-4710.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roggenkamp A, Geiger A M, Leitritz L, Kessler A, Heesemann J. Passive immunity to infection with Yersinia spp. mediated by anti-recombinant V antigen is dependent on polymorphism of V antigen. Infect Immun. 1997;65:446–451. doi: 10.1128/iai.65.2.446-451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roggenkamp A, Neuberger H R, Flugel A, Schmoll T, Heesemann J. Substitution of two histidine residues in YadA protein of Yersinia enterocolitica abrogates collagen binding, cell adherence and mouse virulence. Mol Microbiol. 1995;16:1207–1219. doi: 10.1111/j.1365-2958.1995.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 38.Rosqvist R, Bölin I, Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988;56:2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosqvist R, Forsberg A, Rimpilainen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990;4:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 40.Ruckdeschel K, Roggenkamp A, Schubert S, Heesemann J. Differential contribution of Yersinia enterocolitica virulence factors to evasion of microbicidal action of neutrophils. Infect Immun. 1996;64:724–733. doi: 10.1128/iai.64.3.724-733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rüssmann H, Shams H, Poblete F, Fu Y, Galan J E, Donis R O. Delivery of epitopes by the Salmonella type III secretion system for vaccine development. Science. 1998;281:565–568. doi: 10.1126/science.281.5376.565. [DOI] [PubMed] [Google Scholar]
- 42.Schulze-Koops H, Burkhardt H, Heesemann J, Kirsch T, Swoboda B, Bull C, Goodman S, Emmrich F. Outer membrane protein YadA of enteropathogenic yersiniae mediates specific binding to cellular but not plasma fibronectin. Infect Immun. 1993;61:2513–2519. doi: 10.1128/iai.61.6.2513-2519.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulze-Koops H, Burkhardt H, Heesemann J, von der Mark K, Emmrich F. Plasmid-encoded outer membrane protein YadA mediates specific binding of enteropathogenic yersiniae to various types of collagen. Infect Immun. 1992;60:2153–2159. doi: 10.1128/iai.60.6.2153-2159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simonet M, Richard S, Berche P. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect Immun. 1990;58:841–845. doi: 10.1128/iai.58.3.841-845.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skurnik M, Bölin I, Heikkinen H, Piha S, Wolf-Watz H. Virulence plasmid-associated autoagglutination in Yersinia spp. J Bacteriol. 1984;158:1033–1036. doi: 10.1128/jb.158.3.1033-1036.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sory M-P, Hermand P, Vaerman J P, Cornelis G R. Oral immunization of mice with a live recombinant Yersinia enterocolitica O:9 strain that produces the cholera toxin B subunit. Infect Immun. 1990;58:2420–2428. doi: 10.1128/iai.58.8.2420-2428.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sory M-P, Kaniga K, Goldenberg S, Cornelis G R. Expression of the eukaryotic Trypanosoma cruzi CRA gene in Yersinia enterocolitica and induction of an immune response against CRA in mice. Infect Immun. 1992;60:3830–3836. doi: 10.1128/iai.60.9.3830-3836.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valentine P J, Devore B P, Heffron F. Identification of three highly attenuated Salmonella typhimurium mutants that are more immunogenic and protective in mice than a prototypical aroA mutant. Infect Immun. 1998;66:3378–3383. doi: 10.1128/iai.66.7.3378-3383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogel U, Autenrieth I B, Berner R, Heesemann J. Role of plasmid-encoded antigens of Yersinia enterocolitica in humoral immunity against secondary Y. enterocolitica infection in mice. Microb Pathog. 1993;15:23–36. doi: 10.1006/mpat.1993.1054. [DOI] [PubMed] [Google Scholar]