Abstract
Ductal disease is a broad group encompassing both benign and malignant entities which may overlap clinically and radiologically. Ductal carcinoma in situ (DCIS) is a noninvasive breast malignancy accounting for 20% of newly diagnosed breast cancer cases. It involves malignant epithelial cells confined to the duct(s). Although they are commonly diagnosed incidentally on screening mammography, DCIS may present with nipple discharge or a palpable lump. Benign diseases of the duct include intraductal papilloma and may present similarly with bloody or serous nipple discharge. Imaging evaluation will help in differentiating between the 2 entities and pathological examination will provide the final diagnosis. We present a case of a 72-year-old female who was presented with serous and bloody discharge and histology revealed intermediate grade ductal carcinoma in situ involving an intraductal papilloma.
Keywords: Ductal carcinoma in situ, DCIS, intraductal papilloma
Case report
A 72-year-old female, previously healthy, presented to the outpatient clinic with a history of intermittent spontaneous serous and bloody nipple discharge for the past 2 weeks. She denied any history of trauma or a previous similar episode. There was no significant past medical or surgical history. Her sister developed breast cancer in her 80s. She is married with 3 children that were breastfed.
On local examination of the left breast, there was bloody nipple discharge with mild nodularity in the retroareolar region of the left breast. There were no palpable masses or enlarged left axillary lymph nodes. The right breast was normal.
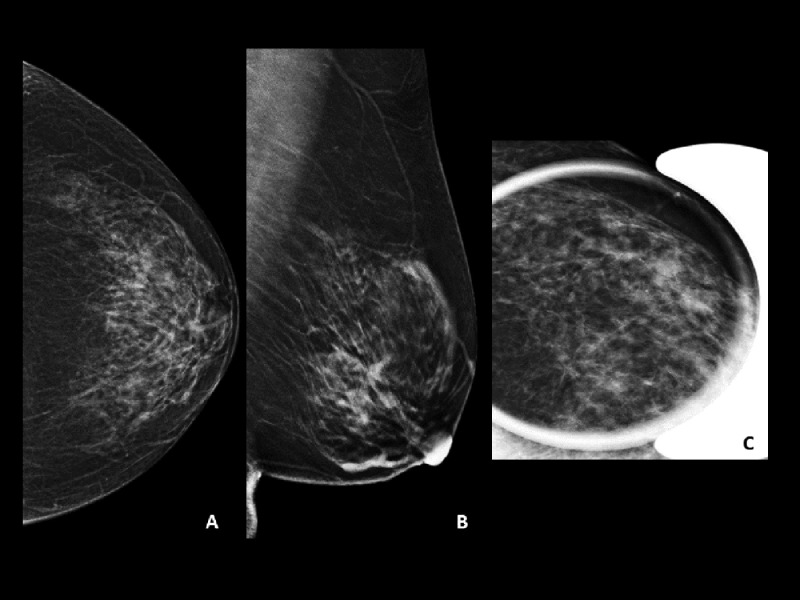
Initial mammography of the left breast had demonstrated round density in the lower outer quadrant (Fig. 1).
Fig. 1.
Breast mammogram (A–C) initial craniocaudal (A) and mediolateral oblique (B) mammograms of the left breast demonstrate a round area of increased density in the lower outer quadrant measuring 1.2 × 1.4 cm with no associated suspicious microcalcifications (arrow). (C) Magnified craniocaudal view of the area of interest. BIRADS 0 was assigned, and ultrasound scan was recommended.
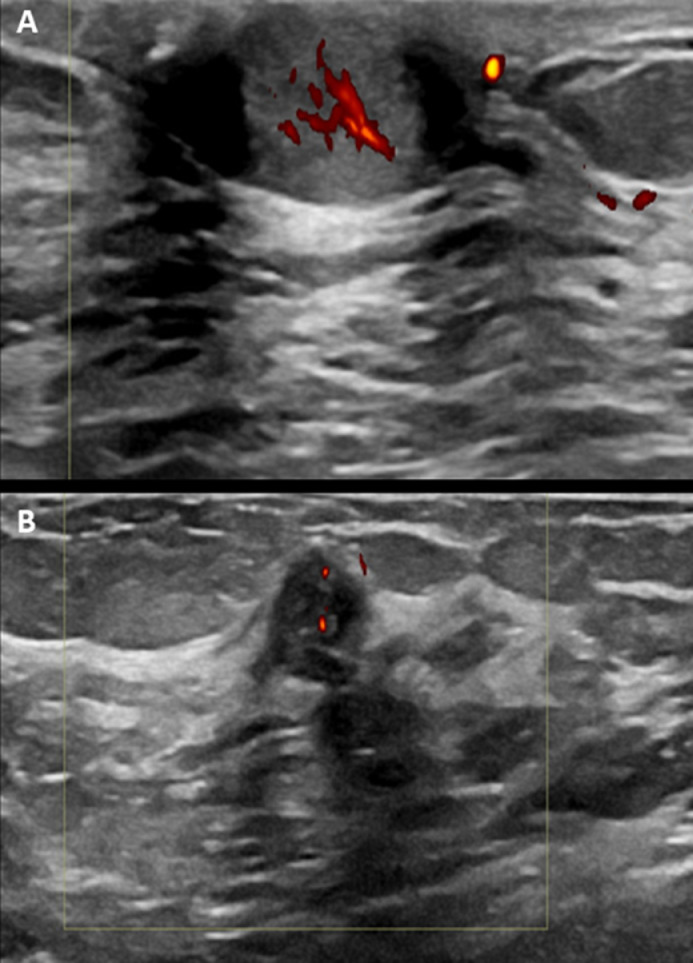
Bilateral breast ultrasound revealed a circumscribed hypoechoic and hypervascular solid lesion located 2 cm away from the nipple (Fig. 2). Findings are correlating to the area of abnormal density identified by mammography and raise the suspicion of malignancy.
Fig. 2.
Transverse and longitudinal ultrasound images (A, B) show a well-demarcated lobulated hypoechoic solid lesion with internal microcystic changes at 3-o'clock measuring 10 mm with nipple involvement. color Doppler sonography demonstrates increased intralesional vascularity.
An ultrasound-guided core needle biopsy was performed on the lesion identified. Histopathology results revealed an intraductal papilloma measuring 5 mm with extensive involvement by ductal carcinoma in situ (DCIS) (intermediate grade). Immunohistochemistry revealed that the lesions were ER+ 99%, PR+.
Discussion
Intraductal papilloma is a common benign lesion characterized by the proliferation of epithelial and myoepithelial cells with a fibrovascular stalk that protrudes into the ductal lumen [1,2]. They are classified as either solitary or multiple papilloma [1,2]. Solitary papilloma arises from the major lactiferous ducts in retroareolar location while multiple papilloma typically arises from the terminal ductal lobular units [3,4]. Multiple papilloma increases the risk of developing malignancy relative to the general population while solitary papilloma rarely does [1,5]. They may have coexisting hyperplasia, atypia, radial scar, or neoplastic lesions including DCIS or invasive carcinoma [1,4,6,7].
Imaging evaluation of intraductal papilloma can be done through mammography, ultrasound, or MRI. Evaluation of small intraductal papilloma is limited on mammography. Large lesions would appear as round or oval lesion with well-circumscribed margins [1]. Benign-looking calcifications can be seen in up to 25% of solitary papillomas [8]. Intraductal papillomas can also be assessed on galactography and they appear as distinct mural-based filling defects with lobulated or smooth contours. Intraductal papillomas can be seen as well-circumscribed solid nodules or mural-based nodules within dilated duct on ultrasonography [6]. Flow within the papilloma resulting from a vascular feeding pedicle may be seen on color Doppler imaging. Small intraductal papillomas on MRI may not be appreciated, but larger papillomas can appear as enhancing nodules with or without intraductal components. It may be challenging to distinguish these nodules from invasive malignancies because their enhancement pattern can be either uniform or irregular with washout or plateau kinetics. 1
The diagnosis of DCIS involving a papilloma can be considered with the presence of multiple papilloma, proliferation of more than 3 mm or larger, or located 3 cm from the nipple [4]. Solitary intraductal papilloma coexisting with DCIS is rare. [9] Histologically, a papilloma with DCIS is diagnosed with residual underlying benign papilloma partially involved with DCIS type of intraductal malignant proliferation [4,10].
Case reported above presented the coexistence of DCIS in solitary papilloma which is an unusual presentation and very few cases have been reported in the literature suggesting the relationship between the two entities [11]. Ultrasound is the mainstay for initial diagnosis and core needle biopsy is gold standard [1,6]. In some cases, particularly in papilloma with DCIS, MR imaging may be used for diagnosis in cases with normal ultrasound or mammographic findings [1].
Conclusion
Although rare, patients with solitary papilloma may have coexisting hyperplasia, atypia, or neoplastic lesions including DCIS as presented in this case. Therefore, surgical excision is required for the possible development of atypia or neoplasia.
Patient consent
A written consent was obtained from the patient for publication of this case and any accompanying images.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Eiada R, Chong J, Kulkarni S, Goldberg F, Muradali D. Papillary lesions of the breast: MRI, ultrasound, and mammographic appearances. AJR Am J Roentgenol. 2012;198(2):264–271. doi: 10.2214/AJR.11.7922. [DOI] [PubMed] [Google Scholar]
- 2.Muttarak M, Lerttumnongtum P, Chaiwun B, Peh WCG. Spectrum of papillary lesions of the breast: clinical, imaging, and pathologic correlation. Am J Roentgenol. 2008;191(3):700–707. doi: 10.2214/AJR.07.3483. [DOI] [PubMed] [Google Scholar]
- 3.Kuehner G, Darbinian J, Habel L, Axelsson K, Butler S, Chang S, et al. Benign papillary breast mass lesions: favorable outcomes with surgical excision or imaging surveillance. Ann Surg Oncol. 2019;26(6):1695–1703. doi: 10.1245/s10434-019-07180-7. [DOI] [PubMed] [Google Scholar]
- 4.Pal SK, Lau SK, Kruper L, Nwoye U, Garberoglio C, Gupta RK, et al. Papillary carcinoma of the breast: an overview. Breast Cancer Res Treat. 2010;122(3):637–645. doi: 10.1007/s10549-010-0961-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tay TKY, Tan PH. Papillary neoplasms of the breast-reviewing the spectrum. Mod Pathol. 2021;34(6):1044–1061. doi: 10.1038/s41379-020-00732-3. [DOI] [PubMed] [Google Scholar]
- 6.Ferris-James DM, Iuanow E, Mehta TS, Shaheen RM, Slanetz PJ. Imaging approaches to diagnosis and management of common ductal abnormalities. Radiographics. 2012;32(4):1009–1030. doi: 10.1148/rg.324115150. [DOI] [PubMed] [Google Scholar]
- 7.Khan S, Diaz A, Archer KJ, et al. Papillary lesions of the breast: to excise or observe? Breast J. 2018;24(3):350–355. doi: 10.1111/tbj.12907. [DOI] [PubMed] [Google Scholar]
- 8.Cardenosa G, Eklund GW. Benign papillary neoplasms of the breast: mammographic findings. Radiology. 1991;181(3):751–755. doi: 10.1148/radiology.181.3.1947092. [DOI] [PubMed] [Google Scholar]
- 9.Kalisher L, Rickert RR, Sharo RJ. Solitary peripheral papilloma of the breast: a radiologic-pathologic correlation of a benign lesion that may mimic breast cancer on mammography. Am J Roentgenol. 1998;171:605–609. doi: 10.2214/ajr.171.3.9725282. [DOI] [PubMed] [Google Scholar]
- 10.Tan PH, Schnitt SJ, van de Vijver MJ, Ellis IO, Lakhani SR. Papillary and neuroendocrine breast lesions: the WHO stance. Histopathology. 2015;66:761–770. doi: 10.1111/his.12463. [DOI] [PubMed] [Google Scholar]
- 11.Takemoto N., Yamamoto H., Shiraishi K., et al. A case of solitary intraductal papilloma of the breast coexisting with ductal carcinoma in situ (DCIS) J Med Ultrasonics. 2007;34:49–52. doi: 10.1007/s10396-006-0128-5. [DOI] [PubMed] [Google Scholar]