Abstract
Pseudoaneurysms of a pulmonary artery branch are a rare complication in cases of penetrating chest trauma. Other more frequent causes are secondary to infections or iatrogenesis. We present the case of a 16-year-old male patient who returns weeks after having sustained a stab wound to the chest, complaining of hemoptysis and chest pain. Imaging studies help detect and characterize a partially thrombosed pseudoaneurysm arising from the artery that supplies the anterior segment of the right upper lobe, with an associated post-traumatic pulmonary arteriovenous fistula. The patient was successfully treated with endovascular arterial embolization. Identifying this entity opportunely has an impact on the survival of this patient, avoiding risk secondary to massive bleeding by making an adequate treatment.
Keywords: Pseudoaneurysm, Pulmonary artery, Arteriovenous fistula, Post-traumatic Injury
Introduction
Pseudoaneurysms of the pulmonary circulation are a potential but very rare complication of chest trauma. The majority occurs in association with penetrating injuries and few cases have been reported in blunt trauma. They must be suspected in cases of persistent opacification on the chest X-ray, which enhances on contrast computed tomography (CT). Hemoptysis is the most common symptom, although patients may also present with dyspnea, chest pain, and hypoxia, and in some cases, they may be asymptomatic.
Case description
A 16-year-old male patient with no remarkable pathologic history admitted to the emergency service with a 5-cm stab wound to the chest, localized on the third intercostal space and right mid clavicular line, associated with massive hemothorax and pulseless electrical activity requiring advanced resuscitation maneuvers, thoracotomy with drainage of 4000 cc, pericardial window which was negative, and cardiac massage. In surgery, a wound to the posterior segment of the right upper lobe was documented, with active bleeding that required lung repair, blood product transfusions, and vasopressor support in the intensive care unit. Follow-up imaging studies of the chest did not show parenchymal opacities, the patient showed clinical improvement and was discharged a few days later. However, 2 months later, the patient returned due to 3 days of a clinical picture of hemoptysis associated with sudden-onset retrosternal chest pain.
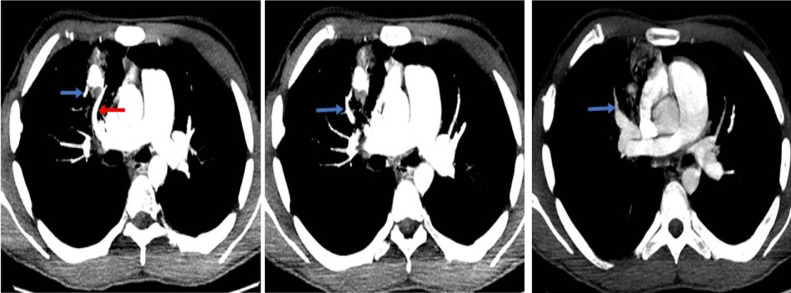
Noncontrast chest CT revealed a nodular opacity in the anterior segment of the right upper lobe measuring 39 mm, surrounded by a focus of ground glass opacity. CT angiography of the chest showed evidence of an abnormal communication between the artery that supplies the anterior segment of the right upper lobe and a branch of the right superior pulmonary vein, forming an arteriovenous fistula. Additionally, a post-traumatic pseudoaneurysm was identified in the segmental artery in question, together with an adjacent ground glass opacity consistent with alveolar hemorrhage (Figs. 1 and 2). The patient was taken to pulmonary arteriography, with findings consistent with those seen on the CT-angiogram. Coil embolization was successful, leading to the resolution of the clinical picture (Fig. 3).
Fig. 1.
CT pulmonary angiography. Axial image (A), sagittal reconstruction (B), axial image lung window (C) and three-dimensional volumetric reconstruction (D). A pseudoaneurysm (yellow arrow in A, B, and D) is identified as a rounded contrast-enhancing structure originating from the anterior wall of the artery to the anterior segment of the right upper lobe (red arrow in A). The neck of the pseudoaneurysm is clearly demonstrated (green arrow in A, B and D). The pseudoaneurysm is surrounded by a focus of ground glass opacity that corresponds to focal alveolar hemorrhage (circle in D).
Fig. 2.
CT pulmonary angiography: Axial images sequence. The artery of the anterior segment of the right upper lobe (red arrow) is the vascular structure from which the pseudoaneurysm originates. Additionally, it presents a drainage vein (blue arrows), a branch of the right superior pulmonary vein, which confirms an abnormal communication between an arterial and a venous structure, configuring a fistula.
Fig. 3.
Pulmonary arteriography. In images A, B and C, progressive opacification of the pseudoaneurysm (yellow arrows) originating from a branch of the artery to the anterior segment of the right upper lobe (red arrows) is identified. There is also opacification of a draining vein (blue arrows) confirming the arteriovenous fistula documented in the CT angiography. In images D, E and F, embolization material (coils) is observed, and it is confirmed that the procedure was successful, finding that there is only opacification of the artery (red arrows), and absence of contrast medium in the pseudoaneurysm and in the vein.
Discussion
Two types of vascular pulmonary injuries can develop after chest trauma: Arteriovenous fistulas and pseudoaneurysms. Both complications are very unusual because low pressures in the pulmonary artery allow for satisfactory occlusion of the injured vessel and because of the existence of low pressure differences between the pulmonary artery and the venous system [1].
Pulmonary artery pseudoaneurysms (PAP) or false post-traumatic aneurysms are a very rare complication in patients sustaining penetrating injuries to the chest. Close to 11% of the patients with hemoptysis taken to angiography show chronic pseudoaneurysms [2], and as of the date of this publication, only 30 cases of pulmonary artery aneurysms have been reported in the world literature [3]. These pseudoaneurysms are a dilation of the outermost layers of the artery, compromising the media and the adventitia, and they develop following an arterial injury where blood flow crosses those layers and creates a hematoma in the vessel wall, giving rise to turbulent flow [4]. The difference between a pseudoaneurysm and an aneurysm is the absence of endothelium in the former. The majority of cases of pulmonary artery pseudoaneurysms reported in the literature were diagnosed weeks to years after the initial trauma, and required either surgical or endovascular treatment [1].
Pulmonary artery pseudoaneurysms can be congenital or acquired, the former being the most prevalent. Acquired pseudoaneurysms are secondary to infections, mostly fungal, due to syphilis or tuberculosis, known as Rasmussen pseudoaneurysms. They can also occur in cases of collagen disease such as Behcet's or Marfan syndrome [5,6]. Post-traumatic aneurysms are false aneurysms because they lack a true wall and are secondary to penetrating injuries, blunt trauma or iatrogenic injury such as a complication from a Swan-Ganz catheter insertion [7].
On CT angiography, pseudoaneurysms appear as a contrast-enhancing nodular structure adjacent to a vascular structure. They are a rounded or oval-shaped saccular dilation of the arterial wall and appear of equal density to that of the intra-arterial contrast medium. Depending on their size, a neck can be identified. Some pseudoaneurysms exhibit hypodense luminal material corresponding to a partial or complete thrombus and, when chronic, they may show calcifications. Because of its high spatial resolution, CT allows to detect small pseudoaneurysms that are not evident on chest X-ray, or which may be asymptomatic.
Although there are no definitive guidelines for the management of traumatic pseudoaneurysms, it is important to analyze their size, as well as patient symptoms and hemodynamic status in order to ascertain the risk of rupture and decide whether to observe the patient or to perform endovascular or surgical repair [8].
In pulmonary arteriovenous fistulas (PAVF) there is an abnormal communication between the arterial and the venous systems of the pulmonary circulation, creating right-to-left shunting. The majority is congenital and, of these, 50% are associated with the Osler-Weber-Rendu syndrome, and usually localize to the lower lobes [9]. Less frequently, fistulas can be acquired, with some cases described following penetrating chest trauma [10]. A retrospective review revealed that the latter only comprise 1% of all pulmonary arteriovenous fistulas [11]. Depending on the number of arterial vessels involved in the defect, they may be classified as simple or complex. Simple PAVFs arise from a single arterial vessel, whereas complex fistulas are fed by 2 or more vessels. From the pathophysiological point, it has been shown that PAVFs can occur following focal vascular injuries that increase angiogenic activity, together with an imbalance between pro-angiogenic and antiangiogenic factors [12]. Also, given the higher pressure in the proximal artery, arterial blood goes to the vein, increasing venous pressure, and reducing peripheral resistance. Systemically, this leads to increased cardiac output and increased heart function [13].
PAVFs are diagnosed between days and years after trauma, depending on when the patient develops symptoms, the most frequent being hemoptysis, followed by chest pain, difficulty breathing and cough. CT angiography is used to detect and characterize arteriovenous fistulas which are seen as early and asymmetric opacifications of the vein during the arterial phase, with an abnormal communication between the artery and the vein.
It is advisable to treat symptomatic fistulas or those with an efferent vessel diameter of more than 3 mm because of the risk of a paradoxic embolic-type cerebrovascular event [14]. Embolization is the technique of choice for the management of fistulas, as it is a less invasive procedure associated with less complications and better outcomes [9,15].
Conclusion
Traumatic injuries of the pulmonary artery and its branches are a diagnostic challenge because of their low rate of occurrence, limiting clinical suspicion. The diagnostic modality of choice is chest CT angiography which has been widely recognized for its excellent performance in detecting and characterizing vascular injuries and determining their relationship with neighboring structures. Each injury has unique radiological characteristics which can be carefully differentiated with the help of CT, hence the importance of becoming familiar with the findings. Radiologists play an essential role in the diagnosis and treatment of arteriovenous fistulas and post-traumatic pseudoaneurysms of the pulmonary circulation.
Patient consent
Consent was received to publish both the clinical history data and the images from the patient.
Footnotes
Competing Interests: The authors have no conflicts of interest to report.
References
- 1.Goel S, Kumar A, Gamanagatti S, Gupta A. Spontaneous resolution of post-traumatic pulmonary artery pseudoaneurysm: report of two cases. Lung India. 2013;30(3):203–205. doi: 10.4103/0970-2113.116262. PMID: 24049255; PMCID: PMC3775200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sbano H., Mitchell A., Ind P., Jackson J. Peripheral pulmonary artery pseudoaneurysms and massive hemoptysis. Am J Roentgenol. 2005;184(4):1253–1259. doi: 10.2214/ajr.184.4.01841253. [DOI] [PubMed] [Google Scholar]
- 3.Shnayderman D, Baginski S, Lea W, Erickson S. Huge traumatic pulmonary artery pseudoaneurysm. Radiol Case Rep. 2017;12(3):467–471. doi: 10.1016/j.radcr.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivera PA, Dattilo JB. Pseudoaneurysm. StatPearls [Internet] 2022 https://www.ncbi.nlm.nih.gov/books/NBK542244/ [Updated 2021 Mar 3] Available from. [PubMed] [Google Scholar]
- 5.Quartey B, Jessie E. Pulmonary artery and vein pseudoaneurysm after gunshot wound to the chest. J Emerg Trauma Shock. 2011;4:313–316. doi: 10.4103/0974-2700.82235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen E., Silva C., Seely J., Chong S., Lee K., Müller N. Pulmonary artery aneurysms and pseudoaneurysms in adults: findings at CT and radiography. Am J Roentgenol. 2007;188(2):W126–W134. doi: 10.2214/ajr.05.1652. [DOI] [PubMed] [Google Scholar]
- 7.Donaldson B &, Nonga Bernadette. Traumatic pseudoaneurysm of the pulmonary artery: case report and review of the literature. Am Surg. 2022;68:414–416. [PubMed] [Google Scholar]
- 8.Abbas AE. Traumatic injury of the pulmonary artery: Transection, rupture, pseudoaneurysm, or dissection? Sometimes semantics do matter. J Thorac Cardiovasc Surg. 2016;152(5):1437–1438. doi: 10.1016/j.jtcvs.2016.07.019. Epub 2016 Jul 28. PMID: 27575243. [DOI] [PubMed] [Google Scholar]
- 9.Arnalich Jiménez M., Ruiz Cobos M., Casanova Espinosa Á., de Santiago Delgado E., Hoyos Vázquez N. Fístula arteriovenosa pulmonar. Rev Patol Respiratoria. 2012;15(1):33–35. [Google Scholar]
- 10.Manganas C, Iliopoulos J, Pang L, Grant PW. Traumatic pulmonary arteriovenous malformation presenting with massive hemoptysis 30 years after penetrating chest injury. Ann Thorac Surg. 2003;76(3):942–944. doi: 10.1016/s0003-4975(03)00527-7. PMID: 12963241. [DOI] [PubMed] [Google Scholar]
- 11.Swanson KL, Prakash UB, Stanson AW. Pulmonary arteriovenous fistulas: Mayo Clinic experience, 1982-1997. Mayo Clin Proc. 1999;74(7):671–680. doi: 10.4065/74.7.671. PMID: 10405695. [DOI] [PubMed] [Google Scholar]
- 12.Goodenberger D.M., Chakinala M. Fishman's Pulmonary Diseases and Disorders. 5th Ed. McGraw Hill; 2015. Pulmonary arteriovenous malformations.https://accessmedicine.mhmedical.com/content.aspx?bookid=1344§ionid=81193071 [Google Scholar]
- 13.Jiménez Varela, Isabel, Arias Gutiérrez, Ernesto Las fístulas arteriovenosas traumáticas. Salus. 2017;21(1):26–29. https://www.redalyc.org/articulo.oa?id=375952385006 [fecha de Consulta 18 de Abril de 2022]. ISSN: 1316-7138. Disponible en. [Google Scholar]
- 14.Raptis DA, Short R, Robb C, Marlow J, Naeem M, McWilliams S, et al. CT Appearance of Pulmonary Arteriovenous Malformations and Mimics. Radiographics. 2022;42(1):56–68. doi: 10.1148/rg.210076. PMID: 34990315. [DOI] [PubMed] [Google Scholar]
- 15.Torralbo J., Bernaldez C., Pérez J. Fístula arteriovenosa pulmonar. A propósito de un caso. Rev Española Patol Torácica. 2021;33(1):62–63. [Google Scholar]