Abstract
Nontypeable Haemophilus influenzae (NTHi) is a common cause of otitis media and respiratory tract infections. Outer membrane proteins (OMP) and lipooligosaccharide (LOS) are major surface antigens of NTHi and potential vaccine candidates. De-O-acylated LOS (dLOS) or oligosaccharide (OS) was coupled to total OMP to form dLOS-OMP and OS-OMP conjugates, while a dLOS-tetanus toxoid (TT) was synthesized for comparison. These conjugates were evaluated in mice and rabbits for immunogenicity. dLOS-OMP elicited a better boostable antibody response against LOS than did dLOS-TT, while OS-OMP was not immunogenic. Formulation of the conjugates with Ribi adjuvant significantly enhanced the immunogenicity of dLOS-OMP and dLOS-TT but not that of OS-OMP. In addition, rabbit antisera elicited by dLOS-OMP but not dLOS-TT (or OMP alone) demonstrated bactericidal activity against 40% of the NTHi strains tested. These results indicate that dLOS is a better derivative of LOS than OS and that OMP is a good carrier for NTHi LOS-based conjugate vaccines.
Nontypeable Haemophilus influenzae (NTHi) is a common cause of otitis media (OM) and respiratory tract infections (6, 28). Unlike H. influenzae type b, for which a highly successful vaccine is available, there is no vaccine against NTHi-induced diseases. Efforts to develop NTHi vaccines have been focused on surface antigens such as outer membrane proteins (OMP), pili/fimbriae, and lipooligosaccharide (LOS) (3, 5, 9, 12, 27, 32). These antigens are believed to play an important role in the interaction of the bacteria with the hosts in vivo (23). OMP and LOS are two major surface antigens that are considered to be potential vaccine candidates because they induced bactericidal antibodies in humans (4, 8) and conferred protection against experimental NTHi OM in animals (12, 20), although both antigens showed antigenic variation among NTHi strains (26, 29).
Previously, two LOS-based protein conjugates were synthesized in our laboratory (15). The conjugates elicited anti-LOS antibodies with bactericidal activity against homologous strains and a large percentage of heterologous strains and conferred protection against experimental NTHi OM in chinchillas. To further improve the immunogenicity and biological activity of the LOS-based conjugate, total OMP were selected as an alternative carrier to explore whether a conjugate with two different surface components from NTHi would serve as a vaccine candidate offering broader and better protection against NTHi infections than either LOS or OMP alone. To investigate the feasibility of such an approach, two different modified LOSs, de-O-acylated LOS (dLOS) and oligosaccharide (OS), were used to covalently couple to the OMP to form dLOS-OMP and OS-OMP. As a control, dLOS-tetanus toxoid (TT) was also synthesized, and the immunological properties of these conjugates were investigated in vitro and in animals.
Purification and characterization of OS, dLOS, and OMP from NTHi.
The conditions for the growth of strain 9274 were described previously (15). LOS was purified from strain 9274 by a modified phenol-water extraction method (14). Two approaches were used for the detoxification of the LOS which was hydrolyzed with acetic acid to produce OS (36) and with hydrazine to produce dLOS (16). The yield was approximately 50% for OS or 60% for dLOS. The purity of dLOS and OS was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by silver staining (33). There was no detectable LOS in 5 μg of dLOS or OS loaded on the gels, indicating that the residual LOS in dLOS or OS preparations was less than 1% compared with that of the LOS standard.
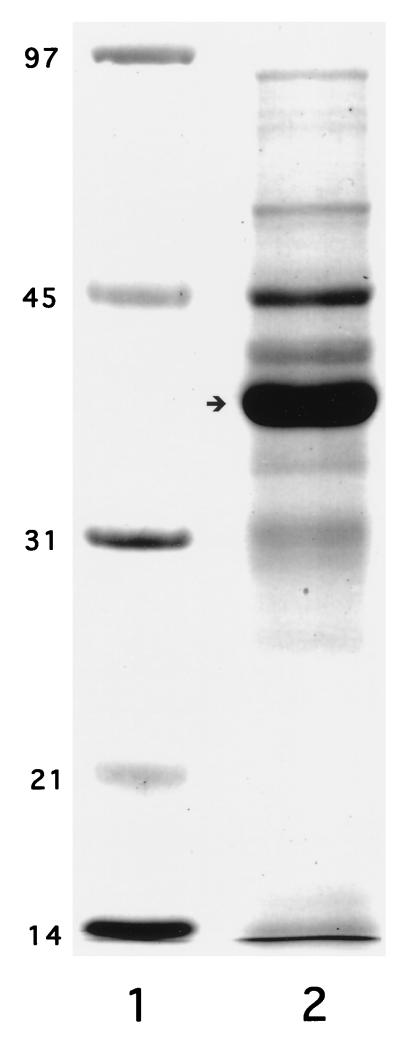
A published method (7) was used for the purification of OMP with modifications. Briefly, strain 12 was grown (15), suspended in 0.1 M Tris buffer (pH 8.5) containing 0.2 mM EDTA (TE), sheared with a blender for 10 min, sonicated with a Labsonic 1000 (B. Braun Biotech Inc., Allentown, Pa.) under conditions of circle for 0.3 s and output 100 for 10 min, and then centrifuged at 120,000 × g at 4°C for 3 h. The resulting pellets were dissolved in TE buffer, incubated at 37°C for 10 min, and purified by a Sephadex G-50 column (1.6 by 85 cm) eluted with 0.02 M Tris buffer (pH 8.5) containing 2 mM EDTA, 1% Na-deoxycholate, and 0.01% NaN3. A peak around the void volume was pooled and designated total OMP (or OMP). The yield of OMP preparation was 0.1 to 0.3% of the wet cell mass. The protein profile of OMP by SDS-PAGE is typical for gram-negative bacteria composed of approximately 20 proteins, 4 to 6 of which are major components (Fig. 1) (24). A major band with an apparent molecular mass of 37 kDa corresponding to P2 or porin (17) accounts for approximately 65% by density. The residual LOS in OMP was 1.4% (wt/wt) by SDS-PAGE and silver staining analysis.
FIG. 1.
Coomassie blue-stained 12% gel of OMP from NTHi strain 12 after SDS-PAGE. Lane 1, molecular weight markers (in thousands); lane 2, OMP (22 μg). Arrow indicates a molecular mass of 37 kDa.
Synthesis and characterization of conjugates.
A method (13) for synthesizing OS-TT was tried first, but OMP was precipitated under acid conditions. Therefore, a method described by Verheul et al. (35) was adopted with modifications. Briefly, OS or dLOS (10 mg/ml) was coupled to cystamine by using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide and sulfo-NHS at pH 4.8. The modified OS or dLOS was incubated in 0.1 M Na2HPO4 buffer (pH 8.1) containing 0.2 M dithiothreitol to generate free SH groups. After the bromoacetylation of OMP or TT with N-succinimidyl bromoacetae, the resulting OMP or TT was further conjugated to OS-SH or dLOS-SH in a ratio of 1 to 5 (wt/wt). After each reaction, three conjugates were synthesized and referred to as OS-OMP, dLOS-OMP, or dLOS-TT.
The compositions and yields of the conjugates were analyzed (Table 1). The weight ratios of OS or dLOS to OMP and dLOS to TT were 0.12, 0.58, and 1.6, respectively. The yields calculated from the modified OS or dLOS were 0.6, 2.1, and 12.6% for OS-OMP, dLOS-OMP, and dLOS-TT. The yield for OMP was approximately 50%. To determine the antigenicity of the conjugates in vitro, a double immunodiffusion assay was used (Fig. 2). dLOS-OMP, dLOS-TT, and LOS formed identical precipitation lines with two monoclonal antibodies against LOS (34), indicating that the conjugates retained the epitopes on LOS. OS and OS-OMP did not form precipitation lines.
TABLE 1.
Composition and yield of conjugates
| Conjugate | Amount (μg/ml)
|
Wt ratio of OS or dLOS to protein | Yielda (%) | |
|---|---|---|---|---|
| OS or dLOS | Protein | |||
| OS-OMP | 36 | 303 | 0.12 | 0.6 |
| dLOS-OMP | 132 | 228 | 0.58 | 2.1 |
| dLOS-TT | 580 | 370 | 1.6 | 12.6 |
Based on the starting amounts of modified OS or dLOS contained in conjugates as measured by the phenol-sulfuric acid method.
FIG. 2.
Double immunodiffusion assay. The central wells contain mouse monoclonal antibodies against 9274 LOS (34). (A) 6245B4; (B) 6347C11. Wells: 1, LOS (1 mg/ml); 2, dLOS (1 mg/ml); 3, dLOS-TT (0.29 mg/ml) (carbohydrate content); 4, TT (0.37 mg/ml); 5, dLOS-OMP (carbohydrate content) (0.132 mg/ml); 6, OMP (0.228 mg/ml). Each well was loaded with a 10-μl sample.
Immunization and antibody production.
Female mice (NIH/Swiss), 5-week-old (10 mice per group), were subcutaneously injected with 5 μg of each conjugate (carbohydrate) alone or with Ribi-700 adjuvant. The mixtures of 5 μg of dLOS and 8 μg of OMP (or 5 μg of TT), designated dLOS + OMP or dLOS + TT, were used as controls. The mice were given three injections at 14-day intervals and bled 14 days after the first injection and 7 days after the second and third injections. Female New Zealand White rabbits weighing 2 to 3 kg (two per group) were given a total of two subcutaneous and intramuscular injections at 28-day intervals with 50 μg of each conjugate alone or with the adjuvant and were bled before and 14 days after each injection. The mixtures of 50 μg of dLOS and 80 μg of OMP or 50 μg of TT were used as controls. Antibody levels in serum were detected by an enzyme-linked immunosorbent assay (ELISA) and expressed as ELISA units (15) or titers (OMP).
In mice, the levels of anti-LOS antibodies elicited by OS-OMP did not increase after three injections (Table 2). However, dLOS-OMP and dLOS-TT elicited significant amounts of LOS antibodies, especially for immunoglobulin G (IgG). There was a 139-fold increase of anti-LOS IgG elicited by dLOS-OMP and a 21-fold increase of anti-LOS IgG elicited by dLOS-TT after three injections. dLOS-OMP elicited higher levels of LOS antibodies than dLOS-TT (P < 0.01). Formulation of the conjugates with Ribi adjuvant enhanced the antibody response in both dLOS-OMP and dLOS-TT but not in the OS-OMP group. Since OS-OMP was not immunogenic in mice, only the other two conjugates were further tested for their immunogenicity in rabbits (Table 3). dLOS-OMP elicited a 115-fold increase of anti-LOS IgG, while dLOS-TT elicited a 27-fold increase after two injections. Formulation of both conjugates with Ribi adjuvant further enhanced the antibody response in rabbits.
TABLE 2.
Murine antibody response to NTHi 9274 LOS elicited by conjugates
| Immunogen | Injection no. | GM (±SD range) ELISA unitsa for:
|
|
|---|---|---|---|
| IgG | IgM | ||
| OS-OMP | 1 | 2 (1–4) | 1 (1–2) |
| 2 | 2 (1–3) | 2 (1–5) | |
| 3 | 1 (1–2) | 1 (1–2) | |
| OS-OMP + adjuvant | 1 | 2 (1–3) | 2 (1–5)+ |
| 2 | 3 (2–7) | 8 (6–12)++ | |
| 3 | 3 (2–5) | 7 (4–12) | |
| dLOS-OMP | 1 | 6 (3–10)+ | 6 (3–10)+ |
| 2 | 269 (60–1,202)++ | 59 (23–148)++ | |
| 3 | 417 (145–1,202) | 16 (6–40) | |
| dLOS-OMP + adjuvant | 1 | 42 (19–91)+ | 27 (10–76)+ |
| 2 | 912 (214–3,891)++ | 132 (38–475)++ | |
| 3 | 5,248 (1,514–18,197) | 47 (14–155) | |
| dLOS + OMP | 1 | 2 (1–3)+ | 3 (1–6) |
| 2 | 7 (3–16)++ | 7 (5–12) | |
| 3 | 5 (2–11) | 8 (4–15) | |
| dLOS-TT | 1 | 3 (1–5)+ | 1 (1–2)+ |
| 2 | 16 (4–71)++ | 9 (4–22)++ | |
| 3 | 63 (16–246) | 3 (1–5) | |
| dLOS-TT + adjuvant | 1 | 275 (123–617)+ | 52 (24–115)+ |
| 2 | 1,122 (447–2,818)++ | 141 (65–309)++ | |
| 3 | 3,020 (1,072–8,511) | 178 (85–372) | |
| dLOS + TT | 1 | 1 (1–2) | 1 (1–2) |
| 2 | 2 (1–4) | 2 (1–4) | |
| 3 | 3 (1–14) | 2 (1–3) | |
Based on a reference serum against strain 9274 (2,400 or 800 U for IgG or IgM) and 9274 LOS as a coating antigen (10 μg/ml). Presera were ≤3 U for IgG and IgM. Symbols: + versus ++, P < 0.01. GM, geometric mean.
TABLE 3.
Rabbit antibody response to NTHi 9274 LOS elicited by conjugates
| Immunogen | Injection no. | GM (range) of ELISA unitsa for:
|
|
|---|---|---|---|
| IgG | IgM | ||
| dLOS-OMP | 1 | 4 (1–27) | 3 (1–9) |
| 2 | 115 (81–243) | 38 (27–81) | |
| dLOS-OMP + adjuvant | 1 | 9 (1–81) | 2 (1–3) |
| 2 | 246 (81–729) | 141 (81–243) | |
| dLOS + OMP | 1 | 2 (1–3) | 1 |
| 2 | 5 (3–9) | 1 | |
| dLOS-TT | 1 | 1 (1–3) | 2 (1–3) |
| 2 | 27 | 5 (3–9) | |
| dLOS-TT + adjuvant | 1 | 5 (3–9) | 2 (1–3) |
| 2 | 141 (81–243) | 81 (27–243) | |
| dLOS + TT | 1 | 1 | 1 (0.3–1) |
| 2 | 2 (1–3) | 1 | |
Based on a reference serum against strain 9274 (720 or 25 U for IgG or IgM) and strain 9274 LOS as a coating antigen. Presera were ≤1 U for IgG or IgM. GM, geometric mean.
All conjugates elicited significant anti-protein IgG with booster responses in mice (Table 4) and rabbits (Table 5) after two or three injections. Formulation of the conjugates with Ribi adjuvant enhanced their IgG antibody responses. Nonconjugated OMP and TT elicited higher IgG antibody levels than those of the conjugates in rabbits but not in mice.
TABLE 4.
Murine antibody response to OMP or TT elicited by conjugates
| Immunogen | Injection no. | GM (±SD range) ELISA unitsa for:
|
|
|---|---|---|---|
| IgG | IgM | ||
| OS-OMP | 1 | 19 (9–41)+ | 2 (1–3)+ |
| 2 | 178 (43–741)++ | 4 (2–6)++ | |
| 3 | 316 (115–891) | 3 (2–5) | |
| OS-OMP + adjuvant | 1 | 37 (14–102)+ | 2 (2–4)+ |
| 2 | 1,698 (398–7,244)++ | 20 (12–33)++ | |
| 3 | 3,467 (1,349–8,913) | 16 (9–28) | |
| dLOS-OMP | 1 | 17 (10–31)+ | 1 (1–2)+ |
| 2 | 912 (407–2,042)++ | 59 (35–100)++ | |
| 3 | 1,000 (631–1,585) | 13 (5–36) | |
| dLOS-OMP + adjuvant | 1 | 65 (31–135)+ | 27 (16–46)+ |
| 2 | 3,162 (1,288–7,763)++ | 72 (51–102)++ | |
| 3 | 3,388 (1,995–5,754) | 53 (30–93) | |
| dLOS + OMP | 1 | 16 (9–28)+ | 3 (2–4)* |
| 2 | 468 (214–1,023)++ | 10 (2–62)** | |
| 3 | 1,259 (575–2,754) | 8 (1–59) | |
| dLOS-TT | 1 | 2 (1–6)+ | 1 (1–2) |
| 2 | 30 (8–110) | 2 (1–3) | |
| 3 | 275 (123–617)++ | 3 (1–7) | |
| dLOS-TT + adjuvant | 1 | 18 (4–83)+ | 8 (6–11)+ |
| 2 | 912 (110–7,586)++ | 24 (9–63)++ | |
| 3 | 5,248 (3,311–8,318) | 27 (12–62) | |
| dLOS + TT | 1 | 7 (4–15)+ | 2 (1–4) |
| 2 | 81 (39–170)++ | 2 (1–4) | |
| 3 | 178 (50–631) | 5 (12–20) | |
Based on a reference serum against OMP (2,430 or 90 U for IgG or IgM) or TT (810 or 30 U for IgG or IgM). OMP (10 μg/ml) or TT (5 μg/ml) was used as a coating antigen. Presera were ≤3 U for each Ig. Symbols: ∗ versus ∗∗, P < 0.05; + versus ++, P < 0.01. GM, geometric mean.
TABLE 5.
Rabbit antibody response to TT or OMP elicited by conjugates
| Immunogen | Injection no. | GM (range) ELISA unitsa or titersb for:
|
|
|---|---|---|---|
| IgG | IgM | ||
| dLOS-TT | 1 | 1 | 1 (0.3–1) |
| 2 | 81 (27–243) | 6 (0.3–1) | |
| dLOS-TT + adjuvant | 1 | 2 (1–3) | 2 (1–3) |
| 2 | 427 (243–729) | 2 (1–3) | |
| dLOS + TT | 1 | 13 (3–27) | 3 |
| 2 | 246 (81–729) | 16 (9–27) | |
| dLOS-OMP | 1 | 43 (30–90) | 44 (30–90) |
| 2 | 540 (270–810) | 62 (30–90) | |
| dLOS-OMP + adjuvant | 1 | 30 | 30 |
| 2 | 1,260 (810–2,430) | 52 (30–90) | |
| dLOS + OMP | 1 | 269 (90–810) | 52 (30–90) |
| 2 | 1,413 (2,430–7,290) | 52 (30–90) | |
Based on a reference serum against TT (720 or 80 U for IgG or IgM) and TT as a coating antigen. Presera were ≤1 U for IgG or IgM.
Due to the high OMP background of rabbit presera, titers are expressed a dilution fold higher than those of presera. OMP was used as a coating antigen. GM, geometric mean.
Bactericidal activity (15).
Mouse antisera elicited by the conjugates were not bactericidal to strain 9274. Only one of four rabbit antisera to dLOS-TT showed a low level of bactericidal activity against strain 9274 at a titer of 1:2. However, all four rabbit antisera to dLOS-OMP showed bactericidal activities against strain 9274 at titers of 1:2 to 1:64. Table 6 shows a comparison between bactericidal activities and levels of antibodies to LOS and OMP among six rabbit antisera elicited by dLOS-OMP or the mixture of dLOS and OMP. A linear regression analysis showed a correlation between LOS IgG (not IgM) levels and bactericidal titers against strain 9274 (r = 0.98, P < 0.001) or strain 12 (r = 0.81, P = 0.048) and no correlation between OMP IgG or IgM levels and bactericidal titers against strain 9274 or strain 12 (P > 0.3) among the rabbit antisera.
TABLE 6.
Correlation between levels of antibodies to conjugates and titers of bactericidal activities
| Antiseruma | Rabbit no. | Units of anti-LOS IgG | Titers of anti-OMP IgG | Bactericidal titerb
|
|
|---|---|---|---|---|---|
| Strain 9274 (dLOS) | Strain 12 (OMP) | ||||
| dLOS-OMP | 1 | 81 | 270 | 1:2 | <1:2 |
| 2 | 243 | 810 | 1:8 | 1:32 | |
| dLOS-OMP + adjuvant | 3 | 81 | 810 | 1:2 | <1:2 |
| 4 | 729 | 2,430 | 1:64 | 1:128 | |
| dLOS + OMP | 5 | 9 | 2,430 | <1:2 | 1:16 |
| 6 | 3 | 7,290 | <1:2 | 1:64 | |
Correlation between levels of serum anti-LOS IgG and bactericidal titers to strain 9274 was significant (P < 0.01).
Highest dilution of antiserum killing >50% of bacteria compared with preserum-containing wells. Strain 9274 is the source of dLOS, while strain 12 is the source of OMP.
The bactericidal activities of the rabbit antisera (no. 2, 4, and 6) were further assayed with 20 strains from the United States and Japan (Table 7). The rabbit antisera elicited by dLOS-OMP alone or with adjuvant demonstrated bactericidal activities to 8 of 20 strains (40%), while the antiserum elicited by the mixture of dLOS and OMP or OMP with Ribi adjuvant showed bactericidal activities to 1 of 20 strains (5%) and 3 of 20 strains (15%), respectively.
TABLE 7.
Bactericidal activities of rabbit antisera elicited by dLOS-OMP or OMP
| Straina | Bactericidal titer
|
|||
|---|---|---|---|---|
| dLOS-OMP (no. 2) | dLOS-OMP with Ribi (no. 4) | OMP + dLOS (no. 6) | OMP with Ribi | |
| F3 | —b | — | — | — |
| F4 | — | — | — | — |
| F5 | — | — | — | — |
| F7 | 1:8 | 1:16 | — | — |
| F16HN | — | — | — | — |
| F25 | — | — | — | — |
| 10A | — | — | — | — |
| 16A | — | — | — | — |
| 18A | — | — | — | — |
| 22HA | 1:8 | 1:32 | — | 1:16 |
| 23 | 1:64 | 1:32 | — | 1:8 |
| 25 | — | — | — | — |
| 27 | 1:4 | 1:16 | — | — |
| 31 | — | — | — | — |
| 41 | 1:4 | 1:8 | — | — |
| 45 | — | — | — | — |
| 46 | — | — | — | — |
| 47 | 1:16 | 1:32 | 1:4 | 1:8 |
| 51 | 1:8 | 1:8 | — | — |
| 55 | 1:4 | 1:16 | — | — |
| Positive rate | 8/20 (40%) | 8/20 (40%) | 1/20 (5%) | 3/20 (15%) |
Twenty clinical isolates from the United States (F3 through 22HA) and Japan (23 through 55).
—, <1:4.
Summary.
The aim of this study was to investigate the effects of OMP as an alternative carrier of immunological properties of LOS-based conjugates. By the method of Verheul et al. (35), in which the reaction pH ranged from 6 to 8, dLOS and OS were coupled to OMP without precipitation. Although the yields for both saccharides were low, more dLOS than OS was bound to OMP. Immunological analysis revealed that OS-OMP was not immunogenic in mice even with Ribi adjuvant, consistent with the observation that NTHi OS-CRM197 (a nontoxic mutant of diphtheria toxin) synthesized by reductive amination was a poor immunogen in mice (10). However, a meningococcal OS-TT conjugate was immunogenic in both mice and rabbits (13). The reason for the poor immunogenicity of the NTHi OS-based conjugates in mice is unclear. Besides the low ratio of OS to OMP (Table 1), one possible interpretation is that the removal of the whole lipid A portion may result in the relaxation of the conformation of the LOS molecule as well as the loss of its adjuvant effect (18).
The immunogenicities of dLOS-OMP and dLOS-TT were compared in mice and rabbits. Both conjugates induced boostable IgG antibody responses against LOS, and the antibody levels elicited by dLOS-OMP were considerably higher than those of dLOS-TT. A possible explanation for the fact that OMP is a better carrier is its mitogenic activity for lymphocytes (22) and stimulatory effect on macrophages (1). OMP may have adjuvant effects, since it is a part of a gram-negative bacterial cell wall including a minor amount of LOS, which has a variety of biological activities (19), such as an effective immunomodulating agent and adjuvant (18, 25).
To investigate the protective capacity of the conjugate-induced antisera, a bactericidal assay was performed with NTHi clinical strains. Rabbit antisera but not mouse antisera showed bactericidal activity, consistent with our previous observations with meningococcal LOS-derived OS-TT and NTHi dLOS-protein conjugates (13, 15). However, with Moraxella catarrhalis dLOS-protein conjugates, 20 to 45% of mouse antisera showed bactericidal activity (11). Many factors may result in the lack of bactericidal antibodies generated by the conjugates in mice. These factors include bacterial strains and species, sources of complement, antibody levels and affinities, and immunization routes. The bactericidal levels elicited by dLOS-OMP varied among rabbit antisera from each group but correlated with the levels of anti-LOS IgG and not the levels of anti-OMP. Two selected antisera elicited by dLOS-OMP also demonstrated bactericidal activities against 8 of 20 NTHi strains, while the antisera elicited by the mixture of dLOS + OMP or OMP showed bactericidal activities in 1 and 3 of 20 strains, respectively. These data indicate that LOS is the major target of bactericidal antibody and that OMP and dLOS have synergetic effects on the dLOS-OMP conjugate. In addition, the adjuvant could enhance the level of bactericidal activity but could not expand the spectrum for dLOS-OMP.
In this study, dLOS-TT exhibited very little bactericidal activity and was not as immunogenic as dLOS-OMP. In contrast, a previously synthesized dLOS-TT (ADH) was very immunogenic (15), and the rabbit antisera demonstrated bactericidal activities against to 45 to 75% of the 20 strains described above, although the levels of bactericidal activities were lower than those of the rabbit sera elicited by dLOS-OMP (data not shown). The immunogenic differences between dLOS-TT and dLOS-TT (ADH) probably resulted from differences in two conjugation methods, different ratios of dLOS to TT (1.6 in dLOS-TT and 0.5 to 1 in dLOS-TT [ADH]), different coupling groups for TT (dLOS was coupled to amino groups of TT by the present method instead of carboxyl groups in dLOS-TT [ADH]), and different distances between dLOS and TT (approximately 15 Å at dLOS-TT and 20 Å at dLOS-TT [ADH]). It has been reported that, besides the size of the carbohydrates and the choice of the carrier protein (2, 21, 30, 31), the ratios of carbohydrate to protein, the use of spacers, the distance between carbohydrates and carrier proteins, and the methods of coupling have significant effects on the immunogenicity of conjugate vaccines. In conclusion, OMP is a good and alternative carrier for NTHi or other conjugate vaccines.
Acknowledgments
We thank M. A. Apicella, S. J. Barenkamp, H. Faden, and G. Mogi for providing strains.
REFERENCES
- 1.Ambrosino D M, Bolon D, Collard H, Van Etten R, Kanchana M V, Finberg R W. Effect of Haemophilus influenzae polysaccharide outer membrane protein complex conjugate vaccine on macrophages. J Immunol. 1992;149:3978–3983. [PubMed] [Google Scholar]
- 2.Anderson P W, Pichichero M E, Stein E C, Porcelli S, Betts R F, Onnuck D M, Korones D, Insel R A, Zahradnik J M, Eby R. Effect of oligosaccharide chain length, exposed terminal group, and hapten loading on the antibody response of human adults and infants to vaccines consisting of Haemophilus influenzae type b capsular antigen unterminally coupled to the diphtheria protein CRM197. J Immunol. 1989;142:2464–2468. [PubMed] [Google Scholar]
- 3.Barenkamp S J. Immunization with high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae modifies experimental otitis media in chinchillas. Infect Immun. 1996;64:1246–1251. doi: 10.1128/iai.64.4.1246-1251.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barenkamp S J, Bordor F F. Development of serum bactericidal activity following nontypeable Haemophilus influenzae acute otitis media. Pediatr Infect Dis J. 1990;9:333–339. doi: 10.1097/00006454-199005000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Brinton C C, Jr, Carter M J, Derber D B, Kar S, Kramarik J A, To A C C, To S C M, Wood S W. Design and development of pilus vaccines for Haemophilus influenzae diseases. Pediatr Infect Dis J. 1989;8:S54–S61. [PubMed] [Google Scholar]
- 6.Coffey J D, Booth H N, Martin A D. Otitis media in the practice of pediatrics. Bacteriological and clinical observations. Pediatrics. 1996;38:25–32. [PubMed] [Google Scholar]
- 7.Frasch C E. Production and control of Neisseria meningitis vaccines. In: Mizrahi A, editor. Bacterial vaccine. New York, N.Y: Alan R. Liss Inc.; 1990. p. 123. [Google Scholar]
- 8.Gnehm H E, Pelton S I, Gulati S, Rice P A. Characterization of antigens from nontypable Haemophilus influenzae recognized by human bactericidal antibodies. Role of Haemophilus outer membrane proteins. J Clin Investig. 1985;75:1645–1658. doi: 10.1172/JCI111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green B A, Farley J E, Quinn-Dey T, Deich R A, Zlotnick G W. The e (P4) membrane protein of Haemophilus influenzae biologic activity of anti-e serum and cloning and sequencing of the structural gene. Infect Immun. 1991;59:3191–3198. doi: 10.1128/iai.59.9.3191-3198.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green B A, Meredith E S, Edwards L, Jones K F. Nontypeable Haemophilus influenzae lipooligosaccharide conjugates as vaccine candidates against NTHi. Vaccine. 1994;94:125–129. [Google Scholar]
- 11.Gu X X, Chen J, Barenkamp S J, Robbins J B, Tsai C M, Lim D J, Battey J. Synthesis and characterization of lipooligosaccharide-based conjugates as vaccine candidates for Moraxella (Branhamella) catarrhalis. Infect Immun. 1998;66:1891–1897. doi: 10.1128/iai.66.5.1891-1897.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu X X, Sun J, Jin S, Barenkamp S J, Lim D J, Robbins J B, Battey J. Detoxified lipooligosaccharide from nontypeable Haemophilus influenzae conjugated to proteins confers protection against otitis media in chinchillas. Infect Immun. 1997;65:4488–4493. doi: 10.1128/iai.65.11.4488-4493.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu X X, Tsai C M. Preparation, characterization, and immunogenicity of meningococcal lipooligosaccharide-derived oligosaccharide protein conjugates. Infect Immun. 1993;61:1873–1880. doi: 10.1128/iai.61.5.1873-1880.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu X X, Tsai C M, Apicella M A, Lim D J. Quantitation and biological properties of released and cell-bound lipooligosaccharide from nontypeable Haemophilus influenzae. Infect Immun. 1995;63:4115–4120. doi: 10.1128/iai.63.10.4115-4120.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu X X, Tsai C M, Ueyama T, Barenkamp S J, Robbins J B, Lim D J. Synthesis, characterization, and immunologic properties of detoxified lipooligosaccharide from nontypeable Haemophilus influenzae conjugated to proteins. Infect Immun. 1996;64:4047–4053. doi: 10.1128/iai.64.10.4047-4053.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta R K, Szu S C, Finkelstein R A, Robbins J B. Synthesis, characterization, and some immunological properties of conjugates composed of the detoxified lipopolysaccharides of Vibro cholerae O1 serotype Inaba bound to cholera toxin. Infect Immun. 1992;60:3201–3208. doi: 10.1128/iai.60.8.3201-3208.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haase E M, Yi K, Morse G D, Murphy T F. Mapping of bactericidal epitopes on the P2 porin protein of nontypeable Haemophilus influenzae. Infect Immun. 1994;62:3712–3722. doi: 10.1128/iai.62.9.3712-3722.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadjipetrou-Kourounakis L, Moller E. Adjuvants influence the immunoglobulin subclass distribution of immune responses in vivo. Scand J Immunol. 1984;19:219–225. doi: 10.1111/j.1365-3083.1984.tb00923.x. [DOI] [PubMed] [Google Scholar]
- 19.Holst O, Ulmer A J, Brade H, Flad H-D, Rietschel E T. Minireview: biochemistry and cell biology of bacterial endotoxins. FEMS Immunol Med Microbiol. 1996;16:83–104. doi: 10.1111/j.1574-695X.1996.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 20.Karasic R B, Trumpp C E, Gnehm H E, Rice P A, Pelton S I. Modification of otitis media in chinchillas rechallenged with nontypeable Haemophilus influenzae and serological response to outer membrane antigens. J Infect Dis. 1985;151:273–279. doi: 10.1093/infdis/151.2.273. [DOI] [PubMed] [Google Scholar]
- 21.Laferriere C A, Sood R K, de Muys J M, Michon F, Jennings H J. The synthesis of Streptococcus pneumoniae polysaccharide-tetanus toxoid conjugates and the effect of chain length on immunogenicity. Vaccine. 1997;15:179–186. doi: 10.1016/s0264-410x(96)00148-x. [DOI] [PubMed] [Google Scholar]
- 22.Liu M A, Friedman A, Oliff A I, Tai J, Martinez D, Deck R R, Shieh J T, Jenkins T D, Donnelly J J, Hawe L A. A vaccine carrier derived from Neisseria meningitidis with mitogenic activity for lymphocytes. Proc Natl Acad Sci USA. 1992;89:4633–4637. doi: 10.1073/pnas.89.10.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu V C, Smith A L. Molecular mechanisms of HI pathogenicity. Antibiot Chemother. 1992;45:30–51. doi: 10.1159/000420999. [DOI] [PubMed] [Google Scholar]
- 24.Loeb M R, Zachary A L, Smith D H. Isolation and partial characterization of outer and inner membranes from encapsulated Haemophilus influenzae type b. J Bacteriol. 1981;145:596–604. doi: 10.1128/jb.145.1.596-604.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louis J A, Lambert P H. Lipopolysaccharides: from immuno-stimulation to autoimmunity. Springer Semin Immunopathol. 1979;2:215–219. [Google Scholar]
- 26.Murphy T F, Apicella M A. Antigenic heterogeneity of outer membrane proteins of nontypeable Haemophilus influenzae is a basis for a serotyping system. Infect Immun. 1985;50:15–21. doi: 10.1128/iai.50.1.15-21.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy T F, Bartos L C, Rice P A, Nelson M B, Dudas K C, Apicella M A. Identification of a 16,600-dalton outer membrane protein on nontypeable Haemophilus influenzae as a target for human serum bactericidal antibody. J Clin Investig. 1986;78:1020–1027. doi: 10.1172/JCI112656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musher D M, Kubitshck K R, Crennaan J, Baughn R E. Pneumonia and acute febrile tracheobronchitis due to H. influenzae. Annu Infect Med. 1983;99:344–350. doi: 10.7326/0003-4819-99-4-444. [DOI] [PubMed] [Google Scholar]
- 29.Patrick C C, Kimura A, Jackson M A, Hermanstorfer L, Hood A, McCracken G H, Jr, Hansen E J. Antigenic characterization of the oligosaccharide portion of the lipooligosaccharide of nontypeable Haemophilus influenzae. Infect Immun. 1987;55:2902–2911. doi: 10.1128/iai.55.12.2902-2911.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peeters C C, Tenbergen-Meekes A M, Evenberg D E, Poolman J T, Zegers B J, Rijkers G T. A comparative study of the immunogenicity of pneumococcal type 4 polysaccharide and oligosaccharide tetanus toxoid conjugates in adult mice. J Immunol. 1991;146:4308–4314. [PubMed] [Google Scholar]
- 31.Seppala I, Makela O. Antigenicity of dextran-protein conjugates in mice. Effect of molecular weight of the carbohydrate and comparison of two modes of coupling. J Immunol. 1989;143:1259–1264. [PubMed] [Google Scholar]
- 32.Sirakova T, Kolattukudy P E, Murwin D, Billy J, Leake E, Lim D, DeMaria T, Bakaletz L O. Role of fimbriae expressed by nontypeable Haemophilus influenzae in pathogenesis of and protection against otitis media and relatedness of the fimbrin subunit to outer membrane protein A. Infect Immun. 1994;62:2002–2020. doi: 10.1128/iai.62.5.2002-2020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai C M, Frasch C E, Rivera E, Hochstein H D. Measurements of lipopolysaccharide (endotoxin) in meningococcal protein and polysaccharide preparations for vaccine usage. J Biol Stand. 1989;17:249–258. doi: 10.1016/0092-1157(89)90017-6. [DOI] [PubMed] [Google Scholar]
- 34.Ueyama T, Gu X X, Tsai C M, Karpas A B, Lim D J. Identification of common lipooligosaccharide types in isolates from patients with otitis media by monoclonal antibodies against nontypeable Haemophilus influenzae 9274. Clin Diagn Lab Immunol. 1999;6:96–100. doi: 10.1128/cdli.6.1.96-100.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verheul A F M, Boons G J P H, Van der Marel G A, Van Boom J H, Jennings H J, Snippe H, Verhoef J, Hoogerhout P, Poolman J T. Minimal oligosaccharide structures required for induction of immune responses against meningococcal immunotype L1, L2, and L3,7,9 lipopolysaccharides determined by using synthetic oligosaccharide-protein conjugates. Infect Immun. 1991;59:3566–3573. doi: 10.1128/iai.59.10.3566-3573.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westphal O, Jann K. Bacterial lipopolysaccharide extraction with phenol:water and further application of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]