Abstract
Background
Coronavirus disease (COVID-19), caused by a betacoronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly evolved into a pandemic since it was first reported in December 2019. thus, SARS-CoV-2 has become a major global public health issue.
Objective
The objective of this work is to compare demographics, comorbidities, clinical symptoms, biology and imaging findings between severe and non-severe COVID-19 patients and to identify clinical and biological risk factors and biomarkers for the development of severe COVID-19 as well as predictive thresholds for severity in order to best rationalize management and decrease the morbidity and mortality caused by this condition.
Patients and methods
This is a single-center retrospective study, from June 25 to December 31, 2021, on 521 patients at the level of the unit COVID-19 of the central laboratory of the Mohammed VI University Hospital Center Oujda, then classified into two groups according to the severity of the disease.
Results
Out of a total of 521 patients, a severe group including 336 cases (64.5%) and a non-severe group with 185 cases (35.5%). Hypertension, diabetes and obesity were noted in the majority of patients. Severe COVID-19 cases had higher C-reactive protein, procalcitonin, D-dimer, ferritin, elevated white blood cell count, and lower lymphocyte count than non-severe cases with a significant difference between the two groups. The areas under the curve (AUC) for C-reactive protein, procalcitonin and D-dimer were 0.886, 0.708, and 0.736 respectively. The optimal thresholds predictive of severity were 105 mg/l for C-reactive protein, 0.13 ng/ml for procalcitonin, 7420/μl for white blood cell count, and 0.55 mg/l for D-dimer.
Conclusion
Comparison of the proportion of clinical, biological and radiological data between severe and non-severe cases of COVID-19, as well as identification of biomarkers for the development of severe form in the present study, will allow optimal streamlining of management with rapid triage of patients.
Keywords: Biomarkers, COVID-19, Risk factor, SARS-CoV-2, Severe group
1. Introduction
COVID-19 (Coronavirus Disease 2019) is the most critical health crisis of the decade with a significant rate of morbidity and mortality worldwide.1 It is an emerging anthropozoonosis-like infectious disease caused by an enveloped, non-segmented, polarity-positive RNA virus belonging to the order Nitrovirals, family Coronaviridae and subgroup Betacoronavirus. First discovered in late December 2019, in the city of Wuhan, China and named SARS-CoV-2 (Severe Acute Respiratory Syndrome CoronaVirus-2) by ICTV (International Committee On Taxonomy of Viruses) due to its phylogenetic proximity to SARS-CoV responsible for the SARS epidemic in 2003.2 The rapidity and extent of viral spread worldwide led the World Health Organization (WHO) to officially declare the SARS-CoV-19 outbreak a pandemic on March 11, 2020.3 , 4 Given the rapid spread of Covid-19, we determined that an updated case analysis could help identify the defining clinical features, concomitant comorbidities, biology and imaging findings of severe cases in our population, as well as compare the proportion of these data between the severe and non-severe group, which is useful for rapid patient triage and improved prognosis. This study aims to investigate and analyze the epidemiological, clinical, biological, and radiological characteristics and prognosis related to mild to severe infection of patients infected with 2019-nCoV during this pandemic to determine appropriate management strategies in advance.
2. Materials and methods
2.1. Participants and data sources
Data collection was retrospective and monocentric. Data were examined by accessing computerized medical records of patients hospitalized in the dedicated COVID-19 wards of the Mohammed VI University Hospital Center (CHU) of Oujda, Morocco, during the period from June 25 to December 31, 2021. Information recorded included demographics, medical history, general, respiratory, digestive and neurosensory symptoms. Laboratory results were collected including standard blood counts (white blood cells, lymphocytes), biochemical markers such as C-reactive protein, procalcitonin, ferritin, d-dimers, urea and creatinine. Additional data collected included chest CT and prognosis (favorable outcome, secondary worsening, or death). We divided our sample into two distinct populations: severe form with 336 cases, and non-severe form, with 185 cases. The criteria of severity in our study based on WHO recommendations were defined as follows: (a) the presence of respiratory symptomatology on admission made of dyspnea, polypnea or desaturation ≤90% with chest imaging signs suggestive of COVID-19 infection and/or (b) acute respiratory distress syndrome (ARDS) secondary worsening and transfer to intensive care unit (ICU) for ventilation and/or (c) death. The criteria of non-severity included general (fever, asthenia, myalgias), respiratory (cough, dyspnea, odynophagia, anosmia, agueusia) and other symptoms without signs of severe COVID-19. hemodynamic repercussions.5
2.2. Inclusion and exclusion criteria
We included in our study all patients with a positive RT-PCR result admitted to the COVID-19 unit at CHU Mohammed VI Oujda and whose records were complete and we excluded from our study patients who were not hospitalized at the CHU Mohammed VI Oujda during the defined period as well as patients whose SARS-CoV-2 infection was not confirmed.
2.3. Statistical methods
All data were analyzed by IBM SPSS (Statistical Package for the Social Sciences) version 23.0. Categorical variables were expressed as frequency and percentage and continuous variables as mean, interquartile range, or median. Multivariate analysis was performed to compare between the two groups of patients with severe and non-severe COVID-19, using a two-tailed Student T test or the Mann-Whitney test. Categorical variables were compared using the CHI-2 test, although Fisher's exact test was used when data were limited. The value of the optimal biomarker cutoffs was calculated using the Receiver Operating Characteristic curve (ROC). The relative risk and confidence interval were defined at 95%. The significance level of p < 0.05 was used to define the significance of the observed differences.
3. Results
3.1. Demographic characteristics
The median age of the patients was 64 years with a range of 17–100 years. The most represented age group in our series was 50–70 years with a percentage of 46.6%, followed by the age group over 70 years with a percentage of 31.9%. The group of patients between 20 and 50 years of age represented 20.9% of cases. While patients under 20 years of age represented only 0.6% of cases. In the severe group, the median age of patients was 66 ± 13.5 years, whereas the median age of patients in the non-severe group was 51 ± 14.6 years. Thus, the median age was significantly lower in the non-severe patients than in the severe patients (p = 0.035), suggesting that middle-aged and elderly people were more susceptible to infections, whereas young healthy adults were less susceptible. The sex ratio (M/F) was 1.76 in the severe patients and 0.36 in the non-severe patients. However, there was a male predominance in the severe group of patients (p = 0.021). The results of the demographic characteristics of our two patient groups are summarized in Table 1 .
Table 1.
Description and statistical analysis of demographic characteristics and comorbidities in severe and non-severe COVID-19 patients hospitalized at Mohammed VI University Hospital, Oujda.
| Features | Total n = 521 | Non-severe group n = 336 | Severe group n = 185 | P value |
|---|---|---|---|---|
| Demographics: | ||||
| Age: median (SD) | 64 ± 16,3 | 51 ± 14,6 | 66 ± 13,5 | 0,035 |
| Gender: N (%) | ||||
| Male | 272(52,2) | 154(45,8) | 118(63,8) | 0,021 |
| Woman | 249(47,8) | 182(54,2) | 67(36,2) | |
| Sex-ratio (M/F) | 0,84 | 1,76 | ||
| Comorbidities: N (%) | ||||
| HTA | 252(48,4) | 106(31,5) | 146(78,9) | 0,028 |
| Diabetes | 1796(37,6) | 88(26,2) | 108(58,4) | 0,005 |
| Obesity | 201(38,6) | 56(16,7) | 145(78,4) | 0,002 |
| Chronic lung disease | 67(12,8) | 35(10,4) | 32(17,3) | 0,019 |
| Cardiovascular pathology | 86(16,5) | 22(6,5) | 64(34,6) | 0,026 |
| Chronic kidney disease | 24(4,6) | 7(2,1) | 17(9,2) | 0,001 |
| Hematological disease | 15(2,9) | 10(3) | 5(2,7) | 0,148 |
| Neoplasia | 12(2,3) | 8(2,4) | 4(2,2) | 0,169 |
| Hypothyroidism | 13(2,5) | 4(1,2) | 9(4,9) | 0,384 |
| Rheumatic pathology | 15(2,9) | 7(2,1) | 8(4,3) | 0,118 |
| Dyslipidemia | 12(2,3) | 5(1,5) | 7(2,7) | 0,231 |
| Neurological pathology | 17(3,3) | 9(2,7) | 8(4,3) | 0,154 |
3.2. Comorbidities
The proportion of comorbidities in severe cases was remarkably higher than in non-severe cases. Statistical analysis showed that in both groups the prevalent comorbidity was arterial hypertension (severe cases: 78.9%, non-severe: 31.5%, p = 0.028), followed by diabetes (severe cases: 58.4%, non-severe: 26.2%, p = 0.005) and obesity (severe cases: 78.4%, non-severe: 16.7%, p = 0.002) 17.3% of severe cases had a chronic pulmonary pathology versus 10.4% with a significant difference p = 0.019. Cardiovascular pathology was found in 34.6% of patients with severe COVID-19 versus 6.5% in the non-severe group (p = 0.026). Chronic kidney disease was present in 9.2% of severe cases versus 2.1% of non-severe cases (p = 0.001). Other pathologies were included in the history of COVID-19 patients with a non-significant difference. These results are summarized in Table 1. In total, 21.1% of patients had no medical history, 33.2% had only one, 24.4% of patients had two, and 21,3% had three or more medical histories.
3.3. Clinical manifestations
Clinical symptoms are summarized in Table 2 . Dry cough (90%), asthenia (82.7%), myalgia (72.7%), fever (63.5%), anosmia-agueusia (54.7%) were the most common symptoms. Dyspnea was present in 50.5% and 75.1% of severe COVID-19 patients. Odynophagia, headache, digestive signs and arthralgia were less frequent. In all, 0.8% of patients had only one symptom, 1.5% had two symptoms, 2.7% had three symptoms and 95% had more than three symptoms.
Table 2.
Description and statistical analysis of the clinical symptoms of COVID-19 in severe and non-severe patients hospitalized at Mohammed VI University Hospital, Oujda.
| Clinical symptoms | Total n = 521 N (%) | Non-severe group n = 336 N (%) | Severe group n = 185 N (%) | P value |
|---|---|---|---|---|
| Cough | 469(90) | 304(90,5) | 165(89,2) | 0,042 |
| Fever | 331(63,5) | 189(56,2) | 142(76,7) | 0,008 |
| Dyspnea | 264(50,7) | 125(37,2) | 139(75,1) | 0,001 |
| Asthenia | 431(82,7) | 277(82,4) | 154(83,2) | 0,026 |
| Myalgias | 379(72,7) | 243(72,3) | 136(73,5) | 0,037 |
| Anosmia-Agueusia | 285(54,7) | 162(48,2) | 123(66,5) | 0,001 |
| Odynophagia | 165(31,7) | 114(33,9) | 51(27,6) | 0,081 |
| Headaches | 184(35,3) | 155(46,1) | 29(15,7) | 0,165 |
| Nausea-Vomiting | 60(11,5) | 35(10,4) | 25(13,5) | 0,179 |
| Diarrhea | 119(22,8) | 76(22,6) | 43(23,2) | 0,367 |
| Arthralgia | 12(2,3) | 5(1,5) | 7(3,8) | 0,088 |
3.4. Biological results
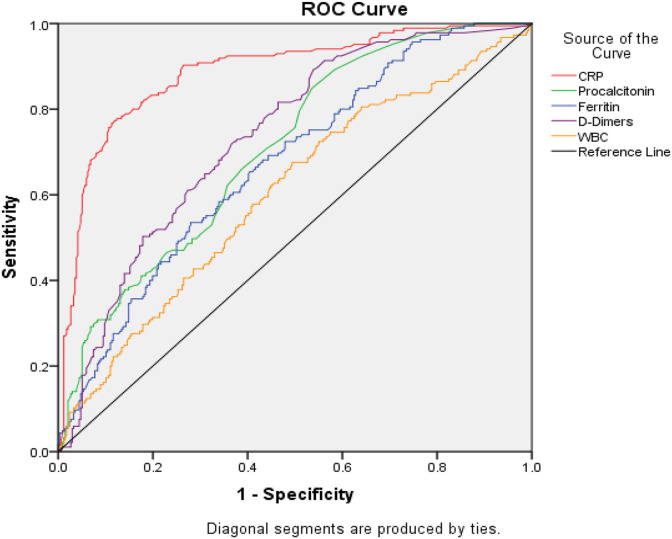
The results of the biological workups obtained are summarized in Table 3 . The median white blood cells count was higher in the severe cases (9 versus 7.9 × 10⁹/L, p = 0.001). Hyper leukocytosis was noted in 38.9% of the severe population. The median lymphocyte count was lower in severe patients than in patients with a non-severe form (0.8 versus 1.1 × 10⁹/L, p = 0.002). Lymphopenia was found in 60% of severe patients, consistent with the main feature of the viral infection. However, median C-reactive protein and procalcitonin were significantly higher in severe patients than in non-severe patients (218 versus 61 mg/L, p = 0.001) and (0.24 versus 0.11 ng/mL, p = 0.024) respectively. The median ferritin level was higher in the severe group (936 versus 519 μg/L) with p = 0.001. Logistic regression analyses identified several predictors of serious outcomes in multivariate analysis, such as C-reactive protein (OR: 2.024,[95% CI: 1.016–2.023], p: 0.001), procalcitonin (OR: 1.063, [95% CI: 1.025–1.084], p: 0.027), ferritin (OR: 1.055, [95% CI: 1.032–1.063], p: 0.042), and D-dimer (OR: 1.975, [95% CI: 1.927–2.026], p: 0.019) were significantly related to patients with severe COVID-19. To study the specificity and sensitivity of the biomarkers, we used the ROC curve (Fig. 1 ), which allowed us to specify the area under the curve of each biomarker, called AUC (Area Under the Curve). The AUC of the curve of the biomarker studied must be greater than or equal to 0.5. Thus, the closer the AUC value is to 1, the better the test studied. As described in Table 4 and Fig. 1, the calculated AUCs are presented as follows: for C-reactive protein (CRP): 0.886, procalcitonin: 0.708, D-dimer: 0.736, ferritin: 0.673 and white blood cells (WBC): 0.603. Biomarkers with AUCs less than or equal to 0.5 were excluded from the next step (lymphocytes). The optimal thresholds predictive of severity were 105 mg/l for C-reactive protein, 0.13 ng/ml for procalcitonin, 669.5 μg/l for ferritin, 7420/μl for white blood cell count, and 0.55 mg/l for D-dimer.
Table 3.
Description and statistical analysis of biological parameters in severe and non-severe COVID-19 patients hospitalized at Mohammed VI University Hospital, Oujda.
| Laboratory results | Total n = 521 Median (Interval) | Non-severe group n = 336 Median (Interval) | Severe group n = 185 Median (Interval) | P value |
|---|---|---|---|---|
| C-reactive protein (mg/l) | 102(0,19–615) | 61(0,19–269) | 218(76–615) | 0,001 |
| Procalcitonin (ng/ml) | 0,15(0,01–66) | 0,11(0,01–15) | 0,24(0,12–66) | 0,024 |
| Ferritin (μg/l) | 659(9,07–40000) | 519(9,07–1981) | 936(122–40000) | 0,001 |
| D-Dimer (mg/l) | 0,28(0,01–85,5) | 0,17(0,01–15,5) | 0,62(0,01–85,5) | 0,062 |
| Creatinine (μmol/l) | 71(26–1179) | 70,2(33,4–675) | 73,4(26,1–1179) | 0,055 |
| Urea (mmol/l) | 5,8(1–46,1) | 5,5(1,5–46,1) | 6,3(1–45,3) | 0,053 |
| Lymphocytes (.109/l) | 1(0,2–6,4) | 1,1(0,2–6,4) | 0,8(0,2–4,5) | 0,002 |
| White blood cells (.109/l) | 7,9(0,3–30,5) | 7,41(0,3–30,5) | 9(0,32-29,3) | 0,001 |
Fig. 1.
Representation of the ROC curve.
Table 4.
Logistic regression adjusted for biological parameters.
| Laboratory results | OR* | 95% CI | P value |
|---|---|---|---|
| C-reactive protein (mg/l) | 2,024 | 1,016–2,023 | 0,001 |
| Procalcitonin (ng/ml) | 1,063 | 1,025–1,084 | 0,027 |
| Ferritin (μg/l) | 1,055 | 1,032–1,063 | 0,042 |
| D-dimer (mg/l) | 1,975 | 1,927–2,026 | 0,019 |
| Creatinine (μmol/l) | 0,029 | 0,003–1,037 | 0,421 |
| Urea (mmol/l) | 0,986 | 0,405–2,396 | 0,387 |
| Lymphopenia (109/l) | 0,042 | 1,014–1,059 | 0,063 |
| White blood cells (.109/l) | 1,021 | 1,018–1,044 | 0,076 |
3.5. Radiological findings
Representative radiological findings in the two groups of patients with severe and non-severe COVID-19 are provided in Table 5 . Of 435 chest scans performed at the time of admission, 83.5% revealed abnormalities suggestive of COVID-19. The most common radiological findings on chest scan were ground-glass opacity (47%), Crazy Paving appearance (43.9%) and parenchymal condensation (42.2%). Pulmonary embolism was found in 24.9% of severe patients compared to 3.3% of non-severe patients, which 44% had high level of D-Dimers. The predominant parenchymal involvement was that of more than 75% in 35.5% of the population.
Table 5.
Description and statistical analysis of radiological abnormalities and lung involvement in severe and non-severe COVID-19 patients hospitalized at Mohammed VI University Hospital, Oujda.
| Imaging results | Total n = 521 N (%) | Non-severe group n = 336 N (%) | Severe group n = 185 N (%) | P value |
|---|---|---|---|---|
| ground-glass opacities | 245(47) | 73(21,7) | 172(93,3) | 0,001 |
| Crazy paving | 229(43,9) | 124(36,9) | 105(56,8) | 0,001 |
| Parenchymal condensation | 220(42,2) | 79(23,5) | 141(76,2) | 0,001 |
| Pulmonary embolism | 57(10,9) | 11(3,3) | 46(24,9) | 0,015 |
| Parenchymal damage: | ||||
| less than 10%. | 37(7,1) | 32(9,5) | 5(2,7) | 0,001 |
| 10–25%. | 68(13) | 48(14,3) | 20(10,8) | |
| 25–50%. | 105(20,1) | 64(19) | 41(22,2) | |
| 50–75% | 143(27,4) | 55(16,4) | 81(43,8) | |
| more than 75% of the total | 185(35,5) | 42(12,5) | 133(71,9) |
3.6. Patient prognosis
6.3% of the patients in the series died from complications of this infection. This mortality rate could be attributed to the fact that they were all hospitalized patients in a third level of health with critical conditions than most of the other patients infected in this COVID-19 pandemic.
4. Discussion
We report in this study a cohort of 521 patients with RT-PCR confirmed 2019-nCoV infection hospitalized at the Mohammed VI University Hospital Oujda and divided into two groups: COVID-19 severe and non-severe. Our study aims to determine the clinical, biological, and imaging characteristics of patients with COVID-19, assess risk factors for severe outcomes, and identify biomarkers and thresholds predictive of severity. Severe cases of COVID-19 increased with age.6 , 7 Laboratory confirmation of SARS-CoV-2 was performed at the unit COVID-19 of the central laboratory of the Mohammed VI University Hospital Center in Oujda. Qualitative detection of viral RNA was performed by the reference technique: real-time RT-PCR according to the WHO protocol, on a nasopharyngeal or oropharyngeal swab. The biological tests taken into account were the first tests performed for each patient at the CHU Mohammed VI Oujda. The measurement of biochemical and hematological markers was performed by methods verified with a very satisfactory coefficient of variation (CV) according to the SFBC (French Society of Clinical Biology) and RICOS, as well as an internal quality control (IQC), an external internal quality control (EQC) and an external quality evaluation (EQE). Our study showed that the median age was in the sixth decade, which was significantly higher in patients with severe symptoms compared to those with non-severe symptoms. This may be due to the fact that older patients are more likely to have underlying diseases that further complicate their condition. Furthermore, our results showed that men were more likely to be severely infected with SARS-CoV-2 than women, which was consistent with the results of several studies.6 , 8 , 9 This observed male predominance could be explained by the action of androgens mediated by TMPRSS2 (transmembrane serine protease 2) which may influence the process of viral infectivity.10 Thus, more intensive surveillance is required when managing elderly and male patients. Previous published studies have shown that patients likely to have a severe course are those with pre-existing medical history such as hypertension, diabetes, obesity, renal failure.7 , 11, 12, 13 This was consistent with the results of our study, where the presence of comorbidities was more frequent in the severe group of patients compared to the non-severe group with a predominance of hypertension followed by diabetes and obesity. (8 , 14 , 15 However, the evaluation of the underlying diseases is fundamental in order to decrease the complications related to SARS CoV-2 infection. Regarding clinical symptoms, they were more manifested in patients in the severe group, including cough, asthenia, fever and dyspnea than in the non-severe group.16, 17, 18 Thus, the onset of clinical symptoms may indicate the severity of the disease. In the present study, multivariate analysis showed that the mean white blood cell count in severe patients was higher than in patients with non-severe symptoms. We also found that the lymphocyte count was significantly lower in severe patients compared with non-severe patients. COVID-19-associated lymphopenia is a common finding in affected patients with significant negative prognostic value. Therefore, lymphocyte depletion could be a good indicator of disease deterioration.19 Previous studies have reached similar conclusions.6 , 20 , 21 The explosive and uncontrolled release of pro-inflammatory cytokines results in a significant increase in the biological parameters of inflammation. An increase in CRP, which is an acute-phase protein synthesized mainly by the liver and rapidly released into the blood after the onset of an inflammatory response, was significantly higher in patients with severe COVID-19.21 , 22 Wang et al. reported that C-reactive protein levels were positively associated with lung injury in COVID-19 patients and may reflect the severity of the condition in the early stages.23 To the same extent, ferritin, procalcitonin, and d-dimer were significantly higher in patients with severe COVID-19 in relation to the explosive and uncontrolled release of these proinflammatory cytokines and are risk factors for the severity of this condition evaluated in our study using logistic regression. This is consistent with other published studies.6 , 22 , 24 Therefore, it is important to compare several biological markers. The predictive threshold values for severity were obtained using ROC curves in our study. Thus, elevated C-reactive protein may be a better biomarker of COVID-19 progression, as well as D-dimer, procalcitonin and ferritin levels that may be useful in predicting the deterioration trend of COVID-19 patients. Our severity thresholds are close to those reported in similar studies, notably, the study by Shi et al., which thresholds were 7.70 × 10⁹/L for white blood cell count, 5.93 × 10⁹/L, and 75.07 mg/L for C-reactive protein25. Nevertheless, special attention should be paid to patients with an increase in these biological parameters.26 In accordance with several studies, lung CT images could manifest different suggestive abnormalities in COVID-19 patients with different time course and disease severity. In our study, the most frequent radiological signs found in severe patients were ground glass opacities, parenchymal condensation and crazy paving. The extension of the lesions was dominated by the critical form by more than 75% in the severe group. This was consistent with similar studies.27, 28, 29, 30 The pathophysiology of pulmonary embolism during SARS-CoV-2 infection is multi-causal. Returning to the clinical and biological timeline of the course of this disease, the rapid worsening of respiratory symptoms is accompanied by an extremely marked elevation of proinflammatory cytokines.24 , 31 , 32 This acute inflammatory phenomenon can affect coagulation and fibrinolysis and amplify hypercoagulability.33 This explains the increased rates of pulmonary emboli during SARS-CoV-2 infection, which were significantly higher in our study in the severe group.34 , 35 Hence the approach adopted by our hospital based on early anticoagulation.
5. Conclusion
There is a significant difference between severe and non-severe COVID-19 patients in terms of demographic characteristics, clinical manifestations, comorbidities, and biological and radiological characteristics. These factors have been associated with disease deterioration and are critical insights that must be carefully considered to reduce mortality from this condition. Furthermore, the determination of biological thresholds predictive of severity and the association of several markers correlated with the epidemiological, clinical, and radiological aspect, offers a broad-spectrum guidance to clinicians to rapidly identify patients with severe COVID-19, classify them, and transport them to specialized centers to initiate appropriate management.
Ethical approval
Access to patient data was authorized by the Mohammed VI University Hospital and approved by the head of the department. Taking into account the retrospective design of this study and the context of emerging infectious diseases, the requirement for patient consent was waived. Data anonymity was respected in our database in accordance with national and international guidelines.
Funding sources
This research did not receive any specific funding from public, commercial, or non-profit funding agencies.
Declaration of competing interest
The authors declare that they have no conflicts of interest in this case series.
Acknowledgements
We would like to thank the medical and paramedical teams of the Mohammed VI University Hospital, Oujda, for their significant involvement in the care of the patients included in our study and for their successful management during this pandemic COVID-19.
References
- 1.Gulati A., Pomeranz C., Qamar Z., et al. A comprehensive review of manifestations of novel coronaviruses in the context of deadly COVID-19 global pandemic. Am J Med Sci. 2020 Jul;360(1):5–34. doi: 10.1016/j.amjms.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu H., Stratton C.W., Tang Y.-W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020 Apr;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.https://www.who.int/news/item/29-06-2020-covidtimeline Listings of WHO’s response to COVID-19 [Internet]. [cited 2022 Jun 28]
- 4.Sohrabi C., Alsafi Z., O'Neill N., et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg Lond Engl. 2020 Apr;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . World Health Organization; 2021. Living Guidance for Clinical Management of COVID-19: Living Guidance; p. 23. November 2021 [Internet] [cited 2022 Jun 28]. Report No.: WHO/2019-nCoV/clinical/2021.2. [Google Scholar]
- 6.Guan W., Ni Z., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020 Mar 17;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goyal P., Choi J.J., Pinheiro L.C., et al. Clinical characteristics of covid-19 in New York city. N Engl J Med. 2020 Jun 11;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen G., Wu D., Guo W., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020 Apr 13;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohamed M.S., Moulin T.C., Schiöth H.B. Sex differences in COVID-19: the role of androgens in disease severity and progression. Endocrine. 2021 Jan;71(1):3–8. doi: 10.1007/s12020-020-02536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Tawfiq J.A., Hinedi K., Ghandour J., et al. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014 Jul 15;59(2):160–165. doi: 10.1093/cid/ciu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013 Sep;13(9):752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajgain K.T., Badal S., Bajgain B.B., Santana M.J. Prevalence of comorbidities among individuals with COVID-19: a rapid review of current literature. Am J Infect Control. 2021 Feb;49(2):238–246. doi: 10.1016/j.ajic.2020.06.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y. Study on evaluation of marine physical education curriculum system in the context of marine power. J Coast Res. 2020 Aug;115(sp1):259–261. doi: 10.2112/JCR-SI115-082.1. [DOI] [Google Scholar]
- 15.Israfil S.M.H., Sarker MdMR., Rashid P.T., et al. Clinical characteristics and diagnostic challenges of COVID−19: an update from the global perspective. Front Public Health. 2021 Jan 11;8 doi: 10.3389/fpubh.2020.567395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H., Shang W., Liu Q., Zhang X., Zheng M., Yue M. Clinical characteristics of 194 cases of COVID-19 in Huanggang and taian, China. Infection. 2020 Oct;48(5):687–694. doi: 10.1007/s15010-020-01440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Y., Ling Y., Bai T., et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020 Jun 1;201(11):1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X., Xu S., Yu M., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020 Jul 1;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Velavan T.P., Meyer C.G. Mild versus severe COVID-19: laboratory markers. Int J Infect Dis. 2020 Jun;95:304–307. doi: 10.1016/j.ijid.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ketfi A., Chabati O., Chemali S., et al. Pan Afr Med J; 2020 Jun 15. Profil clinique, biologique et radiologique des patients Algériens hospitalisés pour COVID-19: données préliminaires. [Internet] [cited 2021 Nov 8];35(Supp 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu K., Fang Y.-Y., Deng Y., et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020 May 5;133(9):1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 Jul 1;180(7):934. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L. C-reactive protein levels in the early stage of COVID-19. Med Maladies Infect. 2020 Jun;50(4):332–334. doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi S., Nie B., Chen X., et al. Clinical and laboratory characteristics of severe and non-severe patients with COVID-19: a retrospective cohort study in China. J Clin Lab Anal. 2021 Jan;35(1) doi: 10.1002/jcla.23692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinot M., Eyriey M., Gravier S., et al. Predictors of mortality, ICU hospitalization, and extrapulmonary complications in COVID-19 patients. Infect Dis Now. 2021 Sep 1;51(6):518–525. doi: 10.1016/j.idnow.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun P., Qie S., Liu Z., Ren J., Li K., Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020 Mar 11 doi: 10.1002/jmv.25735. 10.1002/jmv.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li K., Wu J., Wu F., et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020 Jun;55(6):327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J., Wu X., Zeng W., et al. Chest CT findings in patients with coronavirus disease 2019 and its relationship with clinical features. Invest Radiol. 2020 May;55(5):257–261. doi: 10.1097/RLI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng M.-Y., Lee E.Y.P., Yang J., et al. Imaging profile of the COVID-19 infection: radiologic findings and literature review. Radiol Cardiothorac Imaging. 2020 Feb 1;2(1) doi: 10.1148/ryct.2020200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connors J.M., Levy J.H. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemostasis. 2020;18(7):1559–1561. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the 'Cytokine Storm’ in COVID-19. J Infect. 2020 Jun 1;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson S.P., Darbousset R., Schoenwaelder S.M. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019 Feb 28;133(9):906–918. doi: 10.1182/blood-2018-11-882993. [DOI] [PubMed] [Google Scholar]
- 34.Hékimian G., Lebreton G., Bréchot N., Luyt C.-E., Schmidt M., Combes A. Severe pulmonary embolism in COVID-19 patients: a call for increased awareness. Crit Care. 2020 Jun 2;24(1):274. doi: 10.1186/s13054-020-02931-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gervaise A., Bouzad C., Peroux E., Helissey C. Acute pulmonary embolism in non-hospitalized COVID-19 patients referred to CTPA by emergency department. Eur Radiol. 2020 Nov;30(11):6170–6177. doi: 10.1007/s00330-020-06977-5. [DOI] [PMC free article] [PubMed] [Google Scholar]