Abstract
During the Coronavirus Disease 2019 (COVID-19) pandemic, skin lesions resembling those seen in pernio (chilblains) have been observed in patients with COVID-19 infection. The term “COVID toes” has been used when there is toe involvement. We describe the case of a fully vaccinated, 56-year-old woman with no prior diagnosis of COVID-19 who developed pernio-like lesions many months after being vaccinated. Her skin lesions resolved after treatment with cilostazol, suggesting that this medication may be a viable treatment for pernio in the setting of COVID-19 infection.
COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been associated with certain dermatologic manifestations (Table I ).1 The development of acral skin lesions resembling pernio is one example,1 and is dubbed “COVID toes” in this patient population. We report the case of a COVID-19-vaccinated patient with a likely case of COVID-19 who developed pernio-like skin lesions that resolved after treatment with cilostazol.
Table I.
| Dermatologic manifestation (% of 375 cases) | Description |
|---|---|
| Pseudo-chilblain (19%) | Acral edema/erythema with vesicles or pustules |
| Possibly purpuric | |
| Affecting hands and feet | |
| Other vesicular (9%) | Small monomorphic vesicles |
| May contain blood, become larger or diffuse, and affect trunk and/or limbs | |
| Urticarial (19%) | Either localized to trunk or widespread |
| Rarely palmar | |
| Maculopapular (47%) | May be perifollicular, scaling, or purpuric (punctiform or on larger areas) |
| Infiltrated papular form (pseudovesicular or resembling erythema elevatum diutinum or erythema multiforme) localized to extremities | |
| Livedo/necrosis (6%) | Varying degrees of lesions |
| Areas of truncal or acral ischemia |
Table information sourced from Casas et al., 2020.
Case Report
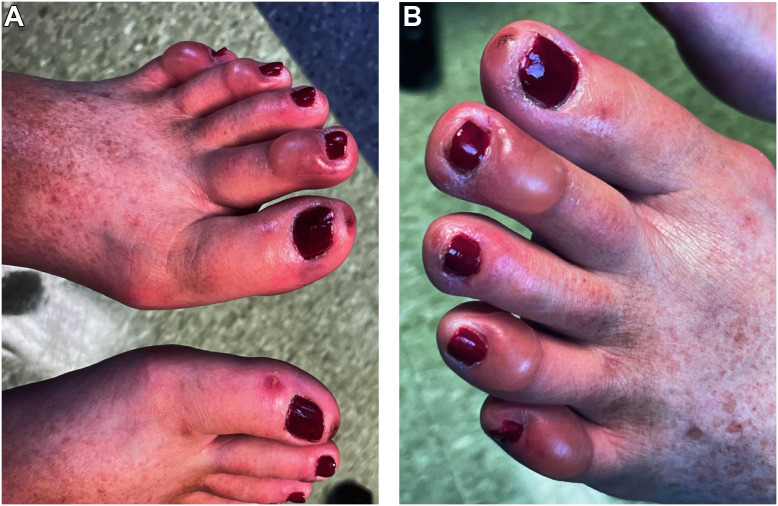
A 56-year-old female with a history of tobacco use disorder, hyperlipidemia, myocardial infarction, and coronary artery disease presented in November for swelling, redness, and a burning, pressure-like pain in the forefoot bilaterally (Fig. 1 ). She reported initial onset of symptoms 2.5 months earlier in August. She began feeling more fatigued, periodically had subjective fevers and diaphoresis, and developed several small, white, painful ulcers on the lateral aspects of her tongue. Treatment included a week course of an oral cephalosporin antibiotic followed by a 2-week course of an oral tetracycline without benefit.
Fig. 1.
(A) Bilateral feet of patient on initial presentation. These are the distal dorsal aspects of the patient's bilateral feet on initial presentation with violaceous lesions at the tip of each great toe and blisters that surround the nail beds. (B)Left foot of patient on initial presentation. This is the distal dorsal aspect of the patient's left foot on initial presentation with violaceous lesions at the tip of the great toe and blisters that surround the nail beds.
Overall, the patient was anxious from chronic pain with normal vital sings. She had no carotid or subclavian bruits. Her lungs were clear to auscultation and heart tones were normal without murmurs, gallop rhythm, or rubs. Palpation and auscultation of the abdomen was unremarkable. Both feet were very tender to palpation and with associated erythema and forefoot edema. They were neither cool nor warm to touch. There was a 1.5 cm violaceous lesion at the tip of each great toe, and blisters were present on the right first and second toes and on the left first, second, fourth, and fifth toes (Fig. 1). She had a normal pulse examination of the upper and lower extremities with absence of truncal rash, livedo reticularis, and vesicular lesions.
Rheumatologic workup, which included antinuclear antibodies, antimyeloperoxidase antibodies, antiproteinase 3 antibodies, human leukocyte antigen- (HLA-) B51, HLA-B27, C-reactive protein, and erythrocyte sedimentation rate , was negative except for C-reactive protein , which was elevated at 21.4 mg/L. There was neither anemia nor leukocytosis. SARS-CoV-2 IgG antibody test was elevated (612.5 AU/mL). She had never been diagnosed with COVID-19 and received her first and second doses of the Pfizer SARS-CoV-2 vaccine about 8 and 7 months prior, respectively. Resting and exercise ankle-brachial indices were normal.
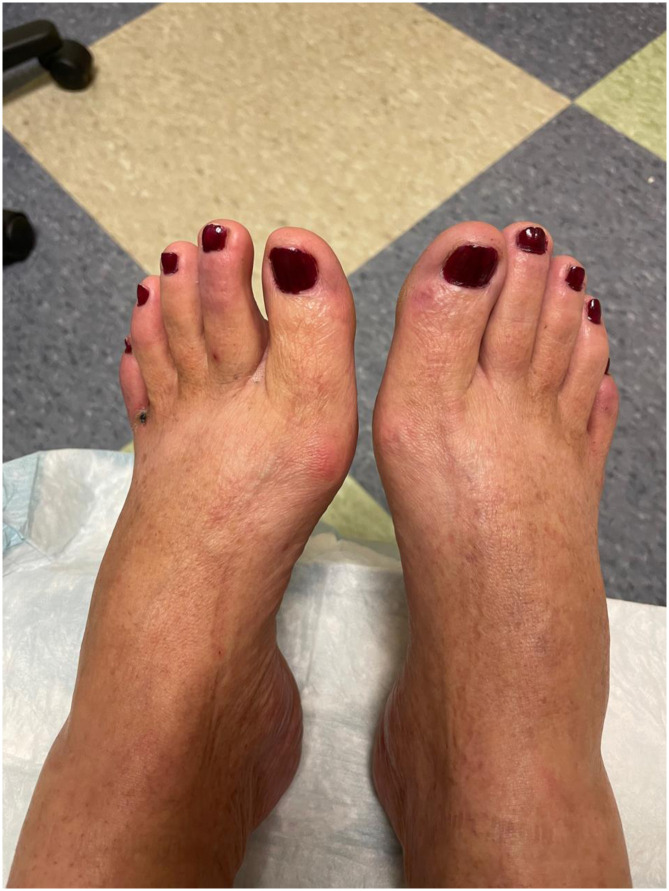
She was started on cilostazol 50 mg twice daily. She had marked improvement in her pain in 1 week and complete resolution of symptoms and healing of the toe wounds within 4 weeks of initiation of therapy. On exam at a 1-month follow-up, she had a normal physical examination (Fig. 2 ).
Fig. 2.
Bilateral feet of patient after completing cilostazol therapy. These are the dorsal aspects of the patient's bilateral feet after completing 4 weeks of treatment with cilostazol. The previously noted violaceous lesions and blisters are resolved.
Discussion
Pernio, or chilblains, typically manifests with pruritic or painful, erythematous, acral papules, or macules that last for more than 24 hr.2 It is suspected to be due to vasospasm of superficial vasculature often caused by exposure to cool temperatures, followed by an inflammatory response.2 Primary pernio is usually seen during the cooler months of late fall and early spring, whereas secondary pernio, associated with systemic disease such as lupus erythematosus is not seasonal.2 Classically, patients have a history of seasonal cold intolerance during cold, moist months with complete resolution once cold weather abates. Chronic pernio presents with a bulbous appearance to the distal aspect of the toes: something we refer to as “lightbulb toes.” We find pernio to be a clinical diagnosis and have not felt dermatopathology to alter the clinical course of these patients. We report the case of a middle-aged woman with pernio-like lesions who had previously received two doses of a COVID-19 vaccine and had no prior diagnosis of COVID-19; however, she had SARS-CoV-2 antibodies and recent symptoms that suggested possible COVID-19 infection. Her pernio-like symptoms resolved after 4 weeks of treatment with cilostazol.
COVID-19 has the capacity to adversely affect multiple organs.3 For example, it is associated with vascular manifestations, including vasculitis and thromboembolism,4 aphthous-like ulcers,5 and dermatologic lesions, some of which resemble chilblains (Table I).1 Furthermore, it is estimated that about 30% of people infected with COVID-19 experience persistent symptoms lasting up to 6 to 10 months, referred to as ‘long COVID’.3 Although our patient was never diagnosed with COVID-19 by an authorized test, her constitutional, oral, and dermatologic signs and symptoms for several months' time suggest that she may have had long COVID. Furthermore, two doses of the Pfizer vaccine are ineffective at preventing laboratory-confirmed COVID-19 infection in 8.7% of patients at 6 months after vaccination.6 Taking this information into account, and our patient's lack of improvement with antibiotics, favoring a viral etiology, it is likely that our patient had an active COVID-19 infection or long COVID at her time of presentation. Measuring serum antibody levels may be a useful tool for confirming previous infection with COVID-19 and further establishing the link between COVID toes and infection with COVID-19.2 During an outbreak of COVID-19 in a nursing home, the median SARS-CoV-2 IgG levels in the residents who were fully vaccinated but tested negative for COVID-19 during the outbreak was 268.7 BAU/mL 3–4 weeks after onset of the outbreak.7 Our patient's levels were lower than this at 87.0 BAU/mL. However, in the same study, nursing home residents who were fully vaccinated and had been diagnosed with COVID-19 during the outbreak had an average antibody level 6.6 times higher than the uninfected group.7 The residents had been vaccinated for 2–4 months when blood samples were gathered to determine antibody levels.7 Our patient was vaccinated about 7 months before determining her antibody titer, suggesting that her humoral immunity may have waned with time, making her more susceptible to infection. Intuitively, our patient's comparatively low antibody titer corroborates her reported history of a lack of known prior COVID infection, and suggests current, acute COVID-19 infection lacking sufficient time to generate a significant titer response.
The Pfizer COVID-19 vaccine has been associated with similar dermatologic manifestations as COVID-19 (Table II ), as well as rare vascular complications (Table III ).8 However, the dermatologic findings typically arise within 1 week of the vaccine and last less than 6 days (Table IV ).9 The fact that our patient did not develop cutaneous symptoms until many months after her last vaccine makes it highly unlikely that these findings were related to the vaccines.
Table II.
Reported cutaneous manifestations of SARS-CoV-2 infection and most commonly reported cutaneous manifestations of COVID-19 vaccines
| Dermatologic description in categories | Associated with COVID-19 infection | Associated with Moderna/Pfizer vaccine |
|---|---|---|
| Vascular complications with dermal evidence (livedo-like, acro-ischemia, necrosis, thromboembolization, chilblain-like eruptions, and petechial/purpuric lesions) | Yes | Yes |
| Maculopapular eruptions (morbilliform, plaques, and pityriasis rosea-like eruption) | Yes | Yes |
| Vesicular eruption (vesicle, bullous, chicken-pox-like, and herpes zoster) | Yes | Yes |
| Erythema (multiformae-like rash, Sweet's syndrome, palmar, and perifollicular) | Yes | |
| Mucosal lesions (enanthema) | Yes | |
| Injection site rash/reaction/urticarial | Yes | |
| Intertriginous or flexural erythema rash | Yes |
Table III.
| COVID-19 Pfizer vaccine | |
|---|---|
| Vascular manifestations | Rate |
| Acquired thrombotic thrombocytopenic purpura | 0.80/1 million |
| Thrombosis | 0.65–3.11/1 million |
Table information sourced from Liu et al., 2022.
Table IV.
| Dermatologic complications of Pfizer COVID-19 vaccine | 1st dose |
2nd dose |
||
|---|---|---|---|---|
| Onset (median days since dose) | Duration (median days since onset) | Onset (median days since dose) | Duration (median days since onset) | |
| Local injection site reaction | 1 | 4 | 1 | 3 |
| Urticaria | 3 | 5 | 2 | 3 |
| Morbilliform rash | 3 | 4.5 | 2 | 2.5 |
| Delayed large local reaction | 7 | 4 | 2 | 3 |
| Erythromelalgia | 7 | 5.5 | 1 | 3 |
Table information sourced from McMahon et al., 2021.
Given the evidence presented above, if we accept that our patient had an active COVID-19 infection or long COVID at the time of her presentation, we can see how our her pernio-like signs and symptoms were due to her infection rather than a coincident case of pernio or other vascular disease. Her symptoms began in August, a month not typically associated with primary pernio.2 She also had no rheumatologic laboratory evidence of a systemic disease that could cause nonseasonal, secondary pernio.2 Our patient's lack of warm, “burning feet” with no cold immersion for relief, and prompt resolution with cilostazol argues against a possible diagnosis of erythromelalgia. Most pseudo-chilblain symptoms associated with COVID-19 arise concurrent with or after the onset of nondermatologic findings (Table V ),1 which is when our patient's cutaneous manifestations began.
Table V.
Temporal relationship of dermatologic signs of COVID-19 compared to other manifestations of COVID-191a
| Onset of dermatologic manifestations | Pseudo-chilblain | Vesicular | Urticarial | Maculopapular | Livedo/necrosis | Total (n) |
|---|---|---|---|---|---|---|
| Before (%) | 7 | 15 | 4 | 5 | 5 | 22 |
| Concurrently (%) | 34 | 56 | 61 | 61 | 86 | 212 |
| After (%) | 59 | 29 | 35 | 34 | 10 | 139 |
| Total (n) | 71 | 34 | 71 | 176 | 21 | 373 |
Table information sourced from Casas et al., 2020.
Laboratory confirmation of COVID-19 antibodies does not necessarily prove cause and effect. In 7 pediatric patients who presented with pernio during the COVID-19 pandemic, SARS-CoV-2 spike protein was identified in the skin lesions despite all the patients having negative SARS-CoV-2 PCR tests.10 This supports a causal relationship between COVID-19 and pernio-like lesions.
In general, pernio is treated conservatively by minimizing exposure to the cold and with smoking cessation. Pharmacological management typically involves the use of topical corticosteroids or oral nifedipine, although data about the efficacy of these therapies is limited.11 Although pseudo-chilblain symptoms of COVID-19 tend to resolve on their own within 20 days (Table VI ),1 The use of cilostazol as a treatment for COVID toes has not previously been reported. Cilostazol can impact vessel tone, platelet activity and rheologic blood flow. In our patient, the prolonged symptoms and prompt resolution after starting cilostazol suggest its benefit to the recovery of the patient.
Table VI.
| Characteristics | Pseudo-chilblain | Vesicular | Urticarial | Maculopapular | Livedo/necrosis |
|---|---|---|---|---|---|
| Duration (days), mean ± SD | 12.7 ± 8 | 10.4 ± 9.3 | 6.8 ± 7.8 | 8.6 ± 6.8 | 9.4 ± 5.4 |
Table information sourced from Casas et al., 2020.
Conclusion
Cilostazol may be a beneficial treatment for patients with pernio-like symptoms in the setting ongoing COVID-19 infection.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
Funding sources: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Casas C.G., Catala A., Hernandez G.C., et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaladonis A., Huang S., Hsu S. COVID toes or pernio? Clin Dermatol. 2020;38:764–767. doi: 10.1016/j.clindermatol.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechner-Scott J., Levy M., Hawkes C., et al. Long COVID or post COVID-19 syndrome. Mult Scler Relat Disord. 2021;55:103268. doi: 10.1016/j.msard.2021.103268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts K.A., Colley L., Agbaedeng T.A., et al. Vascular manifestations of COVID-19 – thromboembolism and microvascular dysfunction. Front Cardiovasc Med. 2020;7:598400. doi: 10.3389/fcvm.2020.598400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandao T.B., Gueiros L.A., Melo T.S., et al. Oral lesions in patients with SARS-CoV-2 infection: could the oral cavity be a target organ? Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;131:e45–e51. doi: 10.1016/j.oooo.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas S.J., Moreira E.D., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller L., Andree M., Ostermann P.N., et al. SARS-CoV-2 infection in fully vaccinated individuals of old age strongly boosts the humoral immune response. Front Med. 2021;8:746644. doi: 10.3389/fmed.2021.746644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu R., Pan J., Zhang C., et al. Cardiovascular complications of COVID-19 vaccines. Front Cardiovasc Med. 2022;9:840929. doi: 10.3389/fcvm.2022.840929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMahon D.E., Amerson E., Rosenbach M., et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deutsch A., Blasiak R., Keyes A., et al. COVID toes: phenomenon or epiphenomenon? J Am Acad Dermatol. 2020;83:e347–e348. doi: 10.1016/j.jaad.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vano-Galvan S., Martorell A. Chilblains. CMAJ. 2021;184:67. doi: 10.1503/cmaj.110100. [DOI] [PMC free article] [PubMed] [Google Scholar]