Abstract
Purpose:
The purpose of the study was to examine the impact of a novel approach to provide diabetes specialty team care to rural patients with type 2 diabetes (T2DM) on clinical outcomes and processes of care.
Methods:
Diabetes Care Network (DCN) provides Veterans with T2DM and elevated A1C an initial 6-week period of remote self-management education and support and medication management by a centrally located team of diabetes specialists. Participants are then co-managed by remote liaisons embedded within rural primary care facilities for the remainder of the 12-month intervention. In this pre-post intervention study, 87 Veterans enrolled in DCN from two different clinical sites had baseline and 12-month post-enrollment A1C, systolic blood pressure, weight, and LDL cholesterol levels collected and compared using paired t-tests.
Results:
Participants were mostly male and white with elevated baseline A1C. Participants from both sites had significant improvement in A1C over the 12-month intervention period compared to an increase in the 12 months prior to enrollment. There were also significant improvements in LDL and systolic blood pressure at one site, with no significant change in weight at either site.
Conclusions:
DCN participants had significant improvement in A1C after not meeting similar goals previously in a robust primary care setting. A technology-enabled collaborative partnership between centrally located diabetes care teams and local liaisons is a feasible approach to enhance access to diabetes specialty care for rural populations.
T2DM affects more than 30 million adults in the United States, with more than 1.4 million new cases diagnosed annually.1 This is an even larger issue for Veterans, who are 2.5 times as likely as non-Veterans to have type 2 diabetes. Diabetes is one of the five most commonly treated conditions in the outpatient setting within the Veterans Health Administration (VA), creating an annual expenditure of $1.5 billion.2 The VA performs better on diabetes care quality measures than commercial, Medicaid, and Medicare health maintenance organizations.3 However, within the VA, 60.5% of Veterans with T2DM and hypertension still do not meet glycemic and blood pressure treatment goals.4 Nationwide, only half of patients with diabetes meet goals for A1C, and this percentage has declined over the last decade.5 Multidisciplinary diabetes specialty team care is one way to help patients reach treatment goals, and is associated with improved diabetes treatment outcomes, quality of care measures, and timely treatment intensification.6–8 One critical component of multidisciplinary diabetes care is diabetes self-management education and support, which improves glycemia, self-management behavior, psychosocial outcomes, value, and quality of care for patients with diabetes.9–12
However, not all patients can easily access diabetes care from a specialty team of providers. Most Veterans with T2DM receive care from the Patient Aligned Care Team (PACT), which is the patient-centered medical home model of primary care within the VA. Veterans who are not meeting diabetes treatment goals can be referred for consultation with a diabetes specialty care team, including an endocrinologist and diabetes care and education specialist (DCES) via a face-to-face clinic visit. Many Veterans reside in rural areas, and travel long distances to see diabetes specialty care teams located in VA medical centers in urban areas.13–15 Similar barriers to diabetes specialty care exist for non-Veteran patients in rural areas, who have limited access to diabetes self-management education and support programs16 and endocrinologists.17 Furthermore, in the face of increasing numbers of patients with T2DM in need of specialty diabetes care, shortages of both diabetes care and education specialists18 and endocrinologists19,20 will exacerbate barriers for patients in rural areas in the future. Therefore, sustainable, structured approaches to disperse team-based diabetes specialty care are needed to improve outcomes for patients who are not meeting treatment goals in the primary care setting.
The chronic care model (CCM) is one potential approach to disseminate comprehensive, proactive, patient-centered care for chronic conditions such as diabetes.21 Within the CCM, elements including community resources, the health system, self-management support, delivery system redesign, decision support, and clinical information systems work together to improve the quality of chronic disease care. These elements have been shown to improve healthcare quality and outcomes when implemented within diabetes care programs.22–25 Built within the CCM framework, Diabetes Care Network (DCN) is a new model of collaborative care between a centrally-located diabetes specialty care team and clinical liaisons embedded within rural primary care clinics of the VA Health System in Western Pennsylvania. This model of care focuses on improving outcomes for rural Veterans with complex diabetes by facilitating chronic co-management through engaged partnerships between a diabetes specialty care team located at the main VA facility and providers at rural clinics located closer to the Veteran’s home. This study aimed to describe the implementation and assess clinical and process outcomes of DCN over the first 12 months of the program.
Research Design & Methodology:
Research Design:
This study is a pre-post study evaluating the impact of a novel approach to diabetes care on clinical outcomes, including A1c, for two cohorts of Veterans with type 2 diabetes from two different clinical sites. As this study evaluates an intervention implemented in real clinical care, it is limited by a lack of control group who did not receive the intervention. However, the change in participant’s A1C had over the 12 months prior to intervention is also presented as a historical comparison.
Delivery System Redesign:
DCN is a multidisciplinary team intervention designed based on elements of the CCM (Figure 1). The diabetes care delivery system for participating sites was redesigned first to facilitate collaborative, remote management of Veterans with T2DM who were not meeting treatment targets. A data-driven, technology-based approach using the electronic medical record was developed to proactively identify Veterans with elevated A1C who were eligible for DCN. Next, to establish the DCN collaborative care model, a centrally located diabetes specialty care team, including a DCES, CRNP, and endocrinologist, built partnerships with clinical liaisons at two participating rural primary care facilities. DCN liaisons differed in their clinical skill sets, with a certified registered nurse practitioner (CRNP) at one site in Erie, PA, and two clinical pharmacy specialists (CPS) at a second site in Butler, PA. As DCN liaisons were embedded within primary care teams at rural facilities, they leveraged existing resources within the patient-centered medical home structure including nutritionists, registered nurses, home-based primary care providers, and home telehealth coordinators to implement individualized diabetes monitoring and support. Telehealth diabetes monitoring included automated transmission of home blood glucose monitoring data to telehealth coordinators when Veterans used the telehealth system. If Veterans did not use the telehealth system, glucometers were downloaded by RNs in clinics. Finally, clinical information systems were adapted to facilitate care coordination and longitudinal decision support between DCN team members and primary care providers; changes included creation of dedicated consult request forms and interfacility communication notes within the electronic medical record, as well as a secure portal for patient enrollment, care coordination, and progress monitoring.
Figure 1:
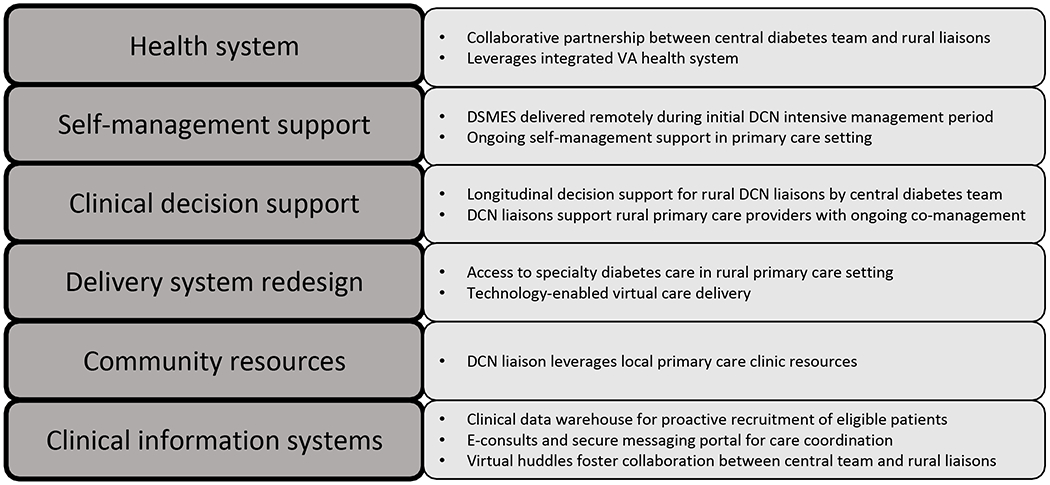
Diabetes Care Network Framework Based in CCM
Inclusion and Exclusion Criteria:
Veterans with T2DM and A1C>9% (75 mmol/mol) who received care at one of the two participating rural primary care locations were eligible. Patients with type 1 diabetes, a history of hypoglycemia unawareness, using an insulin pump or continuous glucose monitor, and those currently or recently under the care of endocrinology were excluded from this intervention.
Intervention:
Recruitment and enrollment occurred over a period of one year beginning in January of 2017. DCN liaisons identified eligible Veterans using electronic medical record data from the VA corporate data warehouse and primary care registry. Patients were enrolled via phone outreach by DCN liaisons. Patients from Erie, PA were managed by PCPs prior to enrollment, while those from Butler, PA were generally already under the care of a CPS for co-management of their type 2 diabetes.
After enrollment, patients received remote intensive management by the central DCN team for 6 weeks. An initial needs assessment, including detailed chart review and telephonic interview of each patient, focused on identifying gaps in therapy and diabetes knowledge and self-management skills.Based on the identified gaps, diabetes self-management support and support (DSMES) was provided telephonically by centrally located DCES. Veterans were educated on the short and long-term importance of diabetes treatment goals and provided with individualized strategies to achieve them. DSMES was complemented by nutrition consultation with a registered dietician when applicable if Veterans were agreeable. Concurrently, the DCN CRNP and endocrinologist optimized diabetes therapeutic regimens to target individualized A1C goals. Therapeutic optimization focused on identifying barriers to optimal medication taking and modified treatment regimens to facilitate medication taking when possible. Initial treatment recommendations were entered as electronic consultation notes into the medical record within 7 days for review by local PCPs and rural liaisons. In this initial phase, the DCN team also identified gaps in diabetes care quality measures, such as immunization, retinal examination, and foot care screening. Rural DCN liaisons then addressed gaps in care locally by scheduling tele-retinal screening, podiatry visits, and immunization in PACT clinics. Finally, all Veterans were offered home telehealth (HT) monitoring services, which allow for automated sharing of home capillary blood glucose monitoring results with PACT clinics.
After the initial 6 weeks, care was transferred to the rural DCN liaison to carry out individualized care plans developed in collaboration with the central DCN team. Patients were monitored on an ongoing basis by rural liaisons, who utilized home telehealth services or in-clinic glucometer downloads, depending on patient preference, to collect home blood glucose data. Rural and central DCN team members collaborated through weekly virtual huddles, when patient self-monitoring and laboratory data were reviewed, and therapeutic regimen recommendations were developed. Ongoing communication between Veterans, local liaisons, and the central team, allowed new issues affecting diabetes treatment such as courses of glucocorticoids, acute illness, planned, and unplanned procedures to be identified in a timely manner and treatment to be adjusted proactively. Ongoing self-management education and support was advanced by the central DCES in collaboration with the remote liaison for each patient based on individualized needs for the duration of intervention.
Outcome measures:
This study assessed the impact of DCN on diabetes outcomes, including A1C, systolic blood pressure, weight, and LDL over 12 months. Clinical data obtained during routine diabetes care was collected through chart review of enrolled Veterans by DCN team members. Patient demographics, baseline medications, laboratory values including A1C and cholesterol, and vital signs were collected at enrollment and tracked as clinically indicated during the intervention. A1C values for 6 and 12 months prior to enrollment in DCN were collected retrospectively to provide historical comparison.
Data analysis:
Outcomes for Butler and Erie cohorts were analyzed separately due to inherent differences in diabetes status and management prior to DCN intervention. Summary statistics were computed for patient demographics, A1C, and comorbidities and presented with mean, standard deviation, and frequency as applicable. For everyone, change scores in systolic blood pressure, weight, and LDL were calculated as month 12 minus baseline value. Paired t-tests were used to assess the significance of these change scores within each cohort. In the absence of a control group for this clinical initiative, the change in A1C over the 12 months prior to DCN enrollment was calculated and presented for comparison to the intervention period. A significance value of 0.05 was used for all tests. Where values were missing, patients were excluded from analysis of that outcome. Stata 16 (StataCorp LLC, College Station, TX) was used for all analyses. This study was determined to be a quality improvement initiative by the VA Pittsburgh IRB.
Results
Baseline characteristics:
In total, 101 patients were identified as eligible, and 87 patients were enrolled over 12 months (January 2017 through December 2017) and completed the 12-month program from the date of enrollment. Of the 14 patients who were eligible but did not complete the DCN program, 9 declined to participate, 3 were transferred to palliative care during the intervention period, 1 moved to another facility and 1 was lost to follow-up. Of the 87 enrolled, missing data was most common for A1C values 6 months prior to enrollment, where 26 Veterans had no A1C values within the target timeframe but was minimal for other timepoints (5 veterans did not have A1C values for 12 months prior to enrollment, and 1 Veteran did not have 12-month follow-up A1C during intervention period).
Thirty-seven Veterans were enrolled from Erie, PA and 50 from Butler, PA. Demographics were similar between the two groups (Table 1). Participants were primarily white (90.8%) males (97.7%) with a mean age 67.2 years. Mean A1C was 10.1% (87 mmol/mol) and was higher in the group recruited from Erie, PA (10.4%, 90 mmol/mol) who were primarily managed by PCPs compared to Butler, PA (9.8%, 84 mmol/mol) where CPS were involved in care at baseline. The most common comorbidities were hypertension (96.6%) and hyperlipidemia (96.6%), while 48.3% of patients had cardiovascular disease and 44.8% had nephropathy. In addition, 37.9% had a mental health diagnosis. Insulin was prescribed to 71.3% of patients, with metformin (60.9%) and sulfonylureas (36.8%) the next most prescribed medications in this cohort. Seven Veterans (8%) participated in home telehealth for remote monitoring of blood glucose and blood pressure at enrollment, and this increased to 19 (22%) at the end of the intervention period. Three (8%) patients in the Erie cohort and 5 (10%) of patients in the Butler cohort received formal nutrition consultation during the intervention; given these small numbers, sub-group analysis was not performed to assess independent effect of nutrition consultation on clinical outcomes.
Table 1:
Baseline characteristics
| Erie (n=37) | Butler (n=50) | |
|---|---|---|
| Sex-Male n (%) | 37 (100%) | 48(96%) |
| Age-Mean (SD) | 68.9 (6.4) | 65.9 (10.3) |
| Race-White n (%) | 35 (94.6%) | 44 (88.0%) |
| Comorbidities n (%) | ||
| Hypertension | 37 (100%) | 47 (94.0%) |
| Hyperlipidemia | 37 (100%) | 47 (94.0%) |
| Mental health diagnosis | 12 (32.4%) | 21 (42.0%) |
| Nephropathy | 22 (59.5%) | 17 (34.0%) |
| Coronary artery disease | 15 (40.5%) | 17 (34.0%) |
| Stroke | 7 (18.9%) | 6 (12.0%) |
| Peripheral arterial disease | 7 (18.9%) | 10 (20.0%) |
| A1C % Mean (SD) | 10.4 (1.8) | 9.8 (0.97) |
| A1C mmol/mol Mean (SD) | 90 (19.7) | 84 (10.6) |
| LDL mg/dL Mean (SD) | 86.9 (35.9) | 90.1 (37.2) |
| Weight lbs Mean (SD) | 228.5 (43.8) | 229.8 (51.4) |
| Systolic blood pressure mmHg | 134.3 (13.9) | 127.7 (17.1) |
| Mean (SD) | ||
| Medications n (%) | ||
| Insulin | 25 (67.6%) | 37 (74%) |
| Metformin | 24 (64.9%) | 29 (58.0%) |
| Sulfonylurea | 16 (43.2%) | 16 (32.0%) |
| GLP-1 receptor agonist | 1 (2.7%) | 1 (2.0%) |
| SGLT2 inhibitor | 1 (2.7%) | 0 (0%) |
Clinical outcomes:
In the Butler cohort, the mean change in A1C from baseline to 12 months during DCN was a decrease of 2.06% (22.5 mmol/mol; 95% CI 1.66,2.45, P<0.001) compared to an increase of 1.21% (13.2 mmol/mol; 95% CI 0.75, 1.67, P<0.001) in the 12 months prior to enrollment (Figure 2). In the Erie cohort, the mean change in A1C from baseline to 12 months during the intervention period was a decrease of 3.03% (33.1 mmol/mol, 95% CI 2.29, 3.76, P<0.001) compared to an increase of 1.08% (12.2 mmol/mol; 95% CI 0.20, 1.96, P=0.02). At 12 months, the Butler cohort had a significant decrease in LDL of 15.90 mg/dL (95% CI 5.96, 25.83, P=0.02), while the mean decrease in LDL in the Erie cohort was not significant (−2.56 mg/dL, 95% CI −16.96, 11.84, P=0.72). In addition, there was a significant reduction in systolic blood pressure with mean decrease of 5.9 mmHg (95% CI 0.38, 11.41, P=0.04) over the intervention period in the Butler cohort, and a mean decrease of −4.14 (95% CI −9.76, 1.49, P=0.14) in the Erie cohort which did not reach statistical significance. Weight decrease did not reach statistical significance in either cohort (Table 2).
Figure 2:
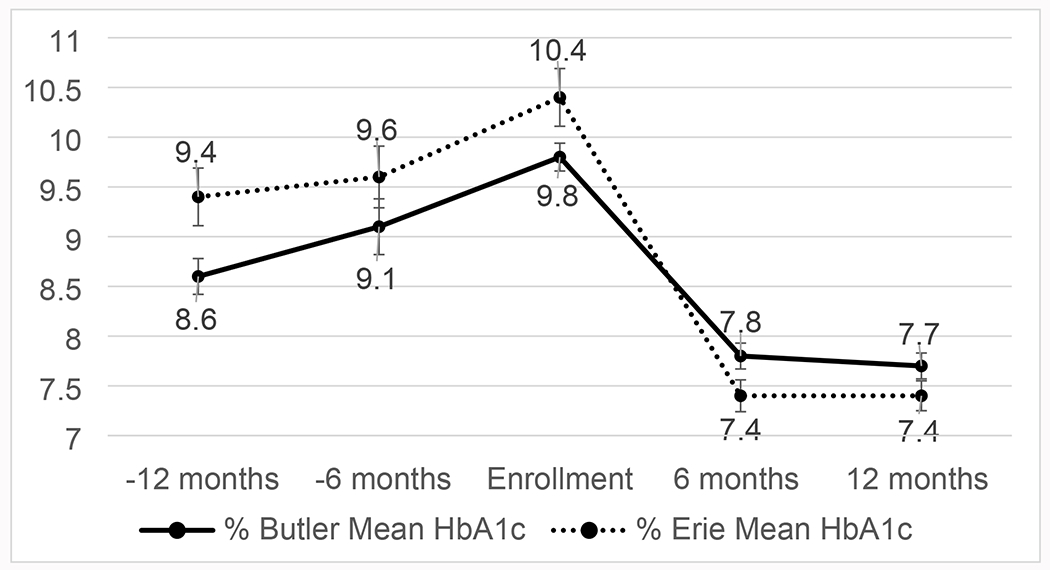
Mean A1C over time
*error bars represent standard error
Table 2:
Clinical outcomes
| Outcome | Erie (n=37) Mean (95% CI) | P-value | Butler (n=50) Mean (95% CI) | P-value |
|---|---|---|---|---|
| A1c change (%) pre-12 months to enrollment; n=81* | 1.08% (0.20, 1.96) | P=0.02 | 1.21% (0.75, 1.67) | P<0.001 |
| A1c change (%) over intervention period; n=86* | −3.03% (−3.76, −2.29) | P<0.001 | −2.06% (−2.45, −1.66) | P<0.001 |
| Weight change (lbs) over intervention period; n=87* | −1.00 (−6.1,4.1) | P=−0.69 | −0.70 (−5.09,3.69) | P=0.75 |
| LDL change (mg/dL) over intervention period; n=85* | −2.56(−16.96, 11.84) | P=0.72 | −15.90(−25.83, −5.96) | P=0.02 |
| Systolic blood pressure (mmHg) change over intervention period; n=86* | −4.14 (−9.76, 1.49) | P=0.14 | −5.90 (−11.41, −0.38) | P=0.04 |
n=number of participants with complete data included in analysis of outcome
P-value for paired t-test of change over 12 months within each cohort
Discussion
This study describes implementation and clinical outcomes of a model of remote collaborative care between a team of centrally located diabetes care specialists and care liaisons embedded within rural clinics. This model of care was designed to provide active, longitudinal support to both patients and primary care providers with guideline-based diabetes care, including DSMES and pharmacologic management. DCN targeted Veterans who would otherwise have significant geographic barriers to accessing diabetes specialty team care. Participants in DCN, who were generally complex patients with multiple comorbid conditions, had significant improvements in A1C over the intervention period. These improvements were especially notable as patients entered the DCN program with elevated A1C while receiving care in the patient-centered medical home structure of the VA, which provides comprehensive team-based primary care at baseline.
With the growth of telemedicine approaches to chronic disease management, there has been significant prior work evaluating technology-facilitated delivery of diabetes specialty team care and DSMES. Telemedicine approaches are rapidly growing as a way to expand access to diabetes specialty care for rural patients and other hard to reach populations.26 Telemedicine-based DSMES interventions are effective at improving glycemia, especially when interventions include patient-generated health data and feedback.27 In addition, diabetes telehealth interventions involving medication titration through remote teleconsultation with endocrinologists and remote monitoring of blood glucose improve A1C.28 While these programs are effective for patients who have internet access which can facilitate virtual video consultation, home blood glucose data transmission, and internet-based education and support, patients who lack internet access or have low technological literacy are unable to benefit from these approaches. Rates of internet access are significantly lower for patients who live in rural areas, as well as racial/ethnic minority and low-income households.29 About one-quarter of Veterans in the United States reside in rural areas, and during the COVID-19 pandemic Veterans in rural areas were less likely to utilize telemedicine to access care.30,31 Thus, approaches to deliver remote diabetes team care which do not rely on patient internet access are needed in order to ensure feasibility for all rural patient populations. In this context, DCN has the advantage of enabling patients to receive remote management and DSMES through telephone and local liaisons and does not require internet-based remote blood glucose monitoring (less than one-quarter of patients were enrolled in home telehealth for transmission blood glucose data at the end of the intervention). With an increasing focus on quality and growth of telehealth, integrated health systems including the VA and others around the country may have increasing interest in investing in interventions such as DCN which remotely deliver diabetes specialty support in the primary care setting. As integrated health systems continue to grow in the United States,32 approaches such as DCN will be increasingly feasible to improve outcomes for patients not meeting treatment goals.
Through an ongoing review of clinical data and active communication with liaisons in the primary care setting, another aim of the DCN model is to overcome clinical inertia in diabetes treatment. Clinical inertia is failure of providers to optimize treatment when patients are not meeting care goals, and includes lack of referral for DSMES, endocrinology consultation, or intensification of pharmacologic treatment.33 Clinical inertia affects more than half of patients with type 2 diabetes who are not meeting treatment goals.34 Addressing clinical inertia requires coordinated efforts at the patient, provider and health system levels.35 The DCN program targets clinical inertia through proactive identification and enrollment of patients who have elevated A1C in the primary care setting. In addition, DCN removes some barriers to DSMES, such as required referral and travel to appointments, which are present in traditional models of diabetes specialty care. The DCN structure allows individualized DSMES to be provided on an ongoing basis remotely and uses local resources embedded within the primary care team. The value of the DCN model lies not only in an improved access to diabetes specialty care for rural patients, but by building relationships between centrally located DCES and local liaisons to advance selfmanagement education and support. With rapid growth of novel therapeutics for diabetes management including continuous glucose monitors, insulin pumps, and new medications, primary care providers may require additional support to navigate and manage these new tools. Scalable interventions such as DCN can facilitate optimization of diabetes treatment regimens with novel therapies and ongoing support to help patients and providers overcome clinical inertia and achieve treatment goals..
Limitations
There are multiple limitations to this study. First, while glycemic data for the 12 months prior to intervention is presented as a historical control, this pre-post study lacks a control group for comparison of outcomes during the intervention period, as it was performed as part of a real-world clinical operations initiative. This limits conclusions about improvements in clinical outcomes being attributed to DCN intervention. Second, the intervention described may have limited generalizability outside of a large integrated health network such as the VA, as it requires a focus on quality of care over volume, and much of the collaborative care would not be compensated in settings with traditional fee-for-service models. Relatedly, we were not able to assess the number of DSMES hours received by each participant as these services were not billed separately, and thus could not discretely examine the specific impact of DSMES on clinical outcomes. Further, a robust unified electronic medical record system and ease of availability of telehealth tools to operationalize this approach may not be available in many non-VA settings. Finally, as is common in studies within VA, the cohort was primarily white and male. Although the demographics of our study population closely match demographics of the surrounding counties according to recent U.S. Census Bureau estimates, this may limit the generalizability of these results.36,37 If DCN were to be implemented in rural areas with more racial and ethnic diversity, the intervention may need to be modified to ensure that the needs of participants of all backgrounds are met. One potential opportunity to enhance outcomes for patients who are members of racial and ethnic minority groups may be adaptation of a peer support component into DCN structure, as peer support integrated with DSMES has been shown to improve HbA1c especially among patients who identify as Hispanic or as members of racial minority groups.38
Conclusion & Future Directions
While the benefits of multidisciplinary diabetes team care are well established, geographic, and other access barriers prevent many patients from reaping the benefits of this approach. Using the Chronic Care Model framework, DCN aims to overcome these barriers by integrating remote diabetes specialty team care, including DSMES and pharmacologic management, into the primary care environment for rural Veterans. Participants in DCN, who were already receiving comprehensive primary care, had significant improvements in glycemia and some other clinical outcomes over the intervention period. In addition, DCN expanded the reach of a centrally located diabetes specialty care team, including DCES, CRNP and endocrinologist, to rural patients without requiring internet access. While further studies with appropriate control comparisons are needed to validate the impact on clinical outcomes, this study demonstrates the potential of using modern tools, including patient data registries and virtual team huddles, to facilitate care partnerships between a centrally located team of diabetes specialists and local care providers. The DCN model is one example of the central role that DCES will play in technology-enabled multidisciplinary collaborative care, which will be foundational to the future of diabetes care within integrated health systems.
Funding:
No external funding was received to support this intervention, as this work was performed as part of a clinical operations initiative. Dr. Zupa’s time on this analysis and publication was supported by by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR001856. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. In addition, the views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Footnotes
Declaration of Conflicting Interests: The Authors declare that there is no conflict of interest.
Prior presentation: This work was presented in limited abstract form at the Association of Diabetes Care and Education Specialists Annual Conference, August 12-15, 2020and the American Diabetes Association 79th Annual Scientific Sessions, June 7-11, 2019.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020: estimates of diabetes and its burden in the United States. Accessed October 12, 2021. https://www.cdc.gov/diabetes/library/features/diabetes-stat-report.html
- 2.Federal Practitioner. Diabetes Mellitus Federal Health Data Trends. Accessed October 10, 2021.https://cdn.mdedge.com/files/s3fs-public/Document/March-2018/fdp_s20-s21.pdf
- 3.Anhang Price R, Sloss EM, Cefalu M, Farmer CM, Hussey PS. Comparing Quality of Care in Veterans Affairs and Non-Veterans Affairs Settings. J Gen Internal Med. 2018;33(10):1631–1638. doi: 10.1007/s11606-018-4433-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rehman H, Akeroyd JM, Ramsey D, et al. Facility-level variation in diabetes and blood pressure control in patients with diabetes: Findings from the Veterans Affairs national database. Clin Cardiol. 2017;40(11):1055–1060. doi: 10.1002/clc.22769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang M, Wang D, Coresh J, Selvin E. Trends in Diabetes Treatment and Control in U.S. Adults, 1999–2018. N Engl J Med. 2021;384(23):2219–2228. doi: 10.1056/NEJMsa2032271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Setji TL, Page C, Pagidipati N, Goldstein BA. Differences in Achieving Hba1C Goals Among Patients Seen by Endocrinologists and Primary Care Providers. Endocrine Practice. 2019;25(5):461–469. doi: 10.4158/EP-2018-0405 [DOI] [PubMed] [Google Scholar]
- 7.Lauffenburger JC, Lewey J, Jan S, Lee J, Ghazinouri R, Choudhry NK. Association of Potentially Modifiable Diabetes Care Factors With Glycemic Control in Patients With Insulin-Treated Type 2 Diabetes. JAMA Network Open. 2020;3(1):e1919645. doi: 10.1001/jamanetworkopen.2019.19645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah BR, Hux JE, Laupacis A, Zinman B, van Walraven C. Clinical Inertia in Response to Inadequate Glycemic Control: Do specialists differ from primary care physicians? Diabetes Care. 2005;28(3):600–606. doi: 10.2337/diacare.28.3.600 [DOI] [PubMed] [Google Scholar]
- 9.Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: A systematic review of the effect on glycemic control. Patient Education and Counseling. 2016;99(6):926–943. doi: 10.1016/j.pec.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 10.Sperl-Hillen J, Beaton S, Fernandes O, et al. Comparative Effectiveness of Patient Education Methods for Type 2 Diabetes: A Randomized Controlled Trial. Archives of Internal Medicine. 2011;171(22):2001–2010. doi: 10.1001/archinternmed.2011.507 [DOI] [PubMed] [Google Scholar]
- 11.Tang TS, Funnell MM, Oh M. Lasting Effects of a 2-Year Diabetes Self-Management Support Intervention: Outcomes at 1-Year Follow-Up. Prev Chronic Dis. 2012;9:E109. doi: 10.5888/pcd9.110313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunisholz KD, Briot P, Hamilton S, et al. Diabetes self-management education improves quality of care and clinical outcomes determined by a diabetes bundle measure. J Multidiscip Healthc. 2014;7:533–542. doi: 10.2147/JMDH.S69000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirsh S, Carey E, Aron DC, et al. Impact of a national specialty e-consultation implementation project on access. Am J Manag Care. 2015;21(12):e648–654. [PubMed] [Google Scholar]
- 14.Saxon DR, Kaboli PJ, Haraldsson B, Wilson C, Ohl M, Augustine MR. Growth of electronic consultations in the Veterans Health Administration. Am J Manag Care. 2021;27(1):12–19. [DOI] [PubMed] [Google Scholar]
- 15.Karajgikar N, Detoya KB, Beattie JN, Lutz-McCain SJ, Boudreaux-Kelly MY, Bandi A. Comparison of E-Consults and Face-to-Face Care on Costs and Glycemic Control among Veterans with Type 2 Diabetes Mellitus. Diabetes. 2018;67(Supp 1):137–LB. doi: 10.2337/db18-137-LB28993341 [DOI] [Google Scholar]
- 16.Rutledge SA, Masalovich S, Blacher RJ, Saunders MM. Diabetes Self-Management Education Programs in Nonmetropolitan Counties — United States, 2016. MMWR Surveill Summ. 2017;66(10):1–6. doi: 10.15585/mmwr.ss6610a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu H, Holt JB, Cheng YJ, Zhang X, Onufrak S, Croft JB. Population-based geographic access to endocrinologists in the United States, 2012. BMC Health Services Research. 2015;15(1):541. doi: 10.1186/s12913-015-1185-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Certification Board for Diabetes Educators. 2022 Count by State of Health Professionals Holding the Certified Diabetes Care and Education Specialist Credential. Accessed July 18, 2022. https://www.cbdce.org/documents/20123/108743/StateCount_0422.pdf/89e2bbcb-d299-1677-2699-9a496ed00b0f?t=1653491589021
- 19.Vigersky RA, Fish L, Hogan P, et al. The clinical endocrinology workforce: current status and future projections of supply and demand. J Clin Endocrinol Metab. 2014;99(9):3112–3121. doi: 10.1210/jc.2014-2257 [DOI] [PubMed] [Google Scholar]
- 20.Malkani S, Keitz SA, Harlan DM. Redesigning Diabetes Care: Defining the Role of Endocrinologists Among Alternative Providers. Curr Diab Rep. 2016;16(12):121. doi: 10.1007/s11892-016-0818-3 [DOI] [PubMed] [Google Scholar]
- 21.Bodenheimer T, Wagner EH, Grumbach K. Improving Primary Care for Patients With Chronic Illness. JAMA. 2002;288(14):1775–1779. doi: 10.1001/jama.288.14.1775 [DOI] [PubMed] [Google Scholar]
- 22.Siminerio LM, Piatt G, Zgibor JC. Implementing the Chronic Care Model for Improvements in Diabetes Care and Education in a Rural Primary Care Practice. Diabetes Educ. 2005;31(2):225–234. doi: 10.1177/0145721705275325 [DOI] [PubMed] [Google Scholar]
- 23.Stellefson M, Dipnarine K, Stopka C. The Chronic Care Model and Diabetes Management in US Primary Care Settings: A Systematic Review. Prev Chronic Dis. 2013;10:E26. doi: 10.5888/pcd10.120180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davy C, Bleasel J, Liu H, Tchan M, Ponniah S, Brown A. Effectiveness of chronic care models: opportunities for improving healthcare practice and health outcomes: a systematic review. BMC Health Serv Res. 2015;15:194. doi: 10.1186/s12913-015-0854-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Valle KL, McDonnell ME. Chronic Care Management Services for Complex Diabetes Management: a Practical Overview. Curr Diab Rep. 2018;18(12):135. doi: 10.1007/s11892-018-1118-x [DOI] [PubMed] [Google Scholar]
- 26.Appuswamy AV, Desimone ME. Managing Diabetes in Hard to Reach Populations: A Review of Telehealth Interventions. Curr Diab Rep. 2020;20(7):28. doi: 10.1007/s11892-020-01310-2 [DOI] [PubMed] [Google Scholar]
- 27.Greenwood DA, Gee PM, Fatkin KJ, Peeples M. A Systematic Review of Reviews Evaluating Technology-Enabled Diabetes Self-Management Education and Support. J Diabetes Sci Technol. 2017;11(5):1015–1027. doi: 10.1177/1932296817713506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tchero H, Kangambega P, Briatte C, Brunet-Houdard S, Retali GR, Rusch E. Clinical Effectiveness of Telemedicine in Diabetes Mellitus: A Meta-Analysis of 42 Randomized Controlled Trials. Telemed e-Health. 2019;25(7):569–583. doi: 10.1089/tmj.2018.0128 [DOI] [PubMed] [Google Scholar]
- 29.Curtis ME, Clingan SE, Guo H, Zhu Y, Mooney LJ, Hser YI. Disparities in digital access among American rural and urban households and implications for telemedicine-based services. J Rural Health. doi: 10.1m/jrh.12614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holder K Veterans in Rural America: 2011-2015. U.S. Census Bureau; Accessed October 1, 2016. https://www.census.gov/content/dam/Census/library/publications/2017/acs/acs-36.pdf
- 31.Ferguson JM, Jacobs J, Yefimova M, Greene L, Heyworth L, Zulman DM. Virtual care expansion in the Veterans Health Administration during the COVID-19 pandemic: clinical services and patient characteristics associated with utilization. J Am Med Inform Assoc. 2021;28(3):453–462. doi: 10.1093/jamia/ocaa284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furukawa MF, Machta RM, Barrett KA, et al. Landscape of Health Systems in the United States. Med Care Res Rev. 2020;77(4):357–366. doi: 10.1177/1077558718823130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabbay RA, Kendall D, Beebe C, et al. Addressing Therapeutic Inertia in 2020 and Beyond: A 3-Year Initiative of the American Diabetes Association. Clinical Diabetes. 2020;38(4):371–381. doi: 10.2337/cd20-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: A systematic review. Diabetes Obes Metab. 2018;20(2):427–437. doi: 10.1111/dom.13088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okemah J, Peng J, Quiñones M. Addressing Clinical Inertia in Type 2 Diabetes Mellitus: A Review. Adv Ther. 2018;35(11):1735–1745. doi: 10.1007/s12325-018-0819-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.United States Census Bureau. Quick Facts; Erie County, Pennsylvania; United States. Accessed May 4, 2022. https://www.census.gov/quickfacts/fact/table/eriecountypennsylvania,US/PST045221
- 37.United States Census Bureau. Quick Facts; Butler County, Pennsylvania; United States. Accessed May 4, 2022. https://www.census.gov/quickfacts/fact/table/butlercountypennsylvania,US/PST045221
- 38.Patil SJ, Ruppar T, Koopman RJ, et al. Peer Support Interventions for Adults With Diabetes: A Meta-Analysis of Hemoglobin A1c Outcomes. The Annals of Family Medicine. 2016;14(6):540–551. doi: 10.1370/afm.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]


