Abstract
Background
Shifts in epidemiological stages of inflammatory bowel disease (IBD) carry implications towards understanding IBD etiology and managing clinical care. We conducted a temporal analysis of the epidemiology of IBD between 1995 and 2016 in the Danish nationwide cohort.
Methods
We used the Danish registers to obtain data on demographics and IBD-related outpatient and inpatient contacts between 1995 and 2016. IBD diagnosis was defined as having ≥2 Crohn’s disease (CD)- or ulcerative colitis (UC)-related registrations within a two-year period. We estimated overall and annual incidence rates and prevalence of CD and UC standardized with respect to age and sex.
Results
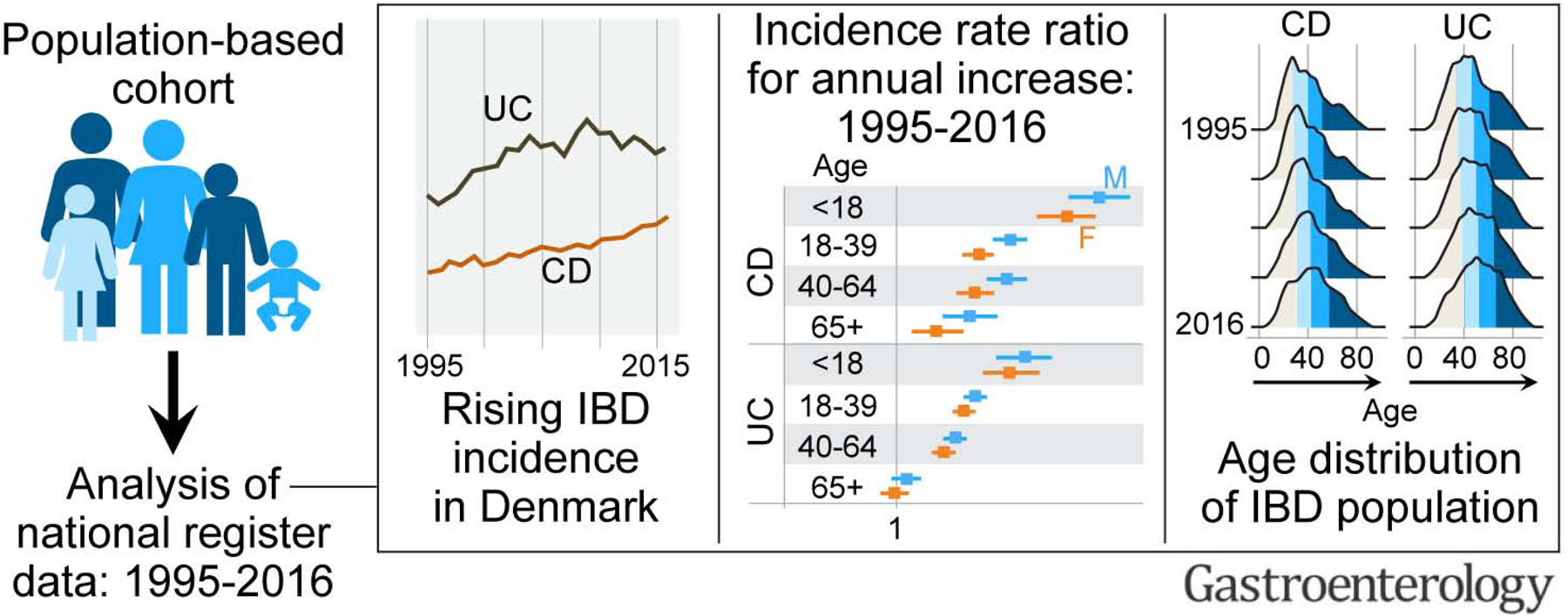
A total of 47,830 individuals met the criteria for IBD diagnosis, of which 33% and 67% were diagnosed with CD and UC, respectively. Between 1995 and 2016, the incidence rate (95% confidence interval) per 100,000 person years rose from 9.1 (8.3, 10.0) to 17.8 (16.8, 19.0) for CD, and from 21.0 (19.8, 22.3) to 28.4 (27.0, 29.8) for UC. The highest increase in CD and UC incidence rates occurred in children and young adults, respectively. The prevalence of IBD doubled from 1995 to 2016; the greatest increase (2.5-fold) was in UC prevalence among individuals >40 years of age. During this period, the median age of the IBD population increased by 6–7 years.
Conclusion
In Denmark, the incidence and prevalence of IBD have increased over the last two decades. The IBD population is shifting towards an older age. These findings have implications towards understanding environmental shifts as well as preparing healthcare systems for an aging IBD population.
Keywords: Crohn’s disease, epidemiology, incidence, inflammatory bowel disease, prevalence
Lay Summary
Using Danish nationwide data with follow up over two decades, we report that IBD incidence and prevalence are increasing and that the IBD population is shifting towards an older age.
Background
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC) is a chronic immune-mediated disease of the gastrointestinal tract, associated with significant morbidity, complications, and substantial costs to healthcare systems and society, and it has no cure.1–3 Previously considered a disease of western countries, the global IBD epidemiology is changing, with reported ongoing steep rise in IBD incidence in eastern, but not western, countries.4 Further, age and sex are likely to influence IBD incidence and prevalence.5, 6
Kaplan and Windsor recently described four epidemiological stages of IBD; emergence, acceleration in incidence, compounding prevalence, and prevalence equilibrium.7 They suggested that western countries are in the compounding prevalence stage, which is characterized by rapidly rising prevalence due to the incidence of IBD outpacing mortality in individuals with IBD. They proposed that with plateauing of IBD incidence and increasing mortality in the aging IBD population, the prevalence of IBD will start to level and lead to the fourth stage; prevalence equilibrium. However, epidemiological data to confirm these stages are lacking.
Robust, unselected, population-based data on IBD diagnosis and demographic variables over time, such as those in the Danish registers, are crucial to characterize temporal trends in IBD epidemiology, and thereby gain insights into IBD etiology, anticipate societal burden due to IBD and prepare for it. The purpose of this study was to analyze the incidence and prevalence of IBD between 1995 and 2016 in the Danish nationwide cohort, including analysis of the impact of age and sex on CD and UC epidemiology, as well as interaction with time. In doing so, we characterize the epidemiological stages of IBD using real world data and substantiate the hypotheses proposed above.
Methods
Study population
The Danish Civil Registration System (CRS) records demographic and vital data on all residents of Denmark and is continuously updated. We used the CRS to obtain information on all residents in Denmark between January 1, 1995 and December 31, 2018.
Outcome assessment
The Danish National Patient Registry (NPR) records all inpatient contacts 1977 onward, and all outpatient contacts 1995 onward. Each outpatient contact is linked with one or more visits and with primary and secondary diagnosis codes using the international classification of diseases (ICD) 8 or 10. For every contact, the most recent diagnosis codes are available. These codes for CD are 563.01, 563.02, 563.08 and 563.09 (ICD-8) and K50 (ICD-10) and those for UC are 563.19 and 569.04 (ICD-8) and K51 (ICD-10). Given the lack of outpatient data between 1977 and 1994, we restricted our analysis to the period between 1995 and 2018.
Defining incident IBD:
To define incident IBD cases, we considered a patient’s registration dates, which refers to either the date of an outpatient visit or the start of an inpatient contact. An outpatient visit is registered under a larger set called “outpatient contact”; each outpatient contact can include one or more outpatient visits (Supplementary Figure 1). Any outpatient visit registered under an outpatient contact and any inpatient contact associated with a primary or secondary IBD diagnosis using ICD 8 or 10 codes were considered IBD related registrations. Emergency room contacts were not included as it is unlikely for IBD diagnosis to be made based on emergency room contact alone without any subsequent follow up visit. Among patients with first IBD related registration between January 1, 1995 and December 31, 2018, we included patients having at least two IBD related registrations within two years. We excluded the years 2017 and 2018 as incident cases were likely to be underestimated during these years given our requirement of two IBD related registrations within two years to define IBD. We considered the date of the first IBD related registration as the date of diagnosis. Individuals who did not live continuously in Denmark for at least 2 years prior to the first IBD related registration were excluded from incidence analysis.
Defining prevalent IBD:
For extracting the IBD population at a specific point in time, i.e., all patients alive at and diagnosed with IBD before that time point, we used the rule described above for including patients whenever their first contact appeared in 1995 or later. For patients with first inpatient contact before 1995, we included patients if they have at least two registrations over any period of time.
Categorization into CD and UC
Each contact can be registered with ≥1 diagnosis codes. If the contact was registered with CD (UC) diagnosis codes only, we considered it as a CD (UC) contact. If both CD and UC diagnosis codes were registered for the same contact, we considered it to be a CD contact because while CD could be misdiagnosed as UC (due to Crohn’s colitis, for example), it is less likely for UC to be misdiagnosed as CD. Based on contact diagnosis codes, we used the following rules to classify patients as having been diagnosed with CD or UC: (1) if the patient had only one IBD-related contact, but containing ≥1 visits, we used the diagnosis code registered for that contact and (2) if the patient had ≥1 IBD-related contacts, then we considered the diagnosis related to the two most recent contacts if they were concordant, or the diagnosis code registered for the majority of the contacts if discordant (UC-CD or CD-UC). If the CD and UC codes were even numbered, then we considered the last code as the diagnosis.
Variables assessment
Using data from the CRS, we determined the date of birth and sex for all individuals who met criteria for IBD diagnosis.
Statistical analysis
We report categorical variables using frequency and proportions, and continuous variables as median (interquartile range, IQR). We estimated annual incidence rates and mid-year prevalence for the period 1995–2016 as the number of incident/prevalent cases divided by the mid-year Danish population size. We used a publicly available database for information on the background population size to enable age- and sex-based standardization.8 Rates were directly standardized with respect to age and sex by the 2016 Danish population, categorizing age as <18, 18–39, 40–64, and ≥65 years and sex as female and male. We used the same categories to determine age-specific and sex-specific incidence rates and prevalence. We used the method proposed by Dobson et al. to calculate confidence intervals for the directly standardized rates.9
We fitted a Poisson regression model to the annual age- and sex-specific number of incident patients with the logarithm of the size of the age- and sex-specific Danish population in the year of interest as the offset for CD and UC separately. To assess the annual change of incidence rates, we estimated the incidence rate ratio related to a one calendar-year increase for CD and UC separately. Considering a Poison regression with the model formulation year*age + year*sex, we obtained estimates for the annual change in age- and sex-specific incidence rates, thus taking into account possible interaction of year with age and with sex. Likelihood ratio tests were used to determine statistical significance of the interaction terms. P values <0.05 were considered statistically significant. We used R software (version 4.1.2) for all analyses and the following R-packages: ggridges for estimating and visualizing the density for age at diagnosis and age of the IBD population, stats for fitting the Poisson models and likelihood ratio tests, and emmeans for extracting incidence rate ratio estimates related to an increase of one calendar-year for all combinations of sex and age group from the fitted Poisson model.
Results
A total of 47,830 individuals met the criteria for IBD diagnosis in the period 1995 to 2016. Of these, 15,583 (32.6%) individuals were diagnosed with CD and 32,247 (67.4%) were diagnosed with UC. Among individuals with CD and UC, 8,764 (56.2%) and 16,888 (52.4%), respectively, were of female sex. The median age at diagnosis (IQR) for CD and UC was 34 (23, 53) and 44 (29, 60) years, respectively. The distribution by age at diagnosis is described in Table 1.
Table 1.
Baseline characteristics of the Danish population-based cohort with inflammatory bowel disease diagnosed between 1995 and 2016
| Characteristic | Crohn’s disease | Ulcerative colitis |
|---|---|---|
| Total (n, %) | 15,583 | 32,247 |
| Median age at diagnosis (IQR), years | 34 (23, 53) | 44 (29, 60) |
| Age at diagnosis, years | ||
| <18 | 1,883 (12.1) | 1,838 (5.7) |
| 18–39 | 7262 (46.6) | 12444 (38.6) |
| 40–64 | 4,497 (28.9) | 11,741 (36.4) |
| ≥65 | 1,941 (12.5) | 6,224 (19.3) |
| Sex | ||
| Female | 8,764 (56.2) | 16,888 (52.4) |
| Male | 6,819 (43.8) | 15,359 (47.6) |
Overall incidence
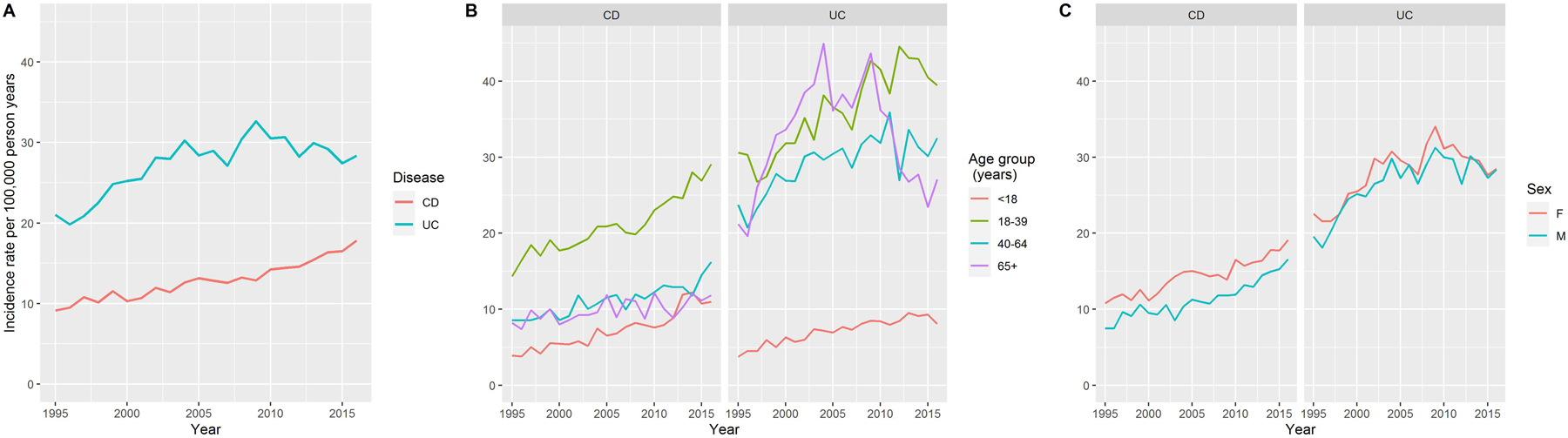
The incidence per 100,000 person years (PY) of CD, while consistently lower than that of UC over the entire study period, increased from 9.1 (95% CI 8.3, 10.0) in 1995 to 17.8 (95% CI 16.8, 19.0) in 2016, corresponding to a 95.2% increase. The incidence per 100,000 PY of UC increased from 21.0 (95% CI 19.8, 22.3) in 1995 to 28.4 (95% CI 27.0, 29.8) in 2016, corresponding to 34.9% increase (Figure 1A).
Figure 1:
Changes in the incidence rates of Crohn’s disease and ulcerative colitis between 1995 and 2016. (A) changes in overall incidence rates (B) changes in age-specific incidence rates and (C) changes in sex-specific incidence rates
Age-specific incidence
The incidence rate of CD was highest in the age group 18–39 years across the studied period, and lowest in the age group <18 years (Figure 1B). The incidence rate of UC varied over the studied period in age groups ≥18 years, and in 2016, it was highest in those 18–39 years of age. Among individuals <18 years of age, UC incidence rate remained lowest through the study period. Upon comparing age-specific CD and UC incidence rates, the latter were higher than the former for all age groups except in those <18 years of age, in whom CD and UC incidence rates were more comparable.
The age-specific incidence rates for both CD and UC increased for individuals in each age category (<18, 18–39, 40–64 and ≥65 years), but with differences in the rates of change (Figure 1B, Supplementary Table 1). Specifically, among individuals <18 years of age, the incidence of CD per 100,000 PY increased from 3.9 (95% CI 2.8, 5.3) in 1995 to 11.0 (95% CI 9.2, 13.1) in 2016 (Supplementary Table 1). This was primarily driven by those diagnosed at 6–18 years of age; the number of individuals <6 years of age diagnosed with IBD in 1995 and 2016 was 6 and 9, respectively. In the 18–39 years age category, CD incidence per 100,000 PY increased from 14.3 (95% CI 12.6, 16.3) in 1995 to 29.1 (95% CI 26.5, 31.9) in 2016. In individuals <18 years of age, UC incidence per 100,000 PY increased from 3.8 (95% CI 2.7, 5.1) in 1995 to 8.1 (95% CI 6.5, 9.9) in 2016. In individuals 18–39 years of age, UC incidence per 100,000 PY increased from 30.6 (95% CI 28.0, 33.4) in 1995 to 39.5 (95% CI 36.4, 42.9) in 2016. The increase in UC incidence rate in older groups was slower compared to that in younger groups.
Sex-specific incidence
The incidence rate of CD among female individuals was consistently higher than that in male individuals over time, while the incidence rate of UC was comparable between the sexes (Figure 1C, Supplementary Table 2). CD incidence per 100,000 PY among female individuals increased from 10.8 (95% CI 9.6, 12.1) in 1995 to 19.1 (95% CI 17.5, 20.8) in 2016, while among male individuals, it increased from 7.5 (95% CI 6.4, 8.6) in 1995 to 16.6 (95% CI 15.1, 18.1) in 2016. The incidence per 100,000 PY of UC among female individuals increased from 22.6 (95% CI 20.8, 24.4) in 1995 to 28.5 (95% CI 26.5, 30.5) in 2016. Among male individuals, UC incidence per 100,000 PY increased from 19.6 (17.8, 21.4) in 1995 to 28.3 (95% CI 26.4, 30.4) in 2016.
When stratifying further by age groups, CD incidence rate was consistently higher in women in the 18–39 years group, and in the 40–64 years age group, although with some variability. UC incidence rate was consistently higher in women in the 18–39 years age group, but comparable between both sexes in younger and older age groups (Supplementary Figure 1).
Estimated annual increase in IBD incidence
The estimated annual increase in IBD incidence by age and sex are presented in Figure 2. There was a consistent increase in incidence of CD in all subgroups; the highest annual increase in incidence was in CD among individuals <18 years of age [incidence rate ratio (IRR) 1.048 (95% CI 1.04, 1.056) and 1.057 (95% CI 1.049, 1.066) for female and male individuals, respectively, Figure 2, Supplementary Table 3). In the age group 18–39 years, the annual increase in CD incidence was lower in female, compared to male, individuals [IRR 1.023 (95% CI 1.019, 1.027) and 1.032 (95% CI 1.027, 1.037), respectively). With respect to UC, while its incidence increased in both female and male individuals in all age groups <65 years of age, among those ≥65 years, there was no statistically significant increase in the estimated annual incidence (IRR 0.999 (95% CI 0.995, 1.004) and 1.003 (95% CI 0.998, 1.007) for female and male individuals, respectively).
Figure 2:
Forest plot depicting estimated incidence rate ratios and 95% confidence intervals for a one-year increase between 1995 and 2016 by age group and sex
Prevalence
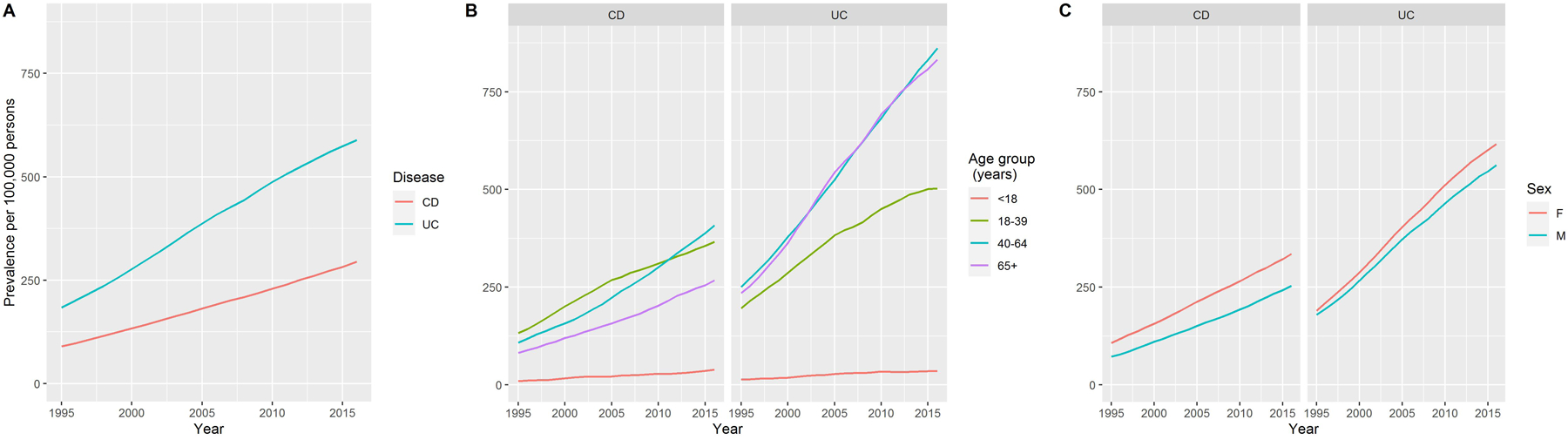
The prevalence per 100,000 persons of CD increased from 90.0 (95% CI 87.4, 92.6) in 1995 to 295 (95% CI 290, 299) in 2016, corresponding to a 2.3-fold increase, and that of UC increased from 184 (95% CI 180, 188) in 1995 to 589 (95% CI 583, 595) in 2016, corresponding to a 2.2-fold increase (Figure 3A).
Figure 3:
Change in the prevalence of Crohn’s disease and ulcerative colitis between 1995 and 2016. (A) changes in overall prevalence (B) changes in age-specific prevalence and (C) changes in sex-specific prevalence
Age-specific prevalence
The age-specific prevalence of IBD and change over time are reported in Figure 3B and Supplementary Table 4. The prevalence of CD was highest in the 18–39 years age group in 1995, but that in the 40–64 years age group became highest in 2016. The prevalence of CD in those <18 years of age remained lowest. With respect to UC, the prevalence was highest in the 40–65 and ≥65 years age groups, lower in 18–39 years, and lowest in <18 years age group. The prevalence of both CD and UC increased in each age group over time, with greatest increase in older age groups. Among individuals 40–64 years of age, CD prevalence per 100,000 persons increased from 108 (95% CI 103, 113) in 1995 to 408 (399, 418) in 2016 and among those ≥65 years of age, it increased from 81.7 (75.6, 88.2) in 1995 to 268 (258, 277) in 2016. The prevalence of UC per 100,000 persons in the age group 40–64 years increased from 251 (243, 259) in 1995 to 861 (848, 875) in 2016 while that among those ≥65 years of age, it increased from 235 (224, 246) in 1995 to 832 (815, 850) in 2016. The most rapid increase occurred in CD prevalence in the <18 years age group; it increased from 10.1 (95% CI 8.3, 12.1) in 1995 to 38.8 (95% CI 35.3, 42.5) in 2016 per 100,000 persons.
Sex-specific prevalence
The prevalence of CD per 100,000 persons in female individuals changed from 108 (95% CI 104, 112) in 1995 to 336 (95% CI 329, 342) in 2016, and among male individuals, it changed from 72.1 (95% CI 68.8, 75.5) in 1995 to 254 (95% CI 248, 260) in 2016 (Figure 3C, Supplementary Table 5). The prevalence of UC per 100,000 persons was also higher among women compared to men; in the former, it changed from 190 (95% CI 185, 195) in 1995 to 616 (95% CI 607, 625) in 2016 and in the latter group, it changed from 179 (95% CI 175, 185) in 1995 to 562 (95% CI 554, 571) in 2016.
Age distribution of IBD population
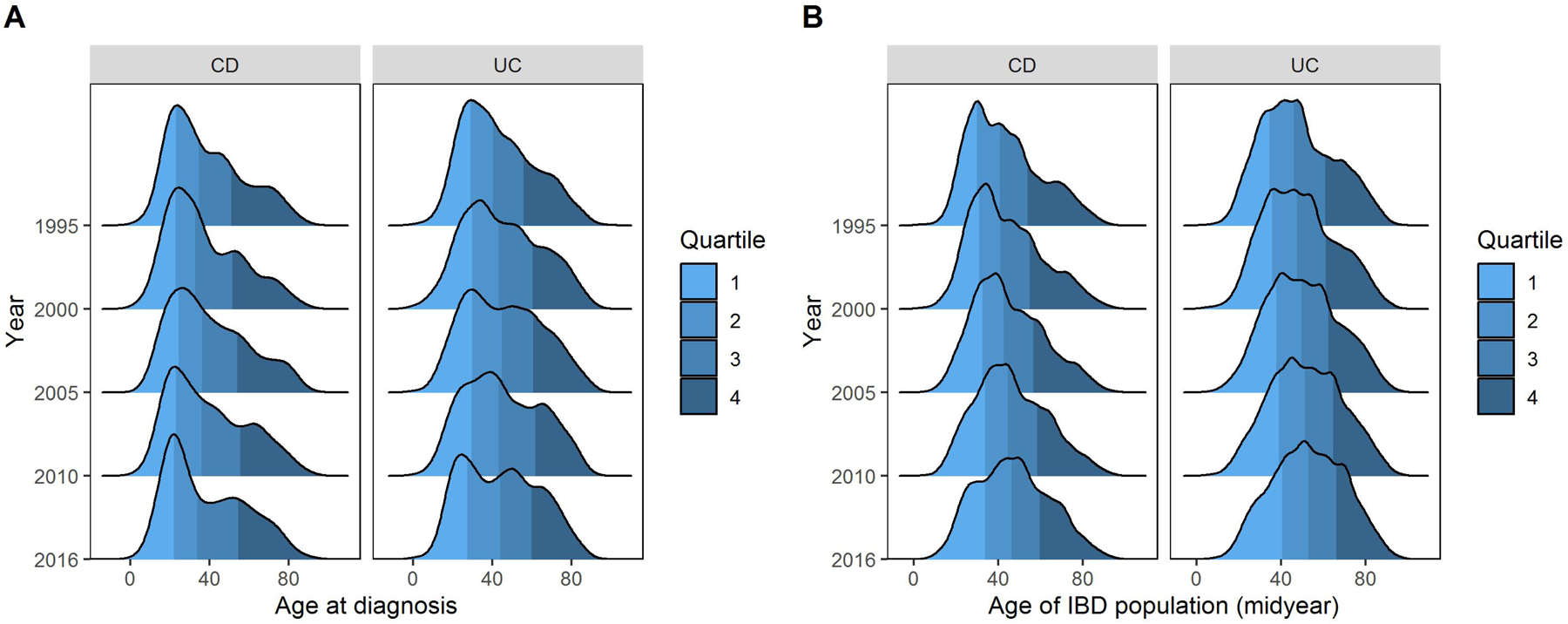
While the overall distribution of age at diagnosis remained consistent for CD, it has changed over time for UC, with an increase in diagnosis at older age, leading to a second peak in the distribution of age at diagnosis (Figure 4A). The median age (IQR) at CD diagnosis in 1995 and in 2016 was 34.4 (23, 50.9) years and 33.8 (21.8, 54.3) years, respectively. The median age at UC diagnosis in 1995 and 2016 was 40.1 (28.8, 55.9) years and 44 (27.4, 59.8) years, respectively.
Figure 4:
Distribution of (A) age at inflammatory bowel disease diagnosis and (B) age of individuals with inflammatory bowel disease between 1995 and 2016
Correspondingly, there have been temporal changes in the age distribution of the IBD population, with the proportion of older individuals with CD and UC on the rise (Figure 4B). In 1995 and 2016, the median age (IQR) of individuals with CD was 40.6 (29.7, 53.7) years and 46.2 (33.5, 59.5), respectively while that of individuals with UC was 45.9 (34.4, 60.6) and 52.7 (40.2, 66.1), respectively. Of all individuals with CD in a given year, the proportion of those ≥40 years of age increased from 51.1% in mid 1995 to 63.4% in mid 2016, while of those with UC, the proportion of individuals ≥40 years increased from 63.5% in mid 1995 to 75.4% in mid 2016. Changes in the proportion of individuals with CD and UC in different age groups are reported in Supplementary Table 6.
Sensitivity analyses
We conducted two sensitivity analyses using two different definitions of the outcome.
First, we included only those individuals for whom the IBD diagnosis had been confirmed on histological analysis in the Danish Pathology Register10 using a previously described histological definition of IBD (Supplementary materials).11, 12 Of 47,830 individuals included in the primary analysis, IBD diagnosis was confirmed on histology in 40,335 individuals. Of these, 27,722 (69%) persons were diagnosed with UC and 12,613 (31%) persons were diagnosed with CD. The incidence rate (95% CI) of UC increased from 17 (15.9, 18.2) per 100,000 person years in 1995 to 25.1 (23.8, 26.4) per 100,000 person years in 2016, corresponding to a 47.7% increase, while the incidence rate of CD increased from 7.2 (6.5, 8) per 100,000 person years in 1995 to 13.9 (13, 14.9) per 100,000 person years in 2016, corresponding to a 92.6% increase (Supplementary Figure 3).
Next, we analyzed temporal trends in the subcohort with concordant diagnoses codes in the two most recent contacts, i.e., we removed patients who had both UC and CD diagnosis codes registered for the two most recent contacts. This subcohort consisted of 46,125 individuals, of which 31,353 (68%) were diagnosed with UC and 14,772 (32%) with CD. The incidence rate (95% CI) of UC increased from 20.3 (19.1, 21.6) per 100,000 person years in 1995 to 27.7 (26.4, 29.1) per 100,000 person years in 2016, corresponding to a 36.7% increase, while the incidence rate of CD increased from 8.8 (8.1, 9.7) per 100,000 person years in 1995 to 17.3 (16.3, 18.4) per 100,000 person years in 2016, corresponding to a 96% increase (Supplementary Figure 4).
Discussion
In this analysis of data from an unselected nationwide population-based cohort over a 22-year period, we report temporal changes in the epidemiology of CD and UC. Our main findings are the following: the incidence rates of CD and UC have increased in the last two decades, with the sharpest increase among children and young adults, respectively. This suggests that we are yet to reach the stage of flattening of the incidence slope, which has been proposed by Kaplan and Windsor.7 The prevalence of CD and UC also rose during the past two decades, especially in older age groups. The median age of the IBD population has increased and IBD patients are now older. These findings are in line with the epidemiological stage of compounding prevalence.7
The rising incidence of IBD is consistent with findings from other epidemiological studies from Denmark.13–15 However, data from other western countries are less clear. In many countries of Europe and North America, the overall IBD incidence is reported to be plateauing or even decreasing.4, 12 This difference could be related, at least in part, to differences in environmental risk factors. For example, smoking and alcohol use are more prevalent in Denmark compared to Sweden where the annual incidence of IBD has decreased by 1–2% per year from 2002 onwards.12, 16 Dietary variables such as ultra-processed foods, sugars and fiber intake also vary in different Nordic countries.16 However, among children and young adults, IBD incidence continue to escalate. In a systematic review of 131 population-based studies from 48 countries, Kuenzig et al. reported the increasing incidence of IBD in individuals <21 years of age, especially in North European countries.17 The increasing incidence in our study is also driven by that in younger age groups. These incidence data are important to understand persistent, and potentially increasingly prevalent nongenetic drivers of IBD; pollution, smoking, and antibiotics have been implicated in IBD pathogenesis and these may be especially relevant during the early life period leading to risk modulation in younger individuals.18, 19 Environmental risk factors for IBD in older and elderly individuals are less well-studied, but antibiotics have been implicated in recent data.20 Importantly, these incidence data herald further increase in IBD prevalence in the coming years suggesting that the health care system should prepare for a higher volume of IBD patients.
Escalating IBD prevalence in older age groups is described previously as compounding prevalence by Coward et al.21 This is especially true for UC. Further, the median age of the IBD population and the proportion of older individuals with IBD are increasing. This shift in patient characteristics from predominantly young individuals, who are more likely to be otherwise healthy, to older individuals, who have greater frailty and frequent comorbid conditions, is likely to impact management of patients, outcomes and healthcare costs.22, 23 IBD therapies warrant additional considerations in older individuals such as adverse effects and interactions with medications.24 This changing landscape of IBD calls for preparation at the physician and healthcare system levels, and adaptation of healthcare delivery models for older patients.25 Further, while IBD was traditionally considered as a disease of young persons, older age should not preclude its inclusion, and especially that of UC, in the differential diagnosis. To better predict the future burden of IBD and prepare for optimal patient care, subsequent studies should investigate temporal trends in IBD disease phenotype and disease course with focus on the older population. These data also emphasize the emerging importance of representation of older and elderly individuals in efficacy and safety trials of new IBD therapies and the need for real world data on IBD in the elderly.
Other findings also warrant consideration. Differences in slopes of CD and UC incidence rates across different age groups are particularly important to understand differences in CD and UC risk factors and etiology. The persistently increasing incidence of IBD, especially that of CD, in children and young adults will continue to contribute to compounding prevalence and societal burden. Higher CD and UC incidence rates in female, compared to male, individuals during young adulthood are consistent with sex-based epidemiological differences reported in other studies.13–15 In a systematic review of population-based studies, similar findings were reported in CD, but not in UC.6 Sex-based differences specific to young adults with IBD may suggest a role of sex hormones on disease pathogenesis and merit further exploration. Interestingly, IBD prevalence was higher in female, compared to male, individuals.
The strengths of this study include a detailed epidemiological analysis, with stringent definitions of IBD, CD and UC, stratified by age and sex, and with long-term follow up data from an unselected population-based cohort with no loss to follow up. Denmark is a high-IBD incidence country with the availability of high-quality register data, making this a suitable cohort to study temporal changes in IBD.4 We used a rigorous definition for IBD diagnosis using ICD-10 codes. We conducted stratified analysis, reporting the impact of age and sex on IBD incidence and prevalence. We conducted additional sensitivity analyses; among those with histologically confirmed IBD, the increase in UC incidence was even higher. Overall, temporal trends were consistent, corroborating the robustness of our outcome definition. Further, over the study period 1995–2016, IBD registration practices and ICD10 codes have remained the same. Therefore, bias in our case definition, if any, would be non-differential, that is, the changes in incidence and prevalence over time are likely to be accurate. Our methodology overcomes some of the limitations of lack of granular data and less rigorous outcome definitions in prior epidemiological analyses from Denmark.13–15 However, our study also has limitations. These include the use of administrative data secondarily for the purpose of research, overlap between CD and UC diagnosis codes, lack of data on disease phenotype and location, and limited generalizability to other populations.
In summary, we report the overall rising incidence and prevalence of IBD from 1995 to 2016 in Denmark with differences by IBD type, age at diagnosis and sex. The increasing incidence of IBD, especially in children and young adults, has implications towards better understanding of persistent, and potentially increasing, environmental IBD risk factors. Increasing prevalence in older age groups and aging of the IBD population marks a shift in IBD-related societal burden and calls for preparation of healthcare systems and rethinking of research strategies to accommodate the needs of this patient group.
Supplementary Material
What you need to know.
BACKGROUND AND CONTEXT
The global IBD epidemiology is changing, with reported rise in IBD incidence in eastern, and compounding prevalence in western countries. However, confirmatory data are lacking.
NEW FINDINGS
Using Danish nationwide data, we report that IBD incidence and prevalence have increased and that the IBD population has shifted towards an older age in the last two decades.
LIMITATIONS
Limitations include the use of administrative data secondarily for the purpose of research, lack of data on disease phenotype and location, and limited generalizability to other populations.
IMPACT
These findings call for preparation of healthcare systems and research strategies to accommodate older patients and carry implications towards better understanding of environmental IBD risk factors.
Acknowledgements:
The authors thank Jill Gregory, Certified Medical Illustrator, Icahn School of Medicine at Mount Sinai, for the graphical abstract illustration.
Funding sources:
Danish National Research Foundation, grant no. DNRF148. MA is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K23DK129762-01). JFC is supported by Mount Sinai (New York) SUCCESS fund.
Conflict of interest statement:
The corresponding author confirms on behalf of all authors that there have been no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated.
MA reports no conflict of interest.
HC reports no conflict of interest.
MB reports no conflict of interest.
JFC reports receiving research grants from AbbVie, Janssen Pharmaceuticals and Takeda; receiving payment for lectures from AbbVie, Amgen, Allergan, Inc. Ferring Pharmaceuticals, Shire, and Takeda; receiving consulting fees from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Celgene Corporation, Eli Lilly, Ferring Pharmaceuticals, Galmed Research, Glaxo Smith Kline, Geneva, Iterative Scopes, Janssen Pharmaceuticals, Kaleido Biosciences, Landos, Otsuka, Pfizer, Prometheus, Sanofi, Takeda, TiGenix,; and hold stock options in Intestinal Biotech Development.
TJ reports no conflict of interest.
KHA reports no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data transparency statement: The study is based on data from the Danish nationwide registers (https://sundhedsdatastyrelsen.dk). The register data is protected by the Danish Act on Processing of Personal Data and are accessed through application to and approval from the Danish Data Protection Agency and the Danish Health Data Authority.
Data Availability Statement:
The data underlying this article are available in the article and in its online supplementary material.
References
- 1.Torres J, Mehandru S, Colombel JF, et al. Crohn’s disease. Lancet 2017;389:1741–1755. [DOI] [PubMed] [Google Scholar]
- 2.Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet 2017;389:1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park KT, Ehrlich OG, Allen JI, et al. The Cost of Inflammatory Bowel Disease: An Initiative From the Crohn’s & Colitis Foundation. Inflamm Bowel Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018;390:2769–2778. [DOI] [PubMed] [Google Scholar]
- 5.Virta LJ, Saarinen MM, Kolho KL. Inflammatory Bowel Disease Incidence is on the Continuous Rise Among All Paediatric Patients Except for the Very Young: A Nationwide Registry-based Study on 28-Year Follow-up. J Crohns Colitis 2017;11:150–156. [DOI] [PubMed] [Google Scholar]
- 6.Shah SC, Khalili H, Gower-Rousseau C, et al. Sex-Based Differences in Incidence of Inflammatory Bowel Diseases-Pooled Analysis of Population-Based Studies From Western Countries. Gastroenterology 2018;155:1079–1089 e3. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2021;18:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Statistics Denmark, 2021.
- 9.Dobson A, Kuulasmaa K, Eberle E, and Scherer J Confidence intervals for weighted sums of Poisson parameters. Statistics in Medicine. Volume 10, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Bjerregaard B, Larsen OB. The Danish Pathology Register. Scand J Public Health 2011;39:72–4. [DOI] [PubMed] [Google Scholar]
- 11.Jess T, Vestergaard MV, Iversen AT, et al. Undiagnosed inflammatory bowel disease among individuals undergoing colorectal cancer screening: a nationwide Danish cohort study 2014–2018. Gut 2022. [DOI] [PubMed] [Google Scholar]
- 12.Forss A, Clements M, Bergman D, et al. A nationwide cohort study of the incidence of inflammatory bowel disease in Sweden from 1990 to 2014. Aliment Pharmacol Ther 2021. [DOI] [PubMed] [Google Scholar]
- 13.Lophaven SN, Lynge E, Burisch J. The incidence of inflammatory bowel disease in Denmark 1980–2013: a nationwide cohort study. Aliment Pharmacol Ther 2017;45:961–972. [DOI] [PubMed] [Google Scholar]
- 14.Nørgård BM, Nielsen J, Fonager K, et al. The incidence of ulcerative colitis (1995–2011) and Crohn’s disease (1995–2012) - based on nationwide Danish registry data. J Crohns Colitis 2014;8:1274–80. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen BA, Fallingborg J, Rasmussen HH, et al. Increase in incidence and prevalence of inflammatory bowel disease in northern Denmark: a population-based study, 1978–2002. Eur J Gastroenterol Hepatol 2006;18:601–6. [DOI] [PubMed] [Google Scholar]
- 16.Matthiessen JAL, Barbieri HE, Borodulin K, Knudsen VK, Kørup K, Thorgeirsdottir H, Trolle E and Fagt S. The Nordic Monitoring System 2011–2014. 2016.
- 17.Kuenzig ME, Fung SG, Marderfeld L, et al. Twenty-first century trends in the global epidemiology of pediatric-onset inflammatory bowel disease: systematic review. Gastroenterology 2022. [DOI] [PubMed] [Google Scholar]
- 18.Agrawal M, Sabino J, Frias-Gomes C, et al. Early life exposures and the risk of inflammatory bowel disease: Systematic review and meta-analyses. EClinicalMedicine 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elten M, Benchimol EI, Fell DB, et al. Residential Greenspace in Childhood Reduces Risk of Pediatric Inflammatory Bowel Disease: A Population-Based Cohort Study. Am J Gastroenterol 2020. [DOI] [PubMed] [Google Scholar]
- 20.Faye AA K;Iversen A;Agrawal M;Faith J;Jean-Frederic C;Jess T Antibiotics as a risk factor for older onset IBD: A population-based cohort study. Journal of Crohn’s and Colitis 2022. [Google Scholar]
- 21.Coward S, Clement F, Benchimol EI, et al. Past and Future Burden of Inflammatory Bowel Diseases Based on Modeling of Population-Based Data. Gastroenterology 2019;156:1345–1353 e4. [DOI] [PubMed] [Google Scholar]
- 22.Kochar B, Jylhävä J, Söderling J, et al. Prevalence and Implications of Frailty in Older Adults with Incident Inflammatory Bowel Diseases: a Nationwide Cohort Study. Clin Gastroenterol Hepatol 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh S, Qian AS, Nguyen NH, et al. Trends in U.S. Health Care Spending on Inflammatory Bowel Diseases, 1996–2016. Inflamm Bowel Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ananthakrishnan AN, Donaldson T, Lasch K, et al. Management of Inflammatory Bowel Disease in the Elderly Patient: Challenges and Opportunities. Inflamm Bowel Dis 2017;23:882–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kochar B, Ufere NN, Ritchie CS, et al. The 5Ms of Geriatrics in Gastroenterology: The Path to Creating Age-Friendly Care for Older Adults With Inflammatory Bowel Diseases and Cirrhosis. Clin Transl Gastroenterol 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.


