Abstract
Monkeypox is the latest reemerging zoonosis worldwide. Anyone is susceptible to contracting this infection; however, the clinical presentation may be atypical in a particularly vulnerable group that identifies as men who have sex with men. Herein, we present two cases of patients diagnosed with monkeypox infection, both of whom were also co-infected with human immunodeficiency virus (HIV) and exhibited different degrees of immunosuppression. Notably, the clinical presentations differed significantly. Patients co-infected with HIV are prone to develop clinical features ranging from barely visible lesions to severe or even fatal disease.
Keywords: Monkeypox, Immunocompromised, HIV, Clinical spectrum
Introduction
Monkeypox has reemerged in recent years. This disease is caused by the monkeypox virus (MPXV), a DNA virus belonging to the Poxviridae family and Orthopoxvirus genus [1], which bears a similarity of 96 % with the variola virus [2] (causative agent of smallpox). As of August 24, 2022, 44,503 confirmed cases had been reported worldwide [3].
Monkeypox is particularly prevalent in a subgroup of patients co-infected with human immunodeficiency virus (HIV), presumably due to transmission via sexual contact [4] and the level of immunosuppression that these patients may show in different stages of their disease. Although monkeypox can occur in any person who has had direct contact with an infected individual, the reasons for this overwhelming prevalence in the aforementioned subgroup remain under debate.
A ”typical” clinical presentation no longer exists for this disease, given the wide spectrum of features reported in the ongoing outbreak that range from localized lesions (e.g., nasal necrosis [5]) to life-threatening generalized dermatoses. The common denominator in all of these cases is some degree of immunosuppression, which is secondary to HIV infection.
Therefore, by presenting two cases of Mexican patients co-infected with monkeypox and HIV, we aimed to discuss the wide variety of signs and symptoms that may occur over the course of this disease, underscoring their clinical differences, which were prompted by differential degrees of immunosuppression, despite sharing a common previous diagnosis.
Case 1
A 32-year-old male sex worker, who identified himself as a man who has sex with men (MSM), came for evaluation at our hospital. During the previous month, he had unprotected sexual intercourse on multiple occasions. The patient had been diagnosed with HIV five years prior and was currently under treatment with bictegravir/emtricitabine/tenofovir alafenamide. The patient denied any other disease. The family history was unremarkable for his present illness.
Twelve days prior to admission, he developed a disseminated maculopapular rash spreading in the cephalocaudal direction and later developed fevers up to 40 °C, often presenting at night, as well as tender cervical and left axillary lymph nodes. Forty-eight hours after symptom onset, he noted multiple pustules, prompting him to seek medical attention at our hospital. The patient was admitted for workup and epidemiological control. Physical examination showed a polymorphic, disseminated dermatosis affecting both lower and upper limbs, characterized by umbilicated, erythematous, violaceous papules with central ulcerations, in addition to pustules with serous content, with a total of 20 lesions scattered throughout the body (Fig. 1, Fig. 2). Biopsy of these lesions revealed non-specific findings, such as hyperkeratosis and mixed inflammation. Hard, tender, left anterior and posterior cervical lymph nodes were noted on palpation and were approximately 3 cm wide. A 2 cm left axillary lymph node with the same features was also noted. The polymerase chain reaction (PCR) test was positive for MPXV. The initial laboratory workup was remarkable for an HIV viral load of 1577 copies/mL and a CD4 + count of 932 cells/mm3; the rest of the laboratory tests were reported to be within normal ranges. Supportive treatment consisted of non-steroidal anti-inflammatory drugs (NSAIDs) and management of fever with acetaminophen. The patient did not develop any bacterial superinfection or other potential complications during hospitalization. Fifteen days from symptom onset, his lesions progressed to the scabbing phase, and he was later discharged on day 17 with the indication of self-isolation at home until the complete resolution of all lesions.
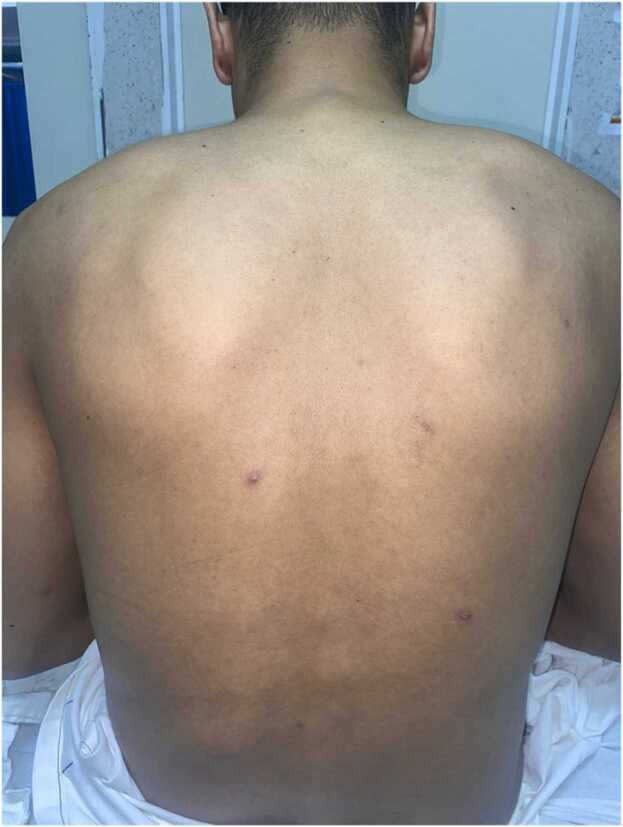
Fig. 1.
Scarce erythematous, papulopustular lesions in the posterior trunk.
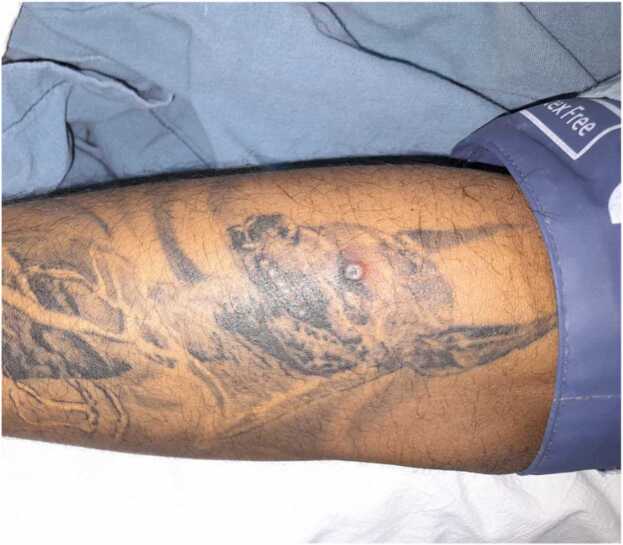
Fig. 2.
Left arm with an erythematous, umbilicated, pustular lesion.
Case 2
A 31-year-old man, who identified as MSM, visited our hospital for medical attention. The patient had been diagnosed with HIV 12 years prior and was taking abacavir/dolutegravir/lamivudine, with poor compliance to treatment. He was also diagnosed with hepatitis C virus (HCV) infection in January 2022 and is not currently under treatment. The patient denied any other illnesses, and his family history was noncontributory.
He began his symptoms eight days prior to admission with a fever up to 39.5 °C, often presenting in the afternoon, as well as enlarged cervical lymph nodes and intermittent holocranial headaches. Twenty-four hours later, the patient developed a papulopustular rash on the face, limbs, and trunk, which was why he came to our hospital for assessment.
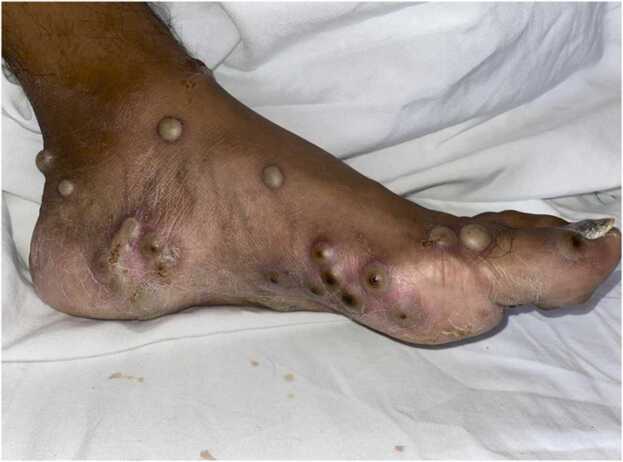
At presentation, his vital signs were within normal range. Physical examination revealed a generalized, polymorphic dermatosis marked by multiple erythemato-squamous papules, with central umbilications and ulcerations, vesicles and pustules with serous content, and honey-colored crusts on the face (Fig. 3, Fig. 4). Both his palms and soles were affected (Fig. 5). The PCR results were positive for MPXV. Laboratory tests were remarkable for an HIV viral load of < 40 copies/mL and a CD4 + count of 167 cells/mm3; the rest of the laboratory tests were reported to be within normal ranges. Supportive care was provided during his hospital stay, namely, beta-lactams due to bacterial superinfection of the lesions, in addition to analgesia, which was initially administered with NSAIDs and then escalated to opioids. Without further complications, the patient was discharged after 11 days of hospital stay and 19 days after symptom onset; home isolation was indicated until the resolution of all lesions.
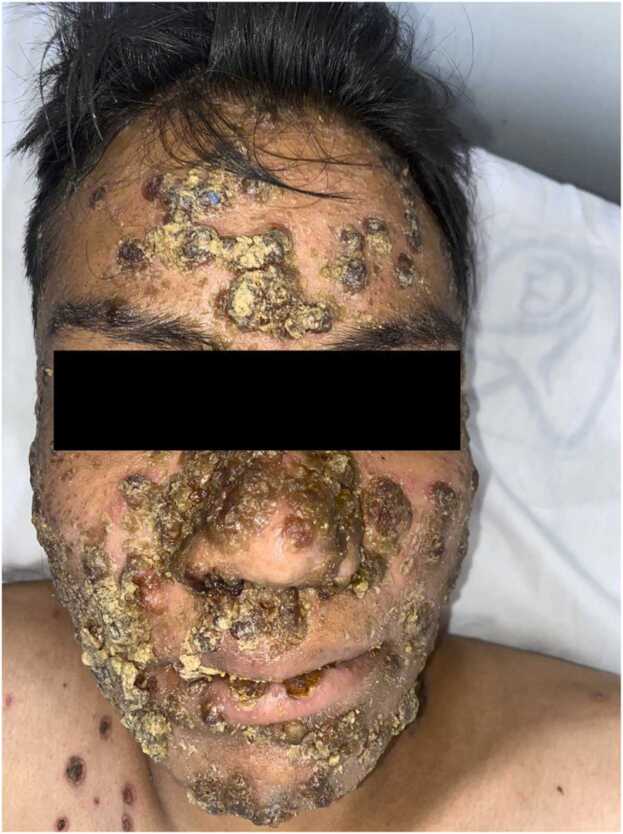
Fig. 3.
Patient #2 with pustular lesions in the face and scalp marked by hematic and honey-colored crusts, suggesting a bacterial superinfection.
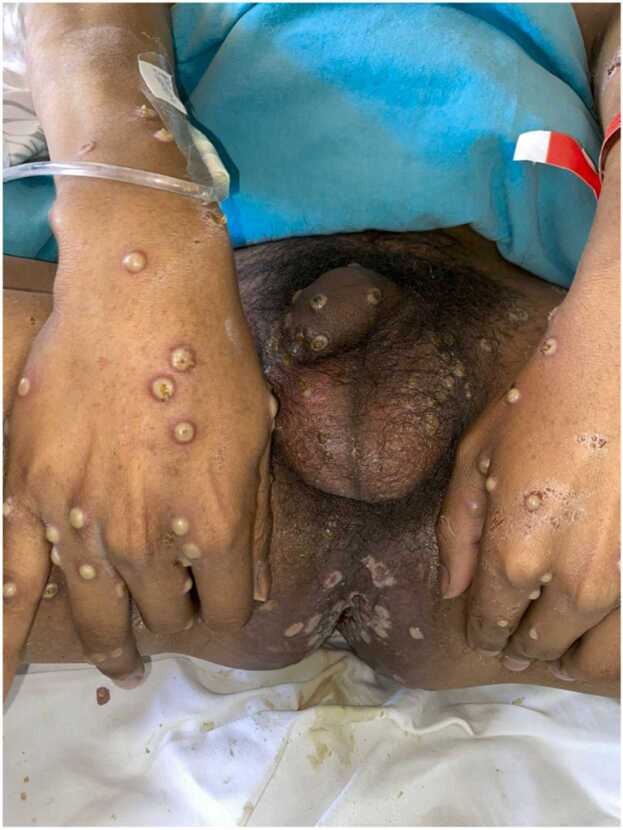
Fig. 4.
Umbilicated, pustular lesions in the hands. Perianal area with multiple shiny-surfaced ulcers and necrotic scabs.
Fig. 5.
Pustular lesions in the soles and ankle with a necrotic center.
Discussion
Monkeypox had been a neglected disease until a few months ago. On May 7, 2022, the index case of the current outbreak was documented [6], and since then, there has been a notorious increase in the number of human monkeypox cases in non-endemic areas, affecting 96 countries in total [3].
The clinical presentation described in outbreaks confined to the African continent consists of fever, lymphadenopathy, and a papulopustular rash that may affect mucous membranes, palms, and soles [7]. This presentation was based on the findings of several observational studies; therefore, manifestations of disease in other ethnic groups may differ in terms of the types and severity of symptoms. In the current outbreak, countries from Europe and the Americas reported a wide range of atypical presentations, such as [8]:
-
•
Scarce lesions, a single lesion, or even none of them
-
•
Development of lesions before or without prodrome
-
•
Lesions confined to the genital and/or perianal areas
-
•
Lesions of asynchronous evolution
In people living with HIV (PLHIV), the clinical presentation can vary owing to an altered immune response. More severe and generalized cases are expected in patients with greater immunocompromise, while more localized cases are expected in patients with better HIV control [9]. Preliminary data from the European Union, England, and the US suggest that for those whose HIV status is known, 28–51 % of MSM who have monkeypox also have HIV [10]. Although they are a vulnerable group in whom the disease can be more aggressive, it is not exclusive to PLHIV; in some cohorts, this subgroup constitutes only 35 %, and the remaining 64.5 % identified as MSM, without being co-infected with HIV [11]. This situation cannot be undermined since there are approximately 38.4 million PLHIV worldwide [12], and most deaths attributed to monkeypox in Africa occurred in these individuals [13].
In the cases presented here, both patients were co-infected with HIV, and one patient was also infected with HCV. The first patient was a male under optimal HIV control, with a CD4 + count within normal values, while the second patient was deemed at stage B3 according to the CD4 + count. In the first patient, the lesions could be easily confused with other diseases, such as molluscum contagiosum, herpes simplex, or even cutaneous drug reactions [7]. Nonetheless, lymphadenopathy is a very specific feature of monkeypox infection [7]. In contrast, the second patient displayed a very suggestive clinical presentation, both in terms of the distribution and morphology of the skin lesions.
To date, deaths attributed to monkeypox infection outside Africa have been negligible, accounting for only five individuals (two in Spain, one in Brazil, Ecuador, and India). However, preventive measures must be reinforced, and seeking medical attention must be encouraged in suspected cases since the stigmatization of this disease, as with many other diseases [14], might cause feelings of guilt and shame in those who contract it, which hinders prompt medical attention and increases the risk of transmission among the population.
Conclusions
Given the current global outbreak of monkeypox, clinicians should have a high index of suspicion owing to the wide range of clinical presentations, especially in immunocompromised patients, whether due to HIV infection or any other condition. Early diagnosis may not significantly influence mortality, but it can certainly prevent wide transmission of this disease and, hopefully, avoid the collapse of healthcare systems worldwide.
Funding
None.
Ethical approval
N/A.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
CRediT authorship contribution statement
Carlos Adrian Pérez Martínez: Care of patient, writing, editing and submit of manuscript. Gustavo Antolín Silva Flores: Care of patient, writing and editing manuscript. Fernando Padilla Santamaría, Lucero Maya Franco: Care of patient and review of manuscript. Floribel Ferman Cano, Luis Alberto García Fierro, Carlos Daniel Sánchez Cárdenas and América Citlali Hernández Magaña: Care of patient and revising of manuscript.
Conflict of interest statement
We declare that there are no conflicts of interest amongst the authors.
Contributor Information
Carlos Adrián Pérez Martínez, Email: carlosadrianperezm.i@gmail.com.
Gustavo Antolin Silva Flores, Email: sdowgus@hotmail.com.
Fernando Padilla Santamaría, Email: fernando.psantamaria23@gmail.com.
Lucero Maya Franco, Email: lucemaf@hotmail.com.
Floribel Ferman Cano, Email: fermancano2804@gmail.com.
Luis Alberto García Fierro, Email: drluisgarciafierro@gmail.com.
Carlos Daniel Sánchez Cárdenas, Email: jefegrillo@gmail.com.
América Citlali Hernández Magaña, Email: ame.hm1312@gmail.com.
References
- 1.Babkin I.V., Babkina I.N., Tikunova N.V. An update of orthopoxvirus molecular evolution. Viruses. 2022;14(2):388. doi: 10.3390/v14020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shchelkunov S.N., Totmenin A.V., Babkin IV, et al. Safronov PF, Ryazankina OI, et al. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 2001;509(1):66–70. doi: 10.1016/s0014-5793(01)03144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.2022 monkeypox outbreak global map. Update. Available from: 〈https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html〉. [Accessed 24 August].
- 4.Multi-country outbreak of monkeypox. Situation update. Available from: 〈https://www.who.int/docs/default-source/coronaviruse/situation-reports/20220810_monkeypox_external_sitrep.pdf?sfvrsn=ccb541dc_5&download=true〉. [Accessed 24 August 2022].
- 5.Boesecke C., Monin M.B., van Bremen K., Schlabe S., Hoffmann C. Severe monkeypox-virus infection in undiagnosed advanced HIV infection. Infection. 2022 doi: 10.1007/s15010-022-01901-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vivancos R., Anderson C., Blomquist P., Balasegaram S., Bell A., Bishop L., et al. Community transmission of monkeypox in the United Kingdom, April to May 2022. Eur Surveill. 2022;27(22) doi: 10.2807/1560-7917.ES.2022.27.22.2200422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCollum A.M., Damon I.K. Human monkeypox. Clin Infect Dis. 2014;58(2):260–267. doi: 10.1093/cid/cit703. [DOI] [PubMed] [Google Scholar]
- 8.Multi-country monkeypox outbreak: situation update. Available from: 〈https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON396〉. [Accessed 24 August 2022].
- 9.Ogoina D., Iroezindu M., James H.I., Oladokun R., Yinka-Ogunleye A., et al. Clinical course and outcome of human monkeypox in Nigeria. Clin Infect Dis. 2020;71(8):e210–e214. doi: 10.1093/cid/ciaa143. [DOI] [PubMed] [Google Scholar]
- 10.O'Shea J., Filardo T.D., Morris S.B., Weiser J., Petersen B., et al. Interim guidance for the prevention and treatment of monkeypox in persons with HIV infection: United States, August 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1023–1028. doi: 10.15585/mmwr.mm7132e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel A., Bilinska J., Tam J.C.H., Da Silva Fontoura D., Mason C.Y., et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378 doi: 10.1136/bmj-2022-072410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization (WHO). Available from: 〈https://www.who.int/es/news-room/fact-sheets/detail/hiv-aids〉. [Accessed 24 August 2022].
- 13.Yinka-Ogunleye A., Aruna O., Dalhat M., Ogoina D., McCollum A., Disu Y., et al. Outbreak of human monkeypox in Nigeria in 2017-18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19(8):872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chew C.C., Lim X.J., Chang C.T., Rajan P., Nasir N., et al. Experiences of social stigma among patients tested positive for COVID-19 and their family members: a qualitative study. BMC Public Health. 2021;21(1):1623. doi: 10.1186/s12889-021-11679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]