Abstract
Purpose:
To report 5-year treatment outcomes in the Primary Tube Versus Trabeculectomy (PTVT) Study.
Design:
Multicenter randomized clinical trial.
Participants:
242 eyes of 242 patients with medically uncontrolled glaucoma and no previous incisional ocular surgery, including 125 patients in the tube group and 117 patients in the trabeculectomy group.
Methods:
Patients were enrolled at 16 Clinical Centers and randomly assigned to treatment with a tube shunt (350-mm2 Baerveldt glaucoma implant) or trabeculectomy with mitomycin C (MMC, 0.4 mg/ml for 2 minutes).
Main Outcome Measures:
The primary outcome measure was the rate of surgical failure, defined as intraocular pressure (IOP) > 21 mmHg or reduced < 20% from baseline, IOP ≤ 5 mmHg, reoperation for glaucoma, or loss of light perception. Secondary outcome measures included IOP, glaucoma medical therapy, and visual acuity.
Results:
The cumulative probability of failure after 5 years of follow-up was 42% in the tube group and 35% in the trabeculectomy group (P = 0.21; hazard ratio = 1.31; 95% confidence interval = 0.86 to 2.01). At 5 years, IOP (mean ± SD) was 13.4 ± 3.5 mmHg in the tube group and 13.0 ± 5.2 mmHg in the trabeculectomy group (P = 0.52), and the number of glaucoma medications was 2.2 ± 1.3 in the tube group and 1.3 ± 1.4 in the trabeculectomy group (P < 0.001).
Conclusions:
Trabeculectomy with MMC and tube shunt surgery produced similar IOPs after 5 years of follow-up in the PTVT Study, but fewer glaucoma medications were required after trabeculectomy. No significant difference in the rate of surgical failure was observed between the two surgical procedures at 5 years.
PRECIS
Tube shunt implantation and trabeculectomy with mitomycin C had similar rates of surgical failure after 5 years of follow-up in the PTVT Study.
The treatment of glaucoma involves lowering intraocular pressure (IOP) to prevent or slow optic nerve damage. Glaucoma surgery is indicated when medical therapy and appropriate laser treatment do not provide adequate IOP reduction. The surgical options for managing glaucoma have expanded exponentially in recent years, however, trabeculectomy and tube shunt surgery remain the most effective operations to decrease IOP. These traditional glaucoma procedures divert aqueous humor to the subconjunctival space, and they generally achieve lower levels of IOP than minimally invasive glaucoma surgery (MIGS) that augments drainage of aqueous through the natural outflow pathway. A dose-response relationship exists between IOP control and glaucomatous progression;1 glaucoma procedures that produce greater IOP reduction are more likely to stabilize the disease.
Practice patterns vary in the surgical treatment of glaucoma. An anonymous survey of the American Glaucoma Society membership in 2016 showed that several different approaches are used in the operative management of primary open-angle glaucoma in eyes without prior ocular surgery.2 Trabeculectomy with mitomycin C (MMC) and tube shunt implantation were the most popular initial incisional glaucoma procedures used in 59% and 23% of patients, respectively. The lack of consensus among glaucoma surgeons regarding the preferred operation for eyes at low risk for filtration failure likely relates to the fact that limited clinical data are available directly comparing trabeculectomy and tube shunts as primary glaucoma procedures.
The Primary Tube Versus Trabeculectomy (PTVT) Study is a multicenter randomized clinical trial comparing the safety and efficacy of tube shunt surgery and trabeculectomy with MMC in eyes without prior incisional ocular surgery. The goal of this investigator-initiated study is to provide information that will assist in surgical decision making in similar patient groups. The methodology and outcomes during the first 3 years of the study have been described in previous publications.3–6 This paper reports 5-year follow-up data on enrolled patients.
METHODS
The study was approved by the Institutional Review Board at each Clinical Center before recruitment was initiated. Written informed consent was obtained from all subjects for both treatment and participation in the research study. The protocol adhered to the Declaration of Helsinki and the Health Insurance Portability and Accountability Act. This study was registered at http://www.clinicaltrials.gov (NCT00666237). The design and methods of the PTVT Study were previously described in detail,6 and they are summarized as follows:
Eligibility Criteria
Patients aged 18 to 85 years who had no previous incisional ocular surgery and inadequately controlled glaucoma with IOP ≥ 18 mmHg and ≤ 40 mmHg on tolerated medical therapy were eligible for the study. Exclusion criteria included no light perception vision, pregnant or nursing women, narrow anterior chamber angle, iris neovascularization or proliferative retinopathy, iridocorneal endothelial syndrome, epithelial or fibrous downgrowth, chronic or recurrent uveitis, steroid-induced glaucoma, severe posterior blepharitis, unwillingness to discontinue contact lens use after surgery, previous cyclodestructive procedure, conjunctival scarring from prior ocular trauma or cicatrizing disease precluding a superior trabeculectomy, functionally significant cataract, need for glaucoma surgery combined with other ocular procedures or anticipated need for additional ocular surgery, unwillingness or inability to give consent, unwillingness to accept randomization, or inability to return for scheduled protocol visits. Only 1 eye of eligible patients was included in the study.
Randomization and Treatment
The PTVT Study was conducted at 16 Clinical Centers (see Appendix for a list of Clinical Centers and Committees in the PTVT Study, available at www.aaojournal.org). Eligibility was independently confirmed at the Statistical Coordinating Center. Patients enrolled in the study were randomly assigned to treatment with a Baerveldt glaucoma implant (Johnson & Johnson Vision, Santa Ana, CA) or trabeculectomy with MMC. Randomization was performed with a permuted block design stratified by age, race, and presence of failed filtering surgery in the non-study eye, as well as Clinical Center. Neither the patient nor the clinician was masked to the randomization assignment.
A 350-mm2 Baerveldt glaucoma implant was placed in the superotemporal quadrant in patients randomized to the tube group. A limbus-based or fornix-based conjunctival flap was dissected, and the end plate was sutured to sclera approximately 10 mm posterior to the limbus. The tube was completely occluded to temporarily restrict flow through the device until encapsulation of the end plate occurred. The surgeon was given the option of fenestrating the tube for early IOP reduction. The tube was inserted into the anterior chamber through a 23-gauge needle track. A patch graft was used to cover the limbal portion of the tube, and the conjunctiva was closed.
Patients randomized to the trabeculectomy group underwent a trabeculectomy with MMC. A limbus-based or fornix-based flap was created, and a fluid-retaining sponge soaked in MMC (0.4 mg/ml) was applied to the superior sclera for 2 minutes. A partial thickness scleral flap was dissected, and a paracentesis was made. A block of limbal tissue was excised underneath the trabeculectomy flap. The scleral flap was reapproximated to the scleral bed with interrupted or releasable 10–0 nylon sutures. The conjunctiva was closed, and Seidel testing was performed at the conclusion of the case.
Patient Visits
Baseline demographic and clinical information was obtained for enrolled patients. Follow-up visits were scheduled at 1 day, 1 week, 1 month, 3 months, 6 months, 1 year, 18 months, 2 years, 3 years, 4 years, and 5 years postoperatively. Data were collected with standardized forms. Additional information was acquired for patients undergoing a reoperation, including the date of surgery, type of procedure, and IOP level and number of glaucoma medications immediately before reoperation.
Outcome Measures
The primary outcome measure in the PTVT Study was the cumulative rate of surgical failure at 1 year, but additional reporting of data was planned for 3 and 5 years during the design of the trial. Secondary outcomes measures included IOP, use of glaucoma medications, and visual acuity (VA). Failure was defined a priori as IOP > 21 mmHg or reduced < 20% below baseline on 2 consecutive follow-up visits after 3 months, IOP ≤ 5 mmHg on 2 consecutive follow-up visits after 3 months, reoperation for glaucoma, or loss of light perception vision. Reoperation for glaucoma was defined as additional glaucoma surgery requiring a return to the operating room, such as placement of a tube shunt or trabeculectomy. Cyclodestruction was also considered a reoperation for glaucoma, regardless of whether it was performed in the clinic or operating room. Interventions performed at the slit lamp, such as needling procedures and laser suture lysis, were not counted as glaucoma reoperations. Eyes that had not failed by the above criteria and were not on supplemental medical therapy were categorized as complete successes, and those receiving glaucoma medications were classified as qualified successes. Investigators were asked to provide a reason for loss of 2 or more lines of Snellen VA from baseline at follow-up visits after 3 months. Study outcomes were regularly reviewed by an independent Safety and Data Monitoring Committee.
Sample Size Calculations
Sample size calculations were performed based on projected differences in failure rates between treatment groups. Enrollment of 88 patients in each treatment group was expected to detect a relative risk of failure of 2.0 at 5 years assuming a 20% failure rate in the lower risk group with a two-sided significance level of 0.05, a power of 0.80, and analysis with Yates corrected chi-square. A total of 242 patients were recruited for the study to allow for a drop-out rate of 6% per year.
Statistical Analysis
Univariate comparisons between treatment groups were performed with the two-sided Student t-test for continuous variables and the Fisher exact or chi-square test (asymptotic, Yates corrected, or exact permutation as appropriate) for categorical variables. Snellen VA measurements were converted to logMAR equivalents for the purpose of data analysis. The time to failure was defined as the time from surgical treatment to reoperation for glaucoma, loss of light perception vision, or the first of 2 consecutive study visits after 3 months in which the patient had persistent hypotony (i.e., IOP ≤ 5 mmHg) or inadequately reduced IOP (i.e., IOP > 21 mmHg or reduced < 20% below baseline). Treatment comparisons of cumulative rate of failure and reoperation for glaucoma were assessed with the stratified Kaplan-Meier survival analysis log-rank test. The design and analysis of data from the PTVT Study were modeled after the Tube Versus Trabeculectomy (TVT) Study. A P-value < 0.05 was considered statistically significant in our analyses.
RESULTS
Recruitment and Retention
A total of 242 eyes of 242 patients were enrolled and underwent surgical treatment between May 2008 and March 2015, including 125 patients in the tube group and 117 patients in the trabeculectomy group. The progress of patients in the study is shown in Figure 1 (available at www.aaojournal.org). During the first 5 years, the visit completion rate was 90.3% in both treatment groups (P = 0.99, chi-square test).
Figure 1.
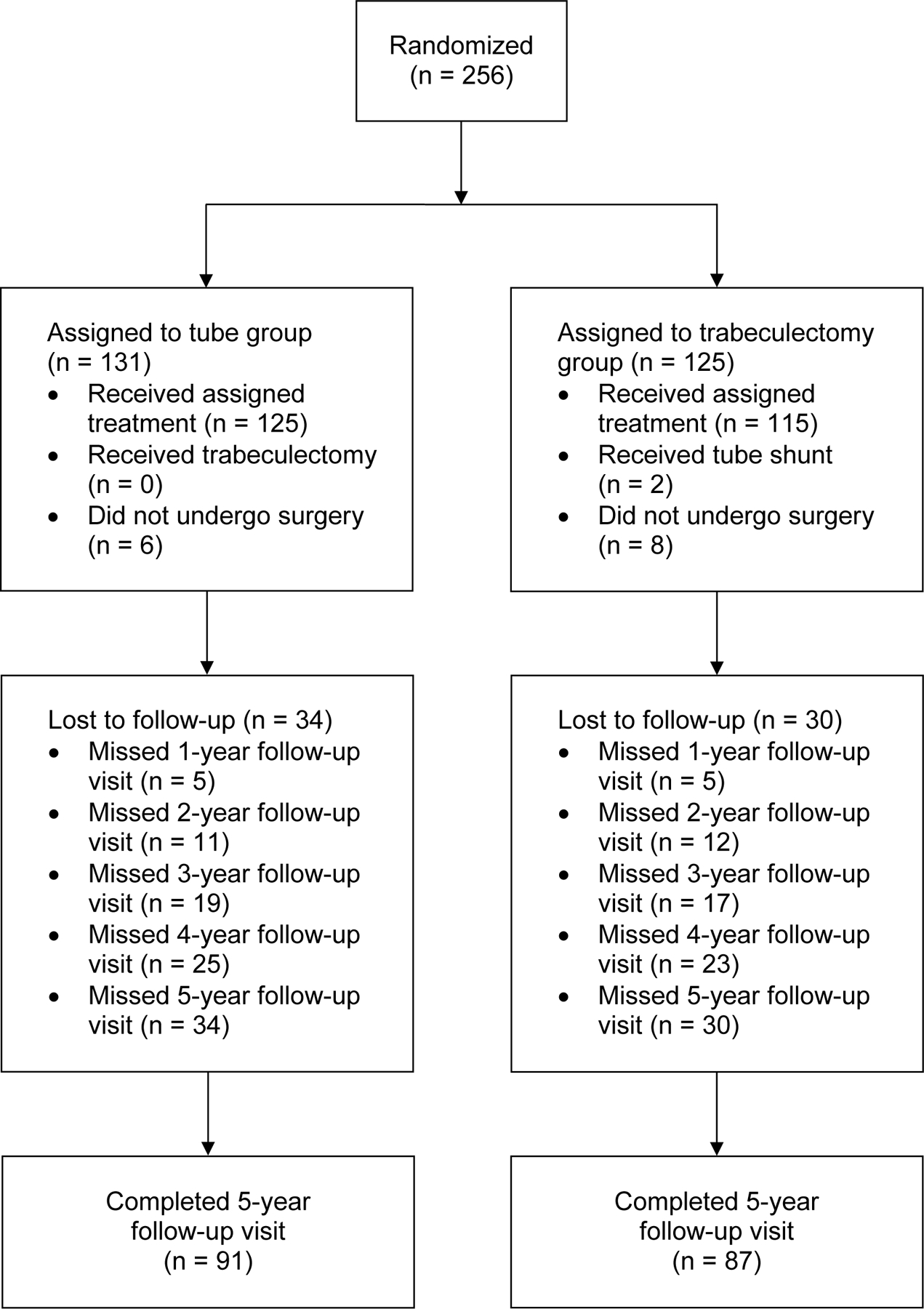
Flowchart of patient progress in the PTVT Study.
Protocol Violations
One patient who was randomized to the trabeculectomy group had laser-assisted in-situ keratomileusis in the study eye and should have been excluded because of previous incisional ocular surgery. Two patients were randomized to the trabeculectomy group but underwent placement of a tube shunt because of surgeon error. All patients were analyzed according to the treatment group to which they were assigned by randomization in an intent-to-treat analysis. None of the 3 patients who violated the study protocol experienced treatment failure or underwent additional ocular surgery.
Baseline Characteristics
The baseline characteristics of the study population are presented in Table 1. The age (mean ± SD) of the study population at enrollment was 61.4 ± 11.8 years, and 160 patients (66%) were male. The IOP (mean ± SD) of the overall study group was 23.6 ± 5.3 mmHg, and the number of glaucoma medications (mean ± SD) was 3.2 ± 1.1. The most common diagnosis was primary open-angle glaucoma in 218 eyes (90%). The mean deviation with Humphrey visual field testing (mean ± SD) was −14.6 ± 9.9 dB. There were no significant differences in any of the demographic or ocular features between treatment groups at enrollment.
Table 1.
Baseline Characteristics of PTVT Study Patients
| Tube Group (n = 125) | Trabeculectomy Group (n = 117) | |
|---|---|---|
| Age (years) | ||
| Mean ± SD | 62.0 ± 11.4 | 60.8 ± 12.3 |
| Median (range) | 62 (28–85) | 61 (21–85) |
|
| ||
| Gender, n (%) | ||
| Male | 84 (67) | 76 (65) |
| Female | 41 (33) | 41 (35) |
|
| ||
| Race, n (%) | ||
| Black | 59 (47) | 57 (49) |
| White | 50 (40) | 45 (39) |
| Hispanic | 9 (7) | 6 (5) |
| Asian | 6 (5) | 7 (6) |
| Other | 1 (1) | 2 (2) |
|
| ||
| Hypertension, n (%) | 63 (50) | 55 (47) |
|
| ||
| Diabetes mellitus, n (%) | 18 (14) | 27 (23) |
|
| ||
| Study eye, n (%) | ||
| Right | 68 (54) | 60 (51) |
| Left | 57 (46) | 57 (49) |
|
| ||
| IOP (mmHg) | ||
| Mean ± SD | 23.3 ± 4.9 | 23.9 ± 5.7 |
| Range | 18–40 | 18–40 |
|
| ||
| Central corneal thickness (microns), mean ± SD | 525 ± 37 | 524 ± 33 |
|
| ||
| Glaucoma medications | ||
| Mean ± SD | 3.1 ± 1.1 | 3.2 ± 1.1 |
| Range | 0–6 | 0–5 |
|
| ||
| Diagnosis, n (%) | ||
| POAG | 109 (87) | 109 (93) |
| CACG | 5 (4) | 3 (3) |
| PG | 4 (3) | 2 (2) |
| PXFG | 4 (3) | 1 (1) |
| Other | 3 (2) | 2 (2) |
|
| ||
| Previous ocular laser treatment, n (%) | ||
| LTP | 34 (27) | 29 (25) |
| LPI | 11 (9) | 2 (2) |
| Other | 9 (7) | 5 (4) |
|
| ||
| ETDRS VA, mean ± SD | 73 ± 20 | 73 ± 20 |
|
| ||
| Snellen VA | ||
| LogMAR mean ± SD | 0.20 ± 0.42 | 0.25 ± 0.51 |
| Median | 20/25 | 20/25 |
| Range | 20/13-HM | 20/13-LP |
|
| ||
| Cataract, n (%) | 76 (61) | 65 (56) |
| Mild | 62 (82) | 50 (77) |
| Moderate | 13 (17) | 14 (22) |
| Severe | 1 (1) | 1 (1) |
|
| ||
| Diplopia, n (%) | 8 (6) | 4 (3) |
|
| ||
| Humphrey visual fields | ||
| MD, mean ± SD | −14.5 ± 10.2 | −14.7 ± 9.7 |
| PSD, mean ± SD | 7.71 ± 3.86 | 8.19 ± 3.57 |
|
| ||
| Stratum, n (%)* | ||
| 1 | 55 (44) | 53 (45) |
| 2 | 8 (6) | 5 (4) |
| 3 | 62 (50) | 59 (50) |
CACG = chronic angle-closure glaucoma; CF = count fingers; ETDRS = Early Treatment Diabetic Retinopathy Study; IOP = intraocular pressure; LP = light perception; LPI = laser iridotomy; LTP = laser trabeculoplasty; MD = mean deviation; PG = pigmentary glaucoma; POAG = primary open-angle glaucoma; PSD = pattern standard deviation; PXFG = pseudoexfoliation glaucoma; SD = standard deviation
Stratum 1 = no failed glaucoma surgery in fellow eye and age ≥ 50 years and not black race; stratum 2 = failed glaucoma surgery in fellow eye; stratum 3 = no failed glaucoma surgery in fellow eye and age < 50 years and/or black race
Treatment Outcomes
The outcomes of randomized patients unadjusted for follow-up time are presented in Table 2 (available at www.aaojournal.org). All patients who completed 5-year follow-up visits and/or experienced prior failure were included in this analysis. Treatment failure had occurred in 49 patients (48%) in the tube group and 38 patients (39%) in the trabeculectomy group at 5 years (P = 0.23, logistic regression analysis adjusted for stratum). In the tube group, 9 patients (9%) were classified as complete successes and 45 (44%) patients were qualified successes. In the trabeculectomy group, 33 patients (34%) were complete successes and 26 patients (27%) were qualified successes. While the overall success rate was similar between treatment groups, the rate of complete success was significantly higher in the trabeculectomy group relative to the tube group (P < 0.001, logistic regression analysis adjusted for stratum).
Table 2.
Treatment Outcomes After 5 Years of Follow-up in the PTVT Study
| Tube Group (n = 103) | Trabeculectomy Group (n = 97) | |
|---|---|---|
| Stratum 1: No failed glaucoma surgery in fellow eye and age ≥ 50 years and not black race | ||
| Failure | 23 (48) | 13 (29) |
| Qualified success | 21 (44) | 13 (29) |
| Complete success | 4 (8) | 19 (42) |
|
| ||
| Stratum 2: Failed glaucoma surgery in fellow eye | ||
| Failure | 3 (50) | 0 |
| Qualified success | 2 (33) | 1 (50) |
| Complete success | 1 (17) | 1 (50) |
|
| ||
| Stratum 3: No failed glaucoma surgery in fellow eye and age < 50 years and/or black race | ||
| Failure | 23 (47) | 25 (50) |
| Qualified success | 22 (45) | 12 (24) |
| Complete success | 4 (8) | 13 (26) |
|
| ||
| Overall group | ||
| Failure* | 49 (48) | 38 (39) |
| Qualified success | 45 (44) | 26 (27) |
| Complete success† | 9 (9) | 33 (34) |
Data presented as number of patients (percentage)
P = 0.23 for the difference in failure rates between treatment groups (logistic regression analysis adjusted for stratum)
P < 0.001 for the difference in complete success rates between treatment groups (logistic regression analysis adjusted for stratum)
Kaplan-Meier survival analysis was also used to compare failure rates between the two treatment groups, and the results are presented in Figure 2. The cumulative probability of failure was 42% in the tube group and 35% in the trabeculectomy group at 5 years (P = 0.21, log rank test adjusted for stratum; hazard ratio = 1.31; 95% confidence interval = 0.86 to 2.01). No significant differences in treatment efficacy were found between strata (P = 0.27, Cox regression analysis).
Figure 2.
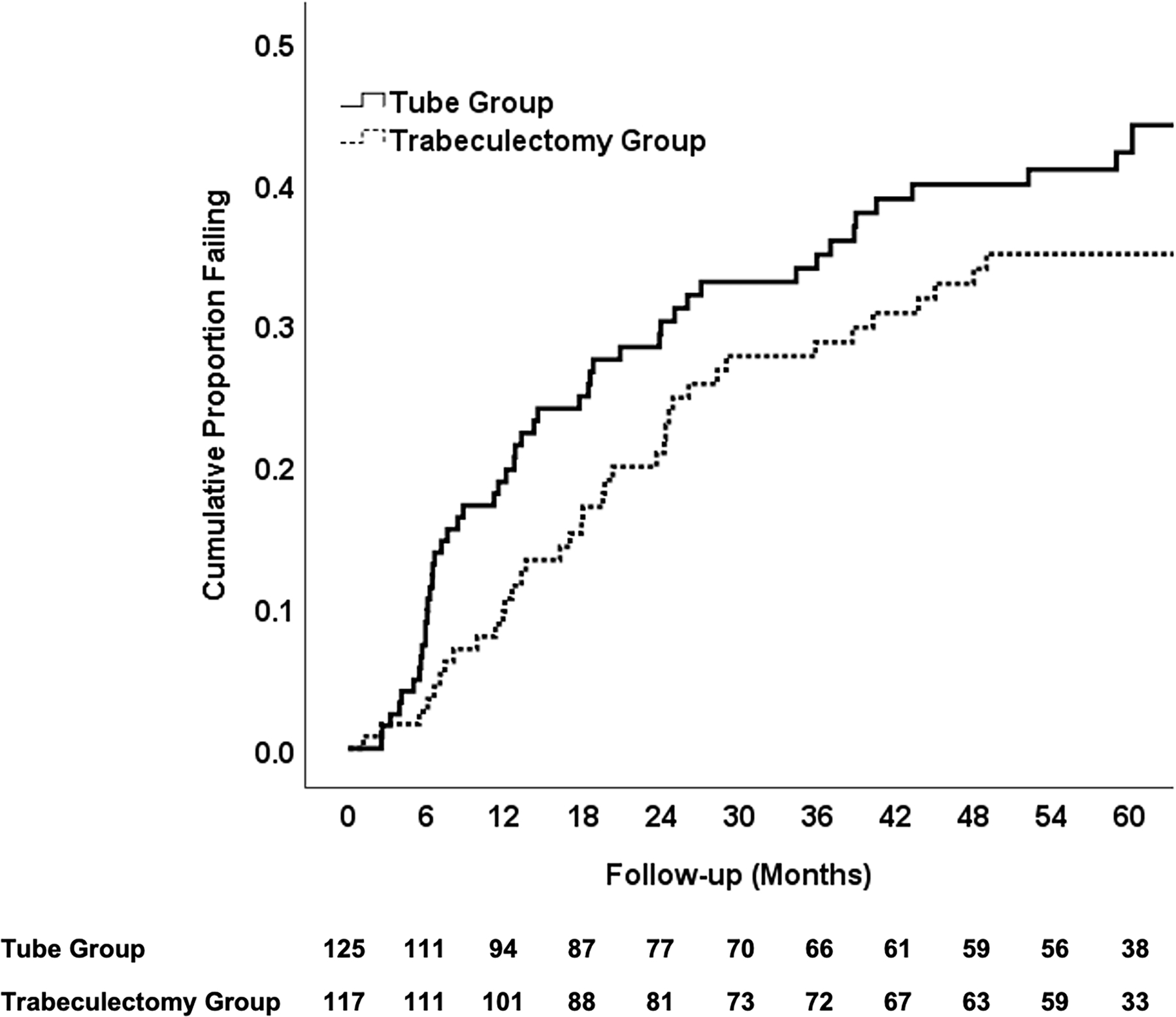
Kaplan-Meier plots showing the cumulative probability of failure in the PTVT Study. The number of patients at risk at each follow-up visit is shown at the bottom.
Figure 3 shows the failure rates for the two treatment groups with alternative outcome criteria. Patients with persistent hypotony, reoperation for glaucoma, or loss of light perception vision were still classified as treatment failures; however, the upper IOP limit distinguishing success from failure was changed. When inadequate IOP reduction was defined as IOP > 17 mmHg or reduced < 20% from baseline on 2 consecutive follow-up visits after 3 months, the cumulative probability of failure at 5 years was 49% in the tube group and 37% in the trabeculectomy group (P = 0.051, log rank test adjusted for stratum; hazard ratio = 1.49; 95% confidence interval = 1.00 to 2.24). When inadequate IOP reduction was defined as IOP > 14 mmHg on 2 consecutive visits after 3 months, the cumulative probability of failure was 64% in the tube group and 57% in the trabeculectomy group at 5 years (P = 0.28, log rank test adjusted for stratum; hazard ratio = 1.21; 95% confidence interval = 0.86 to 1.69).
Figure 3.
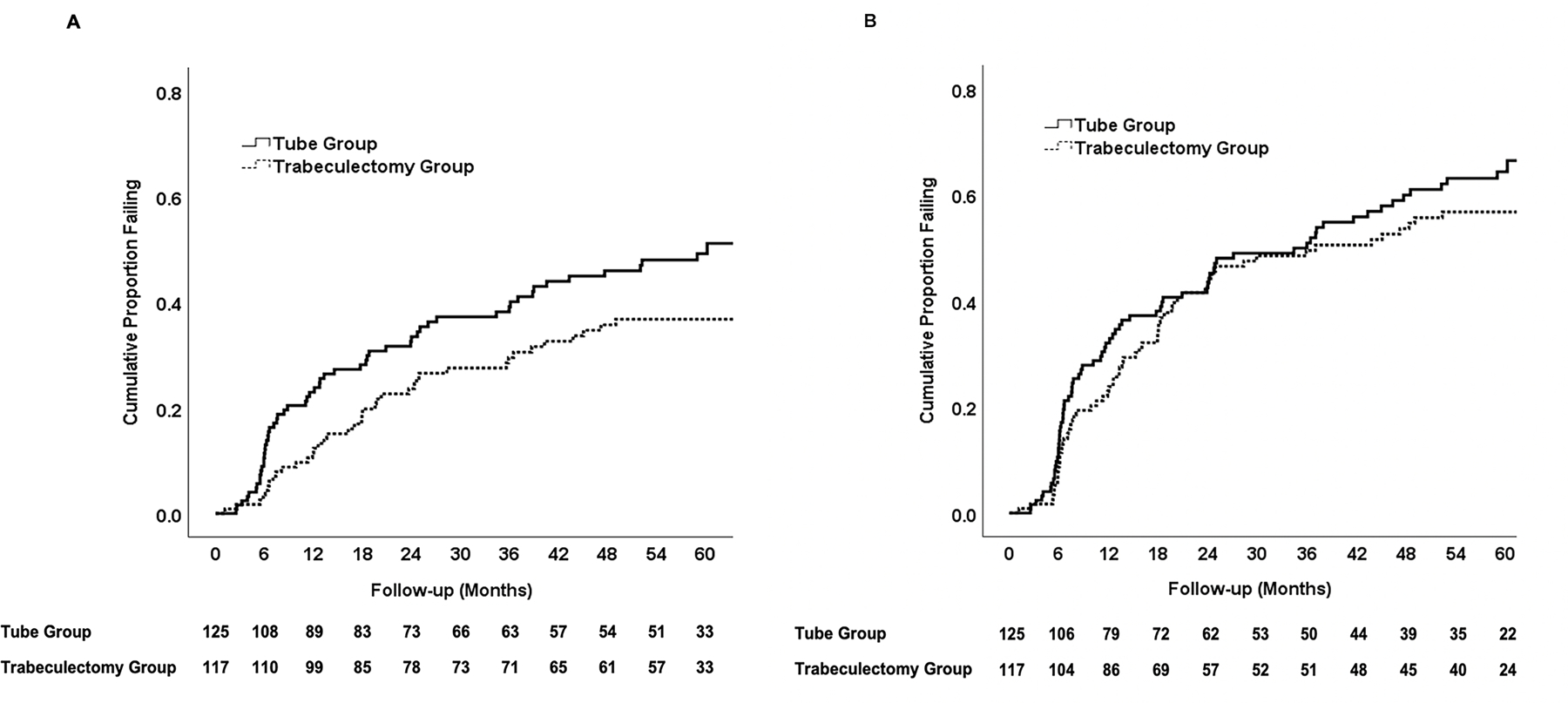
Kaplan-Meier plots showing the cumulative probability of failure in the PTVT Study defining inadequate intraocular pressure (IOP) reduction as IOP > 17 mmHg or reduced < 20% below baseline (A) or IOP > 14 mmHg (B). Inadequate IOP reduction criteria must have been present on 2 consecutive visits after 3 months to qualify as failure. Patients with persistent hypotony, reoperation for glaucoma, and loss of light perception vision are classified as failures. The number of patients at risk at each follow-up visit is shown at the bottom.
The reasons for classification as a treatment failure are listed in Table 3. Inadequate IOP reduction (i.e., IOP > 21 mmHg or reduced < 20% below baseline on 2 consecutive follow-up visits after 3 months) was the most common cause for failure during the first 5 years of follow-up in both treatment groups, occurring in 31 patients in the tube group and 23 patients in the trabeculectomy group. A reoperation for glaucoma was performed in 18 patients in the tube group and 9 patients in the trabeculectomy group before the failure criteria for inadequate IOP reduction were met. Persistent hypotony (i.e., IOP ≤ 5 mmHg on 2 consecutive visits after 3 months) was the cause for treatment failure in 5 patients in the trabeculectomy group. Loss of VA from baseline was seen in all patients with hypotony failure. Loss of light perception vision occurred in 1 patient in the trabeculectomy group. A significant difference in the distribution of reasons for failure was seen between treatment groups with more failures occurring because of glaucoma reoperations in the tube group and more hypotony failures in the trabeculectomy group (P = 0.016, exact permutation chi-square test).
Table 3.
Reasons for Treatment Failure in the PTVT Study
| Tube Group (n = 49) | Trabeculectomy Group (n = 38) | |
|---|---|---|
| Inadequate IOP reduction* | 31 (63) | 23 (61) |
| Reoperation for glaucoma | 18 (37) | 9 (24) |
| Persistent hypotony† | 0 | 5 (13) |
| Loss of light perception | 0 | 1 (3) |
IOP = intraocular pressure
Data are presented as number of patients (percentage)
IOP > 21 mmHg or reduced < 20% below baseline on 2 consecutive follow-up visits after 3 months
IOP ≤ 5 mmHg on 2 consecutive follow-up visits after 3 months
P = 0.016 for the difference in distribution of reasons for failure between treatment groups (exact 4 × 2 permutation chi-square test)
Baseline demographic and clinical characteristics were evaluated as possible predictors for treatment failure and are shown in Table 4. Treatment failures were pooled from both treatment groups for this risk factor analysis. Only lower preoperative IOP was significantly associated with treatment outcome in univariable (P < 0.001, log rank test) and multivariable (P < 0.001, Cox proportional hazards regression analysis) analyses. Stratum, age, gender, race, hypertension, diabetes mellitus, previous ocular laser treatment, preoperative number of glaucoma medications, preoperative Humphrey visual field mean deviation, Clinical Centers, and randomized treatment were not associated with failure either univariably or in a multivariable model. A significant treatment interaction was observed between preoperative IOP and failure. Figure 4 shows Kaplan-Meier survival analysis subdividing patients based on treatment group and preoperative IOP. In the tube group, the cumulative probability of failure was 75% in the 47 patients with baseline IOP < 21 mmHg, 29% in the 47 patients with IOP 21 to 25 mmHg, and 10% in the 31 patients with IOP > 25 mmHg. In the trabeculectomy group, the cumulative probability of failure was 50% in the 44 patient with preoperative IOP < 21 mmHg, 17% in the 37 patients with IOP 21 to 25 mmHg, and 37% in the 36 patients with IOP > 25 mmHg.
Table 4.
Risk Factor Analysis for Failure in the PTVT Study
| Risk Factor | Number (%) | Cumulative Probability of Failure at 5 Years (%)† |
P-value |
|
|---|---|---|---|---|
| Univariable‡ | Multivariable§ | |||
| Stratum* | 0.54 | 0.55 | ||
| 1 | 108 (45) | 35 | ||
| 2 | 13 (5) | 29 | ||
| 3 | 121 (50) | 45 | ||
|
| ||||
| Age | 0.096 | 0.33 | ||
| <50 | 39 (16) | 51 | ||
| 50 to 70 | 142 (59) | 42 | ||
| > 70 | 61 (25) | 27 | ||
|
| ||||
| Gender | 0.56 | 0.95 | ||
| Male | 160 (66) | 39 | ||
| Female | 82 (34) | 40 | ||
|
| ||||
| Race | 0.22 | 0.29 | ||
| Black | 116 (48) | 48 | ||
| White | 95 (39) | 33 | ||
| Other | 31 (13) | 32 | ||
|
| ||||
| Hypertension | 0.50 | 0.80 | ||
| Yes | 118 (49) | 42 | ||
| No | 124 (51) | 38 | ||
|
| ||||
| Diabetes mellitus | 0.57 | 0.65 | ||
| Yes | 45 (19) | 37 | ||
| No | 197 (81) | 40 | ||
|
| ||||
| Previous ocular laser treatment | 0.82 | 0.23 | ||
| Yes | 83 (34) | 41 | ||
| No | 159 (66) | 39 | ||
|
| ||||
| Preoperative IOP (mmHg) | < 0.001 | < 0.001 | ||
| < 21 | 91 (38) | 63 | ||
| 21 to 25 | 84 (35) | 23 | ||
| > 25 | 67 (28) | 28 | ||
|
| ||||
| Preoperative number of glaucoma medications | 0.65 | 0.43 | ||
| 0 to 2 | 57 (24) | 41 | ||
| 3 | 87 (36) | 35 | ||
| > 3 | 98 (41) | 44 | ||
|
| ||||
| Preoperative Snellen VA | 0.81 | 0.96 | ||
| ≥ 20/50 | 207 (86) | 39 | ||
| ≤ 20/60 | 34 (14) | 46 | ||
|
| ||||
| Preoperative HVF MD (dB) | 0.97 | 0.88 | ||
| > −7.0 | 65 (28) | 34 | ||
| −7.0 to −17.0 | 84 (36) | 39 | ||
| < −17.0 | 83 (36) | 43 | ||
|
| ||||
| Clinical centers | 0.32 | 0.15 | ||
| Enrolled ≥ 50% patients | 145 (60) | 36 | ||
| Enrolled < 50% patients | 97 (40) | 45 | ||
|
| ||||
| Treatment | 0.21 | 0.065 | ||
| Tube | 125 (52) | 42 | ||
| Trabeculectomy | 117 (48) | 35 | ||
HVF = Humphrey visual field; IOP = intraocular pressure; MD = mean deviation; VA = visual acuity
Stratum 1 = no failed glaucoma surgery in fellow eye and age ≥ 50 years and not black race; stratum 2 = failed glaucoma surgery in fellow eye; stratum 3 = no failed glaucoma surgery in fellow eye and age < 50 years and/or black race
Kaplan-Meier survival analysis
Log-rank test
Analysis conducted with Cox proportional hazards multiple regression. Randomization stratum and randomized treatment group were included in all models, and other variables in the table were allowed stepwise inclusion.
Figure 4.
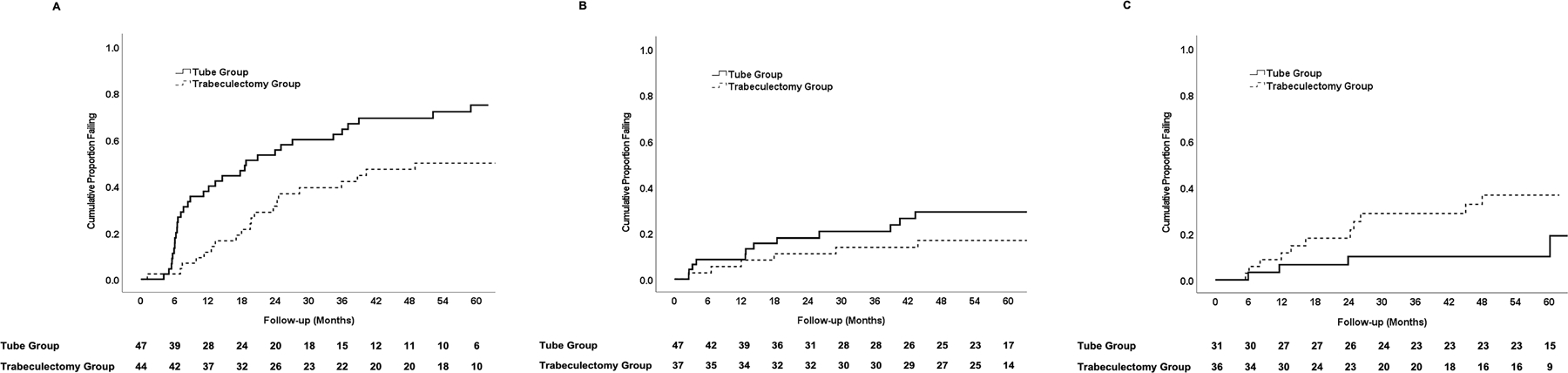
Kaplan-Meier plots showing the cumulative probability of failure in the PTVT Study among patients with preoperative intraocular pressure < 21 mmHg (A), 21–25 mmHg (B), and > 25 mmHg (C). The number of patients at risk at each follow-up visit is shown at the bottom.
Intraocular Pressure
Baseline and follow-up IOP measurements for the tube and trabeculectomy groups are provided in Table 5, and they are also graphically presented in Figures 5 and 6 (available at www.aaojournal.org). Patients who underwent additional glaucoma surgery were censored from analysis after reoperation. At 5 years, IOP (mean ± SD) was 13.4 ± 3.5 mmHg in the tube group and 13.0 ± 5.2 mmHg in the trabeculectomy group (P = 0.52, Student t-test). Among patients who completed 5-year follow-up visits, IOP reduction from baseline (mean ± SD) was 10.2 ± 6.3 mmHg (44%) in the tube group and 10.5 ± 7.4 mmHg (44%) in the trabeculectomy group (P = 0.77, Student t-test). At 5 years, 51 patients (69%) in the tube group and 42 patients (56%) in the trabeculectomy group had an IOP of 14 mmHg or less (P = 0.15, chi-square test).
Table 5.
Intraocular Pressure and Medical Therapy at Baseline and Follow-up in the PTVT Study
| Tube Group | Trabeculectomy Group | P-value* | |
|---|---|---|---|
| Baseline | |||
| IOP (mmHg) | 23.3 ± 4.9 | 23.9 ± 5.7 | 0.35 |
| Glaucoma medications | 3.1 ± 1.1 | 3.2 ± 1.1 | 0.56 |
| N | 125 | 117 | |
|
| |||
| 1 year | |||
| IOP (mmHg) | 13.8 ± 4.1 | 12.4 ± 4.4 | 0.012 |
| Glaucoma medications | 2.1 ± 1.4 | 0.9 ± 1.4 | < 0.001 |
| N | 108 | 105 | |
|
| |||
| 18 months | |||
| IOP (mmHg) | 13.5 ± 4.1 | 12.8 ± 4.7 | 0.33 |
| Glaucoma medications | 2.1 ± 1.3 | 0.8 ± 1.3 | < 0.001 |
| N | 99 | 97 | |
|
| |||
| 2 years | |||
| IOP (mmHg) | 13.6 ± 3.9 | 12.9 ± 5.2 | 0.28 |
| Glaucoma medications | 2.2 ± 1.0 | 1.0 ± 1.5 | < 0.001 |
| N | 99 | 96 | |
|
| |||
| 3 years | |||
| IOP (mmHg) | 13.9 ± 4.2 | 12.1 ± 4.8 | 0.010 |
| Glaucoma medications | 2.1 ± 1.4 | 1.2 ± 1.5 | < 0.001 |
| N | 87 | 88 | |
|
| |||
| 4 years | |||
| IOP (mmHg) | 13.5 ± 3.5 | 13.5 ± 5.4 | 0.98 |
| Glaucoma medications | 2.3 ± 1.4 | 1.3 ± 1.4 | < 0.001 |
| N | 83 | 79 | |
|
| |||
| 5 years | |||
| IOP (mmHg) | 13.4 ± 3.5 | 13.0 ± 5.2 | 0.52 |
| Glaucoma medications | 2.2 ± 1.3 | 1.3 ± 1.4 | < 0.001 |
| N | 74 | 75 | |
IOP = intraocular pressure
Data are presented as mean ± standard deviation
Data censored after a reoperation for glaucoma
Student t-test
Figure 5.
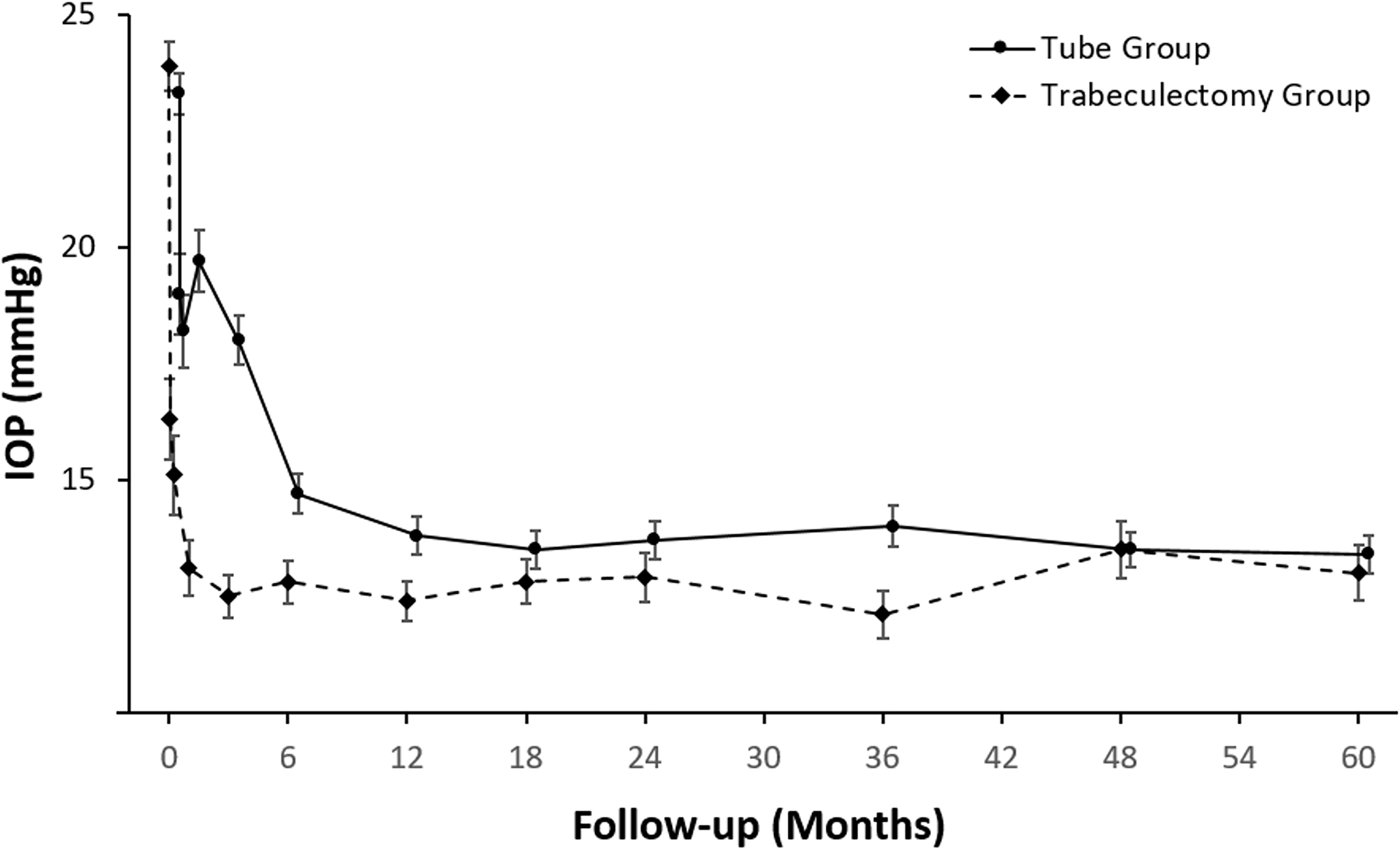
IOP at baseline and follow-up in the PTVT Study. Data are presented as mean ± standard error of the mean. Patients were censored after a reoperation for glaucoma.
Figure 6.
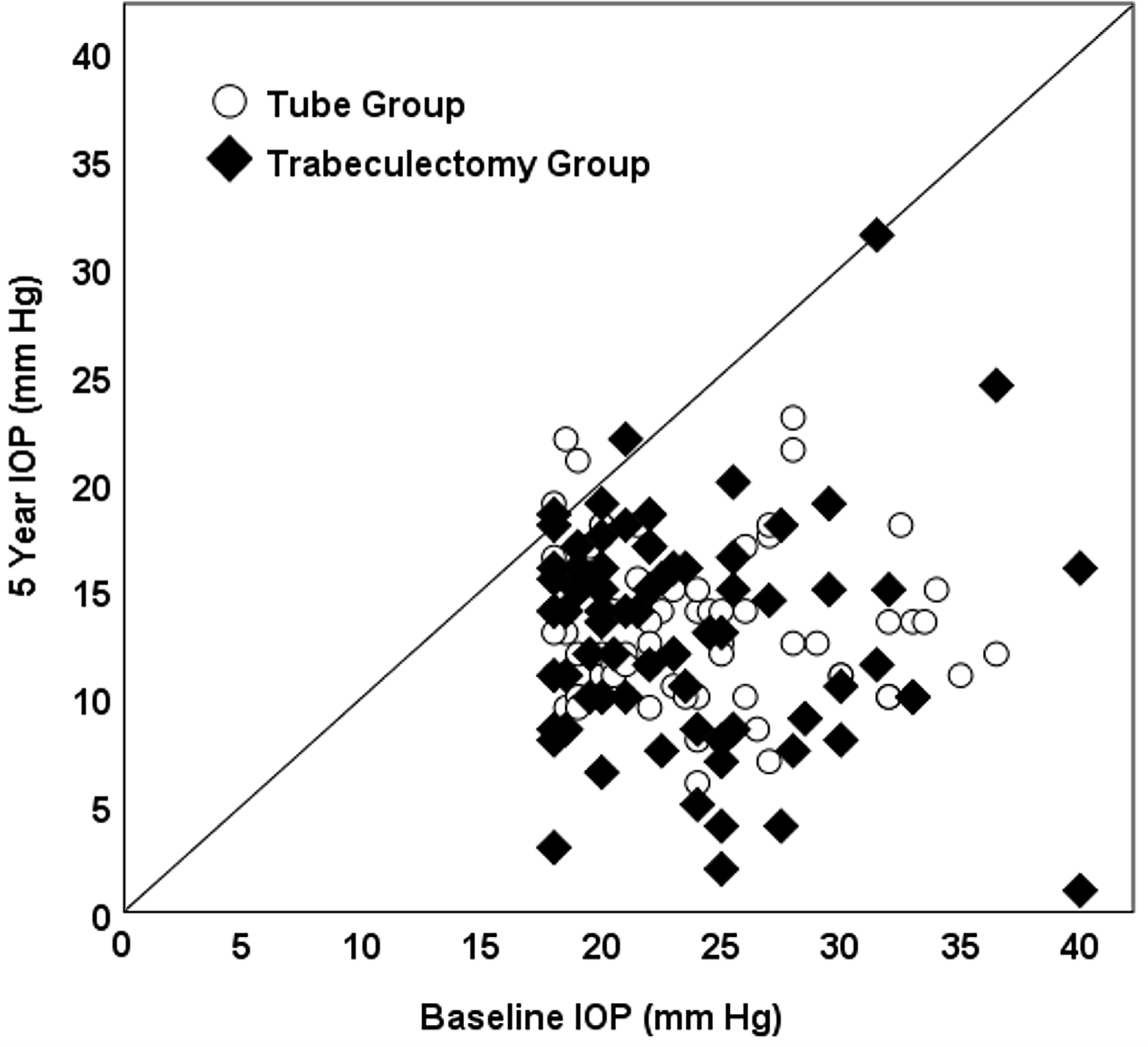
Scatter plot of IOP data in the PTVT Study. Each point represents a patient in the tube group (circle) or trabeculectomy group (diamond) showing the IOP at baseline and 5 years postoperatively. The oblique line indicates no change. Patients were censored after a reoperation for glaucoma.
An analysis was performed carrying the last observation forward, which included the last IOP prior to glaucoma reoperation for patients who had additional glaucoma surgery and the last study visit for patients with missing follow-up. At 5 years, IOP (mean ± SD) was 14.8 ± 5.3 mmHg in the tube group and 13.9 ± 5.8 mmHg in the trabeculectomy group (P = 0.18, Student t-test) with the last observation carried forward. An assessment of IOP was also made for all patients, including those who underwent further surgery for glaucoma. At 5 years, IOP (mean ± SD) was 13.4 ± 3.7 mmHg in the tube group and 13.4 ± 5.3 mmHg in the trabeculectomy group (P = 0.94, Student t-test) considering all medical and surgical management.
Medical Therapy
Table 5 shows the number of glaucoma medications in the tube and trabeculectomy groups at baseline and follow-up. Patients were censored from analysis after a reoperation for glaucoma. At 5 years, the number of glaucoma medications (mean ± SD) was 2.2 ± 1.3 in the tube group and 1.3 ± 1.4 in the trabeculectomy group (P < 0.001, Student t-test). Among patients with qualified success at 5 years, the mean number of glaucoma medications was 2.4 ± 1.0 in the tube group and 2.5 ± 0.7 in the trabeculectomy group (P = 0.94). The number of glaucoma medications (mean ± SD) decreased from baseline by 0.8 ± 1.6 in the tube group and 1.7 ± 1.7 in the trabeculectomy group in patients who completed 5-year follow-up visits (P < 0.001, Student t-test). Significantly greater use of glaucoma medical therapy was observed in the tube group compared with the trabeculectomy group at all follow-up visits.
The number of glaucoma medications (mean ± SD) was 2.3 ± 1.4 in the tube group and 1.4 ± 1.6 in the trabeculectomy group at 5 years (P < 0.001, Student t-test) with the last observation carried forward. When patients who underwent additional glaucoma surgery were included in the analysis, the number of glaucoma medications (mean ± SD) was 2.2 ± 1.3 in the tube group and 1.2 ± 1.4 in the trabeculectomy group (P < 0.001, Student t-test).
Reoperation for Glaucoma
The 5-year cumulative reoperation rate for glaucoma with Kaplan-Meier survival analysis was 18% in the tube group and 10% in the trabeculectomy group (P = 0.15, log-rank test adjusted for stratum). A total of 21 patients in the tube group underwent additional glaucoma surgery, which involved placement of a second tube shunt in 7 patients, transscleral cyclophotocoagulation in 5 patients, endoscopic cyclophotocoagulation and phacoemulsification in 4 patients, trabeculectomy with MMC in 3 patients, insertion of an InnFocus microshunt (Santen Pharmaceuticals Co., Osaka, Japan) with MMC in 1 patient, and implantation of a Xen gel stent (Allergan, Inc., Irvine, CA) with MMC and phacoemulsification in 1 patient. There were 5 patients in the tube group who required 2 glaucoma reoperations. Among the patients who had a second tube shunt, 1 patient subsequently had transscleral cyclophotocoagulation and 2 patients had endoscopic cyclophotocoagulation and phacoemulsification. In the subgroup of patients who had a trabeculectomy with MMC as a glaucoma reoperation, 1 patient later underwent implantation of a second tube shunt and 1 patient had endoscopic cyclophotocoagulation and phacoemulsification as additional glaucoma surgery. There were 12 patients in the trabeculectomy group who had glaucoma reoperations, including tube shunt placement in 10 patients, a repeat trabeculectomy with MMC in 1 patient, and trabeculectomy revision in 1 patient. Phacoemulsification cataract extraction was performed at the time of tube shunt surgery in 4 patients. One patient who had placement of a tube shunt subsequently underwent implantation of a second tube shunt as another glaucoma reoperation.
Because the surgeon was not masked to the treatment assignment, a potential bias existed with respect to the decision to reoperate for glaucoma. To evaluate for selection bias, the IOP levels were compared between treatment groups in patients who underwent glaucoma reoperation, as well as those who failed because of inadequate IOP reduction but did not have additional glaucoma surgery. The IOP (mean ± SD) was 21.3 ± 4.3 mmHg for the 21 patients in the tube group and 22.3 ± 5.7 mmHg for the 12 patients in the trabeculectomy group at the time of reoperation for glaucoma (P = 0.58, Student t-test). The IOP levels were also compared between the 28 patients in the tube group and the 20 patients in the trabeculectomy group who failed because of inadequate IOP reduction but did not undergo additional glaucoma surgery during 5 years of follow-up. In this patient subgroup, the IOP (mean ± SD) was 18.1 ± 3.5 mmHg in the tube group and 19.4 ± 4.1 mmHg in the trabeculectomy group (P = 0.27, Student t-test).
Visual Acuity
Table 6 shows VA results. Among patients who completed 5-year follow-up visits, logMAR Snellen VA (mean ± SD) decreased 0.16 ± 0.37 units (P <0.001, paired t-test) and Early Treatment Diabetic Retinopathy Study (ETDRS) VA (mean ± SD) was reduced by 6 ± 17 letters (P = 0.006, paired t-test) from baseline in the tube group. In the trabeculectomy group, logMAR Snellen VA (mean ± SD) decreased 0.21 ± 0.52 units (P < 0.001, paired t-test) and ETDRS VA (mean ± SD) declined 11 ± 21 letters (P < 0.001, paired t-test) from baseline to the 5-year follow-up visit. No significant differences in Snellen VA (P = 0.24, Student t-test) or ETDRS VA (P = 0.50, Student t-test) were seen between the tube and trabeculectomy groups at 5 years. The changes in Snellen VA (P = 0.43, Student t-test) and ETDRS VA (P = 0.13, Student t-test) from baseline were also similar between treatment groups among patients who completed 5 years of follow-up.
Table 6.
Visual Acuity Results in the PTVT Study
| Tube Group | Trabeculectomy Group | P-value | |
|---|---|---|---|
| ETDRS VA, mean ± SD | |||
| Baseline | 73 ± 20 | 73 ± 20 | 0.96‡ |
| 5 years | 69 ± 23 | 66 ± 27 | 0.50‡ |
| Change* | −6 ± 17 | −11 ± 21 | 0.13‡ |
|
| |||
| Snellen VA, logMAR mean ± SD | |||
| Baseline | 0.20 ± 0.42 | 0.25 ± 0.51 | 0.42‡ |
| 5 years | 0.30 ± 0.54 | 0.42 ± 0.81 | 0.24‡ |
| Change* | 0.16 ± 0.37 | 0.21 ± 0.52 | 0.43‡ |
|
| |||
| Loss of ≥ 2 Snellen lines, n (%)† | 31 (32) | 29 (31) | 1.00§ |
| Glaucoma | 9 | 9 | |
| Cataract | 8 | 8 | |
| Macular disease | 5 | 4 | |
| Other | 2 | 5 | |
| Unknown | 9 | 3 | |
ETDRS = Early Treatment Diabetic Retinopathy Study; SD = standard deviation; VA = visual acuity
Not all patients seen at baseline returned for 5-year visits, so the baseline mean minus the 5-year mean does not equal the change
Some patients had more than 1 reason for decreased vision
Student t-test
Chi-square test
Loss of 2 or more Snellen lines from baseline occurred in 31 patients (32%) in the tube group and 29 patients (31%) in the trabeculectomy group among patients who completed 5-year visits and/or had vision loss at last follow-up (P = 1.00, chi-square test). The examining clinician was asked to provide an explanation for this reduction in VA, which may have included complications that developed after randomized surgical treatment or following additional ocular surgery. The most frequent cause of vision loss after 5 years of follow-up was glaucoma in 9 patients in each treatment group. Vision loss from unoperated cataract occurred in 8 patients in both treatment groups. The etiology of vision loss was unknown in 9 patients in the tube group and 3 patients in the trabeculectomy group. Macular disease produced vision loss in 5 patients in the tube group, including 2 patients with age-related macular degeneration, 2 patients with cystoid macular edema, and 1 patient with an epiretinal membrane. Other causes of vision loss in the tube group were posterior capsular opacification in 1 patient and choroidal effusion in 1 patient. Macular disease caused vision loss in 4 patients in the trabeculectomy group, including 2 patients with hypotony maculopathy, 1 patient with age-related macular degeneration, and 1 patient with cystoid macular edema. Other etiologies of vision loss in the trabeculectomy group were branch retinal vein occlusion in 2 patients, central retinal artery occlusion in 1 patient, dry eye in 1 patient, and retinal detachment in 1 patient.
Sensitivity Analysis
Although the principal analysis was performed on observed data, we also did a sensitivity analysis in which multiple imputation was used to estimate missing IOP and number of glaucoma medications. In the imputed dataset, the averages by study visit and treatment group differed from the observed ones listed in Table 5 by ≤ 0.5 mmHg and ≤ 0.3 medication, except at 4 years when the imputed mean number of medications were higher than observed mean numbers by 0.6 and 0.5 in the tube and trabeculectomy groups, respectively. Some of the differences were slightly more and others slightly less significant with the imputed data. The cumulative probability of failure at 5 years was 45% in the tube group and 35% in the trabeculectomy group (P = 0.15; hazard ratio = 1.33; 95% confidence interval = 0.89 to 1.98), leaving the hazard ratio and associated statistical significance almost unchanged with the imputed data.
DISCUSSION
A shift in glaucoma surgical practice patterns has occurred in recent years. Trabeculectomy has historically been preferred over tube shunt implantation, except in refractory glaucoma at high risk for filtration failure. However, a concern about bleb-related complications has contributed to an expanded use of tube shunts as an alternative to trabeculectomy. Medicare claims data show a 72% decrease in the number of trabeculectomy procedures and a concurrent 410% increase in tube shunt placement between 1994 and 2012.7 Anonymous surveys of the American Glaucoma Society membership have also demonstrated a rise in the use of tube shunts and a decline in the popularity of trabeculectomy in a variety of clinical settings.2,8–10 These surveys also indicate differing opinions regarding the preferred primary incisional procedure for glaucoma. In 2008, the most popular approaches for surgically managing primary open-angle glaucoma in eyes without prior ocular surgery were trabeculectomy with MMC in 74% of patients and implantation of a tube shunt in 11% of patients.10 Selection of tube shunt surgery as a primary procedure increased to 23% in a repeat American Glaucoma Society survey in 2016, and use of trabeculectomy with MMC decreased to 59%.2
The PTVT Study is a multicenter clinical trial that prospectively enrolled patients with medically uncontrolled glaucoma who had not previously undergone incisional ocular surgery and randomized them to treatment with a 350-mm2 Baerveldt glaucoma implant or a trabeculectomy with MMC. The cumulative probability of failure at 5 years was 42% in the tube group and 35% in the trabeculectomy group. The primary outcome measure in the PTVT Study was the rate of surgical failure at 1 year, but additional reporting of data was planned at 3 and 5 years during the design of the trial.3 The higher failure rate in the tube group compared with the trabeculectomy group was statistically significant at 1 year,4 but not at 3 years5 and 5 years. The rate of surgical failure progressively increased in both treatment groups over time. Unfortunately, loss of IOP control during follow-up has been observed with all currently available glaucoma procedures.
Tube shunt surgery and trabeculectomy with MMC were both effective in lowering IOP as a primary glaucoma procedure. Among patients who completed 5-years of follow-up, IOP was reduced by 44% in both treatment groups. Mean IOPs were lower in the trabeculectomy group relative to the tube group throughout 5 years of follow-up, except at 4 years when they were equivalent. The IOP differences were statistically significant at all time points during the first postoperative year and at 3 years, but not at the other time points. The lower pressures in the trabeculectomy group were achieved with significantly fewer glaucoma medications compared with the tube group at all follow-up visits. A majority of patients in both treatment groups had an IOP of 14 mmHg or less at 5 years. This degree of IOP reduction is generally not seen with MIGS. Based on a review of the medical literature in 2008, a panel of glaucoma specialists concluded that low IOP levels generally cannot be attained with tube shunts and the IOP typically settles in the high teens after surgery.11 However, the PTVT Study found a mean IOP of 13.4 mmHg in the tube group at 5 years, and 69% had IOP less than or equal to 14 mmHg. The more favorable results seen in the PTVT Study likely relates to enrollment of a lower risk patient population than prior studies involving tube shunts, which usually included refractory glaucoma (e.g., neovascular glaucoma).
Treatment success was subdivided into complete and qualified successes based on the use of supplemental medical therapy. Although the overall rate of success was similar between the two treatment groups, the rate of complete success was higher in the trabeculectomy group than the tube group. This is consistent with the observed greater use of glaucoma medications by the tube group throughout 5 years of follow-up. These findings suggest that trabeculectomy with MMC is the preferred initial surgical procedure in patients who are nonadherent or poorly tolerant of glaucoma medical therapy.
The ideal measure of success of any glaucoma therapy is the prevention of further glaucomatous optic nerve damage and preservation of visual function. We recognize the difficulty in defining success by an arbitrary IOP level because individuals vary in the susceptibility of their optic nerves to the damaging effect of IOP. Nevertheless, IOP lowering remains the primary goal of all glaucoma therapy at the present time, and no other surrogate measure better reflects therapeutic success for this disease. The outcome measures for the PTVT Study were developed a priori, and our definitions of success and failure are consistent with recommendations from the World Glaucoma Association for the reporting of outcomes from glaucoma surgical trials.12
Results from several multicenter randomized clinical trials have suggested that IOP of 21 mmHg or less may be inadequate to prevent glaucomatous progression in many patients.1,13,14 Planned secondary analyses were performed to determine if the PTVT Study results changed if more stringent IOP criteria were applied to define success. No significant differences in failure rates were seen between treatment groups when the upper IOP level defining success was reduced to 17 mmHg and 14 mmHg, although there was a tendency toward a higher failure rate in the tube group compared with the trabeculectomy group when failure was defined as IOP > 17 mmHg. Because similar results were observed using a broad range of IOP success criteria, the study results seem applicable to patients with the full spectrum of glaucoma from early to advanced disease.
Baseline demographic and clinical factors were explored as possible risk factors for treatment failure. Only lower preoperative IOP was significantly associated with failure in univariable and multivariable analyses. Patients with lower preoperative IOP benefited most from primary trabeculectomy with MMC, and patients with higher preoperative IOP benefited most from primary tube shunt surgery in the PTVT Study. A post hoc analysis of TVT Study data similarly demonstrated that the efficacy of tube shunt surgery relative to trabeculectomy with MMC increased at higher levels of preoperative IOP.6 A risk factor analysis from pooled data from the Ahmed Baerveldt Comparison Study, Ahmed Versus Baerveldt Study, and the tube group of the TVT Study also identified lower preoperative IOP as a significant predictor of tube shunt failure.15 Given the influence of baseline IOP on the outcomes of traditional glaucoma surgery, we believe that it is important to consider preoperative IOP when deciding between tube shunt surgery and trabeculectomy with MMC.
While the overall failure rates were similar between the tube and trabeculectomy groups, the reasons for failure were distributed differently between treatment groups. Inadequate IOP reduction was the most common reason for failure in both treatment groups. Failure because of persistent hypotony occurred more frequently in the trabeculectomy group, and failure due to a glaucoma reoperation was more common in the tube group. Some patients were categorized as treatment failures because of inadequate IOP reduction and subsequently had additional glaucoma surgery. This explains why the total number of patients with reoperations for glaucoma exceeds those who were classified as failures due to a glaucoma reoperation. It has been argued that hypotony may be an acceptable outcome of glaucoma surgery, if it is not associated with vision loss.16 It is noteworthy that all of the patients who failed because of hypotony in the PTVT Study also experienced vision loss.
The rates of reoperation for glaucoma were similar between the two treatment groups. Patients who fail trabeculectomy and require additional glaucoma surgery have historically undergone repeat trabeculectomy or tube shunt placement.2,8–10 However, additional glaucoma surgery in eyes that have failed tube shunt surgery is frequently more complex and usually involves placement of a second tube shunt or cyclodestruction.17–19 Subconjunctival filtering surgery may be a feasible option in some patients who have undergone primary tube shunt implantation. Reoperations for glaucoma in the tube group included trabeculectomy with MMC in 3 patients, InnFocus microshunt implantation with MMC in 1 patient, and a Xen gel stent placement with MMC and phacoemulsification in 1 patient. A retrospective case series reported good surgical results with trabeculectomy with MMC after tube shunt surgery.20 Because investigators in the PTVT Study were not masked to the treatment assignment and the decision to reoperate was left to the surgeon’s discretion, a potential bias existed in performing additional glaucoma surgery. We explored for the possibility that surgeons may have had a higher threshold to perform additional glaucoma surgery in the tube group than the trabeculectomy group. The mean IOPs immediately before reoperation were similar in the tube and trabeculectomy groups, and no significant difference was seen between treatment groups in mean IOPs among patients who failed because of inadequate IOP reduction but did not undergo additional glaucoma surgery. These observations suggest that no selection bias was present for glaucoma reoperations.
Reduction of VA occurred in both treatment groups during 5 years of follow-up. Snellen and ETDRS VA were similar in the tube and trabeculectomy groups at 5 years, and no significant difference in the rates of vision loss were observed between the two groups. Vision loss of 2 or more Snellen lines was most frequently attributed to glaucoma by investigators. The high rate of vision loss from glaucoma in the PTVT Study may relate to the advanced stage of disease of many patients, with an average mean deviation on Humphrey visual field testing of −14.6 decibels in the overall study group at baseline. All patients in the PTVT Study were phakic at enrollment, and cataract progression was common during 5 years of follow-up. Multiple studies have also reported that glaucoma surgery is associated with the development of cataract.21–27 Some of the causes of vision loss, such as age-related macular degeneration and branch retinal vein occlusion, were not directly attributable to the surgical procedures under study.
Previous studies have directly compared trabeculectomy and tube shunt surgery. Panarelli et al retrospectively reviewed the outcomes of Baerveldt implantation and trabeculectomy with MMC in eyes without prior ocular surgery.28 Similar rates of surgical success and complications were observed with both procedures, but trabeculectomy produced greater IOP reduction with use of fewer glaucoma mediations than Baerveldt implantation after 3 years of follow-up. Molteno et al reported results from a prospective, non-randomized study comparing primary trabeculectomy and primary insertion of the Molteno implant (Molteno Ophthalmic Limited, Dunedin, New Zealand).29 The cumulative rate of failure was higher after trabeculectomy relative to Molteno implant placement during 20 years of follow-up, but no significant differences were seen between the two procedures in mean IOP, glaucoma medical therapy, complications, and vision loss.
Selection bias in retrospective and in prospective, non-randomized comparative case series may produce treatment groups with different underlying risk factors for failure. Randomized clinical trials aim to produce study groups that differ only by the treatment received, and they are considered the gold standard for evaluating therapeutic interventions. No significant differences in any of the baseline demographic or ocular characteristics were seen between the tube and trabeculectomy groups in the PTVT Study indicating that randomization was effective in creating two balanced treatment groups.
Wilson et al compared the Ahmed glaucoma valve (New World Medical, Inc., Rancho Cucamonga, CA) to trabeculectomy with or without an antifibrotic agent in a randomized clinical trial.30 Lower mean IOP was observed in the trabeculectomy group, and the Ahmed group had a greater adjunctive medication requirement with a mean follow-up of 9.7 months. The cumulative probability of success was similar between the two treatment groups. This study was conducted in Saudi Arabia and Sri Lanka and included patients with all glaucoma types and some eyes that had undergone previous ocular surgery. A follow-up study continued enrollment in Sri Lanka of patients with primary open-angle glaucoma and primary angle-closure glaucoma without previous ocular surgery.31 With a mean follow-up of 31 months, success rates and mean IOPs were comparable between the trabeculectomy and Ahmed groups. The TVT Study is a multicenter randomized clinical trial that compared Baerveldt implantation to trabeculectomy with MMC in eyes with previous cataract and/or glaucoma surgery.32 The rate of surgical success using survival analysis was higher with tube shunt surgery than trabeculectomy with MMC throughout 5 years of follow-up, although similar mean IOP and use of glaucoma medications were observed with both surgical procedures at 5 years.33 Early postoperative complications occurred more frequently after trabeculectomy with MMC than tube shunt placement, but both procedures had similar rates of late and serious complications after 5 years.26 The differences in results between the PTVT Study and other investigations comparing tube shunts and trabeculectomy with MMC may relate to differences in study design, patient populations, disease stage, surgical technique, length of follow-up, and definitions of success/failure.
The PTVT Study has several limitations. The study population was restricted to patients without previous incisional ocular surgery, and several patient types were ineligible for enrollment. Study results cannot be directly applied to dissimilar patient groups. Patients randomized to the tube group received a 350-mm2 Baerveldt glaucoma implant, and the study results should not be generalized to different implant types. A standard dosage of MMC was used in all trabeculectomy cases based on results from a survey of the American Glaucoma Society membership,10 but it is unclear whether a different dosage may have altered the rate of filtration failure because of fibrosis or hypotony in the trabeculectomy group. Although aspects of both surgical procedures were standardized, some variation in technique occurred because surgeons were allowed sufficient latitude to perform the operations in a manner with which they were comfortable. The patients and investigators were not masked to the randomized treatment assignment, and this is a potential source of bias.
The PTVT Study does not demonstrate clear superiority of one glaucoma operation over the other. Tube shunt implantation and trabeculectomy with MMC both produced average IOPs in the low teens throughout 5 years of follow-up, although fewer supplemental glaucoma medications were required after trabeculectomy. No significant difference in the rate of surgical failure was observed between tube shunt surgery and trabeculectomy with MMC at 5 years. Vision loss occurred at a similar rate after each procedure. The PTVT Study indicates that both tube shunt placement and trabeculectomy with MMC are viable surgical options for managing medically uncontrolled glaucoma in patients without prior incisional ocular surgery.
Even though randomized clinical trials like the PTVT Study offer a high level of evidence-based medicine, surgeon skill and experience with each operation are important additional considerations when selecting a glaucoma surgical procedure that were not evaluated in this study. The recent introduction of MIGS has further expanded the surgical options available for glaucoma patients. These procedures appear to be associated with lower rates of surgical complications than trabeculectomy and tube shunt surgery, but they provide only modest IOP reduction.34 Several multicenter randomized clinical trials involving phacoemulsification and implantation of a MIGS device have shown that approximately two-thirds of the postoperative IOP reduction may be attributed to the cataract extraction and one-third to the MIGS, and these findings are consistent with multiple different MIGS devices.35–38 Unfortunately, a trade-off exists between efficacy and safety with currently available glaucoma operations. Selecting the most appropriate glaucoma procedure involves balancing the risks of complications and the benefits of IOP reduction for an individual patient. Our companion paper describes the postoperative complications encountered in the PTVT Study during 5 years of follow-up and the management of these complications.
Supplementary Material
Acknowledgments
Biostatistical support was provided by research grants from Johnson & Johnson Vision, Santa Ana, California, the National Eye Institute (grant EY014801), National Institutes of Health, Bethesda, Maryland, and Research to Prevent Blindness, Inc., New York, New York.
The sponsors had no role in the design or conduct of this research.
Mr. Feuer has received grant support from Johnson & Johnson Vision. Dr. Ahmed is a consultant for Johnson & Johnson Vision.
Abbreviations/acronyms:
- ETDRS
Early Treatment Diabetic Retinopathy Study
- IOP
intraocular pressure
- MIGS
minimally invasive glaucoma surgery
- MMC
mitomycin C
- PTVT
Primary Tube Versus Trabeculectomy
- TVT
Tube Versus Trabeculectomy
- VA
visual acuity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented as a paper at the American Academy of Ophthalmology Meeting, November 2021, New Orleans, Louisiana
The other authors report no financial interest in the subject matter of this paper.
REFERENCES
- 1.The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol 2000;130:429–440. [DOI] [PubMed] [Google Scholar]
- 2.Vinod K, Gedde SJ, Feuer WJ, et al. Practice preferences for glaucoma surgery: A survey of the American Glaucoma Society. J Glaucoma 2017;26:687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gedde SJ, Chen PP, Heuer DK, et al. The Primary Tube Versus Trabeculectomy (PTVT) Study: Methodology of a multicenter randomized clinical trial comparing tube shunt surgery and trabeculectomy with mitomycin C. Ophthalmology 2018;125:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gedde SJ, Feuer WJ, Shi W, et al. Treatment outcomes in the Primary Tube Versus Trabeculectomy Study after 1 year of follow-up. Ophthalmology 2018;125:650–663. [DOI] [PubMed] [Google Scholar]
- 5.Gedde SJ, Feuer WJ, Lim KS, et al. Treatment outcomes in the Primary Tube Versus Trabeculectomy Study after 3 years of follow-up. Ophthalmology 2020;127:333–345. [DOI] [PubMed] [Google Scholar]
- 6.Gedde SJ, Feuer WJ, Chen PP, et al. Comparing treatment outcomes from the Tube Versus Trabeculectomy and Primary Tube Versus Trabeculectomy Studies. Ophthalmology 2021;128:324–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arora KS, Robin AL, Corcoran KJ, Corcoran SL, Ramulu PY. Use of various glaucoma surgeries and procedures in Medicare beneficiaries from 1994 to 2012. Ophthalmology 2015;122:1615–1624. [DOI] [PubMed] [Google Scholar]
- 8.Chen PP, Yamamoto T, Sawada A, et al. Use of antifibrosis agents and glaucoma drainage devices in the American and Japanese Glaucoma Societies. J Glaucoma 1997;6:192–196. [PubMed] [Google Scholar]
- 9.Joshi AB, Parrish RK, Feuer WF. 2002 survey of the American Glaucoma Society: Practice preferences for glaucoma surgery and antifibrotic use. J Glaucoma 2005;14:172–174. [DOI] [PubMed] [Google Scholar]
- 10.Desai MA, Gedde SJ, Feuer WJ, et al. Practice preferences for glaucoma surgery: A survey of the American Glaucoma Society in 2008. Ophthalmic Surg Lasers Imaging 2011;42:202–208. [DOI] [PubMed] [Google Scholar]
- 11.Minckler DS, Francis BA, Hodapp EA, et al. Aqueous shunts in glaucoma: A report by the American Academy of Ophthalmology. Ophthalmology 2008;115:1089–1098. [DOI] [PubMed] [Google Scholar]
- 12.Heuer DK, Barton K, Grehn F, et al. Consensus on definitions of success. In: Shaarawy TM, Sherwood MB, Grehn F, eds. Guidelines on Design and Reporting of Glaucoma Surgical Trials Amsterdam, The Netherlands: Kugler; 2008:15–24. [Google Scholar]
- 13.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology 2001;108:1943–1953. [DOI] [PubMed] [Google Scholar]
- 14.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002;120:1268–1279. [DOI] [PubMed] [Google Scholar]
- 15.Bowden EC, Choudhury A, Gedde SJ, et al. Risk factors for failure of tube shunt surgery: A pooled analysis. Am J Ophthalmol 2022;240:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tseng VL, Kim CH, Romero PT, et al. Risk factors and long-term outcomes in patients with low intraocular pressure after trabeculectomy. Ophthalmology 2017;124:1457–1465. [DOI] [PubMed] [Google Scholar]
- 17.Saheb H, Gedde SJ, Schiffman JC, et al. Outcomes of glaucoma reoperations in the Tube Versus Trabeculectomy (TVT) Study. Am J Ophthalmol 2014;157:1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaefer JL, Levine MA, Martorana G, et al. Failed glaucoma drainage implant: Long-term outcomes of a second glaucoma drainage device versus cyclophotocoagulation. Br J Ophthalmol 2015;99:1718–1724. [DOI] [PubMed] [Google Scholar]
- 19.Levinson JD, Giangiacomo AL, Beck AD, et al. A comparison of sequential glaucoma drainage device implantation versus cyclophotocoagulation following failure of a primary drainage device. J Glaucoma 2017;26:311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alizadeh R, Akil H, Tan J, Law SK, Caprioli J. Trabeculectomy outcomes after glaucoma drainage device surgery. J Glaucoma 2018;27:133–139. [DOI] [PubMed] [Google Scholar]
- 21.Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol 1998;126:487–497. [DOI] [PubMed] [Google Scholar]
- 22.The AGIS Investigators. The Advanced Glaucoma Intervention Study, 6: Effect of cataract on visual field and visual acuity. Arch Ophthalmol 2000;118:1639–1652. [DOI] [PubMed] [Google Scholar]
- 23.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology 2001;108:1943–1953. [DOI] [PubMed] [Google Scholar]
- 24.The AGIS Investigators. The Advanced Glaucoma Intervention Study: 8. Risk of cataract formation after trabeculectomy. Arch Ophthalmol 2001;119:1771–1779. [DOI] [PubMed] [Google Scholar]
- 25.Hylton C, Congdon N, Friedman D, et al. Cataract after glaucoma filtration surgery. Am J Ophthalmol 2003;135:231–232. [DOI] [PubMed] [Google Scholar]
- 26.Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the Tube Versus Trabeculectomy (TVT) Study during five years of follow-up. Am J Ophthalmol 2012;153:804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Budenz DL, Feuer WJ, Barton K, et al. Postoperative complications in the Ahmed Baerveldt Comparison Study during five years of follow-up. Am J Ophthalmol 2016;163:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panarelli JF, Banitt MR, Gedde SJ, et al. A retrospective comparison of primary Baerveldt implantation versus trabeculectomy with mitomycin C. Ophthalmology 2016;123:789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molteno AC, Bevin TH, Herbison P, Husni MA. Long-term results of primary trabeculectomies and Molteno implants for primary open-angle glaucoma. Arch Ophthalmol 2011;129:1444–1450. [DOI] [PubMed] [Google Scholar]
- 30.Wilson MR, Mendis U, Smith SD, Paliwal A. Ahmed glaucoma valve implant vs trabeculectomy in the surgical treatment of glaucoma: A randomized clinical trial. Am J Ophthalmol 2000;130:267–273. [DOI] [PubMed] [Google Scholar]
- 31.Wilson MR, Mendis U, Paliwal A, Haynatzka V. Long-term follow-up of primary glaucoma surgery with Ahmed glaucoma valve implant versus trabeculectomy. Am J Ophthalmol 2003;136:464–470. [DOI] [PubMed] [Google Scholar]
- 32.Gedde SJ, Schiffman JC, Feuer WJ, et al. The Tube Versus Trabeculectomy Study: Design and baseline characteristics of study patients. Am J Ophthalmol 2005;140:275–287. [DOI] [PubMed] [Google Scholar]
- 33.Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) Study after five years of follow-up. Am J Ophthalmol 2012;153:789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saheb H, Ahmed II. Micro-invasive glaucoma surgery: Current perspectives and future directions. Curr Opin Ophthalmol 2012;23:96–104. [DOI] [PubMed] [Google Scholar]
- 35.Craven ER, Katz LJ, Wells JM, Giamporcaro JE, iStent Study Group. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: Two-year follow-up. J Cataract Refract Surg 2012;38:1339–1345. [DOI] [PubMed] [Google Scholar]
- 36.Vold S, Ahmed II, Craven ER, et al. Two-year COMPASS Trial results: Supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology 2016;123:2103–2112. [DOI] [PubMed] [Google Scholar]
- 37.Samuelson TW, Chang DF, Marquis R, et al. A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: The HORIZON Study. Ophthalmology 2019;126:29–37. [DOI] [PubMed] [Google Scholar]
- 38.Samuelson TW, Sarkisian SR Jr, Lubeck DM, et al. Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in open-angle glaucoma and cataract: Two-year results. Ophthalmology 2019;126:811–821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


