Abstract
Objectives
The objective of this study was to study the association between newborn anthropometrics and childhood cardiovascular risks, and whether newborn anthropometrics mediate the effect of maternal gestational weight gain (GWG) on childhood risks.
Methods
Data of 926 mother-child dyads from the “Hyperglycemia and Adverse Pregnancy Outcomes” study were analyzed. Newborn anthropometrics were treated as predictors and mediators, by using regression model and casual-mediation model, respectively.
Results
Newborn sum of skinfolds (SSF) was associated with childhood diastolic blood pressure (DBP), and pulse wave velocity (PWV) [coefficients (95% CI): 0.13 (0.06 to 0.20); 0.08 (0.004 to 0.15)], whereas newborn ponderal index (PI) was inversely associated with childhood SBP, DBP and PWV [−0.08 (−0.15 to −0.01); −0.08 (−0.14 to −0.008); −0.09 (−0.16 to −0.03)]. Newborn SSF mediated the effects of maternal excessive GWG on childhood SSF and DBP (proportion of total effect 9% and 8%, respectively). In contrast, a significant negative mediation through newborn PI was found for the effect of maternal excessive GWG on childhood DBP (−8%), and its effect on childhood SBP through birthweight (−27%).
Conclusions
Childhood cardiovascular risks are positively associated with newborn SSF, but inversely associated with newborn PI. Newborn SSF mediates the impact of excessive maternal GWG on childhood BP, but birthweight and newborn PI negatively mediated it.
Keywords: Skinfold thickness, birth weight, early childhood risk factors, gestational weight gain
Introduction
Small for gestational age (SGA) infants are at risk of future cardiovascular disease, whereas whether large for gestational age (LGA) infants are also at increased risk remains unclear (1). An inverse association between higher birth weight and future lower blood pressure has been reported in a number of studies (2, 3). Meanwhile, as an important indicator of cardiovascular morbidity and mortality, increased arterial stiffness is linked to risk of hypertension and other diseases, such as stroke. Arterial stiffness is often assessed as the velocity of pulse-wave travel in a defined arterial segment such as the aorta (4). An inverse association between birth weight and later pulse wave velocity (PWV) was found in a cohort in USA (5), but not from another study in the UK (6). Meanwhile, another multi-ethnic study found a significant association between low birth weight and PWV only in Whites, but not in people of color (7).
Previous studies examining the association between newborn anthropometrics and childhood cardiovascular risks were mainly confined to birth weight. Weight at birth consists of different tissue components, among which fat mass, but not fat-free mass, reflects the status of neonatal adiposity. Studies on the association of neonatal adiposity with long-term childhood health are scarce. Neonatal adiposity was found to be associated with higher childhood adiposity (8, 9). Nonetheless, evidence on the association between neonatal adiposity and childhood cardiovascular risks is still lacking.
Increased gestational weight gain (GWG), as a surrogate marker of maternal obesity, has been found to be associated with infant’s weight (10) and adiposity, particularly in women with a healthy BMI (11), as well as with offspring’s cardiovascular risk factors in childhood (12, 13). We recently reported that optimal ranges for maternal GWG, derived from infant’s fat mass (i.e., lowest rates of lean and fat infants), were superior to those based on birth weight alone (i.e., lowest rates of small- and large-for-gestational age infants), for the prediction of childhood cardiovascular risks (14). Increased intake of fat and carbohydrates during pregnancy, as well as decreased late-pregnancy energy expenditure, were associated with greater neonatal fat mass, but not fat-free mass or birth weight (15, 16). Hence, we speculate that newborn adiposity, as compared with birth weight, is a stronger mediator of the association between maternal excessive GWG and childhood cardiovascular risk factors.
Our primary objective was to investigate the associations between various newborn anthropometrics and childhood cardiovascular risk factors. Secondary aims were to explore whether newborn anthropometric measures mediate the effect of maternal GWG on childhood cardiovascular risk factors.
Methods
Study design
This was a secondary analysis on the data from the original Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study and the subsequent follow-up study as an ancillary study to the original HAPO study as endorsed by the HAPO steering committee, carried out in 2000–2006 and 2009–2013, respectively, at the Hong Kong study center. The details of both studies have been described elsewhere (17, 18). In the original HAPO study, pregnant women with a singleton pregnancy underwent a double-blinded 75 g oral glucose tolerance test (OGTT) at 24–32 weeks’ gestation. Mothers who participated in the original HAPO study and returned for the ancillary follow up study with their children at an average of 7 years of age were eligible for this analysis, while children born preterm before 37 weeks of gestation were excluded. (Figure S1)
Mothers’ height was obtained at the first antenatal visit. Self-reported pre-pregnant weight was obtained at either the first antenatal visit or at the enrolment into the HAPO study, while their weight at delivery was measured in early labor upon admission, or before an elective caesarean delivery. We computed the gestational weight gain (GWG) as the difference between maternal pre-pregnant weight and weight at delivery.
Newborn anthropometric measurements were obtained using a standardized validated method by trained research staff within 72 hours of delivery (19). The anthropometric measurements including weight, length, and skinfold thickness were obtained in duplicate. If the results differed by more than a pre-specified amount (i.e., > 10 g for weight, > 0.5 cm for length, or > 0.5 mm for skin folds), a third measurement was performed. For these analyses, the average of the two or three measurements was used. Birth weight was obtained without diaper using a calibrated electronic scale. Skinfold thickness was measured with calipers (Harpenden, Baty, U.K.). Suprailiac skinfold was measured on the left side, just above the iliac crest on a diagonal fold on the mid-axillary line, triceps by taking the vertical fold over the triceps muscle half the distance between the acromion process and olecranon, and subscapular just below the lower angle of the scapula at ∼45° angle to the spine. The skinfolds at all three sites above were summed up to obtain the sum of skinfold (SSF), which reflects the sum of the subcutaneous fat at the respective site (20). Ponderal index (PI) was computed by dividing birth weight by body length cubed.
At the follow up assessment at 7-year of age, demographic data on personal medical history, family history and physical activity were collected using structured questionnaires as previously reported (17). Child’s physical activity were assessed by the Chinese University of Hong Kong: Physical Activity Rating for Children and Youth, which is a one-item activity rating modified from the Jackson Activity Coding and the Godin-Shephard Activity Questionnaire for adolescents (21, 22). Physical activity was graded into three categories, i.e., no exercise, mild exercise, and vigorous exercise on most days, according to an eleven-point score based on the frequency, duration, and intensity of activity.
Children’s standing height without shoes was measured to the nearest 0.1 cm using a Harpenden stadiometer (Holtain Ltd., Crymych, U.K.); body weight (with light clothing) was measured to the nearest 0.1 kg (Tanita physician digital scale, model no. TBF 410; Tanita Corp., Tokyo, Japan). Waist circumference (WC), at the midpoint between the lower ridge of the ribs and the top of the iliac crest, was measured to the nearest 0.1 cm using a nonelastic flexible tape. Hip circumference was measured at the broadest circumference below the waist. Skinfolds were measured at four sites on the right side (biceps, triceps, subscapular, and suprailiac) using a Holtain Tanner/Whitehouse skinfold caliper (Holtain Ltd.). For the biceps and triceps skinfolds, the landmark was determined by measuring the mid distance between the acromion process of the right humerus (shoulder) and the olecranon process (elbow) of the same limb. The mid distance was marked on the skin anteriorly to measure the biceps skinfold and posteriorly to measure the triceps skinfold with the arm by the side of the body. The subscapular and suprailiac skinfolds were measured as described previously. Childhood SSF was the sum of skinfolds at four sites. Childhood BMI percentiles were calculated according to sex- and age-specific references (23).
Children’s carotid-femoral pulse wave velocity (cfPWV) was measured by using the SphygmoCor apparatus (SphygmoCor1 Px, AtCor Medical, Australia) as previously described (24). The cfPWV was obtained with the child lying supine, head supported, and the arms placed by their sides after at least 5 minutes of rest. The measurements were obtained in duplicate. Blood pressure (BP) was measured three times in the nondominant arm by using an Omron T5 BP monitor (Omron Healthcare Co. Ltd., Kyoto, Japan), at 1-min intervals. The average cfPWV and BP were used for analysis. Childhood BP percentiles were calculated according to sex-, age- and height-specific references (25). Children were advised to fast overnight for ≥8 h on the day before the evaluation of arterial stiffness.
Newborn anthropometrics, i.e. body weight, sum of skinfolds (SSF), and ponderal index (PI) at birth, were analyzed both as independent predictors of childhood cardiovascular risk factors and as potential mediators of associations between maternal GWG and childhood cardiovascular risk factors.
Childhood cardiovascular risks were analyzed under two domains: (1) anthropometrics [body weight, BMI percentile, WC, waist to hip circumference ratio (WHR), and SSF] and (2) blood pressure and arterial stiffness [systolic and diastolic blood pressures (SBP and DBP) percentiles, cfPWV].
Statistical analyses
Linear regression analysis was performed to assess the associations of predictors with childhood cardiovascular risk factors. The latter were adjusted for covariates as follows: model 1 – gestational age at birth, child’s sex, age and/or height as appropriate; model 2 – model 1 plus child’s exercise level. Meanwhile, linear regression analysis stratified by child’s sex was also conducted by child’s sex after adjustment for confounders in model 2. Newborn anthropometrics were standardized into z- scores by subtracting the mean and dividing by the standard deviation (SD) from the original HAPO data set. Given that the residuals of the linear regression model had a skewed distribution, as was tested by Q-Q plot, the normal scores transformation method was employed to transform childhood cardiovascular risks to a normal distribution by using the general method in the blom function of the rcompanion R package. The normal scores transformation result in a normal distribution with a mean of 0 and an SD of 1.
Meanwhile, we also explored non-linear associations between newborn anthropometrics and childhood cardiovascular risks by using polynomial regression models with 2 degrees (i.e., quadratic model) and F-test to determine the statistical significance of the quadratic models. We also applied R2 change to assess the superiority of the quadratic model over the linear model, by using an F-test; a significant R2 change implies a better association in the quadratic model.
Model-based causal mediation analyses were performed by using nonparametric bootstrapping method for variance estimation, conducted by mediation R package (26). The model-based causal mediation analysis proceeds in two steps involving two statistical models, namely a mediator model and an outcome model. The mediator model is specified for the conditional distribution of the mediator (newborn anthropometrics) given the predictor (maternal GWG) and a set of covariates. The outcome model is specified for the conditional distribution of the outcome (childhood cardiovascular risk factors) given the predictor, mediator and covariates. These models are fitted separately and then their fitted objects comprise the main inputs to the mediate function, which computes the estimated average causal mediation effects (ACME), i.e., indirect effect, and other quantities of interest under these models and the sequential ignorability assumption. In our analysis, we applied the Institute of Medicine (IOM) recommendations to categorize maternal GWG (predictor) into three categories, i.e., GWG below, within, and exceeding the IOM recommendations (27). Two dummy variables were used to code for the GWG categories. “GWG below the IOM recommendations” and “GWG exceeding the IOM recommendations” were compared with “GWG within the IOM recommendations (reference group)”, respectively, by using linear regression model.
The total effects derived from the causal mediation analysis were adjusted for covariates in model 2 plus covariates during pregnancy, namely paternal BMI; maternal age, parity, gestational hypertension / preeclampsia, smoking and glycemic status in pregnancy [represented by the area under the curve of glucose levels (AUCglu) at OGTT]. The association of maternal GWG with mediators (i.e., birth weight / PI / SSF) was adjusted for the covariates during pregnancy as mentioned previously. (Figure S2)
All statistical analyses were performed by using R (version 4.0.3), which was downloaded at www.r-project.org/. P value <0.05 was used to indicate significance for two-tailed statistical test result.
Results
A total of 926 mother-child dyads were included in the analysis of the associations between newborn anthropometrics with childhood cardiometabolic risks, whereas 905 were included for the mediation analysis (Figure S1). The participants’ baseline characteristics, pregnancy outcomes and childhood cardiovascular risk factors are shown in table S1. The Pearson’s correlation coefficients of birth weight with newborn PI and SSF were 0.51 and 0.62, respectively, and the coefficient between newborn PI and SSF were 0.37 (p <0.001).
Associations between newborn anthropometrics and childhood cardiovascular risk factors
Table 1 showed the associations between the newborn anthropometrics and childhood cardiovascular risks in the two models. Birth weight was associated with childhood body weight, BMI percentile and WC after adjustment for gestational age of birth, child’s sex, age and exercise level. Similarly, newborn PI was associated with childhood body weight, BMI percentile and WHR. However, newborn SSF was associated with childhood SSF, but not their body weight. Newborn PI was found inversely associated with childhood SBP and DBP percentiles, and cfPWV, whereas newborn SSF was positively associated with childhood DBP percentile and cfPWV, after adjustment for gestational age at birth, child’s sex, age and exercise level. Moreover, analysis stratified by child’s sex showed birth weight was associated with childhood SBP percentile in boys but not in girls. In contrast, the association of newborn PI with childhood DBP percentile and cfPWV, as well as the association of newborn SSF with childhood cfPWV was only found in girls but not in boys (table S2).
Table 1.
Associations between newborn anthropometrics z-score and childhood cardiovascular risk factors z-score
| Model 1 | Model 2 | |||
|---|---|---|---|---|
|
| ||||
| β (95% CI) | P | β (95% CI) | P | |
|
| ||||
| Childhood anthropometrics | ||||
| Birth weight | ||||
| Body weight | 0.20 (0.13 to 0.27) | <0.001 | 0.20 (0.14 to 0.27) | <0.001 |
| BMI percentile ∆ | 0.17 (0.10 to 0.24) | <0.001 | 0.17 (0.10 to 0.24) | <0.001 |
| WC | 0.11 (0.04 to 0.18) | 0.001 | 0.12 (0.05 to 0.18) | <0.001 |
| WHR | −0.02 (−0.10 to 0.05) | 0.491 | −0.02 (−0.09 to 0.05) | 0.507 |
| SSF | 0.05 (−0.02 to 0.12) | 0.193 | 0.05 (−0.02 to 0.12) | 0.186 |
| Newborn PI | ||||
| Body weight | 0.08 (0.01 to 0.14) | 0.016 | 0.08 (0.02 to 0.14) | 0.014 |
| BMI percentile ∆ | 0.16 (0.09 to 0.22) | <0.001 | 0.16 (0.09 to 0.23) | <0.001 |
| WC | 0.06 (−0.002 to 0.13) | 0.059 | 0.07 (0.006 to 0.13) | 0.031 |
| WHR | 0.10 (0.03 to 0.16) | 0.004 | 0.10 (0.04 to 0.17) | 0.003 |
| SSF | 0.05 (−0.02 to 0.12) | 0.133 | 0.06 (−0.01 to 0.13) | 0.104 |
| Newborn SSF | ||||
| Body weight | 0.04 (−0.03 to 0.10) | 0.270 | 0.04 (−0.03 to 0.10) | 0.253 |
| BMI percentile ∆ | 0.08 (0.01 to 0.15) | 0.018 | 0.09 (0.02 to 0.15) | 0.017 |
| WC | 0.05 (−0.02 to 0.12) | 0.154 | 0.05 (−0.02 to 0.11) | 0.149 |
| WHR | 0.007 (−0.06 to 0.08) | 0.832 | 0.007 (−0.06 to 0.08) | 0.842 |
| SSF | 0.12 (0.04 to 0.19) | 0.001 | 0.11 (0.04 to 0.18) | 0.002 |
| Childhood blood pressure and arterial stiffness | ||||
| Birth weight | ||||
| SBP percentile ∆ | −0.06 (−0.14 to 0.008) | 0.080 | −0.06 (−0.13 to 0.01) | 0.097 |
| DBP percentile ∆ | −0.006 (−0.08 to 0.07) | 0.870 | −0.003 (−0.07 to 0.07) | 0.941 |
| cfPWV ∇ | −0.01 (−0.06 to 0.09) | 0.717 | 0.01 (−0.06 to 0.09) | 0.698 |
| Newborn PI | ||||
| SBP percentile ∆ | −0.08 (−0.15 to −0.01) | 0.017 | −0.08 (−0.15 to −0.01) | 0.024 |
| DBP percentile ∆ | −0.09 (−0.15 to −0.02) | 0.013 | −0.08 (−0.14 to −0.008) | 0.029 |
| cfPWV ∇ | −0.11 (−0.18 to −0.04) | 0.002 | −0.09 (−0.16 to −0.03) | 0.007 |
| Newborn SSF | ||||
| SBP percentile ∆ | 0.06 (−0.006 to 0.14) | 0.075 | 0.06 (−0.008 to 0.14) | 0.083 |
| DBP percentile ∆ | 0.14 (0.07 to 0.21) | <0.001 | 0.13 (0.06 to 0.20) | <0.001 |
| cfPWV ∇ | 0.08 (0.007 to 0.15) | 0.032 | 0.08 (0.004 to 0.15) | 0.038 |
Model 1 was adjusted for gestational age at birth and child’s sex and age, except as indicated.
Model 2 was adjusted for covariates in model 1 plus child’s exercise level.
Calculated according to sex-, age- and/or height-specific references.
Further adjusted for child’s height.
CI, confidence interval; PI, Ponderal index; SSF, sum of skinfolds; BMI, body mass index; WC, waist circumference; WHR, waist-hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; cfPWV, carotid-femoral pulse wave velocity.
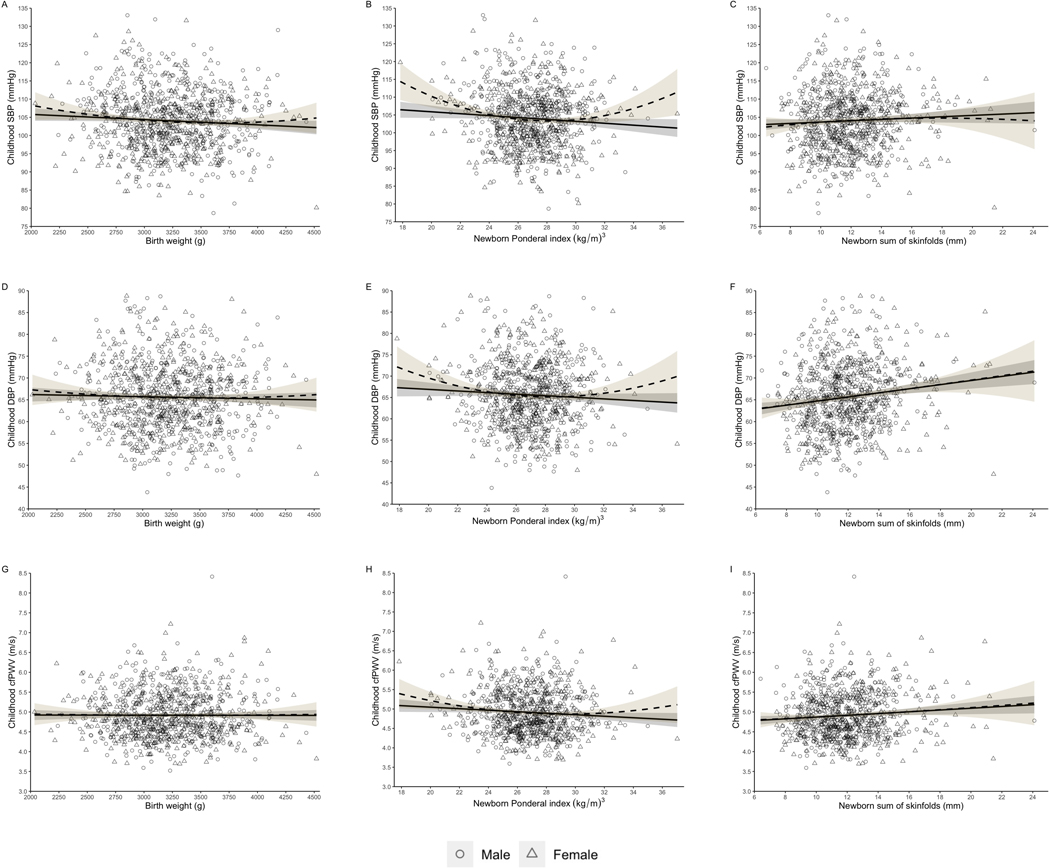
Figure 1 depicted both the linear and quadratic associations of newborn anthropometrics with childhood blood pressure and arterial stiffness. We found better predictions with the quadratic model compared to the linear model, indicated by p values of the test for R square change, in the associations of newborn PI with childhood SBP (adjusted R square: 0.007 vs 0.02, p <0.001), DBP (adjusted R square: 0.03 vs 0.04, p =0.018) and cfPWV (adjusted R square: 0.02 vs 0.05, p =0.039) (Figure 1B, 1E & 1H).
Figure 1.
Linear and quadratic associations between the newborn anthropometrics and childhood cardiovascular risk factors
Childhood cardiovascular risk factors were adjusted for gestational age, child’s age and exercise level. Childhood SBP, DBP and PWV were further adjusted for child’s height.
Circles indicate boys, while triangles indicate girls. Solid line represents linear association, while dashed line represents quadratic association. Grey and yellow area represents 95% confidence intervals of linear model and quadratic model, respectively.
Figure 1 A, D & G depict associations of birth weight with childhood SBP, DBP and PWV, respectively. Figure 1 B, E & H depict associations of newborn PI with childhood SBP, DBP and PWV, respectively. Figure 1 C, F & I depict associations of newborn SSF with childhood SBP, DBP and PWV, respectively.
PI, Ponderal index; SSF, sum of skinfolds; SBP, systolic blood pressure; DBP, diastolic blood pressure; cfPWV, carotid-femoral pulse wave velocity.
Mediation effects of newborn anthropometrics
We further performed model-based causal mediation analysis to investigate the mediation effects by newborn anthropometrics on the impact of maternal GWG on childhood cardiovascular risk factors. The effects of inadequate or excessive maternal GWG on childhood cardiovascular risk factors were shown in table S3. Children born to mothers with excessive GWG had higher BMI percentile, SSF, SBP and DBP percentile at 7-year of age, compared to children born to mothers with adequate GWG. The above childhood cardiovascular risk factors which implied significant total effects were further incorporated into the outcome model. Since no significant effect was observed in the comparison between inadequate and adequate groups, which violated the premise of causal mediation assumption, analysis on the mediation effect of inadequate maternal GWG was omitted.
Table 2 showed significant mediation effect by birth weight of maternal excessive GWG effect on childhood body weight and BMI percentile, after adjustment for covariates during pregnancy and childhood. The proportions of total effect were 13% and 10% respectively. Newborn PI also mediated the effect of maternal excessive GWG on childhood BMI percentile (proportion of total effect 7%), whereas newborn SSF mediated the effect on childhood SSF (proportions of total effects 9%).
Table 2.
The causal mediation analysis on the associations of maternal GWG exceeding the IOM recommendations with childhood cardiovascular risk factors z-score through the newborn anthropometrics z-score
| Direct effect: β (95% CI) | Indirect effect: β (95% CI) | Total effect: β (95% CI) | Proportion mediated (95% CI) | |
|---|---|---|---|---|
|
| ||||
| Childhood anthropometrics | ||||
| Birth weight as mediator | ||||
| Body weight | 0.31 (0.17 to 0.45) § | 0.04 (0.02 to 0.08) § | 0.35 (0.21 to 0.50) § | 0.12 (0.05 to 0.25) § |
| BMI percentile | 0.30 (0.16 to 0.44) § | 0.03 (0.008 to 0.06) ⁑ | 0.33 (0.19 to 0.47) § | 0.10 (0.02 to 0.21) ⁑ |
| SSF | 0.23 (0.09 to 0.38) § | −0.009 (−0.03 to 0.02) | 0.23 (0.08 to 0.37) ‡ | −0.04 (−0.22 to 0.08) |
| Newborn PI as mediator | ||||
| Body weight | 0.33 (0.19 to 0.47) § | 0.005 (−0.01 to 0.02) | 0.34 (0.21 to 0.48) § | 0.02 (−0.03 to 0.07) |
| BMI percentile | 0.29 (0.14 to 0.43) § | 0.02 (0.004 to 0.05) * | 0.31 (0.16 to 0.46) § | 0.08 (0.01 to 0.18) * |
| SSF | 0.22 (0.08 to 0.38) * | 6.8×10−4 (−0.02 to 0.02) | 0.22 (0.08 to 0.38) * | 0.003 (−0.09 to 0.14) |
| Newborn SSF as mediator | ||||
| Body weight | 0.34 (0.21 to 0.49) § | −0.002 (−0.02 to 0.02) | 0.34 (0.20 to 0.49) § | −0.006 (−0.07 to 0.05) |
| BMI percentile | 0.30 (0.16 to 0.45) § | 0.009 (−0.008 to 0.03) | 0.31 (0.17 to 0.46) § | 0.03 (−0.03 to 0.11) |
| SSF | 0.21 (0.06 to 0.38) ‡ | 0.02 (0.001 to 0.04) * | 0.23 (0.08 to 0.39) ‡ | 0.08 (0.005 to 0.29) * |
| Childhood blood pressure | ||||
| Birth weight as mediator | ||||
| SBP percentile | 0.20 (0.04 to 0.35) ⁑ | −0.04 (−0.08 to −0.01) § | 0.16 (0.01 to 0.30) * | −0.27 (−1.6 to −0.04) * |
| DBP percentile | 0.25 (0.11 to 0.39) ‡ | −0.02 (−0.05 to 0.009) | 0.23 (0.10 to 0.37) ‡ | −0.07 (−0.26 to 0.04) |
| Newborn PI as mediator | ||||
| SBP percentile | − | − | 0.14 (−0.01 to 0.30) | − |
| DBP percentile | 0.27 (0.13 to 0.42) § | −0.03 (−0.05 to −0.005) ‡ | 0.25 (0.10 to 0.40) ‡ | −0.10 (−0.29 to −0.02) ⁑ |
| Newborn SSF as mediator | ||||
| SBP percentile | − | − | 0.15 (−0.005 to 0.30) | − |
| DBP percentile | 0.23 (0.08 to 0.38) ‡ | 0.02 (0.004 to 0.05) * | 0.26 (0.12 to 0.40) § | 0.10 (0.01 to 0.28) * |
The mediation model was adjusted for gestational age, paternal BMI maternal age, parity, gestational hypertension/preeclampsia, smoking and glycemic status in pregnancy; child’s sex, age and exercise level.
, total effect was insignificant (p >0.05).
GWG, gestational weight gain; IOM, Institute of Medicine; PI, Ponderal index; SSF, sum of skinfolds; SBP, systolic blood pressure; DBP, diastolic blood pressure; BCF, beta-cell function; IGI, insulinogenic index; ISI, insulin sensitivity index.
P < 0.05;
P<0.01;
P<0.005;
P<0.001.
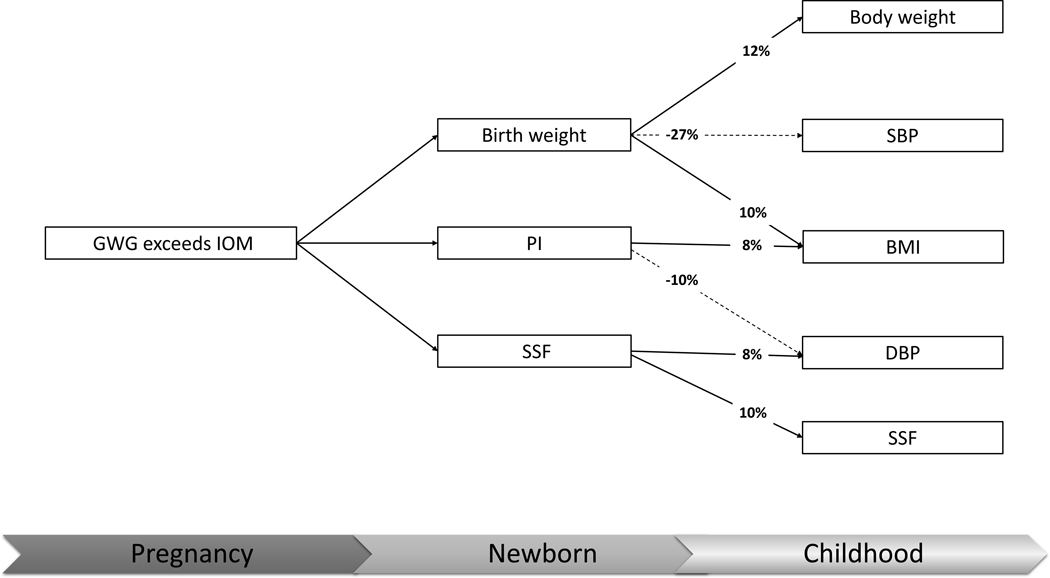
A negative mediation by birth weight of the maternal excessive GWG effect on childhood SBP was observed (proportion of total effect −27%). Similarly, a negative mediation by newborn PI of the effect on childhood DBP percentile was also observed (proportion of total effect −8%). In contrast, newborn SSF was a mediator in the association between maternal excessive GWG and childhood DBP percentile (proportions of total effect 8%). (Table 2 & figure 2)
Figure 2.
The proportion mediated by the newborn anthropometric measurements in the association between maternal excessive pregnancy weight gain and childhood cardiovascular risk factors
The solid line represents positive mediation effect, while the dashed line represents negative mediation effect.
GWG, gestational weight gain; IOM, Institute of Medicine; PI, Ponderal index; SSF, sum of skinfolds; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Discussion
Our study revealed that newborn SSF was positively associated with children’s SSF, BP and PWV at approximately 7 years of age. Whereas newborn PI, most likely representing fat-free mass, was inversely associated with childhood BP and PWV. Newborn SSF mediated the effect of excessive maternal GWG on childhood BP, whereas birth weight or PI negatively mediated the effect. Although our study as well as previous studies (8, 28) continuously find both birth weight and neonatal adiposity are associated with children’s BMI, our results suggest that neonatal adiposity may have a contrasting relationship to birth weight or PI in the association with childhood cardiovascular risks.
Our study provides three novel findings. The first is that newborn SSF is positively associated with the children’s BP and PWV at ∼7 years of age. Our findings suggest that on average, each SD increase in newborn SSF is associated with 0.13 SD (i.e., 10 percentiles in normal distribution) and 0.08 SD (i.e., 6 percentiles in normal distribution) increase in childhood DBP and cfPWV, respectively. In contrast, each SD increase in newborn PI is associated with 0.08 SD decrease in both childhood SBP and DBP, as well as with 0.09 SD (i.e., 7 percentiles in normal distribution) decrease in cfPWV. Moreover, analysis stratified by sex implies that the association of birth weight with childhood SBP is much stronger in boys, whereas the association of newborn SSF with childhood cfPWV is much stronger in girls. Nevertheless, the magnitudes of the associations were all relatively small. Previous studies on the association of neonatal adiposity with children’s BP or arterial stiffness are limited. The Ethiopian infant anthropometry and body composition (iABC) birth cohort failed to observe a significant association between neonatal adiposity and childhood BP. However, the aforementioned study by Wibaek et al. had a much smaller sample size and did not adjust for children’s height (29).
Furthermore, our study also reported that the higher newborn SSF was a mediator in the association between maternal GWG and higher childhood cardiovascular risks. Newborn SSF was recently reported as a mediator in the association between maternal BMI at OGTT and childhood SSF (9). Maternal BMI at OGTT (24–32 weeks gestation) is a surrogate which reflects both maternal pre-pregnancy BMI and the weight obtained through the second trimester. Our study suggests newborn SSF independently mediates the effect of excessive maternal GWG on childhood adiposity, regardless of pre-pregnancy BMI. Moreover, our study shows that the effect of maternal excessive GWG on childhood BP is mediated by newborn SSF. In contrast, birth weight or PI have the inverse effect on childhood BP.
Last, the association of newborn PI with children’s BP and PWV being opposite to the association of SSF was also discovered. Moreover, our study also found a U-shape association of newborn PI with childhood BP and PWV as a slightly better prediction model. Nevertheless, the variance explained by newborn PI remains small. A previous study has found a similar inverse correlation between newborn PI and SBP in male adolescents (30). The Pearson’s correlation coefficients in our study indicate the correlation between newborn PI and SSF is much weaker than their respective associations with birth weight. Further, the intergrowth-21st study found that infant’s PI had a higher association with the child’s fat-free mass rather than fat mass based on the adjusted R square value (0.62 vs 0.43) (31). Hence, we speculate that an infant’s PI may better represent fat free mass or lean rather than adiposity. A reduction of fat-free mass, as well as an accretion of fat mass at birth may both influence the cardiovascular health at childhood.
Although the magnitudes of newborn PI and SSF are relatively small, our study suggests the application of birth weight, which adds up the effects of fat-free mass and fat mass and has been widely used in perinatal care, may attenuate either of the respective effect. In contrast, aiming at higher PI and lower SSF together, may yield promising long-term outcomes. Previous study found that children with high fat mass index have increased metabolic syndrome risk score at 6 years of age, compared to those with low fat mass index, regardless of their lean mass index. Moreover, among girls with high fat mass index, those having high lean mass index had a lower diastolic blood pressure than those having low lean mass index (32). Similarly, studies in adults showed the combination of high fat-free mass and low fat mass was associated with the lowest arterial stiffness and the greatest bone mineral density, whereas sarcopenic obesity posed the highest risk (33). Previous studies showed greater fat-free mass in early infancy might promote later bone mass and muscle mass growth, likely through the complex interplay of growth hormone and Rho guanosine triphosphate hydrolases (GTPases) with insulin-like growth factor 1 (IGF-1) (34, 35).
The present study shares a new insight to assess optimal maternal GWG as a component of antenatal care. The recommendations for optimal GWG, such as the widely-used references proposed by the IOM (27), as well as the recommendations for other populations, such as Japanese (36), were all derived primarily based on infant’s birth weight (i.e., SGA or LGA). However, just as our previous study showed that optimal ranges for GWG derived from newborn fat mass was associated with far more childhood cardiovascular risk factors compared to the optimal ranges derived from birth weight (14). The current study further examines the impact of newborn fat mass and fat-free mass on childhood cardiovascular risks. As supportive evidence, the Health Start Study reported that greater consumption of fat and carbohydrates during pregnancy, and reduced energy expenditure in late-pregnancy, which are independent risk factors associated with excessive maternal GWG, independently increased newborn fat mass, but not fat-free mass or birth weight (15, 16). Therefore, we speculate that targeting optimal ranges for maternal GWG, with the aim to reduce the rate of infant adiposity and increasing fat-free mass may provide more benefits to offspring’s long-term cardiovascular health, compared with targeting those that aim to simply reduce the rate of an infant being LGA.
There were several limitations in the current study. First, environmental factors, such as women’s nutrition and exercise level during pregnancy are relevant to maternal GWG as well as fetal growth. These data unfortunately were not available to us in the HAPO study and the follow-up study. Meanwhile, children’s dietary habits may also influence cardiovascular risks. Mothers’ and offspring’s dietary habits and exercise levels should be recorded for a more comprehensive study in the future. Secondly, the average total newborn’s skinfolds in the current study was approximate 1 mm less than that in another Chinese cohort (37). This may partly because that infants of women with OGTT levels exceeding a certain level (fasting plasma glucose >5.6 mmol/L or 2-hour plasma glucose > 11.1 mmol/L) were excluded for the analysis based on the HAPO study design.
In conclusion, newborn SSF is associated with childhood cardiovascular risks, whereas newborn PI as a potential surrogate of fat-free mass was inversely associated with them. Newborn SSF mediates the impact of maternal excessive GWG on childhood BP, whereas birth weight or newborn PI negatively mediated it. The results of our study suggest the assessment of neonatal body composition, rather than birth weight alone, may improve strategies for development of optimal gestational weight gain.
Supplementary Material
Study Importance.
What is already known about this subject?
Maternal gestational weight gain (GWG) categorized by the recommendations proposed by the Institute of Medicine is associated with childhood cardiovascular risks factors.
Optimal ranges for maternal GWG, derived based on infant’s fat mass, are superior to that based on birth weight, in the prediction of childhood cardiovascular risks.
Birth weight is inversely associated with childhood blood pressure.
What are the new findings in your manuscript?
Newborn sum of skinfolds (SSF) was associated with childhood diastolic blood pressure (DBP), and pulse wave velocity (PWV), whereas newborn ponderal index (PI) was inversely associated with childhood systolic blood pressure (SBP), DBP and PWV.
Newborn SSF significantly mediated the effects of maternal GWG exceeding the Institute of Medicine recommendations on childhood SSF and DBP.
Birth weight and Newborn PI negatively mediated the effect of maternal excessive GWG on childhood SBP and DBP, respectively.
How might your results change the direction of research or the focus of clinical practice?
This study suggests that targeting optimal ranges for maternal GWG with the aim to reduce the rate of infant adiposity may provide more benefits to offspring’s long-term cardiovascular health compared with those which aim to reduce the rate of infant being large for gestation.
Acknowledgements
We thank the HAPO study steering committee for initiating and conducting the original study, and for their kind help and support. We would especially like to thank Prof. A. R. Dyer (Emeritus Professor, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA) for reviewing the manuscript.
Sources of Fundings
The HAPO study was funded by the National Institute of Child Health and Human Development (grant no. R01-HD34242) and the National Institute of Diabetes and Digestive and Kidney Diseases (grant no. R01-HD34243). The HAPO follow-up study at the Hong Kong Centre was supported by the funding from the General Research Fund of the Research Grants Council of the Hong Kong SAR, China (grants CUHK 473408 and, in part, CUHK 471713).
Footnotes
Declaration of interests:
All the authors declared no conflict of interest.
Data availability
The data are not publicly available as they contain information that could compromise the privacy or consent of the research participants.
References
- 1.Nordman H, Jaaskelainen J, Voutilainen R. Birth Size as a Determinant of Cardiometabolic Risk Factors in Children. Horm Res Paediat. 2020;93(3):144–53. [DOI] [PubMed] [Google Scholar]
- 2.Knop MR, Geng TT, Gorny AW, Ding RY, Li CW, Ley SH, et al. Birth Weight and Risk of Type 2 Diabetes Mellitus, Cardiovascular Disease, and Hypertension in Adults: A Meta-Analysis of 7 646 267 Participants From 135 Studies. J Am Heart Assoc. 2018;7(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mu M, Wang SF, Sheng J, Zhao Y, Li HZ, Hu CL, et al. Birth weight and subsequent blood pressure: A meta-analysis. Arch Cardiovasc Dis. 2012;105(2):99–113. [DOI] [PubMed] [Google Scholar]
- 4.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness A Scientific Statement From the American Heart Association. Hypertension. 2015;66(3):698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mzayek F, Sherwin R, Hughes J, Hassig S, Srinivasan S, Chen W, et al. The association of birth weight with arterial stiffness at mid-adulthood: the Bogalusa Heart Study. J Epidemiol Commun H. 2009;63(9):729–33. [DOI] [PubMed] [Google Scholar]
- 6.Miles KL, McDonnell BJ, Maki-Petaja KM, Yasmin Cockcroft JR, Wilkinson IB, et al. The impact of birth weight on blood pressure and arterial stiffness in later life: the Enigma Study. J Hypertens. 2011;29(12):2324–31. [DOI] [PubMed] [Google Scholar]
- 7.Coelho DM, Camelo LV, Giatti L, Chor D, Guimaraes JMN, Mill JG, et al. Racial differences in the association between early socioeconomic position, birth weight, and arterial stiffness in adults from ELSA-Brasil. Ann Epidemiol. 2019;34:45–51. [DOI] [PubMed] [Google Scholar]
- 8.Moore BF, Harrall KK, Sauder KA, Glueck DH, Dabelea D. Neonatal Adiposity and Childhood Obesity. Pediatrics. 2020;146(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Josefson JL, Scholtens DM, Kuang AL, Catalano PM, Lowe LP, Dyer AR, et al. Newborn Adiposity and Cord Blood C-Peptide as Mediators of the Maternal Metabolic Environment and Childhood Adiposity. Diabetes Care. 2021;44(5):1194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes A Systematic Review and Meta-analysis. Jama-J Am Med Assoc. 2017;317(21):2207–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waters TP, Huston-Presley L, Catalano PM. Neonatal Body Composition According to the Revised Institute of Medicine Recommendations for Maternal Weight Gain. J Clin Endocr Metab. 2012;97(10):3648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaillard R, Welten M, Oddy WH, Beilin LJ, Mori TA, Jaddoe VW, et al. Associations of maternal prepregnancy body mass index and gestational weight gain with cardio-metabolic risk factors in adolescent offspring: a prospective cohort study. Bjog. 2016;123(2):207–16. [DOI] [PubMed] [Google Scholar]
- 13.Tam CHT, Ma RCW, Yuen LY, Ozaki R, Li AM, Hou Y, et al. The impact of maternal gestational weight gain on cardiometabolic risk factors in children. Diabetologia. 2018;61(12):2539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Y, Tam CH-T, Yuen LY, Catalano PM, Ma RC-W, Tam WH. Optimal gestational weight gain for Chinese women - analysis from a longitudinal cohort with childhood follow-up. The Lancet Regional Health - Western Pacific. 2021;13:100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crume TL, Brinton JT, Shapiro A, Kaar J, Glueck DH, Siega-Riz AM, et al. Maternal dietary intake during pregnancy and offspring body composition: The Healthy Start Study. American Journal of Obstetrics and Gynecology. 2016;215(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrod CS, Chasan-Taber L, Reynolds RM, Fingerlin TE, Glueck DH, Brinton JT, et al. Physical activity in pregnancy and neonatal body composition: the Healthy Start study. Obstet Gynecol. 2014;124(2 Pt 1):257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tam WH, Ma RCW, Ozaki R, Li AM, Chan MHM, Yuen LY, et al. In Utero Exposure to Maternal Hyperglycemia Increases Childhood Cardiometabolic Risk in Offspring. Diabetes Care. 2017;40(5):679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. New Engl J Med. 2008;358(19):1991–2002. [DOI] [PubMed] [Google Scholar]
- 19.Josefson JL, Nodzenski M, Talbot O, Scholtens DM, Catalano P. Fat mass estimation in neonates: anthropometric models compared with air displacement plethysmography. Brit J Nutr. 2019;121(3):285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Sheridan B, Hod M, et al. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study Associations With Neonatal Anthropometrics. Diabetes. 2009;58(2):453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hui SC, Chan CM, Wong SHS, Ha ASC, Hong YL. Physical activity levels of Chinese youths and the association with physical fitness and demographic variables: The Hong Kong youth fitness study. Res Q Exercise Sport. 2001;72(1):A92–A3. [Google Scholar]
- 22.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10(3):141–6. [PubMed] [Google Scholar]
- 23.Leung SSF, Cole TJ, Tse LY, Lau JTF. Body mass index reference curves for Chinese children. Ann Hum Biol. 1998;25(2):169–74. [DOI] [PubMed] [Google Scholar]
- 24.Tam WH, Ma RCW, Yip GWK, Yang XL, Li AM, Ko GTC, et al. The association between in utero hyperinsulinemia and adolescent arterial stiffness. Diabetes Res Clin Pr. 2012;95(1):169–75. [DOI] [PubMed] [Google Scholar]
- 25.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2):555–76. [PubMed] [Google Scholar]
- 26.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. mediation: R Package for Causal Mediation Analysis. J Stat Softw. 2014;59(5). [Google Scholar]
- 27.Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Rasmussen KM, Yaktine AL, editors. Weight Gain During Pregnancy: Reexamining the Guidelines. The National Academies Collection: Reports funded by National Institutes of Health. Washington (DC)2009. [PubMed] [Google Scholar]
- 28.Tam CH, Wang Y, Luan J, Lee HM, Luk AO, Tutino GE, et al. Non-linear relationship between birthweight and cardiometabolic risk factors in Chinese adolescents and adults. Diabet Med. 2015;32(2):220–5. [DOI] [PubMed] [Google Scholar]
- 29.Wibaek R, Vistisen D, Girma T, Admassu B, Abera M, Abdissa A, et al. Associations of fat mass and fat-free mass accretion in infancy with body composition and cardiometabolic risk markers at 5 years: The Ethiopian iABC birth cohort study. PLoS Med. 2019;16(8):e1002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berkey CS, Gardner J, Colditz GA. Blood pressure in adolescence and early adulthood related to obesity and birth size. Obes Res. 1998;6(3):187–95. [DOI] [PubMed] [Google Scholar]
- 31.Villar J, Puglia FA, Fenton TR, Cheikh Ismail L, Staines-Urias E, Giuliani F, et al. Body composition at birth and its relationship with neonatal anthropometric ratios: the newborn body composition study of the INTERGROWTH-21(st) project. Pediatr Res. 2017;82(2):305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ong YY, Huang JY, Michael N, Sadananthan SA, Yuan WL, Chen LW, et al. Cardiometabolic Profile of Different Body Composition Phenotypes in Children. J Clin Endocr Metab. 2021;106(5):E2015-E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tap L, Kirkham FA, Mattace-Raso F, Joly L, Rajkumar C, Benetos A. Unraveling the Links Underlying Arterial Stiffness, Bone Demineralization, and Muscle Loss. Hypertension. 2020;76(3):629–39. [DOI] [PubMed] [Google Scholar]
- 34.Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. Brit J Pharmacol. 2008;154(3):557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez-Fdez S, Bustelo XR. Rho GTPases in Skeletal Muscle Development and Homeostasis. Cells-Basel. 2021;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morisaki N, Nagata C, Jwa SC, Sago H, Saito S, Oken E, et al. Pre-pregnancy BMI-specific optimal gestational weight gain for women in Japan. Journal of Epidemiology. 2017;27(10):492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fok TF, Hon KL, Ng PC, Wong E, So HK, Lau J, et al. Use of Anthropometric Indices to Reveal Nutritional Status: Normative Data from 10,226 Chinese Neonates. Neonatology. 2009;95(1):23–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are not publicly available as they contain information that could compromise the privacy or consent of the research participants.