Abstract
Background:
Early detection is critical for easing the rising burden of psychiatric disorders. However, the specificity of psychopathological measurements and genetic predictors is unclear among youth.
Methods:
We measured associations between genetic risk for psychopathology (polygenic risk scores (PRS) and family history (FH) measures) and a wide range of behavioral measures in a large sample (n=5204) of early adolescent participants (9–11 years) from the Adolescent Brain and Cognitive Development StudySM. Associations were measured both with and without accounting for shared variance across measures of genetic risk.
Results:
When controlling for genetic risk for other psychiatric disorders, polygenic risk for problematic opioid use (POU) uniquely associated with lower behavioral inhibition. Attention Deficit Hyperactivity Disorder (ADHD), depression (DEP) and attempted suicide (SUIC) PRS shared many significant associations with externalizing, internalizing and psychosis-related behaviors. However, when accounting for all measures of genetic and familial risk these PRS also showed clear, unique patterns of association. Polygenic risk for ASD, BIP, SCZ and attempted suicide uniquely predicted variability in cognitive performance. FH accounted for unique variability in behavior above and beyond PRS and vice versa, with FH measures explaining a greater proportion of unique variability compared to the PRS.
Conclusion:
Our results indicate that, among youth, many behaviors show shared genetic influences; however, there is also specificity in the profile of emerging psychopathologies for individuals with high genetic risk for particular disorders. This may be useful for quantifying early, differential risk for psychopathology in development.
INTRODUCTION
Psychiatric disorders place a huge burden on those affected, their families and society. Identifying risk for psychopathology in developmental samples may offer an opportunity for early detection and intervention. Nearly all psychiatric disorders have a heritable component, with twin heritability estimates ranging from 33–84% across affective, psychotic and developmental disorders1. Lifetime prevalence rates of several disorders are higher among first degree biological relatives of individuals with a psychiatric diagnosis2. Therefore, estimating genetic liability for psychiatric disorders presents one avenue for identifying at risk individuals and probing differential and transdiagnostic risk factors. Here we sought to determine: 1) if increased genetic risk within a large, typically developing sample would be associated with symptoms of psychopathology, related individual difference factors, and cognitive function; and, 2) whether there was any evidence for specificity in behavioral measures predicted by different genetic markers.
Large-sample analyses of results from genome-wide association studies (GWAS) have revealed the highly polygenic architecture of complex behavioral phenotypes, with many variants in the genome additively accounting for substantial heritability, but individually exerting only very small effects. Models using effect sizes at single nucleotide polymorphisms (SNPs) estimated from large-scale independent GWAS, can be used to compute polygenic risk scores (PRS), which estimate an individual’s genetic risk for a trait. Recent powerful, cross-disorder meta-analyses3,4 reveal high genetic correlation and widespread pleiotropy across psychiatric disorders, consistent with overlapping genetic architecture. Indeed, polygenic risk for depression has been shown to positively associate with childhood psychopathology across behavioral domains5.
Family history (FH) is a clinically used factor for predicting psychiatric risk6, yet there have been few direct comparisons of associations between PRS and FH of psychopathology in childhood and adolescence. SNP heritabilities (hSNP2) based on effects across the genome are lower than twin heritabilities, suggesting there are genetic factors driving psychiatric phenotypes which are not fully captured with common variants at current GWAS sample sizes. Indeed, FH likely reflects a complex combination of genetic and environmental factors. Due to the differential information that PRS and FH measures may provide, it is important to determine whether they explain independent or overlapping variance in developmental psychopathology and cognition. For example, in a joint model, PRS and FH of schizophrenia were shown to be independent risk factors for schizophrenia7. Here we aim to further understand the unique contribution of polygenic risk above and beyond FH in a typically developing sample across multiple measures of psychopathology.
For this study we used behavioral and genetic data from 9–11 year-old children from the Adolescent Brain and Cognitive Development (ABCD) StudySM. We generated eight PRS that were trained on large independent datasets. We used these PRS and measures of FH of psychopathology both independently and within the same models to predict a large array of both caregiver and youth-reported behaviors thought to reflect risk for developing psychiatric disorders. Measures included both dimensional and diagnostic assessments of psychopathology, individual difference measures of impulsivity and behavioral approach and inhibition, prodromal psychosis and behaviors associated with mania and prosocial behavior. Given documented associations and genetic overlap between cognitive impairment and schizophrenia and bipolar disorder8,9, we additionally measured associations with cognitive measures from the NIH Toolbox®. Using this approach, we aimed to uncover variability across early signs of psychopathology that are uniquely associated with each genetic/familial predictor. This research is an essential first step in this large longitudinal study to determine whether we can identify early signs of specificity in genetic-behavior associations in development, which can then be tracked to determine their potential predictive power for future diagnoses.
METHODS & MATERIALS
Sample
The ABCD study is a longitudinal study across 21 data acquisition sites in the United States following 11,880 children starting at 9–11 years. This paper uses baseline data from the NIMH Data Archive ABCD Collection Release 2.0.1 (DOI: 10.15154/1504041). The ABCD cohort was recruited to ensure the sample was as close to nationally representative as possible, and therefore exhibits large sociodemographic diversity10. There is an embedded twin cohort and many siblings. As the chosen PRS were trained predominantly on European individuals, the main associations in this study were conducted in a European ancestry sample (n=5204). Supplementary analyses were conducted in those with non-European ancestry (n=3964) and the full sample (n=9168). Table S1 outlines the demographics of the three samples.
ABCD Baseline Mental Health Battery
The Mental Health Battery in ABCD is an extensive battery of questionnaires and semi-structured interviews assessing diagnostic and dimensional measures of psychopathology and individual difference factors. Both youth and their caregivers provided responses at baseline using divergent and overlapping measures. Motivation behind selecting these assessments is outlined elsewhere11. Table S2 lists variables used from the ABCD public release.
DIAGNOSTIC ASSESSMENTS
Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS).
Participants completed a semi-structured, self-administered, computerized version of the validated and reliable KSADS-512. Research Assistants had extensive training to support youth completing this assessment. Caregivers and youth completed modules on depression, bipolar disorder, generalized anxiety disorder, social anxiety disorder, suicidality and sleep. Only caregivers completed psychosis, obsessive-compulsive disorder (OCD), attention deficit hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), conduct disorder (CD), panic disorder and eating disorders modules. Symptom scores were the sum of symptoms endorsed in each module. The total symptom score was a sum across modules.
DIMENSIONAL ASSESSMENTS
Child Behavior Checklist (CBCL).
Caregiver-reported CBCL13 has eight syndrome scales: anxious/depressed, withdrawn/depressed, somatic complaints, social problems, thought problems, attention problems, rule breaking behavior and aggressive behavior, and a total problems score.
General behavior inventory.
Caregiver-report ten-item Mania Scale14 derived from the 73-item General Behavior Inventory (PGBI) for Children and Adolescents15.
Prosocial Behavior Survey.
Caregivers and youth were asked three questions about how helpful and considerate the youth was in general, with summed scores for both caregiver and youth.
Prodromal Questionnaire Brief (PQ-B).
Youth-report measure, modified for use in children in our age range, consisting of a 21-item scale assessing subclinical manifestations of psychosis16,17. The prodromal psychosis severity score is the sum of the number of symptoms endorsed weighted by how distressing the symptoms were.
UPPS-P for children short scale.
Youth-report impulsive behavior scale, which includes five sub-scales that measure four factors of impulsivity: positive and negative urgency, lack of perseverance, premeditation, and sensation seeking18.
Behavioral inhibition and behavioral activation (BISBAS scale).
Youth-report measure of approach and avoidance behaviors19,20 that produces scores for drive, fun seeking, reward responsiveness, and behavioral inhibition.
NIH Toolbox Cognition Battery®.
Widely used battery of cognitive tests that measures a range of different cognitive domains21–23. We analyzed the uncorrected composite scores broadly measuring fluid and crystallized intelligence that are generated from the NIH Toolbox® and have been validated against gold-standard measures24,25. The fluid composite score includes performance on the flanker task, picture sequence memory task, list sorting memory task, pattern comparison processing speed and dimensional change card sort task. The crystallized composite score includes performance on the oral reading recognition task and picture vocabulary task.
Genetic & Familial Measures
Polygenic Risk Scores (PRS)
PRS were estimated from summary statistics for ADHD26, Autism Spectrum Disorder (ASD)27, Bipolar Disorder (BIP)28, Schizophrenia (SCZ)29, Depression (DEP)30, problematic opioid use (POU)31, attempted suicide (SUIC)32 and psychotic experiences (PSYEXP)33. Although we pruned SNPs, the results in the main text result do not apply p-value thresholds when calculating PRS in attempt to guard against overfitting. Figures S1–S6 shows these main results are consistent and often outperform using more stringent p-value thresholds. In supplementary analysis we also present results controlling for polygenic prediction of intelligence34. Additional details of preprocessing genetic data and PRS estimation are in the Appendix S1 of Supporting Information.
Family History Assessment
Caregivers were given a questionnaire asking about family history (FH) of 10 behaviors associated with psychopathology: alcohol use; drug use; depression; mania; psychosis; conduct problems; nerves; seen a therapist; hospitalized for a mental health problem; and, suicide. For each question the caregivers were asked if any blood relative had experienced any of the described behaviors (see Table S3). Importantly, these variables do not indicate clinical diagnoses of these behaviors.
Statistical Analysis
Generalized Linear Models (GLMs) were fit to measure the association between i) each of the 41 behavioral phenotypes and ii) FH and PRS. Univariate models included one independent variable of interest (PRS or FH) in each model (i.e. behavior~PRSi+covariates or behavior~FHi+covariates). Multivariable models included all PRS and FH measures in the same model (i.e. behavior~PRS1+PRS2…+FH1+FH2…+covariates). Fixed nuisance covariates included age, sex, top 10 genetic principal components, household income, highest parental education, and data collection site. ΔR2 was reported as change in R2 from a reduced model (covariates only) to a full model (including the predictor of interest)35. Supplementary analyses were conducted without controlling for socioeconomic status (SES) – i.e. household income and parental education. Family relatedness was controlled for by taking median effect across 100 subsamples of singletons. False discovery (FDR) rate correction was used to determine significance and derive adjusted p-values (p-adj). Although the main results are presented in the European ancestry sample, we also show results from the Full and non-European ancestry samples in Figure S7. Figure S8 displays the discordance between European and non-European ancestry associations motivating our decision to present the European ancestry results in the main text. Additional models were implemented to measure pairwise spearman correlations across all dependent variables (DVs) and independent variables (IVs) in the European ancestry sample after residualizing for the covariates of no interest (Figures S9&S10). Behavioral measures were categorized by behavioral domain (see Table S2) to determine whether associations with each genetic predictor were enriched for measures within domains. See the Supporting Information for further details of statistical analysis.
RESULTS
Unique behavioral associations with PRS across domains
For univariate models (measuring the association between each PRS and each behavior), controlling for SES, the ADHD, DEP and SUIC PRS showed the largest and greatest number of associations across internalizing, externalizing and psychosis-related measures (Figure 1, left panel). The ADHD PRS significantly associated with CBCL rule-breaking (ΔR2=0.0071, p-adj=6.8×10−6), inattentive (ΔR2=0.0063, p-adj=7.3×10−8) and aggressive (ΔR2=0.0031, p-adj=6.8×10−4) behaviors, prodromal psychosis severity (ΔR2=0.0063, p-adj=3.0×10−5), and caregiver reported KSADS oppositional/conduct disorder (ΔR2=0.0041, p-adj=1.3×10−4) and ADHD (ΔR2=0.0030, p-adj=1.2×10−3) symptoms, followed by multiple youth and caregiver reported measures of impulsivity, depression and suicidality symptoms, bipolar and psychosis related measures and developmental social problems. The DEP PRS showed strongest associations with CBCL somatic complaints (ΔR2=0.0053, p-adj=3.3×10−6), KSADS symptoms of oppositional/conduct disorder (ΔR2=0.0039, p-adj=1.9×10−4) and CBCL anxious/depressive (ΔR2=0.0031, p-adj=3.2×10−4), aggressive (ΔR2=0.0030, p-adj=8.4×10−4), and rule-breaking (ΔR2=0.0029, p-adj=5.7×10−3) behaviors. These were followed by caregiver reported KSADS symptoms of suicidality (ΔR2=0.0027, p-adj=2.2×10−3), bipolar disorder (ΔR2=0.0027, p-adj=2.4×10−3) and anxiety (ΔR2=0.0020, p-adj=9.1×10−3) and youth reported KSADS depression symptoms (ΔR2=0.0027, p-adj=2.5×10−3), as well as other measures of negative urgency, developmental social problems, behavioral inhibition and bipolar and psychosis related behaviors. The SUIC PRS showed the strongest significant associations with CBCL rule breaking (ΔR2=0.0065, p-adj=1.7×10−5), aggression (ΔR2=0.0035, p-adj=2.6×10−4), prodromal psychosis severity (ΔR2=0.0041, p-adj=1.0×10−3), CBCL social problems (ΔR2=0.0031, p-adj=2.4×10−3), youth reported depression symptoms (ΔR2=0.0030, p-adj=1.3×10−3), CBCL inattention (ΔR2=0.0022, p-adj=2.3×10−3) and CBCL thought problems (ΔR2=0.0017, p-adj=1.6×10−2).
Figure 1. Univariate (left) and multivariable (right) associations for each behavior predicted by PRS.
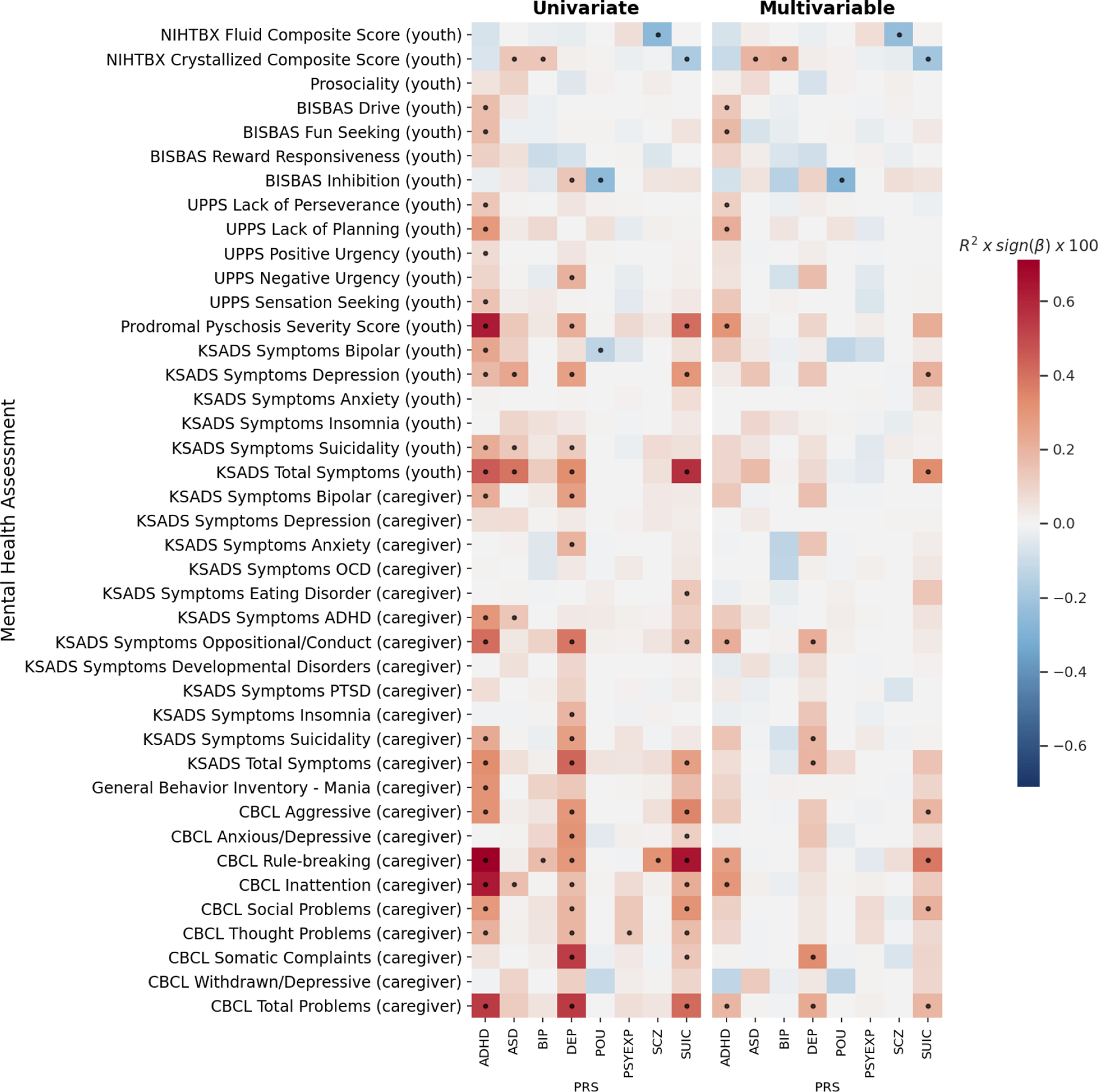
Effect sizes for each association are displayed as the partial variance explained, R2, (as a percentage) multiplied by sign of beta coefficient (red=positive, blue=negative). Response variable for each model is shown on y-axis. In univariate models (left) only a single genetic predictor was included in each model (each cell = 1 model) – i.e. behavior~PRS+covariates. In multivariable models (right) all genetic/familial predictors were included in each model including all PRS and FH measures (each row = 1 model) – i.e. behavior ~ PRS1+PRS2…+FH1+FH2…+covariates. ADHD: Attention Deficit Hyperactivity Disorder, ASD: Autism Spectrum Disorder, BIP: Bipolar Disorder, DEP: Depression, POU: problematic opioid use, PSYEXP: Psychotic Experience, SCZ: Schizophrenia, SUIC: suicide attempt. Dots indicate FDR significant associations.
In addition, the ASD PRS associated with some of the same behaviors as the ADHD, DEP and SUIC PRS, such as youth reported KSADS depression symptoms (ΔR2=0.0024, p-adj=3.9×10−3), suicidality symptoms (ΔR2=0.0013, p-adj=4.0×10−2), and ADHD symptoms (ΔR2=0.0014, p-adj=3.7×10−2), as well as CBCL inattention (ΔR2=0.0016, p-adj=1.0×10−2). The ADHD, ASD, DEP and SUIC PRS were all associated with the youth reported KSADS Total Symptoms score, and the ADHD, DEP and SUIC PRS were also associated with the caregiver reported CBCL Total Problems and KSADS Total Symptoms scores.
The BIP and SCZ PRS were not significantly associated with any bipolar or psychosis-related measures; however, they did significantly associate with CBCL rule-breaking with a smaller effect size compared to ADHD, DEP and SUIC (BIP: ΔR2=0.0017, p-adj=4.0×10−2; SCZ: ΔR2=0.0032, p-adj=3.5×10−3). In contrast, the PSYEXP PRS was significantly associated with CBCL thought problems (ΔR2=0.0013, p-adj=3.5×10−2). The POU PRS significantly negatively associated with youth reported behavioral inhibition (ΔR2=0.0025, p-adj=3.3×10−3) and KSADS bipolar symptoms (ΔR2=0.0013, p-adj=3.9×10−3).
For associations with cognitive performance, the SCZ PRS negatively associated with the fluid composite score from the NIH Toolbox® (ΔR2=0.0026, p-adj=3.2×10−3). Whereas the BIP and ASD PRS positively associated with the crystallized composite score from the NIH Toolbox® (BIP & ASD: ΔR2=0.0014, p-adj=3.6×10−2); and the SUIC PRS negatively associated with the crystallized composite score (ΔR2=0.0018, p-adj=1.7×10−2).
Multivariable models determined the specificity of these associations by covarying for all PRS and FH predictors simultaneously. In these models, PRS associations were attenuated and showed greater specificity for the ADHD, DEP and SUIC PRS (Figure 1, right panel). Each of these PRS predicted unique variability across a different pattern of externalizing, internalizing and psychosis-related measures not predicted by other measures of genetic risk (PRS or FH). For the ASD, BIP and SCZ PRS, only the associations with cognitive performance remained significant in the multivariable models. Controlling for an intelligence PRS attenuated these cognitive associations such that they were no longer significant (Figure S11). For the POU PRS, the negative association with behavioral inhibition remained significant when controlling for other measures of genetic risk (ΔR2=0.0027, p-adj=1.0×10−2) and the magnitude of the effect was not attenuated. The PSYEXP showed no significant associations in the multivariable models.
When not controlling for SES, behavioral associations were slightly larger and the overall pattern of associations was similar (Figure S12 & S13). However, there was an additional significant negative association with the ADHD PRS and the crystallized composite score (ΔR2=0.0021, p-adj=2.5×10−2). Appendix S2 contains regression results from all PRS associations with behavior.
We categorized each behavior into a domain to highlight different types of behavioral measures predicted by each PRS (Figure 2; domains defined in Table S2). Across both univariate and multivariable models, the largest associations with the ADHD PRS were with externalizing and psychosis-related measures; whereas the DEP and SUIC PRS associations encompassed a mix of internalizing and externalizing measures. In multivariable models, the specificity in the unique pattern of behaviors predicted by these PRS across domains was clarified due to the removal of shared variance across the genetic predictors.
Figure 2. Enrichment of PRS associations across behavioral domains.
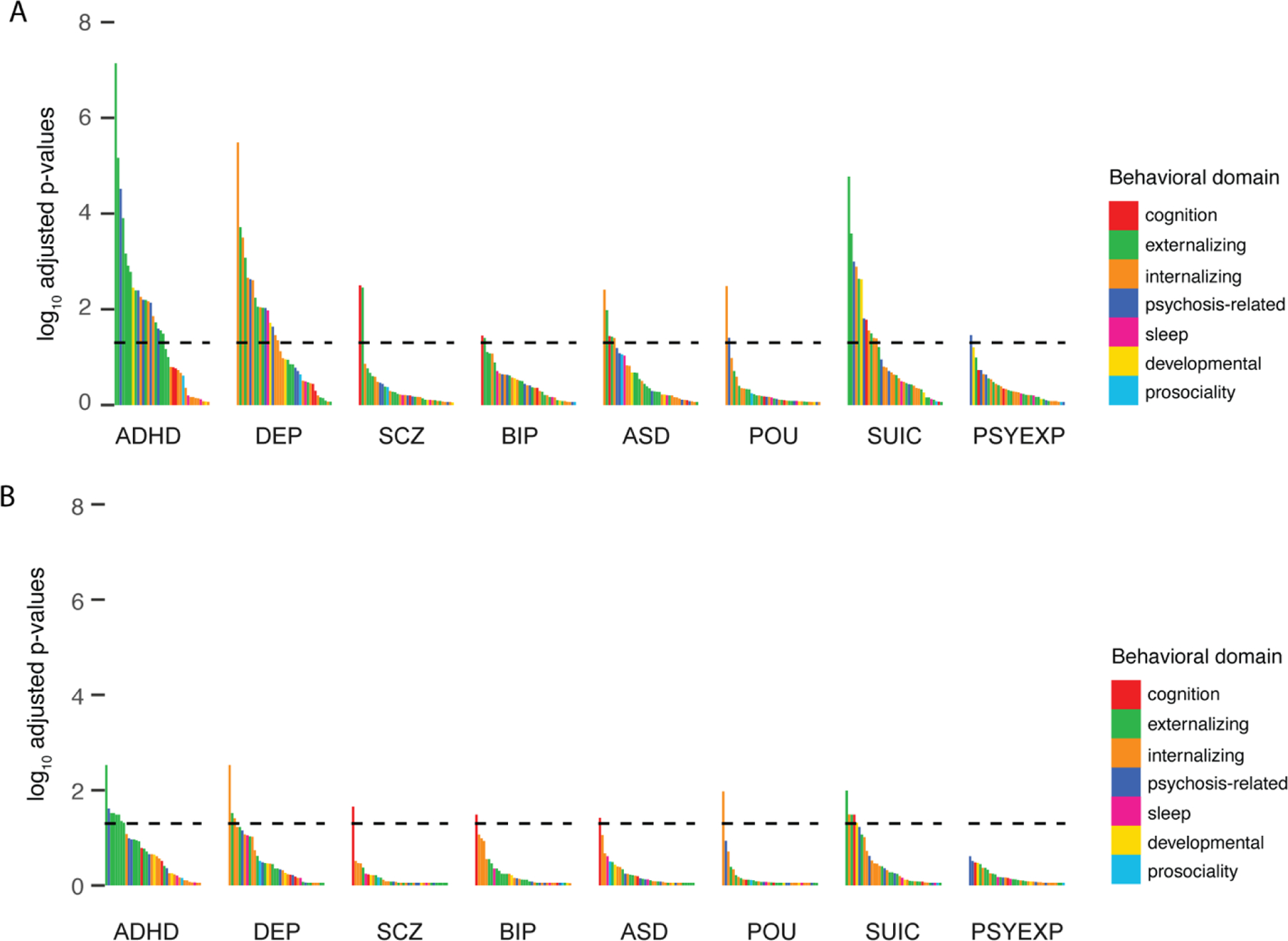
Log(p-adj) for all the associations shown in Figure 1 for: A) univariate and B) multivariable model. Bars are colored by behavioral domain (see Table S4). Horizontal line represents p-adj =0.05.
Unique behavioral associations with FH across domains
Behavioral associations with FH measures were larger than with PRS (Figure 3, left panel) in the univariate models. Given the large number of overlapping univariate associations, we focus on the associations from the multivariable models (i.e. controlling for all other FH and PRS predictors). In multivariable models, FH of conduct problems, depression and anxiety/stress showed the largest effects with some specificity across the behavioral measures (Figure 3, right panel). FH of conduct problems significantly associated with the CBCL subscales particularly with rule-breaking (ΔR2=0.0079, p-adj=2.8×10−5), as well as KSADS symptoms related to both externalizing and internalizing disorders (ΔR2range=0.0022–0.0071), and mania (ΔR2=0.0055, p-adj=1.1×10−3). FH of depression significantly associated with total problems scales from the CBCL (R2=0.0045, p-adj=6.1×10−4) and KSADS (ΔR2=0.0044, p-adj=6.9×10−4), as well as internalizing and externalizing measures across the KSADS and CBCL (ΔR2range=0.0018–0.0039). This pattern was similar to DEP PRS, however, unlike the DEP PRS, FH of depression only associated with caregiver-reported measures in the multivariable models. FH of anxiety/stress showed several associations across domains with the largest effects for caregiver-reported KSADS anxiety symptoms (ΔR2=0.0083, p-adj=9.3×10−7) and the CBCL anxious/depressive subscale (ΔR2=0.0069, p-adj=9.7×10−7).
Figure 3. Univariate (left) and multivariable (right) associations for each behavior predicted by FH.
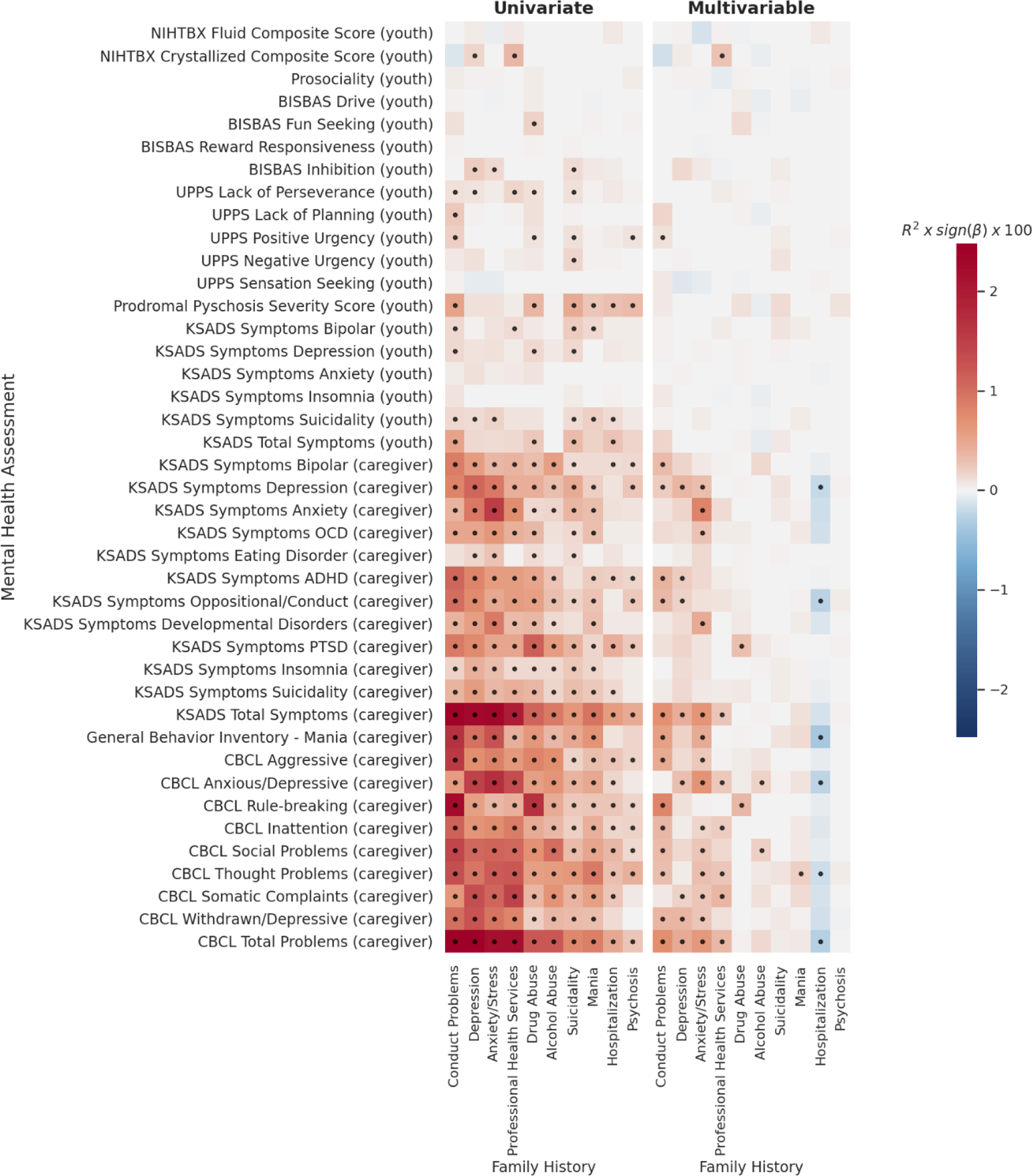
Effect sizes for each association are displayed as the partial variance explained, R2, (as a percentage) multiplied by sign of beta coefficient (red=positive, blue=negative). Response variable for each model is shown on y-axis. In univariate models (left) only a single genetic predictor was included in each model (each cell = 1 model) – i.e. behavior~FH+covariates. In multivariable models (right) all genetic/familial predictors were included in each model including all PRS and FH measures (each row = 1 model) – i.e. behavior ~ PRS1+PRS2…+FH1+FH2…+covariates. Dots indicate FDR significant associations.
FH of use of professional health services was most strongly associated with CBCL somatic complaints (ΔR2=0.0037, p-adj=1.3×10−3), thought problems (ΔR2=0.0026, p-adj=1.0×10−2) and the total problem score (ΔR2=0.0036, p-adj=3.0×10−3), and also showed a positive association with the crystallized composite score (ΔR2=0.0027, p-adj=1.1×10−2). Interestingly, when controlling for all other measures of genetic risk, FH of drug and alcohol abuse associated with differential behaviors, with FH of drug abuse explaining unique variance in CBCL rule-breaking (ΔR2=0.0035, p-adj=1.1×10−2) and KSADS PTSD symptoms (ΔR2=0.0030, p-adj=8.4×10−3), and FH of alcohol abuse explaining unique variance in CBCL social problems (ΔR2=0.0021, p-adj=5.0×10−2) and anxious/depressive behaviors (ΔR2=0.0018, p-adj=3.2×10−2). FH of hospitalization showed several negative associations with caregiver-reported internalizing behaviors, which were positive in the univariate models. This sign flip of effects may be due to collinearity across the genetic risk measures (Figure S10) when used in a single model. Appendix S2 contains regression results from all FH associations with behavior.
For univariate models, the FH measures associated with behaviors across several domains – see Figure 4. These patterns became more specific towards particular domains in multivariable models (controlling for other FH measures and the PRS). For example, FH of depression or anxiety/stress were significantly associated with internalizing behaviors, whereas FH of conduct disorder was significantly associated with externalizing behaviors.
Figure 4. Enrichment of FH associations across behavioral domains.
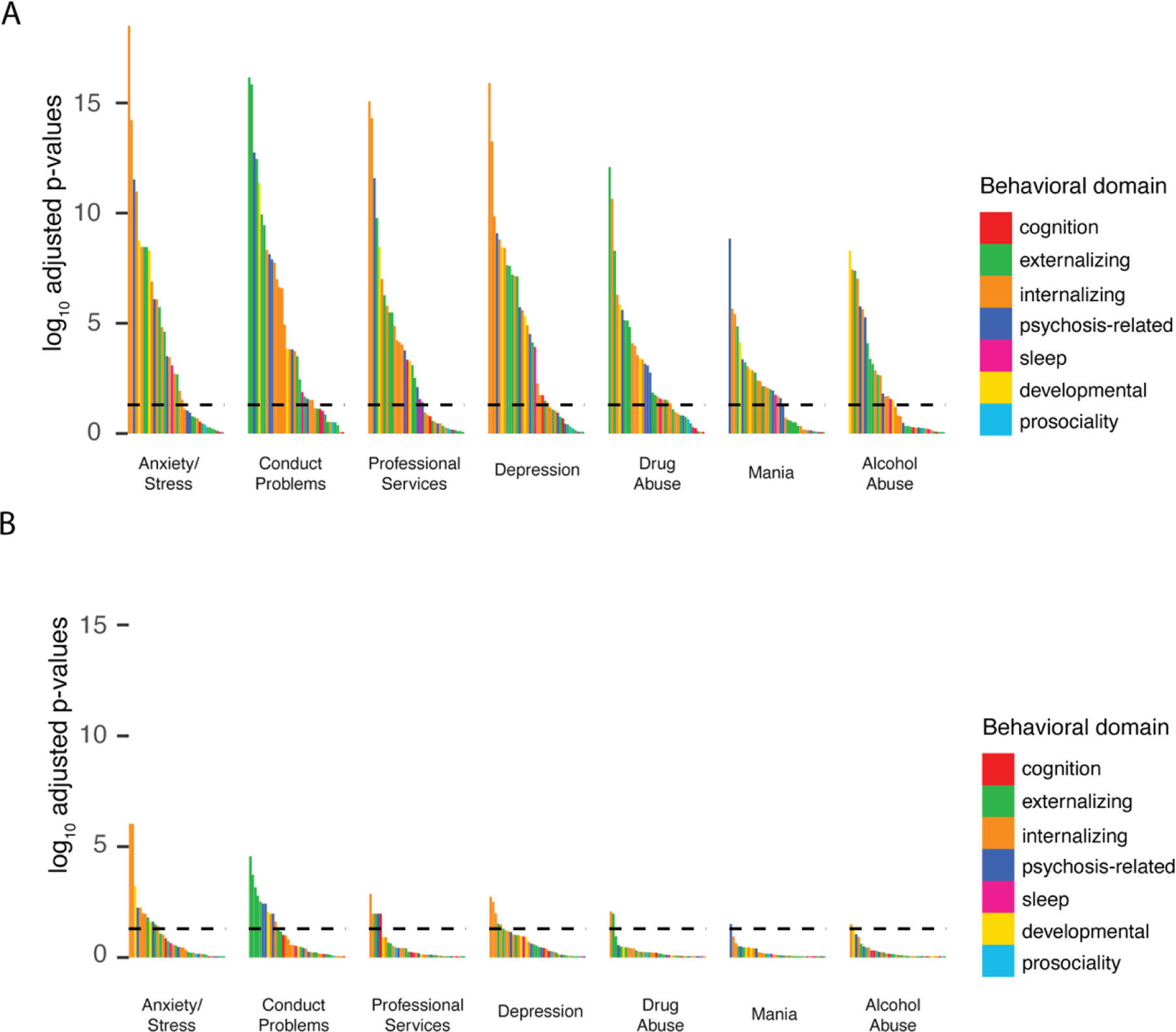
. log(p-adj) for all the associations shown in Figure 1 for: A) univariate and B) multivariable model. Bars are colored by behavioral domain (see Table S4). Horizontal line represents p-adj=0.05.
Total variance in behavior explained by PRS and FH
We quantified the variance in each behavior predicted by the set of PRS and set of FH measures when controlling for the other set of genetic predictors. Table S4 shows that, in all cases, each set independently predicted unique variance over and above the other set of genetic predictors. The maximum variance explained by the FH and PRS measures combined was ΔR2=0.066 of CBCL Total Problems scale, of which ΔR2=0.055 was uniquely predicted by FH and ΔR2=0.0088 was uniquely predicted by PRS. The maximum unique variance explained collectively by PRS was ΔR2=0.011 of the variability in CBCL rule-breaking. These results further demonstrate that PRS and FH predict unique, non-overlapping variance across different domains of behavior in youth with PRS predicting a smaller proportion of variability than FH.
DISCUSSION
Polygenic risk and FH of psychopathology predicted both overlapping and unique variability in behavior across domains in 9–11-year-old youth. Several externalizing and internalizing behaviors were associated with multiple measures of genetic risk highlighting shared genetic influences underlying variability in developmental psychopathology. However, when controlling for shared variance across PRS and FH measures, polygenic risk for ADHD, depression and attempted suicide predicted unique variance across differential externalizing, internalizing and psychosis-related behaviors. Moreover, polygenic risk for problematic opioid use was uniquely negatively associated with behavioral inhibition, and polygenic risk for ASD, BIP, SCZ and attempted suicide uniquely predicted variability in cognitive performance. FH of psychopathology explained additional unique variance in behavior, independent of the PRS, indicating additional genetic and environmental influences on behavior and recapitulating results in adults demonstrating the complementary information provided by PRS and FH7,36. Using combined information across these genetic and familial measures and the dense behavioral phenotyping in the ABCD study, we identified several, specific patterns of behavior associated with genetic risk for psychopathology that may be useful for quantifying early risk across different disorders.
In this developmental, drug-naïve sample, we interestingly found a negative association between polygenic risk for problematic opioid use and behavioral inhibition that remained significant when controlling for all other PRS and FH measures. This behavioral measure is thought to interrogate the behavioral avoidance system that regulates our motivation to move away from something unpleasant19,20. This negative association highlights that children with a high genetic propensity for misuse of prescription opioids, are already showing reduced behavioral avoidance to negative situations at 9–11 years old. The direction of this effect is consistent with associations between behavioral inhibition (using the same scale) and drug and/or alcohol use/abuse reported in adults37,38. The specificity of this association when controlling for polygenic risk for other psychiatric disorders suggests this may be a useful marker specifically for early risk for substance abuse in children.
Of the PRS analyzed, the ADHD, DEP and SUIC PRS showed univariate associations across largely overlapping behavioral measures. All of these PRS predicted variability in externalizing behaviors (e.g. rule-breaking, aggression and conduct problems), internalizing behaviors (e.g. youth reported depression), psychosis-related behaviors (e.g. prodromal psychosis, bipolar symptoms and thought problems), and inattentive and social problems. Given the correlation between behavioral problems in youth, this supports evidence that these frequently comorbid behaviors across different behavioral domains have shared genetic influences5,39. This indicates a common pathway that may contribute to the development of psychopathology. Indeed, suicidality and depression are common across individuals with several different psychiatric disorders and there is evidence that externalizing behaviors in childhood may indicate risk for both externalizing and internalizing disorders in adulthood40. However, there is variability across all the behavioral measures in terms of measurement error, construct validity and endorsement across participants; therefore, these common associations across genetic measures may be biased by the behaviors with the largest signal-to-noise.
Despite this, we did detect some specificity in the behaviors predicted by these different PRS. The ADHD PRS specifically associated with behavioral approach subscales, impulsivity and prodromal psychosis; whereas the DEP PRS associated with somatic complaints and suicidality. Many of the associations between the DEP PRS and internalizing behaviors were no longer significant in the multivariable model likely due to shared variance between the DEP and SUIC PRS. However, there were several specific, unique associations between the SUIC PRS and youth reported depression symptoms, aggression and social problems in these multivariable models. This highlights a complex and unique pattern of behaviors associated with genetic risk for attempting suicide specifically compared to depression. These results may point towards potentially distinct pathways associated with the development of unique profiles of behaviors.
Our results replicated previous findings, with a similar magnitude of effects, showing that ADHD PRS significantly associated with hyperactive and inattentive traits in a developmental sample41,42. Across the PRS, ADHD and ASD were moderately correlated, and when controlling for the other genetic predictors ASD was no longer associated with behavioral problems on the CBCL, and neither ASD or ADHD uniquely predicted ADHD symptoms highlighting the genetic overlap between these disorders in development43. There may be additional factors that contributed to the lack of unique relationship of ASD PRS to youth behaviors. Exclusion criteria of not attending mainstream school classes and an inability to carry out the ABCD protocol would have made low functioning ASD individuals ineligible for the study. Indeed, we did find a unique positive association between cognitive performance and the ASD PRS in our sample. This suggests that the prevalence of ASD symptoms in the ABCD cohort is likely small and restricted to only part of the autism spectrum, which may have greater overlap with ADHD and be associated with higher cognitive functioning. Moreover, rare de novo mutations which are thought to play an important role in ASD44 were not tagged in our analysis.
Interestingly, in our sample, ADHD PRS predicted many bipolar-related behaviors and psychotic-like symptoms. Symptom profiles for pediatric BIP and ADHD are similar and there is high comorbidity across these disorders45. Other studies have shown that childhood ADHD is often premorbid to later development of schizophrenia and relatives of individuals with schizophrenia have higher rates of ADHD than the general population46,47. Given low correlations between ADHD, SCZ and BIP PRS in this study, the ADHD PRS may highlight individuals at risk for developing psychosis-related disorders that may be etiologically distinct from those with high SCZ or BIP scores.
Despite previous studies showing SCZ PRS associating with markers of general psychopathology in adolescence42,48, we did not find any associations of SCZ or BIP PRS with psychopathology; however, we did find a univariate association between PSYEXP and caregiver-reported thought problems. The lack of SCZ/BIP associations with psychopathology in our analysis could be driven by differences in statistical approach, demographics of the samples or the phenotypes measured – which can impact the stability of results across adolescent samples49. The high demands of the study may reduce participation from families with parents or siblings diagnosed with schizophrenia or bipolar disorder; therefore, the prevalence of those with high genetic risk of psychosis may be restricted in this study. Nevertheless, we did identify an expected significant negative association between the SCZ PRS and the fluid composite score from the NIH Toolbox® (which remained after controlling for sociodemographic factors), and an unexpected positive association between BIP and the crystallized composite score from the NIH Toolbox®. Cognitive impairment is a core feature of several psychiatric disorders, particularly those that include psychotic symptoms. Neurodevelopmental studies have highlighted premorbid cognitive impairment across domains in patients with schizophrenia and bipolar disorder50. However, despite this, students who achieve highly academically have been shown to have an increased risk of bipolar disorder51, supporting the positive association found here. Indeed, there is a large genetic overlap across schizophrenia, bipolar disorders and general intelligence8,9, suggesting shared etiological mechanisms affecting psychopathology and cognition. In a supplementary analysis controlling for polygenic risk for intelligence, these cognitive associations were attenuated and no longer significant. This suggests our cohort of individuals with any genetic risk for psychosis may be restricted to those with an overlap in genetic markers also associated with cognition. Studying risk for psychosis in this typically developing sample may therefore by biased towards a specific sub-type psychosis. Future research should aim to probe this further using longitudinal data and comparisons with other large samples enriched for psychosis risk.
There were differences in associations across caregiver and youth reported behaviors, particularly with genetic risk for depression and suicidality. For multivariable models, youth-reported depression symptom scores were more associated with the SUIC and DEP PRS, whilst caregiver-reported depression was associated with a FH of depression. Informant discrepancies between caregiver and child-reported measures have been widely reported52 and we found relatively low correlations between youth and caregiver reported measures. Negative biases from caregivers, particularly due to caregiver depression, can also impact behavioral reports15. An awareness of a history of depression within the youth’s family may have biased the informant’s report about the youth’s depression, possibly generating a stronger relationship between FH of depression and caregiver compared to youth reported measures. Future time points may indicate which informant-reported measure is most predictive of later diagnosis.
FH of anxiety/stress and conduct problems showed the greatest number of associations across different behavioral domains, supporting a role for anxiety and delinquent behavior as transdiagnostic traits. However, there were subtle differences in the pattern of FH-behavior associations across domains, particularly for multivariable models. For example, FH of drug abuse explained unique variance in rule-breaking behaviors, whereas FH of alcohol abuse explained unique variance in social problems and anxious/depressive behaviors – indicating differential behavioral profiles for specific FH’s. Inherent to FH measures are implicit genetic and environmental influences that are difficult to separate. It remains to be seen whether additional variance in behavior explained by FH measures above and beyond PRS reflects environmental or additional genetic influences. Together FH and PRS measures predicted ~7% of the variability in the CBCL Total Problems score. These analyses highlight the utility of measuring multiple markers of genetic risk.
Limitations:
PRS association strength is limited by the phenotype’s heritability and the training sample used53. DEP had the largest discovery sample (Figure S14) and a relatively low SNP heritability, yet displayed some of the largest associations in our sample. This may be due to depression having relatively greater population prevalence compared to the other psychiatric disorders measured, therefore compared to other disorders risk alleles may be well represented in our sample. Correlations between PRS generated in this study were much lower than the genetic correlations determined in the original GWAS, which may be because this cohort is not enriched for individuals with risk alleles. Many psychiatric disorders have increased penetrance during adolescence, therefore the lack of variance in psychopathology symptoms at this age may explain the limited associations between behavior and the SCZ/BIP PRS. Moreover, the GWAS used to produce the PRS in this study were conducted in predominately European ancestry samples. The ABCD sample is demographically diverse, however PRS trained and tested in different ancestry groups do not validly predict phenotypes. This highlights the limited predictive capacity of European-only GWAS for non-European populations and emphasizes the need for conducting GWAS in different ancestry groups. Finally, the magnitude of the genetic-behavior effects detected was very small; the development of psychopathology is complex and genetic risk as estimated with polygenic predictors appears to only account for a small proportion of variability in behavior at this age.
Here we have shown that different PRS and FH measures predicted unique patterns of symptoms of psychopathology, related individual difference factors and cognitive function in a large sample of 9-to-11-year-old children. Unique associations, controlling for other genetic measures, provide encouraging evidence that genetic data may be useful alongside FH in identifying specific risk for psychiatric disorders. Longitudinal analyses will further elucidate the specificity of these associations and may track these patterns of behavior to determine the differential predictive utility for PRS and FH measures.
Supplementary Material
KEY POINTS.
This work quantifies the association between genetic/familial risk and psychopathology in a large socioeconiomically diverse sample of typically developing young adolescents aged 9–11.
We find that genetic risk and family history contribute unique variance across a range of behaviors, with or without controlling for socioeconomic status.
Genetic risk for developing problematic opioid use was associated with lower behavioral inhibition. Genetic liability for depression and attempted suicide showed stronger associations with both internalizing and externalizing symptoms; whereas genetic risk for ADHD showed stronger associations with ADHD symptoms, impulsivity and prodromal psychosis. Additionally, genetic risk for schizophrenia, autism, bipolar disorder and attempted suicide were each uniquely associated with cognitive performance.
Family history for behaviors related to psychopathology displayed associations with many behavioral measures, overall explaining a greater proportion of unique variance compared to genetic risk predictors.
~7% of the variability in a general measure of psychopathology was explained using both genetic risk and family history measures.
This work demonstrates the complimentary information that genetic risk and family history provide in explaining variability in psychopathology at this early age.
ACKNOWLEDGEMENTS
The authors wish to thank the youth and families participating in the Adolescent Brain Cognitive Development (ABCD) Study and all ABCD staff, please see the Supporting Information for full acknowledgment. R.L. was supported by Kavli Innovative Research Grant under award number 2019-1624. C.F. was supported by grant R01MH122688 and RF1MH120025 funded by the National Institute for Mental Health.
A.M.D. reports that he was a Founder of and holds equity in CorTechs Labs, Inc., and serves on its Scientific Advisory Board. He is a member of the Scientific Advisory Board of Human Longevity, Inc. He receives funding through research grants from GE Healthcare to UCSD. The terms of these arrangements have been reviewed by and approved by UCSD in accordance with its conflict-of-interest policies. The remaining authors have declared that they have no competing or potential conflicts of interest.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article:
Conflict of interest statement: See Acknowledgements for full disclosures.
REFERENCES
- 1.Kendler KS Twin studies of psychiatric illness: An update. Archives of General Psychiatry 58, 1005–1014 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Kendler KS, Davis CG & Kessler RC The familial aggregation of common psychiatric and substance use disorders in the National Comorbidity survey: A family history study. Br. J. Psychiatry 170, 541–548 (1997). [DOI] [PubMed] [Google Scholar]
- 3.Anttila V et al. Analysis of shared heritability in common disorders of the brain. Science (80-. ) 360, eaap8757 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee PH et al. Genomic Relationships, Novel Loci, and Pleiotropic Mechanisms across Eight Psychiatric Disorders. Cell 179, 1469–1482.e11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akingbuwa WA et al. Genetic Associations between Childhood Psychopathology and Adult Depression and Associated Traits in 42998 Individuals: A Meta-analysis. JAMA Psychiatry 77, 715–728 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blacker D et al. Psychiatric Genetics: A Survey of Psychiatrists’ Knowledge, Opinions, and Practice Patterns. J. Clin. Psychiatry 66, 821–830 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Lu Y et al. Genetic risk scores and family history as predictors of schizophrenia in Nordic registers. Psychol. Med 48, 1201–1208 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smeland OB et al. Genome-wide analysis reveals extensive genetic overlap between schizophrenia, bipolar disorder, and intelligence. Mol. Psychiatry 25, 844–853 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies G et al. Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat. Commun 9, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garavan H et al. Recruiting the ABCD sample: Design considerations and procedures. Dev. Cogn. Neurosci 32, 16–22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barch DM et al. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Dev. Cogn. Neurosci 32, 55–66 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.KAUFMAN J et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial Reliability and Validity Data. J. Am. Acad. Child Adolesc. Psychiatry 36, 980–988 (1997). [DOI] [PubMed] [Google Scholar]
- 13.Achenbach TM The Achenbach System of Empirically Based Asessment (ASEBA): Development, Findings, Theory and Applications. Burlington, VT Univ. Vermont Res. Cent. Child. Youth Fam (2009). [Google Scholar]
- 14.Youngstrom EA, Frazier TW, Demeter C, Calabrese JR & Findling RL Developing a 10-item mania scale from the Parent General Behavior Inventory for children and adolescents. J. Clin. Psychiatry 69, 831–9 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Youngstrom EA, Findling RL, Danielson CK & Calabrese JR Discriminative validity of parent report of hypomanic and depressive symptoms on the General Behavior Inventory. Psychol. Assess 13, 267–276 (2001). [PubMed] [Google Scholar]
- 16.Ising HK et al. The Validity of the 16-Item Version of the Prodromal Questionnaire (PQ-16) to Screen for Ultra High Risk of Developing Psychosis in the General Help-Seeking Population. Schizophr. Bull 38, 1288–1296 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karcher NR et al. Assessment of the prodromal questionnaire-brief child version for measurement of self-reported psychoticlike experiences in childhood. JAMA Psychiatry 75, 853–861 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whiteside SP, Lynam DR, Miller JD & Reynolds SK Validation of the UPPS impulsive behaviour scale: a four-factor model of impulsivity. Eur. J. Pers 19, 559–574 (2005). [Google Scholar]
- 19.Carver CS & White TL Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J. Pers. Soc. Psychol 67, 319–333 (1994). [Google Scholar]
- 20.Jorm AF et al. Using the BIS/BAS scales to measure behavioural inhibition and behavioural activation: Factor structure, validity and norms in a large community sample. Pers. Individ. Dif 26, 49–58 (1998). [Google Scholar]
- 21.Luciana M et al. Adolescent neurocognitive development and impacts of substance use: Overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Dev. Cogn. Neurosci 32, 67–79 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gershon RC et al. NIH Toolbox for Assessment of Neurological and Behavioral Function. Neurology 80, S2--S6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer CE et al. Distinct Regionalization Patterns of Cortical Morphology are Associated with Cognitive Performance Across Different Domains. Cereb. Cortex 00, 1–16 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akshoomoff N et al. VIII. NIH Toolbox Cognition Battery (CB): composite scores of crystallized, fluid, and overall cognition. Monogr. Soc. Res. Child Dev 78, 119–132 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heaton RK et al. Reliability and validity of composite scores from the NIH toolbox cognition battery in adults. J. Int. Neuropsychol. Soc 20, 588–598 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demontis D et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat. Genet 51, 63–75 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grove J et al. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet 51, 431–444 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mullins N et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat. Genet 53, 817–829 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Consortium, T. S. W. G. of the P. G., Ripke S, Walters JTR & O’Donovan MC Mapping genomic loci prioritises genes and implicates synaptic biology in schizophrenia. medRxiv 2020.09.12.20192922 (2020). doi: 10.1101/2020.09.12.20192922 [DOI] [Google Scholar]
- 30.Howard DM et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci 22, 343–352 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez-Roige S et al. Genome-wide association study of problematic opioid prescription use in 132,113 23andMe research participants of European ancestry. Mol. Psychiatry (2021). doi: 10.1038/s41380-021-01335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullins N et al. Dissecting the Shared Genetic Architecture of Suicide Attempt, Psychiatric Disorders, and Known Risk Factors. Biol. Psychiatry 91, 313–327 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Legge SE et al. Association of Genetic Liability to Psychotic Experiences with Neuropsychotic Disorders and Traits. JAMA Psychiatry 76, 1256–1265 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savage JE et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat. Genet 50, 912–919 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagelkerke NJD A note on a general definition of the coefficient of determination. Biometrika 78, 691–692 (1991). [Google Scholar]
- 36.Agerbo E et al. Risk of Early-Onset Depression Associated with Polygenic Liability, Parental Psychiatric History, and Socioeconomic Status. JAMA Psychiatry 78, 387–397 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franken IHA & Muris P BIS/BAS personality characteristics and college students’ substance use. Pers. Individ. Dif 40, 1497–1503 (2006). [Google Scholar]
- 38.Kimbrel NA, Nelson-Gray RO & Mitchell JT Reinforcement sensitivity and maternal style as predictors of psychopathology. Pers. Individ. Dif 42, 1139–1149 (2007). [Google Scholar]
- 39.Cosgrove VE et al. Structure and etiology of co-occurring internalizing and externalizing disorders in adolescents. J. Abnorm. Child Psychol 39, 109–123 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwong ASF et al. Genetic and Environmental Risk Factors Associated with Trajectories of Depression Symptoms from Adolescence to Young Adulthood. JAMA Netw. Open 2, 1–14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brikell I et al. The contribution of common genetic risk variants for ADHD to a general factor of childhood psychopathology. Mol. Psychiatry 25, 1809–1821 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jansen PR et al. Polygenic scores for schizophrenia and educational attainment are associated with behavioural problems in early childhood in the general population. J. Child Psychol. Psychiatry Allied Discip 59, 39–47 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Pinto R, Rijsdijk F, Ronald A, Asherson P & Kuntsi J The Genetic Overlap of Attention-Deficit/Hyperactivity Disorder and Autistic-like Traits: an Investigation of Individual Symptom Scales and Cognitive markers. J. Abnorm. Child Psychol 44, 335–345 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Satterstrom FK et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 180, 568–584.e23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frías Á, Palma C & Farriols N Comorbidity in pediatric bipolar disorder: Prevalence, clinical impact, etiology and treatment. Journal of Affective Disorders 174, 378–389 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Dalsgaard S et al. Association between attention-deficit hyperactivity disorder in childhood and schizophrenia later in adulthood. Eur. Psychiatry 29, 259–263 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Dalteg A, Zandelin A, Tuninger E & Levander S Psychosis in adulthood is associated with high rates of ADHD and CD problems during childhood. Nord. J. Psychiatry 68, 560–566 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Nivard MG et al. Genetic Overlap Between Schizophrenia and Developmental Psychopathology: Longitudinal and Multivariate Polygenic Risk Prediction of Common Psychiatric Traits During Development. Schizophr. Bull 43, 1197–1207 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akingbuwa WA, Hammerschlag AR, Bartels M & Middeldorp CM Systematic Review: Molecular Studies of Common Genetic Variation in Child and Adolescent Psychiatric Disorders. J. Am. Acad. Child Adolesc. Psychiatry 00, 1–16 (2021). [DOI] [PubMed] [Google Scholar]
- 50.Mollon J & Reichenberg A Cognitive development prior to onset of psychosis. Psychological Medicine 48, 392–403 (2018). [DOI] [PubMed] [Google Scholar]
- 51.MacCabe JH et al. Excellent school performance at age 16 and risk of adult bipolar disorder: National cohort study. Br. J. Psychiatry 196, 109–115 (2010). [DOI] [PubMed] [Google Scholar]
- 52.De Reyes AL & Kazdin AE Informant discrepancies in the assessment of childhood psychopathology: A critical review, theoretical framework, and recommendations for further study. Psychol. Bull 131, 483–509 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Dudbridge F Power and Predictive Accuracy of Polygenic Risk Scores. PLoS Genet 9, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


