Abstract
OBJECTIVE:
To characterize temporal trends and outcomes of delivery hospitalization with maternal CHD.
METHODS:
For this repeated cross-sectional analysis, deliveries to women aged 15–54 years with maternal CHD were identified in the 2000–2018 National Inpatient Sample. Temporal trends in maternal CHD were analyzed using joinpoint regression to estimate the average annual percent change (AAPC) with 95% CIs. The relationship between maternal CHD and several adverse maternal outcomes was analyzed with log linear regression models. Risk for adverse outcomes in the setting of maternal CHD was further characterized based on additional diagnoses of cardiac comorbidity including congestive heart failure, arrhythmia, valvular disease, pulmonary disorders, and history of thromboembolism.
RESULTS:
Of 73,109,790 delivery hospitalizations, 51,841 had a diagnosis of maternal CHD (7.1 per 10,000). Maternal CHD rose from 4.2 to 10.9 per 10,000 deliveries (AAPC 4.8%, 95% CI 4.2%, 5.4%). Maternal CHD deliveries with a cardiac comorbidity diagnosis also increased from 0.6 to 2.6 per 10,000 from 2000 to 2018 (AAPC 8.4%, 95% CI 6.3%, 10.6%). Maternal CHD was associated with severe maternal morbidity (aRR 4.97, 95% CI 4.75, 5.20), cardiac severe maternal morbidity (aRR 7.65, 95% CI 7.14, 8.19), placental abruption (aRR 1.30, 95% 1.21, 1.38), preterm delivery (aRR 1.47, 95% CI 1.43, 1.51), and transfusion (aRR 2.28, 95% CI 2.14, 2.42). Risk for severe morbidity (AAPC 4.7%, 95% CI 2.5%, 6.9%) and cardiac severe morbidity (AAPC 4.7%, 95% CI 2.5%, 6.9%) increased significantly among women with maternal CHD over the study period. The presence of cardiac comorbidity diagnoses was associated with further increased risk.
DISCUSSION:
Maternal CHD is becoming more common among US deliveries. Among deliveries with maternal CHD, risk for severe morbidity is increasing. These findings support that an increasing burden of risk from maternal CHD in the obstetric population.
INTRODUCTION
Advances in treatment of congenital heart disease have resulted in an increasing probability of survival to reproductive age.1,2 Prior studies estimated a prevalence of maternal congenital heart disease (CHD) of 6 per 1000 reproductive-age women in the US and nearly 1 per 1000 delivery hospitalizations.3–5 For some women with maternal CHD, physiologic changes during pregnancy such as increased cardiac output and elevated oxygen consumption may increase risk for arrythmias, heart failure, and other cardiac complications.4,6–10 Population-level risk may be increasing with women with poorer cardiac function and more significant cardiac complications now becoming more likely to attempt pregnancy. From 2014 to 2017 cardiovascular conditions were the leading cause of maternal mortality in the US per the Centers for Disease Control and Prevention’s Pregnancy Mortality Surveillance System.11
There are limited recent national data on outcomes for pregnant women with maternal CHD.4,5,12 Older studies of national data had demonstrated increasing prevalence of maternal CHD during delivery hospitalizations.13 Given that updated nationwide data on outcomes and trends related to deliveries with maternal CHD may be of public health and clinical significance, we performed the following analysis which had the following objectives: (i) to evaluate prevalence and trends of maternal CHD during delivery hospitalizations in the US from 2000 through 2018; (ii) to analyze the risk for severe maternal morbidity associated with maternal CHD; and (iii) to analyze the risk for other adverse outcomes associated with maternal CHD. We additionally sought to determine to what degree cardiac comorbidity including congestive heart failure, arrhythmia, valvular disease, pulmonary disorders, and history of thromboembolism in the setting of maternal CHD was associated with increased risk.
METHODS
Data Source
Data was obtained from the 2000–2018 National (Nationwide) Inpatient Sample (NIS) from the Healthcare Cost and Utilization Project for this repeated cross-sectional analysis.14 The NIS is one of the largest publicly available, all-payer inpatient databases in the United States and approximates a 20% stratified sample of all hospitalizations nationally. More than 7 million hospital stays are included in the NIS annually. In 2018 data from 47 states were included in the NIS.15 Hospitalizations in the NIS can be weighted to be representative of the entire US population; specific NIS weights for trends were applied in this study.16
Given that this study period included the switch (on October 1, 2015) from International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) to International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) coding, billing data from the NIS in the form of both ICD-9-CM and ICD-10-CM codes were included in the analysis.17 For these analyses ICD-9-CM codes were translated to ICD-10-CM codes via an algorithm using the publicly available General Equivalence Mappings provided by the Centers for Medicare and Medicaid Services and the National Center for Health Statistics.18,19
Study Population
We included delivery hospitalizations of women aged 15 to 54 in the NIS from 2000 through 2018. Delivery hospitalizations were identified using algorithms of ICD-9-CM and ICD-10-CM codes that have previously been shown to capture more than 95% of deliveries.20,21 Women with maternal CHD were identified by diagnosis codes (Table S1).12 Women with maternal CHD were further classified based on the presence or absence of additional cardiac comorbidity diagnoses. Cardiac comorbidity included ≥1 of the following diagnoses: congestive heart failure, arrhythmia, valvular disease, pulmonary disorders, and thromboembolism.
Study objectives
This study had three objectives. The first objective was to determine temporal trends in prevalence of maternal CHD diagnoses during delivery hospitalizations. We sought to determine if maternal CHD diagnoses during delivery hospitalizations increased over the 2000 to 2018 study period. We evaluated overall trends in maternal CHD as well as trends in maternal CHD with and without cardiac comorbidity diagnoses.
The second objective was to determine to what degree maternal CHD was associated with risk for severe maternal morbidity. We analyze two composites of severe maternal morbidity: (i) overall severe maternal morbidity as defined by the Centers for Disease Control and Prevention, and (ii) the subset of the CDC severe maternal morbidity consisting of acute cardiac and pulmonary diagnoses. For the first severe maternal morbidity composite outcome, we used CDC criteria excluding transfusion.22 The CDC definition of severe morbidity includes 21 conditions and procedures including shock, stroke, heart failure, and sepsis identified by ICD-9 and ICD-10 codes. Because transfusion is the most common diagnosis in the severe maternal morbidity composite, is unlikely to lead to long-term sequelae, may be a process measure, and is not necessarily representative of large volume transfusion,23 we excluded transfusion from the CDC composite and evaluated risk for the remaining 20 conditions. For the subset of CDC severe maternal morbidity consisting of acute cardiac and pulmonary diagnoses (cardiac severe morbidity) we included: acute myocardial infarction, atrial fibrillation, acute heart failure, pulmonary edema, cardioversion, or ARDS. Risks for each of these two severe maternal morbidity composites (SMM) were analyzed based on the presence or absence of any maternal CHD and trended over the 2000 to 2018 study periods.
The third objective was to determine whether maternal CHD was associated with risk for a range of other adverse pregnancy outcomes including: (i) placental abruption, (ii) preterm delivery, (iii) postpartum hemorrhage, (iv) preeclampsia and gestational hypertension, (v) cesarean delivery, (vi) transfusion, and (vii) stillbirth.
Patient and hospital characteristics
Demographic, medical, obstetric and hospital factors available in the NIS associated with maternal CHD were analyzed. Demographic factors included payer, race and ethnicity, maternal age (categorized as 15–17, 18–24, 25–34, 35–39, and 40–54 years of age), and median household income quartile based on ZIP code. Hospital factors included hospital bed number (small, medium, large) and hospital teaching status (urban teaching, rural, and urban non-teaching). Medical and obstetrical factors were identified using ICD-9-CM and ICD-10-CM codes and included: pregestational diabetes, multiple gestation, chronic hypertension, and obesity.
Statistical Analysis
For the first objective evaluating trends in maternal CHD, we reported the prevalence of delivery hospitalizations by year with maternal CHD. Additionally, we conducted trends analysis over the 2000 to 2018 study period using the National Cancer Institute’s Joinpoint Regression Program (version 4.8.0.1).24,25 This program allows identification of when a trend change is produced and calculates the annual percentage change in rates between trend-change points. The program also estimates the average annual percentage change (AAPC) in the whole period studied. The AAPC is derived by first estimating the underlying joinpoint model that best fits the data. A weighted average is calculated from the slope coefficients of the underlying joinpoint regression line with weights equal to the length of each segment. The AAPC is then calculated by transforming the weighted average of slope coefficients.26,27 We analyzed overall trends for maternal CHD and trends for maternal CHD with an associated cardiac comorbidity diagnosis.
For the second objective analyzing the association between maternal CHD and severe morbidity, we performed unadjusted and adjusted population-weighted log-linear regression models with Poisson distribution and the log link with robust error variance.28 For the unadjusted models, risk was determined based on the presence of maternal CHD. Adjusted models were created which included the aforementioned demographic, medical, and obstetric characteristics. The adjusted risks for women with maternal CHD were then determined. Results are presented as unadjusted (RR) and adjusted risk ratios (aRR) with 95% confidence intervals (CI). Trends in rates of severe morbidity were also analyzed with joinpoint regression.
For the third objective analyzing whether maternal CHD was associated with risk placental abruption, preterm delivery, postpartum hemorrhage, preeclampsia and gestational hypertension, cesarean delivery, stillbirth, and transfusion, we similarly performed unadjusted and adjusted log-linear regression models with Poisson distribution. Risks for these outcomes (along with the severe morbidity outcomes) among women with maternal CHD was additionally calculated based on whether maternal CHD cardiac comorbidity diagnoses such as congestive heart failure, arrhythmia, valvular disease, pulmonary disorders, and history of thromboembolism were present.
Standardized mean difference was used for demographic comparisons based on the presence versus absence of maternal CHD with ≥0.1 (10%) considered to be a meaningful difference.29 Given that the data are de-identified, the analysis was deemed exempt by the University Institutional Review Board. We followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for cross-sectional studies for this analysis.30 All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
An estimated 73,109,790 delivery hospitalizations from 2000 to 2018 were included in the analysis of which 51,841 had a diagnosis of maternal CHD (7.1 per 10,000). From 2000 to 2018, the prevalence of maternal CHD during delivery hospitalizations rose from 4.2 to 10.9 per 10,000 deliveries (Figure 1). The joinpoint model demonstrated an average annual percent change increase of 4.8% (95% CI 4.2%, 5.4%) over the study period. Maternal CHD deliveries with a cardiac comorbidity diagnosis also increased over the study period from 0.6 per 10,000 in 2000 to 2.6 per 10,000 in 2018 (AAPC 8.4%, 95% CI 6.3%, 10.6%).
Figure 1. Trends in the prevalence maternal congenital heart disease diagnoses during delivery hospitalizations.
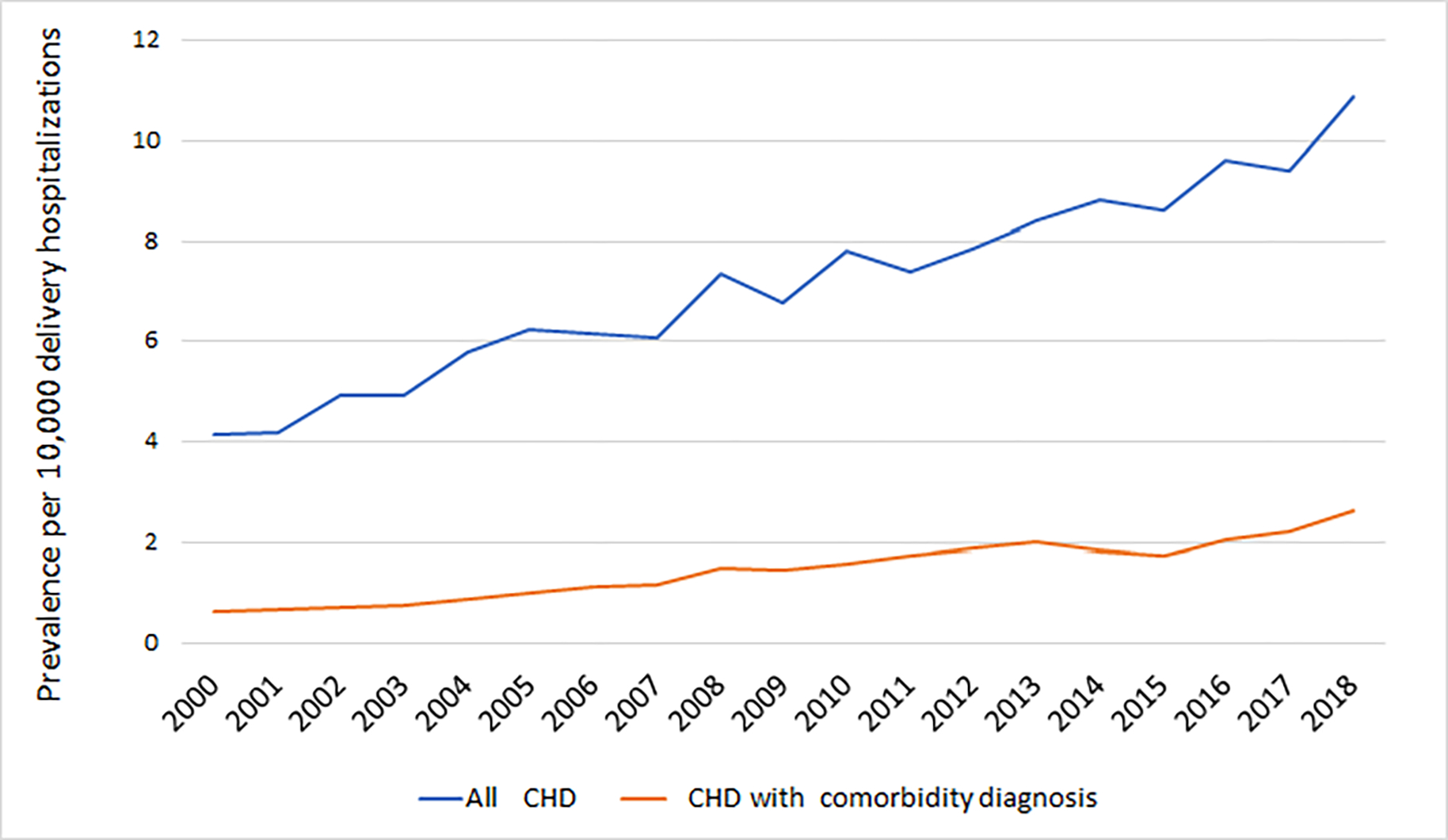
CHD, congenital heart disease. The figure demonstrates the prevalence of all deliveries per year (i) with maternal congenital heart disease, and (ii) maternal congenital heart disease with a cardiac comorbidity. Cardiac comorbidity diagnoses included congestive heart failure, arrhythmia, valvular disease, pulmonary disorders, and thromboembolism.
Evaluating other demographic factors, maternal CHD was more common among deliveries to non-Hispanic White (9.1 per 10,000) than to non-Hispanic Black (5.3 per 10,000) and Hispanic women (5.2 per 10,000) (SMD for maternal race 24.4%). Maternal CHD was more common in the setting of obesity (10.4 per 10,000 deliveries, SMD 8.7%), pre-gestational diabetes (11.1 per 10,000, SMD 4.8%), and chronic hypertension (19.2 per 10,000 deliveries, SMD 15.0%) (Table 1).
Table 1.
Characteristics of the study population
| All deliveries | Deliveries with CHD diagnosis | ||||
|---|---|---|---|---|---|
| Maternal congenital heart disease | ≥1 cardiac comorbidity diagnosis | ||||
| Absent, n (%) | Present, n (%) | Absolute SMD | Absent, n (%) | Present, n (%) | |
| Demographics | |||||
| Year of delivery | |||||
| 2000 | 3,813,256 (5.2%) | 1,593 (3.1%) | 1,346 (3.3%) | 247 (2.3%) | |
| 2001 | 3,748,220 (5.1%) | 1,573 (3.0%) | 1,327 (3.2%) | 246 (2.3%) | |
| 2002 | 3,901,478 (5.3%) | 1,927 (3.7%) | 1,645 (4.0%) | 282 (2.7%) | |
| 2003 | 3,860,150 (5.3%) | 1,912 (3.7%) | 1,616 (3.9%) | 296 (2.8%) | |
| 2004 | 3,996,680 (5.5%) | 2,318 (4.5%) | 1,967 (4.8%) | 352 (3.3%) | |
| 2005 | 4,006,839 (5.5%) | 2,501 (4.8%) | 2,097 (5.1%) | 404 (3.8%) | |
| 2006 | 4,058,003 (5.6%) | 2,500 (4.8%) | 2,042 (5.0%) | 458 (4.3%) | |
| 2007 | 4,325,907 (5.9%) | 2,633 (5.1%) | 2,122 (5.1%) | 511 (4.8%) | |
| 2008 | 4,009,089 (5.5%) | 2,950 (5.7%) | 2,358 (5.7%) | 593 (5.6%) | |
| 2009 | 3,914,417 (5.4%) | 2,649 (5.1%) | 2,073 (5.0%) | 576 (5.5%) | |
| 2010 | 3,682,270 (5.0%) | 2,882 (5.6%) | 2,297 (5.6%) | 585 (5.5%) | |
| 2011 | 3,644,493 (5.0%) | 2,698 (5.2%) | 2,066 (5.0%) | 632 (6.0%) | |
| 2012 | 3,746,736 (5.1%) | 2,940 (5.7%) | 2,230 (5.4%) | 710 (6.7%) | |
| 2013 | 3,724,883 (5.1%) | 3,140 (6.1%) | 2,385 (5.8%) | 755 (7.1%) | |
| 2014 | 3,781,085 (5.2%) | 3,340 (6.4%) | 2,635 (6.4%) | 705 (6.7%) | |
| 2015 | 3,734,835 (5.1%) | 3,230 (6.2%) | 2,575 (6.2%) | 655 (6.2%) | |
| 2016 | 3,778,856 (5.2%) | 3,630 (7.0%) | 2,855 (6.9%) | 775 (7.3%) | |
| 2017 | 3,699,356 (5.1%) | 3,475 (6.7%) | 2,645 (6.4%) | 830 (7.9%) | |
| 2018 | 3,631,394 (5.0%) | 3,950 (7.6%) | 2,990 (7.2%) | 960 (9.1%) | |
| Maternal race | 24.4% | ||||
| Non-Hispanic White | 32,127,323 (44.0%) | 29,222 (56.4%) | 23,415 (56.7%) | 5,807 (54.9%) | |
| Non-Hispanic Black | 8,393,454 (11.5%) | 4,418 (8.5%) | 3,359 (8.1%) | 1,059 (10.0%) | |
| Hispanic | 13,814,858 (18.9%) | 7,179 (13.9%) | 5,582 (13.5%) | 1,597 (15.1%) | |
| Other | 6,588,541 (9.0%) | 3,536 (6.8%) | 2,803 (6.8%) | 733 (6.9%) | |
| Unknown | 12,133,771 (16.6%) | 7,487 (14.4%) | 6,111 (14.8%) | 1,375 (13.0%) | |
| Maternal age | 6.6% | ||||
| 15–19 years old | 17,053,860 (23.3%) | 11,677 (22.5%) | 23,415 (56.7%) | 5,807 (54.9%) | |
| 20–29 years old | 20,386,510 (27.9%) | 14,518 (28.0%) | 3,359 (8.1%) | 1,059 (10.0%) | |
| 30–34 years old | 18,259,847 (25.0%) | 13,250 (25.6%) | 5,582 (13.5%) | 1,597 (15.1%) | |
| 35–39 years old | 8,961,164 (12.3%) | 6,143 (11.9%) | 2,803 (6.8%) | 733 (6.9%) | |
| 40–54 years old | 2,042,006 (2.8%) | 1,485 (2.9%) | 6,111 (14.8%) | 1,375 (13.0%) | |
| Payer | 12.1% | ||||
| Medicare | 422,482 (0.6%) | 792 (1.5%) | 507 (1.2%) | 285 (2.7%) | |
| Medicaid | 30,156,706 (41.3%) | 19,616 (37.8%) | 15,445 (37.4%) | 4,171 (39.5%) | |
| Private Insurance | 38,069,895 (52.1%) | 28,737 (55.4%) | 23,228 (56.3%) | 5,509 (52.1%) | |
| Self-pay | 2,238,596 (3.1%) | 958 (1.9%) | 735 (1.8%) | 222 (2.1%) | |
| No Charge | 134,249 (0.2%) | 47 (0.1%) | 28 (0.1%) | 19 (0.2%) | |
| Other | 1,914,651 (2.6%) | 1,601 (3.1%) | 1,251 (3.0%) | 350 (3.3%) | |
| Unknown | 121,369 (0.2%) | 90 (.2%) | 76 (0.2%) | 14 (0.1%) | |
| ZIP code income quartile | 10.2% | ||||
| Income Quartile 1 | 17,350,802 (23.8%) | 11,616 (22.4%) | 9,003 (21.8%) | 2,613 (24.7%) | |
| Income Quartile 2 | 17,672,246 (24.2%) | 12,263 (23.7%) | 9,729 (23.6%) | 2,535 (24.0%) | |
| Income Quartile 3 | 17,788,278 (24.4%) | 13,241 (25.5%) | 10,707 (25.9%) | 2,534 (24.0%) | |
| Income Quartile 4 | 19,094,137 (26.1%) | 13,968 (26.9%) | 11,226 (27.2%) | 2,743 (26.0%) | |
| Unknown | 1,152,484 (1.6%) | 752 (1.5%) | 606 (1.5%) | 146 (1.4%) | |
| Obstetric and medical factors | |||||
| Obesity | 3,004,877 (4.1%) | 3,119 (6.0%) | 8.7% | 2,342 (5.7%) | 778 (7.4%) |
| Pre-gestational diabetes | 663,626 (0.9%) | 736 (1.4%) | 4.8% | 607 (1.5%) | 129 (1.2%) |
| Chronic hypertension | 1,006,571 (1.4%) | 1,938 (3.7%) | 15.0% | 1,259 (3.1%) | 678 (6.4%) |
| Multiple gestation | 1,312,925 (1.8%) | 1,102 (2.1%) | 2.4% | 849 (2.1%) | 254 (2.4%) |
| Hospital factors | |||||
| Hospital location | 30.6% | ||||
| Rural | 8,147,291 (11.2%) | 3,901 (7.5%) | 3,346 (8.1%) | 555 (5.3%) | |
| Urban non-teaching | 27,460,828 (37.6%) | 12,779 (24.7%) | 10,682 (25.9%) | 2,097 (19.8%) | |
| Urban teaching | 37,222,564 (51.0%) | 34,995 (67.5%) | 27,105 (65.7%) | 7,890 (74.6%) | |
| Missing | 227,264 (0.3%) | 167 (0.3%) | 138 (0.3%) | 29 (0.3%) | |
| Region | 14.7% | ||||
| Northeast | 11,858,021 (16.2%) | 10,402 (20.1%) | 8,506 (20.6%) | 1,897 (17.9%) | |
| Midwest | 15,549,763 (21.3%) | 12,270 (23.7%) | 9,644 (23.4%) | 2,626 (24.8%) | |
| South | 27,647,616 (37.8%) | 17,023 (32.8%) | 13,493 (32.7%) | 3,529 (33.4%) | |
| West | 18,002,548 (24.6%) | 12,147 (23.4%) | 9,627 (23.3%) | 2,520 (23.8%) | |
SMD, standardized mean difference. CHD, congenital heart disease. Additional diagnoses representative of cardiac comorbidity: congestive heart failure, arrhythmia, valvular disease, pulmonary disorders, and history of thromboembolism.
Evaluating likelihood of adverse outcomes, maternal CHD was broadly associated with increased risk (Table 2). In adjusted analyses accounting for demographic, hospital, and medical and obstetric risk factors, increased risks with maternal CHD for several of these adverse outcomes were retained including for: non-transfusion severe maternal morbidity (aRR 4.97, 95% CI 4.75, 5.20), cardiac severe maternal morbidity (aRR 7.65, 95% CI 7.14, 8.19), placental abruption (aRR 1.30, 95% 1.21, 1.38), preterm delivery (aRR 1.47, 95% CI 1.43, 1.51), preeclampsia and gestational hypertension (aRR 1.26, 95% CI 1.22, 1.29), cesarean delivery (aRR 1.20, 95% CI 1.18, 1.22), postpartum hemorrhage (aRR 1.56, 95% CI 1.50, 1.62), transfusion (aRR 2.28, 95% CI 2.14, 2.42), and stillbirth (aRR 1.41, 95% CI 1.30, 1.54).
Table 2.
Adverse outcomes associated with maternal congenital heart disease
| Incidence of outcomes | ||
| CHD present | CHD absent | |
| Adverse outcomes | n, % | n, % |
| Severe maternal morbidity excluding transfusion | 1,835 (3.5%) | 499,315 (0.7%) |
| Cardiac severe maternal morbidity | 820 (1.6%) | 152,964 (0.2%) |
| Placental abruption | 900 (1.7%) | 971,841 (1.3%) |
| Preterm delivery | 4,902 (9.5%) | 4,811,147 (6.6%) |
| Preeclampsia and gestational hypertension | 5,264 (10.2%) | 5,563,433 (7.6%) |
| Cesarean delivery | 19,793 (38.2%) | 22,545,517 (30.9%) |
| Postpartum hemorrhage | 2,465 (4.8%) | 2,180,032 (3.0%) |
| Transfusion | 1,062 (2.1%) | 637,974 (0.9%) |
| Stillbirth | 519 (1.0%) | 513,280 (0.7%) |
| Unadjusted models | ||
| Adverse outcomes | RR (95% CI) | RR (95% CI) |
| Severe maternal morbidity excluding transfusion | 5.18 (4.95, 5.42) | 1.0 (reference) |
| Cardiac severe maternal morbidity | 7.56 (7.05, 8.09) | 1.0 (reference) |
| Placental abruption | 1.31 (1.22, 1.39) | 1.0 (reference) |
| Preterm delivery | 1.44 (1.40, 1.48) | 1.0 (reference) |
| Preeclampsia and gestational hypertension | 1.33 (1.30, 1.37) | 1.0 (reference) |
| Cesarean delivery | 1.24 (1.22, 1.25) | 1.0 (reference) |
| Postpartum hemorrhage | 1.59 (1.53, 1.66) | 1.0 (reference |
| Transfusion | 2.35 (2.21, 2.49) | 1.0 (reference) |
| Stillbirth | 1.42 (1.31, 1.55) | 1.0 (reference) |
| Adjusted models | ||
| Adverse outcomes | aRR (95% CI) | aRR (95% CI) |
| Severe maternal morbidity excluding transfusion | 4.97 (4.75, 5.20) | 1.0 (reference) |
| Cardiac severe maternal morbidity | 7.65 (7.14, 8.19) | 1.0 (reference) |
| Placental abruption | 1.30 (1.21, 1.38) | 1.0 (reference) |
| Preterm delivery | 1.47 (1.43, 1.51) | 1.0 (reference) |
| Preeclampsia and gestational hypertension | 1.26 (1.22, 1.29) | 1.0 (reference) |
| Cesarean delivery | 1.20 (1.18, 1.22) | 1.0 (reference) |
| Postpartum hemorrhage | 1.56 (1.50, 1.62) | 1.0 (reference) |
| Transfusion | 2.28 (2.14, 2.42) | 1.0 (reference) |
| Stillbirth | 1.41 (1.30, 1.54) | 1.0 (reference) |
Estimates in the table demonstrate risk in the presence compared to the absence of maternal congenital heart disease. The adjusted models include all the demographic, obstetric, and medical factors in Table 1 including year of delivery, maternal race, maternal age, payer, ZIP code income quartile, pre-gestational diabetes, chronic hypertension, singleton versus multiple gestation, and obesity.
Evaluating temporal trends in severe maternal morbidity, risk for non-transfusion severe maternal morbidity increased significantly among deliveries with maternal CHD from 2.8% to 5.2% from 2000 to 2018 (AAPC 4.7%, 95% CI 2.5%, 6.9%), but not among deliveries without maternal CHD (AAPC 1.4%, 95% CI −0.2%, 3.0%) (Figure 2A). Cardiac severe morbidity also increased for deliveries with maternal CHD (AAPC 4.7%, 95% CI 2.5%, 6.9%) rising from 1.1 per 1000 deliveries in 2000–2004 to 2.3 per 1000 deliveries in 2015–2018. For deliveries without maternal CHD, cardiac severe maternal morbidity decreased over the study period (AAPC −2.5%, 95% CI −4.3%, −0.6%) (Figure 2B).
Figure 2A. Trends in the incidence non-transfusion severe maternal morbidity based on presence or absence of maternal congenital heart disease.
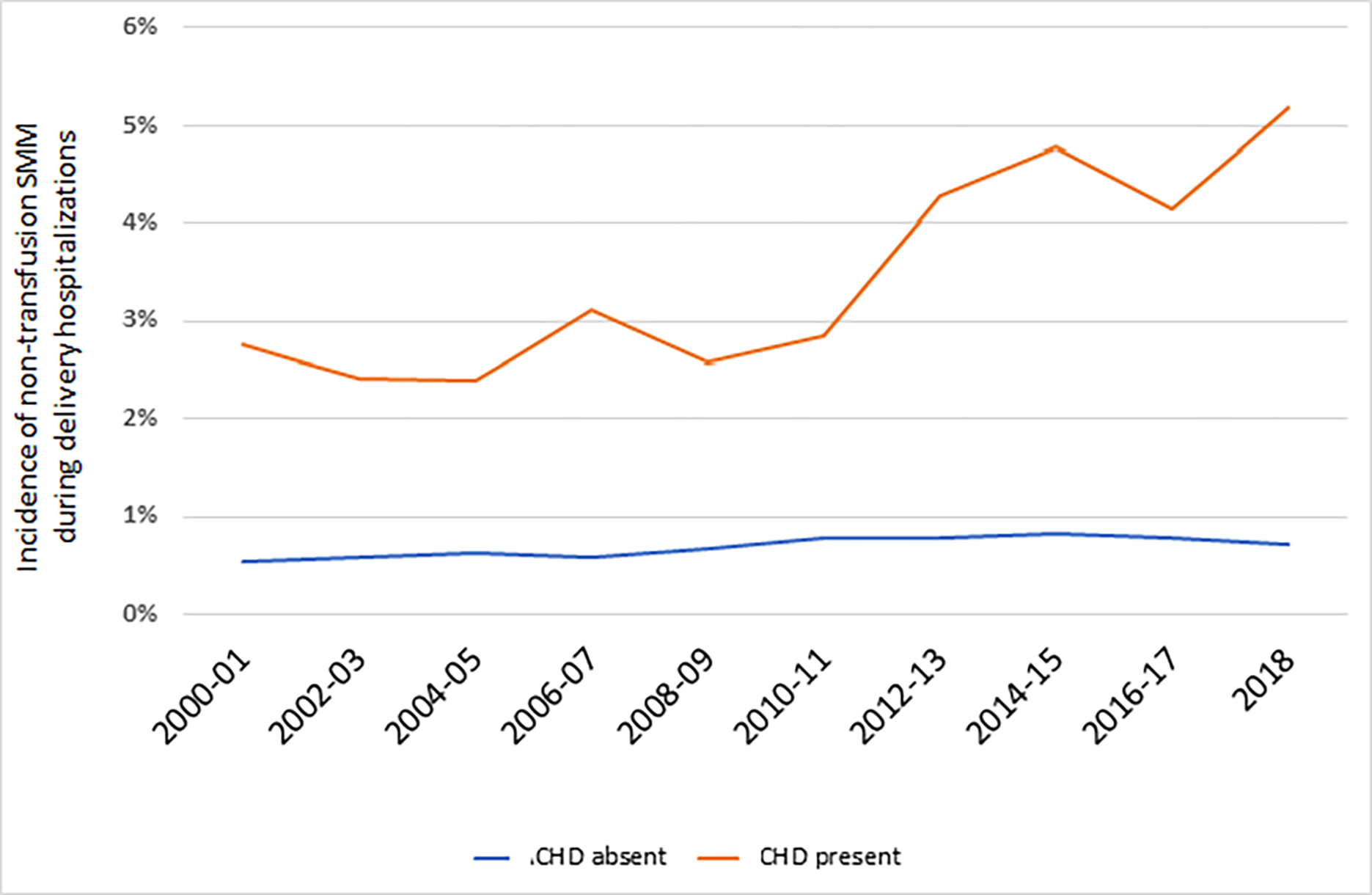
CHD, congenital heart disease. The figure demonstrates the incidence of non-transfusion severe maternal morbidity based on CDC criteria. The average annual percent change (AAPC) for non-transfusion severe maternal morbidity was significant for deliveries with ACHD (AAPC 4.7%, 95% CI 2.5%, 6.9%). For deliveries without maternal CHD, the AAPC was not significant (AAPC 1.4%, 95% CI: −0.2%, 3.0%).
Figure 2B. Trends in the incidence of cardiac severe maternal morbidity based on presence or absence of maternal congenital heart disease.
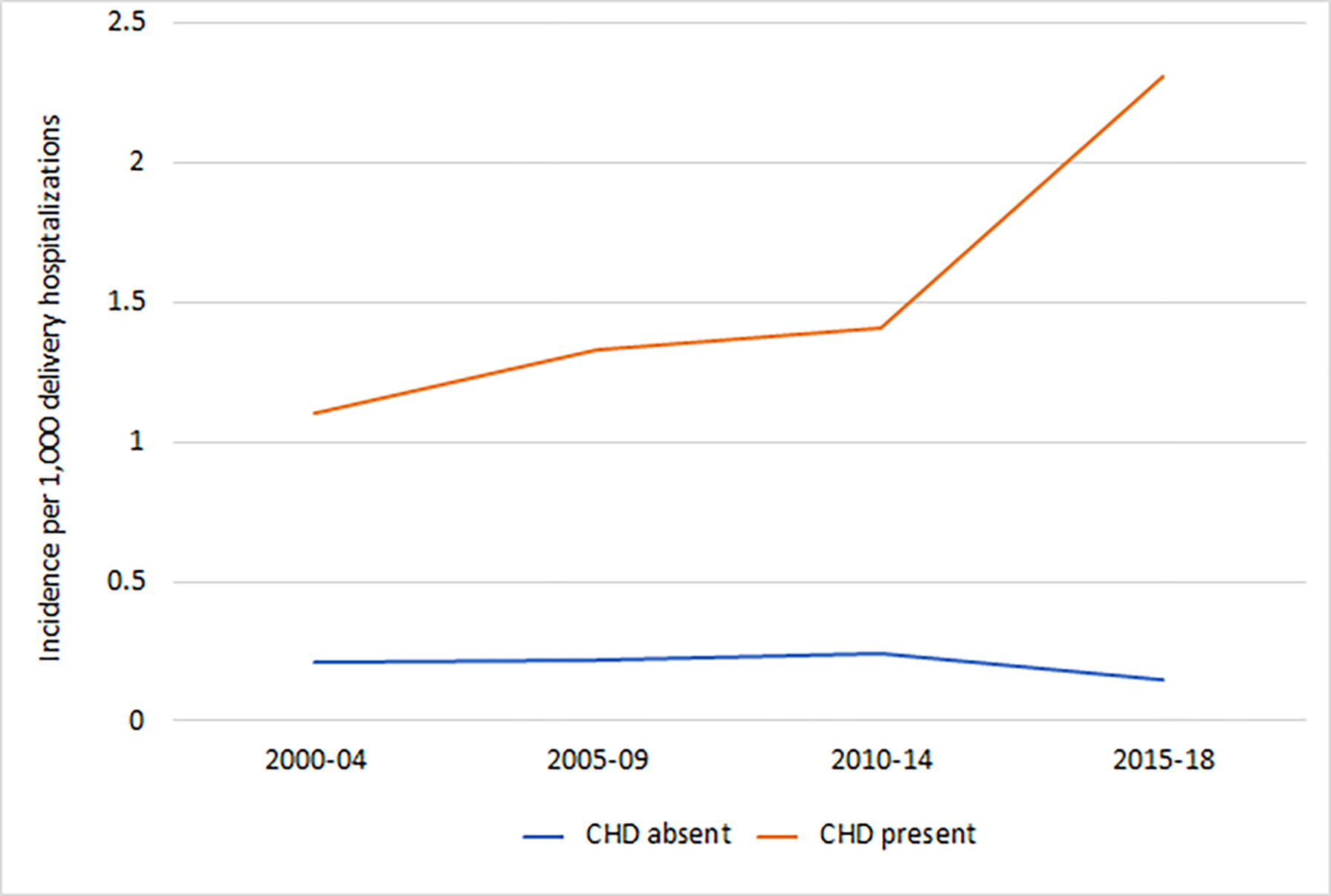
CHD, congenital heart disease. The figure demonstrates the incidence of cardiac severe maternal morbidity. The average annual percent change (AAPC) for cardiac severe maternal morbidity was positive for deliveries with maternal CHD (AAPC 4.7%, 95% CI 2.5%, 6.9%). For deliveries without maternal CHD, the AAPC was negative (AAPC −2.5%, 95% CI −4.3%, −0.6%).
When the analysis was restricted to patients with maternal CHD, unadjusted risk for most adverse outcomes was higher in the presence of cardiac comorbidity diagnoses including congestive heart failure, arrhythmia, valvular disease, pulmonary disorders, and history of thromboembolism (Table 3). This risk persisted in adjusted analyses accounting for demographic, hospital, and medical and obstetric risk factors. Among deliveries of women with CHD the presence of ≥1 cardiac comorbidity diagnoses was associated with increased risk for non-transfusion severe maternal morbidity (aRR 3.40, 95% CI 3.10, 3.73), cardiac severe maternal morbidity (aRR 7.51, 95% CI 6.50, 8.68), placental abruption (aRR 1.38, 95% CI 1.19, 1.60), preterm delivery (aRR 1.46, 95% CI 1.37, 1.55), preeclampsia and gestational hypertension (aRR 1.40, 95% CI 1.31, 1.48), cesarean delivery (aRR 1.20, 95% CI 1.17, 1.24), postpartum hemorrhage (aRR 1.19, 95% CI 1.09, 1.31), and transfusion (aRR 2.02, 95% CI 1.78, 2.29) while risk for stillbirth (aRR 0.69, 95% CI 0.54, 0.87) was lower.
Table 3.
Unadjusted and adjusted risk for associated with cardiac comorbidity diagnosis
| ≥1 cardiac comorbidity diagnosis | No cardiac comorbidity diagnosis | |
| Unadjusted models | Unadjusted RR (95% CI) | Unadjusted, RR (95% CI) |
| Non-transfusion severe maternal morbidity | 3.40 (3.10, 3.73) | 1.0 (reference) |
| Cardiac severe maternal morbidity | 7.51 (6.50, 8.68) | 1.0 (reference) |
| Placental abruption | 1.46 (1.26, 1.69) | 1.0 (reference) |
| Preterm delivery | 1.49 (1.40, 1.59) | 1.0 (reference) |
| Preeclampsia and gestational hypertension | 1.44 (1.35, 1.53) | 1.0 (reference) |
| Cesarean delivery | 1.24 (1.20, 1.28) | 1.0 (reference) |
| Postpartum hemorrhage | 1.26 (1.15, 1.38) | 1.0 (reference) |
| Transfusion | 2.27 (2.00, 2.57) | 1.0 (reference) |
| Stillbirth | 0.72 (0.57, 0.92) | 1.0 (reference) |
| Adjusted models | Adjusted RR (95% CI) | Adjusted, RR (95% CI) |
| Non-transfusion severe maternal morbidity | 3.12 (2.84, 3.42) | 1.0 (reference) |
| Cardiac severe maternal morbidity | 6.79 (5.86, 7.86) | 1.0 (reference) |
| Placental abruption | 1.38 (1.19, 1.60) | 1.0 (reference) |
| Preterm delivery | 1.46 (1.37, 1.55) | 1.0 (reference) |
| Preeclampsia and gestational hypertension | 1.40 (1.31, 1.48) | 1.0 (reference) |
| Cesarean delivery | 1.20 (1.17, 1.24) | 1.0 (reference) |
| Postpartum hemorrhage | 1.19 (1.09, 1.31) | 1.0 (reference) |
| Transfusion | 2.02 (1.78, 2.29) | 1.0 (reference) |
| Stillbirth | 0.69 (0.54, 0.87) | 1.0 (reference) |
The table demonstrates unadjusted and adjusted risks for adverse outcomes among deliveries with maternal congenital heart disease based on whether ≥1 additional diagnoses representative of cardiac comorbidity were present including: congestive heart failure, arrhythmia, valvular disease, pulmonary disorders, and history of thromboembolism. Risk is estimated based on the presence versus absence of cardiac severity. The adjusted models include all the demographic, obstetric, and medical factors in Table 1 including year of delivery, maternal race, maternal age, payer, ZIP code income quartile, pre-gestational diabetes, chronic hypertension, singleton versus multiple gestation, and obesity.
DISCUSSION
Main findings
In this serial cross-sectional study, prevalence of maternal CHD diagnoses during delivery hospitalizations more than doubled over the study period while maternal CHD with cardiac comorbidity diagnoses more than quadrupled. Women with CHD diagnoses had a 5 times higher risk of severe maternal morbidity and more than a 7.5 times higher risk of a cardiac specific severe maternal morbidity compared to women without CHD and were also at a higher risk for the other adverse outcomes evaluated. Additional diagnoses in the setting of maternal CHD including congestive heart failure, arrhythmia, valvular disease, pulmonary disorders, and venous thromboembolism were associated with further increased likelihood of adverse events. These findings suggest that established risk associated with maternal CHD during pregnancy4,5,12,31 may be continuing to increase on a population basis both in absolute and relative terms.
Clinical interpretation
These findings indicate that maternal CHD during pregnancy is a growing cause of adverse maternal outcomes and that optimizing and managing maternal CHD during pregnancy will continue to be an important public health goal. We hypothesize that the increase of patients with maternal CHD observed over the study period is likely due to improved detection, surgery, and medical management of neonatal and pediatric CHD, allowing more women with these diagnoses to become pregnant in adulthood. Maternal CHD outcomes may be improved by preconceptual planning with maternal-fetal medicine, CHD cardiologists, and obstetric anesthesia specialists, close follow up during pregnancy by a multi-disciplinary team, optimizing medical management, regular diagnostic imaging when indicated, detailed delivery planning, referral to experience centers for delivery, and appropriate postpartum follow up.32–35 Given increasing trends in both the number of deliveries with maternal CHD and rising risk, it is likely that maternal CHD will continue to be an important contributor to severe maternal morbidity and maternal mortality in the near future. Findings from the analysis support that risk associated with maternal CHD may be differentiated based on additional diagnoses, further supporting the possibility for risk stratification and referral of appropriate patients to experienced centers. Climbing morbidity and mortality from cardiac causes underscore the need for higher acuity and collaborative care models for maternal CHD. The American Heart Association and the American College of Obstetricians and Gynecologists have highlighted the need for multidisciplinary cardio-obstetrics, delivery centers with higher cardiac volume and experience with high-acuity CHD in pregnancy, and multidisciplinary care that may reduce morbidity.36,37 Maternal cardiac risk may be increasing in the context of other prevalent comorbid conditions that are also becoming more common such as pregestational diabetes, asthma, obesity, and chronic hypertension. Optimal obstetric outcomes for women with CHD may increasingly be dependent on proper management of these comorbid conditions. That cardiac severe morbidity decreased slightly over the study period was an unexpected finding. These results could be secondary to better classification of CHD with ICD-10 billing diagnoses and the high-risk group of women with CHD being better captured in this later iteration.
Strengths and Limitations
There are a number of limitations to consider when evaluating this study. First, the NIS limits analysis to cross-sectional data. It does not allow examination of other longitudinal factors such as outpatient management, hospital re-admittance, medication management, or hospital and emergency encounters. We were thus unable to draw conclusions about other outcomes that occur at different points during pregnancy or in the postpartum period. We cannot analyze antepartum hospitalizations or complications preceding the delivery hospitalization. Second, the NIS uses billing data and clinical data is not available. We were not able to review surgical history, cardiac function, delivery planning, hospital referral planning, functional status, and the role of consultants including maternal fetal medicine, obstetric anesthesia, and CHD cardiologists. We were not able to review clinical complications for criteria such as preeclampsia. Furthermore, billing data is subject to well-known limitations including misclassification and under-ascertainment. Given that we analyzed delivery hospitalizations it is possible that secondary billing diagnoses for cardiac conditions could be under-ascertained if not tied to reimbursement and the population size of deliveries with maternal CHD underestimated. Fourth, the NIS went through two important changes during the study period, first in 2012 when the sampling approach changed and second in 2015 when billing switched from the ICD-9-CM framework to ICD-10-CM billing codes. It is possible that these changes could have affected some of the trends in our analysis. Fifth, because the unit of analysis in the NIS is the individual hospitalization we are not able to account for multiple deliveries occurring to the same woman. Sixth, because of limitations in coding we are not able to disaggregate spontaneous or iatrogenic preterm birth. Seventh, we acknowledge given how commonly cesarean delivery occurs it may not truly represent an adverse event. Eighth, because we cannot link to neonatal outcomes we cannot measure small for gestational age, neonatal death, and other adverse neonatal events. Ninth, because of small numerators, we are unable to estimate risk for specific conditions. Tenth, an important consideration in evaluating maternal cardiac outcomes includes determining to what degree cardiac conditions are diagnosed before delivery. This study is not able to account for whether conditions were diagnosed prior to pregnancy or delivery. Longitudinal claims data could be used to account for the degree to which diagnoses were characterized prior to the delivery hospitalization.
The primary strengths of this study are that our analysis was conducted on a nationally representative cohort, the number of patients included in the study allowed for examination of rare conditions and their association with infrequent outcomes, that the study occurred over a 19-year period, that the analysis included a broad range of outcomes, and that it is appropriate to use administrative data to evaluate disease burden.
Conclusion
This study found that (i) the prevalence of maternal CHD among delivery hospitalizations increased greatly over the study period with rising risk for severe morbidity, (ii) maternal CHD is associated with elevated risk for a broad range of adverse outcomes during delivery hospitalization, and (iii) that maternal CHD cardiac comorbidty diagnoses further characterize risk. These findings support that maternal CHD and associated risk during pregnancy will continue to be of clinical and epidemiological significance and that efforts to improve care should be prioritized.
Supplementary Material
Funding
This research was supported by NIH Short Term Research Training Grant 2T35HL007616-41
Footnotes
Disclosures: Dr. D’Alton has had a leadership role in ACOG II’s Safe Motherhood Initiative which has received unrestricted funding from Merck for Mothers. The other authors did not report any potential conflicts of interest.
IRB Approval: The Columbia University Institutional Review Board (IRB-AAAE8144) deemed this research exempt.
REFERENCES
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association. Circulation. 2018;137(12):e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130(9):749–756. [DOI] [PubMed] [Google Scholar]
- 3.Gilboa SM, Devine OJ, Kucik JE, et al. Congenital Heart Defects in the United States: Estimating the Magnitude of the Affected Population in 2010. Circulation. 2016;134(2):101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson JL, Kuklina EV, Bateman BT, Callaghan WM, James AH, Grotegut CA. Medical and Obstetric Outcomes Among Pregnant Women With Congenital Heart Disease. Obstet Gynecol. 2015;126(2):346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlichting LE, Insaf TZ, Zaidi AN, Lui GK, Van Zutphen AR. Maternal Comorbidities and Complications of Delivery in Pregnant Women With Congenital Heart Disease. J Am Coll Cardiol. 2019;73(17):2181–2191. [DOI] [PubMed] [Google Scholar]
- 6.Elkayam U, Goland S, Pieper PG, Silversides CK. High-Risk Cardiac Disease in Pregnancy: Part I. Journal of the American College of Cardiology. 2016;68(4):396–410. [DOI] [PubMed] [Google Scholar]
- 7.Drenthen W, Pieper PG, Roos-Hesselink JW, et al. Outcome of pregnancy in women with congenital heart disease: a literature review. Journal of the American College of Cardiology. 2007;49(24):2303–2311. [DOI] [PubMed] [Google Scholar]
- 8.Drenthen W, Boersma E, Balci A, et al. Predictors of pregnancy complications in women with congenital heart disease. European heart journal. 2010;31(17):2124–2132. [DOI] [PubMed] [Google Scholar]
- 9.Hayward RM, Foster E, Tseng ZH. Maternal and Fetal Outcomes of Admission for Delivery in Women With Congenital Heart Disease. JAMA Cardiology. 2017;2(6):664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moghbeli N, Pare E, Webb G. Practical assessment of maternal cardiovascular risk in pregnancy. Congenit Heart Dis. 2008;3(5):308–316. [DOI] [PubMed] [Google Scholar]
- 11.The Centers for Disease Control and Prevention. Pregnancy Mortality Surveillance System. Available at: https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm. Accessed August 10, 2021.
- 12.Opotowsky AR, Siddiqi OK, Webb GD. Trends in hospitalizations for adults with congenital heart disease in the U.S. J Am Coll Cardiol. 2009;54(5):460–467. [DOI] [PubMed] [Google Scholar]
- 13.Kuklina E, Callaghan W. Chronic heart disease and severe obstetric morbidity among hospitalisations for pregnancy in the USA: 1995–2006. BJOG. 2011;118(3):345–352. [DOI] [PubMed] [Google Scholar]
- 14.Klebanoff MA, Snowden JM. Historical (retrospective) cohort studies and other epidemiologic study designs in perinatal research. Am J Obstet Gynecol. 2018;219(5):447–450. [DOI] [PubMed] [Google Scholar]
- 15.Cost Healthcare and Project Utilization. Overview of the the National (Nationwide) Inpatient Sample. Accessed February 20, 2021. Available at: https://www.hcup-us.ahrq.gov/nisoverview.jsp.
- 16.Cost Healthcare and Project Utilization. Trend Weights for HCUP NIS Data. Accessed March 20, 2021. Available at: https://www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp.
- 17.The Healthcare Cost and Utilization Project. NIS description of data elements. https://www.hcup-us.ahrq.gov/db/vars/dxn/nisnote.jsp. Accessed February 20, 2021.
- 18.Centers for Medicare & Medicaid Services. ICD-10. Accessed March 20, 2021. Available at: https://www.cms.gov/Medicare/Coding/ICD10/. [PubMed]
- 19.Kinlaw AC. alankinlaw/Easy_ICD9-to-10_GEMs_mapping. https://github.com/alankinlaw/Easy_ICD9-to-10_GEMs_mapping. Published September 24, 2019. Accessed May 31, 2021. [Google Scholar]
- 20.Kuklina E, Whiteman M, Hillis S, Jameieson D, Meikle S, Posner S. An enhanced method for identifying obstetric deliveries: implications for estimating maternal morbidity. Matern Child Health J. 2008;12:469–477. [DOI] [PubMed] [Google Scholar]
- 21.Clapp MA, James KE, Friedman AM. Identification of Delivery Encounters Using International Classification of Diseases, Tenth Revision, Diagnosis and Procedure Codes. Obstet Gynecol. 2020;136(4):765–767. [DOI] [PubMed] [Google Scholar]
- 22.The Centers for Disease Control and Prevention. Severe Maternal Morbidity in the United States. Accessed February 20, 2021. Available at: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/severematernalmorbidity.html.
- 23.Main EK, Abreo A, McNulty J, et al. Measuring severe maternal morbidity: validation of potential measures. Am J Obstet Gynecol. 2016;214(5):643 e641–643 e610. [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute. Joinpoint Trend Analysis Software. https://surveillance.cancer.gov/joinpoint/. Accessed March 20, 2021, 2020.
- 25.Barrio G, Pulido J, Bravo MJ, Lardelli-Claret P, Jimenez-Mejias E, de la Fuente L. An example of the usefulness of joinpoint trend analysis for assessing changes in traffic safety policies. Accid Anal Prev. 2015;75:292–297. [DOI] [PubMed] [Google Scholar]
- 26.National Cancer Institute. Average Annual Percent Change (AAPC) and Confidence Interval. Accessed April 1, 2021. Available at: https://surveillance.cancer.gov/help/joinpoint/setting-parameters/method-and-parameters-tab/apc-aapc-tau-confidence-intervals.
- 27.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. [DOI] [PubMed] [Google Scholar]
- 28.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. [DOI] [PubMed] [Google Scholar]
- 29.Yang D, Dalton J. A unified approach to measuring the effect size between two groups using SAS. SAS Global Forum 2012. Available at: http://support.sas.com/resources/papers/proceedings12/335-2012.pdf. Accessed April 14, 2021. [Google Scholar]
- 30.The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Available at: https://www.equator-network.org/reporting-guidelines/strobe/. Accessed March 28, 2021. [DOI] [PMC free article] [PubMed]
- 31.Ramage K, Grabowska K, Silversides C, Quan H, Metcalfe A. Association of Adult Congenital Heart Disease With Pregnancy, Maternal, and Neonatal Outcomes. JAMA Network Open. 2019;2(5):e193667–e193667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elkayam U, Goland S, Pieper PG, Silversides CK. High-Risk Cardiac Disease in Pregnancy: Part II. J Am Coll Cardiol. 2016;68(5):502–516. [DOI] [PubMed] [Google Scholar]
- 33.Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(12):e81–e192. [DOI] [PubMed] [Google Scholar]
- 34.Baumgartner H, De Backer J, Babu-Narayan SV, et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42(6):563–645. [DOI] [PubMed] [Google Scholar]
- 35.Canobbio MM, Warnes CA, Aboulhosn J, et al. Management of Pregnancy in Patients With Complex Congenital Heart Disease: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2017;135(8):e50–e87. [DOI] [PubMed] [Google Scholar]
- 36.Brown HL, Warner JJ, Gianos E, et al. Promoting Risk Identification and Reduction of Cardiovascular Disease in Women Through Collaboration With Obstetricians and Gynecologists: A Presidential Advisory From the American Heart Association and the American College of Obstetricians and Gynecologists. Circulation. 2018;137(24):e843–e852. [DOI] [PubMed] [Google Scholar]
- 37.Grodzinsky A, Florio K, Spertus JA, et al. Importance of the Cardio-Obstetrics Team. Curr Treat Options Cardiovasc Med. 2019;21(12):84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


