Summary
Formation of neural circuits occurs in a programmed fashion, but proper activity in the circuit is essential for refining the organization necessary for driving complex behavioral tasks. In the retina, sensory deprivation during the critical period of development is well known to perturb organization of the visual circuit making the animals unable to use vision for behavior. However, the extent of plasticity, molecular factors involved and malleability of individual channels in the circuit to manipulations outside of the critical period are not well understood. In this study we selectively disconnected and reconnected rod photoreceptors in mature animals after completion of the retina circuit development. We found that introducing synaptic rod photoreceptor input post-developmentally allowed their integration into the circuit both anatomically and functionally. Remarkably, adult mice with newly integrated rod photoreceptors gained high sensitivity vision even when it was absent from birth. These observations reveal plasticity of the retina circuit organization after closure of the critical period and encourage development of vision restoration strategies for congenital blinding disorders.
Keywords: Vision, retina, synapses, plasticity, critical period, cell adhesion molecules
Graphical Abstract
eTOC Blurb
Cao et al. examine the mechanisms that govern post-developmental connectivity of rod photoreceptors into the retinal circuitry. The study reports that rod synapse formation can be restored in adult mice enabling their rod mediated vision at the behavioral level.
Introduction
A relationship between form and function has long captivated biologists from many angles. In the nervous system, examining this question is particularly relevant for understanding the development and function of neural circuits. Circuits are formed in a stereotypically programmed fashion with defined anatomical and functional properties 1,2. Yet, despite this “hard-wiring”, the activity patterns driven by sensory input are required to refine organization of circuits making them suitable for guiding behavior with precision 3,4. Interestingly, this circuit shaping is thought to be possible only during a specific window in development referred to as the critical period (CP)5,6. The absence of the refining input during development leaves circuits poorly suited for powering specific behavioral tasks and stimulation after closure of the critical period is generally found to be ineffective in correcting these operational deficits 7-13.
Significant progress has been made in defining mechanisms that govern gating of critical periods and circuit plasticity 14-23. There is also a growing consensus that it has far reaching implications for understanding neuropsychiatric process and development of therapeutic interventions 5,24-27. However, many fundamental questions related to the extent of plasticity, molecular factors involved and differences in malleability of individual circuits to manipulations outside of the critical period remain open.
There have been significant advances in elucidating anatomy and functional specification of visual circuitry 28,29. In particular, we now have an exquisitely detailed picture of how vision at the lowest light intensities is orchestrated 30-33. With the world being dark approximately half of the time, an ability to see at night is paramount for survival of most vertebrate species. This process is enabled by highly sensitive rod photoreceptors 34-37. To reliably transmit stimuli generated in response to single photons of light, rods must relay their signals via a dedicated high gain channel known as primary rod pathway 38-40. In this circuit, operating near the absolute visual threshold, rods synaptically communicate with their postsynaptic partners, the rod ON-bipolar neurons 41,42. Disruption of this process leads to congenital night blindness illustrating that the primary rod pathway is indispensable for a highly stereotypic night vision 43-64. However, how hard wired and plastic rod photoreceptor synapses are for enabling behaviorally relevant vision is an open question. Answering it will have significant bearing not only for understanding fundamental neurobiology of circuitry development but also for translational efforts aimed at restoring vision lost to degenerative process.
Examining plasticity of the photoreceptor wiring may have implications for understanding CP in the vertebrate visual system, where it was originally defined 9,13. Complete deprivation from retina inputs during early development leads to profound disorganization of the visual circuit in the brain and inability of animals to rely on vision for guiding behavior following CP closure 7,8,10-12,65,66. Interestingly, clinical evidence from humans suffering from correctable vision-depriving conditions from early childhood indicates that restoration of visual function could be achieved past CP closure 67. This suggests capacity for post-developmental plasticity of the higher order visual circuit allowing it to handle visual information when retina inputs were not completely obliterated. However, studies examining the boundaries of CP plasticity in driving behavioral vision with circuit precision have been lacking.
In this work we relied on advanced molecular genetics to manipulate synaptic connectivity of rods, selectively switching their communication on and off post-development while studying the impact on signal propagation along primary rod pathway and vision at the behavioral level. Our results reveal remarkable post-developmental plasticity of physical rod photoreceptor wiring in adult visual system which could be exploited therapeutically.
Results
Cell adhesion mechanisms of mature photoreceptors for maintaining synaptic alignment and transduction of visual signals.
Probing the plasticity of circuit organization and its contribution to behavior requires an ability to molecularly manipulate the configuration of the light input at precise developmental stages without compromising the viability or activity of the photoreceptors and their signaling capacity. In searches for a critical molecular factor for such manipulation, our attention was drawn to a cell adhesion molecule of rods, Elfn1. Its deletion in mice has been shown to selectively and completely abolish synaptic communication of rods without compromising their viability while preserving cone function and transduction of the light signals via secondary pathways 68. Thus, manipulations with Elfn1 may allow for specific isolation of the primary rod pathway’s contribution to vision. To date, however, it was only shown to be required for the assembly of the circuit early in development.
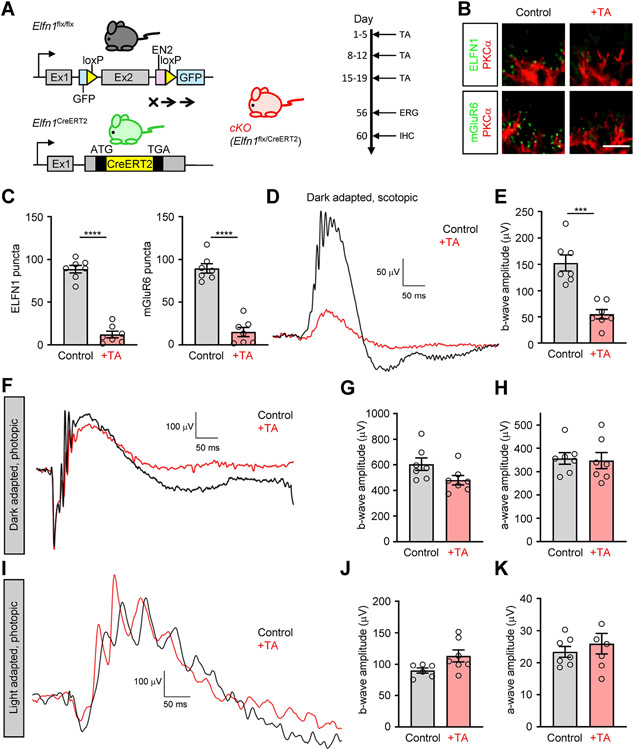
To test the utility of E1fn1 for manipulating circuit configuration post-developmentally, we first probed whether Elfn1 continues to function after development is complete. To enable such studies, two modified Elfn1 alleles were used. First, we developed a conditional driver line knocking in tamoxifen-sensitive Cre recombinase (CreERT2) under the control of endogenous Elfn1 promoter (Elfn1CreERT2). We confirmed the activity of the Cre recombinase specifically in rods upon induction with tamoxifen by crossing the line with the Ai14 reporter strain (Figure S1). We then crossed it with mice carrying a conditional Elfn1flx allele to obtain gene-autonomous conditional Elfn1flx/CreERT2 mice (cKO). In this model, Elfn1 loss was induced in adult mice (P21) by repeated injections of tamoxifen (Figure 1A). Mice not receiving tamoxifen served as controls and had readily detectable Elfn1 and mGluR6 at rod synapses (Figure 1B). Importantly, treatment with tamoxifen dramatically reduced Elfn1 levels which was accompanied by a severe reduction in mGluR6 accumulation at synapses (Figure 1B,C). Consistent with these observations, we found that transmission of synaptic signals of rods to ON-RBC was nearly abolished as evidenced by blunted ERG b-wave, barely detectable in mice with lowest Elfn1/mGluR6 content (Figure 1D,E). These deficits were specific to rod synaptic transmission and did not impact transduction of the cone generated signals to ON-CBC as photopic b-wave amplitudes in both dark adapted mice (Figure 1F,G) and mice exposed to rod-suppressing light background (Figure 1I,J) were not affected by the tamoxifen treatment. Neither did we notice any deficits in the ability of the photoreceptors to generate a light response as judged by intact a-waves (Figure 1H,K). Together, these results indicate that loss of Elfn1 in fully developed retinas leads to selective disintegration of rod synaptic communication suggestive of the role Elfn1 in synaptic maintenance in mature retinas.
Figure 1. Analysis of post-developmental elimination of Elfn1.
(A) Schematic of the strategy for conditional elimination of Elfn1 in the retina. A conditional knockout allele for Elfn1 (Elfn1flx/flx) was generated by flanking exon 2 with loxP sites. Elfn1flx/flx mice were crossed with a tamoxifen-inducible Cre strain Elfn1CreER2 under the the control of endogenous Elfn1 promoter to generate conditional Elfn1flx/CreER2 (cKO) mice. The 2-month-old cKO mice were administered tamoxifen (TA) for three five-day injections to induce ELFN1 loss. One-month post-administration, electroretinograms were recorded, then immunohistochemistry analyses were performed. TA, tamoxifen.
(B) Elfn1 and mGluR6 localization in rods of cKO mice. Retina cross-sections were double-immunostained with antibodies against Elfn1(green) or mGluR6 (green) with rod ON-BC marker PKCα (red) (Scale bar, 5 μm.)
(C) Quantification of Elfn1 and mGluR6 in cKO mice. Synaptic puncta were counted and averaged from six images per retina collected from seven separate mice in each condition.
(D) Analysis of rod synaptic transmission in cKO mice by ERG. Representative traces of responses to a scotopic flash of 0.001 cd*s/m2 (~0. 6R* per rod) are shown.
(E) Quantification of b-wave ERG amplitudes in cKO mice treated with or without tamoxifen under scotopic conditions.
(F) Representative ERG traces to a photopic flash of 100 cd*s/m2 (~58,000 R*/rod) to activate both rod and cone pathways.
(G) Quantification of b-wave ERG amplitudes in cKO mice treated with or without tamoxifen under photopic conditions.
(H) Quantification of a-wave ERG amplitudes in cKO mice treated with or without tamoxifen under photopic conditions.
(I) Representative ERG traces to a photopic flash of 100 cd*s/m2 (~58,000 R*/rod) under a 32 cd*s/ m2 (~18,500 R*/rod/s) light background to activate the cone pathway only.
(J) Quantification of b-wave ERG amplitudes in cKO mice treated with or without tamoxifen under light-adapted photopic conditions.
(K) Quantification of a-wave ERG amplitudes in cKO mice treated with or without tamoxifen under light-adapted photopic conditions. Data are represented as mean ± SEM. n=7, ***P < 0.001, ****P < 0.0001, unpaired t-test.
See also Figure S1.
Post-developmental rescue of Elfn1 expression restores synaptic connectivity and transmission of rods.
Having established a role for Elfn1 in the development and maintenance of synaptic connections of rods, we next asked the central question related to the plasticity of rod connectivity and whether its restoration was possible in mature retinas where rods had never been connected to their postsynaptic ON-BC partners. To technically enable these studies, we generated a conditional rescue allele (Elfn1CR/CR) by inverting the exon 2 of the Elfn1 gene relative to its normal endogenous orientation and flanking it with loxP sites to allow Cre-induced restoration of normal gene organization (Figure S2A). We found complete elimination of Elfn1 immunoreactivity in rod synapses of Elfn1CR/CR retinas, accompanied by loss of mGluR6, (Figure S2B, C). This was accompanied by predicted loss of rod synaptic transmission as evidenced by complete loss of scotopic ERG b-wave (Figure S2D, E). Thus, our Elfn1CR allele is a complete null and phenocopies the effects of the global Elfn1 KO 68. Validating the approach, we bred Elfn1CR mice with the Pcdh21Cre line, a constitutive driver active in photoreceptors (Figure S2A) in order to achieve the developmental conditional rescue of Elfn1 in photoreceptors (pCR). Indeed, we observed that retinas of pCR mice contained both Elfn1 and mGluR6 synaptic puncta (Figure S2B, C). Furthermore, synaptic transmission was also rescued in pCR mice (Figure S2D, E) indicating the general success of genetic rescue strategy of Elfn1 expression and rod synaptic organization early in development.
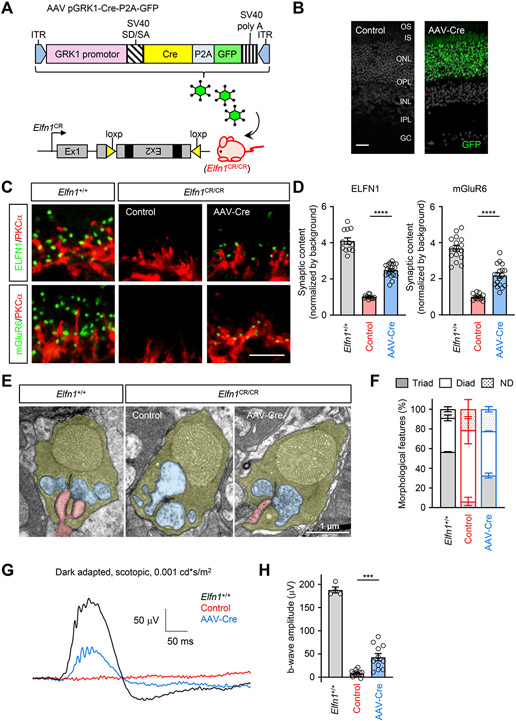
To test the impact of Elfn1 restoration on synaptic organization after the closure of developmental window we turned to a viral-mediated expression of Cre recombinase in rods of two-month-old Elfn1CR/CR mice (Figure 2A, B). Subretinal injection of AAV8-hGRK1-Cre-P2A-GFP led to efficient transduction of photoreceptors and resulted in prominent restoration of synaptic Elfn1 in rods accompanied by re-appearance of mGluR6 (Figure 2C, D) as well as other key synaptic signaling molecules (Figure S3A) at the dendritic tips. Pre-synaptic molecules not affected by Elfn1 elimination were also unaffected by its restoration with AAV-Cre indicating specificity of the effect (Figure S3B).
Figure 2. Analysis of post-developmental rescue of Elfn1.
(A) Schematic illustration of the strategy for the conditional rescue of Elfn1 in the retina. The mice carrying the conditional rescue Elfn1 allele (Elfn1CR/CR) are obtained by inverting the exon 2 of the Elfn1 gene relative to their normal endogenous orientation and are flanked with loxP66 and loxP71 sequences inserted in an inverted orientation relative to each other. The restoration of Elfn1 is achieved by subretinal injection of rAAV using human rhodopsin kinase 1 promoter construct to provide photoreceptor specificity for Cre recombinase expression. ITR, inverted terminal repeat; GRK1 promotor, human rhodopsin kinase 1 promoter; SV40 SD/SA, SV40 splice donor/splice acceptor.
(B) Retina cross-section showing GFP induction specifically in the photoreceptor. OS, outer segment; IS, inner segment; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GC, ganglion cells. (Scale bar, 25 μm.)
(C) Analysis of the effect of Elfn1 restoration on synaptic content of mGluR6 in rod terminals. Retina cross-sections were double-immunostained with antibodies against Elfn1 (green) or mGluR6 (green) and rod ON-BC marker PKCα (red). Scale bar, 5 μm.
(D) Quantification of Elfn1 and mGluR6 content in rod ON-BC synapses. Mean fluorescence intensity from 10 puncta per retina section was normalized by background. Data are from three images per retina collected from four to six separate mice and represented as mean ± SEM; n = 12 to 18. ****P < 0.0001, one-way ANOVA followed by Tukey's multiple comparisons.
(E) Analysis of synaptic morphology by electron microscopy. Photoreceptor axonal terminals are colored in yellow, processes of horizontal cells in blue, and ON-BC dendrites in pink.
(F) Quantification of synaptic organization features. Rod terminals containing ON-BC dendrites were designated as triads and devoid of ON-BC processes: diads. 188-366 rod terminals from two separate mice were analyzed for each condition. UN, undetermined.
(G) Representative ERG traces elicited by a scotopic flash of 0.001 cd*s/m2 (~0.6 R*/rod) to activate the primary rod pathway only in Elfn1CR/CR mice with subretinal injection of AAV-Cre or control virus.
(H) Quantification of b-wave ERG amplitudes under scotopic conditions. Data are represented as mean ± SEM. n=4-13, ***P < 0.001, one-way ANOVA followed by Tukey's multiple comparisons.
See also Figures S2 and S3.
Importantly, ultrastructural examination revealed that AAV-Cre expression further restored normal morphology of rod synapses: while invaginating rod ON-BC dendrites were absent in control Elfn1CR/CR, they were quantitatively detectable in rod spherules following AAV-mediated Cre delivery (Figure 2E, F). Overall, these results reveal that Elfn1 re-expression in mature retinas where synaptic contacts of rods had not been developed leads to post-developmental synapse formation and accumulation of receptors mediating the synaptic communication.
We next asked the question whether anatomically restored synapses are functional by evaluating light responses of the retina by ERG. We found that AAV-Cre injected mice showed robust b-waves, albeit lower than in wild-type mice, upon activating rods only with dim scotopic flashes (Figure 2G, H). This was in stark contrast with completely absent scotopic b-waves in control animals lacking Elfn1 (Figure 2G, H). Notably, AAV-mediated Cre expression in rods did not affect the processing of signals by cone photoreceptors and synaptic transmission of cone signals as evidenced by no differences between the groups in either a-waves of b-waves upon photopic stimulation with or without rod suppressing light background (Figure S3C-H). Thus, we conclude that connecting rods into mature circuits after the completion of development permits functional transduction of light evoked signals.
Mice with post-developmentally restored rod wiring fully regain scotopic vision.
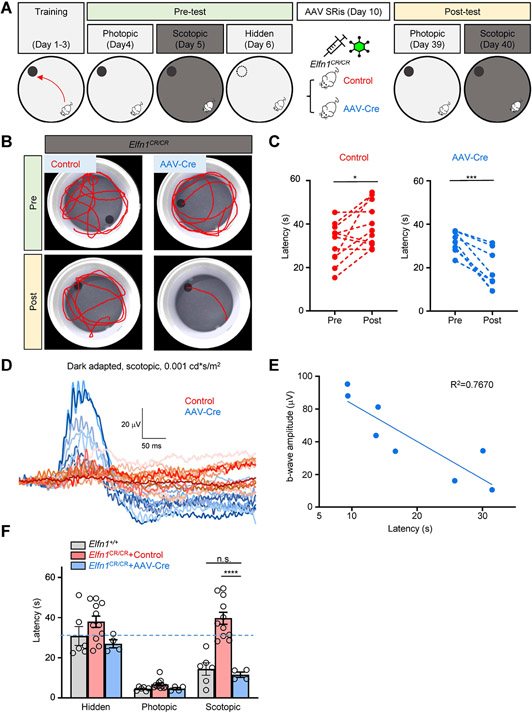
An ability to restore functional synaptic connectivity of rods in mature mice allows us to address the central question: can restoration of rod wiring support behavior guided by vision? We investigated this issue using a water-maze navigational task where animals must rely on vision to locate an escape platform. First, Elfn1CR/CR mice were trained to locate a visible escape platform under photopic conditions when mice do not rely on rod inputs for vision (Figure 3A). Mice showed steady improvement in their performance plateauing after 3 days of training when they swam directly to the platform with an average escape latency of 5.47±0.47 s (Figure S4A,B). The same level of performance was observed in WT mice with unperturbed visual function (5.41±0.57 s). In control experiments, the platform was hidden and the mice had to locate it by randomly sampling the space without relying on vision. This substantially prolonged the escape latencies with an average of 36.19±2.05 s. Again, this was no different from the performance of the WT mice in the same task (30.84±4.67 s). Thus, the difference in escape latencies between visible and hidden platform can serve as a behavioral measure of visual function. Notably, when the mice were evaluated under scotopic conditions requiring the rod pathway to be active, the escape latencies of Elfn1CR/CR mice, but not those of WT, were no different than those observed for the hidden platform, indicating that at the outset, mice lacking rod synaptic inputs have completely lost visual function in a dim light environment (Figure S4A,B). Next, the animals were split into two cohorts with each receiving either AAV-Cre or control AAV8-hGRK1-GFPvector delivered by subretinal injections (Figure 3A). When mice were evaluated for the behavior four weeks later, we found that all of the AAV-Cre injected animals showed decreased escape latencies under the scotopic conditions (Figure 3B,C). In contrast, mice injected with the AAV-GFP control showed no improvement in their behavior. Both groups performed equally well in a photopic lighting environment (Figure 3F).
Figure 3. The restoration of Elfn1 in Elfn1CR/CR mice enables scotopic vision.
(A) Schematic of the timeline of AAV subretinal treatment and evaluating mouse visual sensitivity to light by a water maze task. Photopic condition (bright gray): 100 cd*s/m2, Scotopic condition (dark gray): 0.001 cd*s/m2; SRis, subretinal injections.
(B) Swimming tracks of representative animals during sessions at the scotopic condition (0.001 cd*s/m2) before and after AAV subretinal injections.
(C) Summary of platform finding latencies at the scotopic condition (0.001 cd*s/m2) before and after AAV subretinal injections. Data are represented as mean±SEM, n=8-11, *p<0.05, ***p < 0.001, paired t-test.
(D) ERG traces elicited by a scotopic flash of 0.001 cd*s/m2 (~0.6 R*/rod) to activate the primary rod pathway only in Elfn1CR/CR mice with subretinal injection of AAV-Cre (blue) or control virus (red).
(E) Correlation of finding platform latency and b-wave ERG amplitudes in AAV-Cre treated Elfn1CR/CR mice. Quantification of data presented in Figs. 3C and 3D.
(F) Summary of platform finding latencies after AAV subretinal injections. Statistical analysis was performed using two-way ANOVA test followed by Turkey's multiple comparisons test. Data are represented as mean± SEM, n=4 to 11, ****p < 0.0001.
See also Figure S4.
Interestingly, we noted significant variability in the extent of the behavioral improvement in AAV-Cre injected group. To understand the nature of this variability we performed ERG on all subjects and noted similar variability in the extent of the b-wave magnitude (Figure 3D). Plotting the amplitudes of scotopic b-waves against the escape latencies in the behavioral task revealed a strong correlation (Figure 3E), indicating that restoration of the visual function is likely limited by the degree of the functional improvement in the synaptic transmission which in turn depends on the efficiency of the AAV transduction in individual mice. Thus, to isolate the impact of reconnecting the rods on vision, we focused on mice with the largest degree of functional rescue, restricting our analysis to mice with ERG b-wave that reached at least 25% of the normally sized response in the model without Elfn1 elimination (as in Figure 1). Analysis of the behavior in these mice revealed complete restoration of visually guided behavior under a scotopic light environment (Figure 3F). Indeed, their visual task performance was equivalent to that of WT mice or to mice evaluated under the photopic conditions. Overall, these results indicate that restoring synaptic inputs of rod photoreceptors fully enables behavioral vision in adult mice which had not previously had any rod-mediated vision.
Recognition of rod synaptic terminals by ON-bipolar dendrites occurs independently from the synapse formation.
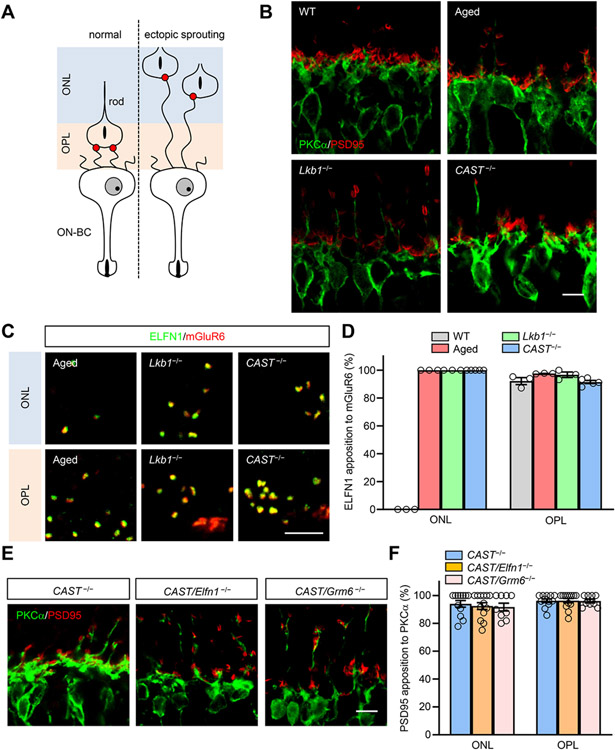
Our finding that the formation and function of rod synapses can be readily rescued post-development raises questions about the mechanisms underlying plasticity of the processes involved in aligning the contacts between rods and their synaptic partners, the ON-BC neurons. To address this, we turned to experiments where we studied the relationship between synapse formation and alignment of rod axons with ON-BC dendrites by cell-cell recognition. First, we analyzed the positioning of the rod synaptic contacts in retinas with misplaced positioning of rod axonal spherules. Normally, rod axons terminate in the outer plexiform layer (OPL) where they meet ON-BC dendrites to make synaptic contacts (Figure 4A,B). However, under certain conditions, rod spherules retract into the outer nuclear layer (ONL) inducing well-documented sprouting of ON-BC dendrites into this area 69-73. We reasoned that if synapse formation plays a role in this process, Elfn1/mGluR6 pairing would be found at the sites of the ectopic contacts. We used three different models that induce ectopic sprouting: aged retinas, knockout of central metabolic regulator LKB1 and elimination of the active zone protein CAST (Figure 4B). We found similar levels of stochastic ON-BC dendritic sprouting in all of these models (Figure 4B,D). In all of these cases, axons retracted to ONL marked by presynaptic marker PSD95 in ribbons (Figure 4B). Importantly, double-staining experiments revealed that Elfn1 was localized in retracted axonal terminals in direct apposition to postsynaptic receptor mGluR6 at the tips of the sprouting dendritic tips in all models (Figure 4C, D). Thus, trans-synaptic interaction of mGluR6-Elfn1 can occur upon displaced matching of ON-BC dendrites with prematurely terminating axons highlighting the plasticity of the synapse formation process.
Figure 4. Lamina displacement of axono-dendritic matching preserves trans-synaptic Elfn1-mGluR6 alignment.
(A) Schematic of the rod to ON-BC processes in the normal and ectopically spouting retina. ON-BC to photoreceptor synapses form in the wild-type retinas strictly in the outer plexiform layer (OPL). However, under the conditions that induce axonal retraction, ON-BC dendrites extend all the way to the outer nuclear layer (ONL) to locate the synaptic partner.
(B) Rod bipolar ectopic processes in old wild-type, LKB1-deficient and CAST knockout retinas. Retina cross-sections were double-stained with antibodies against rod ON-BC marker PKCα (green) and pre synaptic marker PSD95 (red). (Scale bar, 5 μm.)
(C) Elfn1-mGluR6 trans-synaptic complex persists at ectopic sites. High magnification of OPL and ONL synapse stained with Elfn1 (green) and mGluR6 (red). (Scale bar, 2.5 μm.)
(D) Statistical analysis of Elfn1 puncta apposed to mGluR6 puncta in OPL and ONL synapse. Data are presented as mean ± SEM. n=3-5.
(E) Elimination of Elfn1 or mGluR6 has no effect on dendritic sprouting in the CAST KO retinas. Retina cross-sections were double immuno-stained with antibodies against PSD95 (red) and PKCα (green). (Scale bar, 2.5 μm.)
(F) Statistical analysis of RBC dendritic tips apposed to the presynaptic terminal in OPL and ONL synapse. Data are presented as mean ± SEM. n=9-13.
Since Elfn1-mGluR6 trans-synaptic contact is essential for establishing the rod to rod-ON-BC synapse, we next asked whether the dendritic sprouting can be prevented by eliminating synapse formation. To address this question, we studied the effect of Elfn1 or mGluR6 loss which both prevent synapse formation on dendritic sprouting in the CAST−/− model. We found ectopic sprouting was no different between the retinas with a singular elimination of CAST and when CAST was concurrently eliminated with either Elfn1 or mGluR6 (Figure 4E,F). This indicates that seeking of rod axons by ON-bipolar dendrites and physical matching of both processes occurs independently from the synapse formation driven by trans-synaptic mGluR6-Elfn1 interaction.
Synaptic targeting of Elfn1 is autonomous and independent of synaptic assembly
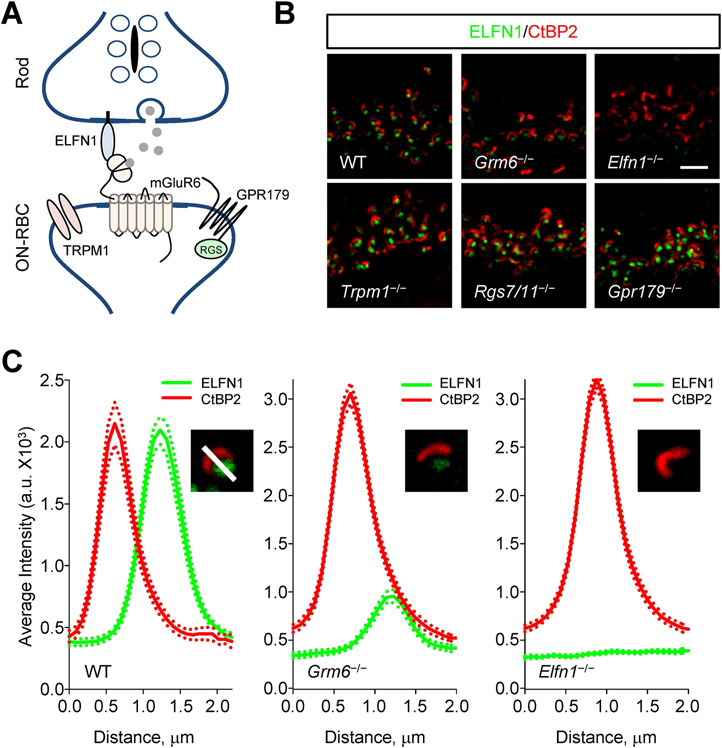
Given the trans-synaptic nature of interactions involving Elfn1 we next inquired whether its positioning at the axonal terminals of rods depends on the components of the postsynaptic compartment in ON-BC. Our strategy was to systematically delete key components of postsynaptic compartment in mice to study their impact on Elfn1 localization (Figure 5A). Eliminating TRPM1, the principal ion channel that mediates depolarizing response of ON-BC did not affect synaptic targeting of Elfn1 (Figure 5B, S5). Similarly, knockout of RGS proteins RGS7 and RGS11 that constitute the GAP complex that plays an essential role in generating postsynaptic response in ON-BC also failed to affect synaptic accumulation of Elfn1 (Figure 5B, S5). Furthermore, no effect was observed upon deletion of the orphan receptor, GPR179 (Figure 5B), which engages in trans-synaptic contacts with Pikachurin-dystroglycan complex in rod terminals 74.
Figure 5. The presynaptic targeting of Elfn1 is independent of the postsynaptic machinery.
(A) Schematic of molecule organization of the first visual synapse.
(B) Effect of eliminating key postsynaptic molecules on expression and localization of Elfn1. Retina cross-sections were double-stained with antibodies against Elfn1 (green) and presynaptic marker CtBP2 (red). Scale bar, 2.5 μm.
(C) Analysis of Efln1 localization in postsynaptic receptor mGluR6 knockout retina. Plots show quantification of relative fluorescence intensity distributions from 10 synaptic puncta. Dashed lines are plots of respective SEM values.
See also Figure S5.
We previously reported that elimination of mGluR6 compromises the stability of Elfn1 reducing its expression level as assessed by Western blotting 68. This leaves the question whether Elfn1 is still targeted to synapses in the absence of mGluR6 despite its reduced expression. To answer this question, we performed a quantitative analysis of Elfn1 distribution in the synaptic photoreceptor layer of retinas lacking mGluR6 (Figure 5C). We found that in Grm6−/− retinas, despite the reduction in concentration, Elfn1 could still be concentrated in ribbon synapses (Figure 5C). Thus, the ability of Elfn1 to accumulate at rod synapses is independent from postsynaptic organization and its interaction with mGluR6. Since photoreceptor to ON-BC synapses also fail to form in retinas lacking mGluR6, we further conclude that presynaptic targeting of Elfn1 is independent of physical synapse formation.
Discussion
In this study, we achieve selective post-developmental manipulation of rod photoreceptor synaptic connectivity. With targeted genetic manipulations we were able to disconnect rods from their exclusive synaptic partners (ON-BC neurons) and then to physically reconnect them in mature retinas. This enabled us to address one of the central questions pertaining to plasticity in the organization of the visual circuit- can introduction of highly sensitive retina inputs absent from birth successfully drive visually guided behavior in adult animals? We report that connecting rods in adult mice not only resulted in their physical wiring with ON-bipolar neurons and enabled functional synaptic transmission but also remarkably resulted in emergence of dim light vision not present in the animals at any point before.
The acquisition of visual information first occurs in the retina followed by further processing in the lateral geniculate nucleus (LGN) and visual cortex. Current dogma posits that visual activity and experience play essential roles in visual circuit development. Since Huber and Weisel demonstrated the response of visual cortex neurons to light stimulation was shifted when one eye was deprived of vision in cats during the first few months of life 9,13, this vision-dependent critical period for ocular dominance columns has been extensively studied in various mammalian species 7,8,10-12. The establishment of synaptic connections is thought to be complete before eye opening and occur independently from light inputs, although dark rearing can block the refinement of retinal ganglion cell (RGC) responses 75,76. Dark rearing also disrupts cone-mediated but not rod-mediated response 77. Furthermore, blockage of depolarizing ON-BCs by applying glutamate analogue to developing cat retinas delay the stratification of ON and OFF ganglion cell dendrites 78.
While prior studies manipulated entire visual inputs, we investigated more circuit selective intervention by disconnecting only high sensitivity rod inputs. It is known that rod and cone pathways converge in the retina by the time they reach output neurons: retina ganglion cells 79. Thus, our results indicate that a generalized pathway, independent of rod photoreceptor activity, may be sufficient for entrainment of higher-order brain structures permitting development of cortical organization with plasticity to then receive additional inputs with distinct characteristics. This in turn enables higher-order vision that guides complex behavioral tasks. These observations give hope to vision restoration approaches for congenital disorders that target adults with gene replacement strategies. Indeed, viral gene transfer studies indicate that propagation of photoreceptor signals could be restored in other models of congenital stationary night blindness featuring synaptic dysfunctions 80-83. This progress paves the way for asking several exciting questions pertaining to the organization of the visual circuitry. For example, dissecting the contribution of rod specific rescue in the absence of functioning cones or how central nervous system in higher visual centers handles the post-developmental plasticity of the photoreceptor inputs are important questions. Furthermore, exploring whether viral expression of Elfn1 for its key role in rod photoreceptor communication could be generally beneficial in correcting synaptic deficiencies observed in a variety of models and conditions will be of interest.
Our study also clarifies the mechanistic basis behind the ability of rods to rewire following the completion of retina circuit development. We report that, regardless of physical synapse formation, rod photoreceptor axonal terminals maintain close proximity and likely contacts with the dendrites of the ON-BC. This allows us to conclude that the process of axono-dendritic matching for the photoreceptor-bipolar wiring is independent of the establishment of the synaptic connectivity between these cells. As photoreceptors develop, they send axons that terminate specifically in the outer plexiform layer (OPL) of the retina. Then ON-BCs extend their dendrites initially all the way through the photoreceptor layer followed by their pruning, restricting their terminating points at the OPL 84,85. Because axonal dendritic matching co-insides with synaptogenesis, it was unclear how these processes interact and whether synapse formation is essential for guiding dendritic processes to axonal terminals.
The identification of Elfn1 as a critical factor for synapse formation provided an excellent opportunity to answer this question. We found that Elfn1 is targeted to axonal terminals independently from the mGluR6-containing postsynaptic complex and elimination of either mGluR6 or Elfn1, while preventing synaptogenesis, had no impact on axono-dendritic pairing between rods and ON-BC. This leads to a conclusion that selective recognition of postsynaptic partners by rod photoreceptors occurs independently from their synapse formation. Several cell adhesion molecules have been identified to facilitate pairing of pre- and postsynaptic neurites and guiding them to the same lamina 86-88. In the retina, these include repulsive guidance cue semaphorin 6A (Sema6A) and its receptor Plexin A4 (PlexA4) 89-91, cadherin family protein Dscams/Sidekicks 86,87,92,93 and transmembrane proteins MEGF10/11 94,95. The identity of the molecular factor(s) mediating matching of photoreceptor and ON-BC processes is currently unknown. Its identification and relationship with synaptogenic Elfn1 function will be of significant interest.
STAR METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dr. Kirill Martemyanov (kmartemyanov@ufl.edu).
Materials availability
The Mouse lines generated in this study are available upon request from the lead contact.
Data and code availability
Original data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Trpm1−/−, Rgs7−/− Rgs11−/−, Gpr139−/−, Lkb1−/−, Elfn1−/−, CAST−/−, Elfn1flx/flx, Grm6−/− mouse lines were described previously 68,70,96-103. Reporter line Ai14 (B6;129S6-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J) was obtained from the Jackson Laboratory. Elfn1CreERT2 mouse was generated by recombination replacing of exon 2 of Elfn1 gene with CreERT2 cassette. The specificity and efficiency of Cre recombination in Elfn1CreERT2 lines are evaluated by crossing with Ai14 strain. Conditional targeting of Elfn1 was achieved by flanking exon 2 with LoxP sites (Elfn1flx/flx). The resulting mice were crossed with Elfn1CreERT2 line to generate cKO strain (Elfn1CreERT2/flx). The mice carrying the Elfn1CR allele are achieved by inverting the exon 2 of Elfn1 gene relative to their normal endogenous orientation and are flanked with loxP66 and loxP71 sequences inserted in an inverted orientation relative to each other. The resulting mice were crossed with photoreceptor-specific Cre driver line Pcdh21Cre 104,105 to produce Elfn1CR/CRPcdh21Cre strain (pCR).
To induce Cre expression, tamoxifen (Sigma, St. Louis, MO), dissolved in ethanol, then mixed with corn oil. The ethanol was removed by evaporator (SAVANT ISS110, Thermo Scientific).75 mg/kg was administered by intraperitoneal injection with a 27 gauge smooth tip needle. Tamoxifen was administered once daily for one five-consecutive day section or three five-consecutive day sections. Mice used in the study were 1–3 months old and were maintained on a diurnal 12 h light/dark cycle. Procedures involving mice strictly followed NIH guidelines and were approved by the Institutional Animal Care and Use Committee at Scripps Florida.
METHOD DETAILS
Antibodies and genetic constructs
Generation of rabbit anti-RGS11106, sheep anti-TRPM1, sheep anti-mGluR6 and rabbit anti-Elfn1 antibodies were described previously 68,96. Rabbit anti-CaV1.4 73 was a generous gift from Dr. Amy Lee (University of Iowa, IA). Rabbit anti-LRIT3107 was a generous gift from Dr. Christina Zeits (Institut de la Vision). Commercial antibodies were: mouse anti-CtBP2 (612044, BD Biosciences), mouse anti-PKCα (ab11723; Abcam), rabbit anti-PSD95 (D27E11, Cat# 3450S, Cell Signaling Technology).
Adeno associated virus (AAV8)- based vector containing three surface-exposed proteosomal avoidance mutations (Y447F, Y733F and T494V) was packaged with a construct containing the human rhodopsin kinase 1 (hGRK1) promoter driving Cre recombinase, followed by a P2A cleavage site and green fluorescent protein (GFP)(pTR-hGRK1-Cre-P2A-GFP). Control vectors (packaged in the same capsid) contained hGRK1 driving GFP. Use of the hGRK1 promoter ensures photoreceptor specificity for Cre recombinase and GFP expression 108.
Subretinal Injections
Two-month-old mice were anesthetized by 100 mg/kg Ketamine and 10 mg/kg Xylazine. A small incision in the sclera near the cornea through the skin using the tip of a sharp 30-gauge needle. An injection needle was carefully inserted into the eyeball through the incision to the subretinal space between RPE and retina. 1 μl rAAV virus (5× 1010) was slowly injected into the subretinal space. The mice were warmed up by a heat blanket until they recovered from the anesthetic before returned to their mother. ERG was performed five weeks after surgery.
Immunohistochemistry
Dissected eyecups were fixed for 15 min in 4% paraformaldehyde, cryoprotected with 30% sucrose in PBS for 2 h at room temperature, and embedded in optimal cutting temperature medium. Twelve-micron thick frozen sections were cut, mounted onto slides and blocked in PBS with 0.1% Triton X-100 and 10% donkey serum for 1 hour. They then were incubated with the primary antibody in PBS with 0.1% Triton X-100 and 2% donkey serum for at least 1 hour. After four washes with PBS with 0.1% Triton (PT), sections were incubated with fluorophore-conjugated secondary antibodies in PT for 1 h. After four washes, sections were mounted in Fluoromount (Sigma). Images were taken with Zeiss LSM 780/880 confocal microscope. Quantitative analysis of puncta immunofluorescence from confocal images was performed using Metamorph software.
Evaluation of vision by behavioral water maze task
Mouse visual behavior was assessed using a water maze task with a visible escape platform. The method for assessing visual function in this experiment is principled on a Morris water maze 109, and previous reports describing evaluation of mouse vision by a swimming-based task 110. Mice are natural swimmers, and this task exploits their innate inclination to escape from water to a solid substrate. This task uses an ability of a mouse to see a visible platform with a timed escape from water, as an index of its visual ability. Before testing, mice readily learned to swim to the visible escape-platform and performance usually plateaued at around 10 seconds within 15 trials for all treated groups. Mice that did not learn the task, e.g. performance did not improve or plateau for at least the last 3 or more consecutive trials or had any visible motor deficits were discarded from the experiment. Visually-guided behavior was tested at 100and 0.001 cd/m2 and timed-performances from 20 trials (4 sessions of 5 trials each) for each mouse at each light-intensity were averaged. Uniform room luminance settings were stably achieved by an engineered adjustable light-source and constantly monitored with a luminance meter LS-100 (Konica Minolta). To be certain that we were measuring the mice’s visual ability only and not memory, the platform was placed pseudo-randomly in the water tank and all external visual cues were eliminated.
Electroretinography (ERG)
Electroretinograms were recorded by using the UTA system and a Big-Shot Ganzfeld (LKC Technologies). Mice (6~8 weeks old) were dark-adapted (≥6 h) and prepared for recordings using a dim red light. Mice were anesthetized with an i.p. injection of ketamine and xylazine mixture containing 100 and 10 mg/kg, respectively. All procedures were approved by the Institutional Animal Care and Use Committee at the Scripps Florida Research Institute. The pupil was dilated with 10% phenylephrine hydrochloride (Akorn), followed by the application of 0.5% Tropicamide (Akorn). Recordings were performed with a gold loop electrode supplemented with contact lenses to keep the eyes immersed in solution. The reference electrode was a stainless steel needle electrode placed subcutaneously in the neck area. The mouse body temperature was maintained at 37 °C by using a heating pad controlled by ATC 1000 temperature controller (World Precision Instruments). ERG signals were sampled at 1 kHz and recorded with 0.3-Hz low-frequency and 300-Hz high-frequency cut-offs.
Full field white flashes were produced by a set of LEDs (duration < 5 ms) for flash strengths ≤2.5 cd· s/m2 or by a Xenon light source for flashes > 2.5 cd· s/m2 (flash duration < 5 ms). Given increased mortality of mice lacking Elfn1 undergoing anesthesia and experimental design pairing ERG recordings with behavioral evaluation, ERG responses were elicited only by two flashes at fixed intensities to stimulate rods only (0.001 cd*s/m2) and all photoreceptors (100 cd*s/m2). Ten trials were averaged for responses evoked by flashes 0.001 cd*s/m2, single flash responses were recorded for 100 cd*s/m2. To allow for recovery, interval times between single flashes were as follows: 5 s for 0.001 cd*s/m2. Rod-saturating light background of 32 cd/m2 was administered for recording cone-only ERGs. Ten trials elicited from 100 cd*s/m2 flashes were averaged at an interval recovery time of 1 second between flashes.
ERG traces were analyzed using the EM LKC Technologies software and Microsoft Excel. The a-wave amplitude was calculated as the value difference between the onset of light flash and the peak of the deflection wave for both dark and light-adapted ERG. The b-wave amplitude was calculated from the bottom of the a-wave response to the peak of the b-wave for both dark and light-adapted ERG.
Electron Microscopy
Eyes were enucleated, cleaned of extra-ocular tissue, and pre-fixed for 15 min in 100 mM cacodylate-buffered half-Karnovsky's fixative containing 2mM calcium chloride. Then the eyecups were hemisected along the vertical meridian and fixed overnight in the same fixative. The specimens were rinsed with cacodylate buffer and postfixed in 2% osmium tetroxide in water for 1 hour, en-block stained with 1% uranyl acetate for 25 minutes, then gradually dehydrated in an increasing ethanol and acetone series (30–100%), and embedded in Durcupan ACM resin (Sigma Aldrich). Blocks were cut with 70 nm-thickness and were stained with 3% uranyl acetate and 0.5% lead citrate. Sections were examined in a Tecnai G2 spirit BioTwin (Thermo Fisher Scientific, Waltman, MA, United States) transmission electron microscope at 100 kV accelerating voltage. Images were captured with a Veleta CCD camera (Olympus, Tokyo, Japan) operated by TIA software (Thermo Fisher Scientific, Waltman, MA, United States).
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistics were performed using GraphPad Prism 9 software. Unless otherwise noted, at least three independent samples were used for statistical comparisons. Statistical significance on bar graphs and box plots was expressed as * P<0.05, ** P<0.01, *** P<0.001, and **** P<0.0001. Not significant (n.s.) was indicated where appropriate. Data were expressed as mean ± SEM with all data points provided representing n.
Supplementary Material
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-RGS11 | Martemyanov Lab 106 | N/A |
| Rabbit anti-LRIT3 | Dr. Christina Zeits 107 |
N/A |
| Sheep anti-TRPM1 | Martemyanov Lab 96 | N/A |
| Sheep anti-mGluR6 | Martemyanov Lab 96 | N/A |
| Rabbit anti-ELFN1 (NTR) | Martemyanov Lab 68 | N/A |
| Rabbit anti-CaV1.4 | Dr. Amy Lee 73 | N/A |
| Mouse anti-CtBP2 | BD Biosciences | Cat#612044; RRID:AB_399431 |
| Rabbit anti-PSD95 | Cell Signaling Technology | Cat# 3450S; RRID:AB_2292883 |
| Mouse anti-PKCα | Abcam | Cat#ab11723; RRID:AB_298510 |
| Bacterial and virus strains | ||
| AAV8-hGRK1-Cre-P2A-GFP | This paper | N/A |
| AAV8-hGRK1-GFP | This paper | N/A |
| Critical commercial assays | ||
| Antigen Retrieval Sampler | R&D System | Cat#CTS016 |
| Experimental Models: Organisms/Strains | ||
| Mouse: Lrit3−/− | Reference 101 | N/A |
| Mouse: GPR179−/− | Reference 102 | N/A |
| Mouse: Rgs7/11−/− | Reference 97 | N/A |
| Mouse: Elfn1−/− | Reference 68 | N/A |
| Mouse: Trpm1−/− | Reference 103 | N/A |
| Mouse: Grm6−/− | Riken Bioresource Center | Cat# RBRC05552, RRID:IMSR_RBRC05552 |
| Mouse: Elfn1flx/flx | Reference 100 | N/A |
| Mouse: Elfn1CR/CR | This paper | N/A |
| Mouse: Elfn1CreERT2 | This paper | N/A |
| Mouse: Elfn1flx/CreERT2 | This paper | N/A |
| Mouse: Elfn1CR/CRPcdh21Cre | This paper | N/A |
| Mouse: LKB1−/− | Reference 70 | N/A |
| Mouse: CAST−/− | Reference 98 | N/A |
| Mouse: CAST−/− Elfn1−/− | This paper | N/A |
| Mouse: CAST−/− Grm6−/− | This paper | N/A |
| Mouse: Ai14 | The Jackson Laboratory | RRID:IMSR_JAX:007908 |
| Software and algorithms | ||
| EM LKC Technologies software | LKC Technologies | http://www.lkc.com/products/compliance-pack/ |
| Zen2.1 SP1 (Black) | Carl Zeiss | RRID:SCR_013672 |
| MetaMorph image analysis software | Molecular Devices | RRID:SCR_002368 |
| EthoVision XT | Noldus | RRID:SCR_000441 |
| GraphPad Prism9 | GraphPad Software, Inc | RRID:SCR_002798 |
Highlights.
Cell adhesion molecule ELFN1 is essential for maintenance of rod synapses
Reactivation of ELFN1 expression in adult mice allows rods to form synapses
Post-developmentally rescued rod synapses are functional
Mice gain dim light vision upon restoration of rod connectivity in adult retinas
Acknowledgements
We thank Ms. Natalia Martemyanova for performing the genetic crosses needed to obtain mice utilized in these studies. We thank Light Microscopy Core in Max Planck Florida Institute for Neuroscience for supporting image acquisition. This work was supported by NIH grants EY018139 and EY028033 (KAM), EY024280 (SEB), EY030458 and EY02798 (MAS), and by Japan Society for the Promotion of Science KAKENHI grant15H04272 (TO).
Inclusion and diversity Statement
We support inclusive, diverse, and equitable conduct of research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare no competing interests.
References
- 1.Bargmann CI & Marder E From the connectome to brain function. Nat Methods 10, 483–490 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Tau GZ & Peterson BS Normal development of brain circuits. Neuropsychopharmacology 35, 147–168, doi: 10.1038/npp.2009.115 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hooks BM & Chen C Circuitry Underlying Experience-Dependent Plasticity in the Mouse Visual System. Neuron 106, 21–36, doi: 10.1016/j.neuron.2020.01.031 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sengpiel F & Kind PC The role of activity in development of the visual system. Curr Biol 12, R818–826, doi: 10.1016/s0960-9822(02)01318-0 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Marin O Developmental timing and critical windows for the treatment of psychiatric disorders. Nat Med 22, 1229–1238, doi: 10.1038/nm.4225 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Hooks BM & Chen C Critical periods in the visual system: changing views for a model of experience-dependent plasticity. Neuron 56, 312–326, doi: 10.1016/j.neuron.2007.10.003 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Fagiolini M, Pizzorusso T, Berardi N, Domenici L & Maffei L Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res 34, 709–720, doi: 10.1016/0042-6989(94)90210-0 (1994). [DOI] [PubMed] [Google Scholar]
- 8.Gordon JA & Stryker MP Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci 16, 3274–3286 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hubel DH & Wiesel TN The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol 206, 419–436, doi: 10.1113/jphysiol.1970.sp009022 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hubel DH, Wiesel TN & LeVay S Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci 278, 377–409, doi: 10.1098/rstb.1977.0050 (1977). [DOI] [PubMed] [Google Scholar]
- 11.Issa NP, Trachtenberg JT, Chapman B, Zahs KR & Stryker MP The critical period for ocular dominance plasticity in the Ferret's visual cortex. J Neurosci 19, 6965–6978 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LeVay S, Wiesel TN & Hubel DH The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol 191, 1–51, doi: 10.1002/cne.901910102 (1980). [DOI] [PubMed] [Google Scholar]
- 13.Wiesel TN & Hubel DH Single-Cell Responses in Striate Cortex of Kittens Deprived of Vision in One Eye. J Neurophysiol 26, 1003–1017, doi: 10.1152/jn.1963.26.6.1003 (1963). [DOI] [PubMed] [Google Scholar]
- 14.Desai NS, Cudmore RH, Nelson SB & Turrigiano GG Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci 5, 783–789, doi: 10.1038/nn878 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Goel A & Lee HK Persistence of experience-induced homeostatic synaptic plasticity through adulthood in superficial layers of mouse visual cortex. J Neurosci 27, 6692–6700, doi: 10.1523/JNEUROSCI.5038-06.2007 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maffei A, Nelson SB & Turrigiano GG Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci 7, 1353–1359, doi: 10.1038/nn1351 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Liu S, Rao Y & Daw N Roles of protein kinase A and protein kinase G in synaptic plasticity in the visual cortex. Cereb Cortex 13, 864–869, doi: 10.1093/cercor/13.8.864 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Bear MF, Kleinschmidt A, Gu QA & Singer W Disruption of experience-dependent synaptic modifications in striate cortex by infusion of an NMDA receptor antagonist. J Neurosci 10, 909–925 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beaver CJ, Ji Q, Fischer QS & Daw NW Cyclic AMP-dependent protein kinase mediates ocular dominance shifts in cat visual cortex. Nat Neurosci 4, 159–163, doi: 10.1038/83985 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Rao Y et al. Reduced ocular dominance plasticity and long-term potentiation in the developing visual cortex of protein kinase A RII alpha mutant mice. Eur J Neurosci 20, 837–842, doi: 10.1111/j.1460-9568.2004.03499.x (2004). [DOI] [PubMed] [Google Scholar]
- 21.Maffei A, Nataraj K, Nelson SB & Turrigiano GG Potentiation of cortical inhibition by visual deprivation. Nature 443, 81–84, doi: 10.1038/nature05079 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Tropea D et al. Gene expression changes and molecular pathways mediating activity-dependent plasticity in visual cortex. Nat Neurosci 9, 660–668, doi: 10.1038/nn1689 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Majdan M & Shatz CJ Effects of visual experience on activity-dependent gene regulation in cortex. Nat Neurosci 9, 650–659, doi: 10.1038/nn1674 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Millan MJ et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov 15, 485–515, doi: 10.1038/nrd.2016.28 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Kalia A et al. Development of pattern vision following early and extended blindness. Proc Natl Acad Sci U S A 111, 2035–2039, doi: 10.1073/pnas.1311041111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veenstra-VanderWeele J & Warren Z Intervention in the context of development: pathways toward new treatments. Neuropsychopharmacology 40, 225–237, doi: 10.1038/npp.2014.232 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hubener M & Bonhoeffer T Neuronal plasticity: beyond the critical period. Cell 159, 727–737, doi: 10.1016/j.cell.2014.10.035 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Prasad S & Galetta SL Anatomy and physiology of the afferent visual system. Handb Clin Neurol 102, 3–19, doi: 10.1016/B978-0-444-52903-9.00007-8 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Bence M & Levelt CN Structural plasticity in the developing visual system. Prog Brain Res 147, 125–139, doi: 10.1016/S0079-6123(04)47010-1 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Burns ME & Arshavsky VY Beyond counting photons: trials and trends in vertebrate visual transduction. Neuron 48, 387–401 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Burger CA, Jiang D, Mackin RD & Samuel MA Development and maintenance of vision's first synapse. Dev Biol 476, 218–239, doi: 10.1016/j.ydbio.2021.04.001 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gregg RG, Ray TA, Hasan N, McCall MA & Peachey NS in G Protein Signaling Mechanisms in the Retina (eds Martemyanov Kirill A. & Sampath Alapakkam P.) 67–79 (Springer; New York, 2014). [Google Scholar]
- 33.Vardi N & Dhingra A in G Protein Signaling Mechanisms in the Retina (eds Martemyanov Kirill A. & Sampath Alapakkam P.) 81–97 (Springer; New York, 2014). [Google Scholar]
- 34.Schneeweis DM & Schnapf JL Photovoltage of rods and cones in the macaque retina. Science 268, 1053–1056, doi: 10.1126/science.7754386 (1995). [DOI] [PubMed] [Google Scholar]
- 35.Tamura T, Nakatani K & Yau KW Calcium feedback and sensitivity regulation in primate rods. J Gen Physiol 98, 95–130, doi: 10.1085/jgp.98.1.95 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rieke F & Baylor DA Origin of reproducibility in the responses of retinal rods to single photons. Biophys J 75, 1836–1857, doi: 10.1016/S0006-3495(98)77625-8 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rieke F Mechanisms of single-photon detection in rod photoreceptors. Methods Enzymol 316, 186–202, doi: 10.1016/s0076-6879(00)16724-2 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Bloomfield SA & Dacheux RF Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res 20, 351–384, doi: 10.1016/s1350-9462(00)00031-8 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Sharpe LT & Stockman A Rod pathways: the importance of seeing nothing. Trends Neurosci 22, 497–504 (1999). [DOI] [PubMed] [Google Scholar]
- 40.Dacheux RF & Raviola E The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci 6, 331–345 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor WR & Smith RG Transmission of scotopic signals from the rod to rod-bipolar cell in the mammalian retina. Vision Res 44, 3269–3276 (2004). [DOI] [PubMed] [Google Scholar]
- 42.DeVries SH & Baylor DA Synaptic circuitry of the retina and olfactory bulb. Cell 72 Suppl, 139–149, doi: 10.1016/s0092-8674(05)80033-9 (1993). [DOI] [PubMed] [Google Scholar]
- 43.Zeitz C, Robson AG & Audo I Congenital stationary night blindness: an analysis and update of genotype-phenotype correlations and pathogenic mechanisms. Progress in retinal and eye research 45, 58–110, doi: 10.1016/j.preteyeres.2014.09.001 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Gal A, Orth U, Baehr W, Schwinger E & Rosenberg T Heterozygous missense mutation in the rod cGMP phosphodiesterase beta-subunit gene in autosomal dominant stationary night blindness. Nat Genet 7, 64–68, doi: 10.1038/ng0594-64 (1994). [DOI] [PubMed] [Google Scholar]
- 45.Bech-Hansen NT et al. Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet 19, 264–267, doi: 10.1038/947 (1998). [DOI] [PubMed] [Google Scholar]
- 46.Strom TM et al. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat Genet 19, 260–263, doi: 10.1038/940 (1998). [DOI] [PubMed] [Google Scholar]
- 47.Bech-Hansen NT et al. Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat Genet 26, 319–323, doi: 10.1038/81619 (2000). [DOI] [PubMed] [Google Scholar]
- 48.Audo I et al. Whole-exome sequencing identifies mutations in GPR179 leading to autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet 90, 321–330, doi: 10.1016/j.ajhg.2011.12.007 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Audo I et al. TRPM1 is mutated in patients with autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet 85, 720–729, doi: 10.1016/j.ajhg.2009.10.013 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bijveld MM et al. Genotype and phenotype of 101 dutch patients with congenital stationary night blindness. Ophthalmology 120, 2072–2081, doi: 10.1016/j.ophtha.2013.03.002 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Devi S et al. Metabotropic glutamate receptor 6 signaling enhances TRPM1 calcium channel function and increases melanin content in human melanocytes. Pigment Cell Melanoma Res 26, 348–356, doi: 10.1111/pcmr.12083 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dryja TP et al. Night blindness and abnormal cone electroretinogram ON responses in patients with mutations in the GRM6 gene encoding mGluR6. Proc Natl Acad Sci U S A 102, 4884–4889, doi:0501233102 [pii] 10.1073/pnas.0501233102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacobi FK et al. Phenotypic expression of the complete type of X-linked congenital stationary night blindness in patients with different mutations in the NYX gene. Graefes Arch Clin Exp Ophthalmol 240, 822–828, doi: 10.1007/s00417-002-0562-z (2002). [DOI] [PubMed] [Google Scholar]
- 54.Leroy BP et al. A common NYX mutation in Flemish patients with X linked CSNB. Br J Ophthalmol 93, 692–696, doi:bjo.2008.143727 [pii] 10.1136/bjo.2008.143727 (2009). [DOI] [PubMed] [Google Scholar]
- 55.Li Z et al. Recessive mutations of the gene TRPM1 abrogate ON bipolar cell function and cause complete congenital stationary night blindness in humans. Am J Hum Genet 85, 711–719, doi: 10.1016/j.ajhg.2009.10.003 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Connor E et al. Congenital stationary night blindness associated with mutations in GRM6 encoding glutamate receptor MGluR6. Br J Ophthalmol 90, 653–654, doi:90/5/653 [pii] 10.1136/bjo.2005.086678 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pusch CM et al. The complete form of X-linked congenital stationary night blindness is caused by mutations in a gene encoding a leucine-rich repeat protein. Nat Genet 26, 324–327, doi: 10.1038/81627 (2000). [DOI] [PubMed] [Google Scholar]
- 58.Scholl HP et al. Slow and fast rod ERG pathways in patients with X-linked complete stationary night blindness carrying mutations in the NYX gene. Invest Ophthalmol Vis Sci 42, 2728–2736 (2001). [PubMed] [Google Scholar]
- 59.Sergouniotis PI et al. Retinal structure, function, and molecular pathologic features in gyrate atrophy. Ophthalmology 119, 596–605, doi: 10.1016/j.ophtha.2011.09.017 (2012). [DOI] [PubMed] [Google Scholar]
- 60.van Genderen MM et al. Mutations in TRPM1 are a common cause of complete congenital stationary night blindness. Am J Hum Genet 85, 730–736, doi: 10.1016/j.ajhg.2009.10.012 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu X et al. Sequence variations of GRM6 in patients with high myopia. Molecular vision 15, 2094–2100 (2009). [PMC free article] [PubMed] [Google Scholar]
- 62.Zeitz C et al. Mutations in GRM6 cause autosomal recessive congenital stationary night blindness with a distinctive scotopic 15-Hz flicker electroretinogram. Invest Ophthalmol Vis Sci 46, 4328–4335, doi: 10.1167/iovs.05-0526 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Zeitz C et al. Novel mutations in CACNA1F and NYX in Dutch families with X-linked congenital stationary night blindness. Mol Vis 11, 179–183 (2005). [PubMed] [Google Scholar]
- 64.Zeitz C et al. Whole-exome sequencing identifies LRIT3 mutations as a cause of autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet 92, 67–75, doi: 10.1016/j.ajhg.2012.10.023 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harwerth RS, Smith EL 3rd, Crawford ML & von Noorden GK Behavioral studies of the sensitive periods of development of visual functions in monkeys. Behav Brain Res 41, 179–198, doi: 10.1016/0166-4328(90)90107-p (1990). [DOI] [PubMed] [Google Scholar]
- 66.Emerson VF, Chalupa LM, Thompson ID & Talbot RJ Behavioural, physiological, and anatomical consequences of monocular deprivation in the golden hamster (Mesocricetus auratus). Exp Brain Res 45, 168–178, doi: 10.1007/BF00235776 (1982). [DOI] [PubMed] [Google Scholar]
- 67.Scheiman MM et al. Randomized trial of treatment of amblyopia in children aged 7 to 17 years. Arch Ophthalmol 123, 437–447, doi: 10.1001/archopht.123.4.437 (2005). [DOI] [PubMed] [Google Scholar]
- 68.Cao Y et al. Mechanism for Selective Synaptic Wiring of Rod Photoreceptors into the Retinal Circuitry and Its Role in Vision. Neuron 87, 1248–1260, doi: 10.1016/j.neuron.2015.09.002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.tom Dieck S et al. Deletion of the presynaptic scaffold CAST reduces active zone size in rod photoreceptors and impairs visual processing. J Neurosci 32, 12192–12203, doi: 10.1523/JNEUROSCI.0752-12.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Samuel MA et al. LKB1 and AMPK regulate synaptic remodeling in old age. Nat Neurosci 17, 1190–1197, doi: 10.1038/nn.3772 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zabouri N & Haverkamp S Calcium channel-dependent molecular maturation of photoreceptor synapses. PLoS One 8, e63853, doi: 10.1371/journal.pone.0063853 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mansergh F et al. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum Mol Genet 14, 3035–3046, doi: 10.1093/hmg/ddi336 (2005). [DOI] [PubMed] [Google Scholar]
- 73.Liu X et al. Dysregulation of Ca(v)1.4 channels disrupts the maturation of photoreceptor synaptic ribbons in congenital stationary night blindness type 2. Channels 7, 514–523, doi: 10.4161/chan.26376 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Orlandi C et al. Transsynaptic Binding of Orphan Receptor GPR179 to Dystroglycan-Pikachurin Complex Is Essential for the Synaptic Organization of Photoreceptors. Cell Rep 25, 130–145 e135, doi: 10.1016/j.celrep.2018.08.068 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tian N & Copenhagen DR Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron 39, 85–96, doi: 10.1016/s0896-6273(03)00389-1 (2003). [DOI] [PubMed] [Google Scholar]
- 76.Di Marco S, Nguyen VA, Bisti S & Protti DA Permanent functional reorganization of retinal circuits induced by early long-term visual deprivation. J Neurosci 29, 13691–13701, doi: 10.1523/JNEUROSCI.3854-09.2009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dunn FA, Della Santina L, Parker ED & Wong RO Sensory experience shapes the development of the visual system's first synapse. Neuron 80, 1159–1166, doi: 10.1016/j.neuron.2013.09.024 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bodnarenko SR & Chalupa LM Stratification of ON and OFF ganglion cell dendrites depends on glutamate-mediated afferent activity in the developing retina. Nature 364, 144–146, doi: 10.1038/364144a0 (1993). [DOI] [PubMed] [Google Scholar]
- 79.Zhang C, Kolodkin AL, Wong RO & James RE Establishing Wiring Specificity in Visual System Circuits: From the Retina to the Brain. Annu Rev Neurosci 40, 395–424, doi: 10.1146/annurev-neuro-072116-031607 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Hasan N et al. Presynaptic Expression of LRIT3 Transsynaptically Organizes the Postsynaptic Glutamate Signaling Complex Containing TRPM1. Cell Rep 27, 3107–3116 e3103, doi: 10.1016/j.celrep.2019.05.056 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Varin J et al. Substantial restoration of night vision in adult mice with congenital stationary night blindness. Mol Ther Methods Clin Dev 22, 15–25, doi: 10.1016/j.omtm.2021.05.008 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miyadera K et al. Targeting ON-bipolar cells by AAV gene therapy stably reverses LRIT3-congenital stationary night blindness. Proc Natl Acad Sci U S A 119, e2117038119, doi: 10.1073/pnas.2117038119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scalabrino ML et al. Intravitreal delivery of a novel AAV vector targets ON bipolar cells and restores visual function in a mouse model of complete congenital stationary night blindness. Hum Mol Genet 24, 6229–6239, doi: 10.1093/hmg/ddv341 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morgan JL, Dhingra A, Vardi N & Wong RO Axons and dendrites originate from neuroepithelial-like processes of retinal bipolar cells. Nat Neurosci 9, 85–92 (2006). [DOI] [PubMed] [Google Scholar]
- 85.Reese BE Developmental plasticity of photoreceptors. Progress in brain research 144, 3–19, doi: 10.1016/s0079-6123(03)14401-9 (2004). [DOI] [PubMed] [Google Scholar]
- 86.Yamagata M & Sanes JR Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature 451, 465–469, doi: 10.1038/nature06469 (2008). [DOI] [PubMed] [Google Scholar]
- 87.Yamagata M, Weiner JA & Sanes JR Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell 110, 649–660, doi: 10.1016/s0092-8674(02)00910-8 (2002). [DOI] [PubMed] [Google Scholar]
- 88.Xiao T et al. Assembly of lamina-specific neuronal connections by slit bound to type IV collagen. Cell 146, 164–176, doi: 10.1016/j.cell.2011.06.016 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matsuoka RL et al. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature 470, 259–263, doi: 10.1038/nature09675 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suto F et al. Interactions between plexin-A2, plexin-A4, and semaphorin 6A control lamina-restricted projection of hippocampal mossy fibers. Neuron 53, 535–547, doi: 10.1016/j.neuron.2007.01.028 (2007). [DOI] [PubMed] [Google Scholar]
- 91.Suto F et al. Plexin-a4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance. J Neurosci 25, 3628–3637, doi: 10.1523/JNEUROSCI.4480-04.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fuerst PG, Koizumi A, Masland RH & Burgess RW Neurite arborization and mosaic spacing in the mouse retina require DSCAM. Nature 451, 470–474, doi: 10.1038/nature06514 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fuerst PG et al. DSCAM and DSCAML1 function in self-avoidance in multiple cell types in the developing mouse retina. Neuron 64, 484–497, doi: 10.1016/j.neuron.2009.09.027 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kay JN, Chu MW & Sanes JR MEGF10 and MEGF11 mediate homotypic interactions required for mosaic spacing of retinal neurons. Nature 483, 465–469, doi: 10.1038/nature10877 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ray TA et al. Formation of retinal direction-selective circuitry initiated by starburst amacrine cell homotypic contact. Elife 7, doi: 10.7554/eLife.34241 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cao Y, Posokhova E & Martemyanov KA TRPM1 forms complexes with nyctalopin in vivo and accumulates in postsynaptic compartment of ON-bipolar neurons in mGluR6-dependent manner. J Neurosci 31, 11521–11526, doi: 10.1523/JNEUROSCI.1682-11.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cao Y et al. Regulators of G protein signaling RGS7 and RGS11 determine the onset of the light response in ON bipolar neurons. Proc Natl Acad Sci U S A 109, 7905–7910, doi: 10.1073/pnas.1202332109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hagiwara A et al. Cytomatrix proteins CAST and ELKS regulate retinal photoreceptor development and maintenance. J Cell Biol 217, 3993–4006, doi: 10.1083/jcb.201704076 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peachey NS et al. GPR179 is required for depolarizing bipolar cell function and is mutated in autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet 90, 331–339, doi: 10.1016/j.ajhg.2011.12.006 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cao Y et al. Interplay between cell-adhesion molecules governs synaptic wiring of cone photoreceptors. Proc Natl Acad Sci U S A 117, 23914–23924, doi: 10.1073/pnas.2009940117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Neuille M et al. Lrit3 deficient mouse (nob6): a novel model of complete congenital stationary night blindness (cCSNB). PLoS One 9, e90342, doi: 10.1371/journal.pone.0090342 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peachey NS et al. GPR179 Is Required for Depolarizing Bipolar Cell Function and Is Mutated in Autosomal-Recessive Complete Congenital Stationary Night Blindness. Am J Hum Genet 90, 331–339, doi: 10.1016/j.ajhg.2011.12.006 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shen Y et al. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci 29, 6088–6093, doi:29/19/6088 [pii] 10.1523/JNEUROSCI.0132-09.2009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rattner A et al. A photoreceptor-specific cadherin is essential for the structural integrity of the outer segment and for photoreceptor survival. Neuron 32, 775–786, doi: 10.1016/s0896-6273(01)00531-1 (2001). [DOI] [PubMed] [Google Scholar]
- 105.Boland MJ et al. Adult mice generated from induced pluripotent stem cells. Nature 461, 91–94, doi: 10.1038/nature08310 (2009). [DOI] [PubMed] [Google Scholar]
- 106.Cao Y et al. Retina-specific GTPase accelerator RGS11/G beta 5S/R9AP is a constitutive heterotrimer selectively targeted to mGluR6 in ON-bipolar neurons. J Neurosci 29, 9301–9313, doi:29/29/9301 [pii] 10.1523/JNEUROSCI.1367-09.2009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Neuille M et al. LRIT3 is essential to localize TRPM1 to the dendritic tips of depolarizing bipolar cells and may play a role in cone synapse formation. Eur J Neurosci 42, 1966–1975, doi: 10.1111/ejn.12959 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alexander JJ et al. Restoration of cone vision in a mouse model of achromatopsia. Nat Med 13, 685–687, doi: 10.1038/nm1596 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Morris R Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11, 47–60 (1984). [DOI] [PubMed] [Google Scholar]
- 110.Prusky GT, West PW & Douglas RM Behavioral assessment of visual acuity in mice and rats. Vision Res 40, 2201–2209 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.