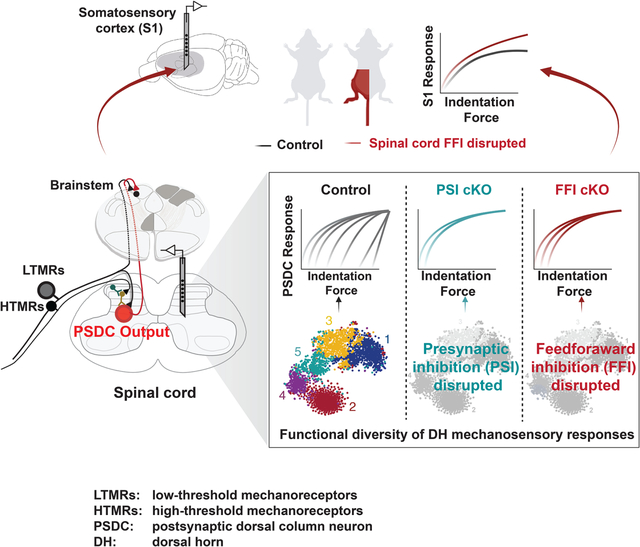
Summary
The encoding of touch in the spinal cord dorsal horn (DH) and its influence on tactile representations in the brain are poorly understood. Using a range of mechanical stimuli applied to the skin, large scale in vivo electrophysiological recordings, and genetic manipulations, here we show that neurons in the mouse spinal cord DH receive convergent inputs from both low- and high-threshold mechanoreceptor subtypes and exhibit one of six functionally distinct mechanical response profiles. Genetic disruption of DH feedforward or feedback inhibitory motifs, comprised of interneurons with distinct mechanical response profiles, revealed an extensively interconnected DH network that enables dynamic, flexible tuning of postsynaptic dorsal column (PSDC) output neurons and dictates how neurons in primary somatosensory cortex respond to touch. Thus, mechanoreceptor subtype convergence and nonlinear transformations at the earliest stage of the somatosensory hierarchy shape how touch of the skin is represented in the brain.
Graphical Abstract
In Brief
At the earliest stage of the somatosensory pathway, the spinal cord dorsal horn neurons exhibit functionally diversity where they receive and integrate convergent inputs from non-noxious touch stimuli; the interconnected network and local dynamics at dorsal horn shape output neuron diversity and tactile representations in the somatosensory cortex.
Introduction
Fundamental to understanding the neurobiological basis of touch sensation is knowing how signals from primary mechanosensory neurons are combined and transformed as they ascend the somatosensory neuraxis to encode salient features of mechanical stimuli.
The mechanosensory neurons associated with glabrous (non-hairy) skin include Aβ rapidly adapting low-threshold mechanoreceptors (LTMRs) that innervate either Meissner or Pacinian corpuscles (Aβ RA1- and Aβ RA2-LTMRs, respectively), Aβ slowly adapting (SA)-LTMRs that associate with Merkel cells, and A- and C-fiber high-threshold mechanoreceptor (HTMR) subtypes1–6 that form free nerve endings in the skin. In response to sustained skin indentation, Aβ RA-LTMRs fire at the stimulus onset and offset, whereas Aβ SA-LTMRs fire during the onset and sustained phase but lack an OFF response5–8. While Aβ LTMRs can entrain to mechanical vibrations, the activation thresholds of Aβ RA-LTMRs are uniquely frequency dependent: Aβ RA1-LTMRs optimally encode mechanical vibrations in the ‘flutter’ range (20–100Hz) and Aβ RA2-LTMRs encode high-frequency vibrations, from 100 to over 500Hz5,6,9,10. In comparison, A- and C-fiber HTMRs require high forces for activation and are typically slowly adapting3,11–13. Thus, tactile stimuli can activate distinct combinations of mechanoreceptor subtypes to produce unique ensembles of impulses propagating from the skin to engage the central touch circuitry. Mechanosensory pathways conveying touch signals form the periphery to the brain include: 1) the “direct dorsal column pathway”, which carries Aβ LTMR signals from the skin directly to the brainstem dorsal column nuclei (DCN) where second order neurons project to the somatosensory thalamus and other higher brain regions; 2) the “indirect dorsal column pathway”, which conveys mechanosensory signals from the spinal cord dorsal horn (DH) to the DCN via post-synaptic dorsal column (PSDC) neurons; and 3) the anterolateral pathway, which transmits signals from the spinal cord to the somatosensory thalamus, lateral parabrachial nucleus, and other brain regions that process nociceptive and affective aspects of somatosensation.
How and where in the CNS are signals from peripheral mechanoreceptor subtypes combined to generate central representations of touch? Recent studies indicate that tactile responses in somatosensory cortex (S1), thalamus, and DCN reflect subcortical convergence of Aβ LTMR subtype signals14–16. Thus, the emerging view is that signals from physiologically diverse mechanosensory neuron types converge early in the somatosensory hierarchy to generate complex tactile feature representations. Indeed, the spinal cord DH may serve as the initial locus of mechanoreceptor subtype signal integration. In support of this idea, the majority of Aβ LTMR synapses are localized to the DH, with relatively few residing in the DCN1,17–24. Moreover, most if not all other cutaneous somatosensory neuron types, including hairy skin-innervating C-LTMRs, Aδ-LTMRs, and HTMRs synapse exclusively in the DH25–28. Therefore, most mechanoreceptor synapses reside within the DH and not the DCN. In addition, recent anatomical and in vitro electrophysiological analyses point to crosstalk of peripheral mechanosensory channels within the DH20,25,26. Moreover, classic studies in the cat indicate that output neurons of the deep DH, the PSDC projection neurons, are exquisitely mechanically sensitive, have large, complex receptive fields (RFs), and can exhibit RF plasticity29–33. It is also noteworthy that the DH is predominantly comprised of locally projecting, morphologically and physiologically diverse inhibitory and excitatory interneuron types primed to support sensory input computations18,20,34–38. Despite this, the in vivo response properties of DH neuron types, the nature and extent of mechanoreceptor subtype convergence within the DH, and the contributions of DH mechanosensory coding to tactile representations at higher levels of the somatosensory hierarchy are largely unknown.
Here, we found that the DH has a highly interconnected network architecture, receiving extensively convergent LTMR and HTMR signals and transforming them into a diverse range of PSDC output channels. Thus, the DH flexibly shapes PSDC signals that ascend via the indirect dorsal column pathway to the DCN where they combine with unmodified Aβ LTMR signals of the direct dorsal column pathway to dictate how touch of the body is represented in the brain.
Results
Functional diversity of mechanosensory responses in the DH
A number of recent studies have begun to define the molecular, cellular, and synaptic architecture of the spinal cord DH using transcriptomic profiling and electrophysiological and morphological approaches20,34,37,39–46. We sought to complement and extend these prior analyses using large scale in vivo electrophysiology to address: (1) the in vivo mechanical response properties of DH neurons, including genetically defined interneurons and PSDC output neurons; (2) the contributions of LTMR subtypes, HTMRs, and local circuit motifs in shaping these responses; and (3) the contribution of DH mechanosensory processing to responses S1 and somatosensory behaviors. We developed a preparation for in vivo spinal cord multielectrode array (MEA) electrophysiology and recorded simultaneously from dozens of lumbar DH neurons while delivering well-controlled mechanical stimuli to the plantar hindpaw surface of urethane anesthetized mice (Figures 1A, S1B–C). Step indentations spanning mechanical activation thresholds of both LTMRs and HTMRs were used to assess force intensity tuning of DH neurons residing within spinal cord laminae I-V and allow for direct comparisons to responses of primary afferent mechanoreceptors (Figure S1A, D). Indentations within this force range did not evoke a nociceptive behavioral response in awake mice15. For spatial RF measurements, the same step indentations were applied to different locations across the paw. In addition, vibratory stimuli of varying force amplitudes and frequencies were delivered to the RF center. A total of 5060 single unit recordings from 142 mice were included in initial stages of this analysis (Figures 1C, S1E).
Figure 1. Functional diversity of mechanosensory responses in DH neurons.
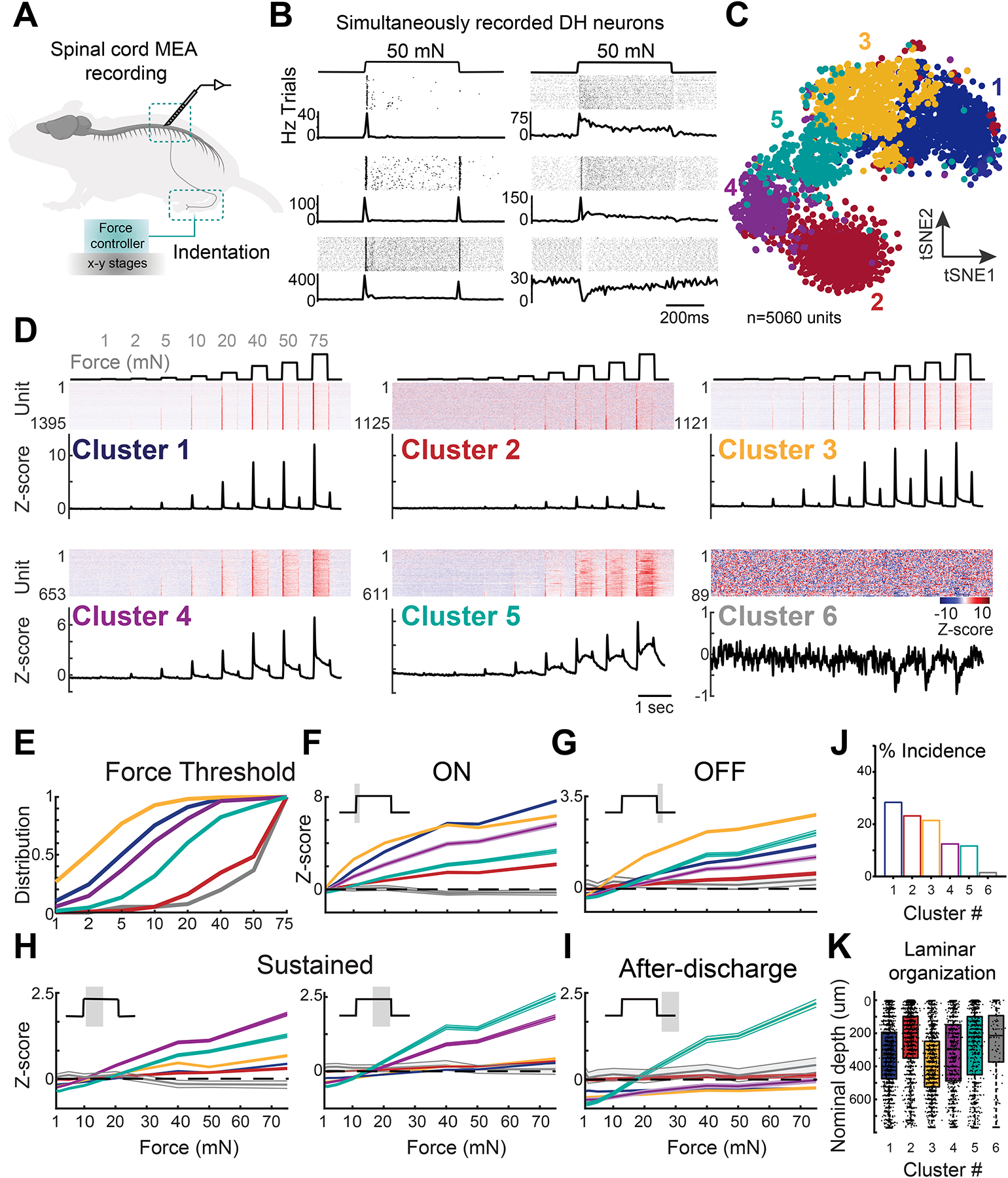
A. Experimental configuration.
B. Example raster plots and PSTHs from simultaneously recorded DH neurons during 50 mN step indentations. Top: spike raster; bottom: average PSTH.
C. Functional clusters shown in tSNE.
D. Z-scored firing rates for DH units corresponding to 6 principal functional classes. Top: force traces aligned to the heatmaps of Z-scored firing rates for each functional group. Units are sorted by sensitivity to step indentations. Bottom: average PSTHs for each functional group.
E. Cumulative distribution of response thresholds across functional groups.
F-I. Mean baseline-subtracted firing rates (±SEM) for DH units in each functional group at step indentation onset (ON, 10–50 ms after step onset), offset (OFF; 10–50ms after step offset); sustained (50–250 and 250–500 ms after step onset) and after-discharge (50–250 ms after step offset). See Table S1 for statistical analyses.
J. Percentage incidence for each functional group.
K. Summary boxplots of laminar location for DH units in each functional group.
Remarkably, the vast majority of lumbar DH neurons recorded (92%) responded to innocuous force steps with a broad range of functional response profiles (Figure 1B). We used unsupervised clustering to assess the extent of this functional diversity (Figure 1C, S1E). This analysis divided DH units into six principal functional groups distinguished by their responses across the full range of indentation intensities (Figure 1D–I) and abundance (Figure 1J). A similar fraction of broad response types was consistent across experiments (Figure S1F).
DH neurons across the six functional clusters differed in their sensitivity to step indentations, with sensitivities and sustained responses tiling mechanical force space (Figure 1E, H). DH neurons in cluster 3 were the most sensitive, with 30% responding to 1mN, the lowest force amplitude tested (Figure 1D, E). Neurons in clusters 2 and 6 were the least sensitive, exhibiting minimal responses at lower forces. Cluster 6 harbors neurons uniquely inhibited by high intensity force steps. DH functional clusters were also distinguished by their spiking patterns at the onset (ON), offset (OFF), and sustained portion of step indentations (Figure 1D, F–I). Units in clusters 1 and 2 exhibited transient response profiles, with robust spiking during the ON phase and little to no response during the sustained portion of the step (Figure 1D, F–H). Cluster 1 neurons often lacked an OFF response (Figure 1D, G). Neurons in clusters 3, 4 and 5, on the other hand, displayed prominent sustained responses that were most pronounced at higher forces (Figure 1D, H). DH functional types also differed in their spontaneous firing rates (Figure S1H). Surprisingly, neurons within different functional groups were broadly distributed across laminar depth (Figure 1K), although insensitive units tended to reside more superficially, and more sensitive units tended to reside deeper in the DH (Figures 1K, S1I).
RF analysis revealed that RF areas varied across DH functional cluster types and were exclusively excitatory with no inhibitory surround (Figure 2A–C; S2A–C). Additionally, the most sensitive DH neurons tended to have the largest RFs. Indeed, while units in cluster 3, the most sensitive cluster, exhibited the largest RFs at 10mN (median 14.75mm2), units in cluster 2, with some of the highest activation thresholds, had the smallest RFs (median 2.34 mm2; Figure 2B, C). It is notable that RFs for virtually all DH units in the dataset and representing all excitatory functional clusters are considerably larger than individual Aβ RA-LTMR and Aβ SA-LTMR RFs, even at the lowest forces used, suggesting a high degree of convergence of primary mechanosensory neuron signals onto individual DH neurons.
Figure 2. Receptive fields and vibration tuning of DH neuron functional types.
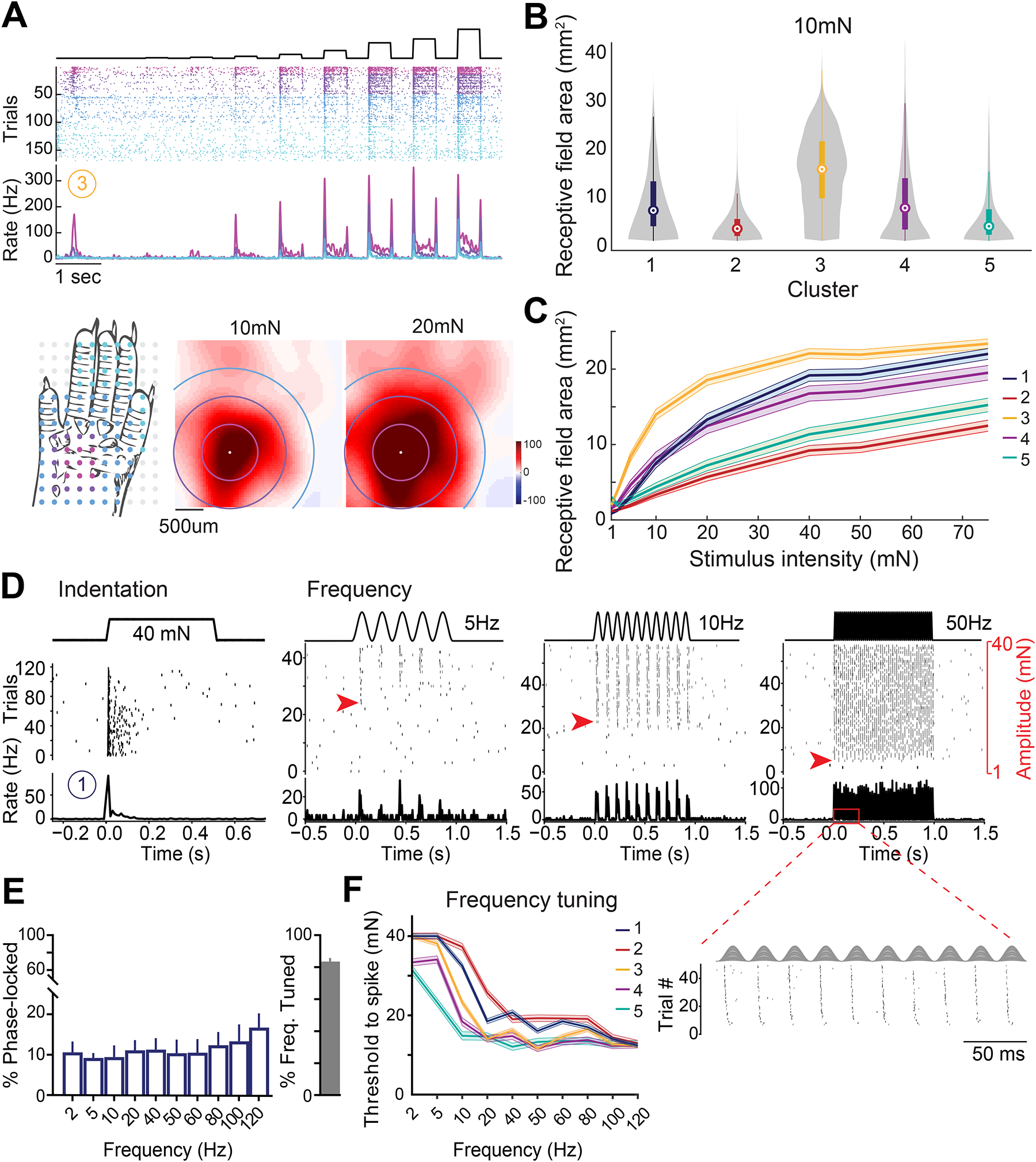
A. Example DH receptive field organization. Top: spike raster and PSTH from a representative DH unit assigned to cluster 3. Color-coded trials and corresponding PSTHs show responses to indentations applied to different locations across the paw. Bottom: stimulus locations superimposed on a hindpaw schematic (left), and RF area at 10 and 20mN step indentations. Colored circles correspond to trials and PSTH in the raster plot.
B. Excitatory RF areas at 10mN for units across five functional groups.
C. Average RF areas across force intensities. Shaded areas: SEM.
D. Example DH neuron with phase-locking and vibration tuning assigned to functional cluster 1. Red arrows: force activation threshold decreasing with increasing vibration frequency. Inset: evoked spikes phase-locked to the periodic cycle of the 50Hz vibration.
E. Left: percentage of phase-locked DH units across vibratory frequencies; Right: percentage of frequency-tuned DH neurons (83.9±2.3 %). Bars: mean ± SEM.
F. Frequency tuning curves across DH functional clusters, plotted as median ±SEM.
See Table S1 for statistical analyses. See also Figure S2; Table S1.
We also investigated how DH neurons encode mechanical vibrations (Figure 2D–F). Only a small subset of mechanically sensitive DH neurons (~15%) entrained their firing to vibrations up to 120Hz (Figures 2D, E; S2E). In contrast, the sensitivity of most DH neurons across all clusters increased (i.e., force thresholds decreased) as the frequency of vibration increased, to 120Hz (Figures 2D, E, F; S2D). These findings suggest that most DH neurons receive convergent input, either directly or indirectly, from Meissner corpuscle Aβ RA1-LTMRs and Pacinian corpuscle Aβ RA2-LTMRs, most sensitive to 20–100 Hz and 100–500 Hz vibratory stimuli, respectively6.
Genetically defined DH neuron subtypes map onto functionally defined clusters.
To determine whether the properties of known genetically defined DH interneurons align with particular functional clusters, we combined in vivo MEA recordings with optical tagging of genetically defined DH interneuron types (Example shown in Figure 3A–C). Thus, we selectively recorded from (1) broad DH neuron populations subdivided based on neurotransmitter identity and (2) a sampling of previously described morphologically and physiologically distinct excitatory and inhibitory DH interneuron types (Figure 3D–J). The in vivo response properties and RFs of genetically labeled DH interneurons were then compared to those of DH functional types defined by unbiased clustering.
Figure 3. Genetically defined interneurons map onto DH neuron functional types.
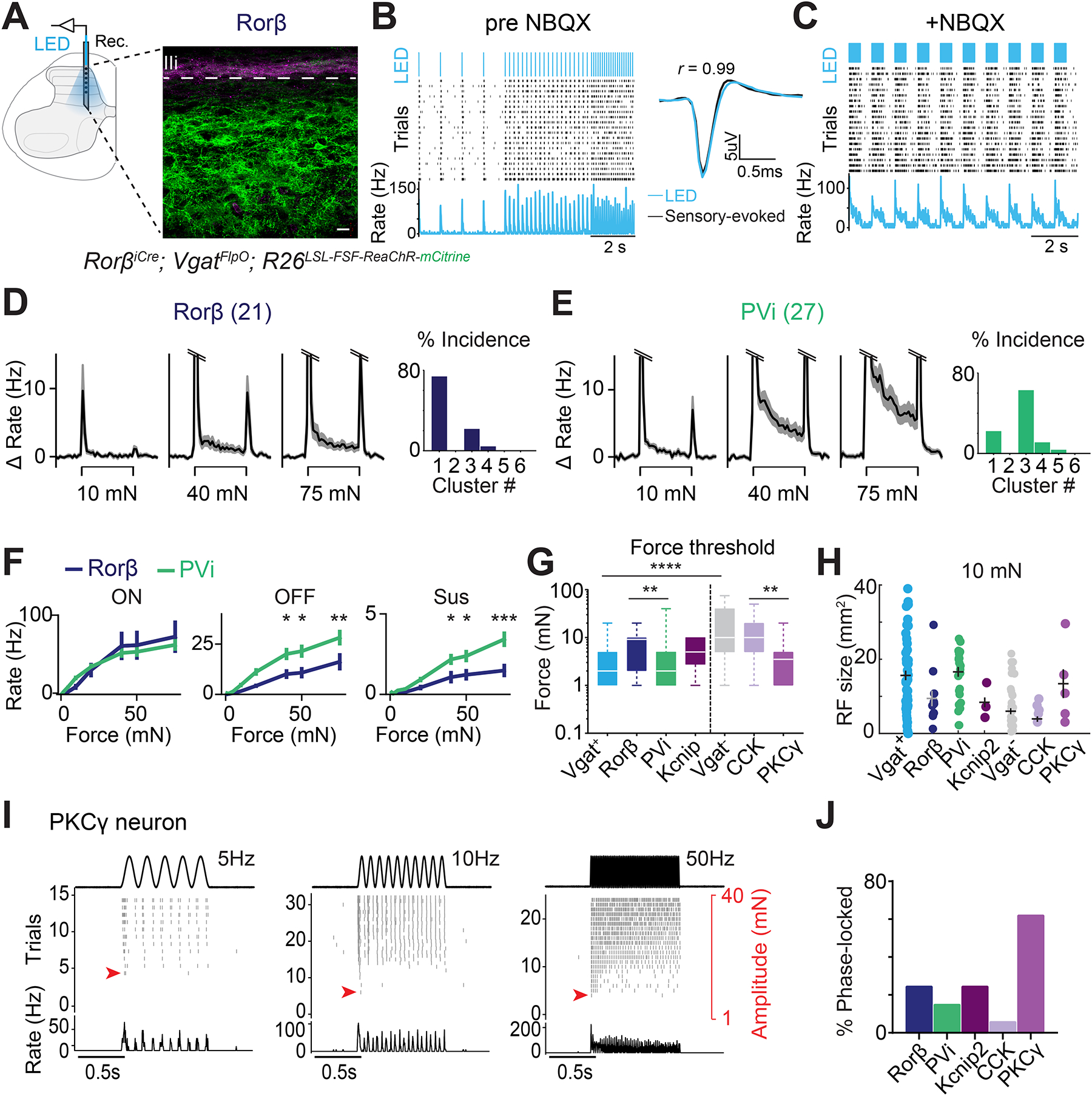
A. Left: in vivo experimental set-up. Right: sagittal spinal cord section at the recording site shown in the schematic (left) from a RorβiCre; VgatFlpO; R26LSL-FSF-ReaChR-mCitrine mouse (green, ReaChR; purple, IB4). Scale bar: 40 μm.
B. Optotagging protocol. Left: light-evoked spike raster and PSTH for an example optotagged Rorβ unit; Right: average indentation- and light-evoked waveforms. Tagged neurons display high correlation between light-evoked and sensory-evoked waveforms.
C. Light-evoked spike raster and PSTH after NBQX (5mM) was applied to the surface of the cord at the end the recording.
D. Indentation-evoked response profiles (mean firing rate ± SEM) of optotagged Rorβ neurons (n=21 units; 5 mice). The ON response is truncated at 15Hz. Right: cluster assignment for tagged Rorβ units.
E. As in (D), but for tagged PVi units (PV2aCre; VgatFlpO; R26LSL-FSF-ReaChR-mCitrine; n=27; 4 mice).
F. Mean baseline-subtracted firing rates (± SEM) for tagged Rorβ and PVi neuron at the onset (ON), offset (OFF), and sustained (Sus) portion of the step indentation. Two-way ANOVA with post hoc Sidak’s test; ON: (F [1, 328] = 0.009453, p = 0.9226); OFF: (F [1, 328] = 22.34, p <0.0001); Sus: (F [1, 328] = 22.14, p <0.0001): ***p < <0.0001, **p < 0.001, *p < 0.05.
G. Response thresholds for optotagged DH neurons. Dashed line separates inhibitory and excitatory interneurons. H= 17.63; P <0.005, Kruskal-Wallis H test with post hoc Dunn’s test. ****p < 0.0001, *p < 0.05.
H. RF areas for optotagged DH neurons at 10mN force steps.
I. Example of a phase-locked PKCγ unit, shown at selected frequencies.
J. Percentage phase-locked units for optotagged DH interneurons.
See also Figure S3.
To compare broadly across inhibitory and excitatory populations, both GABAergic and glycinergic interneurons were optotagged using VgatiCre; R26LSL-ChR2 mice (Figures S3E, F). Blocking excitatory transmission in the spinal cord to suppress recurrent excitation at the end of optotagging experiments allowed for unambiguous identification of tagged units (Figure 3A–C; S3A–D). We found that DH inhibitory interneurons were more sensitive and produced more sustained responses at higher indentation forces than simultaneously recorded putative excitatory DH units (Figures 3G, S3E, F). Inhibitory interneurons also exhibited larger RFs than their excitatory interneuron counterparts (Figure 3H). Despite these differences, there was substantial heterogeneity among inhibitory and excitatory neuron response profiles, as illustrated by functional cluster assignments of both subtypes (Figures S3E, F).
We next sampled five genetically defined DH interneuron populations using interneuron specific Cre-driver lines and either ChR2 or ReaChR actuator lines47,48 (Figures 3A–J, S3A–D; Methods) to assess their in vivo responses to mechanical stimuli. Each population predominantly mapped onto one or more of the six functional response profiles (Figure 1D, E, S3G–K). Optotagged Rorβ inhibitory interneurons had transient responses to indentation, with little to no sustained response and modest OFF responses at low forces, and thus the majority mapped onto DH functional cluster 1 (Figure 3D, F). In contrast, tagged PV inhibitory interneurons (PVi) exhibited pronounced sustained and OFF responses to step indentations, low force thresholds and large RFs (Figure 3E–H) and thus were mostly assigned to DH functional cluster 3 (Figure 3E). Inhibitory Kcnip2 interneurons had a distinct cluster assignment, reflecting their unique sustained firing patterns, high spontaneous firing rates, and small RFs (Figures 3G, H, and S3I, L). In contrast CCK excitatory lineage neurons exhibited some of the smallest RFs (Figure 3H). Interestingly, optotagged PKCγ neurons, a subset of spatially restricted DH excitatory interneurons, had homogeneous response properties with higher sensitivity and larger RFs compared to other excitatory interneuron populations (Figures 3G, H, and S3K). Thus, while functional cluster 2 may be comprised exclusively of excitatory interneurons (Figure S3E–K), clusters 1 and 3 both harbor distinct combinations of genetically labeled excitatory and inhibitory interneuron subtypes (Figure 3D–E, S3G–K). Interestingly, 60% of PKCγ excitatory interneurons were phase-locked to vibratory stimuli, compared to only 6% of the broad CCK excitatory interneuron population (Figure 3I, J). On the other hand, each of the five optotagged interneuron populations exhibited vibration frequency-dependent sensitivity (Figure S3M). Together, these findings point to a broadly distributed arrangement of mechanically sensitive DH neurons that fall into six principal functional clusters, likely corresponding to one or more genetically defined interneuron types with distinct intrinsic physiological, morphological, and synaptic properties.
LTMR and HTMR signal convergence shapes touch-evoked responses in DH neurons
To assess the contributions of mechanoreceptor subtype inputs to the range of DH neuron physiological response profiles, we next used sensory neuron loss-of-function and gain-of-function manipulations in conjunction with in vivo MEA recordings. We first asked whether DH neuron responsivity to skin indentation is dependent on the mechanosensory ion channel Piezo249. Remarkably, DH neurons of mice in which Piezo2 was deleted from all neurons below cervical level 2 (Cdx2-Cre; Piezo2flox/flox mice50) displayed minimal responses to indentation steps up to 75mN, while pinch-evoked responses were intact (Figure S4A–D). We next assessed the contributions of Aβ RA- and Aβ SA-LTMRs to distinct DH responses using genetic ablation strategies that result in loss of Meissner corpuscles and their associated Aβ RA-LTMRs (AdvillinCre; TrkBflox/flox mice51 referred to as TrkBcKO) or loss of both Meissner corpuscles/Aβ RA-LTMRs and Merkel cells, required for normal Aβ SA-LTMR responses (AdvillinCre; TrkBflox/flox; Atohflox/flox referred to as DKO15). DH neurons from DKO mice exhibited dramatically decreased sensitivity compared to control littermates (Figure 4A–G), and showed no ON responses to indentation forces below 20 mN. Similarly, DH neuron sensitivity and ON responses were diminished, albeit to a lesser degree, in TrkBcKO mice. In addition, OFF responses were virtually absent at low forces and reduced at higher force intensities in both TrkBcKO and DKO mice (Figure 4B, C, E, F). DH units from DKO mice also lacked phase-locked responses at most vibration frequencies tested (Figure 4H, J). A few DH units in DKO mice did exhibit phase-locking at 120Hz (Figure 4H), presumably because high frequency skin vibrations >100Hz are transduced by intact Aβ RA2-LTMRs associated with Pacinian corpuscles. Consistent with this, both TrkBcKO and DKO mice lacked frequency tuning below 80Hz but did exhibit normal responsivity in the Pacinian range, above 80Hz (Figure 4I).
Figure 4. Decreased DH neuron sensitivity in the absence of Aβ RA-LTMR and Aβ SA-LTMR signals.
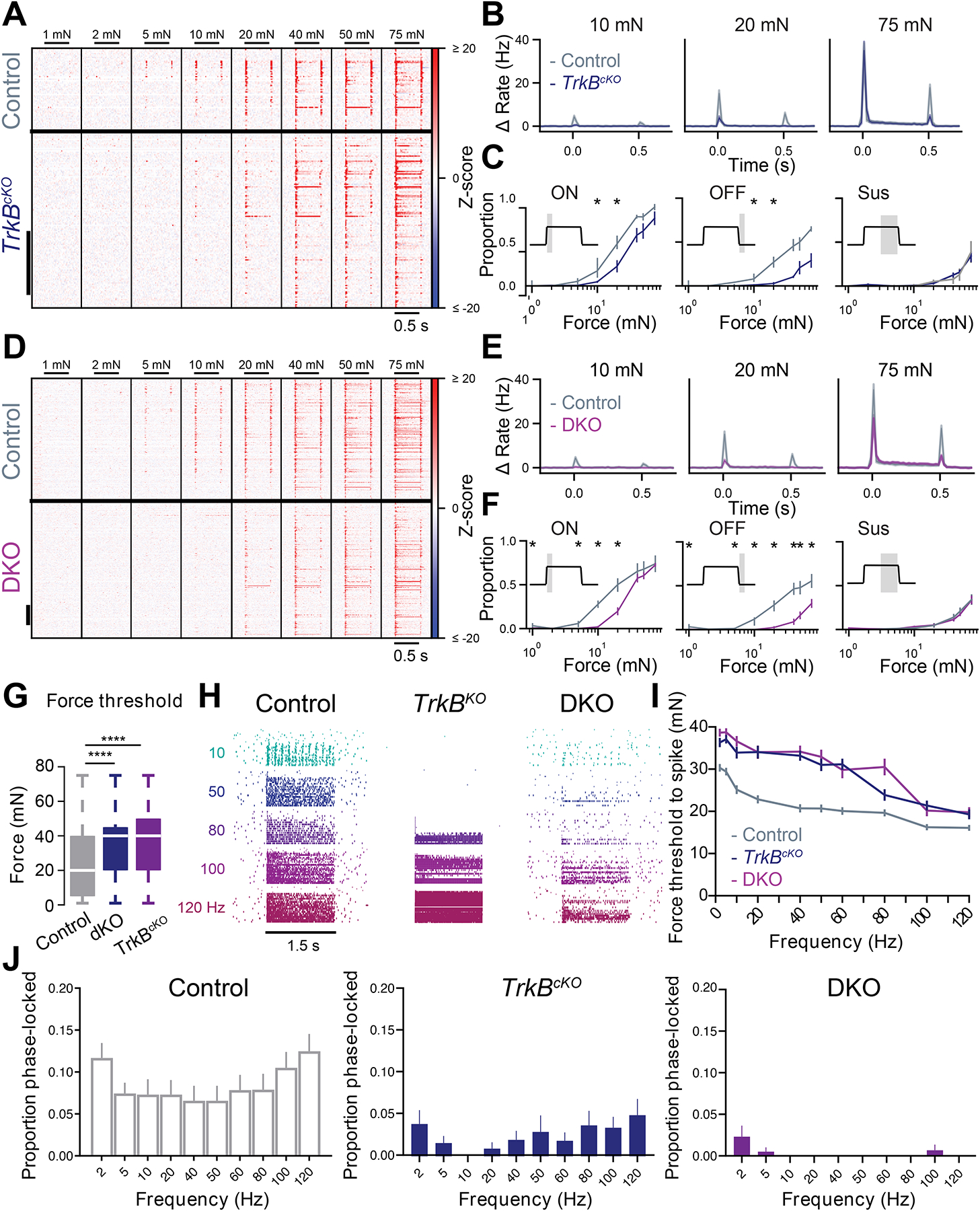
A. Indentation-evoked Z-scored firing rates for DH units from littermate control (TrkBflox/flox; top; n=72 units, 3 mice) and AdvillinCre;TrkBflox/flox animals (TrkBcKO; bottom; n=142 units, 4 mice). Units are sorted by depth. Scale bar: 50 units.
B. Mean baseline-subtracted firing rates (± SEM) to 10, 20, and 75mN step indentations from control (grey) and TrkBcKO animals (dark blue).
C. Proportion of units from control (grey) and TrkBcKO animals (dark blue) responding at the onset (left), offset (middle) and sustained (right) phase of step indentations for each force. * p < 0.05 for comparisons between units in TrkBflox/flox and TrkBcKO mice, two-proportions Z test.
D. As in A, for DH units in littermate controls (top; n=328 units; from 5 recordings in 4 mice) and DKO mice (AdvillinCre;TrkBflox/flox; Atoh1flox/flox; bottom; n=436 units; from 8 recordings in 4 mice).
E. As in B, for littermate control (grey) and DKO (purple) DH units.
F. As in C, for littermate controls (grey) and DKO (purple) DH units.
G. Response thresholds from all units in control, TrkBcKO and DKO animals. ****p < 0.0001, Mann-Whitney U tests.
H. Example responses to mechanical vibrations varying in frequency and amplitude (ordered by frequency, then amplitude) in control, TrkBcKO, and DKO animals.
I. Frequency- threshold tuning curves from control, TrkBcKO, and DKO animals.
J. Proportion of phase-locked units in control (left), TrkBcKO (middle), and DKO (right) animals.
See also Figure S4.
Interestingly, the fraction of DH units spiking during the ON and sustained portions at forces >20mN was comparable between TrkBcKO, DKO, and control mice (Figure 4C, F). This finding suggests that LTMR and HTMR signals converge onto the majority, and possibly all, mechanically sensitive DH neurons. To further address this, we next generated Nav1.8Cre; Piezoflox/flox mice in which Piezo2 is deleted from a large fraction of small- and medium-diameter neurons, including many HTMRs, but not from Aβ RA- and Aβ SA-LTMRs (Figure S4E–F). As predicted, DH neurons in Nav1.8Cre; Piezoflox/flox mice exhibited no changes in mechanical response thresholds compared to littermate controls (Figure S4J). However, these mutants did exhibit diminished firing at indentation forces at and above 10mN during the sustained portion of step indentations (Figure S4G–H). This reduction is modest and likely an underestimation of the HTMR contribution due to incomplete Piezo2 deletion in Nav1.8Cre; Piezoflox/flox mice (Figure S4E–F).
To assess the sufficiency of select LTMR subtypes and HTMRs for DH neuron activation across the functional cluster types, we next compared DH neuron responses to (1) mechanical stimuli and (2) optogenetic stimuli applied to the same skin region to selectively activate one or more somatosensory neuron types (Figure 5A). Genetic driver lines were used to express ReaChR in select populations of sensory neurons (see Methods), and brief light pulses applied to the skin triggered one action potential in a few sensory neurons15. Strikingly, selective optogenetic activation of either Aβ RA- or Aβ SA-LTMRs evoked short-latency responses in the majority of DH units (Figure 5B–C). Furthermore, “optogenetic RFs” of DH neurons, mapped using patterned optogenetic stimuli to selectively activate Aβ RA- or Aβ SA-LTMRs across the skin, were compared to the mechanical RFs of Aβ RA- or Aβ SA-LTMRs (Figure 5D), allowing us to compute homotypic convergence estimates. On average, individual DH neurons receive convergent inputs from seven or more of these Aβ-LTMR subtypes (Figure 5D).
Figure 5. Extensive convergence of LTMR and HTMR signals across DH neuron functional types.
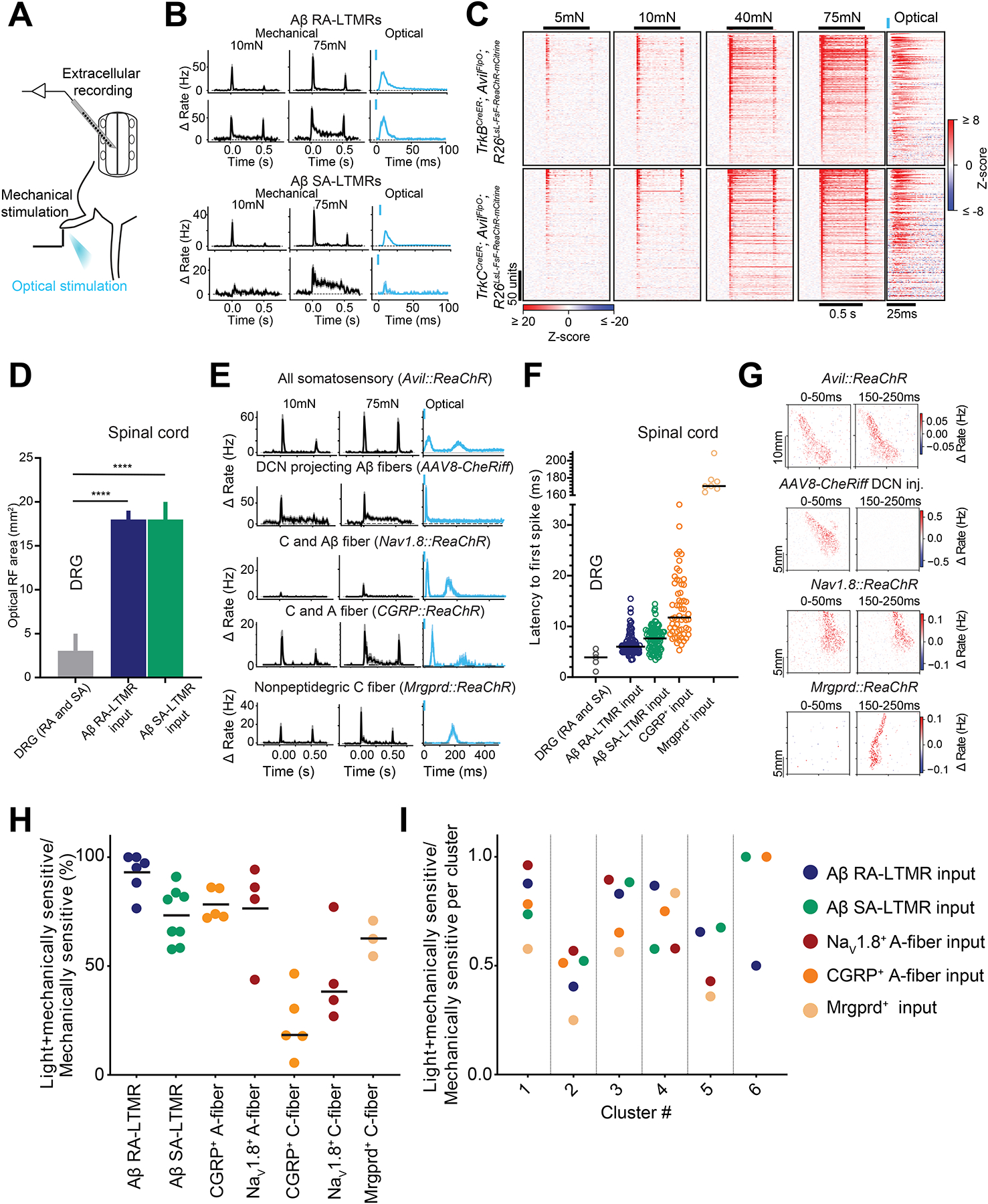
A. Experimental configuration.
B. Mechanical and optically-evoked mean PSTHs (±SEM) in example DH units from TrkBCreER; AdvillinFlpO; R26LSL-FSF-ReaChR-mCitrine (Aβ RA-LTMRs; top) and TrkCCreER; AdvillinFlpO; R26LSL-FSF-ReaChR-mCitrine mice (Aβ SA-LTMRs; bottom).
C. Z-scored indentation- and optically-evoked responses in mechanically sensitive DH units from TrkBCreER; AdvillinFlpO; R26LSL-FSF-ReaChR-mCitrine (top) and TrkCCreER; AdvillinFlpO; R26LSL-FSF-ReaChR-mCitrine mice (bottom). Units are sorted by the magnitude of ON response. Note the timescale difference between mechanical and optical heatmaps.
D. Summary optical RFs in DRG and DH neurons. Bars: median with 95% CI. ****p < 0.0001, Mann-Whitney U tests.
E. As in B, in animals expressing excitatory opsins in all somatosensory (AdvillinFlpO; R26FSF-ReaChR-mCitrine); Aβ-DCN projecting Aβ fibers (AAV8-CheRiff DCN injection), Nav1.8+ expressing C fibers (Nav1.8Cre; R26LSL-ReaChR-mCitrine); CGRP+ HTMRs (CGRPCreER; R26LSL-ReaChR-mCitrine) and MRGPRD+ nonpeptidergic C fibers (MrgprdCreER; R26LSL-ReaChR-mCitrine).
F. Optically-evoked spike latencies in DRG neurons and DH neurons following optical cutaneous activation of distinct sensory neuron types. Bars: mean.
G. Optically-evoked RFs with both A- and C-fiber components in DH neurons.
H. Proportion of mechanically sensitive units that respond to cutaneous optical activation of distinct sensory neuron types. Each marker represents one animal; markers are color-coded by genotype. Bars: mean.
I. Proportion of DH units firing in response to cutaneous optical activation of distinct LTMR and HTMR subtypes per DH functional group. Dotted lines separate adjacent functional clusters. Aβ-RA LTMR input (n=205 units; 6 mice); Aβ SA-LTMR input (n=215 units; 8 mice); Nav1.8 input (n=101 units; 4 mice); CGRP input (n=99 units; 5 mice); Mrgprd input (n=45 units; 3 mice).
Cutaneous optogenetic stimulation of DCN-projecting Aβ-fiber sensory neurons (Figure 5E; Methods) evoked only short latency responses in virtually all mechanically sensitive DH neurons. In contrast, cutaneous optogenetic activation of all somatosensory neurons (using Advillin-FlpO; R26FSF-ReaChR mice) elicited both short and long latency responses (Figure 5E). Similar short and long latency responses were observed following electrical stimulation of the skin and were indicative of A- and C-fiber inputs, respectively (Figure S5L). Broad optogenetic activation of HTMRs and other sensory neuron types, using both Nav1.8Cre lineage and CGRPCreER driver lines, also triggered both short (A-fiber) and long (C-fiber; >100ms) latency responses (Figure 5E, F) in the majority of DH neurons (Figure 5H). In fact, as observed for Aβ RA-, Aβ SA-LTMRs, and DCN-projecting Aβ-fiber sensory neurons, optogenetic activation A-fiber CGRP+ DRG neurons, which are HTMRs3,52–54, evoked spiking in the majority of DH neurons and across all functional classes (Figure 5H, I). Intriguingly, optical RFs for A- and C-fiber evoked responses overlapped somatotopically for the majority of DH units (Figure 5G). Finally, optogenetic activation of Mrgprd+ HTMRs evoked exclusively long-latency responses (Figure 5E–G), as expected for these C-fiber neurons, and across DH units corresponding to all functional cluster types (Figure 5H, I). Together, these primary sensory neuron loss-of-function and gain-of-function manipulations suggest that parallel signals from physiologically distinct Aβ LTMR subtypes and HTMRs differentially combine to shape the mechanical responses of DH neurons broadly, across all functional cluster types.
Sensory-evoked feed-forward and feedback inhibitory circuit motifs exert broad control over DH neuron responses
To begin to define the role of local DH circuit motifs in shaping the responses of DH interneurons and output neurons, we next assessed synaptic connectivity between primary afferents and PSDC output neurons, and the contributions of Rorβ and PVi inhibitory interneurons to feedforward and feedback inhibition in acute spinal cord slices (Figure 6A). Photostimulation of Aβ RA- or Aβ SA-LTMR central terminals evoked large monosynaptic excitatory postsynaptic currents (EPSCs) (Figure 6A–C; S5A) as well as strychnine-sensitive polysynaptic inhibitory postsynaptic currents (IPSCs) in PSDC neurons through feedforward inhibition (FFI) in the local DH circuit (Figure 6B–E and Figure S5A). Moreover, optogenetic activation of Nav1.8+ sensory neuron terminals evoked smaller, polysynaptic EPSCs in PSDC neurons (Figure S5B). On the other hand, optogenetic stimulation of Rorβ interneurons evoked strong monosynaptic IPSCs (Figure 6F, G) and, in current clamp experiments, directly suppressed the activity and excitability of neighboring PSDCs (Figure 6H; S5C). Consistent with this, Rorβ interneurons form inhibitory synaptic contacts onto the perisomatic compartment of PSDC neurons (Figure S5H). In contrast to this, optogenetic activation of PVi neurons failed to evoke direct, monosynaptic IPSCs in PSDC neurons (Figure 6K).
Figure 6. Sensory-evoked feed-forward and feedback inhibitory circuit motifs exert broad control over DH neuron responses.
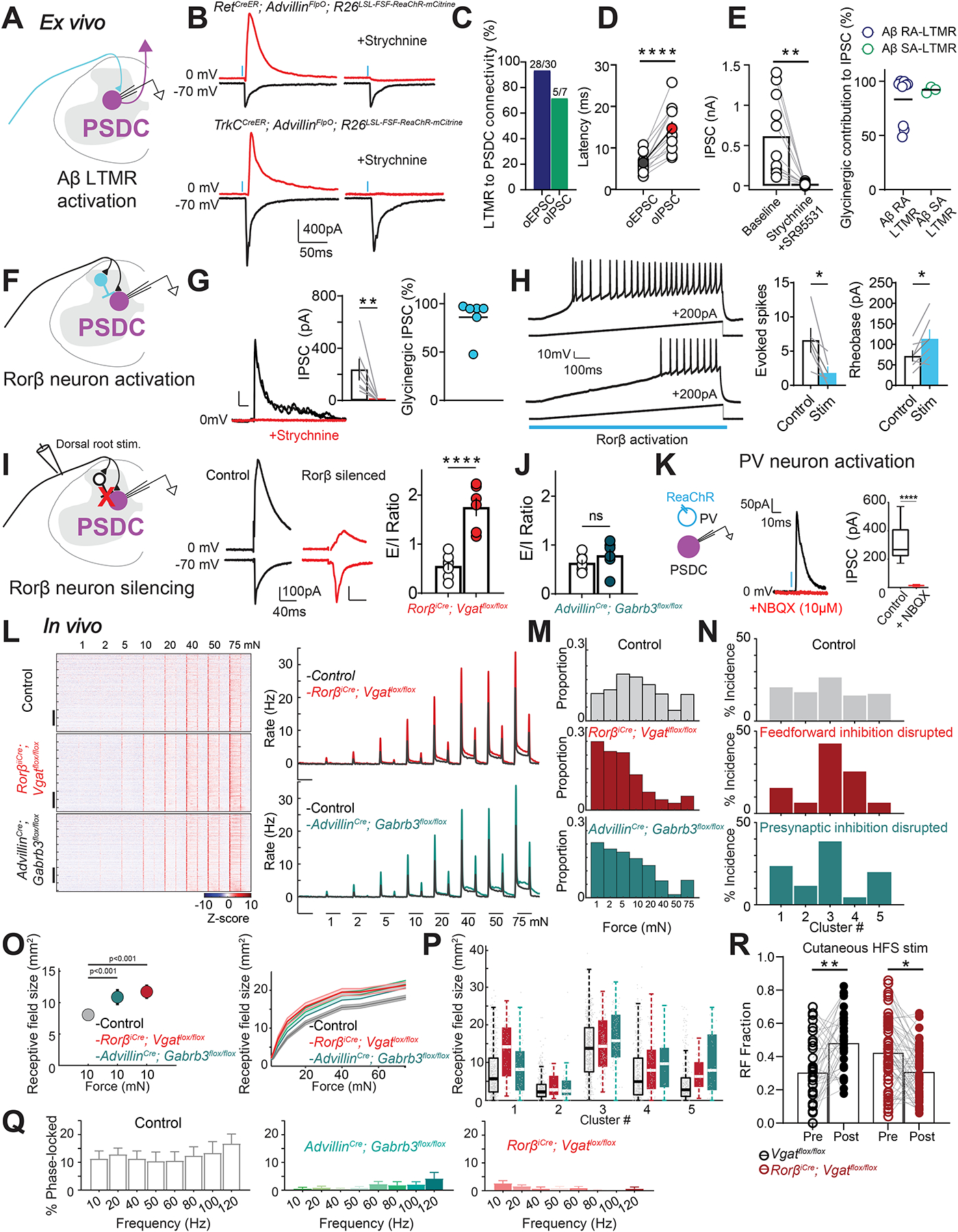
A. Recording configuration.
B. Optical excitatory postsynaptic currents (oEPSCs) and polysynaptic inhibitory postsynaptic currents (oIPSCs) in PSDC neurons evoked by optogenetic activation of Aβ RA-LTMR (in RetCreER; AdvillinFlpO; R26LSL-FSF-ReaChR∷mCitrine mice) and Aβ SA-LTMR synaptic terminals (in TrkCCreER; AdvillinFlpO; R26LSL-FSF-ReaChR∷mCitrine mice) under baseline conditions and following strychnine (2μM) application.
C. Connectivity rates for Aβ RA-LTMR and Aβ SA-LTMR oEPSCs to PSDC neurons.
D. Onset latencies for opto-evoked Aβ RA-LTMR PSCs to PSDC neurons (n=14 PSDCs; 8 mice). P < 0.0001; Paired t test. Colored symbols: means (±SEM).
E. Left: Aβ RA-LTMR evoked oIPSC amplitudes before and after strychnine, and in some cases (n=3) of co-application of strychnine and SR95531 (10uM) for the same PSDCs shown in D. Bars: means. P < 0.01; Paired two tailed t-test. Right: percentage of IPSC component blocked by strychnine (subsequent SR95531 application blocked remaining component). Horizontal lines: means. P= 0.4818; Mann-Whitney U test.
F. Schematic of experimental set-up.
G. Light-evoked IPSCs from Rorβ neurons to PSDCs are blocked by 2μM strychnine (n=7 PSDCs; 5 mice). Left: representative glycinergic oIPSCs in PSDC neurons; Middle: oIPSC amplitudes before and after strychnine. P < 0.05; Paired t test. Right: contribution of glycine and GABA to Rorβ-mediated IPSCs onto PSDC neurons. Strychnine insensitive IPSC component was blocked by SR95531.
H. Current clamp recordings in PSDC neurons in response to 200pA current injection ramps paired with 1s optical activation of Rorβ neurons. Left: top trace: current injection evoked firing; bottom trace: response in the same PSDC when neighboring Rorβ neurons are coincidentally activated. Middle: Evoked spikes at 100pA current injection ± optical activation Rorβ neurons. P < 0.05; Paired t test; n=6 neurons. Right: Rheobase ± Rorβ neuron activation. P < 0.05; Paired t test; n=6 neurons.
I. Left: Schematic of experimental set-up. Rorβ silencing in spinal cord slices from RorβiCre; Cdx2FlpO; R26LSL-FSF-TeTox and RorβiCre; Vgatflox/flox mice. Middle: representative sensory-evoked EPSCs and IPSCs to PSDC neurons in littermate controls (black) and Rorβ silenced mice (red). Right: E/I ratios in Rorβ silenced mice (n=6 PSDCs; 6 mice) compared to littermate controls (n=7; 6 mice). Bars: mean ± SEM. P < 0.001; Mann-Whitney U test.
J. E/I ratios in PSDCs from AdvillinCre; Gabrb3flox/flox mice (n=7 PSDCs; 3 mice) and control littermates (n=5; 4 mice). Bars: mean ± SEM. P > 0.05; Mann-Whitney U test.
K. Left: Experimental design. Middle: representative IPSC evoked by optogenetic activation of PV interneurons under baseline condition and following NBQX application (red). Right: average IPSC amplitudes before and after NBQX (n=5 PSDCs; 3 mice).
L. Right: Z-scored firing rates for DH units from control (top, n=486 units; 14 mice), FFI cKO (RorβiCre; Vgatflox/flox; middle, n=474 units; 12 mice), and PSI cKO mice (AdvillinCre; Gabrb3flox/flox; bottom, n=507 units; 12 mice). Units are sorted by depth. Scale bar: 100 units. Left: baseline subtracted PSTHs (control: grey, FFI cKO: red, PSI cKO; teal).
M. Indentation threshold distribution in control (grey), FFI cKO (red), and PSI cKO mice (teal).
N. Incidence of DH functional clusters in control (grey), FFI cKO (red) and PSI cKO mice (teal).
O. RF area at 10mN in units from control (grey), FFI cKO (red) and PSI cKO (teal) mice. P < 0.001; Mean ±SD of bootstrapped RFs. Right: Mean RFs (±SEM, shaded areas) across all force intensities in control (grey), FFI cKO (red) and PSI cKO (teal) animals.
P. RFs at 10mN across 5 functional clusters in control (grey), FFI cKO (red), and PSI cKO animals.
Q. Phase-locked vibration responses in DH units from FFI cKO (red) and PSI cKO mice (teal).
R. RFs at 10mN following cutaneous HFS in controls (black; 2 mice), and in mice lacking FFI (red; 2 mice). WT: P < 0.01; FFI cKO: P < 0.05; Paired two tailed t -test. See also Figures S5 and S6.
In related experiments, we used dorsal root electrical stimulation20,55,56 to measure sensory-evoked EPSCs and polysynaptic IPSCs in PSDC neurons in acute slices from mice in which different inhibitory interneuron subtypes and circuit motifs were silenced (see Methods). Inhibiting Rorβ interneurons using two different chronic silencing strategies strongly attenuated Aβ-fiber evoked glycinergic FFI onto PSDC neurons, resulting in a 3-fold increase in E-I conductance ratios (Figure 6I). In addition, acute optogenetic silencing of Rorβ neurons suppressed 50% of FFI onto PSDC neurons without affecting monosynaptic excitatory transmission from sensory afferents (Figure S5E). We also assessed the role of presynaptic inhibition (PSI) of primary afferent terminals in sensory-evoked EPSCs to PSDC neurons, which is mediated in part by axo-axonic synapses formed by PVi inhibitory interneurons onto primary afferent terminals20,57. Genetic deletion of presynaptic GABAA receptors in somatosensory neurons using AdvillinCre; Gabrb3flox/flox mice56,58 did not alter sensory-evoked E/I ratios in PSDC neurons (Figure 6J).
We next assessed the contributions of sensory-evoked inhibition in shaping in vivo DH interneuron responses to mechanical stimuli. Disrupting Rorβ neuron-mediated FFI or GABAAR-mediated PSI caused a dramatic increase in sensitivity and spiking responses of DH neurons to step indentations (Figures 6L–M; S5I). Moreover, while loss of either form of inhibition shifted DH response profiles into a more sensitive range (Figure 6M), DH response cluster diversity was differentially altered following FFI and PSI disruption (Figure 6N). In complementary experiments, we found that acute optogenetic silencing of Rorβ or PVi interneurons increased sensitivity and indentation evoked spiking in a subset of neighboring DH units (Figure S5F, G).
Inactivation of Rorβ-mediated FFI and GABAAR-mediated PSI similarly increased RFs across all recorded DH neurons (Figure 6O) and cluster types (Figures 6P). However, while RFs measured at the ON response were significantly larger in both the FFI and PSI mutants (Figure 6O), RFs measured during the sustained and OFF portions of indentation steps in the FFI mutants, but not the PSI mutants, differed from controls (Figure S5J). Moreover, DH neuron phase-locking to vibratory stimuli was nearly abolished in the absence of FFI or PSI (Figure 6Q).
Finally, the potential contribution of one form of sensory-evoked inhibition, Rorβ interneuron mediated FFI, to high frequency stimulation (HFS) evoked plasticity of DH responses was tested. Remarkably, cutaneous HFS electrical stimulation evoked rapid and marked increases in both sustained responses and RF properties across a large cohort of DH neurons in control mice, but not in mice lacking Rorβ neuron mediated FFI (Figures 6R, S5K–M). Related to this, in spinal cord slice recordings, HFS of dorsal roots triggered robust long-term potentiation (LTP) of Aβ sensory neuron synapses onto PSDC output neurons, and this form of plasticity was virtually absent in slices from mice lacking Rorβ interneuron mediated glycinergic FFI (Figure S5N).
Together, these findings indicate that FFI and PSI, and thus the interneurons mediating these distinct forms of synaptic inhibition, including interneurons assigned to clusters 1 and 3, shape the mechanical responses of most and possibly all DH neurons, across all functional cluster types. This finding supports a model in which DH interneurons are functionally inter-dependent: interneurons of one functional type shape the mechanical responses and RFs of other DH interneuron types.
Moreover, DH neuron response properties and RFs across functional cluster types flexibly adjust to high frequency volleys of sensory inputs, and at least one DH inhibitory motif, glycinergic FFI mediated by Rorβ interneurons of cluster 1, is required for this widespread sensory-evoked plasticity.
PSDC output signals reflect convergent mechanosensory inputs and are shaped by distinct modes of DH synaptic inhibition
We next tested the hypothesis that DH output signals conveyed by PSDC neurons reflect extensive convergence of mechanoreceptor inputs and the inter-dependency of DH circuit components found here. Thus, we recorded from PSDC neurons using optogenetic tagging and antidromic activation strategies that enabled in vivo measurements of PSDC tactile-evoked responses in conjunction with sensory neuron and DH neuron functional manipulations (Figures 7A, S6A–F; Methods). Overall, tagged PSDC neurons exhibited a surprisingly high diversity of response properties and sensitivities to mechanical step indentations (Figures 7B, Control, S6K). PSDC neurons also had typically large cutaneous RFs (Figure S6I). While phase-locking to mechanical vibrations exceeding 20Hz was absent (Figure S6J), PSDCs did display vibration frequency-dependent sensitivity (Figure S6J). Importantly, selective optogenetic activation of Aβ RA- or Aβ SA-LTMRs evoked action potentials in most PSDC neurons tested, demonstrating extensive convergent input from Aβ LTMR subtypes (Figure S6G, H).
Figure 7. DH synaptic inhibition shapes response properties and receptive fields of PSDC output neurons and neurons in S1.
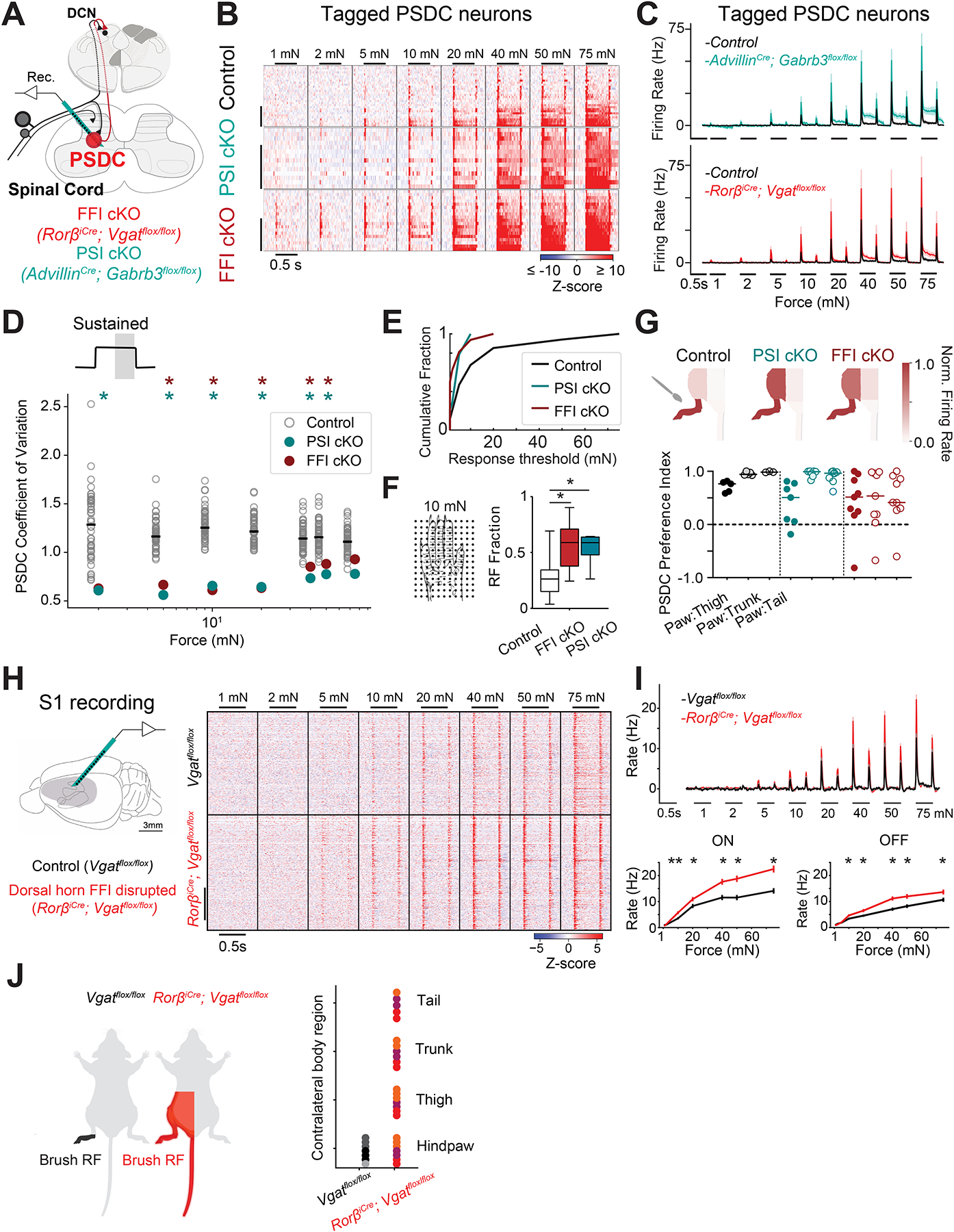
A. Schematic of experimental set-up.
B. Heatmaps of z-scored firing rates for tagged PSDCs in control (n=34; N=18 mice); FFI cKO (RorβiCre; Vgatflox/flox; n=17; 6 mice) and PSI cKO animals (AdvillinCre; Gabrb3flox/flox; n=14; 5 mice). Units sorted by sustained response magnitude.
C. Baseline-subtracted mean firing rates (±SEM) of tagged PSDCs (black traces; control mice, red traces; FFI cKO mice, teal traces; PSI cKO mice).
D. C.V. of sustained response magnitudes at indentation steps between 2 to 75mN in FFI cKO (red markers; n=17); PSI cKO (teal markers; n=14) and 50 representative sets of 14 randomly sampled PSDCs from control mice (grey markers). Asterisks: parameters for which the value in FFI cKO and PSI cKO PSDC neurons was lower than that for WT PSDC neurons in <0.5% of 10,000 subsamples (p < 0.05).
E. Indentation threshold cumulative distribution in control PSDCs compared to FFI cKO or PSI cKO PSDCs. WT vs. FFI PSDCs: P < 0.005; WT vs. PSI PSDCs: P < 0.05; Mann-Whitney U test.
F. RF fraction of skin area mapped with 10mN force steps in control vs. FFI cKO and PSI cKO PSDCs. P < 0.01; Mann-Whitney U test.
G. Brush-evoked RFs in PSDCs from control (grey), FFI cKO (red) and PSI cKO (teal) animals. Top: Firing rates normalized to the maximum brush-evoked response for example PSDCs from control, FFI cKO and PSI cKO mice. Bottom: Body region preference index for a subset of PSDC units from control (n=5), FFI cKO (n=9) or PSI cKO animals (n=7). H=6.56; P < 0.05; Kruskal-Wallis H test with post hoc Dunn’s test, P<0.05.
H. Left: Recording configuration for S1 in vivo electrophysiology. Right: Heatmaps of z-scored firing rates in hindpaw S1 from control Vgatflox/flox (top; n=335 units from 7 recording in 3 mice) and RorβiCre; Vgatflox/flox mice (bottom; n=373 units from 7 recordings in 3 mice).
I. Top: Mean baseline-subtracted firing rates (±SEM) across all control (grey) and FFI cKO (red) units. Bottom: baseline-subtracted mean firing rate (±SEM) at the step onset (left) and offset (right) in units from wild-type (grey) and FFI cKO (red) animals.
J. Left: schematic of brush-evoked RFs in hindpaw S1 units from control and FFI CKO animals. Right: Brush-evoked responses across multiple body regions in control (dark grey hues) and FFI cKO mice (red hues). Multiple recordings from the same animal are represented with markers of the same color within a genotype. See also Figures S6 and S7.
Disrupting either Rorβ neuron-mediated FFI or GABAAR mediated PSI decreased mechanical thresholds and increased indentation-evoked spiking in PSDC neurons (Figures 7B, C, E). Spiking responses at the ON and OFF portions of step indentations were elevated across the entire force range, demonstrating that both FFI and PSI gate Aβ RA- and Aβ SA-LTMR drive onto PSDC neurons (Figures 7B, C, S7A). Responsivity during the sustained phase of step indentations was also strongly elevated at indentation forces that recruit HTMR input, and to a greater extent in mice lacking PSI than in mice lacking FFI (Figures 7B, C and S7A). Furthermore, a prominent after-discharge following step indentations was observed in mice lacking PSI, but not FFI, indicating that PSI uniquely contributes to PSDC response kinetics and temporal filtering of mechanosensory inputs (Figure 7B, C). Moreover, PSDC RFs were substantially larger in the absence of FFI or PSI (Figures 7F, G, S7C). Finally, while PSDC neurons in control mice exhibited highly diverse response properties and sensitivities, as observed in the heat maps of individual PSDC responses (Figure 7B) and quantified using a coefficient of variation analysis of the entire population (Figure 7D, S7B), PSDCs in mice lacking FFI or PSI had considerably less variation in their mechanical response thresholds, response profiles, and RFs (Figures 7D, E, S7B, C). Thus, distinct modes of synaptic inhibition that control mechanical responses across all DH interneuron types are also crucial in shaping the wide diversity of responses in PSDC neurons.
DH outputs dictate tactile responses at supraspinal regions
Because PSDC neurons convey tactile information from the DH to the DCN, and as a group exhibited heightened sensitivity and larger RFs following both FFI and PSI disruption, we hypothesized that loss of FFI or PSI in the DH may lead to: (1) increased sensitivity and response amplitudes to skin indentation in downstream neurons, (2) touch-evoked RF expansion, and (3) altered behavioral responses to tactile stimuli. Because the genetic manipulations used to disrupt FFI are restricted to the spinal cord (Figures S7F, G), we next recorded touch-evoked responses in S115 of awake RorβiCre; Vgatflox/flox mice and Vgatflox/flox littermate controls. We found that selective disruption of DH FFI increased S1 neuron spiking at the ON and OFF phase, without altering their transient response profiles (Figures 7H, I, S7D, E). We also observed a striking alteration of RF properties of individual S1 units in the absence of DH FFI. While individual units from hindlimb S1 responded most robustly to hindlimb skin stroking in control mice, S1 units from RorβiCre; Vgatflox/flox mice responded comparably to stroke stimuli applied across the entire contralateral lower body (Figure 7J). Moreover, behavioral measurements showed that disruption of Rorβ neuron mediated FFI caused tactile over-reactivity (Figure S7I), deficits in texture discrimination (Figure S7J), and an alteration in a sunflower seed handling behavioral assay that assesses the integrity of sensory-motor exchange and dexterous use of the forepaws (Figure S7K). Together, these findings indicate that mechanoreceptor signal processing in the DH shapes touch-evoked responses and topographic body representation at supraspinal levels as well as somatosensory behaviors.
Discussion
Here we combined large-scale in vivo electrophysiology and a range of functional manipulations to assess spinal cord DH interneuron and output neuron mechanical response type diversity and function. We observed six mechanically sensitive DH neuron functional types based on their sensitivities, tonic and mechanically evoked firing patterns, and RF areas and shapes. A sampling of previously identified genetically labeled interneuron subtypes showed that they map mainly onto one or a few of the functional classes. Our findings from perturbation experiments support a model in which functionally distinct DH interneuron types receive extensively convergent LTMR and HTMR inputs and form a highly inter-connected network architecture that serves to flexibly shape a diversity of response properties of PSDC outputs. We also provide evidence that mechanosensory signal transformations within the intricate, interconnected DH network dictate how mechanical forces acting on the skin are represented in the brain.
Extensive LTMR subtype and HTMR convergence in the dorsal horn
Several lines of evidence point to a remarkably high degree of Aβ LTMR subtype signal convergence onto the majority of DH neuron functional types. First, the majority of mechanically sensitive DH units across all functional response types have large and spatially complex RFs when probed with 10mN force steps, which preferentially activate LTMRs. Since the RFs of Aβ RA- and Aβ SA-LTMRs themselves are considerably smaller than even the smallest DH neuron RFs, DH RFs measured at low indentation forces likely reflect homotypic and/or heterotypic convergence of signals from many individual Aβ RA- and Aβ SA-LTMRs. Second, genetic disruption of Aβ RA- and Aβ SA-LTMR signals resulted in loss of responsivity to 10mN indentations across all mechanically sensitive DH units, indicating broad contribution of these Aβ LTMR types to virtually all DH responses in the low force range. Third, cutaneous optogenetic stimuli that selectively evoked single action potentials in Aβ RA- or Aβ SA-LTMRs15 was sufficient to drive spiking across the majority of DH neurons representing all functional response types, and in PSDC projection neurons. Related to this, the vast majority of DH neurons across all functional response types exhibited broad vibration tuning. Together with the observation that DH units from TrkBcKO mice lack vibration tuning in the Meissner, but not in the Pacinian range, these findings suggest that most DH neurons receive input, either directly or indirectly, from both Meissner corpuscle Aβ RA1-LTMRs and Pacinian corpuscle Aβ RA2-LTMRs. Collectively, these findings demonstrate extensive LTMR subtype signal convergence and non-linear transformations giving rise to distinct DH interneuron and output neuron firing patterns. Moreover, sensory neuron perturbation experiments also revealed that most DH neurons across all functional response types receive inputs from HTMRs, either directly or indirectly. The surprisingly high degree of LTMR and HTMR convergence is also reflected at the level of PSDC neurons. Thus, one consequence of extensive LTMR and HTMR signal convergence in the DH is that the indirect dorsal column pathway encodes mechanical stimuli over a wider dynamic range of intensities, as compared to the direct dorsal column pathway.
Feedforward and feedback inhibitory motifs broadly shape tactile-evoked responses in the DH, revealing a highly interconnected network architecture
How are LTMR and HTMR inputs to the DH transformed into output signals conveyed to other CNS regions, including the DCN? Our findings support a model in which the DH is an interconnected network of circuit elements, with each principal interneuron type uniquely contributing to the responses of other interneuron and output neuron populations. Rorβ interneurons, mapped predominantly to functional class 1, mediate sensory-evoked glycinergic FFI onto PSDC neurons. On the other hand, PVi inhibitory interneurons, which fall mostly into functional class 3, participate in GABAAR-dependent PSI20,57,59. Both FFI and PSI govern the sensitivity of neurons across all DH neuron functional types and PSDC output neurons. Importantly, these distinct modes of inhibition differentially modulate DH outputs, either directly or indirectly: for example, PSI, but not FFI, governs temporal filtering of PSDC responses to step indentation, through preventing after-discharge spiking following the offset of step indentations. Moreover, FFI has a more widespread effect on PSDC RF size, compared to PSI, and this RF expansion is amplified to an even greater extent in S1. These findings point to a highly inter-dependent DH network model in which interneurons of distinct functional classes, and the circuit motifs they engage, cooperate to shape responses across all DH interneuron types and PSDC outputs.
The DH and its role in mechanotransmission
What is the functional significance of the highly interconnected DH circuitry and its broadly convergent LTMR and HTMR inputs? One clue stems from the finding that PSDCs exhibit highly heterogeneous responses to tactile stimuli. This functional diversity is reminiscent of the broad, heterogeneous tuning and RF properties of output neurons of other early sensory processing areas, including retinal ganglion cells60–63. A second clue comes from the observation that PSDC response properties become remarkably similar in mice lacking FFI or PSI. Indeed, in the absence of the FFI or PSI motifs, virtually all PSDC neurons responded to the lowest indentation forces, exhibited pronounced sustained firing patterns, particularly in the HTMR range, and displayed extremely large RFs. As such, proper DH network function is essential for setting a wide range of sensitivities, response properties, and RFs across the PSDC population. A third clue comes from our observation that bursts of high frequency afferent stimulation evoked changes in the response properties and RFs of neurons across the DH, consistent with the observation of activity-dependent changes in PSDC RF properties29. Relevant to this, afferent stimulation-evoked changes in DH neuron response properties and activity-dependent potentiation of Aβ LTMR to PSDC synapses are both absent in mice lacking Rorβ neuron-mediated FFI. That the DH is modulated by top-down control via corticospinal inputs is also of interest when considering models of DH network function. Collectively, these observations lead us to propose a model in which the highly interconnected DH circuitry, with its broadly convergent LTMR and HTMR inputs, functions to enable a wide, flexible range of PSDC output neuron sensitivities, firing patterns and RF properties, modifiable by sensory experience and internal state. Future experiments will test how mechanosensory processing in the DH and the resultant variable, modifiable PSDC output signals conveyed by the indirect dorsal column pathway combine with unmodified LTMR signals carried by the direct dorsal column pathway to shape the brain’s representations of myriad features of the physical world.
Limitations of the study
Here, we investigated how DH neurons encode tactile features using mechanical stimuli and anaesthetized mice. Future studies should address DH neuron response profiles, and the contributions of FFI and PSI inhibitory circuit motifs, during naturalistic behaviors in awake mice. Also, while we defined a role for Rorβ neurons in sensory-evoked FFI onto PSDC projection neurons, additional DH inhibitory interneurons mediate FFI onto other DH neuron types. Future studies should test the contributions of the range of inhibitory and excitatory interneuron types that underlie FFI, PSI, and feed-forward excitation, preferably using next generation genetic tools and acute perturbations in awake, behaving mice.
STAR METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, David Ginty (david_ginty@hms.harvard.edu).
Material availability
This study did not generate new unique reagents.
Data and code availability
All data reported in this study will be shared by the lead contact upon request.
All original code is available in this paper’s supplemental information.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and subject details
All experimental procedures were approved by the Harvard Medical School Institutional Care and Use Committee (IACUC) and were performed in compliance with the Guide for Animal Care and Use of Laboratory Animals. Animals were housed in a temperature- and humidity-controlled facility and were maintained on a 12-hour light/dark cycle.
Mouse Lines.
The following published mouse lines were used: VgatiCre (JAX#016962; Ref. 65), RorβiCre (JAX#023526; Ref. 66), RorβCreER (JAX#030290; Ref. 20), Pvalb2a-Cre (JAX#012358; Ref. 67); Kcnip2-CreER (JAX#030385; Ref. 20), CCKiCre (JAX#012706; Ref. 68), PKCγCreER (JAX#030289; Ref. 20), Lbx1FlpO; Ref. 69, Vgat-2A-FlpO (JAX#029591; Ref. 70), Gad2T2A-NLS-mCherry (JAX#023140; Ref. 71), Cdx2-Cre; Ref. 72, Cdx2-NSE-FlpO (JAX#030288; Ref. 20), AdvillinCre; Ref. 73, AdvillinFlpO (JAX#032027; Ref. 34), TrkBCreER; Ref. 74, RetCreER; Ref. 75, TrkCCreER; Ref. 17, Nav1.8Cre (JAX #036564; Ref. 76), CalcaCreER; Ref. 77, Calca-FlpE; Ref. 34; MrgprdCre; Ref. 78, MrgprdCreER (JAX#031286; Ref. 27), R26LSL-FSF-ReaChR-mCitrine (JAX#024846; Ref. 48), R26LSL-ChR2-YFP (JAX#012569; Ref. 79), R26LSL-eNpHR3.0-YFP (JAX#014539; Ref. 79), R26LSL-synaptophysin-tdTomato (JAX#012570; Ref. 79), TauLSL-mGFP-i-NLS-lacZ (JAX#021162; Ref. 80), Vgatflox (JAX#012897; Ref. 81), Gabrb3flox (JAX#008310; Ref. 82), TrkBflox (JAX#022362; Ref. 83), Atoh1flox (JAX#008681; Ref. 84), Piezo2flox (JAX #027720; Ref. 85), RC∷PFtox; Ref. 86. Animals were maintained on mixed C57bl/J6, 129S1/SvImJ and CD1 backgrounds and included both males and females. C57Bl/J6 were obtained from Jackson Laboratories (JAX #000664).
Table S1.
Genetic crosses and tamoxifen labeling strategy used in this study.
| Strategy | Tamoxifen Age | Tamoxifen administration | Tamoxifen Dose (mg) |
|---|---|---|---|
| Rorβ CreER ; R26 LSL-synaptophysin-tdTomato | P21-P25 | intraperitoneal injection | 5mg (1mg/day) |
| Kcnip CreER ; R26 LSL -ChR2 | P21-P25 | intraperitoneal injection | 5mg (1mg/day) |
| PKCγCreERT2; Lbx1FlpO; R26LSL-FSF-ReaChR PKCγCreERT2; R26 | P21-P25 | intraperitoneal injection | 5mg (1mg/day) |
| Ret CreERT2 ; Advillin FlpO ; R26 LSL-FSF-ReaChR | E10.5-E11.5 | Oral gavage | 3mg |
| Ret CreERT2 ; R26 LSL-FSF-ReaChR | E10.5 | Oral gavage | 3mg |
| TrkC CreERT2 ; Advillin FlpO ; R26 LSL-FSF-ReaChR | E12.5 | Oral gavage | 3mg |
| TrkB CreERT2 ; Advillin FlpO ; R26 LSL-FSF-ReaChR | P3 | intraperitoneal injection | 0.5mg |
| TrkB CreERT2 ; R26 LSL-ReaChR | P3 | intraperitoneal injection | 0.5mg |
| CGRP CreER ; R26 LSL-ChR2 | P9 | intraperitoneal injection | 1mg |
| Mrgprd CreERT2 ; R26 LSL-ReaChR | P9 | intraperitoneal injection | 1mg |
Tamoxifen treatment
Tamoxifen was dissolved in ethanol (20mg/ml), mixed with an equal volume of sunflower seed oil (Sigma), vortexed for 5–10min and centrifuged under vacuum for 30min for ethanol removal. The solution was kept at −80°C and delivered via oral gavage to pregnant females for embryonic treatment (E10.5-E12.5, as specified in Table S1), or via intraperitoneal injection for postnatal treatment (P15-P25, as specified above). For spinal cord interneurons, time-points were chosen to label adult interneuron populations with defined anatomical and physiological signatures20. No changes in health or behavior were observed in tamoxifen treated animals compared to nontamoxifen treated animals.
METHOD DETAILS
In vivo spinal cord multielectrode array electrophysiology
Adult (>6 weeks) animals were administered dexamethasone (2 mg/kg IP) 1–2 hours prior to recording to prevent tissue swelling, and were anesthetized with urethane (1.5 g/kg, Sigma). During surgery, isoflurane (1%) was administered but removed prior MEA recordings, and surgical plane of anesthesia was confirmed throughout the recording. The temperature of the animal was monitored and maintained (TC-344B, Warner Instruments) between 35 −37.5°C using a thermoelectric heater (C3200–6145, Honeywell) embedded in castable cement (Aremco). The hair surrounding the dorsal hump was shaved; a skin incision was made over the spinal segments T13 to L6, and the surrounding tissue was removed exposing the spinal column. An incision was made between vertebrae and tendons to allow for spinal clamp placement. The vertebrae above the recording site were stabilized using custom clamps to prevent movement. All tissue was cleared from vertebra and intervertebral space with forceps and spring scissors. The vertebrae between L4 and L5, were retracted to expose the dorsal surface of the spinal cord, the dura was removed and the surface of the cord bathed in mineral oil/submerged in saline solution. A 32-channel silicon probe (Neuronexus A1×32-Poly3-5mm-25s-177-A32 or Cambridge Neurotech ASSY-37 H4 optrode) was inserted into the hindlimb representation region of the dorsal horn (medial L4-L5 spinal levels) and advanced up to ~700 μm below the dorsal surface under visual guidance. Once positioned in a region where firing in many units could be evoked by brushing the hindpaw, the MEA was kept in place for 20 minutes to ensure a stable recording. Signals were amplified, filtered (0.1 – 7.5 kHz bandpass), and digitized (20 kHz) using a headstage amplifier and recording controller (Intan Technologies RHD2132 and Recording Controller). Data acquisition was controlled with open-source software (Intan Technologies Recording Controller version 2.07).
The hindpaw was stabilized with the plantar surface facing upwards, and stroking of the glabrous hindpaw was used a search stimulus to confirm probe placement. If cutaneous receptive fields were not on the glabrous hindpaw the probe was removed and reinserted in a new location. A 150–200 μm diameter Teflon-tipped indenter was controlled by a dual-mode force controller (Aurora Scientific 300C-I) and used to stimulate the glabrous hindpaw. For mapping receptive field (RF) areas, the position of the indenter was controlled with two linear translation piezo stages and a stage controller (Physik Instrumente U-521.24 and C-867.2U2). The position, force, and displacement of the indenter were commanded with custom-written Matlab scripts controlling a Nidaq board (National Instruments, NI USB 6259). Force steps were applied atop the minimum force required to keep the indenting probe in contact with the skin.
In a subset of experiments, electrodes were coated with DiI (Thermo Fisher) after the recording was completed, re-inserted in the spinal cord to the same coordinates and allowed to stabilize for 10–20 minutes. Animals were then anesthetized with isoflurane and transcardially perfused with PBS followed by 4% PFA for post hoc identification of the electrode track.
Optical stimulation of genetically defined DH interneurons
To record from genetically defined DH interneuron populations, we used an optical tagging strategy in mice expressing excitatory opsins in specific DH neuron types (as specified in figure legends). We identified optotagged units by delivering 1–20ms pulses of blue light (4–10 mW/mm2 at fiber tip) to the surface of the spinal cord through optical fibers (200μm core diameter; NA = 0.66) attached to Cambridge Neurotech ASSY-37 H4 optrodes. Light was delivered from a 470 nm LED (M470F3, Thorlabs) or a 150 mW, 488 nm fiber coupled laser (OBIS LX #SKU 1220124, Coherent). Because most dorsal horn interneurons are glutamatergic, at the end of each experiment we applied ~25–50μL NBQX (5 mM, Tocris, dissolved in H2O) to the surface of the cord to block recurrent excitation. Efficient block of glutamatergic synaptic transmission was determined by testing whether DH neuronal responses to brush stimulation or indentation were abolished, typically 10–20 minutes following NBQX application. Neurons that responded to optical stimulation both before and after NBQX application were considered optotagged. A modified stimulus-associated spike latency test (SALT; Ref.87, 15) was additionally used to confirm short light-evoked spike latencies (<5ms) and low first spike jitter in optotagged units. We also verified the validity of optotagging by comparing the average peak-aligned sensory-evoked waveform with average light evoked waveform using Pearson’s correlation coefficient (r >0.9).
In vivo dorsal column antidromic stimulation
In some experiments PSDC neurons were identified through antidromic activation of their dorsal column axons at cervical levels C1/C2. A laminectomy was performed to expose cervical spinal cord prior to lumbar dorsal horn recordings, and the region was sealed off with mineral oil. After measuring tactile responses, a bipolar electrode (platinum-irridium 250 μm spacing, FHC) was placed on the surface of the dorsal column at cervical levels C1-C2. Single stimuli were applied at 0.1Hz frequency while evoked spikes were monitored in the lumbar spinal cord. This strategy activated both Aβ fiber axons and axons of PSDC neurons ascending via the dorsal column to the DCN. Therefore, evoked spikes could be measured in the majority of dorsal horn units that responded to low-threshold mechanical stimulation of the skin. To isolate antidromically activated PSDC units, ~50μL NBQX (5 mM, Tocris, dissolved in H2O) was applied to the surface of the cord to block excitatory synaptic transmission from dorsal horn Aβ fiber synapses. Efficient block of glutamatergic synaptic transmission was confirmed by testing whether DH neuronal responses to brush stimulation or indentation were abolished, typically 10–20 minutes following NBQX application. Units that responded to antidromic stimulation with reliable and precisely timed antidromic spikes (spike latencies and jitter <5ms) in the presence of NBQX were considered PSDC units.
In vivo cortical recordings in awake mice
Primary somatosensory cortex (S1) MEA recordings were performed as described previously15. Briefly, a craniotomy spanning hindpaw S1 was performed at least 24h prior to the recording sessions (coordinates: 0.60 mm posterior and 1.65 mm lateral to bregma). For S1 MEA recordings of awake mice, a 32-channel silicon probe (Neuronexus A1×32-Poly2-5mm-50s-177-OA32) was inserted into hindpaw S1 and the tip of the probe was advanced to 1100 mm below the dura.
Shortly after the probe was inserted into the brain, we confirmed probe placement in hindpaw S1 by gently brushing the skin of the animal with a fine paintbrush while monitoring spikes from multiple channels. If RFs were not on the glabrous paw, the probe was removed from the brain, moved to a new location within the craniotomy, and reinserted. Otherwise, the paw was tethered over a circular aperture (7.6 mm diameter) in an acrylic platform that supported the animal. A 0.5-mm diameter, cylindrical, Teflon-tipped indenting probe was controlled by a dual-mode force controller (Aurora Scientific 300C-I) and was used to stimulate the paw through the aperture. The position, force, and displacement of the indenter were commanded with the same custom Matlab (version 2017a) scripts controlling a Nidaq board (National Instruments, NI USB 6259) used for spinal cord recordings, described above. For indentation-evoked responses, regular spiking and fast spiking units were combined and sorted by depth (Figure 7H–I). For multiregional RF mapping, we gently brushed the entire body of the animal. Regions of the body that elicited an increase in spiking to brushing were documented and included in the overall RF map.
In vivo DRG electrophysiology
In vivo recordings were made from L4 DRGs using the same preparation as previously described15,17,50 and a subset of the data presented here (Figure 5F and Figure S1C) originated from previously published recordings15. Briefly, mice were treated with urethane (1 g/kg body weight) and anesthesia was supplemented with 1–2% isoflurane during the laminectomy. The L4 DRG was exposed via a dorsal incision and laminectomy. The exposed DRG was immersed in external solution containing (in mM) 140 NaCl, 3.1 KCl, 0.5 KH2PO4, 6 glucose, 1.2 CaCl2, 1.2 MgSO4 (pH adjusted to 7.4 with NaOH) and the same solution was used to fill glass pipettes with a 20–30 μm tip diameter. Extracellular action potentials were measured using a Multiclamp 700A amplifier (Axon Instruments) operating in the voltage clamp configuration. The pipette voltage was set so that no current was flowing through the amplifier at baseline. The data was digitized at 40 kHz with a Digidata 1550a (Molecular Devices), low-pass filtered at 10 kHz (four-pole Bessel filter), and acquired using pClamp (Molecular Devices, Version 10). For MEA recordings, MEAs were inserted into the L4 DRG (H4–32ch, Cambridge Neurotech). MEA signals were high-pass filtered at 200 Hz, amplified (RHD2132) and acquired at 20kHz (RHD2000, Intantech) for offline processing.
Spike sorting
Open-source software (JRCLUST version 3.2.2;88 was used to automatically sort action potentials into clusters, manually refine clusters, and classify clusters as single or multi units. Drift monitoring was performed during acquisition and experiments with detectable changes in spike waveforms were discarded. The voltage traces were filtered with a differentiation filter of order 3. Frequency outliers were removed with a threshold of 10 median absolute deviations (MADs). Action potentials were detected with a threshold of 4.5 times the standard deviation of the noise. Action potentials with similar times across sites were merged and then sorted into clusters with a density-based-clustering algorithm89 (clustering by fast search and find of density peaks) with cutoffs for log10(r) at −3 and log10(d) at 0.6. Clusters with a waveform correlation greater than 0.99 were automatically merged. Outlier spikes (> 6.5 MADs) were removed from each cluster.
To isolate putative single units, manual cluster curation was performed with JRCLUST split and merge tools. Clusters were classified as single units if (1) the waveforms were large with respect to baseline; (2) there was a clear refractory period in the cross-correlogram (interspike intervals > 1 ms); (3) waveforms were clearly distinct from nearby clusters. Spikes event times for clusters classified as single units were exported and processed in Python.
Analysis of DH response properties and unbiased clustering
Feature extraction and clustering
Automated unsupervised clustering was performed on DH single unit response profiles to 500 ms step indentations of increasing intensities from 1mN to 75mN. For dataset collection, step indentations were applied either across the entire hindpaw (N= 106 mice; see Receptive field mapping below), or at manually determined RF centers for the majority of units on the probe (N= 36 mice). Units with spiking below the 1st spiking percentile and units with no response threshold (for which only baseline firing was detected) were excluded from this analysis. The baseline firing rate was measured during the 1s interval before each indentation trial, and analysis was performed after the baseline firing rate was subtracted from stimulus-evoked responses.
We first extracted a minority of DH units inhibited by step indentations (i.e., units with ON and early sustained responses below their baseline firing rate at 40–75mN; n = 89). These neurons comprise the inhibitory functional cluster, cluster 6. We used principal component analysis (PCA) on the remaining dataset of 4971 units to extract features of indentation-evoked signals. The extracted features resembled classically defined temporal response profiles such as ON, OFF and sustained responses, with the first two principal components (PC1 and PC2) resembling RA- and SA-LTMR responses respectively (Figure S1D). We then performed k-medoid clustering using the number of PCs that account for 90% of variance in the data (n=503). Three different distance metrics (Euclidian, cosine and correlation distance) were tested, and cosine distance was chosen because this metric resulted in better cluster quality. To find the optimal number of clusters, silhouette values were calculated, and a local maximum determined (K= 5), with the addition of the inhibitory cluster, K=6 for the complete dataset. Peristimulus time histograms (PSTHs) with 10 ms time bins were generated to show the average response across all units within a functional cluster. PSTHs are shown in 10 ms in all figures, unless otherwise stated.
Response feature analysis
Response properties of DH units across the six principal functional response types were then computed based on raw spike counts. Step indentation were subdivided into 5 distinct time windows to monitor different aspects of neuronal responses. The ON response was defined as the firing rate evoked during the first 50 ms after the stimulus onset, Early Sustained response, as 50–200 ms following ON response; Late Sustained response, as 0–200 ms before stimulus offset; OFF response, as the first 50 ms after stimulus offset and after-discharge response as 50–200 ms after stimulus offset.
Thresholds for all units were determined by bootstrapping the baseline firing rate 1000 times to generate 95% confidence intervals, and detecting the smallest stimulus within the ON/OFF/Early Sustained or Late Sustained response windows that exceeded the upper bound or drops below the lower bound.
We quantified sensitivity and response magnitude by (1) quantifying the maximum Z-scored firing rate within the 5 time intervals described above at all step indentations, and (2) by determining the fraction of units that responded to each force step (Figures 4, S4 and 5). A unit was determined to be responsive if it produced |z-scored firing rate| ≥ 3 between 10 and 50 ms after the onset or offset of the step indentation, or |z-scored firing rate| ≥ 1 during the sustained portion of the step.
Mechanical and optical stimuli
Step indentations
The amplitude of the ramp and hold indentation was 1mm, and their overall duration was 10 s, with on and off and ramps lasting 25 ms and separated by a 500 ms interval. Indentations were presented for a minimum of 30 trials at a unit’s hotspot or at multiple locations across the paw. In the glabrous skin conditional knockout mice (AdvillinCre; TrkBflox/flox and AdvillinCre; TrkBflox/flox; Atohflox/flox; Figures 4 and S4), step indentations were restricted to plantar pedal pads and digital pads to avoid small hairs in the center of the mouse hindpaw glabrous skin90.
Receptive field mapping
To measure receptive fields, step indentations of increasing forces were applied twice to randomized locations across the hind paw in a 6 ×6 mm grid with 500 μm spacing between each stimulation site. In some cases, grid spacing was modified, as specified in the figure legends. The average grid area was 36mm2. Receptive field area was calculated at forces delivered at and above each unit’s response threshold. To compute receptive fields, we first centered on each grid location and pooled all adjacent sites in a 3 × 3 grid into a larger spatial bin. For each response type, we then computed the bootstrap mean response over all 3 × 3 sites for 1000 bootstrap samples to establish 95% confidence interval. If the lower/upper bound of the CI was greater/smaller than the mean baseline firing rate of that bin, an excitatory/inhibitory response was assigned to that center site. The excitatory/inhibitory receptive field size was then calculated as the fraction of sites that had an excitatory/inhibitory response multiplied by the probed area, whereas the RF fraction was simply the fraction of sites that had an excitatory/inhibitory response.
To measure responses at the receptive field hotspot, the average firing rate across all forces and all grid locations was computed, and grid locations with the largest responses (5% of the grid with the highest responsivity) were determined. The neuron’s hotspot was defined as the location on the skin that produced the highest firing rate when stimulated. 36 locations closest to the RF hotspot were selected, and responses across these sites were averaged, creating a PSTH, to represent responsivity at the hotspot. When response profile clustering was performed at the hotspot, only the data collected at hot spot grid locations was used.
To map receptive fields beyond glabrous hindpaw at locations where force-controlled step indentation delivery was not feasible, we used a hand-held brush head (5/0 Round Princeton Art & Brush Co., Blick) mounted to a strain gauge force sensor (MBL (BL341AH) 25 gram Model MBL load cell, Sensotec-Honeywell) connected to an amplifier (DMD-465WB, Omega). Stroke was delivered for 60 s to a given body region with 5 s inter-trial intervals. Mean baseline subtracted firing rate was measured for each body region and used to compute a preference index in a subset of PSDC units. PSDC body region preference index was computed as (FRpaw − FRthigh)/(FRpaw + FRthigh); (FRpaw − FRtrunk)/(FRpaw + FRtrunk); (FRpaw − FRtail)/(FRpaw + FRtail)); related to Figure 7.
Mechanical vibration stimuli and analysis
Vibratory stimuli were delivered to manually determined receptive field hotspots at intensities ranging from 1mN to 40mN and at 10 frequencies (2Hz to 120Hz). Frequency and amplitude of sinusoidal step vibrations, lasting 1s, were presented in a randomized order, separated by 1.5s interstimulus interval for a total of 250 trails. Units were determined to be vibrationally responsive if they fired action potentials at rates above baseline to at least two frequencies delivered at the 40mN intensity. The threshold for frequency tuned units is the lowest force evoking responses above baseline to three consecutive sine waves at each frequency. Entrainment (phase-locking) was determined at intensities between 15mN and 40mN and at all frequencies. Entrained units responded with at least 0.5 spikes/cycle and displayed precise spike timing within a particular part of the sine wave. This was determined using a permutation test comparing actual spike times to randomized spike times.
Optical skin stimulation and analysis
Pulses of light were generated every 100ms or 500ms using a 300mW, 445 nm laser (CST-H-445–300, Ultralasers, Inc.). A minimum of 5000 light pulses were directed to the paw through two galvanometer scan mirrors (GVS201, Thorlabs) and an F-lens (FTH100-1064, Thorlabs), which focused the light to a 30 mm diameter spot. The intensity was modulated by inserting neutral density filters into the light path between the laser and the scan mirrors. Pulses were 1 ms in duration and the location of each pulse was randomized but confined to a 20 × 20 mm area that included the glabrous hind paw skin region. The location and timing of the light pulses were controlled using voltage signals generated with Matlab (2017b, Mathworks) and a National Instruments system (NI USB 6259). Z-scored firing rate was calculated in 1-ms bins using the baseline mean and standard deviation in the 10 ms preceding each laser pulse. Units were determined to be responsive to optical stimuli if the absolute value of the Z-scored firing rate exceeded 2.58 (98% confidence interval) within 25 ms after the laser pulse for A-fiber activation, or within 200 ms for C-fiber activation.
Optical RF measurements
Optical RFs for DH and DRG neurons were computed with 1mm2 spatial bins. Baseline-subtracted optically-evoked firing rate across these 1mm2 subregions was normalized to the maximum optically-evoked firing rate. Bins with responses > 0.5 of the maximum-normalized response were included in the overall RF area for each unit (calculated as the sum of the binarized subregions). DRG optical RFs originated were analyzed from previously published recordings15.
PSDC retrograde labeling
Animals (P13–15 for slice physiology experiments; 4–6weeks for in vivo electrophysiology; P21-P30 for histology) were anesthetized with isoflurane and placed in a stereotaxic frame. The head was tilted 30° forward. Puralube ointment was applied to the eyes. The hair over the neck and caudal scalp was removed using a clipper, and the skin was sanitized using betadine. An incision was made in the midline of the back skin at the cervical level to expose neck muscles and local anesthetic (0.5% lidocaine) was applied to the incision site. Neck muscles were removed to expose the brainstem. A small incision was made on the dura to expose the DCN. The following retrograde tracers were injected into the DCN using a glass pipette under visual guidance: Adeno-Associated Virus (AAV9- hSyn-CheRiff-TdT, titer 2.73E+15 in 0.9% saline, Boston Children’s viral core; AAV2retro-hSyn-Chronos-GFP; Boston Children’s viral core titer 1.3 E+13 in 0.9% saline, Boston Children’s viral core), Rabies Virus (RabV-deltaG-GFP, titer 5.84E+7 – 9.48E+8 IU/mL, Boston Children’s viral core), or cholera toxin subunit B (CTB; 2 mg/ml in PBS, Invitrogen). Once penetrating the surface, a small volume (30–50 nL) of tracer was injected at multiple locations in the DCN (200–400 nl total volume). The pipette was then removed, and overlying muscle and skin was stitched together with sutures. Animals were administered analgesic (Buprenex SR, 0.1 mg/kg) and monitored post-operatively. At the appropriate time point (4 weeks following AAV injections or 3–7 days following CTB or RabV injections), mice were used for electrophysiology experiments or transcardially perfused for tissue harvest.
Spinal cord slice preparation
Mice were briefly anesthetized with isoflurane, and intracardially perfused with ice-cold oxygenated choline solution (ACSF) prior to spinal cord removal. The isolated spinal cord was embedded in low-melting agarose (Sigma Aldrich), and transverse slices (300 μm) with dorsal roots attached were prepared from lumbar levels (L3-L5) using a Leica vibrating blade microtome (Leica VT1200S). Spinal cord slices were prepared in ice-cold oxygenated choline solution containing (in mM): 92 Choline Chloride, 2.5 KCL, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 2.5 Glucose, 5 Sodium Ascorbate, 2 Thiourea, 3 Sodium Pyruvate, 10 MgSO4 7H2O, 0.5mM CaCl2 2H2O. Slices recovered at 34°C for 30min in HEPES holding solution equilibrated with 95% O2, 5% CO2 containing (in mM): 86 NaCl, 2.5 KCl, 1.2 NaH2PO4, 35 NaHCO3, 20 HEPES, 25 glucose, 5 NaAscorbate, 2 Thio Urea, 3 Na Pyruvate, 1 MgSO4 7H2O, 2 CaCl2 (pH 7.3, osmolarity 305; Ting et al., 2014), and were held in the same HEPES solution at room temperature until use.
Acute slice recordings
Spinal cord slices were transferred to a submerged recording chamber at room temperature and continuously perfused with ACSF containing (in mM): 2.5 CaCl2, 1 NaH2PO4, 119 NaCl, 2.5 KCl, 1.3 MgSO4 7H2O, 26 NaHCO3, 25 dextrose, and 1.3 Na ascorbate, saturated with 95% O2, 5% CO2 at a rate of ~1–2 ml/min. Cells were visualized using infrared differential interference contrast and fluorescence microscopy. Whole cell voltage-clamp recordings of retrogradely labeled PSDCs in laminae IV-V were obtained under visual guidance using a 40x objective. Whole cell voltage clamp recordings were obtained using an internal solution containing (in mM): 135 CsMeSO3, 4 ATP-Mg2+, 0.3 GTP-Na+, 1 EGTA, 3.3 QX-314(Cl− salt), 8 Na2-Phoshocreatine and 10 HEPES. Synaptic currents were evoked with electrical stimulation of dorsal roots using a suction electrode at Aβ fiber strength (<=25 mA, 20–100μs; Nakatsuka et al., 2000; Torsney and MacDermott, 2006). For isolation of sensory-evoked EPSCs and feedforward IPSCs, PSDC neurons were voltage-clamped alternatively at the reversal potential for synaptic inhibition (−70mV) and excitation (0mV). To activate ChR2 in acute slices, LED whole field illumination was used through a water immersion 40x objective. Aβ-LTMR axon terminals were stimulated with brief pulses (1–5ms) of blue light (473 nm, 5mW). Optically-evoked IPSCs (oIPSCs) were blocked by inhibitory or excitatory transmission blockers as specified in the figure legends.
For plasticity experiments, sensory-evoked glutamatergic EPSCs in PSDCs were isolated using pharmacological antagonists of GABAARs and glycine receptors (10 μM bicuculline, 1 μM strychnine). High-frequency stimulation (HFS; two 1 s trains at 100 Hz, intertrain interval 20 s, at 1.5 times test current intensity) was delivered after a stable 10 min baseline.
Current-clamp recordings were obtained with an internal solution containing (in mM): 135 K-Gluconate, 10 NaCl, 2 MgCl2, 0.5 EGTA, 10 HEPES, 2 Mg-ATP, 0.3 Na-GTP. Input resistance and access resistance were monitored continuously throughout each experiment and cells were excluded from analysis if these values changed by more than 10% during the experiment or if the resting membrane potential was higher than 50 mV. Data were acquired using a Multiclamp 700B amplifier, a Digidata 1440A acquisition system, and pClamp 10 software (Molecular Devices). Sampling rate was 10 kHz, and data were low-pass filtered at 3 kHz. No correction for junction potential was applied.
Spinal cord immunohistochemistry of free-floating sections
Mice (P30-P35) were anesthetized with CO2 and perfused with 5–10mL modified Ames Media (Sigma) in 1x PBS, followed by 20–40 mL of 4% paraformaldehyde (PFA) in PBS at room temperature (RT). Vertebral columns (including spinal cords and dorsal root ganglia) were dissected and were post-fixed in 4% PFA at 4°C for 2–16 hr. Lumbar spinal cord sagittal sections (100–150 μm) were cut on a vibrating blade microtome (Leica VT100S) and processed for immunohistochemistry as described previously (Hughes et al., 2012; Abraira et al., 2017). Briefly, tissue samples were rinsed in 50% ethanol/water solution for 30 min to allow for enhanced antibody penetration. Three washes in high salt Phosphate Buffer Saline (HS PBS) were conducted each lasting 10 min. The tissue was then incubated in primary antibodies in high salt Phosphate Buffer Saline containing 0.3% Triton X-100 (HS PBSt) for 48–72 hr at 4°C. Primary antibodies used goat anti-mCherry (1:1000, AB0040, Sicgen), rabbit anti-GFP (1:1000, A-11122, Thermo Fisher Scientific) and mouse anti-gephyrin (1:1000; Synaptic Systems). The tissue was washed in HS PBSt, then incubated in a secondary antibody solution in HS PBSt overnight at 4°C. Secondary antibodies included species-specific Alexa Fluor 405, 488, 546, and 647 conjugated IgGs (Life Technologies). Tissue sections were then mounted on glass slides, coverslipped with Fluoromount Aqueous Mounting Medium (Sigma) and stored at 4°C.
Immunohistochemistry of frozen tissue sections
Brains and vertebral columns, including spinal cords and dorsal root ganglia, were removed from perfused mice and post-fixed in 4% PFA at 4 °C overnight. Tissues were washed in 1× PBS for over 3 h, and brains and spinal cords were finely dissected out from the rest of the tissue. Brain and spinal cord tissues were cryoprotected in 30% sucrose at 4 °C for 2 days, embedded in OCT (1437365, Fisher), frozen using dry ice and stored at −80 °C. Coronal brain sections and transverse spinal cord sections (30–40 μm) were cryosectioned on a cryostat (Leica). Spinal cord sections were collected on glass slides (12-550-15, Fisher), and brain sections were collected on glass slides or in 1× PBS. Sections were washed three times for 5 min with 1× PBS containing 0.1% Triton X-100 (0.1% PBST), incubated with blocking solutions (0.1% PBST containing 5% normal goat serum (S-1000, Vector Labs) or normal donkey serum (005-000-121, Jackson ImmunoResearch) for 1h at RT, incubated with primary antibodies diluted in blocking solutions at 4 °C overnight, washed three times for 10 min each with 0.1% PBST, incubated with secondary antibodies diluted in blocking solutions at 4 °C overnight, washed again four times for 10 min each with 0.1% PBST and mounted with Fluoromount-G (Southern Biotech). For spinal cord sections, IB4 (1:500; Alexa 647 conjugated, L21411, Molecular Probes) was incubated together with secondary antibodies. Primary antibodies used include goat anti-mCherry (1:1,000, AB0040, Sicgen), chicken anti-GFP (1:1,000, GFP-1020, Aves Labs), rabbit anti-GFP (1:1,000, A-11122, Thermo Fisher Scientific), mouse anti-NeuN (1:1,000, MAB377, Millipore). Secondary antibodies included Alexa 488–conjugated donkey anti-chicken antibodies, Alexa 546–conjugated donkey anti-goat antibodies and Alexa 647–conjugated donkey anti-mouse antibodies. All secondary antibodies were purchased from Thermo Fisher Scientific and Jackson ImmunoResearch Labs, and used at 1:500 dilution.
DH synaptic connectivity analysis
Synaptic connectivity analysis between Rorβ and PSDC neurons was performed in RorβCreER; R26LSL-synaptophysin-tdTomato mice in which PSDC neurons were retrogradely labeled with dG-RV-GFP (see above), as previously described (Abraira et al. 2017). In addition to the genetically encoded presynaptic marker synaptophysin, the inhibitory postsynaptic marker gephyrin was used in this analysis. A total of 4 animals were used.
Z stack images of spinal cord slices were taken on a Zeiss LSM 700 confocal microscope using a 40X oil-immersion lens (Zeiss; Plan-Apochromat 40X/NA 1.40) and scanned at a z-separation of 0.5 μm. Images were taken in DH lamina IIiv-IV, which was defined as between the lamina IIiv border (marked by IB4 binding) and 250 μm below that border. Imaging parameters (laser power, gain/offset, averaging, dwell time, etc.) were consistent across animals.
For analysis, images were first pre-processed using ImageJ: using the channel of PSDC labeling, two masks were generated – one using a standardized threshold for signal in this channel and a second by expanding this first mask by 1 μm in all dimensions. These masks were then used to isolate pre- and post-synaptic labeling by multiplying these channels (using the Image Calculator function) with the expanded and non-expanded masks, respectively. Next, images were analyzed using the Cell Counter ImageJ plugin to determine the proportion of inhibitory reporter terminals that apposed a gephyrin-immunoreactive puncta. Puncta were counted as a function of location: cell body, proximal neurite (within the first 50 μm) or distal neurite (beyond the first 50 μm).
In situ hybridization
Detection of Piezo2, NaV1.8, CGRP, Mrgprd and NFH transcripts in lumbar DRG neurons was performed by fluorescent in situ hybridization, as previously described50,91. Briefly, lumbar DRG ganglia were rapidly dissected from euthanized mice, frozen in dry-ice and stored at −80°C until further processing. DRGs were cryosectioned at a thickness of 20 μm and RNA was detected using RNAscope (Advanced Cell Diagnostics) according to the manufacturer’s protocol. The following probes were used: Mm Piezo2 exons 43–45 (Cat# 439971-C3), NaV1.8 (Cat#: 426011-C2), Calca (Cat#:420361), Mm-Mrgprd (Cat#: 417921), Mm-Nefh (Cat#: 443671-C2). Sections were mounted in FluoroMount-G (Fisher 0100–01) and imaged on a Zeiss LSM 700 confocal microscope using a 10x or 20x objective.
Detection of Rorβ and Slc32a1 transcripts in lumbar spinal cord neurons was performed as previously described (Choi et al., 2020). Briefly, lumbar spinal cords were dissected immediately embedded in OCT, and frozen in dry-ice. Spinal cord tissue was cryosectioned (20 μm) and mRNA was detected as described for lumbar DRGs. The RNA scope catalogue probes were used to detect Rorβ (Cat#: 444271-C2) and Slc32a1 (Cat#: 319191) mRNA.
Behavior
To assess the contribution of dorsal horn Rorβ neurons to somatosensory behaviors, three complementary intersectional inactivation strategies were used: (1) RorβCreER in conjunction with Cdx2-NSE-FlpO for restricted FlpO expression to the spinal cord and the dual recombinase tetanus toxin line RC:PFtox; (2) RorβCreER; Vgatflox/flox and (3) RorβiCre; Vgatflox/flox for selective block of GABA/glycine release from either adult (2) or inhibitory lineage Rorβ+ neurons (3). The RorßCreER driver line captures the adult Rorß population, but is not 100% efficient. On the other hand, the RorßiCre; vGatflox/flox intersectional approach excludes lineage-labeled excitatory Rorß neurons in the spinal cord, as well Rorβ+ neurons in S1 known to be exclusively excitatory, and is more penetrant (as it recapitulates the locomotor hindlimb impairments reported in Rorβ−/− mice).
Prepulse Inhibition (PPI) Assay
Tactile PPI Assay was used as a measurement of hairy skin sensitivity, as described previously (Orefice et al., 2016). Briefly, using a San Diego Instruments startle reflex system (SR-LAB Startle Response System), mice were tested in a cylindrical chamber within a soundproof chamber. For tactile PPI, a prepulse of an air puff was administered to the hairy skin of the back. The air puff was delivered at a constant intensity (0.9 PSI), at varying intervals before the startle pulse, ranging from 50ms to 1s. A tone pre-stimulus (ranging from 68 dB to 80 dB, for 20ms), followed by startle tone stimulus (120 dB, 20 ms) version of the PPI assay (acoustic PPI) was done as a control. Acoustic PPI was done with background noise set at 65dB, while tactile PPI had background noise at 75dB to ensure the air puff prepulse could not be heard. Startle reflex was quantitated using an accelerometer measuring the amplitude of movement of the animal.
Texture NORT
Texture NORT was used to measure texture discrimination in glabrous skin, as previously described (Orefice et al., 2016). On day 1 and 2 of behavioral testing, animals were individually habituated to an empty testing chamber (40 cm × 40 cm × 40 cm) under dim lighting for 10 min. Following habituation, animals were tested on color/shape NORT (day 3) and texture NORT (day 4). During the learning phase, the animal explored the testing chamber, in which two identical objects spaced equidistant from each other and the chamber walls, for 10 min. The animal was returned to the home cage for a 5 min retention period, during which the chamber and objects were thoroughly cleaned with 70% ethanol and one of the objects was replaced with a novel object. After the 5 min period, the animal was returned to the chamber for the 10 min exploration of the testing phase. Both the learning and testing phases were video-recorded from above. Custom MATLAB scripts were used to track animal position in the chamber and calculate the amount of time the animal spent investigating the objects. For color/shape NORT, the objects were wooden blocks differing in shape and color. In texture NORT, the objects were plexiglass cubes (4 cm3) that were visually identical but varied in texture (rough or smooth). Animals were whisker plucked three days before the start of habituation. The preference for the novel object was calculated based on the time exploring both objects.
Sunflower Seed Assay
To test the role of feed-forward and presynaptic inhibition in a fine sensorimotor behavior, we used a Sunflower Seed Handling Assay, as described previously (Neubarth et al., 2020). To habituate animals, one week prior to testing one to two tablespoons of black oil sunflower seeds (Bio-Serv, S5137-1, Wagner’s, 76025) were added to the floor of the animals’ home cage for five consecutive days. If animals did not recognize seeds as a food source, then a teaspoon of seeds were cracked before adding to the cage floor.
Habituation to the behavior chamber and handling began two days prior to sunflower seed testing. Animals were habituated to the behavior room environment and investigator handling by undergoing tail inking on habituation day 1. To ink the tail, each animal was gently lifted and placed on the cage wire food hopper facing away from the investigator. Firmly holding the tail midway from the tail base, a blue permanent soy ink marker was rolled across the tail forming parallel lines to indicate identifying ear notch numbers. Once inked animals were gently transferred back into the home cage to await test chamber habituation.
The test chamber was constructed of a black matte acrylic wall and three optically clear walls, 10 in (l) × 8 in (w) × 8 in (h), 0.25 in thick, centered on a white matte acrylic floor under diffuse warm white light (2700K). Three digital USB 2.0 CMOS video cameras mounted on camera sliders were positioned on each clear side of the test chambers. One additional overview camera was mount directly above the test chamber.
Two days prior to testing and during testing seeds were withheld from the home cage to encourage foraging and seed eating in the test chamber. Animals were not food restricted for this assay. On habituation day 1 and day 2, animals were removed from the home cage and placed in an empty test chamber resting on the white matte acrylic floor. Each animal was given 2–3 seeds while freely exploring the test chamber for 20 minutes. Following the exploration animals were then returned to their home cages. Testing began on day 3. Animals were transferred from their home cage and placed in the test chamber and allowed to explore the chamber for 5 minutes. Following acclimation, 2–3 seeds were placed on the floor of the test chamber and seed eating activity was recorded. At the completion of the seed eating test animals were removed from the test chamber and returned to their home cage. Chambers were reset and cleaned with unscented soapy water, wiped down with ddH2O and dried. Animals that failed to eat seeds after 20 minutes were returned to their home cage and the test rescheduled. This schedule was repeated until each animal fully deshelled and consumed multiple seeds. Seeds that were partially deshelled/consumed or discarded were not counted.
Behaviors were measured by defined seed deshelling and eating actions: (i) Seed peeling and deshelling—the act of grasping and holding the sunflower seed between the forepaws, clamping the upper and lower incisors into the shell surface, and applying downward force (dip) that pushed the shell away from the head and teeth toward the floor. This action resulted in a systematic peeling of the shell to expose the seed kernel. Animals unable to maintain a firm grip on, or fully grasp, the shell would typically adapt by touching, tapping, resting and/or bracing the seed against the floor between the forepaws. Animals also “tucked” the shell against their abdomen, holding the shell between the forepaws, clamping their upper and lower incisors onto the shell surface pulling their heads backward away from the shell and forepaws to peel off sections of the shell exposing the seed kernel. (ii) Touch-taps—the act of touching and/or holding and/or bracing the seed shell between the forepaws and the floor during seed peeling. (iii) Dip—the act of holding the seed shell between forepaws, clamping shell between incisors, and applying downward force to peel off sections of shell. (iv) Rotate—the act of or ability to change and/or manipulate shell orientation within the forepaws. (v) Rocking—the act of grasping the shell between the forepaws with the shell firmly between incisors, using forepaws to “rock” the shell side to side between the incisors to bite into the shell to peel and expose the seed kernel.
Quantification and statistical analysis
Statistical tests were conducted using the SciPy stats module (Python 3.8.5) or GraphPad Prism. Both non-parametric tests and parametric tests were used, depending on data normality, for comparing two independent groups (Mann-Whitney U test or Student’s t test), and multiple groups (Kruskal-Wallis H test/one-way ANOVA or two-way ANOVA for multiple groups with multiple timepoints). All statistically tests performed are indicated in the figure legends or supplemental statistics table. p < 0.05 was considered significant. Additional details on sample sizes and statistical tests for each experiment can be found in the figure legends, main text, and the supplemental statistics table S1.
Supplementary Material
Figure S1. Functional characterization of mechanosensory responses in DH neurons. Related to Figure 1.
A. In vivo MEA recordings from L4 DRG neurons in anaesthetized mice. Spike raster plots and peristimulus time histograms (PSTHs) aligned to 50 mN indentation step from example Aβ RA-LTMR (top) and Aβ SA-LTMR (bottom). PSTHs are 10ms bins.
B. Example DiI-coated MEA in L4 spinal DH from a mouse expressing ChR2-YFP in Mrgdprd+ polymodal nociceptors (MrgdprdCreER; R26LSL-ChR2-EYFP) terminating in lamina IIi also labeled with IB4 (green, EYFP; blue, IB4 binding protein; red, DiI coating probe tract). Scale bar, 100μm.
C. Average waveform and corresponding autocorrellogram for example DH unit.
D. Top: Average maximum-normalized responses (±SEM) for Aβ SA-LTMRs (n = 11 neurons) and Aβ RA-LTMRs (n = 13 neurons) at the ON, OFF, and sustained portion of step indentations. Bottom: Average maximum-normalized firing rate (±SEM; shaded regions) for all mechanically sensitive DH neurons.
E. Unsupervised clustering pipeline for identifying DH functional response profiles. Left: Heatmap of individual DH units sorted by their response thresholds to step indentations across all experiments. Top, force traces ranging in intensity from 1 to 75 mN. Bottom, grand average z-scored PSTH. First, units with decreased firing rates to step indentations, the ‘inhibitory group’, were separated from the overall dataset. PCA analysis was then used to extract features of indentation-evoked responses from the remaining dataset. Inset shows PC1 and PC2 accounting for 25% of variance in the data, and resembling responses with transient or sustained components. Finally, the number of PCs accounting for 90% of the variance (n=503) were used for k-mediods clustering. This approach yielded 6 principal functional clusters: 5 excitatory clusters are shown in TSNE space and cluster 6 is comprised of the ‘inhibitory group’.
F. Occurrence of units sorted into the 6 principal clusters across experiments.
G. Proportion of unit response thresholds within each principal cluster (median is denoted as dashed red line).
H. Summary boxplots of spontaneous firing rates for DH units in each functional group Boxplots: median (line), quartiles (box), minimum and maximum (whiskers).
I. Nominal depth vs. laminar distribution for each functional group. Data represent cluster means ± SD. Spearman correlation coefficient for correlation over individual units r= 0.1676; P = 2.83*10−30.
Figure S2. Functional response profiles at RF hotspot and vibrotactile features. Related to Figure 2.
A. Spike raster plot of DH unit linear RF organization with trials in the RF hotspot colored in red (top). Mean PSTHs for RF hotspot (red) and RF surround (black) shown below. Note the increase in firing rate from RF surround to RF hotspot.
B. Functional clustering was performed as described (see Methods; Figure S1 D) at the RF hotspot. For each cluster, a heatmap of all included DH units responding to indentation is shown above the average z-scored PSTH. Heatmaps are sorted by force thresholds to step indentations.
C. Percentage of DH units used in RF hotspot clustering per functional cluster.
D. Percentage incidence of DH units that are frequency tuned across the 6 functional clusters.
E. Percentage incidence of phase-locked DH units across the 6 functional clusters.
Figure S3. Functional characterization of distinct DH interneuron types. Related to Figure 3.
A. Light-evoked first spike latency probability distribution from the optotagged Rorβ unit in B (left) and non-tagged neighboring unit (right).
B. Histogram of optical test (SALT) P values for optically tagged Rorβ units and simultaneously recorded non-tagged units (n=21; 5 mice; p < 0.01, blue for tagged units; p=1; grey for non-tagged units).
C. Raster plot and PSTH of the same Rorβ unit in the absence or presence of NBQX (5 mM) applied to the surface of the cord to block excitatory synaptic transmission.
D. Left: Summary light-evoked firing before and after NBQX application. Optotagged units (blue) had similar optically evoked firing rates in the two conditions (Two-sided paired t test). Right: Summary mechanically-evoked firing before and after NBQX application. Mechanically-evoked spikes were blocked in both Rorβ optotagged units (blue) and non-tagged units (grey; p < 0.01; Two-sided paired t test). N = 3 mice.
E. Left: Mechanical responses to a series of step indentations for all optotagged inhibitory DH units (top) and average PSTH (bottom) from VgatiCre; R26LSL-ChR2 mice. Right: Assignment of optotagged Vgat+ interneurons to functional response clusters.
F. As in (D), for simultaneously recorded putative excitatory DH interneurons (left), and cluster assignment (right).
G-K. As in (D), for genetically distinct inhibitory interneurons (F-H): Rorβ (using RorβiCre; VgatFlpO; R26LSL-FSF-ReaChR-mCitrine mice); PVi (PV2aCre; VgatFlpO; R26LSL-FSF-ReaChR-mCitrine); Kcnip2 (Kcnip2CreER; R26ChR2-YFP); and excitatory interneurons (I-J): CCK (CCKiCre; R26ChR2-YFP) and PKCγ (PKCγCreER; R26ChR2-YFP and PKCγCreER; Lbx1FlpO; R26LSL-FSF-ReaChR-mCitrine).
L. Spontaneous firing rates for all optotagged DH units.
M. Frequency- threshold tuning curves for genetically distinct interneuron populations.
Figure S4. DH neuron responses in Piezo2 conditional knockout animals. Related to Figure 4.
A. Mean firing rates in response to mechanical and optical activation of glabrous hindpaw in all DH units from littermate controls (Piezo2flox/flox; AdvillinFlpO R26LSL-FSF-ReaChR; top; n= units, 3 mice) and Cdx2-Cre; Piezo2flox/flox; AdvillinFlpO R26LSL-FsF-ReaChR animals (bottom; 3 mice). Units are sorted by depth. Note the timescale difference between mechanical and optical heatmaps. Scale bar: 50 units.
B. Mechanical and optical responses to skin stimulation in example DH units from control (left) and Cdx2-Cre; Piezo2flox/flox; AdvillinFlpO R26LSL-FSF-ReaChR animals, showing both short and long-latency spikes to optical stimulation of the skin.
C. Left: Summary of indentation-evoked firing rates in control (N=4) and Cdx2-Cre; Piezo2flox/flox; AdvillinFlpO; R26LSL-FSF-ReaChR; Cdx2-Cre; Piezo2flox/flox (N=2) animals. Each dot represents one animal. Bars: mean. Right: Percentage of units responding to indentation and optical stimulation. Few units in Piezo2 mutants responded to indentation, however optical stimulation of the skin elicited similar amounts of responsivity in control and Cdx2-Cre; Piezo2flox/flox; AdvillinFlpO; R26LSL-FSF-ReaChR animals. P < 0.05, Mann-Whitney U test.
D. Example raster plot and PSTH of DH unit form Piezo2 cKO mouse showing no response to stroke on the skin, and robust response to noxious pinch.
E. Representative images of Piezo2 and Nav1.8 smRNA-FISH from L1-L6 DRGs in Nav1.8Cre; Piezo2flox/flox animals (bottom), or Piezo2flox/flox littermates (top).
F. Quantification of the percentage overlap between Piezo2 and DRG markers Nav1.8, Mrgdprd, CGRP, and NFH. Multiple lumbar DRGs from three Nav1.8Cre; Piezo2flox/flox and three Piezo2flox/flox littermates were quantified. ***p<0.0001; **p<0.005; Unpaired t test.
G. Z-scored firing rates of all dorsal horn units from littermate control (Piezo2flox/flox; top, N=3) and Nav1.8-Cre; Piezo2flox/flox (Nav1.8 cKO; bottom, N=3). Units are sorted by depth. Scale bar: 50 units.
H. Grand mean baseline-subtracted firing rates (± SEM) to 10, 20 and 75mN step indentations across all units from control (black) and Nav1.8 cKO (red) animals.
I. Proportion of units from control (black) and Nav1.8-Cre; Piezo2flox/flox animals (red) responding at the ON (left), OFF (middle) and sustained (right) phase of step indentation across all forces tested. * p < 0.05, two-proportions Z test.
J. Response thresholds from all units in control, and Nav1.8-Cre; Piezo2flox/flox animals. Bar: median with 95% CI. n.s., Mann-Whitney U test.
Figure S5. Sensory-evoked feed-forward and feedback inhibitory circuit motifs exert broad control over DH neuron responses. Related to Figure 6.
A. Optogenetic activation of Aβ-RA LTMR terminals evokes monosynaptic EPSCs and feedforward inhibition onto PSDC neurons. Left: Application of AMPA receptor antagonist NBQX (10 μM) blocks both oEPSC and oIPSC. Right: Example EPSCs under baseline conditions and following co-application of voltage-gated Na+ channel blocker TTX and voltage-gated K+ channel blocker 4-aminopyridine (4AP).
B. Top: Example EPSC in a PSDC neuron evoked by optogenetic activation of Nav1.8+ sensory terminals. Bottom: Connectivity rate for NaV1.8+ oEPSCs to PSDCs (left) and onset latencies for opto-evoked primary afferent EPSCs onto PSDC neurons (right).
C. Summary current clamp recordings from PSDC neurons in response to current injection ramps paired with a 1s optical activation of Rorβ neurons (blue; n=6 PSDCs).
D. Example current clamp recording from eNpHR3.0-expressing Rorβ neuron. Light activation of eNpHR3.0 confirms silencing of spiking. Rheobase trace is shown in red.
E. Top: Voltage-clamp recording from PSDCs isolates direct sensory-evoked EPSC (black) and polysynaptic IPSC (orange) at different holding potentials in spinal cord slices from RorβiCre; R26LSL- eNpHR3.0-YFP mice. Bottom: Summary of normalized EPSC and IPSC amplitudes with (ON) and without (OFF) suppression of Rorβ neurons. N=3 mice; n=3 PSDCs; p>0.1 EPSC; p < 0.05 IPSC; Paired two-tailed t test.
F. In vivo MEA recording from PViCre; R26LSL- eNpHR3.0-YFP mice. Right: raster and PSTH for example PVi interneuron suppressed by eNpHR3.0-YFP, and quantification of the firing rate suppression in putative PVi interneurons (n=6 units; one mouse). Left: example PSTH to 10mN step indentation without (black) and with (orange) acute PVi silencing, and quantification of the effect of PVi suppression on indentation-evoked spiking at the onset of step indentation in neighboring units on the probe (n=12 units). This effect was observed in 12 out of 48 units.
G. In vivo MEA recording from RorβiCre; R26LSL- eNpHR3.0-YFP mice. Left: Raster and PSTH for example Rorβ interneuron suppressed by eNpHR3.0-YFP, and quantification of firing rate suppression in all putative Rorβ interneurons (n=14 units; 5 mice). Right: Example PSTH to 10mN step indentation without (black) and with (orange) acute Rorβ silencing, and quantification of the effect of Rorβ suppression on indentation evoked spiking at the ON response (n=7 units; 5 mice) in neighboring units on the probe. This effect was observed in 7 out of 28 units.
H. Confocal images showing synaptophysin-tdTomato expression driven by RorβCreER, and PSDC neurons (green) retrogradely labeled from DCN with glycoprotein (G) gene-deleted rabies virus (ΔG-GFP-RV). Co-labeling with gephyrin (blue) was used to determine axo-dendritic contacts from Rorβ+ inhibitory interneurons to PSDCs (arrowheads), quantified across the LTMR-RZ (right).
I. Median (±95% CI) firing rates for DH units in littermate control (white), FFI cKO (red) and PSI cKO (teal) mice in response to 1mN-75mM step indentations at the ON, OFF, sustained, and after-discharge periods.
J. Average RF areas at 10mN force steps measured during the sustained (left) and OFF (right) phase of step indentations in control, FFI cKO and PSI cKO mice. **p < 0.01; *p < 0.05, standard deviation of bootstrapped RFs.
K. Loss of LTP at Aβ-PSDC synapses in spinal cord slices form RorβiCre; Cdx2FlpO; RC:PFtox mice. Averaged EPSC amplitudes before and after high frequency dorsal root afferent stimulation (arrow) in PSDC neurons from control (n=3 PSDCs, 3 mice) and Rorβ silenced mice (n=3 PSDCs, 3 mice). Insets: Averaged EPSCs from single experiments before and 20 min after HFS.
L. Left: experimental design. Right: Raster plot and PSTH of DH unit in response to cutaneous electrical stimulation. Both short latency and long latency spikes were evoked, indicative of A and C fiber input.
M. DH indentation responses to 75mN force steps before and after cutaneous HFS stimulation in control (black) and FFI cKO (red) mice.
N. Summary firing rates evoked during ON and sustained phases of 75mN step indentations before and after cutaneous HFS stimulation in DH units from control (black), and FFI cKO mice. **p < 0.01; ****p < 0.0001, Mann-Whitney test.
Figure S6. In vivo identification and mechanical response properties of PSDC neurons. Related to Figure 7.
A. Left: Schematic of PSDC retrograde viral labeling and recording configuration. This strategy results in excitatory opsin expression in both PSDCs and Aβ DCN projecting sensory neurons. Right: transverse lumbar spinal cord section with retrogradely labeled PSDCs and Aβ LTMR terminals (green), and IB4+ terminals (blue).
B. Left: Example light-evoked raster plot and PSTH of PSDC neuron in the presence of NBQX (5mM) blocking excitatory synaptic transmission from direct pathway sensory neurons. Middle: summary light-evoked spike latencies in putative PSDC units. Right: Mechanically evoked responses for simultaneously recorded DH units before and after NBQX application to the surface of the cord. Mechanically-evoked spikes were blocked in both putative PSDC optotagged units (blue) and non-tagged units (grey; P < 0.01; Two-sided paired t test).
C. Left: Dorsal column antidromic stimulation strategy. Right: Raster plot and PSTH in response to electrical antidromic stimulation of dorsal column axons at cervical levels C1-C3. Putative PSDC units continued to spike with short latencies and jitter in the presence of NBQX.
D. Boxplots for all tagged PSDC neurons show low first spike latency and small jitter to DC antidromic stimulation.
E. Summary of antidromic spike rates in putative PSDC neurons (purple) and neighboring units (grey) before and after NBXQ application. P < 0.0001; two-sided paired t test.
F. Summary of firing rates in response to skin stroke before and after NBQX application confirms excitatory synaptic transmission blocking efficiency (mean: orange). P < 0.0001; Two-sided paired t test.
G. Tagged PSDC neurons receive direct input from Aβ-RA and Aβ-SA LTMRs. Mechanical and optically- evoked PSTHs (mean ± SEM) in example putative PSDC unit from TrkBCreER; AdvillinFlpO; R26LSL-FSF-ReaChR-mCitrine (top) and from TrkCCreER; AdvillinFlpO; R26LSL-FSF-ReaChR-mCitrine (bottom) mice.
H. Proportion of mechanically sensitive PSDC units that respond to optical activation of distinct sensory neuron types in glabrous hindpaw. Each marker represents one animal. Bars: mean.
I. Example PSDC spatiotemporal RF organization.
J. Left: Example responses to mechanical vibrations in tagged PSDC neurons (bottom), and simultaneously recorded non-tagged DH units (top). Right: Phase-locked responses and vibration frequency tuning across all recorded PSDCs (purple) and simultaneously recorded nontagged units (black).
K. PSDC cluster assignment based on responses to step indentations.
Figure S7. DH outputs shape tactile responses in S1 and somatosensory behaviors. Related to Figure 7.
A. Baseline-subtracted mean firing rates in tagged PSDC neurons from control, FFI cKO and PSI cKO mice at the ON, OFF, and the sustained portion of the indentation step. Error bars: SEM.
B. C.V. of ON and OFF response magnitudes at indentation force steps between 2 to 75mN, measured from PSDC neurons in FFI cKO (red markers; n=17); PSI cKO (teal markers; n=14) and 50 representative sets of 14 randomly sampled PSDC neurons from control mice (grey markers).
C. RF fraction of skin area mapped with increasing force steps in control PSDC neurons vs. PSDC neurons from FFI cKO and PSI cKO animals.
D-E. Fraction (± SEM) of control and FFI cKO S1 units that produce a response at the ON (left), OFF (right) and sustained phase of the step indentation for each force. *P< 0.05; two-proportions Z test.
F. Cross-sections through brain, brainstem and lumbar spinal cords from RorβiCre; TauLSL-nlslacZ; gad2-mCherry animals. Colocalization of the inhibitory marker gad2 (red) with Rorβ lineage neurons (green) was found in lumbar spinal cord (right), but not at other sites of the somatosensory pathway including DCN, somatosensory thalamus or S1. Inhibitory Rorβ+ neurons were also found in amygdala and striatum. Filled arrowheads: Rorβ+ neurons that do not express gad2; asterisk: double-positive neurons.
G. Left: transverse lumbar DH sections from Vgatflox/flox and RorβiCre; Vgatflox/flox animals. Rorβ (green) and Vgat (red) mRNA molecules were detected with gene-specific RNA scope probes. Empty arrowheads: double-positive neurons; filled arrowheads: Rorβ+ neurons that do not express Vgat. Right: Quantification of the percentage overlap between Rorβ and Vgat markers. Multiple LTMR-RZ sections were quantified from three Vgatflox/flox littermate control and three RorβiCre; Vgatflox/flox mice. P<0.001; Unpaired t test.
H. Genetic strategies used for selective silencing of spinal cord inhibitory Rorβ neurons. Right, silencing Rorβ neurons in RorβiCre; Vgatflox/flox causes characteristic hindlimb hyperflexion locomotor phenotype, previously described37,64, demonstrating efficiency of the silencing strategy.
I. Percentage inhibition of the startle response to a 125-dB noise, when the startle response was preceded by a light air puff (0.9PSI) in control littermates and Rorβ silenced mice. *P < 0.05; Unpaired t test.
J. Discrimination indices for texture NORT and shape NORT in control littermates and Rorβ silenced mice. Positive value indicates preference for the novel object compared to the familiar object. *P < 0.05; Unpaired t-test.
K. Sunflower seed assay reveals distinct sensory-motor phenotypes in DH FFI cKO (green) compared to PSI cKO (red) animals. **P< 0.01 and ****p < 0.0001; Unpaired t test.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat polyclonal anti-mCherry | Sicgen | Cat# AB0040, RRID:AB_2333093 |
| Rabbit polyclonal anti-GFP | Thermo Fisher Scientific | Cat# A-11122, RRID:AB_221569 |
| Chicken polyclonal anti-GFP | Aves Labs | Cat# GFP-1020, RRID:AB_10000240 |
| Mouse monoclonal anti-gephyrin | Synaptic Systems | Cat# 147 011C5, RRID:AB_2810214 |
| Mouse monoclonal anti-NeuN | Millipore | Cat# MAB377, RRID:AB_2298772 |
| IB4 (Alexa-647 conjugated) | Molecular Probes | Cat# L21411, RRID:AB_2314665 |
| Donkey anti-Goat IgG Alexa Fluor 546 | Thermo Fisher Scientific | Cat# A-11056, RRID:AB_2534103 |
| Donkey anti-Rabbit IgG Alexa Fluor 488 | Thermo Fisher Scientific | Cat# A-21206, RRID:AB_2535792 |
| Donkey anti-Chicken IgG Alexa Fluor 488 | Jackson ImmunoResearch Labs | Cat# 703-545-155, RRID:AB_2340375 |
| Donkey anti-Mouse IgG Alexa Fluor 488 | Thermo Fisher Scientific | Cat# A-21202, RRID:AB_141607 |
| Donkey anti-Mouse IgG Alexa Fluor 488 | Thermo Fisher Scientific | Cat# A32766, RRID:AB_2762823 |
| Bacterial and virus strains | ||
| AAV9- hSyn-CheRiff-TdT | Boston Children’s viral core | N/A |
| AAV2retro-hSyn-Chronos-GFP | Boston Children’s viral core | N/A |
| RabV-deltaG-GFP | Boston Children’s viral core | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Tamoxifen | Sigma | Cat# T5648–1g |
| Sunflower seed oil | Sigma | Cat# S5007 |
| Urethane | Sigma | Cat# U2500 |
| Isofluorane | Henry Schein | Cat# 029405 |
| NBQX disodium salt | Tocris | Cat# 1044 |
| SR95531 | Tocris | Cat# 1262/10 |
| Strychnine hydrochloride | Sigma | Cat# S8753 |
| Tetrodotoxin citrate | Tocris | Cat# 1069 |
| 4-aminopyridine (4AP) | Tocris | Cat# 0940 |
| Vybrant DiI Cell-Labeling Solution | Thermo Fisher Scientific | Cat# V22885 |
| Paraformaldehyde, reagent grade, crystalline | Millipore Sigma | Cat# P6148–500G |
| Critical commercial assays | ||
| RNAscope Fluorescent Multiplex Assay | ACD Bio | Cat# 320850 |
| RNAscope Protease III & Protease IV Reagents | ACD Bio | Cat# 322340 |
| RNAscope Probe Diluent | ACD Bio | Cat# 300041 |
| Experimental models: Organisms/strains | ||
| Mouse: VgatiresCre | The Jackson Laboratory | RRID:IMSR_JAX:016962 |
| Mouse: RorβiresCre | The Jackson Laboratory | RRID:IMSR_JAX:023526 |
| Mouse: RorβCreER | The Jackson Laboratory | RRID:IMSR_JAX:030290 |
| Mouse: Pvalb2a-Cre | The Jackson Laboratory | RRID:IMSR_JAX:012358 |
| Mouse: Kcnip2-CreER | The Jackson Laboratory | RRID:IMSR_JAX:030385 |
| Mouse: CCKiresCre | The Jackson Laboratory | RRID:IMSR_JAX:012706 |
| Mouse: PKCγCreER | The Jackson Laboratory | RRID:IMSR_JAX:030289 |
| Mouse: Lbx1FlpO | Bourane et al., 2015 | N/A |
| Mouse: Vgat-2A-FlpO | The Jackson Laboratory | RRID:IMSR_JAX:029591 |
| Mouse: Gad2T2A-NLS-mCherry | The Jackson Laboratory | RRID:IMSR_JAX:023140 |
| Mouse: Cdx2-Cre | Coutaud and Pilon, 2013 | N/A |
| Mouse: Cdx2-NSE-FlpO | Abraira et al., 2017 | RRID:IMSR_JAX:030288 |
| Mouse: AdvillinCre | Hasegawa et al., 2007 | RRID:IMSR_JAX:032536 |
| Mouse: AdvillinFlpO | Choi et al., 2020 | N/A |
| Mouse: TrkBCreER | Rutlin et al., 2015 | MGI:5616440 |
| Mouse: RetCreER | Luo et al., 2009 | N/A |
| Mouse: TrkCCreER | Bai et al., 2015 | N/A |
| Mouse: Nav1.8Cre | The Jackson Laboratory | RRID:IMSR_JAX:036564 |
| Mouse: CalcaCreER | Song et al., 2012 | MGI:5460801 |
| Mouse: Calca-FlpE | Choi et al., 2020 | N/A |
| Mouse: MrgprdCre | Rau et al., 2009 | MGI:3852395 |
| Mouse: MrgprdCreER | Olson et al., 2017 | RRID:IMSR_JAX:031286 |
| Mouse: R26LSL-ChR2-YFP | Madisen et al., 2012 | RRID:IMSR_JAX:012569 |
| Mouse: R26LSL-eNpHR3.0-YFP | Madisen et al., 2012 | RRID:IMSR_JAX:014539 |
| Mouse: R26LSL-synaptophysin-tdTomato | Madisen et al., 2012 | RRID:IMSR_JAX:012570 |
| Mouse: TauLSL-mGFP-ires-NLS-lacZ | Hippenmeyer et al., 2005 | RRID:IMSR_JAX:021162 |
| Mouse: Vgatflox | The Jackson Laboratory | RRID:IMSR_JAX:012897 |
| Mouse: Gabrb3flox | The Jackson Laboratory | RRID:IMSR_JAX:008310 |
| Mouse: TrkBflox | Liu et al., 2012 | RRID:IMSR_JAX:022362 |
| Mouse: Atoh1flox | Shroyer et al., 2007 | RRID:IMSR_JAX:008681 |
| Mouse: Piezo2flox | Woo et al., 2014 | RRID:IMSR_JAX:027720 |
| Mouse: RC::PFtox | Kim et al., 2009 | N/A |
| Oligonucleotides | ||
| Mm-Piezo2-E43-E45-C3 | ACD Bio | Cat# 439971-C3 |
| Mm-Scn10a-C2 | ACD Bio | Cat# 426011-C2 |
| Mm-Calca-tv2tv3 | ACD Bio | Cat# 420361 |
| Mm-Mrgprd | ACD Bio | Cat# 417921 |
| Mm-Nefh | ACD Bio | Cat#: 443671-C2 |
| Mm-Rorβ | ACD Bio | Cat# 444271-C2 |
| Mm-Slc32a1 | ACD Bio | Cat# 319191 |
| Software and algorithms | ||
| JRCLUST | Jun et al., 2017 | https://github.com/JaneliaSciComp/JRCLUST |
| MATLAB | Mathworks | https://www.mathworks.com/products/matlab.html; RRID: SCR_001622 |
| Python | Van Rossum and Drake, 1995 | https://www.python.org/ |
| Clampex 10 | Molecular Devices | https://www.moleculardevices.com; RRID: SCR_011323 |
| ImageJ | Schneider et al., 2012 | https://imagej.nih.gov/ij/ |
| Other | ||
| Galvo mirrors | Cambridge Technologies | 6210H |
| F-theta lens | Thorlabs | FTH100-1064 |
| Multielectrode arrays | Cambridge Neurotech | ASSY-37 H4 |
| Multielectrode arrays | Neuronexus | A1×32-Poly3-5mm-25s-177-A32 and A1×32-Poly2-5mm-50s-177-OA32 |
| RHD USB interface board Intan Technologies C3100 | RHD USB interface board Intan Technologies C3100 | RHD USB interface board Intan Technologies C3100 |
| Patch-clamp amplifier | Molecular Devices | Multiclamp 700A |
| Acquisition system | Molecular Devices | Digidata 1440A |
Highlights.
Dorsal horn (DH) neurons exhibit one of six mechanical response profiles
DH neurons receive extensively convergent inputs from LTMR subtypes and HTMRs
The DH is a highly interconnected network that enables flexible tuning of its outputs
Mechanosensory processing in the DH shapes tactile representations in cortex
Acknowledgements
We thank O. Mazor and P. Gorelik (HMS Research Instrumentation Core), T. Javaluyas, K. Clausel, S. Tsan, M. Yee, and M. DeLisle for technical assistance, and members of the Ginty lab for helpful comments on the manuscript. This work was supported by The Mahoney Neuroscience Institute Fund (AMC), The Ellen and Melvin Gordon Center for the Cure and Treatment of Paralysis (AMC), NSF GRFP DG1745303 (GR), a Stuart & Victoria Quan Fellowship (S-YT), NIH grants MH125776 and NS089521 (CDH), NS119739 (AJE), and NS097344 and AT011447 (DDG), The Hock E. Tan and Lisa Yang Center for Autism Research (DDG), and the Lefler Center for Neurodegenerative Disorders (DDG). DDG is an HHMI investigator. This article is subject to HHMI’s Open Access to Publications policy. HHMI lab heads have previously granted a nonexclusive CC BY 4.0 license to the public and a sublicensable license to HHMI in their research articles. Pursuant to those licenses, the author-accepted manuscript of this article can be made freely available under a CC BY 4.0 license immediately upon publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
References
- 1.Perl ER (1968). Myelinated afferent fibres innervating the primate skin and their response to noxious stimuli. J Physiol 197, 593–615. 10.1113/jphysiol.1968.sp008576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bessou P, and Perl ER (1969). Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol 32, 1025–1043. 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- 3.Arcourt A, Gorham L, Dhandapani R, Prato V, Taberner FJ, Wende H, Gangadharan V, Birchmeier C, Heppenstall PA, and Lechner SG (2017). Touch Receptor-Derived Sensory Information Alleviates Acute Pain Signaling and Fine-Tunes Nociceptive Reflex Coordination. Neuron 93, 179–193. 10.1016/j.neuron.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 4.Hill RZ, and Bautista DM (2020). Getting in Touch with Mechanical Pain Mechanisms. Trends Neurosci 43, 311–325. 10.1016/j.tins.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Handler A, and Ginty DD (2021). The mechanosensory neurons of touch and their mechanisms of activation. Nat Rev Neurosci 22, 521–537. 10.1038/s41583-021-00489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson KO (2001). The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol 11, 455–461. 10.1016/s0959-4388(00)00234-8. [DOI] [PubMed] [Google Scholar]
- 7.Owens DM, and Lumpkin EA (2014). Diversification and specialization of touch receptors in skin. Cold Spring Harb Perspect Med 4. 10.1101/cshperspect.a013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horch KW, Tuckett RP, and Burgess PR (1977). A key to the classification of cutaneous mechanoreceptors. J Invest Dermatol 69, 75–82. 10.1111/1523-1747.ep12497887. [DOI] [PubMed] [Google Scholar]
- 9.Mountcastle VB, Talbot WH, Darian-Smith I, and Kornhuber HH (1967). Neural basis of the sense of flutter-vibration. Science 155, 597–600. 10.1126/science.155.3762.597. [DOI] [PubMed] [Google Scholar]
- 10.Freeman AW, and Johnson KO (1982). Cutaneous mechanoreceptors in macaque monkey: temporal discharge patterns evoked by vibration, and a receptor model. J Physiol 323, 21–41. 10.1113/jphysiol.1982.sp014059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koltzenburg M, Stucky CL, and Lewin GR (1997). Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol 78, 1841–1850. 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- 12.Dubin AE, and Patapoutian A (2010). Nociceptors: the sensors of the pain pathway. J Clin Invest 120, 3760–3772. 10.1172/JCI42843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgess PR, and Perl ER (1967). Myelinated afferent fibres responding specifically to noxious stimulation of the skin. J Physiol 190, 541–562. 10.1113/jphysiol.1967.sp008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pei YC, Denchev PV, Hsiao SS, Craig JC, and Bensmaia SJ (2009). Convergence of submodality-specific input onto neurons in primary somatosensory cortex. J Neurophysiol 102, 1843–1853. 10.1152/jn.00235.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emanuel AJ, Lehnert BP, Panzeri S, Harvey CD, and Ginty DD (2021). Cortical responses to touch reflect subcortical integration of LTMR signals. Nature 600, 680–685. 10.1038/s41586-021-04094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suresh AK, Greenspon CM, He Q, Rosenow JM, Miller LE, and Bensmaia SJ (2021). Sensory computations in the cuneate nucleus of macaques. Proc Natl Acad Sci U S A 118. 10.1073/pnas.2115772118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai L, Lehnert BP, Liu J, Neubarth NL, Dickendesher TL, Nwe PH, Cassidy C, Woodbury CJ, and Ginty DD (2015). Genetic Identification of an Expansive Mechanoreceptor Sensitive to Skin Stroking. Cell 163, 1783–1795. 10.1016/j.cell.2015.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abraira VE, and Ginty DD (2013). The sensory neurons of touch. Neuron 79, 618–639. 10.1016/j.neuron.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown AG (1982). The dorsal horn of the spinal cord. Q J Exp Physiol 67, 193–212. 10.1113/expphysiol.1982.sp002630. [DOI] [PubMed] [Google Scholar]
- 20.Abraira VE, Kuehn ED, Chirila AM, Springel MW, Toliver AA, Zimmerman AL, Orefice LL, Boyle KA, Bai L, Song BJ, et al. (2017). The Cellular and Synaptic Architecture of the Mechanosensory Dorsal Horn. Cell 168, 295–310 e219. 10.1016/j.cell.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown AG (1968). Cutaneous afferent fibre collaterals in the dorsal columns of the cat. Exp Brain Res 5, 293–305. 10.1007/BF00235904. [DOI] [PubMed] [Google Scholar]
- 22.Brown PB, Gladfelter WE, Culberson JC, Covalt-Dunning D, Sonty RV, Pubols LM, and Millecchia RJ (1991). Somatotopic organization of single primary afferent axon projections to cat spinal cord dorsal horn. J Neurosci 11, 298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown AG, Rose PK, and Snow PJ (1978). Morphology and organization of axon collaterals from afferent fibres of slowly adapting type I units in cat spinal cord. J Physiol 277, 15–27. 10.1113/jphysiol.1978.sp012257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petit D, and Burgess PR (1968). Dorsal column projection of receptors in cat hairy skin supplied by myelinated fibers. J Neurophysiol 31, 849–855. 10.1152/jn.1968.31.6.849. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, et al. (2011). The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 147, 1615–1627. 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuehn ED, Meltzer S, Abraira VE, Ho CY, and Ginty DD (2019). Tiling and somatotopic alignment of mammalian low-threshold mechanoreceptors. Proc Natl Acad Sci U S A 116, 9168–9177. 10.1073/pnas.1901378116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olson W, Abdus-Saboor I, Cui L, Burdge J, Raabe T, Ma M, and Luo W (2017). Sparse genetic tracing reveals regionally specific functional organization of mammalian nociceptors. Elife 6. 10.7554/eLife.29507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zylka MJ, Rice FL, and Anderson DJ (2005). Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron 45, 17–25. 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Brown AG, Brown PB, Fyffe RE, and Pubols LM (1983). Receptive field organization and response properties of spinal neurones with axons ascending the dorsal columns in the cat. J Physiol 337, 575–588. 10.1113/jphysiol.1983.sp014643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown AG, and Fyffe RE (1981). Form and function of dorsal horn neurones with axons ascending the dorsal columns in cat. J Physiol 321, 31–47. 10.1113/jphysiol.1981.sp013970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown AG, Noble R, and Riddell JS (1986). Relations between spinocervical and post-synaptic dorsal column neurones in the cat. J Physiol 381, 333–349. 10.1113/jphysiol.1986.sp016330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noble R, and Riddell JS (1989). Descending influences on the cutaneous receptive fields of postsynaptic dorsal column neurones in the cat. J Physiol 408, 167–183. 10.1113/jphysiol.1989.sp017453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noble R, and Riddell JS (1988). Cutaneous excitatory and inhibitory input to neurones of the postsynaptic dorsal column system in the cat. J Physiol 396, 497–513. 10.1113/jphysiol.1988.sp016974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi S, Hachisuka J, Brett MA, Magee AR, Omori Y, Iqbal NU, Zhang D, DeLisle MM, Wolfson RL, Bai L, et al. (2020). Parallel ascending spinal pathways for affective touch and pain. Nature 587, 258–263. 10.1038/s41586-020-2860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paixao S, Loschek L, Gaitanos L, Alcala Morales P, Goulding M, and Klein R (2019). Identification of Spinal Neurons Contributing to the Dorsal Column Projection Mediating Fine Touch and Corrective Motor Movements. Neuron 104, 749–764 e746. 10.1016/j.neuron.2019.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gatto G, Bourane S, Ren X, Di Costanzo S, Fenton PK, Halder P, Seal RP, and Goulding MD (2021). A Functional Topographic Map for Spinal Sensorimotor Reflexes. Neuron 109, 91–104 e105. 10.1016/j.neuron.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koch SC, Del Barrio MG, Dalet A, Gatto G, Gunther T, Zhang J, Seidler B, Saur D, Schule R, and Goulding M (2017). RORbeta Spinal Interneurons Gate Sensory Transmission during Locomotion to Secure a Fluid Walking Gait. Neuron 96, 1419–1431 e1415. 10.1016/j.neuron.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moehring F, Halder P, Seal RP, and Stucky CL (2018). Uncovering the Cells and Circuits of Touch in Normal and Pathological Settings. Neuron 100, 349–360. 10.1016/j.neuron.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grudt TJ, and Perl ER (2002). Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol 540, 189–207. 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russ DE, Cross RBP, Li L, Koch SC, Matson KJE, Yadav A, Alkaslasi MR, Lee DI, Le Pichon CE, Menon V, and Levine AJ (2021). A harmonized atlas of mouse spinal cord cell types and their spatial organization. Nat Commun 12, 5722. 10.1038/s41467-021-25125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sathyamurthy A, Johnson KR, Matson KJE, Dobrott CI, Li L, Ryba AR, Bergman TB, Kelly MC, Kelley MW, and Levine AJ (2018). Massively Parallel Single Nucleus Transcriptional Profiling Defines Spinal Cord Neurons and Their Activity during Behavior. Cell Rep 22, 2216–2225. 10.1016/j.celrep.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haring M, Zeisel A, Hochgerner H, Rinwa P, Jakobsson JET, Lonnerberg P, La Manno G, Sharma N, Borgius L, Kiehn O, et al. (2018). Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat Neurosci 21, 869–880. 10.1038/s41593-018-0141-1. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg AB, Roco CM, Muscat RA, Kuchina A, Sample P, Yao Z, Graybuck LT, Peeler DJ, Mukherjee S, Chen W, et al. (2018). Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science 360, 176–182. 10.1126/science.aam8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osseward PJ 2nd, Amin ND, Moore JD, Temple BA, Barriga BK, Bachmann LC, Beltran F Jr., Gullo M, Clark RC, Driscoll SP, et al. (2021). Conserved genetic signatures parcellate cardinal spinal neuron classes into local and projection subsets. Science 372, 385–393. 10.1126/science.abe0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu Y, and Perl ER (2005). Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (laminae I and II). J Neurosci 25, 3900–3907. 10.1523/JNEUROSCI.0102-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polgar E, Fowler JH, McGill MM, and Todd AJ (1999). The types of neuron which contain protein kinase C gamma in rat spinal cord. Brain Res 833, 71–80. 10.1016/s0006-8993(99)01500-0. [DOI] [PubMed] [Google Scholar]
- 47.Lin JY, Knutsen PM, Muller A, Kleinfeld D, and Tsien RY (2013). ReaChR: a redshifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci 16, 1499–1508. 10.1038/nn.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hooks BM, Lin JY, Guo C, and Svoboda K (2015). Dual-channel circuit mapping reveals sensorimotor convergence in the primary motor cortex. J Neurosci 35, 4418–4426. 10.1523/JNEUROSCI.3741-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Begay V, Coste B, Mainquist J, et al. (2014). Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516, 121–125. 10.1038/nature13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lehnert BP, Santiago C, Huey EL, Emanuel AJ, Renauld S, Africawala N, Alkislar I, Zheng Y, Bai L, Koutsioumpa C, et al. (2021). Mechanoreceptor synapses in the brainstem shape the central representation of touch. Cell 184, 5608–5621 e5618. 10.1016/j.cell.2021.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neubarth NL, Emanuel AJ, Liu Y, Springel MW, Handler A, Zhang Q, Lehnert BP, Guo C, Orefice LL, Abdelaziz A, et al. (2020). Meissner corpuscles and their spatially intermingled afferents underlie gentle touch perception. Science 368. 10.1126/science.abb2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghitani N, Barik A, Szczot M, Thompson JH, Li C, Le Pichon CE, Krashes MJ, and Chesler AT (2017). Specialized Mechanosensory Nociceptors Mediating Rapid Responses to Hair Pull. Neuron 95, 944–954 e944. 10.1016/j.neuron.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCarthy PW, and Lawson SN (1990). Cell type and conduction velocity of rat primary sensory neurons with calcitonin gene-related peptide-like immunoreactivity. Neuroscience 34, 623–632. 10.1016/0306-4522(90)90169-5. [DOI] [PubMed] [Google Scholar]
- 54.Hoheisel U, Mense S, and Scherotzke R (1994). Calcitonin gene-related peptide-immunoreactivity in functionally identified primary afferent neurones in the rat. Anat Embryol (Berl) 189, 41–49. 10.1007/BF00193128. [DOI] [PubMed] [Google Scholar]
- 55.Torsney C, and MacDermott AB (2006). Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci 26, 1833–1843. 10.1523/JNEUROSCI.4584-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orefice LL, Zimmerman AL, Chirila AM, Sleboda SJ, Head JP, and Ginty DD (2016). Peripheral Mechanosensory Neuron Dysfunction Underlies Tactile and Behavioral Deficits in Mouse Models of ASDs. Cell 166, 299–313. 10.1016/j.cell.2016.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boyle KA, Gradwell MA, Yasaka T, Dickie AC, Polgar E, Ganley RP, Orr DPH, Watanabe M, Abraira VE, Kuehn ED, et al. (2019). Defining a Spinal Microcircuit that Gates Myelinated Afferent Input: Implications for Tactile Allodynia. Cell Rep 28, 526–540 e526. 10.1016/j.celrep.2019.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zimmerman AL, Kovatsis EM, Pozsgai RY, Tasnim A, Zhang Q, and Ginty DD (2019). Distinct Modes of Presynaptic Inhibition of Cutaneous Afferents and Their Functions in Behavior. Neuron 102, 420–434 e428. 10.1016/j.neuron.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hughes DI, Sikander S, Kinnon CM, Boyle KA, Watanabe M, Callister RJ, and Graham BA (2012). Morphological, neurochemical and electrophysiological features of parvalbumin-expressing cells: a likely source of axo-axonic inputs in the mouse spinal dorsal horn. J Physiol 590, 3927–3951. 10.1113/jphysiol.2012.235655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baden T, Berens P, Franke K, Roman Roson M, Bethge M, and Euler T (2016). The functional diversity of retinal ganglion cells in the mouse. Nature 529, 345–350. 10.1038/nature16468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanes JR, and Masland RH (2015). The types of retinal ganglion cells: current status and implications for neuronal classification. Annu Rev Neurosci 38, 221–246. 10.1146/annurevneuro-071714-034120. [DOI] [PubMed] [Google Scholar]
- 62.Milner ES, and Do MTH (2017). A Population Representation of Absolute Light Intensity in the Mammalian Retina. Cell 171, 865–876 e816. 10.1016/j.cell.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Emanuel AJ, Kapur K, and Do MTH (2017). Biophysical Variation within the M1 Type of Ganglion Cell Photoreceptor. Cell Rep 21, 1048–1062. 10.1016/j.celrep.2017.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andre E, Gawlas K, and Becker-Andre M (1998). A novel isoform of the orphan nuclear receptor RORbeta is specifically expressed in pineal gland and retina. Gene 216, 277–283. 10.1016/s0378-1119(98)00348-5. [DOI] [PubMed] [Google Scholar]
- 65.Vong L, Ye C, Yang Z, Choi B, Chua S Jr., and Lowell BB (2011). Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154. 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harris JA, Hirokawa KE, Sorensen SA, Gu H, Mills M, Ng LL, Bohn P, Mortrud M, Ouellette B, Kidney J, et al. (2014). Anatomical characterization of Cre driver mice for neural circuit mapping and manipulation. Front Neural Circuits 8, 76. 10.3389/fncir.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13, 133–140. 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, et al. (2011). A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013. 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bourane S, Grossmann KS, Britz O, Dalet A, Del Barrio MG, Stam FJ, Garcia-Campmany L, Koch S, and Goulding M (2015). Identification of a spinal circuit for light touch and fine motor control. Cell 160, 503–515. 10.1016/j.cell.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daigle TL, Madisen L, Hage TA, Valley MT, Knoblich U, Larsen RS, Takeno MM, Huang L, Gu H, Larsen R, et al. (2018). A Suite of Transgenic Driver and Reporter Mouse Lines with Enhanced Brain-Cell-Type Targeting and Functionality. Cell 174, 465–480 e422. 10.1016/j.cell.2018.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peron SP, Freeman J, Iyer V, Guo C, and Svoboda K (2015). A Cellular Resolution Map of Barrel Cortex Activity during Tactile Behavior. Neuron 86, 783–799. 10.1016/j.neuron.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 72.Coutaud B, and Pilon N (2013). Characterization of a novel transgenic mouse line expressing Cre recombinase under the control of the Cdx2 neural specific enhancer. Genesis 51, 777–784. 10.1002/dvg.22421. [DOI] [PubMed] [Google Scholar]
- 73.Hasegawa H, Abbott S, Han BX, Qi Y, and Wang F (2007). Analyzing somatosensory axon projections with the sensory neuron-specific Advillin gene. J Neurosci 27, 14404–14414. 10.1523/JNEUROSCI.4908-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rutlin M, Ho CY, Abraira VE, Cassidy C, Bai L, Woodbury CJ, and Ginty DD (2015). The Cellular and Molecular Basis of Direction Selectivity of Adelta-LTMRs. Cell 160, 1027. 10.1016/j.cell.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 75.Luo W, Enomoto H, Rice FL, Milbrandt J, and Ginty DD (2009). Molecular identification of rapidly adapting mechanoreceptors and their developmental dependence on ret signaling. Neuron 64, 841–856. 10.1016/j.neuron.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nassar MA, Stirling LC, Forlani G, Baker MD, Matthews EA, Dickenson AH, and Wood JN (2004). Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci U S A 101, 12706–12711. 10.1073/pnas.0404915101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song H, Yao E, Lin C, Gacayan R, Chen MH, and Chuang PT (2012). Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc Natl Acad Sci U S A 109, 17531–17536. 10.1073/pnas.1207238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rau KK, McIlwrath SL, Wang H, Lawson JJ, Jankowski MP, Zylka MJ, Anderson DJ, and Koerber HR (2009). Mrgprd enhances excitability in specific populations of cutaneous murine polymodal nociceptors. J Neurosci 29, 8612–8619. 10.1523/JNEUROSCI.1057-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ 3rd, Gu X, Zanella S, et al. (2012). A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci 15, 793–802. 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, and Arber S (2005). A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol 3, e159. 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tong Q, Ye CP, Jones JE, Elmquist JK, and Lowell BB (2008). Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci 11, 998–1000. 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferguson C, Hardy SL, Werner DF, Hileman SM, Delorey TM, and Homanics GE (2007). New insight into the role of the beta3 subunit of the GABAA-R in development, behavior, body weight regulation, and anesthesia revealed by conditional gene knockout. BMC Neurosci 8, 85. 10.1186/1471-2202-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Y, Rutlin M, Huang S, Barrick CA, Wang F, Jones KR, Tessarollo L, and Ginty DD (2012). Sexually dimorphic BDNF signaling directs sensory innervation of the mammary gland. Science 338, 1357–1360. 10.1126/science.1228258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shroyer NF, Helmrath MA, Wang VY, Antalffy B, Henning SJ, and Zoghbi HY (2007). Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology 132, 2478–2488. 10.1053/j.gastro.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 85.Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, et al. (2014). Piezo2 is required for Merkel-cell mechanotransduction. Nature 509, 622–626. 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim JC, Cook MN, Carey MR, Shen C, Regehr WG, and Dymecki SM (2009). Linking genetically defined neurons to behavior through a broadly applicable silencing allele. Neuron 63, 305–315. 10.1016/j.neuron.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kvitsiani D, Ranade S, Hangya B, Taniguchi H, Huang JZ, and Kepecs A (2013). Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature 498, 363–366. 10.1038/nature12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jun JJ, Mitelut C, Lai C, Gratiy SL, and, A. CA, Harris TD (2017). Real-time spike sorting platform for high-density extracellular probes with ground-truth validation and drift correction. bioRxiv. 10.1101/101030 [DOI] [Google Scholar]
- 89.Rodriguez A, and Laio A (2014). Machine learning. Clustering by fast search and find of density peaks. Science 344, 1492–1496. 10.1126/science.1242072. [DOI] [PubMed] [Google Scholar]
- 90.Walcher J, Ojeda-Alonso J, Haseleu J, Oosthuizen MK, Rowe AH, Bennett NC, and Lewin GR (2018). Specialized mechanoreceptor systems in rodent glabrous skin. J Physiol 596, 4995–5016. 10.1113/JP276608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sharma N, Flaherty K, Lezgiyeva K, Wagner DE, Klein AM, and Ginty DD (2020). The emergence of transcriptional identity in somatosensory neurons. Nature 577, 392–398. 10.1038/s41586-019-1900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schneider CA, Rasband WS, and Eliceiri KW (2012). NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9, 671–675. 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Functional characterization of mechanosensory responses in DH neurons. Related to Figure 1.
A. In vivo MEA recordings from L4 DRG neurons in anaesthetized mice. Spike raster plots and peristimulus time histograms (PSTHs) aligned to 50 mN indentation step from example Aβ RA-LTMR (top) and Aβ SA-LTMR (bottom). PSTHs are 10ms bins.
B. Example DiI-coated MEA in L4 spinal DH from a mouse expressing ChR2-YFP in Mrgdprd+ polymodal nociceptors (MrgdprdCreER; R26LSL-ChR2-EYFP) terminating in lamina IIi also labeled with IB4 (green, EYFP; blue, IB4 binding protein; red, DiI coating probe tract). Scale bar, 100μm.
C. Average waveform and corresponding autocorrellogram for example DH unit.
D. Top: Average maximum-normalized responses (±SEM) for Aβ SA-LTMRs (n = 11 neurons) and Aβ RA-LTMRs (n = 13 neurons) at the ON, OFF, and sustained portion of step indentations. Bottom: Average maximum-normalized firing rate (±SEM; shaded regions) for all mechanically sensitive DH neurons.
E. Unsupervised clustering pipeline for identifying DH functional response profiles. Left: Heatmap of individual DH units sorted by their response thresholds to step indentations across all experiments. Top, force traces ranging in intensity from 1 to 75 mN. Bottom, grand average z-scored PSTH. First, units with decreased firing rates to step indentations, the ‘inhibitory group’, were separated from the overall dataset. PCA analysis was then used to extract features of indentation-evoked responses from the remaining dataset. Inset shows PC1 and PC2 accounting for 25% of variance in the data, and resembling responses with transient or sustained components. Finally, the number of PCs accounting for 90% of the variance (n=503) were used for k-mediods clustering. This approach yielded 6 principal functional clusters: 5 excitatory clusters are shown in TSNE space and cluster 6 is comprised of the ‘inhibitory group’.
F. Occurrence of units sorted into the 6 principal clusters across experiments.
G. Proportion of unit response thresholds within each principal cluster (median is denoted as dashed red line).
H. Summary boxplots of spontaneous firing rates for DH units in each functional group Boxplots: median (line), quartiles (box), minimum and maximum (whiskers).
I. Nominal depth vs. laminar distribution for each functional group. Data represent cluster means ± SD. Spearman correlation coefficient for correlation over individual units r= 0.1676; P = 2.83*10−30.
Figure S2. Functional response profiles at RF hotspot and vibrotactile features. Related to Figure 2.
A. Spike raster plot of DH unit linear RF organization with trials in the RF hotspot colored in red (top). Mean PSTHs for RF hotspot (red) and RF surround (black) shown below. Note the increase in firing rate from RF surround to RF hotspot.
B. Functional clustering was performed as described (see Methods; Figure S1 D) at the RF hotspot. For each cluster, a heatmap of all included DH units responding to indentation is shown above the average z-scored PSTH. Heatmaps are sorted by force thresholds to step indentations.
C. Percentage of DH units used in RF hotspot clustering per functional cluster.
D. Percentage incidence of DH units that are frequency tuned across the 6 functional clusters.
E. Percentage incidence of phase-locked DH units across the 6 functional clusters.
Figure S3. Functional characterization of distinct DH interneuron types. Related to Figure 3.
A. Light-evoked first spike latency probability distribution from the optotagged Rorβ unit in B (left) and non-tagged neighboring unit (right).
B. Histogram of optical test (SALT) P values for optically tagged Rorβ units and simultaneously recorded non-tagged units (n=21; 5 mice; p < 0.01, blue for tagged units; p=1; grey for non-tagged units).
C. Raster plot and PSTH of the same Rorβ unit in the absence or presence of NBQX (5 mM) applied to the surface of the cord to block excitatory synaptic transmission.
D. Left: Summary light-evoked firing before and after NBQX application. Optotagged units (blue) had similar optically evoked firing rates in the two conditions (Two-sided paired t test). Right: Summary mechanically-evoked firing before and after NBQX application. Mechanically-evoked spikes were blocked in both Rorβ optotagged units (blue) and non-tagged units (grey; p < 0.01; Two-sided paired t test). N = 3 mice.
E. Left: Mechanical responses to a series of step indentations for all optotagged inhibitory DH units (top) and average PSTH (bottom) from VgatiCre; R26LSL-ChR2 mice. Right: Assignment of optotagged Vgat+ interneurons to functional response clusters.
F. As in (D), for simultaneously recorded putative excitatory DH interneurons (left), and cluster assignment (right).
G-K. As in (D), for genetically distinct inhibitory interneurons (F-H): Rorβ (using RorβiCre; VgatFlpO; R26LSL-FSF-ReaChR-mCitrine mice); PVi (PV2aCre; VgatFlpO; R26LSL-FSF-ReaChR-mCitrine); Kcnip2 (Kcnip2CreER; R26ChR2-YFP); and excitatory interneurons (I-J): CCK (CCKiCre; R26ChR2-YFP) and PKCγ (PKCγCreER; R26ChR2-YFP and PKCγCreER; Lbx1FlpO; R26LSL-FSF-ReaChR-mCitrine).
L. Spontaneous firing rates for all optotagged DH units.
M. Frequency- threshold tuning curves for genetically distinct interneuron populations.
Figure S4. DH neuron responses in Piezo2 conditional knockout animals. Related to Figure 4.
A. Mean firing rates in response to mechanical and optical activation of glabrous hindpaw in all DH units from littermate controls (Piezo2flox/flox; AdvillinFlpO R26LSL-FSF-ReaChR; top; n= units, 3 mice) and Cdx2-Cre; Piezo2flox/flox; AdvillinFlpO R26LSL-FsF-ReaChR animals (bottom; 3 mice). Units are sorted by depth. Note the timescale difference between mechanical and optical heatmaps. Scale bar: 50 units.
B. Mechanical and optical responses to skin stimulation in example DH units from control (left) and Cdx2-Cre; Piezo2flox/flox; AdvillinFlpO R26LSL-FSF-ReaChR animals, showing both short and long-latency spikes to optical stimulation of the skin.
C. Left: Summary of indentation-evoked firing rates in control (N=4) and Cdx2-Cre; Piezo2flox/flox; AdvillinFlpO; R26LSL-FSF-ReaChR; Cdx2-Cre; Piezo2flox/flox (N=2) animals. Each dot represents one animal. Bars: mean. Right: Percentage of units responding to indentation and optical stimulation. Few units in Piezo2 mutants responded to indentation, however optical stimulation of the skin elicited similar amounts of responsivity in control and Cdx2-Cre; Piezo2flox/flox; AdvillinFlpO; R26LSL-FSF-ReaChR animals. P < 0.05, Mann-Whitney U test.
D. Example raster plot and PSTH of DH unit form Piezo2 cKO mouse showing no response to stroke on the skin, and robust response to noxious pinch.
E. Representative images of Piezo2 and Nav1.8 smRNA-FISH from L1-L6 DRGs in Nav1.8Cre; Piezo2flox/flox animals (bottom), or Piezo2flox/flox littermates (top).
F. Quantification of the percentage overlap between Piezo2 and DRG markers Nav1.8, Mrgdprd, CGRP, and NFH. Multiple lumbar DRGs from three Nav1.8Cre; Piezo2flox/flox and three Piezo2flox/flox littermates were quantified. ***p<0.0001; **p<0.005; Unpaired t test.
G. Z-scored firing rates of all dorsal horn units from littermate control (Piezo2flox/flox; top, N=3) and Nav1.8-Cre; Piezo2flox/flox (Nav1.8 cKO; bottom, N=3). Units are sorted by depth. Scale bar: 50 units.
H. Grand mean baseline-subtracted firing rates (± SEM) to 10, 20 and 75mN step indentations across all units from control (black) and Nav1.8 cKO (red) animals.
I. Proportion of units from control (black) and Nav1.8-Cre; Piezo2flox/flox animals (red) responding at the ON (left), OFF (middle) and sustained (right) phase of step indentation across all forces tested. * p < 0.05, two-proportions Z test.
J. Response thresholds from all units in control, and Nav1.8-Cre; Piezo2flox/flox animals. Bar: median with 95% CI. n.s., Mann-Whitney U test.
Figure S5. Sensory-evoked feed-forward and feedback inhibitory circuit motifs exert broad control over DH neuron responses. Related to Figure 6.
A. Optogenetic activation of Aβ-RA LTMR terminals evokes monosynaptic EPSCs and feedforward inhibition onto PSDC neurons. Left: Application of AMPA receptor antagonist NBQX (10 μM) blocks both oEPSC and oIPSC. Right: Example EPSCs under baseline conditions and following co-application of voltage-gated Na+ channel blocker TTX and voltage-gated K+ channel blocker 4-aminopyridine (4AP).
B. Top: Example EPSC in a PSDC neuron evoked by optogenetic activation of Nav1.8+ sensory terminals. Bottom: Connectivity rate for NaV1.8+ oEPSCs to PSDCs (left) and onset latencies for opto-evoked primary afferent EPSCs onto PSDC neurons (right).
C. Summary current clamp recordings from PSDC neurons in response to current injection ramps paired with a 1s optical activation of Rorβ neurons (blue; n=6 PSDCs).
D. Example current clamp recording from eNpHR3.0-expressing Rorβ neuron. Light activation of eNpHR3.0 confirms silencing of spiking. Rheobase trace is shown in red.
E. Top: Voltage-clamp recording from PSDCs isolates direct sensory-evoked EPSC (black) and polysynaptic IPSC (orange) at different holding potentials in spinal cord slices from RorβiCre; R26LSL- eNpHR3.0-YFP mice. Bottom: Summary of normalized EPSC and IPSC amplitudes with (ON) and without (OFF) suppression of Rorβ neurons. N=3 mice; n=3 PSDCs; p>0.1 EPSC; p < 0.05 IPSC; Paired two-tailed t test.
F. In vivo MEA recording from PViCre; R26LSL- eNpHR3.0-YFP mice. Right: raster and PSTH for example PVi interneuron suppressed by eNpHR3.0-YFP, and quantification of the firing rate suppression in putative PVi interneurons (n=6 units; one mouse). Left: example PSTH to 10mN step indentation without (black) and with (orange) acute PVi silencing, and quantification of the effect of PVi suppression on indentation-evoked spiking at the onset of step indentation in neighboring units on the probe (n=12 units). This effect was observed in 12 out of 48 units.
G. In vivo MEA recording from RorβiCre; R26LSL- eNpHR3.0-YFP mice. Left: Raster and PSTH for example Rorβ interneuron suppressed by eNpHR3.0-YFP, and quantification of firing rate suppression in all putative Rorβ interneurons (n=14 units; 5 mice). Right: Example PSTH to 10mN step indentation without (black) and with (orange) acute Rorβ silencing, and quantification of the effect of Rorβ suppression on indentation evoked spiking at the ON response (n=7 units; 5 mice) in neighboring units on the probe. This effect was observed in 7 out of 28 units.
H. Confocal images showing synaptophysin-tdTomato expression driven by RorβCreER, and PSDC neurons (green) retrogradely labeled from DCN with glycoprotein (G) gene-deleted rabies virus (ΔG-GFP-RV). Co-labeling with gephyrin (blue) was used to determine axo-dendritic contacts from Rorβ+ inhibitory interneurons to PSDCs (arrowheads), quantified across the LTMR-RZ (right).
I. Median (±95% CI) firing rates for DH units in littermate control (white), FFI cKO (red) and PSI cKO (teal) mice in response to 1mN-75mM step indentations at the ON, OFF, sustained, and after-discharge periods.
J. Average RF areas at 10mN force steps measured during the sustained (left) and OFF (right) phase of step indentations in control, FFI cKO and PSI cKO mice. **p < 0.01; *p < 0.05, standard deviation of bootstrapped RFs.
K. Loss of LTP at Aβ-PSDC synapses in spinal cord slices form RorβiCre; Cdx2FlpO; RC:PFtox mice. Averaged EPSC amplitudes before and after high frequency dorsal root afferent stimulation (arrow) in PSDC neurons from control (n=3 PSDCs, 3 mice) and Rorβ silenced mice (n=3 PSDCs, 3 mice). Insets: Averaged EPSCs from single experiments before and 20 min after HFS.
L. Left: experimental design. Right: Raster plot and PSTH of DH unit in response to cutaneous electrical stimulation. Both short latency and long latency spikes were evoked, indicative of A and C fiber input.
M. DH indentation responses to 75mN force steps before and after cutaneous HFS stimulation in control (black) and FFI cKO (red) mice.
N. Summary firing rates evoked during ON and sustained phases of 75mN step indentations before and after cutaneous HFS stimulation in DH units from control (black), and FFI cKO mice. **p < 0.01; ****p < 0.0001, Mann-Whitney test.
Figure S6. In vivo identification and mechanical response properties of PSDC neurons. Related to Figure 7.
A. Left: Schematic of PSDC retrograde viral labeling and recording configuration. This strategy results in excitatory opsin expression in both PSDCs and Aβ DCN projecting sensory neurons. Right: transverse lumbar spinal cord section with retrogradely labeled PSDCs and Aβ LTMR terminals (green), and IB4+ terminals (blue).
B. Left: Example light-evoked raster plot and PSTH of PSDC neuron in the presence of NBQX (5mM) blocking excitatory synaptic transmission from direct pathway sensory neurons. Middle: summary light-evoked spike latencies in putative PSDC units. Right: Mechanically evoked responses for simultaneously recorded DH units before and after NBQX application to the surface of the cord. Mechanically-evoked spikes were blocked in both putative PSDC optotagged units (blue) and non-tagged units (grey; P < 0.01; Two-sided paired t test).
C. Left: Dorsal column antidromic stimulation strategy. Right: Raster plot and PSTH in response to electrical antidromic stimulation of dorsal column axons at cervical levels C1-C3. Putative PSDC units continued to spike with short latencies and jitter in the presence of NBQX.
D. Boxplots for all tagged PSDC neurons show low first spike latency and small jitter to DC antidromic stimulation.
E. Summary of antidromic spike rates in putative PSDC neurons (purple) and neighboring units (grey) before and after NBXQ application. P < 0.0001; two-sided paired t test.
F. Summary of firing rates in response to skin stroke before and after NBQX application confirms excitatory synaptic transmission blocking efficiency (mean: orange). P < 0.0001; Two-sided paired t test.
G. Tagged PSDC neurons receive direct input from Aβ-RA and Aβ-SA LTMRs. Mechanical and optically- evoked PSTHs (mean ± SEM) in example putative PSDC unit from TrkBCreER; AdvillinFlpO; R26LSL-FSF-ReaChR-mCitrine (top) and from TrkCCreER; AdvillinFlpO; R26LSL-FSF-ReaChR-mCitrine (bottom) mice.
H. Proportion of mechanically sensitive PSDC units that respond to optical activation of distinct sensory neuron types in glabrous hindpaw. Each marker represents one animal. Bars: mean.
I. Example PSDC spatiotemporal RF organization.
J. Left: Example responses to mechanical vibrations in tagged PSDC neurons (bottom), and simultaneously recorded non-tagged DH units (top). Right: Phase-locked responses and vibration frequency tuning across all recorded PSDCs (purple) and simultaneously recorded nontagged units (black).
K. PSDC cluster assignment based on responses to step indentations.
Figure S7. DH outputs shape tactile responses in S1 and somatosensory behaviors. Related to Figure 7.
A. Baseline-subtracted mean firing rates in tagged PSDC neurons from control, FFI cKO and PSI cKO mice at the ON, OFF, and the sustained portion of the indentation step. Error bars: SEM.
B. C.V. of ON and OFF response magnitudes at indentation force steps between 2 to 75mN, measured from PSDC neurons in FFI cKO (red markers; n=17); PSI cKO (teal markers; n=14) and 50 representative sets of 14 randomly sampled PSDC neurons from control mice (grey markers).
C. RF fraction of skin area mapped with increasing force steps in control PSDC neurons vs. PSDC neurons from FFI cKO and PSI cKO animals.
D-E. Fraction (± SEM) of control and FFI cKO S1 units that produce a response at the ON (left), OFF (right) and sustained phase of the step indentation for each force. *P< 0.05; two-proportions Z test.
F. Cross-sections through brain, brainstem and lumbar spinal cords from RorβiCre; TauLSL-nlslacZ; gad2-mCherry animals. Colocalization of the inhibitory marker gad2 (red) with Rorβ lineage neurons (green) was found in lumbar spinal cord (right), but not at other sites of the somatosensory pathway including DCN, somatosensory thalamus or S1. Inhibitory Rorβ+ neurons were also found in amygdala and striatum. Filled arrowheads: Rorβ+ neurons that do not express gad2; asterisk: double-positive neurons.
G. Left: transverse lumbar DH sections from Vgatflox/flox and RorβiCre; Vgatflox/flox animals. Rorβ (green) and Vgat (red) mRNA molecules were detected with gene-specific RNA scope probes. Empty arrowheads: double-positive neurons; filled arrowheads: Rorβ+ neurons that do not express Vgat. Right: Quantification of the percentage overlap between Rorβ and Vgat markers. Multiple LTMR-RZ sections were quantified from three Vgatflox/flox littermate control and three RorβiCre; Vgatflox/flox mice. P<0.001; Unpaired t test.
H. Genetic strategies used for selective silencing of spinal cord inhibitory Rorβ neurons. Right, silencing Rorβ neurons in RorβiCre; Vgatflox/flox causes characteristic hindlimb hyperflexion locomotor phenotype, previously described37,64, demonstrating efficiency of the silencing strategy.
I. Percentage inhibition of the startle response to a 125-dB noise, when the startle response was preceded by a light air puff (0.9PSI) in control littermates and Rorβ silenced mice. *P < 0.05; Unpaired t test.
J. Discrimination indices for texture NORT and shape NORT in control littermates and Rorβ silenced mice. Positive value indicates preference for the novel object compared to the familiar object. *P < 0.05; Unpaired t-test.
K. Sunflower seed assay reveals distinct sensory-motor phenotypes in DH FFI cKO (green) compared to PSI cKO (red) animals. **P< 0.01 and ****p < 0.0001; Unpaired t test.
Data Availability Statement
All data reported in this study will be shared by the lead contact upon request.
All original code is available in this paper’s supplemental information.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


