Abstract
Osteoporosis is an age-related systemic skeletal disease leading to bone mass loss and microarchitectural deterioration. It affects a large number of patients, thereby economically burdening healthcare systems worldwide. The low bioavailability and complications, associated with systemic drug consumption, limit the efficacy of anti-osteoporosis drugs currently available. Thus, a combination of therapies, including local treatment and systemic intervention, may be more beneficial over a singular pharmacological treatment. Hydrogels are attractive materials as fillers for bone injuries with irregular shapes and as carriers for local therapeutic treatments. They exhibit low cytotoxicity, excellent biocompatibility, and biodegradability, and some with excellent mechanical and swelling properties, and a controlled degradation rate. This review reports the advantages of hydrogels for adjuvants loading, including nature-based, synthetic, and composite hydrogels. In addition, we discuss functional adjuvants loaded with hydrogels, primarily focusing on drugs and cells that inhibit osteoclast and promote osteoblast. Selecting appropriate hydrogels and adjuvants is the key to successful treatment. We hope this review serves as a reference for subsequent research and clinical application of hydrogel-based delivery systems in osteoporosis therapy.
Keywords: hydrogel, delivery system, osteoporosis, osteoclast, osteoblast, local treatment
1 Introduction
Osteoporosis is an age-related systemic skeletal disease leading to bone mass loss and microarchitectural deterioration, leading to increased bone fragility and susceptibility to fracture (Compston et al., 2019). The World Health Organization, based on standard deviation scores for bone mineral density (BMD), set the criteria to diagnose osteoporosis as a BMD T-score of –2.5 or less (Kanis, 1994). The prevalence of osteoporosis was found to be higher for postmenopausal women (32.1%) compared to men aged 50 years or older (6.9%) (Wang L. et al., 2021). In addition, the onset of vertebral compressive fractures among men and women affected by osteoporosis and aged 80 years or older is nearly 40%, leading to a high rate of mortality, and in turn increased healthcare costs (Kado et al., 2003; Burge et al., 2007; Schousboe, 2016; Wang L. et al., 2021). In fact, nearly six million osteoporosis-related fractures are expected to occur annually by 2050, accounting for an expenditure of over $ 25.43 billion (Si et al., 2015).
Despite the intrinsic self-repairing properties of bone tissue, its regeneration is hindered in the complex osteoporotic pathological environment; therefore, severe bone defects often require targeted treatments promoting bone formation or anti-resorptive therapies (Zhao et al., 2020; Macías et al., 2021). Pharmacological therapy is commonly used for high-risk patients in the absence of contraindications, such as bisphosphonate (BP) drugs (Khosla et al., 2012; McClung et al., 2013), receptor activator of nuclear factor κB ligand (RANKL) inhibitors (Rachner et al., 2011), hormone-replacement (Cauley et al., 2003), selective estrogen-receptor modulators (Ettinger et al., 1999), and parathyroid hormone-related protein (Neer et al., 2001; Miller et al., 2016). Despite anti-resorptive effects and reduced fracture risks, the low bioavailability and the high risk of complications linked to the systemic use of these drugs limit their application (Khan et al., 2015; Tan et al., 2016). For example, the very low bioavailability of alendronate (0.6%) may cause renal dysfunction, hypocalcemia, osteonecrosis of the jaw, and esophageal ulceration after excessive use (Khan et al., 2015; Posadowska et al., 2015; Hosny and Rizg, 2018; Nafee et al., 2018). Thus, the development of a local delivery system assumes a high practical significance in the clinical treatment of osteoporosis.
Hydrogels are cross-linking hydrophilic polymer chains arranged in 3D networks, exhibiting low cytotoxicity and excellent biocompatibility and biodegradability. Due to their unique properties and high similarity to living tissues (Ossipov et al., 2021; Zheng et al., 2021), hydrogels have gained increasing attention for various biomedical applications, such as wound dressing and tissue engineering (Rinker et al., 2014; Norouzi et al., 2016; Solana Muñoz et al., 2018; Ghavimi et al., 2019; Ning et al., 2019). However, their use in bone regeneration is often hampered due to a lack of mineralization. Thus, hydrogels are loaded with anti-osteoporosis adjuvants to form a delivery system that can effectively compensate for this deficiency. In recent years, the development of hydrogel-related materials to treat osteoporosis has garnered significant interest, and given the encouraging results obtained, for instance, a locally-applied treatment was found to inhibit peri-implant bone resorption, while enhancing peri-implant bone formation and implant stability (Fu et al., 2016; Kettenberger et al., 2017). In this review, we summarize the preclinical research literature on the local treatment of osteoporosis with hydrogel-based delivery systems developed in the last decade, including different hydrogels and adjuvants. Hence, we hope to provide a valid starting point for subsequent research and clinical application of hydrogel-based delivery systems for osteoporosis therapy.
2 Hydrogel carriers
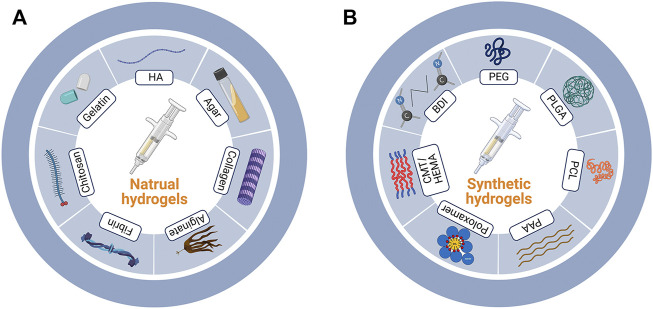
According to the types of the components, here in this review, we grouped the hydrogels into nature-based ones and synthetic ones (Figure 1). Besides, composite materials containing several types of hydrogels were also discussed. The detailed discussion is listed as follows.
FIGURE 1.
Nature-based and synthetic hydrogels. (A) Major components of nature-based and (B) synthetic hydrogels. HA, hyaluronic acid; PEG, polyethylene glycol; PLGA, poly (lactic-co-glycolic acid); PCL, polycaprolactone; PAA, polyacrylic acid; CMT/HEMA, carboxy methyl tamarind/hydroxyethyl methacrylate; BDI, butane-diisocyanate.
2.1 Nature-based hydrogels
Nature-based hydrogels are derived from cross-linking components derived from nature. These are characterized with low cytotoxicity, excellent biocompatibility, and biodegradability (Table 1; Ito et al., 2012; Ito and Otsuka, 2013; Jiang et al., 2014; Ye et al., 2014; Lee et al., 2015; Cancian et al., 2016; Ma et al., 2016; Pelled et al., 2016; Kim et al., 2017; Kim et al., 2018; Nafee et al., 2018; Solana Muñoz et al., 2018; Ghavimi et al., 2019; Liang et al., 2019; Ning et al., 2019; Ossipov et al., 2021; van Houdt et al., 2021; Yilmaz et al., 2021). The commonly used materials include hyaluronic acid (HA), chitosan, collagen, gelatin, alginate, fibrin, agar and so on.
TABLE 1.
Components and characteristics of nature-based hydrogels.
| Hydrogels | Literatures | Characteristics |
|---|---|---|
| HA, Ca2+ | Ossipov et al. (2021), van Houdt et al. (2021) | self-healing, two drugs released independently in response to acidic and thiol-containing microenvironments |
| HA, β-TCP | Lee et al. (2015) | porous β-TCP microspheres |
| Chitosan, β-glycerophosphate | Nafee et al. (2018) | Thermoreversibility |
| Chitosan, hydroxyapatite, carbon nanotubes | Cancian et al. (2016) | thermosensitive, higher resistance to compression, controlled release of protein drugs |
| Chitosan, cellulose nanocrystal | Ghavimi et al. (2019) | mechanical strength similar to vertebral bone, osteoinductivity |
| Collagen, hydroxyapatite, genipin | Ma et al. (2016) | improved mechanical property, higher gel content, lower swelling ratio, and tunable degradation behaviors against collagenase |
| Collagen I | Jiang et al. (2014) | NA |
| Methacrylated gelatin | Ning et al. (2019) | prolonged drug release (>10 days) |
| Gelatin, HPA | Kim et al. (2018) | NA |
| Calcium alginate | Ye et al. (2014) | NA |
| Alginate, CaP | Ito et al. (2012) | slow-releasing drug reservoir, protective coating, pH buffer |
| Ito and Otsuka, (2013) | ||
| Silk fibroin | Liang et al. (2019) | NA |
| Fibrin | Kim et al. (2017) | commercially available |
| Pelled et al. (2016) | ||
| Agar, hydroxyapatite | Solana Muñoz et al. (2018) | screw augmentation effect |
| Silica-quince seed mucilage | Yilmaz et al. (2021) | osteogenesis |
HA, hyaluronic acid; β-TCP, β-tricalcium phosphate; HPA, hydroxyphenyl propionic acid; CaP, calcium phosphate; NA, not available.
2.1.1 Hyaluronic acid
HA is a natural mucopolysaccharide acidic polymer and a main component of the extracellular matrix. It is widely distributed on the market as a hydrogel carrier (Kettenberger et al., 2017) owing to its excellent biocompatibility, high amenability, and multifunctional properties (Qiu et al., 2021), which make it an optimal candidate for tissue engineering as well. Ossipov et al. (2021) reported an injectable and self-healing HA hydrogel that independently released two different drugs in response to acidic and thiol-containing microenvironments. Except for the hydrogel carrier itself, the binding sites available on each individual component must also be considered when designing hydrogels. For instance, the BP prodrug is conjugated to HA via a self-immolative disulfide linker, that is, stable in the blood plasma and cleavable in the cytoplasm; the resulting HA-linked BP ligands reversibly bind Ca2+ ions and form coordination hydrogels (Ossipov et al., 2021).
2.1.2 Chitosan or chitin
Chitosan, a linear and semi-crystalline polysaccharide, is a direct derivative of chitin, which is the second most abundant natural polymer after cellulose (Friedman and Juneja, 2010; Gong et al., 2022). Chitosan has excellent biocompatibility, biodegradability, adsorption capacity, anti-bacterial properties, and thermosensitive properties (Cancian et al., 2016; Nafee et al., 2018). It can be easily produced through acylation, alkylation, and carboxylation reactions (Shariatinia, 2019). However, chitosan-based hydrogels often fail to meet the mechanical strength requirements of bone fillers. The poor mechanical strength of chitosan-based hydrogels can be compensated by incorporating several types of nanofillers, i.e., carbon nanotubes and cellulose nanocrystals, which is particularly important for treating managing osteoporotic vertebral compression fractures (Cancian et al., 2016; Ghavimi et al., 2019; Vitale et al., 2022).
2.1.3 Collagen or gelatin
Gelatin is a type of protein obtained by the partial hydrolysis of collagen; both have similar homologies. Collagen has a rod-like triple-helical structure, which is partially separated and broken when it is partially hydrolyzed to make gelatin (Veis and Cohen, 1960). Collagen-based hydrogels formed under physiological conditions using genipin as a cross-linker exhibited markedly improved mechanical properties, higher gel content, lower swelling ratio, and tunable degradation behaviors against collagenase (Ma et al., 2016). In addition, Ning et al. (2019) reported that the methacrylated gelatin-based hydrogel prolonged the release of the adjuvant (>10 days).
2.1.4 Alginate
Alginate, derived from algae, is a linear copolymer composed of beta-d-mannuronic acid and C-5-epimer alpha-l-guluronic acid. An alginate solution gels when exposed to Ca2+ and other bivalent cations as these cations strongly bind to the G residues of the alginate molecule. Therefore, the calcium alginate gel is considered a three-dimensional network of molecules with cross-links between the G residues of other long-chain molecules through the action of Ca2+ (Bjerkan et al., 2004). The drug-release kinetics from hydrogels are commonly controlled by the network properties and drug–network interactions (Ossipov et al., 2021). The alginate gel sometimes degrades too rapidly under acidic conditions, such as the area around the osteoporotic bones. Thus, it has been reported that adding amorphous CaP powders positively affected dissociation rate (Ito et al., 2012; Ito and Otsuka, 2013), owing to the pH buffering mechanism inside the gel, thereby allowing for a controlled drug release.
2.2 Synthetic hydrogels
Synthetic hydrogels are covalently cross-linked with synthetic materials (Sanyasi et al., 2014; Fu et al., 2016; Tan et al., 2016; Neuerburg et al., 2019; Li et al., 2020; Zhao et al., 2020; Wang X. et al., 2021; Chen et al., 2021) (Table 2). Apart from the excellent mechanical and swelling properties, some of them allow for a controlled drug release (Zheng et al., 2021).
TABLE 2.
Components and characteristics of synthetic hydrogels.
| Hydrogels | Literatures | Characteristics |
|---|---|---|
| Poloxamer 407 | Chen et al. (2021) | commercially available, thermosensitive |
| Wang et al. (2021) | ||
| Tan et al. (2016) | ||
| Fu et al. (2016) | ||
| BDI | Neuerburg et al. (2019) | commercially available, thermosensitive |
| Tetra-PEG | Li et al. (2020) | biocompatible, rapid gel formation, excellent injectability |
| PAA, nano-hydroxyapatite, sodium carbonate | Zhao et al. (2020) | initial morphology and mechanical properties maintained under physiological conditions, good primary stability, biocompatibility, bioactivity, and osteoconductivity |
| CMT/HEMA | Sanyasi et al. (2014) | effective adhesion, growth and further clustering of bone precursor cells, without any cytotoxicity |
BDI, butane-diisocyanate; PEG, polyethylene glycol; PAA, polyacrylic acid; CMT/HEMA, carboxy methyl tamarind/hydroxyethyl methacrylate.
2.2.1 Poloxamer 407 and butane-diisocyanate
Some commercially available products, such as Poloxamer 407 and butane-diisocyanate, can be directly used as hydrogels to carry adjuvants and are mainly used to study the function of targeted drugs (Neuerburg et al., 2019; Chen et al., 2021). Other commonly used synthetic materials used to produce hydrogels are reported below.
2.2.2 Polyethylene glycol
PEG, also known as a macrogol, is a type of nontoxic and water-soluble polymer with unique hydrophilicity and electrical neutrality. It consists of chemically active hydroxyl groups at both ends, thereby promoting the conjugation with other functional groups (Alper and Pashankar, 2013; Shi et al., 2021). It can rapidly form biocompatible gels and is easily injectable; therefore, it is considered an ideal hydrogel carrier (Li et al., 2020).
2.2.3 Poly lactic-co-glycolic acid
PLGA is a biodegradable polymeric compound formed by polymerizing lactic acid and glycolic acid, which are by-products of human metabolic pathways. Therefore, it is nontoxic, except in those individuals suffering from lactose deficiency. The co-polymerization between PLGA and the carried drug may prolong the release time; this confirms that the release of the drugs from the carrier depends on the degradation of the hydrogel and the decomposition of the drug complex (Liu et al., 2017; Peng et al., 2017). Additionally, PLGA can co-polymerize with PEG to form new polymers for hydrogel-based delivery systems (Liu et al., 2017).
2.2.4 CHAp-polyacrylic acid
CHAp-PAA is the term used to refer to a supramolecular hydrogel composed of nano-hydroxyapatite, sodium carbonate, and polyacrylic acid (PAA) (Zhao et al., 2020); owing to the high mineralization, such hydrogel can be used as a scaffold to treat bone defects in osteoporotic individuals. Because of the biomineral composition, the hydrogel can mimic the chemical composition and structural characteristics of natural bones, while achieving mechanical stability, biocompatibility, and osteogenesis without delivering any additional therapeutic agents or stem cells (Zhao et al., 2020).
2.2.5 Carboxy methyl tamarind/hydroxyethyl methacrylate
CMT/HEMA (ratio of 1:10) is a hydrogel with a surface that promotes the adhesion of bone precursor cells and efficient growth of bone tissues (Sanyasi et al., 2014). It is highly compatible with bone cells (RAW264.7) and sensitive to neuronal (Neuro2a) and human umbilical vein endothelial (HUVEC) cells (Sanyasi et al., 2014).
2.3 Composite hydrogels
While nature-based hydrogels are unable to withstand the pressure at the site of the bone injury because of their poor mechanical properties, their synthetic counterparts generally exhibit poor biocompatibility, lack interactions with targeted cells, and tend to cause adverse reactions in the body (Zheng et al., 2021). Composite hydrogels that combine the advantages of natural and synthetic hydrogels have been proposed to overcome these individual drawbacks (Utech and Boccaccini, 2016; Gačanin et al., 2017; Kettenberger et al., 2017; Kim et al., 2018; Segredo-Morales et al., 2018; García-García et al., 2019; Ghavimi et al., 2019; Akbari et al., 2020; Bai et al., 2020; García-García et al., 2020; Gilarska et al., 2021; Yoon et al., 2021); some of these composite hydrogels with their respective characteristics are reported in Table 3. However, given the treatment required to treat osteoporosis, a prolonged drug release time was the main aim while designing the target composite hydrogel, as discussed below.
TABLE 3.
Components and characteristics of composite hydrogels.
| Hydrogels | Literatures | Characteristics |
|---|---|---|
| Hydroxypropyl chitin, HA | Yu et al. (2020b) | thermosensitive, tunable biodegradable property, long-term sustained drug release (>28 days) with considerable structure stability, compatibility, osteoconductive potential |
| Hydroxypropyl chitin, alginate, Ca2+ | Yu et al. (2020a) | thermosensitive, long-term sustained drug release (>28 days) with conformation stability, biocompatible, osteoconductive potential |
| Chitosan, glycerophosphate, gelatin | Akbari et al. (2020) | High gel strength, slow drug release rate, promotive effect on differentiation of osteoblasts |
| Biopolymeric collagen/chitosan/hyaluronic acid matrix, amine group-functionalized silica particles decorated with apatite, genipin | Gilarska et al. (2021) | lack systemic toxicity, particularly useful for the repair of small osteoporotic bone defects |
| HA, PVA, hydroxyapatite | Kettenberger et al. (2017) | commercially available |
| Gellan gum, PLGA | Posadowska et al. (2015) | low release rate |
| N-chitosan, ADH, HA-ALD | Bai et al. (2020) | self-healable, injectable, and biodegradable, functions and structures retained after external damage |
| Alginate, PLGA | García-García et al. (2020) | injectable, lower porosity, water absorption capacity and bone repair compared with its solid sponge state |
| Alginate, PLGA-PEG-PLGA, Ca2+ | Liu et al. (2017) | thermosensitive, controlled drug release |
| Chitosan, collagen, 2-hidroxipropil γ-ciclodextrin, nanoparticles of hydroxyapatite, PLGA | García-García et al. (2019) | controlled drug release |
| Human serum albumin, ssDNA, PEG | Gačanin et al. (2017) | biocompatible, biodegradability, rapid gelation under physiological conditions, self-healing, spatiotemporally controlled release of active proteins |
| Gelatin, PNIPAM, PDMS | Yoon et al. (2021) | highly interconnected, dense channel networks |
| PF127, T1307, CD, PLGA, PLA | Segredo-Morales et al. (2018) | thermoresponsive |
| mPEG-PLGA | Peng et al. (2017) | extended drug release, biocompatibility |
| PLGA, PCL, capryol 90 | Hosny and Rizg, (2018) | higher bioavailability, extended drug release (>3 months) |
| HA, PF127 | Akbari et al. (2020) | commercially available |
HA, hyaluronic acid; PVA, polyvinyl alcohol; PLGA, poly (lactic-co-glycolic acid); N-chitosan, N-carboxyethyl chitosan; ADH, adipic acid dihydrazide; HA-ALD, hyaluronic acid-aldehyde; PEG, polyethylene glycol; PNIPAM, poly (N-isopropylacrylamide); PDMS, polydimethylsiloxane; PF127, Pluronic F127; T1307, Tetronic 1307; CD, α-cyclodextrin; PLA, poly lactic acid; PCL, polycaprolactone.
The combination of natural hydrogels showed interesting results, as reported in several studies (Akbari et al., 2020; Yu et al., 2020a; Yu et al., 2020b) investigating the combination of chitin and chitosan for this scope; the resulting composite materials showed a good adsorption capacity coupled with a longer drug release time. Yu et al. combined hydroxypropyl chitin and HA or alginate, which exhibited a prolonged (28 days) and controlled drug release and considerable structure stability, and the studies showed a higher ALP activity, calcium expression and extracellular calcium deposition without inflammation and immune responses, indicating its potential for osteoconductive applications (Yu et al., 2020a; Yu et al., 2020b).
For composite hydrogels formed by the combination of natural and synthetic, or synthetic and synthetic components, PLGA is often added to prolong the drug release time (Posadowska et al., 2015; Liu et al., 2017; Peng et al., 2017; Hosny and Rizg, 2018; García-García et al., 2019). PLGA is commonly used in such composite systems to form microsphere structures. In the study by García-García et al. (2019), the hydrogel core of a sandwich-like system composed of the chitosan-collagen complex, 2-hidroxipropil-ciclodextrina and hydroxyapatite nanoparticles, and the addition of PLGA-based microspheres controlled the release of the adjuvants. The system placed in the defect easily adapted to the shape; after 12 weeks, approximately 50% of the defect was refilled with new tissue. Hosny and Rizg, (2018) adopted a Box–Behnken experimental design while using the Statgraphics® software to develop an in situ hydrogel. The composite material, composed of PLGA, PCL, and the lipid surfactant capryol®90, exhibited a high bioavailability and extended drug release (>3 months), which aided in minimizing the side effects of several anti-osteoporosis drugs (Hosny and Rizg, 2018).
Several other composite hydrogels have been developed based on different application requirements. The circulatory system is the major route used to deliver drugs. Thus, a highly interconnected and dense channel network can be achieved by combining gelatin, poly (N-isopropylacrylamide), and polydimethylsiloxane. This composite material overcame the 200 μm diffusion limit of any 3D hydrogel (Yoon et al., 2021) and aided the recovery of the endocrine function. Moreover, it led to a full endometrium regeneration in the osteoporotic models, while effectively suppressing the side effects observed with the synthetic hormone treatment and preventing the representative aftereffects of menopause (Yoon et al., 2021). Lately, the self-healing capacity reported for some types of hydrogels has also received attention (Gačanin et al., 2017; Bai et al., 2020). Adipic acid dihydrazide (ADH), which is also a cross-linking agent promoting the formation of relatively stable hydrazone links from aldehydes (Bystrický et al., 1999) is one such example. It is cross-linked with N-carboxyethyl chitosan (N-chitosan) and hyaluronic acid-aldehyde (HA-ALD) in situ to form an injectable and self-healing supramolecular hydrogel. This composite hydrogel exhibited a remarkable self-healing capacity and retained its structural integrity after it was subjected to external damage (Bai et al., 2020).
3 Loaded anti-osteoporosis adjuvants
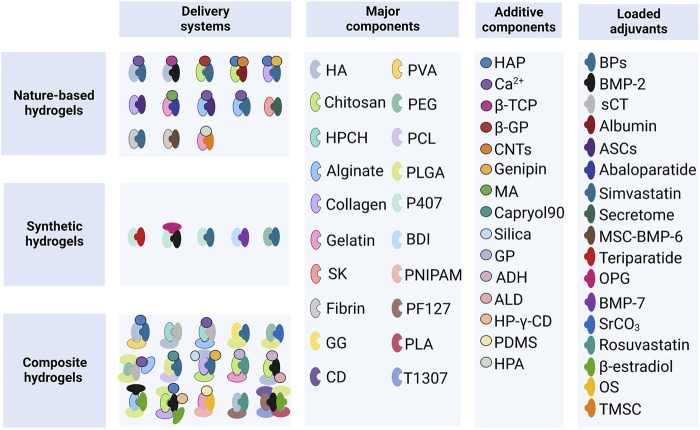
The human skeleton is composed of the cortical and cancellous bone (the main part of the vertebrae). Bone remodeling is a process in which osteoclastic bone resorption and osteoblastic bone formation are regulated to achieve a dynamic balance in young adults (Hattner et al., 1965). The process of aging is associated with a negative remodeling balance, resulting in bone mass loss and disruption of the bone microarchitecture (Compston et al., 2019). The genetic factors account for 50%–85% of the normal variance in bone mass. The general signal pathways in osteoporosis include the receptor activator of nuclear factor κB (RANK), RANKL, osteoprotegerin (OPG), bone morphogenic protein (BMP), and Wingless-related integration site (Wnt) (Rachner et al., 2011). As discussed below, the loaded adjuvants mainly inhibit osteoclast formation or promote osteoblasts formation; several systems composed of the hydrogel-based carrier and adjuvant have been investigated thus far (Figure 2).
FIGURE 2.
Hydrogel-based delivery systems. HA, hyaluronic acid; PVA, polyvinyl alcohol; HPCH, hydroxypropyl chitin; PEG, polyethylene glycol; PCL, polycaprolactone; SK, silk fibroin; GG, gellan gum; PLGA, poly (lactic-co-glycolic acid); P407, Poloxamer 407; BDI, butane-diisocyanate; PNIPAM, poly (N-isopropylacrylamide); PF127, Pluronic F127; T1307, Tetronic 1307; CD, α-cyclodextrin; PLA, poly-lactic acid; HAP, hydroxyapatite; β-TCP, β-tricalcium phosphate; β-GP, β-glycerophosphate; CNTs, carbon nanotubes; MA, methacrylic anhydride; GP, glycerophosphate; ADH, adipic acid dihydrazide; ALD, aldehyde; HP-γ-CD, 2-hidroxipropil γ-cyclodextrin; PDMS, polydimethylsiloxane; HPA, hydroxyphenyl propionic acid; BPs, bisphosphonates; BMP-2, bone morphogenetic protein-2; sCT, salmon calcitonin; ASCs, adipose-derived stem cells; MSC-BMP6, porcine mesenchymal stem cells overexpressing the BMP6 gene; OPG, osteoprotegerin; BMP-7, bone morphogenetic protein-7; OS, ovarian spheroids; TMSC, tonsil-derived mesenchymal stem cells.
3.1 Inhibition of osteoclastic bone resorption
3.1.1 Osteoclast differentiation pathways and bone resorption function
To treat osteoporosis successfully, it is important to inhibit the formation, differentiation, and resorption functions of osteoclasts. Osteoclasts are a special type of terminally differentiated cell deriving from a family of mononuclear macrophages in the blood. They can be fused by their mononuclear progenitor cells in various ways to form a multinuclear giant cell, where the RANKL and macrophage colony-stimulating factor (M-CSF) play a crucial role (Rachner et al., 2011). Tumor necrosis factor superfamily 11 (TNFSF11), the gene encoding RANKL, is abundantly expressed by osteoblasts, bone marrow stromal cells, and T and B lymphocytes (Rachner et al., 2011). As a homotrimeric type II transmembrane protein, RANKL can be released from the cell membrane upon decomposition of several extracellular proteases, including disintegrin and metalloprotease (Nagy and Penninger, 2015). RANK and OPG are the two main receptors of RANKL; such receptors are also known as tumor necrosis factor receptor superfamily member 11A (TNFRSF11A) and TNFRSF11B, respectively (Nagy and Penninger, 2015).
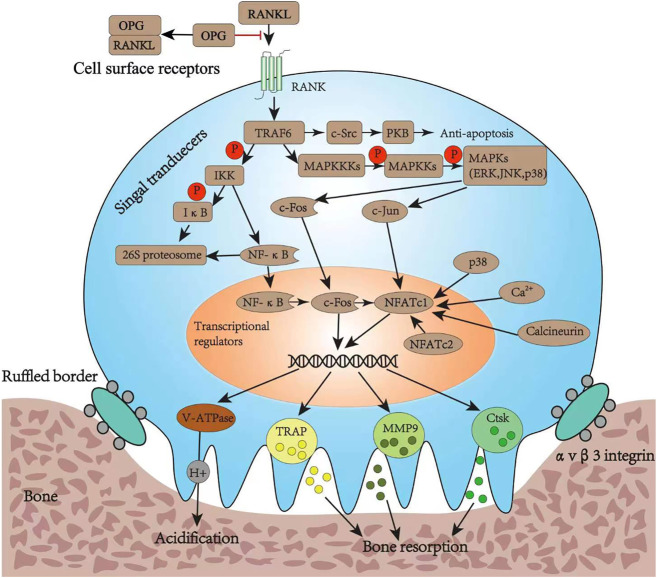
When secreted RANKL binds to the membrane-binding receptor RANK on the precursor of osteoclasts, it causes the RANK receptor to polymerize into a trimer that recruits several junction molecules, including tumor necrosis factor receptor-associated factor 6 (TRAF6) (Armstrong et al., 2002). The recruitment of TRAF6 leads to the activation of a variety of signaling pathway cascades, including inhibitor of nuclear factor κB kinase (IKK), mitogen-activated protein kinase (MAPK) family, and cellular Src kinase (c-Src), which enable osteoclasts to differentiate, survive, polarize, and have absorptive activity. The MAPK pathway is composed of three types of molecules: MAPK (including ERK, JNK, and p38), MAPK kinase (MAPKK or MEK), and MAPKK kinase (MAPKKK or MEKK) (Lee et al., 2018). The activation of MAPKs induces the nuclear translocation of c-Fos and c-Jun, while nuclear factor-κB (NF-κB), derived from the IKK pathway, up-regulates c-Fos in the nucleus upon nuclear translocation. The c-Src activates the anti-apoptotic program through protein kinase B. The nuclear factor of activated T-cells cytoplasmic 1 (NFATc1) is a critical transcription factor for osteoclast differentiation. Upon the initial activation through NF-κB and NFATc2, it is then upregulated by the action of c-Jun, p38, c-Fos, calcineurin, and calcium ions (Zhao et al., 2010). Finally, the up-regulated c-Fos and NFATc1 synergistically promote the expression of osteoclast-specific genes, such as tartrate-resistant acid phosphatase (TRAP), cathepsin K (CtsK), matrix metalloproteinase-9 (MMP-9), and vesicle-type ATPase (V-ATPase) V0 domain d2 subunit (Teitelbaum, 2000; Boyle et al., 2003; Nagy and Penninger, 2015). Bone degradation includes polarization, acidification, and protein breakdown; during these steps, the ruffled bone edge formed by osteoclasts plays an important role. In fact, it secretes protons (H+) into the bone resorption space through the V-ATPase and proteases such as TRAP, CtsK, and MMP-9; while the former degrade the bone minerals, the latter deteriorate the organic bone components (Georgess et al., 2014) (Figure 3).
FIGURE 3.
Osteoclast differentiation pathways and its bone resorption function. RANKL, ligand of receptor activator of nuclear factor κB; RANK, receptor activator of nuclear factor κB; OPG, osteoprotegerin; TRAF6, tumor necrosis factor receptor-associated factor 6; IKK, inhibitor of nuclear factor κB kinase; IκB, inhibitor of nuclear factor kappa B; NF-κB, nuclear factor-κB; PKB, protein kinase B; MAPKKK, mitogen-activated protein kinase; MAPKK, mitogen-activated protein kinase; MAPK, mitogen-activated protein kinase; ERK, extracellular regulated protein kinase; JNK, c-Jun N-terminal kinase; NFATc1, nuclear factor of activated T cell cytoplasmic 1; NFATc2, nuclear factor of activated T cell cytoplasmic 2; V-ATPase, vacuolar H (+) ATPase; TRAP, tartrate-resistant acid phosphatase; MMP9, matrix metalloproteinase 9; Ctsk, cathepsin K.
3.1.2 Adjuvants commonly used to inhibit osteoclasts
The binding between RANKL and RANK, and the following signaling cascade play an important role in osteoclast differentiation and survival. The inhibition of these pathways has become a feasible target for the systematic or local treatment of osteoporosis (Matsumoto and Endo, 2021). Targeting extracellular pathways, OPG-loaded composite hydrogels capable of controlling the release of OPG can inhibit the binding of RANKL and RANK; therefore, the osteoclastic activation is reduced, while promoting bone regrowth and osseointegration in osteoporotic defects (Wang X. et al., 2021). Alendronate, which is widely used to alleviate osteoporosis by inhibiting osteoclasts, is one of the most common drugs loaded on hydrogels (Posadowska et al., 2015; Nafee et al., 2018; Li et al., 2020; Gilarska et al., 2021; Jiang et al., 2022); such a complex would likely inhibit the rate-limiting step in the cholesterol biosynthesis pathway, essential for osteoclast function (Fisher et al., 1999). In addition, it was observed that the loading of zoledronate does not affect its action; in fact, the inhibition of the degradation of the mineralized hydrogel and the resorption of the peri-implant bone are effectively carried out by the loaded and unbound zoledronate (Kettenberger et al., 2017).
3.2 Promotion of osteogenesis
3.2.1 Osteogenic differentiation pathways
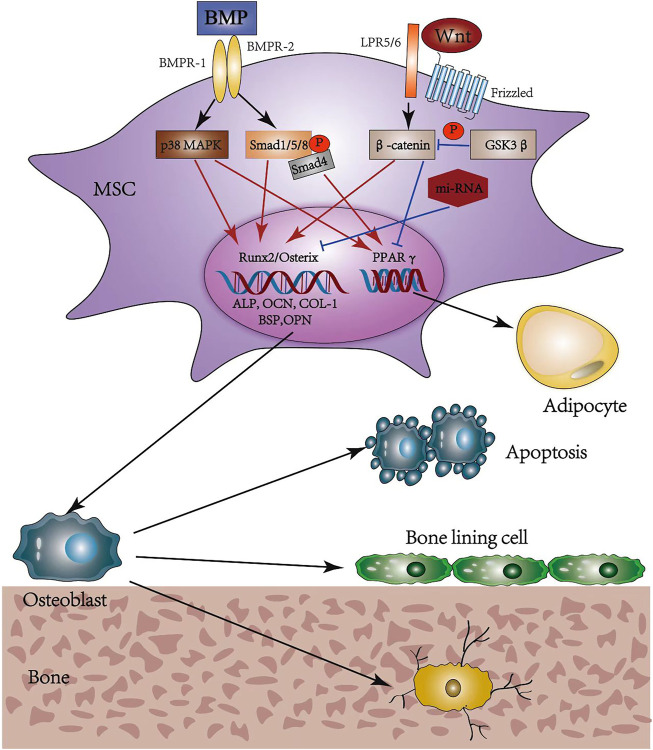
During osteoporosis development, bone marrow mesenchymal stem cells (MSCs) promote the depletion of osteoblasts while increasing the amount of adipocytes, thereby resulting in a slower bone formation rate and improved marrow fat accumulation (Moerman et al., 2004; Li et al., 2015). The specific differentiation direction is precisely regulated by factors in the signaling pathways, transcription factors, and microRNAs. Among numerous studies of signaling pathways, the wingless and int-1 (Wnt) classes and BMP represent two critical signaling pathways (Hu et al., 2018).
The canonical Wnt signaling, also called Wnt/β-catenin, is essential for determining the fate of osteoblast cells. It binds a seven-transmembrane-spanning frizzled protein (Frz) receptor with the low-density lipoprotein receptor-related protein (LRP) 5/6 co-receptor to prevent the phosphorylation and degradation of β-catenin (Kim et al., 2013). Then, β-catenin translocates into the nucleus to promote osteogenesis while inhibiting adipogenesis, regulated by MSCs (Etheridge et al., 2004; Shen et al., 2011; Yuan et al., 2016). Moreover, this function may be achieved by inducing the expression of runt-related transcription factor-2 (runx-2) and osterix, and inhibiting peroxisome proliferation-activated receptor γ (PPARγ) (Bennett et al., 2005; Kang et al., 2007). BMP is the collective name for a series of transforming growth factor-β (TGF-β) family members and operates through either canonical or non-canonical BMP signaling (Chen et al., 2012) upon binding to bone morphogenetic protein receptor I (BMPR-I) and BMPR-II. The canonical BMP signaling induces phosphorylation of Smad1/5/8, which translocates into the nucleus upon the formation of complexes with Smad4; the non-canonical BMP signaling occurs mainly through the p38 MAPK pathway (Chen et al., 2012). Both signaling can regulate the target gene expression of runx-2, osterix, and PPARγ, showing dual roles in inducing osteogenic and adipogenic differentiation of MSCs (Kang et al., 2009). Several studies (Wang et al., 1993; Gori et al., 1999) indicate that a high BMP-2 concentration accelerates osteoblast differentiation, while adipocyte formation is promoted at low concentrations and in the presence of BMP-4 (Tang et al., 2004) (Figure 4).
FIGURE 4.
Osteogenic differentiation pathways and possible fates of osteoblasts. MSC, mesenchymal stem cell; BMP, bone morphogenic protein; BMPR, bone morphogenic protein receptor; MAPK, mitogen-activated protein kinase; Smad, small mothers against decapentaplegic; Wnt, wingless and int-1; LRP, low density lipoprotein receptor-related protein; GSK, glycogen synthase kinase; Runx2, runt-related transcription factor 2; ALP, alkaline phosphatase; OCN, osteocalcin; COL-1, collagen-1; BSP, bone sialoprotein; OPN, osteopontin; PPARγ, peroxisome proliferation-activated receptor γ.
3.2.2 Adjuvants commonly used to promote osteogenesis
Since a high concentration of BMP-2 promotes osseointegration, it is one of the most common drugs loaded in hydrogel scaffolds (Lee et al., 2015; Segredo-Morales et al., 2018; García-García et al., 2019; Bai et al., 2020; García-García et al., 2020; Wang et al., 2021b; van Houdt et al., 2021). BMP-7 or BMP-6 loaded hydrogels have been used in local treatment, are potent stimulators of osteogenesis, and can reduce the risk of further osteoporosis-associated secondary fractures (Pelled et al., 2016; Neuerburg et al., 2019). Alternatively, Rosuvastatin is a popular drug that promotes osteogenic differentiation of MSCs in the model of osteoporosis by the Wnt/β-catenin signaling (Wang et al., 2019; Akbari et al., 2020). Simvastatin can also promote osteogenesis, and the underlying mechanism appears to involve a higher expression of BMP-2 (Ito et al., 2012; Fu et al., 2016; Tan et al., 2016; Zhu et al., 2021). In addition, carrying Si or Sr ions has been shown to promote osteogenic differentiation, with the former being controlled by upregulating the expression of the osteogenesis-related genes (Peng et al., 2017; Yilmaz et al., 2021). Hydrogels can also be directly loaded with MSCs, showing potential as a supplement or alternative to the current therapies proposed (Kim et al., 2018; Chang et al., 2019). Adipose-derived stem cells are also able to promote osteogenesis and inhibit adipogenesis of osteoporotic MSCs through activation of the BMP-2/BMP receptor-type IB signal pathway in the local delivery system (Jiang et al., 2014; Ye et al., 2014). Interestingly, it was observed that the treatment of drug-loaded hydrogels by extracorporeal shockwaves can promote bone formation by upregulating the alkaline phosphatase activity, mineralization, and expression of runx-2, type-I collagen, osteocalcin, and osteopontin (Chen et al., 2021).
3.3 Hormone analogs and osteo immunomodulators
The use of hormones and their analogs often play an important role in osteoporosis treatment, triggering complex physiological mechanisms, which have effects on osteoclasts and osteoblasts. Abaloparatide, as an analog of the human recombinant parathyroid hormone-related protein (PTHrp) that selectively binds to the RG conformation of the parathyroid hormone type one receptor, may represent a successful option for postmenopausal women affected by osteoporosis (Hattersley et al., 2016; Miller et al., 2016). Its role as a drug in hydrogel scaffold has also been proven (Ning et al., 2019). The hydrogel system containing calcitonin effectively reduced serum calcium levels, while promoting the reconstruction of bone trabecula (Liu et al., 2017; Yu et al., 2020b). Estrogens have also been tested for drug delivery in hydrogel carriers; for instance, 17β-estradiol is often locally delivered along with BMP (Segredo-Morales et al., 2018; García-García et al., 2019). In addition, hormone autocrination by vascularized hydrogel delivery of ovary spheroids (VHOS) to treat ovarian dysfunctions is successively conducted. The VHOS implantation effectively suppresses the side effects usually observed with synthetic hormone treatment, such as tissue overgrowth, hyperplasia, cancer progression, and deep vein thrombosis (Yoon et al., 2021). Moreover, recent studies have emphasized the use of immune cells in bone regeneration, giving rise to a new research field termed “osteoimmunology” (Liu et al., 2018; Fan et al., 2021). Among various innate immune cells, macrophages are one of the most vital effectors; as an example, they are the earliest cells approaching the implant area upon surgery. In the study of Jin et al. (2019) biomimetic hierarchical intrafibrillarly mineralized collagen loading IL-4 potently induced osteogenesis by promoting CD68+CD163+ M2 macrophage polarization in response to the critical-sized bone defects. In terms of other immune cells, hydroxyapatite nanorods with different aspect ratios could regulate osteogenesis through the modulation of T cells and IL-22 during bone regeneration (Yu et al., 2022).
4 Perspective and conclusion
As osteoporosis is a systemic disease, its local treatment has often been underestimated in the past; however, a hydrogel-adjuvant delivery system might lead to significant advantages in some specific cases, including local bone augment in surgery to prevent screw loosening or accelerating local bone healing after fracture. The inherent advantages of using natural hydrogels, i.e., good biocompatibility, cannot offset their lacking in mechanical properties; therefore, the modification of these natural components or the addition of synthetic ones is usually inevitable. The resulting composite hydrogel would ideally have excellent mechanical and swelling properties, controlled degradation and release rate, high drug-loading capacity, low cytotoxicity, and high biocompatibility. Regarding the loaded adjuvants, they should effectively promote osteoblasts while inhibiting the formation of osteoclasts. Despite the tremendous progress made in the field of tissue engineering over the past several decades, the passage from basic research to clinical application remains a critical challenge. The detection of the ideal hydrogel-adjuvant system has the potential to ease such a transition, but it requires the joint efforts of clinicians and researchers.
Acknowledgments
We would like to thank Furong Li for the assistant of figure visualization.
Author contributions
YG, YB, and YL conceptualized the work. XG, BZ, ZQ, and DW developed the investigation. YG drafted the original manuscript. DH, BH, LK, and LY reviewed and edited the manuscript. WH created the artwork. LY acquired funding. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (82260181), National Natural Science Foundation of China (82070909), Key project of Natural Science Basic Research Plan of Shaanxi Province (2022JZ-43), and Medical Research Project of Xi’an Science and Technology Bureau (22YXYJ0083).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Akbari V., Rezazadeh M., Ebrahimi Z. (2020). Comparison the effects of chitosan and hyaluronic acid-based thermally sensitive hydrogels containing rosuvastatin on human osteoblast-like MG-63 cells. Res. Pharm. Sci. 15 (1), 97–106. 10.4103/1735-5362.278719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper A., Pashankar D. S. (2013). Polyethylene glycol: A game-changer laxative for children. J. Pediatr. Gastroenterol. Nutr. 57 (2), 134–140. 10.1097/MPG.0b013e318296404a [DOI] [PubMed] [Google Scholar]
- Armstrong A. P., Tometsko M. E., Glaccum M., Sutherland C. L., Cosman D., Dougall W. C. (2002). A RANK/TRAF6-dependent signal transduction pathway is essential for osteoclast cytoskeletal organization and resorptive function. J. Biol. Chem. 277 (46), 44347–44356. 10.1074/jbc.M202009200 [DOI] [PubMed] [Google Scholar]
- Bai H., Zhao Y., Wang C., Wang Z., Wang J., Liu H., et al. (2020). Enhanced osseointegration of three-dimensional supramolecular bioactive interface through osteoporotic microenvironment regulation. Theranostics 10 (11), 4779–4794. 10.7150/thno.43736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C. N., Longo K. A., Wright W. S., Suva L. J., Lane T. F., Hankenson K. D., et al. (2005). Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc. Natl. Acad. Sci. U. S. A. 102 (9), 3324–3329. 10.1073/pnas.0408742102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerkan T. M., Bender C. L., Ertesvåg H., Drabløs F., Fakhr M. K., Preston L. A., et al. (2004). The Pseudomonas syringae genome encodes a combined mannuronan C-5-epimerase and O-acetylhydrolase, which strongly enhances the predicted gel-forming properties of alginates. J. Biol. Chem. 279 (28), 28920–28929. 10.1074/jbc.M313293200 [DOI] [PubMed] [Google Scholar]
- Boyle W. J., Simonet W. S., Lacey D. L. (2003). Osteoclast differentiation and activation. Nature 423 (6937), 337–342. 10.1038/nature01658 [DOI] [PubMed] [Google Scholar]
- Burge R., Dawson-Hughes B., Solomon D. H., Wong J. B., King A., Tosteson A. (2007). Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J. Bone Min. Res. 22 (3), 465–475. 10.1359/jbmr.061113 [DOI] [PubMed] [Google Scholar]
- Bystrický S., Machová E., Malovíková A., Kogan G., Malovikova A. (1999). Determination of the cross-linking effect of adipic acid dihydrazide on glycoconjugate preparation. Glycoconj. J. 16 (11), 691–695. 10.1023/a:1007103309053 [DOI] [PubMed] [Google Scholar]
- Cancian G., Tozzi G., Hussain A. A., De Mori A., Roldo M. (2016). Carbon nanotubes play an important role in the spatial arrangement of calcium deposits in hydrogels for bone regeneration. J. Mat. Sci. Mat. Med. 27 (8), 126. 10.1007/s10856-016-5740-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauley J. A., Robbins J., Chen Z., Cummings S. R., Jackson R. D., LaCroix A. Z., et al. (2003). Effects of estrogen plus progestin on risk of fracture and bone mineral density: The women's Health initiative randomized trial. Jama 290 (13), 1729–1738. 10.1001/jama.290.13.1729 [DOI] [PubMed] [Google Scholar]
- Chang Y., Cho B., Kim S., Kim J. (2019). Direct conversion of fibroblasts to osteoblasts as a novel strategy for bone regeneration in elderly individuals. Exp. Mol. Med. 51 (5), 1–8. 10.1038/s12276-019-0251-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Deng C., Li Y. P. (2012). TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 8 (2), 272–288. 10.7150/ijbs.2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Xia C., Shi B., Chen C., Yang C., Mao G., et al. (2021). Extracorporeal shock wave combined with teriparatide-loaded hydrogel injection promotes segmental bone defects healing in osteoporosis. Tissue Eng. Regen. Med. 18 (6), 1021–1033. 10.1007/s13770-021-00381-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston J. E., McClung M. R., Leslie W. D. (2019). Lancet 393 (10169), 364–376. 10.1016/s0140-6736(18)32112-3 [DOI] [PubMed] [Google Scholar]
- Etheridge S. L., Spencer G. J., Heath D. J., Genever P. G. (2004). Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells. Stem Cells 22 (5), 849–860. 10.1634/stemcells.22-5-849 [DOI] [PubMed] [Google Scholar]
- Ettinger B., Black D. M., Mitlak B. H., Knickerbocker R. K., Nickelsen T., Genant H. K., et al. (1999). Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: Results from a 3-year randomized clinical trial. Multiple outcomes of raloxifene evaluation (MORE) investigators. Jama 282 (7), 637–645. 10.1001/jama.282.7.637 [DOI] [PubMed] [Google Scholar]
- Fan L., Guan P., Xiao C., Wen H., Wang Q., Liu C., et al. (2021). Exosome-functionalized polyetheretherketone-based implant with immunomodulatory property for enhancing osseointegration. Bioact. Mat. 6 (9), 2754–2766. 10.1016/j.bioactmat.2021.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J. E., Rogers M. J., Halasy J. M., Luckman S. P., Hughes D. E., Masarachia P. J., et al. (1999). Alendronate mechanism of action: Geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro . Proc. Natl. Acad. Sci. U. S. A. 96 (1), 133–138. 10.1073/pnas.96.1.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M., Juneja V. K. (2010). Review of antimicrobial and antioxidative activities of chitosans in food. J. Food Prot. 73 (9), 1737–1761. 10.4315/0362-028x-73.9.1737 [DOI] [PubMed] [Google Scholar]
- Fu X., Tan J., Sun C. G., Leng H. J., Xu Y. S., Song C. L. (2016). Intraosseous injection of simvastatin in poloxamer 407 hydrogel improves pedicle-screw fixation in ovariectomized minipigs. J. Bone Jt. Surg. 98 (22), 1924–1932. 10.2106/jbjs.15.00937 [DOI] [PubMed] [Google Scholar]
- Gačanin J., Kovtun A., Fischer S., Schwager V., Quambusch J., Kuan S. L., et al. (2017). Spatiotemporally controlled release of rho-inhibiting C3 toxin from a protein-DNA hybrid hydrogel for targeted inhibition of osteoclast formation and activity. Adv. Healthc. Mat. 6 (21), 1700392. 10.1002/adhm.201700392 [DOI] [PubMed] [Google Scholar]
- García-García P., Reyes R., Pérez-Herrero E., Arnau M. R., Évora C., Delgado A. (2020). Alginate-hydrogel versus alginate-solid system Efficacy in bone regeneration in osteoporosis. Mater. Sci. Eng. C 115, 111009. 10.1016/j.msec.2020.111009 [DOI] [PubMed] [Google Scholar]
- García-García P., Reyes R., Segredo-Morales E., Pérez-Herrero E., Delgado A., Évora C. (2019). PLGA-BMP-2 and PLA-17β-estradiol microspheres reinforcing a composite hydrogel for bone regeneration in osteoporosis. Pharmaceutics 11 (12), 648. 10.3390/pharmaceutics11120648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgess D., Machuca-Gayet I., Blangy A., Jurdic P. (2014). Podosome organization drives osteoclast-mediated bone resorption. Cell adh. Migr. 8 (3), 192–204. 10.4161/cam.27840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavimi S. A. A., Lungren E. S., Faulkner T. J., Josselet M. A., Wu Y., Sun Y., et al. (2019). Inductive co-crosslinking of cellulose nanocrystal/chitosan hydrogels for the treatment of vertebral compression fractures. Int. J. Biol. Macromol. 130, 88–98. 10.1016/j.ijbiomac.2019.02.086 [DOI] [PubMed] [Google Scholar]
- Gilarska A., Hinz A., Bzowska M., Dyduch G., Kamiński K., Nowakowska M., et al. (2021). Addressing the osteoporosis problem-multifunctional injectable hybrid materials for controlling local bone tissue remodeling. ACS Appl. Mat. Interfaces 13 (42), 49762–49779. 10.1021/acsami.1c17472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y., Zhang B., Yan L. (2022). A preliminary review of modified polymethyl methacrylate and calcium-based bone cement for improving properties in osteoporotic vertebral compression fractures. Front. Mat. 9, 912713. 10.3389/fmats.2022.912713 [DOI] [Google Scholar]
- Gori F., Thomas T., Hicok K. C., Spelsberg T. C., Riggs B. L. (1999). Differentiation of human marrow stromal precursor cells: Bone morphogenetic protein-2 increases OSF2/CBFA1, enhances osteoblast commitment, and inhibits late adipocyte maturation. J. Bone Min. Res. 14 (9), 1522–1535. 10.1359/jbmr.1999.14.9.1522 [DOI] [PubMed] [Google Scholar]
- Hattersley G., Dean T., Corbin B. A., Bahar H., Gardella T. J. (2016). Binding selectivity of abaloparatide for PTH-type-1-receptor conformations and effects on downstream signaling. Endocrinology 157 (1), 141–149. 10.1210/en.2015-1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattner R., Epker B. N., Frost H. M. (1965). Suggested sequential mode of control of changes in cell behaviour in adult bone remodelling. Nature 206 (983), 489–490. 10.1038/206489a0 [DOI] [PubMed] [Google Scholar]
- Hosny K. M., Rizg W. Y. (2018). Quality by design approach to optimize the formulation variables influencing the characteristics of biodegradable intramuscular in-situ gel loaded with alendronate sodium for osteoporosis. PLoS One 13 (6), e0197540. 10.1371/journal.pone.0197540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Yin C., Zhao F., Ali A., Ma J., Qian A. (2018). Mesenchymal stem cells: Cell fate decision to osteoblast or adipocyte and application in osteoporosis treatment. Int. J. Mol. Sci. 19 (2), 360. 10.3390/ijms19020360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Otsuka M. (2013). Application of calcium phosphate as a controlled-release device. Biol. Pharm. Bull. 36 (11), 1676–1682. 10.1248/bpb.b13-00383 [DOI] [PubMed] [Google Scholar]
- Ito T., Saito M., Uchino T., Senna M., Iafisco M., Prat M., et al. (2012). Preparation of injectable auto-forming alginate gel containing simvastatin with amorphous calcium phosphate as a controlled release medium and their therapeutic effect in osteoporosis model rat. J. Mat. Sci. Mat. Med. 23 (5), 1291–1297. 10.1007/s10856-012-4597-3 [DOI] [PubMed] [Google Scholar]
- Jiang M., Wang X., Liu H., Zhou L., Jiang T., Zhou H., et al. (2014). bone formation in adipose-derived stem cells isolated from elderly patients with osteoporosis: A preliminary study. Cell Biol. Int. 38 (1), 97–105. 10.1002/cbin.10182 [DOI] [PubMed] [Google Scholar]
- Jiang W., Hou F., Gu Y., Saiding Q., Bao P., Tang J., et al. (2022). Local bone metabolism balance regulation via double-adhesive hydrogel for fixing orthopedic implants. Bioact. Mat. 12, 169–184. 10.1016/j.bioactmat.2021.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S. S., He D. Q., Luo D., Wang Y., Yu M., Guan B., et al. (2019). A biomimetic hierarchical nanointerface orchestrates macrophage polarization and mesenchymal stem cell recruitment to promote endogenous bone regeneration. ACS Nano 13 (6), 6581–6595. 10.1021/acsnano.9b00489 [DOI] [PubMed] [Google Scholar]
- Kado D. M., Duong T., Stone K. L., Ensrud K. E., Nevitt M. C., Greendale G. A., et al. (2003). Incident vertebral fractures and mortality in older women: A prospective study. Osteoporos. Int. 14 (7), 589–594. 10.1007/s00198-003-1412-5 [DOI] [PubMed] [Google Scholar]
- Kang Q., Song W. X., Luo Q., Tang N., Luo J., Luo X., et al. (2009). A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 18 (4), 545–558. 10.1089/scd.2008.0130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S., Bennett C. N., Gerin I., Rapp L. A., Hankenson K. D., Macdougald O. A. (2007). Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/Enhancer-binding protein α and peroxisome proliferator-activated receptor γ. J. Biol. Chem. 282 (19), 14515–14524. 10.1074/jbc.M700030200 [DOI] [PubMed] [Google Scholar]
- Kanis J. A. (1994). Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. Osteoporos. Int. 4 (6), 368–381. 10.1007/BF01622200 [DOI] [PubMed] [Google Scholar]
- Kettenberger U., Luginbuehl V., Procter P., Pioletti D. P. (2017). In vitro and in vivo investigation of bisphosphonate-loaded hydroxyapatite particles for peri-implant bone augmentation. J. Tissue Eng. Regen. Med. 11 (7), 1974–1985. 10.1002/term.2094 [DOI] [PubMed] [Google Scholar]
- Khan A. A., Morrison A., Hanley D. A., Felsenberg D., McCauley L. K., O'Ryan F., et al. (2015). Diagnosis and management of osteonecrosis of the jaw: A systematic review and international consensus. J. Bone Min. Res. 30 (1), 3–23. 10.1002/jbmr.2405 [DOI] [PubMed] [Google Scholar]
- Khosla S., Bilezikian J. P., Dempster D. W., Lewiecki E. M., Miller P. D., Neer R. M., et al. (2012). Benefits and risks of bisphosphonate therapy for osteoporosis. J. Clin. Endocrinol. Metab. 97 (7), 2272–2282. 10.1210/jc.2012-1027 [DOI] [PubMed] [Google Scholar]
- Kim B. S., Shkembi F., Lee J. (2017). In vitro and in vivo evaluation of commercially available fibrin gel as a carrier of alendronate for bone tissue engineering. Biomed. Res. Int. 2017, 1–10. 10.1155/2017/6434169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G., Park Y. S., Lee Y., Jin Y. M., Choi D. H., Ryu K. H., et al. (2018). Tonsil-derived mesenchymal stem cell-embedded in situ crosslinkable gelatin hydrogel therapy recovers postmenopausal osteoporosis through bone regeneration. PLoS One 13 (7), e0200111. 10.1371/journal.pone.0200111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W., Kim M., Jho E. H. (2013). Wnt/β-catenin signalling: From plasma membrane to nucleus. Biochem. J. 450 (1), 9–21. 10.1042/bj20121284 [DOI] [PubMed] [Google Scholar]
- Lee J. H., Baek H. R., Lee K. M., Zheng G. B., Shin S. J., Shim H. J. (2015). Effects of ovariectomy and corticosteroid-induced osteoporosis on the osteoinductivity of rhBMP-2 in a segmental long-bone defect model. Tissue Eng. Part A 21 (15-16), 2262–2271. 10.1089/ten.TEA.2014.0659 [DOI] [PubMed] [Google Scholar]
- Lee K., Seo I., Choi M. H., Jeong D. (2018). Roles of mitogen-activated protein kinases in osteoclast biology. Int. J. Mol. Sci. 19 (10), 3004. 10.3390/ijms19103004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. J., Cheng P., Liang M. K., Chen Y. S., Lu Q., Wang J. Y., et al. (2015). MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J. Clin. Invest. 125 (4), 1509–1522. 10.1172/jci77716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Zhou J., Zhang M., Ma Y., Yang Y., Han X., et al. (2020). Long-term delivery of alendronate through an injectable tetra-PEG hydrogel to promote osteoporosis therapy. Biomater. Sci. 8 (11), 3138–3146. 10.1039/d0bm00376j [DOI] [PubMed] [Google Scholar]
- Liang M., Liu W., Peng Z., Lv S., Guan Y., An G., et al. (2019). The therapeutic effect of secretome from human umbilical cord-derived mesenchymal stem cells in age-related osteoporosis. Artif. Cells Nanomed. Biotechnol. 47 (1), 1357–1366. 10.1080/21691401.2019.1596945 [DOI] [PubMed] [Google Scholar]
- Liu W., Li J., Cheng M., Wang Q., Yeung K. W. K., Chu P. K., et al. (2018). Zinc-modified sulfonated polyetheretherketone surface with immunomodulatory function for guiding cell fate and bone regeneration. Adv. Sci. (Weinh). 5 (10), 1800749. 10.1002/advs.201800749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Chen X., Li S., Guo Q., Xie J., Yu L., et al. (2017). Calcitonin-loaded thermosensitive hydrogel for long-term antiosteopenia therapy. ACS Appl. Mat. Interfaces 9 (28), 23428–23440. 10.1021/acsami.7b05740 [DOI] [PubMed] [Google Scholar]
- Ma X., He Z., Han F., Zhong Z., Chen L., Li B. (2016). Preparation of collagen/hydroxyapatite/alendronate hybrid hydrogels as potential scaffolds for bone regeneration. Colloids Surfaces B Biointerfaces 143, 81–87. 10.1016/j.colsurfb.2016.03.025 [DOI] [PubMed] [Google Scholar]
- Macías I., Alcorta-Sevillano N., Infante A., Rodríguez C. I. (2021). Cutting edge endogenous promoting and exogenous driven strategies for bone regeneration. Int. J. Mol. Sci. 22 (14), 7724. 10.3390/ijms22147724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T., Endo I. (2021). RANKL as a target for the treatment of osteoporosis. J. Bone Min. Metab. 39 (1), 91–105. 10.1007/s00774-020-01153-7 [DOI] [PubMed] [Google Scholar]
- McClung M., Harris S. T., Miller P. D., Bauer D. C., Davison K. S., Dian L., et al. (2013). Bisphosphonate therapy for osteoporosis: Benefits, risks, and drug holiday. Am. J. Med. 126 (1), 13–20. 10.1016/j.amjmed.2012.06.023 [DOI] [PubMed] [Google Scholar]
- Miller P. D., Hattersley G., Riis B. J., Williams G. C., Lau E., Russo L. A., et al. (2016). Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: A randomized clinical trial. Jama 316 (7), 722–733. 10.1001/jama.2016.11136 [DOI] [PubMed] [Google Scholar]
- Moerman E. J., Teng K., Lipschitz D. A., Lecka-Czernik B. (2004). Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-γ2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell 3 (6), 379–389. 10.1111/j.1474-9728.2004.00127.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafee N., Zewail M., Boraie N. (2018). Alendronate-loaded, biodegradable smart hydrogel: A promising injectable depot formulation for osteoporosis. J. Drug Target. 26 (7), 563–575. 10.1080/1061186x.2017.1390670 [DOI] [PubMed] [Google Scholar]
- Nagy V., Penninger J. M. (2015). The RANKL-RANK story. Gerontology 61 (6), 534–542. 10.1159/000371845 [DOI] [PubMed] [Google Scholar]
- Neer R. M., Arnaud C. D., Zanchetta J. R., Prince R., Gaich G. A., Reginster J. Y., et al. (2001). Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. Overseas. Ed. 344 (19), 1434–1441. 10.1056/nejm200105103441904 [DOI] [PubMed] [Google Scholar]
- Neuerburg C., Mittlmeier L. M., Keppler A. M., Westphal I., Glass Ä., Saller M. M., et al. (2019). Growth factor-mediated augmentation of long bones: Evaluation of a BMP-7 loaded thermoresponsive hydrogel in a murine femoral intramedullary injection model. J. Orthop. Surg. Res. 14 (1), 297. 10.1186/s13018-019-1315-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning Z., Tan B., Chen B., Lau D. S. A., Wong T. M., Sun T., et al. (2019). Precisely controlled delivery of abaloparatide through injectable hydrogel to promote bone regeneration. Macromol. Biosci. 19 (6), e1900020. 10.1002/mabi.201900020 [DOI] [PubMed] [Google Scholar]
- Norouzi M., Nazari B., Miller D. W. (2016). Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov. Today 21 (11), 1835–1849. 10.1016/j.drudis.2016.07.006 [DOI] [PubMed] [Google Scholar]
- Ossipov D. A., Lüchow M., Malkoch M. (2021). Differentiating Co-delivery of bisphosphonate and simvastatin by self-healing hyaluronan hydrogel formed by orthogonal "clicks": An iIn-vVitro assessment. Polym. (Basel) 13 (13), 2106. 10.3390/polym13132106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelled G., Sheyn D., Tawackoli W., Jun D. S., Koh Y., Su S., et al. (2016). BMP6-Engineered MSCs induce vertebral bone repair in a pig model: A pilot study. Stem Cells Int. 2016, 1–8. 10.1155/2016/6530624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S., Lai Z. T., Hong D. W., Chu I. M., Lai P. L. (2017). Controlled release of strontium through neutralization reaction within a methoxy(polyethylene glycol)-polyesterc hydrogel. J. Appl. Biomater. Funct. Mat. 15 (2), e162–e169. 10.5301/jabfm.5000313 [DOI] [PubMed] [Google Scholar]
- Posadowska U., Parizek M., Filova E., Wlodarczyk-Biegun M., Kamperman M., Bacakova L., et al. (2015). Injectable nanoparticle-loaded hydrogel system for local delivery of sodium alendronate. Int. J. Pharm. X. 485 (1-2), 31–40. 10.1016/j.ijpharm.2015.03.003 [DOI] [PubMed] [Google Scholar]
- Qiu Y., Ma Y., Huang Y., Li S., Xu H., Su E. (2021). Current advances in the biosynthesis of hyaluronic acid with variable molecular weights. Carbohydr. Polym. 269, 118320. 10.1016/j.carbpol.2021.118320 [DOI] [PubMed] [Google Scholar]
- Rachner T. D., Khosla S., Hofbauer L. C. (2011). Osteoporosis: Now and the future. Lancet 377 (9773), 1276–1287. 10.1016/s0140-6736(10)62349-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinker T. E., Hammoudi T. M., Kemp M. L., Lu H., Temenoff J. S. (2014). Interactions between mesenchymal stem cells, adipocytes, and osteoblasts in a 3D tri-culture model of hyperglycemic conditions in the bone marrow microenvironment. Integr. Biol. 6 (3), 324–337. 10.1039/c3ib40194d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyasi S., Kumar A., Goswami C., Bandyopadhyay A., Goswami L. (2014). A carboxy methyl tamarind polysaccharide matrix for adhesion and growth of osteoclast-precursor cells. Carbohydr. Polym. 101, 1033–1042. 10.1016/j.carbpol.2013.10.047 [DOI] [PubMed] [Google Scholar]
- Schousboe J. T. (2016). Epidemiology of vertebral fractures. J. Clin. Densitom. 19 (1), 8–22. 10.1016/j.jocd.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Segredo-Morales E., García-García P., Reyes R., Pérez-Herrero E., Delgado A., Évora C. (2018). Bone regeneration in osteoporosis by delivery BMP-2 and PRGF from tetronic-alginate composite thermogel. Int. J. Pharm. X. 543 (1-2), 160–168. 10.1016/j.ijpharm.2018.03.034 [DOI] [PubMed] [Google Scholar]
- Shariatinia Z. (2019). Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 263, 131–194. 10.1016/j.cis.2018.11.008 [DOI] [PubMed] [Google Scholar]
- Shen L., Glowacki J., Zhou S. (2011). Inhibition of adipocytogenesis by canonical WNT signaling in human mesenchymal stem cells. Exp. Cell Res. 317 (13), 1796–1803. 10.1016/j.yexcr.2011.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Zhang J., Zhao M., Tang S., Cheng X., Zhang W., et al. (2021). Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale 13 (24), 10748–10764. 10.1039/d1nr02065j [DOI] [PubMed] [Google Scholar]
- Si L., Winzenberg T. M., Jiang Q., Chen M., Palmer A. J. (2015). Projection of osteoporosis-related fractures and costs in China: 2010-2050. Osteoporos. Int. 26 (7), 1929–1937. 10.1007/s00198-015-3093-2 [DOI] [PubMed] [Google Scholar]
- Solana Muñoz J., Kettenberger U., Procter P., Pioletti D. P. (2018). Non-setting, injectable biomaterials containing particulate hydroxyapatite can increase primary stability of bone screws in cancellous bone. Clin. Biomech. (Bristol, Avon. 59, 174–180. 10.1016/j.clinbiomech.2018.09.023 [DOI] [PubMed] [Google Scholar]
- Tan J., Fu X., Sun C. G., Liu C., Zhang X. H., Cui Y. Y., et al. (2016). A single CT-guided percutaneous intraosseous injection of thermosensitive simvastatin/poloxamer 407 hydrogel enhances vertebral bone formation in ovariectomized minipigs. Osteoporos. Int. 27 (2), 757–767. 10.1007/s00198-015-3230-y [DOI] [PubMed] [Google Scholar]
- Tang Q. Q., Otto T. C., Lane M. D. (2004). Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. U. S. A. 101 (26), 9607–9611. 10.1073/pnas.0403100101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum S. L. (2000). Bone resorption by osteoclasts. Science 289 (5484), 1504–1508. 10.1126/science.289.5484.1504 [DOI] [PubMed] [Google Scholar]
- Utech S., Boccaccini A. R. (2016). A review of hydrogel-based composites for biomedical applications: Enhancement of hydrogel properties by addition of rigid inorganic fillers. J. Mat. Sci. 51 (1), 271–310. 10.1007/s10853-015-9382-5 [DOI] [Google Scholar]
- van Houdt C. I. A., Koolen M. K. E., Lopez-Perez P. M., Ulrich D. J. O., Jansen J. A., Leeuwenburgh S. C. G., et al. (2021). Regenerating critical size rat segmental bone defects with a self-healing hybrid nanocomposite hydrogel: Effect of bone condition and BMP-2 incorporation. Macromol. Biosci. 21 (8), e2100088. 10.1002/mabi.202100088 [DOI] [PubMed] [Google Scholar]
- Veis A., Cohen J. (1960). Reversible transformation of gelatin to the collagen structure. Nature 186, 720–721. 10.1038/186720a0 [DOI] [PubMed] [Google Scholar]
- Vitale M., Ligorio C., McAvan B., Hodson N. W., Allan C., Richardson S. M., et al. (2022). Hydroxyapatite-decorated Fmoc-hydrogel as a bone-mimicking substrate for osteoclast differentiation and culture. Acta Biomater. 138, 144–154. 10.1016/j.actbio.2021.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B. X., Li K. P., Yu T., Feng H. Y. (2019). Rosuvastatin promotes osteogenic differentiation of mesenchymal stem cells in the rat model of osteoporosis by the Wnt/β-catenin signal. Eur. Rev. Med. Pharmacol. Sci. 23 (22), 10161–10168. 10.26355/eurrev_201911_19586 [DOI] [PubMed] [Google Scholar]
- Wang E. A., Israel D. I., Kelly S., Luxenberg D. P. (1993). Bone morphogenetic protein-2 causes commitment and differentiation in C3Hl0T1/2 and 3T3 cells. Growth factors. 9 (1), 57–71. 10.3109/08977199308991582 [DOI] [PubMed] [Google Scholar]
- Wang L., Yu W., Yin X., Cui L., Tang S., Jiang N., et al. (2021a). Prevalence of osteoporosis and fracture in China: The China osteoporosis prevalence study. JAMA Netw. Open 4 (8), e2121106. 10.1001/jamanetworkopen.2021.21106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Li Z., Wang Z., Liu H., Cui Y., Liu Y., et al. (2021b). Incorporation of bone morphogenetic protein-2 and osteoprotegerin in 3D-printed Ti6Al4V scaffolds enhances osseointegration under osteoporotic conditions. Front. Bioeng. Biotechnol. 9, 754205. 10.3389/fbioe.2021.754205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X., Zhang P., Xue S., Xu Y., Tan J., Liu G. (2014). Adipose-derived stem cells alleviate osteoporosis by enchancing osteogenesis and inhibiting adipogenesis in a rabbit model. Cytotherapy 16 (12), 1643–1655. 10.1016/j.jcyt.2014.07.009 [DOI] [PubMed] [Google Scholar]
- Yilmaz H. D., Cengiz U., Arslan Y. E., Kiran F., Ceylan A. (2021). From a plant secretion to the promising bone grafts: Cryogels of silicon-integrated quince seed mucilage by microwave-assisted sol-gel reaction. J. Biosci. Bioeng. 131 (4), 420–433. 10.1016/j.jbiosc.2020.11.008 [DOI] [PubMed] [Google Scholar]
- Yoon H. J., Lee Y. J., Baek S., Chung Y. S., Kim D. H., Lee J. H., et al. (2021). Hormone autocrination by vascularized hydrogel delivery of ovary spheroids to rescue ovarian dysfunctions. Sci. Adv. 7 (18), eabe8873. 10.1126/sciadv.abe8873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Lian R., Liu L., Liu T., Bi C., Hong K., et al. (2022). Biomimetic hydroxyapatite nanorods promote bone regeneration via accelerating osteogenesis of BMSCs through T cell-derived IL-22. ACS Nano 16, 755–770. 10.1021/acsnano.1c08281 [DOI] [PubMed] [Google Scholar]
- Yu P., Liu Y., Jin R., Zhang P., Ding C., Jiang X., et al. (2020a). Thermosensitive polysaccharide hydrogel as a versatile platform for prolonged salmon calcitonin release and calcium regulation. ACS Biomater. Sci. Eng. 6 (7), 4077–4086. 10.1021/acsbiomaterials.0c00591 [DOI] [PubMed] [Google Scholar]
- Yu P., Xie J., Chen Y., Liu J., Liu Y., Bi B., et al. (2020b). A thermo-sensitive injectable hydroxypropyl chitin hydrogel for sustained salmon calcitonin release with enhanced osteogenesis and hypocalcemic effects. J. Mat. Chem. B 8 (2), 270–281. 10.1039/c9tb02049g [DOI] [PubMed] [Google Scholar]
- Yuan Z., Li Q., Luo S., Liu Z., Luo D., Zhang B., et al. (2016). PPARγ and wnt signaling in adipogenic and osteogenic differentiation of mesenchymal stem cells. Curr. Stem Cell Res. Ther. 11 (3), 216–225. 10.2174/1574888x10666150519093429 [DOI] [PubMed] [Google Scholar]
- Zhao Q. X., Wang X., Liu Y. H., He A. M., Jia R. K. (2010). NFATc1: Functions in osteoclasts. Int. J. Biochem. Cell Biol. 42 (5), 576–579. 10.1016/j.biocel.2009.12.018 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Li Z., Jiang Y., Liu H., Feng Y., Wang Z., et al. (2020). Bioinspired mineral hydrogels as nanocomposite scaffolds for the promotion of osteogenic marker expression and the induction of bone regeneration in osteoporosis. Acta Biomater. 113, 614–626. 10.1016/j.actbio.2020.06.024 [DOI] [PubMed] [Google Scholar]
- Zheng Z., Yu C., Wei H. (2021). Injectable hydrogels as three-dimensional network reservoirs for osteoporosis treatment. Tissue Eng. Part B Rev. 27 (5), 430–454. 10.1089/ten.TEB.2020.0168 [DOI] [PubMed] [Google Scholar]
- Zhu J., Zhang C., Jia J., Wang H., Leng H., Xu Y., et al. (2021). Osteogenic effects in a rat osteoporosis model and femur defect model by simvastatin microcrystals. Ann. N. Y. Acad. Sci. 1487 (1), 31–42. 10.1111/nyas.14513 [DOI] [PubMed] [Google Scholar]