Summary
Background
The diagnosis of heart failure with preserved ejection fraction (HFpEF) remains challenging. Exercise-stress testing is recommended in case of uncertainty; however, this approach is time-consuming and costly. Since preserved EF does not represent normal systolic function, we hypothesized comprehensive cardiovascular magnetic resonance (CMR) assessment of cardiac hemodynamic forces (HDF) may identify functional abnormalities in HFpEF.
Methods
The HFpEF Stress Trial (DZHK-17; Clinicaltrials.gov: NCT03260621) prospectively recruited 75 patients with exertional dyspnea, preserved EF (≥50%) and signs of diastolic dysfunction (E/e’ ≥8) on echocardiography. Patients underwent rest and exercise-stress right heart catheterisation, echocardiography and CMR. The final study cohort consisted of 68 patients (HFpEF n = 34 and non-cardiac dyspnea n = 34 according to pulmonary capillary wedge pressure (PCWP)). HDF assessment included left ventricular (LV) longitudinal, systolic peak and impulse, systolic/diastolic transition, E-wave deceleration as well as A-wave acceleration forces. Follow-up after 24 months evaluated cardiovascular mortality and hospitalisation (CVH) – only two patients were lost to follow-up.
Findings
HDF assessment revealed impairment of LV longitudinal function in patients with HFpEF compared to non-cardiac dyspnoea (15.8% vs. 18.3%, p = 0.035), attributable to impairment of systolic peak (38.6% vs 51.6%, p = 0.003) and impulse (20.8% vs. 24.5%, p = 0.009) forces as well as late diastolic filling (−3.8% vs −5.4%, p = 0.029). Early diastolic filling was impaired in HFpEF patients identified at rest compared with patients identified during stress only (7.7% vs. 9.9%, p = 0.004). Impaired systolic peak was associated with CVH (HR 0.95, p = 0.016), and was superior to LV global longitudinal strain assessment in prediction of CVH (AUC 0.76 vs. 0.61, p = 0.048).
Interpretation
Assessment of HDF indicates impairment of LV systolic ejection force in HFpEF which is associated with cardiovascular events.
Funding
German Centre for Cardiovascular Research (DZHK).
Keywords: HFpEF, Cardiovascular magnetic resonance, Hemodynamic force, Deformation imaging, Strain
Abbreviations: AUC, area under the curve; bSSFP, balanced steady state free precession; CI, confidence intervals; CMR, cardiovascular magnetic resonance; CV, chamber view; CVH, cardiovascular hospitalisation; GLS/GCS/GRS, global longitudinal/circumferential/radial strain; EDV/ESV, End diastolic/systolic volume; Ees, End-systolic elastance; EF, Ejection fraction; FT, Feature tracking; HDF, hemodynamic force; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; IQR, interquartile ranges; IVPG, intraventricular pressure gradient; LV, Left ventricular; LAS, Long axis strain; LAVI, Left atrial volume index; LAX, Long axis; NCD, Non cardiac dyspnoea; NTproBNP, N-terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association; PCWP, Pulmonary capillary wedge pressure; PVL, Pressure volume loops; RHC, right heart catheterisation; RMS, Root mean square; RV, Right ventricular; SAX, Short axis; SV, Stroke volume
Research in context.
Evidence before this study
Heart failure with preserved ejection fraction (HFpEF) is associated with both systolic and diastolic functional impairment. During early stages of disease, functional alterations are unmasked using exercise-stress testing, the reference standard of which is exercise right heart catheterisation (RHC). Previously, exercise-stress cardiovascular magnetic resonance (CMR) imaging has shown high diagnostic accuracy compared to RHC. CMR-derived hemodynamic force assessment (HDF) aims to quantify functional changes at rest. However, to date, data on HDF remains inconclusive with 4D flow assessment reporting no impairment in HFpEF as opposed to assessment based on cine sequences showing impaired systolic function.
Added value of this study
The present study reports left ventricular (LV) functional quantification (hemodynamic forces) based on LV geometry, endocardial tissue movement as well as aortic and mitral valve orifice areas to detect functional alterations in HFpEF at rest. This approach may represent an alternative non-invasive test on routinely acquired cine sequences at rest, compared to an exercise-stress CMR protocol limited to highly specialised centres.
Implications of all the available evidence
Measurement of hemodynamic forces enables earlier detection of subtle LV functional alterations compared to volumetric or conventional deformation assessment in HFpEF patients.
Introduction
In an aging western population, heart failure with preserved ejection fraction (HFpEF) can be observed in approximately 5% of the population aged ≥60 years and has become more prevalent than heart failure with reduced ejection fraction (HFrEF).1 Due to the diverse pathophysiology and late onset of symptoms it remains challenging to reliably diagnose HFpEF at an early stage,2 subsequently delaying therapeutic intervention and efforts to prevent cardiac remodelling.3, 4, 5 With the emergence of new treatments to improve both cardiac remodelling and outcome, early diagnosis is becoming more important.6,7 To date, exercise-stress testing via echocardiography or invasive right heart catheterisation (RHC) is recommended in case of uncertainty.8 However, echocardiographic assessments during exercise-stress remain prone to reduced image quality,9 whilst RHC carries the risk associated with an invasive procedure. The HFpEF Stress trial demonstrated feasibility of novel real-time cardiovascular magnetic resonance (CMR) imaging to conduct exercise-stress testing in CMR imaging.10 Despite its high diagnostic accuracy, exercise-stress CMR is only available in highly specialised centres, and as such the observable impact of this novel technique is limited.11
Invasive assessments employing conductance catheters allow the assessment of left ventricular (LV) pressure and volume relationships.12 Using pressure volume loops (PVL), impaired diastolic stiffness at rest has been demonstrated in HFpEF. This causes inability to cope with higher hemodynamic demands during exercise, ultimately resulting in reduced stroke volume and higher end-diastolic pressure during exercise-stress.13 Advances in non-invasive imaging aim for estimation of intraluminal pressure gradients based on 4D flow assessment14, 15, 16, 17, 18 as well as LV geometry, endocardial tissue movement and aortic and mitral valve orifice areas.19,20 Whilst still in the realms of research, improved non-invasive functional assessments at rest would allow a broader availability of diagnostic testing.7 We hypothesized CMR derived hemodynamic force (HDF) assessment may identify cardiac dysfunction in HFpEF as defined by post-capillary pulmonary hypertension in PCWP compared to patients without and may therefore improve diagnostic and prognostic accuracy.
Methods
This is a substudy from the HFpEF-Stress Trial.10,21 The HFpEF Stress trial prospectively recruited 75 patients who presented as in–or outpatients with preserved ejection fraction (EF ≥ 50%), signs of diastolic dysfunction (E/e’ ≥8) and exertional dyspnea (NYHA class ≥ II), between August 2017 and September 2019,10 Fig. 1. Exclusion criteria included common contraindications for CMR (e.g. glomerular filtration rate <30 ml/min),22 and other causes of dyspnoea of both pulmonary (tested on spirometry) or cardiac (cardiomyopathies, significant coronary artery or valvular heart disease) origins.10 All patients underwent rest and exercise-stress assessments by simultaneous RHC and echocardiography, as well as CMR imaging within 24 h.
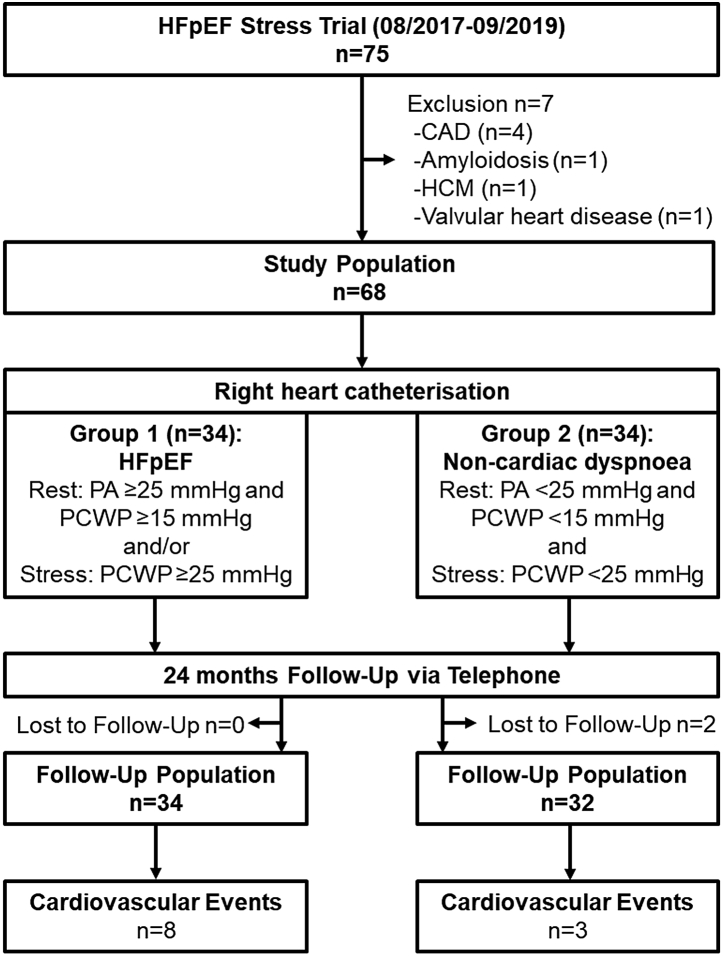
Fig. 1.
Study Flow-Chart. HFpEF, heart failure with preserved ejection fraction; CAD, coronary artery disease, HCM, hypertrophic cardiomyopathy; PA, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure.
Exercise-stress was conducted using supine bicycle ergometry. At an average rotation speed of 50–60 rotations per minute, a 5 Watt increasing ramp protocol was used to stress patients, until an average heart rate of 100 beats/min was achieved before starting data acquisition. If necessary, the workload was adjusted to maintain heart rates between 100 and 110 beats/min. Hemodynamic changes (blood pressure and heart rate) at rest and during exercise-stress are given in Table S1. Data acquisition was performed in stable sinus rhythm only. HFpEF was defined based on elevated pulmonary capillary wedge pressures (PCWP) of ≥15 mmHg at rest or ≥25 mmHg during exercise-stress RHC assessments according to current guideline recommendations.8 Otherwise patients were classified as non-cardiac dyspnoea in the absence of evidence pointing towards cardiovascular disease. A telephone follow-up consultation was conducted 24 months after initial recruitment (Fig. 1).21 Primary clinical endpoints were cardiovascular mortality and admission for congestive heart failure (cardiovascular hospitalisation (CVH)). The study was funded by the German Centre for Cardiovascular Research (DZHK-17).
Ethics
The study was approved by the local ethics committee at the University Medical Center Goettingen (35/8/15). All patients gave written informed consent before participation. The study was conducted according to the principles of the Helsinki Declaration.
Cardiovascular magnetic resonance imaging
Myocardial function was assessed by conventional electrocardiogram-triggered balanced steady state free precession (bSSFP) cine sequences at rest. bSSFP cine sequences included long axis (LAX) 2-, 3- and 4-chamber views (CV) and a short axis (SAX) stack covering the entire heart. Post processing was performed using commercially available software by Medis (QMass® and HDF module, Medical Imaging Systems, Leiden, Netherlands), TomTec (2D CPA MR, Cardiac Performance Analysis, TomTec Imaging Systems, Unterschleissheim, Germany) and OsiriX MD (Pixmeo SARL, CH-1233 Bernex, Switzerland).
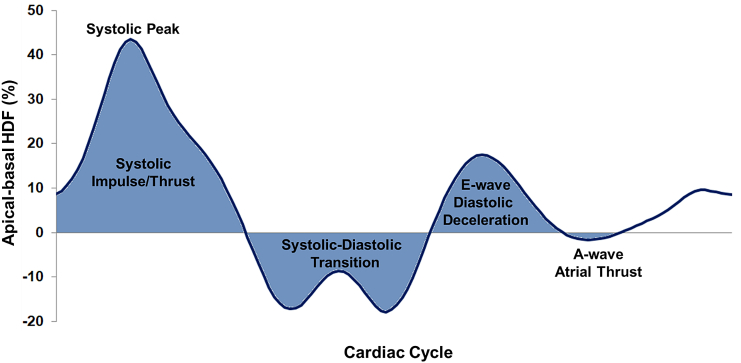
LV HDF was calculated for apical-to-basal (longitudinal direction) movements from deformation imaging of 2-, 3- and 4-CV with assessments of mitral and aortic in/outflow valve width (Fig. 2). HDF represents the integral of pressure gradients over the LV, normalized to LV volume and blood specific weight, thus reported as percentage of gravity acceleration. HDF calculations have previously been described elsewhere.14,19,20,23 The diameter of the aortic valve was assessed in 3 CV orientation, the diameter of the mitral valve was calculated from the average of the 2 and 4 CV diameter. LV contours were manually traced at end-systole (ES) and end-diastole (ED). The tracking algorithm was then applied to propagate the contours though the entire cardiac cycles. Results were visually reviewed; and if required, corrections were made to the initial manual contours only prior to reapplying the tracking algorithm. HDF calculations comprised the following parameters: firstly, overall HDF strength is given as the root mean square (RMS) of the longitudinal hemodynamic force over the entire cardiac cycle, considering both absolute values (regardless of whether these values were positive or negative). Secondly, systolic function was obtained and assessed by peak systolic HDF and the systolic impulse. Thirdly, systolic–diastolic transition is described by LV deceleration and LV suction. Lastly, diastolic function comprised of early diastolic filling/diastolic deceleration, late diastolic filling/atrial thrust and their proportion to one and another (early diastolic filling/late diastolic filling). Systolic peak HDF was assessed as the peak of the HDF curve. Parameters with either a positive or negative value (systolic impulse, transition and early/late diastolic filling) are given as average values and were calculated from the area under the curve (AUC) normalised to the respective time interval. Despite a negative sign for atrial thrust in HDF analyses, the index for diastolic deceleration/atrial thrust considered its absolute value only (this is analogous to echocardiographic assessments).
Fig. 2.
Left ventricular hemodynamic force in apical-basal direction. The graph shows an exemplary hemodynamic force (HDF) curve for the apical-basal motion of the left ventricle.
Volumetric analyses were performed from SAX cine images and comprised of LV end-diastolic/systolic and stroke volumes (EDV/ESV/SV), LV EF and myocardial mass estimation. Deformation imaging was conducted on LAX cine sequences for LV global longitudinal strain (GLS) assessment as well as on SAX cine sequence for global circumferential and radial strain measurements (GCS & GRS respectively).24 In line with HDF analyses, LV borders were manually traced at ES and ED prior to application of the semi-automatic tracking algorithm. Similarly, after visual revision, corrections were made to the initial manual contours only if deemed necessary by the observer. In that case, the tracking algorithm was reapplied.
LV long axis strain (LAS)25 was assessed on 2 and 4 CV real-time rest and exercise-stress cine sequences.26 The distance between the middle of a line connecting the origins of the mitral leaflets and the epicardial apical border was measured in end-diastole and end-systole. The difference was then divided by the end-diastolic length to account for LAS in per cent. Assessments were performed in OsiriX MD (Pixmeo SARL, CH-1233 Bernex, Switzerland).
Statistical analyses
Categorical variables are shown as frequencies with associated percentages and were compared by applying the chi-squared test. Continuous variables are reported with median values and corresponding interquartile ranges (IQR), and were compared applying the Mann–Whitney U test (asymptotic and exact). Spearman's rank coefficients were used to test for correlation after checking for normal distribution using the Shapiro–Wilk test. Predictors for the primary endpoint were identified by univariate Cox regression analyses, reported as hazard ratios (HR) with 95% confidence intervals (CI) as well as Kaplan Meier curves with associated log-rank testing. Two-tailed p-values <0.05 were considered statistically significant. Predictors for the presence of HFpEF were identified by area under the receiver operating characteristic curve (AUC) analyses which are reported with 95% CI. AUCs comparisons were calculated using the method proposed by DeLong et al.27 Reproducibility was assessed in 5 randomly selected HFpEF and NCD patients using intraclass correlation coefficients based on absolute agreement.
A sample size calculation was conducted prior to study enrolment based on 42 HFpEF and 28 NCD patients resulting in a power in excess of 80% to demonstrate that the area under the ROC curve is larger than 0.65 at an one-sided significance level of 2.5% given the true AUC is at least 0.82.28 Analyses were performed using SPSS version 26.0 (IBM, Armonk, New York, USA). There was no correction for multiple testing.
Role of funders
The study received funding from and was carried out using clinical-scientific infrastructure of the DZHK (German Centre for Cardiovascular Research). The DZHK had no role in study design, data collection, analyses or interpretation as well as writing of reports.
Results
Study population
The final study population in the follow-up from the HFpEF Stress Trial consisted of 68 patients (HFpEF n = 34, non-cardiac dyspnoea (NCD) n = 34). Two NCD patients had been lost to follow-up. After 24 months of follow-up, 1 patient had died from a non-cardiac cause not associated with a cardiovascular event and eleven patients (HFpEF n = 8, NCD n = 3, p = 0.123) were hospitalised for cardiovascular reasons. Admissions for elective procedures were not included. Baseline characteristics of the study population are reported in Table 1, parts of these characteristics have been published previously.10 All study participants were white Caucasian. HFpEF patients had both a higher H2FPEF score29 (body mass index >30 kg/m2, ≥2 antihypertensive drugs, atrial fibrillation, pulmonary artery systolic pressure >35 mmHg, >60 years, E/e’ >9; 5 vs 3, p = 0.003) and HFA-PEFF score8 (Heart Failure Association diagnostic algorithm score 5.5 vs 4, p < 0.001). HDF values dichotomised according to the HFA_PEFF Score are given in supplementary Table S2. On RHC, both PCWP at rest and during exercise-stress were statistically significantly increased in HFpEF patients (p < 0.001), whilst the cardiac index was preserved at rest but impaired during exercise-stress (p = 0.022). 56% (n = 19/34) HFpEF patients were diagnosed during stress only. There were no differences on spiroergometric testing comparing HFpEF to NCD (VO2 18.0 IQR 16.1–20.7 vs 20 IQR 17.0–23.7 ml/(kg∗min), p = 0.102) and O2 Pulse (14.7 IQR 12.4–18.6 vs 15.0 IQR 13.4–17.7, p = 0.621).
Table 1.
Patient characteristics.
| Variable | HFpEF n = 34 |
NCD n = 34 |
Significance p |
|---|---|---|---|
| Age (years) | 69 (67, 77) | 66 (52, 73) | 0.034 |
| Sex male/female | 9/25 | 15/19 | 0.128 |
| Ethnicity – white Caucasian | 34 | 34 | |
| NYHA class | 21 × II, 13 × III | 27 × II, 7 × III | 0.110 |
| Atrial fibrillation | 16 | 5 | 0.004 |
| H2FPEF score | 5.0 (3.0, 6.3) | 3.0 (2.0, 5.0) | 0.003 |
| HFA-PEFF score | 5.5 (3.8, 6.0) | 4.0 (2.0, 4.0) | <0.001 |
| Cardiovascular risk factors | |||
| Active smoking | 4 | 5 | 0.720 |
| Hypertension | 27 | 27 | 1.000 |
| Hyperlipoproteinemia | 21 | 21 | 1.000 |
| Diabetes | 5 | 5 | 1.000 |
| Body mass index (kg/m2 BSA) | 28.7 (26.8, 33.2) | 27.6 (25.2, 32.3) | 0.339 |
| Laboratory testing | |||
| NT-proBNP (ng/l) | 255 (102, 606) | 75 (50, 134) | <0.001 |
| Echocardiographya | |||
| E/e' rest | 12.5 (9.7, 13.3) | 9.15 (7.5, 10.7) | <0.001 |
| E/e' stress | 13.8 (10.8, 15.9) | 11.0 (10.0, 14.0) | 0.120 |
| LAVI (ml/m2 BSA) | 43.8 (36.6, 54.2) | 36.2 (29.2, 41.1) | 0.001 |
| TAPSE (mm) | 24 (21.2, 27.2) | 22.5 (20.5, 25.7) | 0.335 |
| PAPsys (mmHg) | 28 (23.5, 33.1) | 22.8 (19.6, 24.7) | 0.001 |
| Right heart catheterisation | |||
| PCWP rest (mmHg) | 13 (11, 18) | 8 (6, 10) | |
| PCWP stress (mmHg) | 27 (26, 31) | 18 (11, 22) | |
| Cardiac Index rest (l/m2 BSA) | 2.9 (2.4, 3.2) | 2.9 (2.6, 3.4) | 0.663 |
| Cardiac Index stress (l/m2 BSA) | 5.2 (3.7, 6.1) | 5.8 (4.7, 6.7) | 0.022 |
NCD, non-cardiac dyspnoea; NYHA, New York Heart Association; LAVI, left atrial volume index; TAPSE, tricuspid annular plane systolic excursion; PAPsys, systolic pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; BSA, body surface area. Categorical parameters are reported in absolutes numbers and were compared using the Chi-squared test whilst continuous parameters are presented as medians with interquartile ranges and were compared by using the Mann–Whitney U test. Bold p-values indicate statistical significance below 0.05.
Numbers differ for echocardiographic assessments shown for HFpEF/NCD (E/e’ stress n = 30/20; TAPSE n = 31/28; PAPsys n = 30/26), table adapted from Ref. 10
CMR derived functional alterations and prognostic implications
CMR derived functional parameters are shown in Table 2, Table 3 (and Tables S3 and S4 based on Mann–Whitney U exact test as well as Table S5 reporting mean differences comparing the subgroups of NCD, HFpEF Stress and HFpEF rest). Systolic function, assessed by LV systolic peak HDF, correlated with PCWP at rest (r = −0.29, p = 0.016) and during exercise-stress (r = −0.39, p = 0.001), as well as left atrial volume index (LAVI) (r = −0.42, p < 0.001), Table S6. Amongst the diastolic parameters, the best correlation to PCWP was diastolic deceleration/atrial thrust, both at rest (r = 0.33, p = 0.007) and stress (r = 0.28, p = 0.024). Atrial thrust alone showed better correlation to both echocardiographic functional (E/e, r = 0.26, p = 0.033) and morphological (LAVI, r = 0.32, p = 0.008) measurements and serum biomarker N-terminal prohormone of brain natriuretic peptide (NTproBNP, r = 0.32, p = 0.008).
Table 2.
CMR derived LV function.
| Variable | HFpEF n = 34 |
NCD n = 34 |
Mean difference (95% CI) | Significance p |
|---|---|---|---|---|
| Cardiovascular magnetic resonance volumetry | ||||
| LV EDV | 68.3 (60.7, 77.3) | 68.5 (57.4, 76.8) | −0.8 (−8.1, 6.5) | 0.741 |
| LV ESV | 19.6 (14.8, 25.9) | 20.4 (14.8, 24.3) | 0.5 (−3.2, 4.1) | 0.917 |
| LV SV | 49.6 (42.1, 54.5) | 46.7 (40.1, 53.0) | −1.3 (−6.2, 3.7) | 0.447 |
| LV EF | 69.0 (66.3, 76.1) | 69.0 (65.0, 75.6) | −0.6 (−4.0, 2.8) | 0.731 |
| LV Mass | 57.0 (51.0, 66.9) | 55.6 (50.4, 72.0) | 1.7 (−4.4, 7.8) | 0.932 |
| Cardiovascular magnetic resonance deformation imaging | ||||
| FT LV GLS | −19.9 (−18.8, −22.5) | −21.0 (−19.0, −23.2) | −0.8 (−2.3, 0.7) | 0.194 |
| FT LV GCS | −35.2 (−30.9, −39.0) | −34.9 (−30.7, −36.9) | 0.0 (−11.0, 10.9) | 0.516 |
| FT LV GRS | 66.2 (57.7, 74.2) | 63.4 (56.5, 70.1) | −5.7 (−14.1, 2.7) | 0.275 |
| LV LAS rest | 13.5 (11.4, 15.4) | 13.9 (12.0, 15.9) | 0.7 (−0.6, 2.1) | 0.542 |
| LV LAS exercise-stress | 14.9 (12.5, 18.0) | 18.9 (15.7, 21.7) | 4.0 (2.1, 6.0) | <0.001 |
| Cardiovascular magnetic resonance hemodynamic force | ||||
| LV longitudinal force (RMS) | 15.8 (13.4, 19.6) | 18.3 (14.8, 27.3) | 5.5 (1.6, 9.4) | 0.035 |
| Systolic peak | 38.6 (29.3, 50.5) | 52.4 (38.5, 67.4) | 18.6 (8.2, 29.0) | 0.002 |
| Systolic impulse | 20.8 (14.4, 27.5) | 25.3 (20.0, 39.1) | 9.0 (3.8, 14.3) | 0.006 |
| LV systolic/diastolic transition | −10.6 (−7.3, −14.6) | −10.9 (−7.3, −14.3) | −0.5 (−3.7, 2.7) | 0.961 |
| Diastolic deceleration | 9.1 (7.1, 11.3) | 7.1 (6.0, 9.0) | −1.0 (−3.3, 1.2) | 0.044 |
| Atrial thrust | −3.8 (−2.1, −6.3) | −5.4 (−3.7, −7.7) | −1.8 (−4.6, 1.0) | 0.029 |
| Diastolic deceleration/atrial thrust | 1.9 (1.2, 3.3) | 1.3 (0.7, 1.9) | 2.3 (−4.0, 8.7) | 0.018 |
NCD, non-cardiac dyspnoea; LV, left ventricular; EDV, end-diastolic volumel; ESV, end-systolic volume; SV, stroke volume; EF, ejection fraction; FT, feature-tracking; GLS/GCS/GRS, global longitudinal/circumferential/radial strain; LAS, long axis strain; RMS, root mean square; HDF, hemodynamic force. Volumes are given in ml/m2 body surface area (BSA), mass in g/m2 BSA, strain and HDF values in %. Independent continuous parameters are presented as medians with interquartile ranges and were compared by using the Mann–Whitney U test (asymptotic). Bold p-values indicate statistical significance. ∗conventional CMR cardiac function parameters adapted fromRef. 10
Table 3.
Hemodynamic force HFpEF.
| Variable | NCD n = 34 |
p-value NCD vs Stress |
HFpEF stress n = 19 |
p-value Stress vs Rest |
HFpEF rest n = 15 |
|---|---|---|---|---|---|
| Cardiovascular magnetic resonance hemodynamic force | |||||
| LV longitudinal force (RMS) | 18.3 (14.8, 27.3) | 0.194 | 16.3 (13.8, 21.1) | 0.271 | 15.7 (12.0, 16.0) |
| Systolic peak | 52.4 (38.5, 67.4) | 0.024 | 41.5 (30.3, 53.2) | 0.430 | 36.8 (25.3, 49.7) |
| Systolic impulse | 25.3 (20.0, 39.1) | 0.038 | 21.6 (15.9, 27.8) | 0.493 | 20.6 (12.8, 26.2) |
| LV Systolic/diastolic transition | −10.9 (−7.3, −14.3) | 0.469 | −11.3 (−9.1, −14.8) | 0.190 | −8.9 (−5.7, −13.3) |
| Diastolic deceleration | 7.1 (6.0, 9.0) | 0.003 | 9.9 (8.8, 11.8) | 0.004 | 7.7 (5.5, 9.1) |
| Atrial thrust | −5.4 (−3.7, −7.7) | 0.512 | −5.1 (−2.3, −9.5) | 0.096 | −3.0 (−2.0, −4.4) |
| Diastolic deceleration/atrial thrust | 1.3 (0.7, 1.9) | 0.292 | 1.6 (0.9, 2.3) | 0.142 | 2.2 (1.5, 4.1) |
HFpEF stress refers to patients identified by the means of right heart catheterisation (RHC) during exercise-stress only (≥25 mmHg pulmonary capillary wedge pressure (PCWP)), whilst HFpEF rest refers to patients which were identified according to RHC thresholds (≥15 mm Hg PCWP) at rest.
NCD, non-cardiac dyspnoea; RMS: root mean square, LV, left ventricular. Independent continuous parameters are presented as medians with interquartile ranges and were compared by using the Mann–Whitney U test (asymptotic). Bold p-values indicate statistical significance. HDF values are given in %.
HFpEF patients showed impaired global LV longitudinal force (15.8 IQR 13.4, 19.6 vs. 18.3 IQR 14.8, 27.3, p = 0.035) compared with NCD patients. The decline in LV function can be attributed to both systolic (peak 38.6 IQR 29.3, 50.5 vs 52.4 IQR 38.5, 67.4, p = 0.002 and impulse 20.8 IQR 14.4, 27.5 vs. 25.3 IQR 20.0, 39.1, p = 0.006) and late diastolic impairment (atrial thrust −3.8 IQR-2.1, −6.3 vs −5.4 IQR -3.7, −7.7, p = 0.029) Table 2, Fig. 3, Fig. 4.
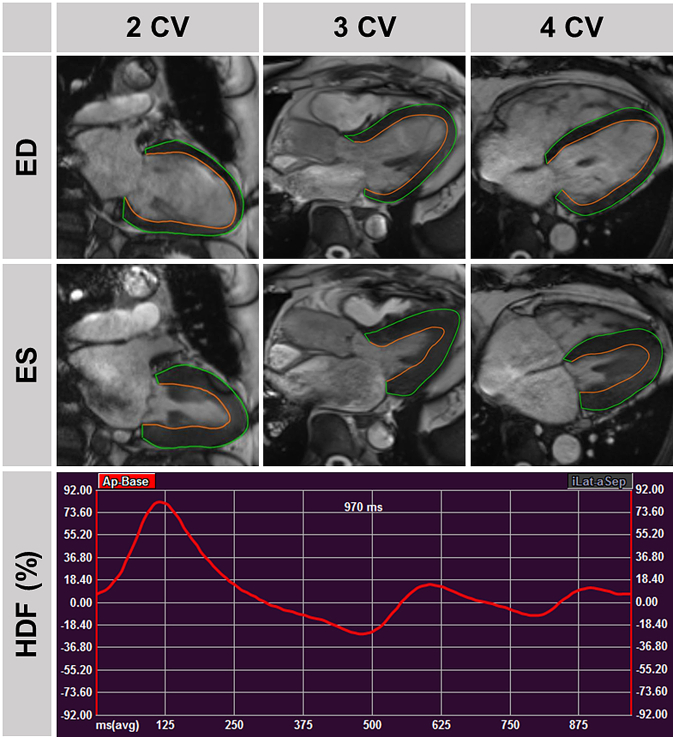
Fig. 3.
Hemodynamic Force in non-cardiac dyspnea. Top: Traced left ventricular contours in 2/3/4 chamber view (CV) orientations in end-diastole and -systole in a patient with non-cardiac dyspnea and no cardiovascular hospitalisation during follow-up. Bottom: Associated hemodynamic force (HDF) curve.
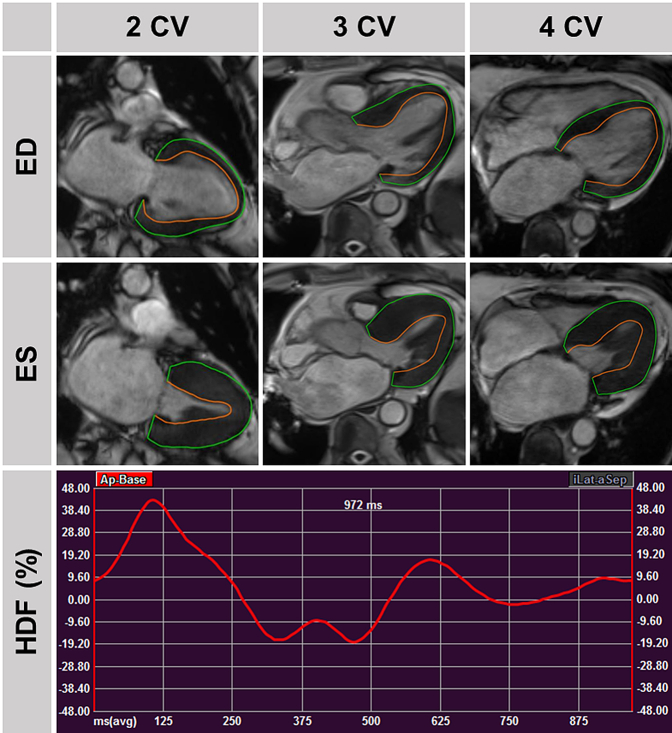
Fig. 4.
Hemodynamic Force in Heart Failure with preserved Ejection Fraction. Top: Traced left ventricular contours in 2/3/4 chamber view (CV) orientations in end-diastole and -systole in a patient with heart failure and preserved ejection fraction as well as cardiovascular hospitalisation during follow-up. Bottom: Associated hemodynamic force (HDF) curve.
HFpEF patients identified by stress thresholds only show impaired systolic function compared with NCD, (peak 41.5 IQR 30.3, 53.2 vs 52.4 IQR 38.5, 67.4, p = 0.024 and impulse. 21.6 IQR 15.9, 27.8 vs 25.3 IQR 20.0, 39.1, p = 0.038) whilst diastolic deceleration in these patients was increased (9.9 IQR 8.8, 11.8 vs 7.1 IQR 6.0, 9.0, p = 0.003). Comparing HFpEF patients identified during stress to HFpEF patients identified at rest reveals no decrease in systolic function, but a decline in diastolic function is seen (early diastolic filling 9.9 IQR 8.8, 11.8 vs 7.7 IQR 5.5, 9.1, p = 0.004) Table 3. As a result, HFpEF patients showed a higher early/late diastolic filling ratio compared with NCD (1.9 IQR 1.2, 3.3 vs. 1.3 IQR 0.7, 1.9, p = 0.018). Overall, the reproducibility of HDF analyses was excellent (ICC ≥0.89) and better for systolic than diastolic parameters (ICC ≥0.95 vs. ≥0.89), Table S7.
In contrast to HDF assessments, conventional LV volumetric indices (p ≥ 0.447) and LV feature tracking (FT)-based deformation imaging (p ≥ 0.194) revealed no statistically significant differences in LV function when comparing HFpEF and NCD patients.
Diagnostic and prognostic accuracy
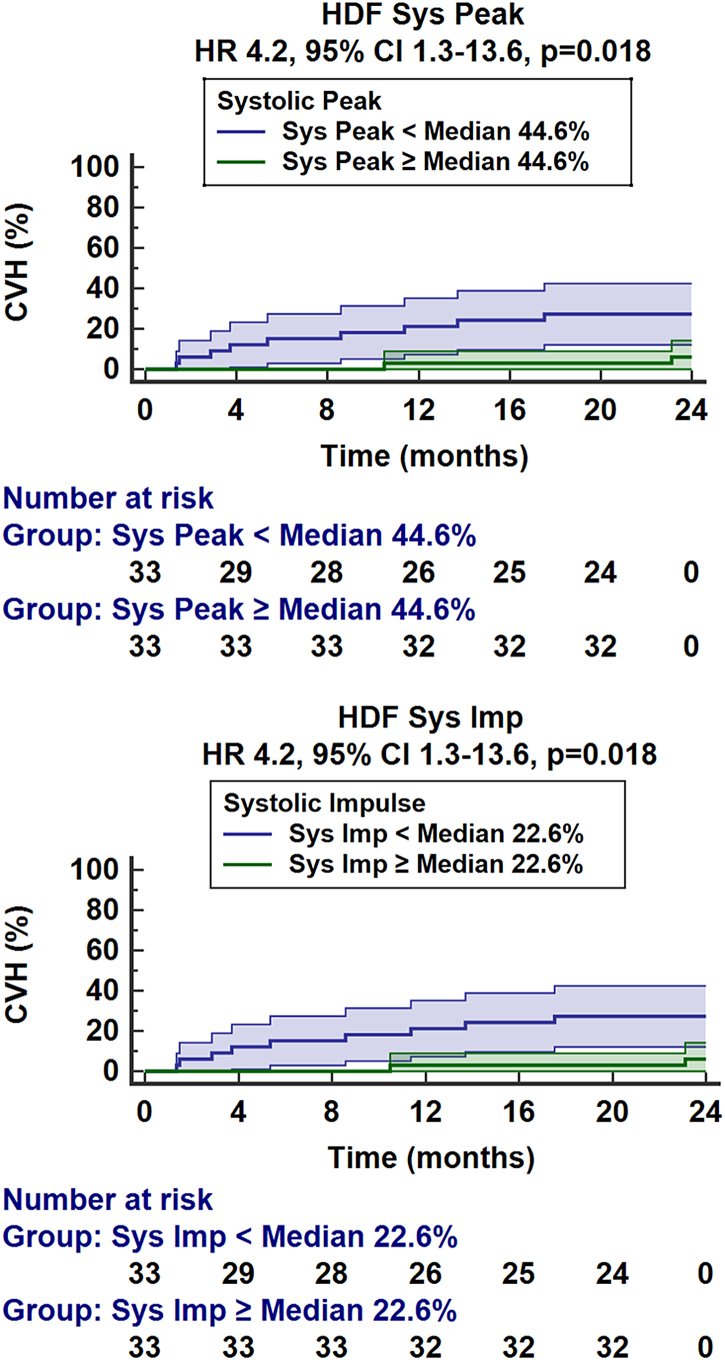
Systolic peak HDF outperformed LV LAS at rest in detecting HFpEF (AUC 0.72 CI 0.60–0.84 vs. 0.55 CI 0.41–0.68, p = 0.022) and achieved a similar value compared to LV LAS at stress (AUC 0.72 CI 0.60–0.84 vs. 0.76 CI 0.65–0.88, p = 0.573), Table S8 and Fig. S1. Systolic peak HDF showed a numerically higher diagnostic accuracy for the detection of HFpEF compared with LV GLS, which did not reach statistical significance (AUC 0.72 CI 0.60–0.84 vs. 0.59 CI 0.46–0.73, (p = 0.118). Furthermore, impairment of systolic peak or impulse was associated with higher rates of CVH (HR 0.95 CI 0.91–0.99 and 0.90 CI 0.82–0.98 respectively, p = 0.016 for both, Table 4). This is also seen on the Kaplan Meier curves (p = 0.018 for both, Fig. 5). Superior prognostic implications are seen for systolic peak HDF over LV GLS (AUC 0.76 CI 0.62–0.90 vs. 0.61 CI 0.44–0.78, p = 0.048). Area under the receiver operating characteristic curve analyses are shown in supplementary Table S3.
Table 4.
Prognostic implication of different LV function parameters.
| Variable | Hazard ratio (95% CI) |
Significance p |
|---|---|---|
| Cardiovascular magnetic resonance volumetry | ||
| LV EDV | 1.01 (0.97–1.05) | 0.775 |
| LV ESV | 1.01 (0.94–1.09) | 0.779 |
| LV SV | 1.01 (0.95–1.07) | 0.827 |
| LV EF | 0.97 (0.89–1.06) | 0.539 |
| Cardiovascular magnetic resonance deformation imaging | ||
| FT LV GLS | 1.10 (0.93–1.31) | 0.259 |
| FT LV GCS | 0.97 (0.92–1.04) | 0.409 |
| FT LV GRS | 1.02 (0.98–1.06) | 0.391 |
| LV LAS rest | 0.83 (0.67–1.02) | 0.081 |
| LV LAS exercise-stress | 0.92 (0.80–1.06) | 0.258 |
| Cardiovascular magnetic resonance hemodynamic force | ||
| LV longitudinal force (RMS) | 0.89 (0.79–1.01) | 0.063 |
| Systolic peak | 0.95 (0.91–0.99) | 0.016 |
| Systolic impulse | 0.90 (0.82–0.98) | 0.016 |
| LV systolic/diastolic transition | 1.01 (0.91–1.12) | 0.917 |
| Diastolic deceleration | 1.00 (0.89–1.13) | 0.977 |
| Atrial thrust | 1.02 (0.93–1.12) | 0.682 |
LV, left ventricular; EDV, end-diastolic volume; ESV, end-systolic volume; SV, stroke volume; EF, ejection fraction; FT, feature-tracking; GLS/GCS/GRS, global longitudinal/circumferential/radial strain; LAS, long axis strain; RMS, root mean square; HDF, hemodynamic force. Volumes are given in ml/m2 body surface area, strain and HDF values in %. Hazard rations for the occurrence of cardiovascular events were calculated using Cox regression analyses. Bold p-values indicate statistical significance.
Fig. 5.
Cardiovascular hospitalisation. Left: The graph shows the percentage of patients with cardiovascular hospitalisation (CVH) in patients with systolic peak hemodynamic force (HDF) assessed by cardiovascular magnetic resonance HDF analyses above or below the median. Right: The graph shows the percentage of patients with CVH in patients with a systolic impulse in HDF analyses above or below the median.
Discussion
HFpEF is defined according to preserved LV systolic function assessed by volumetric measurement. However, the novel advances in deformation imaging post-processing used in this study shed new light on LV dysfunction assessment in HFpEF. Firstly, HDF analyses were able to detect systolic impairment in HFpEF where volumetric and FT-based deformation imaging did not. Secondly, HDF systolic function quantification analyses were better predictors of cardiovascular hospitalisation and superior for risk assessment compared to conventional FT deformation imaging.
CMR is the reference standard for cardiac function assessment based on volumetric analyses.30 However, from a volumetric point of view, LV function in HFpEF is, by definition, preserved. Beyond volumetric assessments, CMR allows the assessment of myocardial contractility and relaxation.24 Indeed, FT LV GLS correlates with invasive assessments of the LV relaxation time constant, Tau.31 Park et al.32 reported impaired LV function based on deformation imaging in HFpEF. Whilst in the present study population LV GLS was numerically smaller in the HFpEF population, we were unable to detect statistically significant differences in LV function using FT deformation imaging. However, in contrast to the study population by Park et al.32 based on 1335 HFpEF individuals, the present HFpEF populations accounted for 34 HFpEF and 34 NCD patients only. Consequently, from a statistical point of view, requirements for sensitivity and specificity of LV GLS are more demanding in the present population to detect subtle differences. Importantly and despite the small study population, HDF analyses were able to detect systolic impairment in the present population.
The HFpEF-Stress Trial demonstrated the distinct importance of exercise-stress to induce cardio-pulmonary congestion and unmask cardiac functional failure for early diagnosis in HFpEF.10 The fact that 56% of HFpEF patients were exclusively identified by exercise-stress testing, with a median NTproBNP level of only 255 ng/l in laboratory testing, is suggestive of an early stage of diastolic dysfunction in the present population. This is further supported by data from spiroergometric assessment with peak O2 uptake falling in the range where additional exercise-tests would be advised.33 However, whilst lower natriuretic peptide levels are associated with better diastolic function, cardiac remodelling is likely already present, impacting both overall cardiac function and outcome.34 In fact, microvascular disease and tissue remodelling play an early role in disease pathophysiology and progression in HFpEF.35,36 Fibrosis and collagen deposition can be appreciated from increased extracellular volume on CMR.37 Furthermore, compared to HFpEF patients with an LVEF >60%, HFpEF patients with an LVEF of 50–60% have increased extracellular volumes paralleled by decreased systolic contractility appreciated from PVL.38 This highlights the importance of an early diagnosis, suggestive that early collagen deposition and degrading LV systolic function can be at different stages whilst still having a preserved LVEF. Indeed, HDF analyses in this study were able to detect subtle differences in LV function at rest, highlighting the precision of HDF assessment for LV function quantification. In contrast, LV LAS at rest was unable to detect LV impairment; diagnostic accuracy was vastly improved during exercise-stress testing, thus confirming the importance of exercise-stress imaging in HFpEF.8 Interestingly, HDF systolic force analyses exceeded the value of LV LAS at rest to detect HFpEF and showed similar worth when compared with LV LAS exercise-stress imaging. Furthermore, impaired systolic peak and impulse HDF were associated with a higher rate of CVH. Park et al.32 previously reported an association of impaired LV GLS to all-cause mortality and heart failure hospitalisation in their large HFpEF population. Within this study population, the value of LV GLS for risk prediction was distinctly less pronounced. Indeed, the accuracy of HDF systolic force exceeded the value of LV GLS for CVH prediction appreciated from AUC comparison. However, it should be noted that none of the cohort died from a cardiovascular cause in the follow-up period.
Advances in non-invasive CMR imaging techniques have allowed us to move on from the need for invasive intraventricular pressure gradient (IVPG) assessments.39 Whilst sophisticated 4D flow CMR techniques have merit, they are limited to specialised centres.15,17 This study demonstrates that it is possible to assess HFpEF in more detail using routinely acquired bSSFP cine imaging based on LV geometry, endocardial tissue movement as well as aortic and mitral valve orifice areas.14,19,20 Invasive PVL, the current reference-standard for cardiac hemodynamic assessments, with derived LV end-systolic elastance (Ees) as a reference for contractility, indicates increased contractility in HFpEF at rest.40 This stands in contradiction to other measurements of reduced systolic function.41 This mismatch has been attributed to the susceptibility of Ees to changes in LV geometry caused by concentric myocardial remodelling and ventricular stiffening.42 Though systolic function is more mildly impaired in HFpEF compared with HFrEF,42 this mild impairment is associated with increased mortality.41 Indeed, within this study population, systolic impairment emerged as the most prominent change. However, whilst Ees is influenced by the overall LV geometry, this is also taken into consideration for HDF calculation. As such, systolic functional quantification showed the highest correlation to PCWP during exercise-stress.
bSSFP-derived data confirms a gap between healthy volunteers and HFpEF within the systolic aspect of the HDF curve, whilst the diastolic aspect of both curves seemingly overlap.23 In contrast, a recent study reporting 4D flow-derived data did not detect systolic nor diastolic functional differences between HFpEF and the control group.43 However, that particular study classified the cardiac cycle as having one systolic and one diastolic phase; instead, this research addresses specific phases within systole and diastole. This may explain why subtle differences were detected within different functional phases in our study. Within our population, early diastolic filling was comparable between both HFpEF and NCD patients, potentially due to the overall early stage of diastolic dysfunction in our population. This is contrasted with the findings previously seen in patients via invasive PVL assessment.13 On the one hand side HDF analyses were able to demonstrate reduced early diastolic filling in HFpEF patients diagnosed at rest compared with HFpEF patients which were only identified by exercise-stress thresholds. On the other hand side HFpEF patients diagnosed at rest showed similar diastolic functional values compared to NCD. Whilst HDF analyses may thus be able to quantify different phases of diastolic function, in this small patient population at a relatively early disease stage, HDF analyses emerge not sensitive enough to quantify functional alterations as seen in PVL.
Beyond LV function, impaired atrial function has come to the fore in HFpEF.40,44 HDF analyses demonstrated impaired late diastolic filling by failing atrial thrust and subsequently increased early/late diastolic filling ratio in HFpEF compared to NCD which is in line with data from echocardiography.45 Whilst atrial thrust is statistically significantly correlated to LAVI, this correlation is low, suggesting further value of thrust quantification beyond sole morphologic assessment. As such, HDF analyses may allow non-invasive monitoring of declining diastolic dysfunction. Consequently, advancements in CMR post-processing enabled HDF analyses to sensitively detect LV systolic and diastolic dysfunction at rest. Future developments in HDF analyses should incorporate exercise-stress imaging to allow further insight into stress-induced cardiac congestion and myocardial functional failure.
Study limitations
The HFpEF stress trial was a single centre feasibility study to evaluate real-time exercise-stress testing in CMR. Consequently, conclusions based on that study population are single centre experiences only with limited statistical power given the small size of the study population. All eligible patients undergoing echocardiography were approached. However, without appropriate randomization, the decision to participate in the study may be affected by an inherent selection bias. Furthermore, limitations of the case–control setting apply. Disease progression in HFpEF may be slower compared to HFrEF and given that more than half of the patients studied were diagnosed according to stress thresholds only, a follow-up of 24 months may be too short for progressive cardiac remodelling leading to adverse cardiovascular events. Despite this, eleven events were noted and distinct differences for LV functional parameters could be identified. Invasively obtained PVL were not assessed within this study, and as such no invasive validation was possible. Furthermore, reference-standard non-invasive 4D flow measurements were not performed to compare the assessment of HDF between both methods.
Conclusion
HDF analyses reveal a particular perspective on the intracardiac pressure dynamics and indicate impairment of LV ejection force beyond the capabilities of volumetric and strain analyses in HFpEF.
Contributors
SJB and AS designed the study protocol, performed data acquisition, performed statistical analyses and drafted the manuscript. SJB and AS had full access to the underlying data. HU and SFR performed data acquisition and analysis. AS, TL, RJC, RE and GH revised the manuscript and participated in the scientific discussion during the study. All authors read and approved the final manuscript.
Data sharing statement
Regarding data availability, we confirm that all relevant data are within the paper and all data underlying the findings are fully available without restriction from the corresponding author at the University Medical Centre Goettingen for researchers who meet the criteria for access to confidential data.
Declaration of interests
The authors declare no competing interests in relation to the present manuscript.
Acknowledgements
Funding: German Centre for Cardiovascular Research (DZHK).
Footnotes
Clinical Trial Registration: Clinicaltrials.gov, NCT03260621.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2022.104334.
Appendix A. Supplementary data
References
- 1.van Riet E.E.S., Hoes A.W., Wagenaar K.P., Limburg A., Landman M.A.J., Rutten F.H. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail. 2016;18(3):242–252. doi: 10.1002/ejhf.483. [DOI] [PubMed] [Google Scholar]
- 2.Paulus W.J., Tschöpe C., Sanderson J.E., et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28(20):2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 3.Pitt B., Pfeffer M.A., Assmann S.F., et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 4.Ravassa S., Trippel T., Bach D., et al. Biomarker-based phenotyping of myocardial fibrosis identifies patients with heart failure with preserved ejection fraction resistant to the beneficial effects of spironolactone: results from the Aldo-DHF trial. Eur J Heart Fail. 2018;20(9):1290–1299. doi: 10.1002/ejhf.1194. [DOI] [PubMed] [Google Scholar]
- 5.Lewis G.A., Dodd S., Clayton D., et al. Pirfenidone in heart failure with preserved ejection fraction: a randomized phase 2 trial. Nat Med. 2021;27(8):1477–1482. doi: 10.1038/s41591-021-01452-0. [DOI] [PubMed] [Google Scholar]
- 6.Anker S.D., Butler J., Filippatos G., et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 7.Schulz A., Schuster A. Visualizing diastolic failure: non-invasive imaging-biomarkers in patients with heart failure with preserved ejection fraction. eBioMedicine. 2022 doi: 10.1016/j.ebiom.2022.104369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pieske B., Tschöpe C., de Boer R.A., et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) Eur Heart J. 2019;40(40):3297–3317. doi: 10.1093/eurheartj/ehz641. [DOI] [PubMed] [Google Scholar]
- 9.Obokata M., Kane G.C., Reddy Y.N.V., Olson T.P., Melenovsky V., Borlaug B.A. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive-echocardiographic study. Circulation. 2017;135(9):825–838. doi: 10.1161/CIRCULATIONAHA.116.024822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Backhaus S.J., Lange T., George E.F., et al. Exercise-stress real-time cardiac magnetic resonance imaging for non-invasive characterisation of heart failure with preserved ejection fraction: the HFpEF stress trial. Circulation. 2021;143:1484–1498. doi: 10.1161/CIRCULATIONAHA.120.051542. [DOI] [PubMed] [Google Scholar]
- 11.Borlaug B.A., Nelson M.D. Real-time cardiac magnetic resonance imaging: a new spin on the evaluation of HFpEF. Circulation. 2021;143(15):1499–1501. doi: 10.1161/CIRCULATIONAHA.120.053026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baan J., van der Velde E.T., de Bruin H.G., et al. Continuous measurement of left ventricular volume in animals and humans by conductance catheter. Circulation. 1984;70(5):812–823. doi: 10.1161/01.cir.70.5.812. [DOI] [PubMed] [Google Scholar]
- 13.Westermann D., Kasner M., Steendijk P., et al. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117(16):2051–2060. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 14.Pedrizzetti G., Arvidsson P.M., Töger J., et al. On estimating intraventricular hemodynamic forces from endocardial dynamics: a comparative study with 4D flow MRI. J Biomech. 2017;60:203–210. doi: 10.1016/j.jbiomech.2017.06.046. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson J., Bolger A.F., Ebbers T., Carlhäll C.-J. Assessment of left ventricular hemodynamic forces in healthy subjects and patients with dilated cardiomyopathy using 4D flow MRI. Physiol Rep. 2016;4(3) doi: 10.14814/phy2.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriksson J., Zajac J., Alehagen U., Bolger A.F., Ebbers T., Carlhäll C.-J. Left ventricular hemodynamic forces as a marker of mechanical dyssynchrony in heart failure patients with left bundle branch block. Sci Rep. 2017;7(1):2971. doi: 10.1038/s41598-017-03089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arvidsson P.M., Töger J., Carlsson M., et al. Left and right ventricular hemodynamic forces in healthy volunteers and elite athletes assessed with 4D flow magnetic resonance imaging. Am J Physiol Heart Circ Physiol. 2017;312(2):H314–H328. doi: 10.1152/ajpheart.00583.2016. [DOI] [PubMed] [Google Scholar]
- 18.Töger J., Arvidsson P.M., Bock J., et al. Hemodynamic forces in the left and right ventricles of the human heart using 4D flow magnetic resonance imaging: phantom validation, reproducibility, sensitivity to respiratory gating and free analysis software. PLoS One. 2018;13(4) doi: 10.1371/journal.pone.0195597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedrizzetti G. On the computation of hemodynamic forces in the heart chambers. J Biomech. 2019;95 doi: 10.1016/j.jbiomech.2019.109323. [DOI] [PubMed] [Google Scholar]
- 20.Faganello G., Collia D., Furlotti S., et al. A new integrated approach to cardiac mechanics: reference values for normal left ventricle. Int J Cardiovasc Imag. 2020;36(11):2173–2185. doi: 10.1007/s10554-020-01934-1. [DOI] [PubMed] [Google Scholar]
- 21.Backhaus S.J., Rösel S.F., Schulz A., et al. RT-CMR imaging for noninvasive characterization of HFpEF: medium-term outcomes of the HFpEF stress trial. JACC Cardiovasc Imag. 2022;15(5):943–945. doi: 10.1016/j.jcmg.2021.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Kramer C.M., Barkhausen J., Flamm S.D., Kim R.J., Nagel E. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson. 2013;15:91. doi: 10.1186/1532-429X-15-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapinskas T., Pedrizzetti G., Stoiber L., et al. The intraventricular hemodynamic forces estimated using routine CMR cine images: a new marker of the failing heart. JACC Cardiovasc Imag. 2019;12(2):377–379. doi: 10.1016/j.jcmg.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Schuster A., Hor K.N., Kowallick J.T., Beerbaum P., Kutty S. Cardiovascular magnetic resonance myocardial feature tracking: concepts and clinical applications. Cardiovasc Imaging. 2016;9(4) doi: 10.1161/CIRCIMAGING.115.004077. [DOI] [PubMed] [Google Scholar]
- 25.Schuster A., Backhaus S.J., Stiermaier T., et al. Fast manual long-axis strain assessment provides optimized cardiovascular event prediction following myocardial infarction. Eur Heart J Cardiovasc Imag. 2019;20(11):1262–1270. doi: 10.1093/ehjci/jez077. [DOI] [PubMed] [Google Scholar]
- 26.Uecker M., Zhang S., Voit D., Karaus A., Merboldt K.-D., Frahm J. Real-time MRI at a resolution of 20 ms. NMR Biomed. 2010;23(8):986–994. doi: 10.1002/nbm.1585. [DOI] [PubMed] [Google Scholar]
- 27.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837. [PubMed] [Google Scholar]
- 28.Obuchowski N.A. Sample size calculations in studies of test accuracy. Stat Methods Med Res. 1998;7(4):371–392. doi: 10.1177/096228029800700405. [DOI] [PubMed] [Google Scholar]
- 29.Reddy Y.N.V., Carter R.E., Obokata M., Redfield M.M., Borlaug B.A. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138(9):861–870. doi: 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pennell D.J. Cardiovascular magnetic resonance. Circulation. 2010;121(5):692–705. doi: 10.1161/CIRCULATIONAHA.108.811547. [DOI] [PubMed] [Google Scholar]
- 31.Ito H., Ishida M., Makino W., et al. Cardiovascular magnetic resonance feature tracking for characterization of patients with heart failure with preserved ejection fraction: correlation of global longitudinal strain with invasive diastolic functional indices. J Cardiovasc Magn Reson. 2020;22(1):42. doi: 10.1186/s12968-020-00636-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park J.J., Park J.-B., Park J.-H., Cho G.-Y. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol. 2018;71(18):1947–1957. doi: 10.1016/j.jacc.2018.02.064. [DOI] [PubMed] [Google Scholar]
- 33.Guazzi M., Wilhelm M., Halle M., et al. Exercise testing in heart failure with preserved ejection fraction: an appraisal through diagnosis, pathophysiology and therapy - a clinical consensus statement of the Heart Failure Association and European Association of Preventive Cardiology of the European Society of Cardiology. Eur J Heart Fail. 2022;24(8):1327–1345. doi: 10.1002/ejhf.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verbrugge F.H., Omote K., Reddy Y.N.V., Sorimachi H., Obokata M., Borlaug B.A. Heart failure with preserved ejection fraction in patients with normal natriuretic peptide levels is associated with increased morbidity and mortality. Eur Heart J. 2022;43:1941–1951. doi: 10.1093/eurheartj/ehab911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Severino P., D'Amato A., Prosperi S., et al. Myocardial tissue characterization in heart failure with preserved ejection fraction: from histopathology and cardiac magnetic resonance findings to therapeutic targets. Int J Mol Sci. 2021;22(14) doi: 10.3390/ijms22147650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nair N. Epidemiology and pathogenesis of heart failure with preserved ejection fraction. Rev Cardiovasc Med. 2020;21(4):531–540. doi: 10.31083/j.rcm.2020.04.154. [DOI] [PubMed] [Google Scholar]
- 37.Rommel K.-P., von Roeder M., Latuscynski K., et al. Extracellular volume fraction for characterization of patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2016;67(15):1815–1825. doi: 10.1016/j.jacc.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Rosch S., Kresoja K.-P., Besler C., et al. Characteristics of heart failure with preserved ejection fraction across the range of left ventricular ejection fraction. Circulation. 2022;146:506–518. doi: 10.1161/CIRCULATIONAHA.122.059280. [DOI] [PubMed] [Google Scholar]
- 39.Guerra M., Brás-Silva C., Amorim M.J., Moura C., Bastos P., Leite-Moreira A.F. Intraventricular pressure gradients in heart failure. Physiol Res. 2013;62(5):479–487. doi: 10.33549/physiolres.932531. [DOI] [PubMed] [Google Scholar]
- 40.Melenovsky V., Borlaug B.A., Rosen B., et al. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49(2):198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 41.Borlaug B.A., Lam C.S.P., Roger V.L., Rodeheffer R.J., Redfield M.M. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54(5):410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borlaug B.A., Paulus W.J. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32(6):670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arvidsson P.M., Nelsson A., Magnusson M., Smith J.G., Carlsson M., Arheden H. Hemodynamic force analysis is not ready for clinical trials on HFpEF. Sci Rep. 2022;12(1) doi: 10.1038/s41598-022-08023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Roeder M., Rommel K.-P., Kowallick J.T., et al. Influence of left atrial function on exercise capacity and left ventricular function in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.116.005467. [DOI] [PubMed] [Google Scholar]
- 45.Mitter S.S., Shah S.J., Thomas J.D. A Test in Context: E/A and E/e' to Assess Diastolic Dysfunction and LV Filling Pressure. J Am Coll Cardiol. 2017;69(11):1451–1464. doi: 10.1016/j.jacc.2016.12.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.