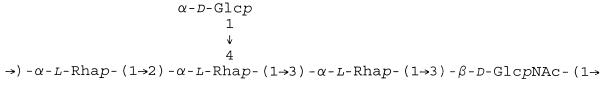Abstract
Seroepidemiological data and a clinical trial with a Shigella sonnei O-specific polysaccharide (O-SP)–Pseudomonas aeruginosa recombinant exoprotein A (rEPA) conjugate provide evidence that a critical level of immunoglobulin G (IgG) lipopolysaccharide (LPS) antibodies in serum confers protection against shigellosis. We evaluated the immunogenicity of conjugates whose carrier proteins and O-SPs were treated with succinic anhydride (SA), which reacts with amino groups at neutral pH to form amide-linked carboxyls (succinylation). Conjugates were synthesized with either of two genetically inactivated medically useful toxins, the diphtheria protein CRM9 or rEPA, bound to the O-SP of Shigella flexneri type 2a. Conjugates composed of the succinylated protein, succinylated O-SP, or both succinylated components were administered to mice by a clinically relevant scheme, and their levels of serum IgG anti-LPS and anti-proteins were assayed 7 days after the second and third injections. CRM9 served as a more immunogenic carrier than rEPA. Conjugates composed of succinylated components were more immunogenic than the conjugates composed of the native components. SA treatment of both the carrier protein and the O-SP did not confer an advantage over the succinylated protein alone. Conjugates prepared with native proteins, in general, elicited slightly higher levels of IgG protein antibodies than conjugates composed of the SA-treated proteins.
Shigellosis remains a serious and common disease that is a major cause of growth retardation and death in children of developing countries and a problem under conditions of crowding, as in chronic-care institutions, among army recruits, and in refugee camps. (4, 12, 15, 18, 20, 23, 28, 37, 46). Under such conditions, a vaccine may be the only means of preventing shigellosis.
Shigella flexneri type 2a, the most common cause of shigellosis in developing countries, is now resistant to most antibiotics. The development of vaccines has been difficult because shigellae are inhabitants of and pathogens for humans only, and there is no consensus about the mechanism(s) of immunity (35, 36, 47). We proposed that a critical level of immunoglobulin G (IgG) antibody to the O-specific polysaccharide (O-SP) domain of the lipopolysaccharide (LPS) in serum confers immunity to Shigella by inactivating the inoculum on the intestinal epithelium (35, 36). This hypothesis provides an explanation for the age-related incidence of shigellosis and the type-specific immunity it confers (17, 33, 35, 36). Newborns, infants, and adults are relatively resistant compared to children, who have a high incidence of shigellosis (15, 17, 35). A vaccine for shigellosis, therefore, will have to confer immunity to young children (3, 35). The O-SPs of Shigella combine with specific antibodies but are nonimmunogenic, due to their comparatively low molecular weights (haptens). Covalent binding to proteins converts the O-SP to an immunogen (8–10, 27, 35, 44). Conjugates of Shigella dysenteriae type 1, S. flexneri type 2a, and Shigella sonnei were safe and immunogenic in young adults; the latter two were also safe and immunogenic in children 4 to 7 years old (3, 9, 10, 44). In a double-blinded, randomized, vaccine-controlled study, an S. sonnei O-SP conjugate showed an efficacy of 74% (P = 0.006) against shigellosis in Israeli army recruits (10). This conjugate also prevented shigellosis occurring within 1 to 17 days after vaccination, albeit at a lower rate (43% efficacy, P = 0.04), indicating that a conjugate could be useful in controlling epidemics. Efficacy was related to the level of conjugate-induced IgG anti-LPS in serum (10).
The immunogenicity of saccharide components has been related to their molecular weights, the density of the saccharides on the carrier, and the intactness of the carrier protein (1, 2, 8, 13, 38, 43). The O-SP of S. flexneri type 2a is a linear-branched copolymer of a pentasaccharide (6, 7, 24–26) (Fig. 1). Isolated by the acid hydrolysis of LPS, the O-SP of S. flexneri type 2a also contains the core region with residues of aminoethanol and 8-ketooctanoic acid (24, 29). The O-SP was activated with cyanogen bromide and treated with adipic acid dihydrazide (ADH) to form an adipic hydrazide derivative (AH) (8, 38). 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) forms an amide linkage between the hydrazide of the O-SP and the carboxyl of proteins (21). This synthesis is accompanied by side reactions that include the formation of amide bonds between the ɛ-amino groups of lysines and adjacent carboxyls of the protein (intramolecular cross-linking) and adjacent proteins (intermolecular cross-linking).
FIG. 1.
O-SP of S. flexneri type 2a.
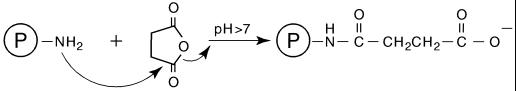
Succinic anhydride or dihydro-2,5-furandione (SA) reacts rapidly with the ɛ-amino groups of lysines and the α-amino groups of the N termini of proteins at pH 7 to 8, forming an amide bond by replacing the amino group with a carboxyl (19, 30, 34) (Fig. 2). SA also reacts, to a lesser extent, with tyrosyl, histidyl, cysteinyl, and threonyl side chains that hydrolyze rapidly at alkaline pH. The by-product of SA hydrolysis is succinic acid. Theoretically, the conversion of the ɛ-amino groups of lysines and the α-amino groups of N termini following succinylation of the protein should reduce EDC-induced intra- and intermolecular amide formation. The additional carboxyls should also facilitate binding of AH–O-SP derivatives to the protein. Similarly, SA reacts with amino groups of the core to increase the number of carboxyls, thus facilitating the formation of AH derivatives of the O-SP. SA treatment has been shown to inactivate diphtheria and tetanus toxins and stabilize the resultant toxoids against aggregation (40).
FIG. 2.
The action of succinic anhydride upon the amino groups of a protein.
To study the effect of SA upon the immunogenicity of S. flexneri type 2a O-SP conjugates, either of two genetically inactivated toxins, the diphtheria protein CRM9 or Pseudomonas aeruginosa recombinant exoprotein A (rEPA), was used as a carrier (5, 22, 42, 44).
The O-SP of S. flexneri type 2a had less than 1% each of protein and nucleic acids: its molecular mass was ∼25 kDa, and its 13C nuclear magnetic resonance spectrum was identical to published data (25, 26, 44). rEPA was prepared as previously described (5). CRM9, a cross-reacting nontoxic mutant protein of tox+ Corynebacterium diphtheriae, was cultivated from C7 (β)(tox-201,tox-9) (strain 7), and CRM9 was purified as described previously (42). CRM9 was further purified by precipitation with 75% (NH4)2SO4 and chromatography on a Superdex 200 column in 50 mM sodium phosphate (pH 7.4). CRM9 showed an identity reaction with diphtheria toxin by double immunodiffusion in 0.9% agarose with anti-diphtheria toxin and had a molecular mass of 63 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown) (22).
EDC, SA, MES (2-[N-morpholino]-ethanesulfonic acid) hydrate, MES sodium salt, trinitrobenzenesulfonic acid (TNBS), and thimerosal were from Sigma Chemical Co., St. Louis, Mo.; ADH, CNBr, and acetonitrile were from Sigma-Aldrich, Milwaukee, Wis.; CL-4B Sepharose, Sephadex G-50, STREAMLINE DEAE, and Superdex 200 were from Pharmacia, Piscataway, N.J.; and a YM-10 membrane (10,000 molecular weight cutoff) was from Amicon, Beverly, Mass.
The protein and saccharide components of the S. flexneri type 2a O-SP conjugates were assayed as previously described (8, 16, 41). Derivatization with AH was measured by the TNBS assay (38). ADP ribosyl transferase activity, with purified diphtheria toxin as a standard, was assayed as previously described (32, 42). Succinylation was measured indirectly by the reduction in amino groups of the protein with lysine as the standard (16). Endotoxin was assayed with Limulus polyphemus amoebocyte lysate (BioWhittaker, Walkersville, Md.) with Escherichia coli LPS (U.S. Food and Drug Administration) as a standard. Alison O’Brien, Uniformed Services University of the Health Sciences, assayed residual diphtheria toxin activity by using the Vero cell assay (32). The control was S. dysenteriae type 1 Shiga toxin. The 50% cytotoxic dose was 9.5 × 10−14 M for diphtheria toxin, 1.1 × 10−9 M for CRM9, and 2.0 × 10−8 M for S. flexneri type 2a O-SP–CRM9SA (the succinylated form of CRM9).
Preliminary experiments defined the conditions that allowed succinylation of the proteins while their antigenicity was retained (measured by double immunodiffusion with goat anti-P. aeruginosa exotoxin A or rabbit anti-diphtheria toxin). SA was added to the proteins (10 mg/ml of saline) at room temperature and mixed vigorously, and the pH was maintained at 7.2 to 7.5 with 0.5 M NaOH in a pH-stat for 20 min. The reaction mixture was passed through a 1- by 50-cm Sephadex G-50 column in 0.2 M NaCl, and the void volume peak was pooled and concentrated. Various ratios (wt/wt) of SA to rEPA (1:2, 1:4, 1:5, and 1:10) and of SA to CRM9 (1:1, 1:4, 1:10, and 1:20) were evaluated. Reduced reactivity of the succinylated proteins with their antisera was related to the amount of SA in the reaction mixture.
S. flexneri type 2a O-SP (20 mg/ml of H2O) was mixed with SA (0.3 mg/mg of O-SP), and the pH was maintained at 7.0 with 0.2 M NaOH for 30 min. The reaction mixture was dialyzed extensively against water at 4°C and freeze-dried. The succinylated S. flexneri type 2a O-SP was derivatized with ADH by using EDC as described previously (8).
AH derivatization of O-SP ranged from 1.34 to 1.67%; succinylation increased the level of AH about twofold, to a range of 2.24 to 3.46%. The reaction of SA with the O-SP was probably limited to the ethanolamine in the core (24, 29). O-SP and the succinylated O-SP (O-SPSA) yielded an identity reaction by double immunodiffusion with anti-LPS serum (data not shown).
To evaluate the effect of the succinylation of the protein and/or O-SP on the immunogenicity of the conjugates, we prepared different conjugates under similar conditions: O-SP–rEPA, O-SP–rEPASA, O-SPSA–rEPA, O-SPSA–rEPASA, O-SP–CRM9, O-SP–CRM9SA, O-SPSA–CRM9, and O-SPSA–CRM9SA. The S. flexneri type 2a O-SP and the proteins, native or succinylated, were made at a concentration of 20 mg/ml in 0.1 M MES buffer (pH 5.8). With stirring, 0.05 M EDC was added, and the pH was maintained at 5.8 by the addition of 0.1 M HCl in a pH-stat for 4 h at room temperature. The reaction mixture was dialyzed against 6 liters of 0.2 M NaCl with three changes over 48 h, applied to a 1- by 90-cm CL-4B Sepharose column in 0.2 M NaCl, and the void volume peak was pooled.
Female Swiss Webster mice, 5 weeks old, were injected subcutaneously with 2.5 μg of the saccharide of the conjugates at days 0, 14, and 28. Groups of 10 mice were exsanguinated either 7 days after the second injection or 7 days after the third injection (38). IgG and IgM anti-LPS and IgG anti-protein levels in serum were measured by enzyme-linked immunosorbent assay (ELISA) as described previously (8, 27). The results (in ELISA units [EUs]) are expressed as geometric means (GMs). Levels that were less than the sensitivity of the ELISA were assigned one-half of that level. Comparisons of the GMs were performed with an unpaired t test or a Wilcoxon test.
Table 1 shows that the composition of the conjugates was similar, with O-SP/protein ratios (wt/wt) of about 1 (range, 0.39 to 2.65). Three conjugates had ratios of 0.39, 1.7, and 2.65, probably due to variability in the synthetic process.
TABLE 1.
Composition and immunogenicity in mice of S. flexneri type 2a O-SP–protein conjugatesa
| Polysaccharide and carrier protein | Conjugate composition
|
Levels (EU/ml of serum) of:
|
|||
|---|---|---|---|---|---|
| SA/protein | O-SP/protein (wt/wt) | Anti-LPS
|
IgG anti-protein | ||
| IgGb | IgM | ||||
| O-SP–CRM9 | None | 0.86 | 0.93 A | 1.70 | 1.50 |
| O-SP–CRM9SA | 1:10 | 1.70 | 8.27 B | 10.1 | 0.30 |
| O-SP–CRM9SA | 1:10 | 0.39 | 3.21 C | 28.3 | 2.40 |
| O-SPSA–CRM9 | None | 1.11 | 5.24 D | 2.63 | 3.72 |
| O-SPSA–CRM9 | None | 0.81 | 2.59 E | 6.17 | 8.51 |
| O-SPSA–CRM9SA | 1:10 | 2.65 | 6.02 | 2.53 | 2.16 |
| O-SPSA–CRM9SA | 1:10 | 1.34 | 5.45 | 6.17 | 2.0 |
| O-SP–rEPA | None | 0.86 | 0.34 F | 8.40 | 724 |
| O-SP–rEPASA | 1:5 | 0.60 | 0.25 G | 16.4 | 385 |
| O-SPSA–rEPA | None | 0.92 | 0.60 H | 5.04 | 4.95 |
| O-SPSA–rEPASA | 1:5 | 1.16 | 2.38 | 7.62 | 18.6 |
Groups of 10 5-week-old mice were injected subcutaneously with 2.5 μg of O-SP in 0.1 ml of saline on days 0, 14, and 28. Serum samples were taken 7 days after the second and third injections, assayed for IgG and IgM anti-LPS and IgG anti-protein by ELISA (see Materials and Methods), and expressed as geometric mean ELISA units with a standard antiserum arbitrarily assigned a value of 100 EU. Antibody levels only after the third injection are depicted.
B versus A, P = 0.01; B versus F, P = 0.005; B versus G, P = 0.0008; C versus G, P = 0.03; D versus H, P < 0.05; D, E versus H, P < 0.05.
In preliminary experiments, none of the conjugates elicited anti-LPS in serum after the first injection and only low levels were elicited after the second. All, however, elicited booster responses after the third injection (Table 1). CRM9 served as a more immunogenic carrier for IgG anti-LPS than rEPA (0.93 versus 0.34, P was not significant [NS]). Higher levels of anti-LPS were also elicited by succinylated O-SPs (5.24 and 2.59 versus 0.60, P < 0.05). Similarly, conjugates prepared with CRM9SA were more immunogenic than those with rEPASA (8.27 versus 0.25, P = 0.0008; and 3.21 versus 0.25, P = 0.03).
Consistently, but not always significantly, for conjugates of CRM9, SA treatment of CRM9, O-SP, or both elicited higher levels of IgG anti-LPS than of the native components (8.27 versus 0.93, P = 0.01; 5.24 and 6.02 versus 0.93, P < 0.05; and 3.21, 2.59, and 5.45 versus 0.93, P was NS). Similarly, succinylated CRM9, with the native or succinylated O-SP, elicited higher levels of IgM anti-LPS than the native components. Similar levels of anti-LPS were induced by conjugates prepared with rEPA with either native or succinylated O-SP. The conjugate prepared with succinylated O-SP and succinylated rEPA (O-SPSA–rEPASA) elicited a higher level of IgG anti-LPS (2.38) than O-SPSA–rEPA (0.60, P was NS), O-SP–rEPASA (0.25, P = 0.01) or O-SP–rEPA (0.34, P was NS). O-SP–rEPASA elicited the highest level of IgM anti-LPS (16.4).
CRM9 and rEPA were each assayed relative to its standard antiserum and assigned 100 EU (the concentration was unknown). Therefore, the antibody values cannot be compared between the two proteins. Conjugates prepared with CRM9 elicited higher antibody levels (average GM, 4.57) than those of the succinylated protein (average GM, 1.71) (Table 1). rEPA bound to the native S. flexneri type 2a O-SP elicited higher antibody levels than O-SP–rEPASA (724 versus 385). O-SPSA–rEPASA, in contrast, elicited higher levels of antibodies than O-SPSA–rEPA (18.6 versus 4.95); these levels were considerably lower than those elicited by the conjugates prepared with the native O-SP.
As was shown for Haemophilus influenzae type b conjugates, there was no relation between the levels of protein and LPS antibodies (39).
Conjugates composed of SA-treated components elicited higher levels of IgG anti-LPS than conjugates prepared with the native components. CRM9, rEPA, and tetanus toxin become more soluble when treated with low levels of SA (unpublished data) (40). The addition of carboxyls to the O-SP and to the proteins is easily assayed (16). The by-product, succinic acid, is a nontoxic metabolite. Although the synthetic scheme is different from that used for S. flexneri type 2a O-SP, preliminary experiments have shown that conjugates of S. sonnei O-SP bound to succinylated rEPA or CRM9 were more immunogenic than those prepared with the native proteins. Accordingly, treatment with SA may be applicable to other proteins and amino-containing saccharides.
O-SPs of group B Shigella have the same tetrasaccharide backbone: type 2a specificity is conferred by the side chain of glucose linked α(1→4) to the middle rhamnose (7, 25, 26). Based upon serological data with typing antisera, it is unlikely that our conjugates will elicit significant cross-protection against other types of group B Shigella (6, 14, 31). Accordingly, it may be necessary to add another group B type in some areas to achieve a more complete effect upon shigellosis (31).
To our knowledge, S. flexneri type 2a O-SP is the only polysaccharide conjugate that induces booster responses in adults as well as in children (3, 9, 44). Seroepidemiological data and clinical evaluations of S. sonnei-rEPA in young adults indicate that it is the level of IgG anti-LPS in serum that correlates with immunity to shigellosis (10–12, 35, 36). It can be predicted, therefore, that enhancing the immunogenicity of our Shigella conjugates will increase both the level and duration of their efficacy.
Prevention of shigellosis is considered a priority by the World Health Organization (47). Because of their high degree of homology, Shigella and E. coli should be considered as a single genus (35, 45). Should conjugates be shown to be effective for infections caused by Shigella, principles derived from their study will likely be applicable to enteric diseases caused by E. coli strains, such as serotype O157:H7 (27).
REFERENCES
- 1.Anderson P, Pichichero M E, Insel R A. Immunogens consisting of oligosaccharides from the capsule of Haemophilus influenzae type b coupled to diphtheria toxoid or the toxin protein CRM197. J Clin Investig. 1985;76:52–59. doi: 10.1172/JCI111976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson P W, Pichichero M E, Stein E C, Porcelli S, Betts R F, Connuck D M, Korones D, Insel R A, Zahradnik J M, Eby R. Effect of oligosaccharide chain length, exposed terminal group, and hapten loading on the antibody response of human adults and infants to vaccines consisting of Haemophilus influenzae type b capsular antigen uniterminally coupled to the diphtheria protein CRM197. J Immunol. 1989;142:2464–2468. [PubMed] [Google Scholar]
- 3.Ashkenazi S, Passwell J H, Harlev E, Miron D, Dagan R, Farzan N, Ramon R, Majadly F, Bryla D A, Karpas A B, Robbins J B, Schneerson R the Israel Pediatric Shigella Study Group. Safety and immunogenicity of Shigella sonnei and Shigella flexneri 2a O-specific polysaccharide conjugates in children. J Infect Dis. 1999;179:1565–1568. doi: 10.1086/314759. [DOI] [PubMed] [Google Scholar]
- 4.Black R E, Brown K H, Becker S. Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics. 1984;73:799–805. [PubMed] [Google Scholar]
- 5.Blumentals I I, Kelly R M, Gorziglia M, Kaufman J B, Shiloach J. Development of a defined medium and two-step culturing method for improved exotoxin A yields from Pseudomonas aeruginosa. Appl Environ Microbiol. 1987;53:2013–2020. doi: 10.1128/aem.53.9.2013-2020.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlin N I A, Lindberg A A. Monoclonal antibodies specific for O-antigenic polysaccharides of Shigella flexneri: clones binding to II, II:3,4, and 7,8 epitopes. J Clin Microbiol. 1983;18:1183–1189. doi: 10.1128/jcm.18.5.1183-1189.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlin N I A, Lindberg A A, Bock K, Bundle D R. The Shigella flexneri O-antigenic polysaccharide chain. Nature of the biological repeating unit. Eur J Biochem. 1984;139:189–194. doi: 10.1111/j.1432-1033.1984.tb07993.x. [DOI] [PubMed] [Google Scholar]
- 8.Chu C, Liu B, Watson D, Szu S, Bryla D, Shiloach J, Schneerson R, Robbins J B. Preparation, characterization, and immunogenicity of conjugates composed of the O-specific polysaccharide of Shigella dysenteriae type 1 (Shiga’s bacillus) bound to tetanus toxoid. Infect Immun. 1991;59:4450–4458. doi: 10.1128/iai.59.12.4450-4458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen D, Ashkenazi S, Green M, Lerman Y, Slepon R, Robin G, Orr N, Taylor D N, Sadoff J C, Chu C-Y, Shiloach J, Schneerson R, Robbins J B. Safety and immunogenicity of investigational Shigella conjugate vaccines in Israeli volunteers. Infect Immun. 1996;64:4074–4077. doi: 10.1128/iai.64.10.4074-4077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen D, Ashkenazi S, Green M S, Gdalevich M, Robin G, Slepon R, Yavzori M, Orr N, Block C, Ashkenazi I, Shemer J, Taylor D N, Hale T L, Sadoff J C, Pavliakova D, Schneerson R, Robbins J B. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet. 1997;349:155–159. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- 11.Cohen D, Green M S, Block C, Slepon R, Ofek I. Prospective study of the association between serum antibodies to lipopolysaccharide O antigen and the attack rate of shigellosis. J Clin Microbiol. 1991;29:386–389. doi: 10.1128/jcm.29.2.386-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen D, Green M S, Block C, Rouach T, Ofek T. Serum antibodies to lipopolysaccharide and natural immunity to shigellosis in an Israeli military population. J Infect Dis. 1988;157:1068–1071. doi: 10.1093/infdis/157.5.1068. [DOI] [PubMed] [Google Scholar]
- 13.Dintzis R Z, Okajima M, Middleton M H, Greene G, Dintzis H M. The immunogenicity of soluble haptenated polymers is determined by molecular mass and hapten valence. J Immunol. 1989;143:1239–1244. [PubMed] [Google Scholar]
- 14.Edwards P R, Ewing W H. Identification of Enterobacteriacae. 3rd ed. Minneapolis, Minn: Burgess Publishing Co.; 1972. [Google Scholar]
- 15.Ferreccio C, Prado V, Ojeda A, Cayyazo M, Abrego P, Guers L, Levine M M. Epidemiologic patterns of acute diarrhea and endemic Shigella infections in children in a poor periurban setting in Santiago, Chile. Am J Epidemiol. 1991;134:614–627. doi: 10.1093/oxfordjournals.aje.a116134. [DOI] [PubMed] [Google Scholar]
- 16.Fields R. The measurement of amino groups in proteins and peptides. Biochem J. 1971;124:581–590. doi: 10.1042/bj1240581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Floyd T M, Higgins A R, Kader M A. Studies in shigellosis. V. The relationship of age to the incidence of Shigella infections in Egyptian children, with special reference to shigellosis in the newborn and in infants in the first six months of life. Am J Trop Med Hyg. 1956;5:119–130. doi: 10.4269/ajtmh.1956.5.119. [DOI] [PubMed] [Google Scholar]
- 18.Goma Epidemiology Group. Public health impact of Rwandan refugee crisis: what happened in Goma, Zaire, in July, 1994? Lancet. 1995;345:339–344. [PubMed] [Google Scholar]
- 19.Gounaris A D, Perlmann G E. Succinylation of pepsinogen. J Biol Chem. 1967;242:2739–2745. [PubMed] [Google Scholar]
- 20.Henry F J, Alam N, Aziz K M S, Rahaman M M. Dysentery, not watery diarrhoea, is associated with stunting in Bangladeshi children. Hum Nutr Clin Nutr. 1987;41:243–249. [PubMed] [Google Scholar]
- 21.Hoare D G, Koshland D E., Jr A method for the quantitative modification and estimation of carboxylic acid groups in proteins. J Biol Chem. 1967;242:2447–2453. [PubMed] [Google Scholar]
- 22.Hu V W, Holmes R K. Single mutation in the A domain of diphtheria toxin results in a protein with altered membrane insertion behavior. Biochim Biophys Acta. 1987;902:24–30. doi: 10.1016/0005-2736(87)90132-5. [DOI] [PubMed] [Google Scholar]
- 23.Hughes J M, Rouse J D, Barada F A, Guerrant R L. Etiology of summer diarrhea among the Navajo. Am J Trop Med Hyg. 1980;29:613–619. doi: 10.4269/ajtmh.1980.29.613. [DOI] [PubMed] [Google Scholar]
- 24.Jansson P-E, Lindberg A A, Lindberg B, Wollin R. Structural studies on the hexose region of the core in lipopolysaccharides from Enterobactericeae. Eur J Biochem. 1981;115:571–577. doi: 10.1111/j.1432-1033.1981.tb06241.x. [DOI] [PubMed] [Google Scholar]
- 25.Jansson P E, Kenne L, Wehler T. A 2D-1H-n.m.r. study of some Shigella flexneri O-polysaccharides. Carbohydr Res. 1987;166:271–282. doi: 10.1016/0008-6215(87)80063-0. [DOI] [PubMed] [Google Scholar]
- 26.Kenne L, Lindberg B, Petersson K, Katzenellenbogen E, Romanowska E. Structural studies of Shigella flexneri O-antigens. Eur J Biochem. 1978;91:279–284. doi: 10.1111/j.1432-1033.1978.tb20963.x. [DOI] [PubMed] [Google Scholar]
- 27.Konadu E Y, Parke J C, Jr, Tran H T, Bryla D A, Robbins J B, Szu S C. Investigational vaccine for Escherichia coli O157: phase 1 study of O157 O-specific polysaccharide-Pseudomonas aeruginosa recombinant exoprotein A (rEPA) conjugates in adults. J Infect Dis. 1998;177:383–387. doi: 10.1086/514203. [DOI] [PubMed] [Google Scholar]
- 28.Lee L A, Shapiro C N, Hargrett-Bean N, Tauxe R V. Hyperendemic shigellosis in the United States; a review of surveillance data for 1967–1988. J Infect Dis. 1991;164:894–900. doi: 10.1093/infdis/164.5.894. [DOI] [PubMed] [Google Scholar]
- 29.Mayer H, Schmidt G. The occurrence of three different lipopolysaccharide cores in shigella and their relationship to known enterobacterial core types. Zentbl Bakteriol Abt 1 Orig A. 1973;224:345–354. [PubMed] [Google Scholar]
- 30.Merck & Co., Inc. The Merck Index. 12th ed. Rahway, N.J: Merck & Co., Inc.; 1996. Compound 9039; p. 1515. [Google Scholar]
- 31.Noriega F R, Liao F M, Maneval D R, Ren S, Formal S B, Levine M M. Strategy for cross-protection among Shigella flexneri serotypes. Infect Immun. 1999;67:782–788. doi: 10.1128/iai.67.2.782-788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oku Y, Yutsudo T, Hirayama T, O’Brien A D, Takeda Y. Purification and some properties of a Vero toxin from a human strain of Escherichia coli that is immunologically related to Shiga-like toxin II (VT2) Microb Pathog. 1989;6:113–122. doi: 10.1016/0882-4010(89)90014-4. [DOI] [PubMed] [Google Scholar]
- 33.Passwell J H, Freier S, Shor R, Farzam N, Block C, Lison M, Shiff E, Ashkenazi S. Shigella lipopolysacchride antibodies in pediatric populations. Pediatr Infect Dis J. 1995;14:859–865. doi: 10.1097/00006454-199510000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Riordan J F, Vallee B L. Succinylcarboxypeptidase. Biochemistry. 1964;11:1768–1774. doi: 10.1021/bi00899a032. [DOI] [PubMed] [Google Scholar]
- 35.Robbins J B, Chu C-Y, Schneerson R. Hypothesis for vaccine development: protective immunity to enteric diseases caused by nontyphoidal salmonellae and shigellae may be conferred by serum IgG antibodies to the O-specific polysaccharide of their lipopolysaccharides. Clin Infect Dis. 1992;15:346–361. doi: 10.1093/clinids/15.2.346. [DOI] [PubMed] [Google Scholar]
- 36.Robbins J B, Schneerson R, Szu S C. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171:1387–1398. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 37.Ronsman C, Bennish M L, Wierzba T. Diagnosis and management of dysentery by community health workers. Lancet. 1988;ii:552–555. doi: 10.1016/s0140-6736(88)92669-4. [DOI] [PubMed] [Google Scholar]
- 38.Schneerson R, Barrera O, Sutton A, Robbins J B. Preparation, characterization and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980;152:361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneerson R, Robbins J B, Parke J C, Jr, Bell C, Schlesselman J J, Sutton A, Wang Z, Schiffman G, Karpas A, Shiloach J. Quantitative and qualitative analyses of serum antibodies elicited in adults by Haemophilus influenzae type b and pneumococcus type 6A capsular polysaccharide-tetanus toxoid conjugates. Infect Immun. 1986;52:519–528. doi: 10.1128/iai.52.2.519-528.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwendeman S P, Costantino H R, Gupta R K, Siber G R, Klibanov A M, Langer R. Stabilization of tetanus and diphtheria toxoids against moisture-induced aggregation. Proc Natl Acad Sci USA. 1995;92:11234–11238. doi: 10.1073/pnas.92.24.11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shields R, Burnett W W. Determination of protein-bound carbohydrate in serum by a modified anthrone method. Anal Chem. 1960;32:885–886. [Google Scholar]
- 42.Shiloach J, Kaufman J B. The combined use of expanded bed volume adsorption and gradient elution for capture and partial purification of mutant diphtheria toxin (CRM9) from Corynebacterium diphtheriae. Sep Sci Technol. 1999;34:29–40. [Google Scholar]
- 43.Szu S C, Li X, Schneerson R, Vickers J H, Bryla D, Robbins J B. Comparative immunogenicities of Vi polysaccharide-protein conjugates composed of cholera toxin or its B subunit as a carrier bound to high- or lower-molecular-weight Vi. Infect Immun. 1989;57:3823–3827. doi: 10.1128/iai.57.12.3823-3827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor D N, Trofa A C, Sadoff J, Chu C, Bryla D, Shiloach J, Cohen D, Ashkenazi S, Lerman Y, Egan W, Schneerson R, Robbins J B. Synthesis, characterization, and clinical evaluation of conjugate vaccines composed of the O-specific polysaccharides of Shigella dysenteriae type 1, Shigella flexneri type 2a, and Shigella sonnei (Plesiomonas shigelloides) bound to bacterial toxoids. Infect Immun. 1993;61:3678–3687. doi: 10.1128/iai.61.9.3678-3687.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor D V, Echeverria P, Sethabutr O, Pitarangsi C, Leksomboon U, Blacklow N R, Rowe B, Gross R, Cross J. Clinical and microbiological features of Shigella and enteroinvasive Escherichia coli infections detected by DNA hybridization. J Clin Microbiol. 1988;26:1362–1366. doi: 10.1128/jcm.26.7.1362-1366.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor D N, Houston R, Schlim D R, Bhaibulaya M, Ungar B L, Echeverria P. Etiology of diarrhea among travelers and foreign residents in Nepal. JAMA. 1988;260:1245–1248. [PubMed] [Google Scholar]
- 47.World Health Organization. Guidelines for the control of epidemics due to Shigella dysenteriae type 1. WHO/CDD/SER/88.12. Geneva, Switzerland: World Health Organization; 1988. [Google Scholar]