Abstract
Pseudomonas aeruginosa is a gram-negative opportunistic pathogen that is cytotoxic towards a variety of eukaryotic cells. To investigate the effect of this bacterium on macrophages, we infected J774A.1 cells and primary bone-marrow-derived murine macrophages with the P. aeruginosa strain PA103 in vitro. PA103 caused type-III-secretion-dependent killing of macrophages within 2 h of infection. Only a portion of the killing required the putative cytotoxin ExoU. By three criteria, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling assays, cytoplasmic nucleosome assays, and Hoechst staining, the ExoU-independent but type-III-secretion-dependent killing exhibited features of apoptosis. Extracellular bacteria were capable of inducing apoptosis, and some laboratory and clinical isolates of P. aeruginosa induced significantly higher levels of this form of cell death than others. Interestingly, HeLa cells but not Madin-Darby canine kidney cells were susceptible to type-III-secretion-mediated apoptosis under the conditions of these assays. These findings are consistent with a model in which the P. aeruginosa type III secretion system transports at least two factors that kill macrophages: ExoU, which causes necrosis, and a second, as yet unidentified, effector protein, which induces apoptosis. Such killing may contribute to the ability of this organism to persist and disseminate within infected patients.
Pseudomonas aeruginosa has long enjoyed a prominent place among bacteria that inhabit hospital environments and cause nosocomial infections. This pathogen utilizes an impressive arsenal of weapons to persist and disseminate within the hostile environment of the human body (32). As is the case with an increasing number of gram-negative pathogens, a type III secretion system that appears to contribute to virulence in a number of animal models has been recently identified in P. aeruginosa (10). This system secretes several effector proteins that have interesting effects on host cells. ExoY is an adenylate cyclase secreted by some strains of P. aeruginosa (39) and is homologous to the adenylate cyclases of Bordetella pertussis and Bacillus anthracis. ExoS and ExoT are two ADP-ribosylating proteins that are 76% identical, although ExoT has only 0.2% of the in vitro activity of ExoS (38). ExoU (also called PepA) is a putative cytotoxin that is necessary for full virulence of P. aeruginosa (6, 14). Both ExoS and ExoU cause cytotoxicity in epithelial cells, as evidenced by changes in cell morphology and decreased viability (6, 12, 14, 27, 30). Recent reports suggest that in addition to killing epithelial cells, the P. aeruginosa type III secretion system is also involved in the killing of macrophages (5, 33). Whether additional P. aeruginosa effector proteins exist and may act as cytotoxins is not known.
Interestingly, the type III systems of several other bacteria secrete proteins that induce apoptosis in macrophages. For example, IpaB of Shigella spp. (40), YopJ/P of Yersinia spp. (24, 25), and SipB of Salmonella spp. (16) have all been implicated as mediators of apoptosis. Both IpaB and SipB are thought to induce apoptosis by activation of caspase-1, while YopP/J may inhibit NF-κB-mediated pathways (29, 34). At least in the case of Shigella, apoptosis is not an artifact of tissue culture conditions. This phenomenon has been observed in cell lines infected with clinical rather than laboratory isolates (13), in the Peyer’s patches of experimentally infected rabbits (42), and in the rectal mucosae of patients with shigellosis (19).
In this report, we demonstrate that P. aeruginosa is capable of inducing apoptosis in macrophages and some epithelial cells. This process is type III dependent but is ExoU independent, suggesting the existence of an additional type III secreted effector protein capable of injuring macrophages. The ability of P. aeruginosa to kill macrophages by multiple mechanisms may assist in the neutralization of these important components of the immune system and thus contribute to this bacterium’s ability to persist and perhaps disseminate in the host.
P. aeruginosa PA103 is cytotoxic toward J774A.1 cells.
To investigate the effect of P. aeruginosa on macrophages, we microscopically examined J774A.1 cells infected with strain PA103 (Table 1) in vitro. Bacteria were grown for 17 h to stationary phase in Luria-Bertani broth at 37°C in the absence of shaking. Immediately prior to infection, the bacteria were diluted to exponential growth phase with the appropriate tissue culture medium and their concentration was determined by measurement of the optical density at 600 nm. All concentrations were checked by plating serial dilutions of samples onto agar plates and counting the number of CFU following incubation at 37°C for 20 h. J774A.1 cells were grown for 2 to 3 days on acid-etched glass coverslips to approximately 70% confluency. Cells were washed and infected with bacteria at a multiplicity of infection (MOI) of approximately 80. Infections were carried out in minimal essential medium (MEM) with Hank’s salts (Sigma Chemical Co., St. Louis, Mo.) supplemented with 3.5% sodium bicarbonate and 20 mM HEPES buffer (designated MEM-lite) at 37°C in room air for 2 h. Cells were washed and treated with amikacin (400 μg/ml) (Sigma Chemical Co.) for 1 h at 37°C to kill bacteria. Cells were then stained with a 2 μM concentration of the dead cell stain ethidium homodimer-1 (Molecular Probes, Inc., Eugene, Oreg.) and 5 μM concentration of the live cell stain calcein AM (Molecular Probes, Inc.) in MEM-lite for 30 min at 37°C. Cells were then washed, and coverslips were mounted and visualized by using a fluorescence microscope. Addition of PA103 resulted in significant killing of J774A.1 cells (data not shown).
TABLE 1.
Bacterial strains used in this study
| Straina | Description | Reference and source |
|---|---|---|
| PA103 | Wild-type, cytotoxic isolate | 22 |
| PA103exsA::Ω | Mutant of PA103 defective in expression of type III secretion genes | 11 |
| PA103pscJ::Tn5 | Mutant of PA103 defective in production of PscJ, a putative component of the bacterial type III secretion apparatus | 15, 20 |
| PA103pcrG::Tn5 | Mutant of PA103 defective in secretion of PopB and PopD, putative components of the type III translocation complex | 15, 20 |
| PA103exoU::Tn5 | Mutant of PA103 defective iin production of ExoU | 14, 20 |
| PA103exoU::Tn5 (pAH807) | PA103exoU::Tn5 harboring a plasmid containing an intact copy of the exoU gene and its chaperone, SpcU | 6, 14, 20 |
| PA103tox::Ω | Mutant of PA103 defective in production of exotoxin A | 21 |
| PAK | P. aeruginosa laboratory strain | 37 |
| 6294 | P. aeruginosa hyperinvasive corneal isolate | 9 |
| PAO1 | P. aeruginosa laboratory strain | 17 |
| 388 | P. aeruginosa laboratory strain | 2, 18 |
| 617000 | P. aeruginosa respiratory isolate | 14 |
| M90T | Wild-type S. flexneri serotype 5 | 41; a generous gift from Arturo Zychlinsky |
| BS176 | Plasmid-cured derivative of M90T | 41; a generous gift from Arturo Zychlinsky |
Tn5 refers to a Tn5 transposon modifed to contain the gentamicin resistance gene (20).
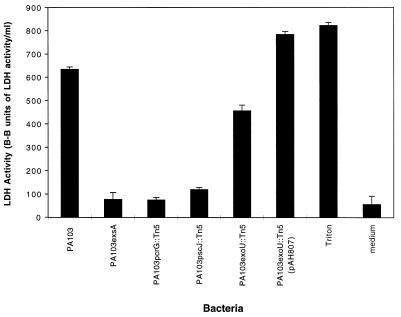
Lactate dehydrogenase (LDH) release assays (Sigma Chemical Co.) were used to quantify J774A.1 cell killing by PA103. This enzyme is normally in the cytoplasm of eukaryotic cells, and its release into the culture medium correlates with cell lysis. J774A.1 cells were seeded into 24-well plastic trays (approximately 4 × 104 cells/well), grown for 3 days, washed, and infected with bacteria at an MOI of approximately 80 in MEM-Lite for 3 h. LDH release into the medium was determined according to the manufacturer’s instructions. The media of J774A.1 cell cultures infected with PA103 consistently contained significantly more LDH than the media of uninfected cell cultures (Fig. 1). Triton X-100, a detergent that lyses all cells, was used as a positive control. PA103 killing of J774A.1 cells clearly increased in a time-dependent manner, with significant killing first being noted after 2 h of infection (data not shown). These results confirmed that P. aeruginosa is cytotoxic towards a macrophage-like cell line.
FIG. 1.
LDH release by J774A.1 cells infected with PA103 or mutants defective in type III secretion. Cells were infected for 3 h at an MOI of approximately 80. Mutants with defects in the type III secretion transcriptional activator (PA103exsA::Ω), the secretion apparatus (PA103pscJ::Tn5), or the translocation apparatus (PA103pcrG::Tn5) were noncytotoxic, whereas an exoU mutant (PA103exoU::Tn5) exhibited intermediate cytotoxic capacity. A plasmid (pAH807) containing an intact ExoU-encoding gene and its chaperone complemented the exoU mutant to a phenotype of full cytotoxicity. Error bars represent standard errors of the means for experiments performed in triplicate.
Cytotoxicity to J774A.1 cells is type III secretion dependent.
To investigate whether macrophage killing required an intact type III secretion system, a set of previously characterized isogenic transposon insertion mutants was used (14, 15, 20) (Table 1). The parent strain from which these mutants were derived, PA103, secretes the following type III secreted proteins: ExoU, ExoT, PopB (also known as PepB), and PopD (also known as PepD). PA103 does not contain the ExoS- or ExoY-encoding genes (8, 39). PA103pscJ::Tn5 is a mutant with a transposon inserted in the gene encoding PscJ, a putative outer membrane protein and component of the type III secretion apparatus. This mutant has a global type III secretion defect. On the other hand, a mutant with a transposon inserted in the PcrG gene, at the beginning of the operon encoding PopB and PopD, is defective in in vitro secretion of PopB and PopD but not other type III secreted proteins, such as ExoT and ExoU. PopB and PopD are P. aeruginosa type III secreted proteins that are thought to form the translocation complex, which translocates effector proteins into the host cell cytoplasmic compartment. Consistent with the notion that PA103pcrG::Tn5 has a defect in the type III translocation complex is the absence of ExoU-mediated cytotoxicity toward epithelial cells infected with this mutant even though abundant amounts of ExoU are secreted in vitro (20). A mutant with a transposon inserted in the ExoU structural gene, PA103exoU::Tn5, secretes ExoT, PopB, and PopD but not ExoU, suggesting that the type III secretion defect of this mutant is limited to expression and secretion of ExoU. Finally, PA103exsA::Ω, a mutant in which an omega cassette replaces the exsA gene (11), does not secrete ExoT, ExoU, PopB, or PopD (38). PA103exsA::Ω lysates also lack these proteins even when the bacteria are grown under low-calcium conditions that normally trigger their production. These findings are consistent with the role of ExsA as the transcriptional activator of the P. aeruginosa type III system. In summary, the phenotypes of these mutants suggest the following: PA103pscJ::Tn5 has a defective type III secretion apparatus, PA103pcrG::Tn5 has a defective translocation complex, PA103exsA::Ω fails to express any of the known type III secreted proteins, and PA103exoU::Tn5 is defective in ExoU production but has an otherwise intact type III secretion system. Although many of these mutants have not been completely characterized with regard to polarity, they do have protein secretion phenotypes consistent with the expected defects (14, 15, 20). We have used them as tools to study the P. aeruginosa type III secretion system.
These isogenic mutants of PA103 were first used to determine the role of the P. aeruginosa type III secretion system in the killing of J774A.1 cells (Fig. 1). After 3 h of infection, LDH release assays indicated that PA103 killed significant numbers of J774A.1 cells. In contrast, the three type-III-secretion-defective mutants, PA103exsA::Ω, PA103pscJ::Tn5, and PA103pcrG::Tn5, did not, suggesting that a functional type III secretion system is essential for the killing of J774A.1 cells by P. aeruginosa PA103.
PA103 killing of Madin-Darby canine kidney (MDCK) epithelial cells is almost totally dependent upon the type III secreted effector protein ExoU (20). We wished to determine if this was also true of PA103 killing of macrophage-like J774A.1 cells. Infection of these cells for 3 h with PA103exoU::Tn5 resulted in less killing than observed following infection with wild-type PA103 but significantly more killing than produced by PA103 mutants with global type III secretion defects (Fig. 1). Cytotoxicity was restored by complementation of the exoU mutant with a construct (pAH807) containing an intact copy of the exoU gene and its chaperone, SpcU (6, 7, 14). An isogenic mutant of PA103 with a transposon inserted in the 3′ portion of the ExoU gene has been previously shown to secrete a truncated form of ExoU (14). This mutant, which is defective in cytotoxicity toward MDCK epithelial cells (14, 20), also caused an intermediate level of killing when used to infect J774A.1 cells (data not shown). Together, these findings suggest that, in addition to ExoU, one or more distinct effector proteins secreted by the PA103 type III system are involved in the killing of J774A.1 cells.
P. aeruginosa PA103 induces apoptosis of J774A.1 cells.
Apoptosis and necrosis are distinct forms of cell death (3, 23) that can be distinguished by morphologic and biochemical methods. To determine the mechanism of J774A.1 cell injury by PA103, cells were grown and infected in Dulbecco’s Modified Eagle Medium (DMEM) with 4.5 g of glucose/liter and 0.584 g of glutamine/liter (University of California at San Francisco [UCSF] Cell Culture Facility) supplemented with 10% heat-inactivated fetal calf serum (Gibco-BRL, Gaithersburg, Md.). Six hours after infection cells were fixed, permeabilized, and stained by using a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay (In Situ Cell Death Detection kit—Fluorescein; Roche Diagnostics Corp., Indianapolis, Ind.) according to the manufacturer’s directions. Numerous J774A.1 cells incubated with PA103 showed positive TUNEL staining, indicating that infected cells were undergoing apoptosis (data not shown). In contrast, very few cells exposed to medium alone showed evidence of apoptosis.
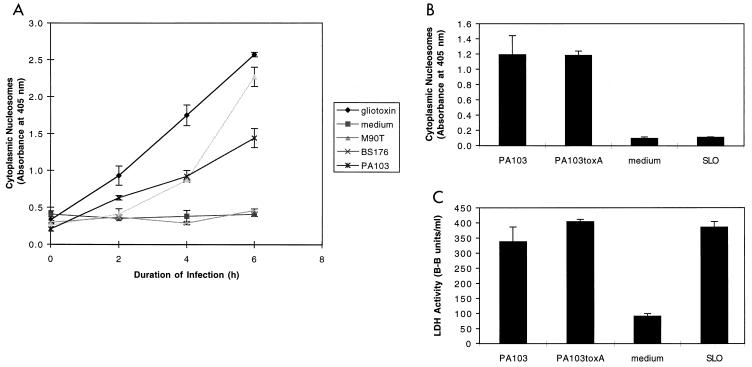
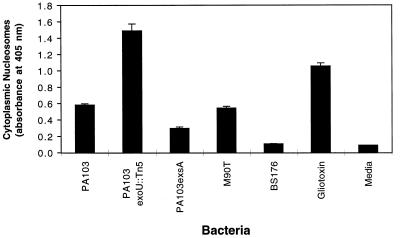
A commercially available enzyme-linked immunosorbent assay (ELISA) that detects cytoplasmic DNA fragments bound to histones (Cell Death Detection ELISAPLUS; Roche Diagnostics Corp.) was used to quantify the level of apoptosis occurring in infected cells. A positive result in this assay requires two features of apoptosis: DNA fragmentation and nuclear membrane breakdown. J774A.1 cells were seeded onto 96-well plastic trays (approximately 104 cells/well) and grown for 20 h at 37°C in 5% CO2. Cells were then washed and either infected in DMEM with 4.5 g of glucose/liter, 0.584 g of glutamine/liter, and 10% fetal bovine serum at 37°C in 5% CO2 or exposed to a 5 μM concentration of the apoptosis-inducing agent gliotoxin. Following infection, apoptosis was measured by using the Cell Death Detection ELISAPLUS according to the manufacturer’s instructions. PA103-infected J774A.1 cells showed increasing levels of apoptosis with longer infections and increasing MOI (range, 1 to 200; Fig. 2A and data not shown). Several positive and negative controls were assayed. J774A.1 cells underwent apoptosis following exposure to gliotoxin, an NF-κB inhibitor known to cause programmed cell death (28, 36). Cells exposed to medium alone exhibited only background levels of apoptosis. J774A.1 cells infected with Shigella flexneri M90T under identical conditions (MOI of 80) also underwent apoptosis in a time-dependent manner. This wild-type S. flexneri strain harbors a virulence plasmid encoding a type III secretion system that induces apoptosis of J774A.1 cells (41). In contrast, S. flexneri BS176, which is identical to M90T except that it lacks the virulence plasmid (41), caused only background levels of apoptosis.
FIG. 2.
Apoptosis of J774A.1 cells infected with PA103. (A) PA103 caused apoptosis of J774A.1 cells in a time-dependent manner as measured by the quantitative assay ELISAPlus. Cells were infected at an MOI of approximately 160. Positive controls included gliotoxin (5 μM) and the virulent S. flexneri strain M90T. Negative controls included medium and the avirulent S. flexneri strain BS176. (B) Apoptosis-inducing capacity of PA103, PA103exsA::Ω, PA103tox::Ω, and SLO, as measured by the quantitative ELISA. Cells were infected for 6 h at an MOI of approximately 160 or exposed to SLO at a concentration of 37.5 μg/ml. (C) Cytotoxicity-inducing capacity of PA103, PA103exsA::Ω, PA103tox::Ω, and SLO, as measured by LDH release assays. Infection conditions were similar to those described in panel B. The ELISA for apoptosis is clearly capable of distinguishing necrotic cell death (SLO) from apoptotic cell death (PA103). Furthermore, exotoxin A does not contribute to PA103-induced apoptosis under the conditions of this assay. Error bars represent standard errors of the means for experiments performed in triplicate.
To ensure that the quantitative ELISA was actually measuring apoptosis and not background DNA fragmentation resulting from necrosis, we wished to use the assay on a negative control that caused necrosis but not apoptosis. For this purpose, we chose the group A streptococcal pore-forming toxin streptolysin O (SLO). SLO kills eukaryotic cells by lysis but does not cause apoptosis. SLO (purchased from Suchharit Bhakdi, Johannes Gutenberg Universitat Mainz, Mainz, Germany) was added to cell cultures (final concentration, 37.5 μg/ml) in place of bacteria. The quantitative ELISA measured only background levels of apoptosis associated with J774A.1 cells treated with SLO for 6 h even though LDH release assays confirmed that significant necrosis was occurring at this time point (Fig. 2B and C). In contrast, cells infected with PA103 were associated with both cytotoxicity and apoptosis.
PA103 secretes exotoxin A, a non-type-III-secreted protein that has been reported to cause apoptosis (26). To determine whether this protein contributed to killing under the conditions of these assays, J774A.1 cells were infected for 6 h with an isogenic mutant of PA103, PA103tox::Ω, that was defective in exotoxin A production. This mutant caused levels of both cytotoxicity and apoptosis similar to those caused by wild-type PA103 (Fig. 2B and C), indicating that exotoxin A does not contribute to either of these phenotypes under the conditions of these assays.
Apoptosis of J774A.1 cells requires an intact type III secretion pathway but is independent of ExoU.
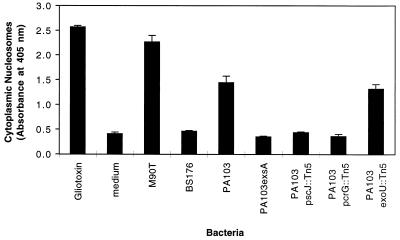
The quantitative ELISA for apoptosis was used to investigate the dependence of PA103 apoptosis upon type III secretion. PA103 was associated with significant amounts of apoptosis after infection of J774A.1 cells for 6 h (Fig. 3). In contrast, infections with PA103exsA::Ω, PA103pscJ::Tn5, and PA103pcrG::Tn5, mutants with defects in the type III secretion pathway, resulted in only background levels of apoptosis. These findings confirm that an intact type III secretion pathway is necessary for induction of apoptosis in PA103-infected J774A.1 cells. Interestingly, the exoU mutant was associated with wild-type levels of apoptosis. Thus, this putative effector protein, which is associated with necrosis of J774A.1 cells, does not play a role in the induction of apoptosis in these cells. Taken together, these results suggest that a type III secreted protein other than ExoU mediates apoptosis of J774A.1 cells infected with PA103.
FIG. 3.
Apoptosis of J774A.1 cells infected with type III secretion mutants of PA103. J774A.1 cells were infected for 6 h at an MOI of approximately 160 and then tested for apoptosis by using the quantitative ELISA for apoptosis. Bacterial mutants with type III secretion pathway defects were associated with significantly less apoptosis than wild-type PA103. Interestingly, PA103exoU::Tn5, which has an intact secretion pathway but does not secrete the effector protein ExoU, was associated with wild-type levels of apoptosis, indicating that this factor is not necessary for induction of programmed cell death. Error bars represent standard errors of the means for experiments performed in triplicate.
The dependence of J774A.1 cell apoptosis upon a type III secreted factor other than ExoU was confirmed by using Hoechst staining. Confluent J774A.1 cells were harvested, diluted, seeded onto 8-well chamber slides (approximately 2 × 104 cells/well), and grown for 20 h at 37°C in 5% CO2. Cells were washed and infected in DMEM containing 4.5 g of glucose/liter, 0.584 g of glutamine/liter, and 10% heat-inactivated fetal calf serum for 8 h at 37°C in 5% CO2 at an MOI of approximately 160. Cells were then stained for 10 min with Hoechst stain 33342 (Sigma Chemical Co.) (10 μg/ml), fixed for 30 min in formaldehyde, washed with phosphate-buffered saline, and examined by fluorescence microscopy. Cells infected with PA103exoU::Tn5 for 8 h had condensed nuclei typical of apoptotic cells and similar to those of gliotoxin-treated cells (data not shown). In contrast, cells infected with PA103exsA::Ω, a type-III-secretion-defective mutant, contained fewer condensed nuclei, similar to cells exposed to medium alone (data not shown). These findings confirmed those obtained by using the quantitative ELISA for apoptosis and suggested that a type III secreted factor other than ExoU caused apoptosis of J774A.1 cells. The identity of this second cytotoxic factor (or factors) is unknown, although in the case of PA103 it is not ExoS or ExoY, since this strain does not produce either of these proteins (8, 39).
Phagocytosis of P. aeruginosa by macrophages is not necessary for induction of apoptosis.
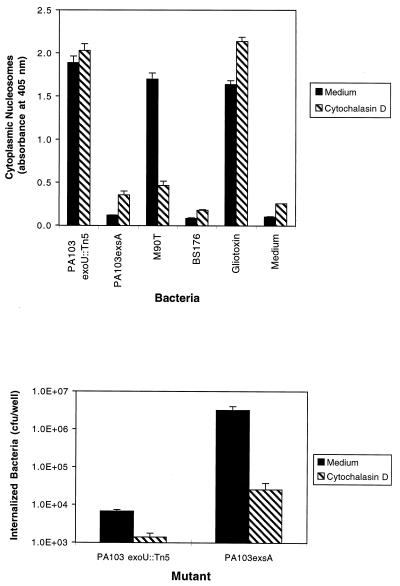
The results outlined above suggested that the type III secretion system of P. aeruginosa transports a factor that causes apoptosis of J774A.1 cells. This factor may be secreted by extracellular bacteria bound to the surface of these macrophage-like cells or by internalized bacteria following phagocytosis. Cytochalasin D, an inhibitor of actin polymerization (35), was used to block phagocytosis of bacteria in experiments designed to distinguish between these two possibilities. J774A.1 cells were pretreated with 5 μg of cytochalasin D (Sigma Co.) per ml for 30 min and then infected with bacteria in the presence of the same concentration of cytochalasin D for 6 h (Fig. 4A). Since only internalized S. flexneri cells cause type-III-secretion-mediated apoptosis (41), these bacteria were used as controls. As expected, the wild-type S. flexneri strain M90T induced significant amounts of apoptosis in J774A.1 cells in the absence but not in the presence of cytochalasin D. BS176, an S. flexneri strain that lacks a type III secretion system, did not cause significant levels of programmed cell death in the presence or the absence of cytochalasin D. In contrast, PA103exoU::Tn5 induced nearly equal amounts of apoptosis in the presence and absence of cytochalasin D, suggesting that extracellular P. aeruginosa cells are capable of inducing apoptosis. (PA103exoU::Tn5 was used in these experiments to avoid the confounding effect of J774A.1 cell death due to ExoU-mediated necrosis.) As expected, PA103exsA::Ω caused very little apoptosis in the presence or in the absence of cytochalasin D.
FIG. 4.
Effect of cytochalasin D on P. aeruginosa-induced apoptosis of J774A.1 cells. (A) Quantitative ELISAs for apoptosis on J774A.1 cells infected with either PA103exoU::Tn5 (an exoU mutant that induces apoptosis) or PA103exsA::Ω (a mutant with a generalized defect in type III secretion that does not induce apoptosis). Gliotoxin (5 μM), medium, and the S. flexneri strains M90T and BS176 were used as controls. Cells were infected for 6 h at an MOI of approximately 160 in the presence or absence of 5 μg of cytochalasin D/ml. The ability of M90T to induce apoptosis was significantly inhibited by the addition of cytochalasin D, since this bacterium must be internalized to cause apoptosis. In contrast, the ability of P. aeruginosa PA103exoU::Tn5 to cause apoptosis was unaffected by cytochalasin D. (B) Effect of cytochalasin D on internalization of P. aeruginosa by J774A.1 cells. The number of internalized bacteria was significantly reduced in the presence of cytochalasin D. These results suggest that, unlike S. flexneri, extracellular P. aeruginosa causes type-III-mediated apoptosis. Error bars represent standard errors of the means for experiments performed in triplicate.
Aminoglycoside-exclusion internalization assays were used to confirm that cytochalasin D significantly decreased the number of internalized bacteria without affecting levels of programmed cell death. Confluent J774A.1 cells were harvested, diluted, seeded onto 12-mm-diameter Transwell filters (Corning Costar Corp.) (approximately 4 × 104 cells/well), and grown for 20 h at 37°C in 5% CO2. Cells were then washed and pretreated for 30 min with either cytochalasin D (5 μg/ml) or medium. Infections were performed in DMEM containing 4.5 g of glucose/liter, 0.584 g of glutamine/liter, and 10% heat-inactivated fetal calf serum at 37°C in 5% CO2 for 3 h at an MOI of approximately 150 in either the presence or absence of cytochalasin D (5 μg/ml). The J774A.1 cells were then washed and incubated in medium for 2 h at 37°C in the presence of 400 μg of amikacin (Sigma Chemical Co.) per ml to kill extracellular bacteria. J774A.1 cells were then washed four times with medium and lysed by vortexing with glass beads in the presence of 0.25% Triton X-100. Lysates were diluted and plated on Luria-Bertani agar and incubated at 37°C for 17 h to quantify the number of CFU of viable internalized bacteria. Cytochalasin D inhibited the internalization of PA103exoU::Tn5 without significantly affecting the amount of apoptosis caused by these bacteria (Fig. 4). Note that PA103exsA::Ω was internalized in far greater numbers than PA103exoU::Tn5. This has previously been shown by using infected epithelial cells and may reflect the type III secretion of a factor that inhibits bacterial internalization by eukaryotic cells (8, 9, 15). This factor is thought to be secreted by PA103exoU::Tn5 but not PA103exsA::Ω, which has a generalized defect in type III secretion. Taken together, these results suggest that, as is the case with Salmonella and Yersinia (4, 24), the P. aeruginosa proapoptotic factor is effectively secreted and targeted by bacteria located on the surfaces of macrophages. This is in contrast to Shigella spp., which must be internalized to cause apoptosis (41).
Type-III-secretion-dependent apoptosis occurs in primary murine macrophages.
It is possible that J774A.1 cells, which are immortalized cells, differ from primary macrophages in such a way that they alone are susceptible to the proapoptotic effects of the PA103 type III secretion system. To examine whether PA103-induced apoptosis occurred in nonimmortalized macrophages, quantitative ELISAs for apoptosis were performed on infected primary macrophages. Murine bone-marrow-derived macrophages were obtained from 6- to 15-week-old BALB/c mice (Bantin and Kingman, Inc., Fremont, Calif.). Mice were euthanized, and marrow cells were collected from both femurs. Cells were grown for 4 days in RPMI 1640 medium supplemented with 25 mM HEPES (UCSF Cell Culture Facility), 10% heat-inactivated fetal calf serum, and 20% filtered conditioned media from L929 cell cultures. After a washing to remove nonadherent cells, fresh medium was added, and cells were grown for an additional 24 h prior to being used in assays. Macrophages were infected in vitro at an MOI of approximately 320 with PA103 or type-III-secretion-defective mutants and tested for apoptosis. As was seen with J774A.1 cells, PA103 caused significant levels of apoptosis in these cells, although longer infection times (12 to 15 h as opposed to 6 h) were required (Fig. 5). At these longer infection times, wild-type PA103 had already killed the majority of cells, most likely by ExoU-mediated necrosis, so few apoptotic cells remained (data not shown). However, experiments using PA103exoU::Tn5 clearly showed that apoptosis was occurring in these cells. As was the case for J774A.1 cells, apoptosis was dependent upon an intact type III secretion system but not ExoU. These results indicate that the type III secretion system of P. aeruginosa causes apoptosis of primary murine macrophages as well as immortalized macrophage-like cells.
FIG. 5.
Quantitative ELISAs for apoptosis of primary bone-marrow-derived macrophages infected with PA103 and isogenic type III secretion mutants. Macrophages were infected for 13 h at an approximate MOI of 320 with P. aeruginosa PA103 or isogenic mutants with type III secretion defects or with S. flexneri M90T or BS176. As was the case with J774A.1 cells, apoptosis of P. aeruginosa-infected primary macrophages required an intact type III secretion pathway but not ExoU. Samples infected with PA103 exhibited significantly less apoptosis than those infected with PA103exoU::Tn5, an isogenic mutant that did not secrete ExoU, because secretion of this protein was associated with significant necrosis over the 13-h course of the experiment. Thus, fewer cells were present at the end of the assay to show signs of apoptosis. Error bars represent standard errors of the means for experiments performed in triplicate.
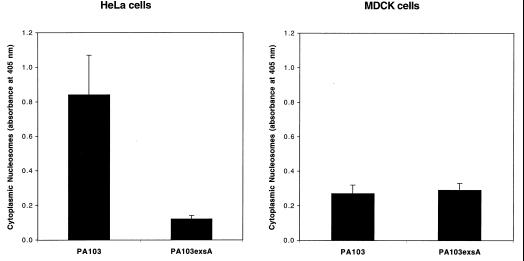
P. aeruginosa causes apoptosis of HeLa cells but not MDCK cells.
Cell types other than macrophages were also examined for susceptibility to PA103-mediated apoptosis. HeLa cells, a human epithelioid cell line, were grown and infected in DMEM with 1 g of glucose/liter, 0.584 g of glutamine/liter, and 110 mg of sodium pyruvate (UCSF Cell Culture Facility) per liter, supplemented with 5% heat-inactivated fetal calf serum. Infections were performed at an MOI of approximately 160. Quantitative ELISAs for apoptosis indicated that PA103 induced significant amounts of programmed cell death in HeLa cells (Fig. 6A). Furthermore, this killing required an intact type III secretion system, as evidenced by the absence of significant apoptosis in HeLa cells infected with PA103exsA (Fig. 6A). Not all cell types, however, were susceptible to PA103-associated apoptosis under the conditions of these assays. MDCK cells, a canine epithelial cell line, were grown and infected in MEM with Earle’s salts and 0.584 g of glutamine (UCSF Cell Culture Facility) per liter, containing 5% heat-inactivated fetal calf serum. MDCK cells were resistant to type III-mediated apoptosis following 6-h infections under the conditions of these assays (MOI of 40, Fig. 6B, and MOI of 80, data not shown). This result accords with the findings of Apodaca et al. (1), who also were unable to detect apoptotic killing of MDCK cells by P. aeruginosa. Thus, susceptibility to type-III-secretion-mediated apoptosis may vary with cell type but is not limited to macrophages.
FIG. 6.
Quantitative ELISAs for apoptosis of HeLa cells (A) and MDCK cells (B) infected with wild-type PA103 or PA103exsA::Ω, a mutant with a generalized defect in type III secretion. HeLa cells were infected for 6 h at an MOI of approximately 160. MDCK cells were infected for 6 h at an MOI of approximately 40. HeLa cells were susceptible to type III-mediated apoptosis, whereas MDCK cells were not. Error bars represent standard errors of the means for experiments performed in triplicate.
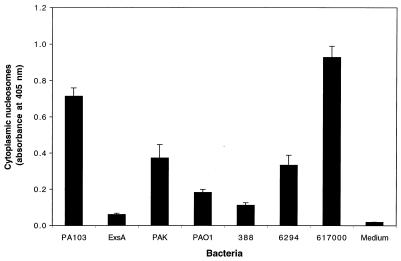
P. aeruginosa strains differ in their abilities to cause apoptosis.
Other strains of P. aeruginosa were tested for the ability to induce apoptosis to ensure that this phenotype was not unique to PA103. Laboratory strains (PAK, PAO1, and 388), a human respiratory isolate (617000), and a human corneal isolate (6294) were used to infect J774A.1 cells for 6 h. The cells were then examined for evidence of apoptosis by using quantitative ELISAs (Fig. 7). The strains differed markedly in their abilities to induce programmed cell death. Strain 617000 caused higher levels of apoptosis than PA103, while other strains, such as 388, were associated with levels only slightly greater than those induced by PA103exsA::Ω, the mutant with a generalized defect in type III secretion. These results indicated that the apoptosis induction phenotype is not unique to strain PA103 and that it may be a variable trait among P. aeruginosa strains and isolates. Whether these strains cause apoptosis by a type-III-secretion-mediated mechanism cannot be determined from the results of these experiments.
FIG. 7.
Quantitative ELISAs for apoptosis of J774A.1 cells infected with one of several different P. aeruginosa strains. PAK, PAO1, and 388 are commonly used laboratory strains. 6294 is a hyperinvasive corneal isolate, and 617000 is a respiratory isolate. These strains were used to infect J774A.1 cells for 6 h at an MOI of approximately 160, after which cells were tested for evidence of apoptosis by using quantitative ELISAs. Strains varied markedly in their abilities to induce programmed cell death. Error bars represent standard errors of the means for experiments performed in triplicate.
Taken together, these findings indicate that P. aeruginosa joins a growing list of bacteria, including Yersinia, Salmonella, and Shigella spp., that kill macrophages by a type-III-secretion-mediated mechanism. This is not surprising given the important role that macrophages play in the host immune response. Neutralization of these cells may allow bacterial pathogens such as P. aeruginosa to persist or disseminate. Although other explanations are possible, the results presented here are consistent with a model in which the P. aeruginosa type III secretion system transports distinct factors that kill macrophages by different mechanisms: apoptosis or necrosis. The secretion of multiple factors that damage macrophages may explain why this pathogen so frequently causes severe infections in hosts with preexisting defects in other arms of the immune system, such as neutropenic patients (31). A better understanding of the different mechanisms used by P. aeruginosa to subvert host cells will likely lead to new therapeutic interventions designed to block these processes.
Acknowledgments
We thank members of the Engel laboratory for reading the manuscript and for scientific advice. We acknowledge Elizabeth Prescott and Sidong Huang for help with some experiments. We also thank Arturo Zychlinsky for kindly providing the Shigella strains and Ben Kelly for help in harvesting bone-marrow-derived macrophages.
This work was supported by grants from the University-Wide AIDS Research Program (J.N.E.), the NIH (J.N.E. [R01 AI42806] and A.R.H. [K08 AI001524]), the American Lung Association (J.N.E.), and the Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation Fellowship (A.R.H.). J.N.E. is a career investigator of the American Lung Association.
REFERENCES
- 1.Apodaca G, Bomsel M, Lindstedt R, Engel J, Frank D, Mostov K, Wiener-Kronish J. Characterization of Pseudomonas aeruginosa-induced MDCK cell injury: glycosylation defective host cells are resistant to bacterial killing. Infect Immun. 1995;63:1541–1551. doi: 10.1128/iai.63.4.1541-1551.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjorn M J, Pavlovskis O R, Thompson M R, Iglewski B H. Production of exoenzyme S during Pseudomonas aeruginosa infection in burned mice. Infect Immun. 1979;24:837–842. doi: 10.1128/iai.24.3.837-842.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buja L M, Eigenbrodt M L, Eigenbrodt E H. Apoptosis and necrosis: basic types and mechanisms of cell death. Arch Pathol Lab Med. 1993;117:1208–1214. [PubMed] [Google Scholar]
- 4.Chen L M, Kone K, Galan J E. Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol. 1996;21:1101–1115. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- 5.Coburn J, Frank D. Macrophages and epithelial cells respond differently to the Pseudomonas aeruginosa type III secretion system. Infect Immun. 1999;67:3151–3154. doi: 10.1128/iai.67.6.3151-3154.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finck-Barbancon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish J P, Fleiszig S M J, Wu C, Mende-Mueller L, Frank D. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 7.Finck-Barbancon V, Yahr T L, Frank D W. Identification and characterization of SpcU, a chaperone required for efficient secretion of the ExoU cytotoxin. J Bacteriol. 1998;180:6224–6231. doi: 10.1128/jb.180.23.6224-6231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleiszig S M J, Wiener-Kronish J P, Miyazaki H, Vallas V, Mostov K, Kanada D, Sawa T, Yen T S B, Frank D. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65:579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleiszig S M J, Zaidi T S, Preston M J, Grout M, Evans D J, Pier G B. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect Immun. 1996;64:2288–2294. doi: 10.1128/iai.64.6.2288-2294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank D. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol Microbiol. 1997;26:621–629. doi: 10.1046/j.1365-2958.1997.6251991.x. [DOI] [PubMed] [Google Scholar]
- 11.Frank D W, Nair G, Schweizer H. Construction and characterization of chromosomal insertional mutations of the Pseudomonas aeruginosa exoenzyme S trans-regulatory locus. Infect Immun. 1994;62:554–563. doi: 10.1128/iai.62.2.554-563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frithz-Lindsten E, Du Y, Rosqvist R, Forsberg A. Intracellular targeting of exoenzyme S of Pseudomonas aeruginosa via type III-dependent translocation induces phagocytosis resistance, cytotoxicity and disruption of actin microfilaments. Mol Microbiol. 1997;25:1125–1139. doi: 10.1046/j.1365-2958.1997.5411905.x. [DOI] [PubMed] [Google Scholar]
- 13.Guichon A, Zychlinsky A. Clinical isolates of Shigella species induce apoptosis in macrophages. J Infect Dis. 1997;175:470–473. doi: 10.1093/infdis/175.2.470. [DOI] [PubMed] [Google Scholar]
- 14.Hauser A R, Kang P J, Engel J. PepA, a novel secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol Microbiol. 1998;27:807–818. doi: 10.1046/j.1365-2958.1998.00727.x. [DOI] [PubMed] [Google Scholar]
- 15.Hauser A R, Kang P J, Fleiszig S J M, Mostov K, Engel J. Defects in type III secretion correlate with internalization of Pseudomonas aeruginosa by epithelial cells. Infect Immun. 1998;66:1413–1420. doi: 10.1128/iai.66.4.1413-1420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hersh D, Monack D M, Smith M R, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding caspase-1. Proc Natl Acad Sci USA. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holloway B W, Krishnapillai V, Morgan A F. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979;43:73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iglewski B H, Sadoff J, Bjorn M J, Maxwell E S. Pseudomonas aeruginosa exoenzyme S: an adenosine diphosphate ribosyltransferase distinct from toxin A. Proc Natl Acad Sci USA. 1978;75:3211–3215. doi: 10.1073/pnas.75.7.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Islam D, Veress B, Bardhan P K, Lindberg A A, Christensson B. In situ characterization of inflammatory responses in the rectal mucosae of patients with shigellosis. Infect Immun. 1997;65:739–749. doi: 10.1128/iai.65.2.739-749.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang P J, Hauser A R, Apodaca G, Fleiszig S, Wiener-Kronish J, Mostov K, Engel J N. Identification of Pseudomonas aeruginosa genes required for epithelial cell injury. Mol Microbiol. 1997;24:1249–1262. doi: 10.1046/j.1365-2958.1997.4311793.x. [DOI] [PubMed] [Google Scholar]
- 21.Kudoh I, Wiener-Kronish J P, Hashimoto S, Pittet J-F, Frank D. Exoproduct secretions of P. aeruginosa strains influence severity of alveolar epithelial injury. Am J Physiol. 1994;267(5 Pt. 1):L551–L556. doi: 10.1152/ajplung.1994.267.5.L551. [DOI] [PubMed] [Google Scholar]
- 22.Liu P V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. II. Effects of lecithinase and protease. J Infect Dis. 1966;116:112–116. doi: 10.1093/infdis/116.1.112. [DOI] [PubMed] [Google Scholar]
- 23.Majno G, Joris I. Apoptosis, oncosis, and necrosis: an overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 24.Mills S D, Boland A, Sory M-P, van der Smissen P, Kerbourch C, Finlay B B, Cornelis G R. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monack D M, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morimoto H, Bonavida B. Diphtheria toxin and Pseudomonas A toxin-mediated apoptosis: ADP-ribosylation of elongation factor-2 is required for DNA fragmentation and cell lysis and synergy with tumor necrosis factor-α. J Immunol. 1992;149:2089–2094. [PubMed] [Google Scholar]
- 27.Olson J C, McGuffie E M, Frank D W. Effects of differential expression of the 49-kilodalton exoenzyme S by Pseudomonas aeruginosa on cultured eukaryotic cells. Infect Immun. 1997;65:248–256. doi: 10.1128/iai.65.1.248-256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pahl H L, Krauss B, Schulze-Osthoff K, Decker T, Traenckner E B-M, Vogt M, Myers C, Parks T, Warring P, Muhlbacher A, Czernilofsky A-P, Baeuerle P. The immunosuppressive fungal metabolite gliotoxin specifically inhibits transcription factor NF-κB. J Exp Med. 1996;183:1829–1840. doi: 10.1084/jem.183.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmer L E, Hobbie S, Galan J E, Bliska J B. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNR-alpha production and downregulation of the MAP kinases p38 and JNK. Mol Microbiol. 1998;27:953–965. doi: 10.1046/j.1365-2958.1998.00740.x. [DOI] [PubMed] [Google Scholar]
- 30.Pederson K J, Barbieri J T. Intracellular expression of the ADP-ribosyltransferase domain of Pseudomonas aeruginosa exoenzyme S is cytotoxic to eukaryotic cells. Mol Microbiol. 1998;30:751–759. doi: 10.1046/j.1365-2958.1998.01106.x. [DOI] [PubMed] [Google Scholar]
- 31.Pollack M. Infections due to Pseudomonas species and related organisms. In: Fauci A S, Braunwald E, Isselbacher K J, Wilson J D, Martin J B, Kasper D L, Hauser S L, Longo D L, editors. Harrison’s principles of internal medicine. 14th ed. New York, N.Y: McGraw-Hill; 1998. pp. 943–950. [Google Scholar]
- 32.Salyers A A, Whitt D D. Bacterial pathogenesis: a molecular approach. Washington, D.C: ASM Press; 1994. [Google Scholar]
- 33.Sawa T, Yahr T, Ohara M, Kurahashi K, Gropper M A, Wiener-Kronish J P, Frank D W. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat Med. 1999;5:392–398. doi: 10.1038/7391. [DOI] [PubMed] [Google Scholar]
- 34.Schesser K, Spiik A-K, Dukuzumuremyi J-M, Neurath M F, Petersson S, Wolf-Watz H. The yopJ locus is required for Yersinia-mediated inhibition of NF-κB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol Microbiol. 1998;28:1067–1079. doi: 10.1046/j.1365-2958.1998.00851.x. [DOI] [PubMed] [Google Scholar]
- 35.Tanenbaum S W. Cytochalasin: biochemical and cell biological aspects. Amsterdam, The Netherlands: North-Holland; 1978. [Google Scholar]
- 36.Waring P, Eichner R D, Mullbacher A, Sjaarda A. Gliotoxin induces apoptosis in macrophages unrelated to its antiphagocytic properties. J Biol Chem. 1988;263:18493–18499. [PubMed] [Google Scholar]
- 37.Whitchurch C B, Mattick J S. Characterization of a gene, pilU, required for twitching motility but not phage sensitivity in Pseudomonas aeruginosa. Mol Microbiol. 1994;13:1079–1081. doi: 10.1111/j.1365-2958.1994.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 38.Yahr T L, Barbieri J T, Frank D W. Genetic relationship between the 53- and 49-kilodalton forms of exoenzyme S from Pseudomonas aeruginosa. J Bacteriol. 1996;178:1412–1419. doi: 10.1128/jb.178.5.1412-1419.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yahr T L, Vallis A J, Hancock M K, Barbieri J T, Frank D W. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci USA. 1998;95:13899–13904. doi: 10.1073/pnas.95.23.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zychlinsky A, Kenny B, Menard R, Prevost M-C, Holland I B, Sansonetti P J. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol Microbiol. 1994;11:619–627. doi: 10.1111/j.1365-2958.1994.tb00341.x. [DOI] [PubMed] [Google Scholar]
- 41.Zychlinsky A, Prevost M-C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 42.Zychlinsky A, Thirumalai K, Arondel J, Cantey J R, Aliprantis A O, Sansonetti P J. In vivo apoptosis in Shigella flexneri infections. Infect Immun. 1996;64:5357–5365. doi: 10.1128/iai.64.12.5357-5365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]