Abstract
Chemerin is a hormone produced mainly by adipose tissue and liver. We have recently shown that it is locally produced in the reproductive tract in hens, particularly at the magnum level, leading to its accumulation in the egg albumen. We have also determined that chemerin is necessary for egg fertilization, embryo development, and angiogenesis within the chorio-allantoic membrane in chicken species. We, therefore, hypothesize that chemerin, widely present in various gallinacean species, could be a marker of egg fertility in this animal order. To demonstrate this, we used a model close to the hen: the pheasant. By RT-qPCR, we have shown that chemerin and its three receptors CMKLR1, GPR1, and CCRL2 are expressed in the reproductive tract of females. In addition, chemerin is also produced predominantly in the magnum and accumulates in the egg albumen as determined by immunoblot. We then compared two lines of pheasants with different reproductive characteristics: the F11 and F22 breeds. F22 lays more eggs than F11, but have significantly lower fertility and hatchability rates. In addition, F22 exhibit a significantly lower amount of chemerin protein in their magnum (P < 0.01) and in the egg albumen (P < 0.0001) compared to F11. Finally, we observed a positive correlation between the chemerin amount in the albumen of F11 eggs and the hatching rate of the eggs (r = 0.5; P = 0.04) as well as a negative correlation between the chemerin quantity in the albumen of F22 eggs and the rate of unfertilized eggs (r = −0.37; P = 0.04). Finally, chemerin system (ligand and receptors) is also expressed within embryo annexes (chorioallantoic and amniotic membranes) during incubation. These data demonstrate an interspecies conservation of chemerin production in the magnum, its accumulation in the egg albumen and its possible use as a marker for determining the quality of eggs in term of fertility and embryo development.
Key words: pheasant, chemerin, female reproduction, oviduct, egg albumen
INTRODUCTION
Humans have been raising and domesticating animals for thousands of years and this anthropic intervention has obvious effects on domestic species evolution compare to their wild ancestors. Domestication and breeding of wild species have also a huge impact on animals and notably on their biological traits such as metabolism and reproduction, compare to their wild counterparts (Pérez-Enciso et al., 2017; Nynca et al., 2020; van der Horst and Maree, 2022). The link between these two last physiological characteristics is very narrow and it is well known that they can interact bilaterally, like in birds species (Estienne et al., 2020; Bernardi et al., 2021). Liver and adipose tissue are endocrine organs able to produce hormones that influence physiological parameters such as reproduction (Mattern et al., 2014). In mammals, chemerin is one of these hormones synthetized by these 2 organs and this adipo/hepatokine is also involved not only in reproduction but also in inflammation, angiogenesis, homeostasis, immune system (Sleeman et al., 2000; Bozaoglu et al., 2007; Mattern et al., 2014). Its synthesis is quite ubiquitous but local production is variable according to the considered organ or tissue (Rourke et al., 2013; Kennedy and Davenport, 2018). In mammals, pre-pro-chemerin is submitted to an enzymatic truncation of its 20 amino acids signal peptide and then is released within the extracellular compartment as an inactive pro-chemerin constituted of 143 amino acids (Zabel et al., 2005). This pro-chemerin is then hydrolyzed by the enzymatic cleavage of 5 to 7 amino acids from its carboxyl end in the extracellular compartment. Two neutrophil serine proteases, elastase and cathepsin G, eliminate 6 and 7 amino acids, respectively, to generate an active form (Mattern et al., 2014). Plasmin and tryptase are also able to cleave 5 amino acids from the carboxyl end after cleavage of the carboxy-terminal lysine by carboxypeptidases N and B, which also lead to active chemerin (Bondue et al., 2011). In rodent, different tissues, specific isoforms of chemerin have been identified depending on proteases that make the cleavage (Helfer and Wu, 2018). Chemerin exerts its main biological functions through binding to its main G protein-coupled receptor chemokine-like receptor 1 (CMKLR1)(Gantz et al., 1996). This adipokine is also able to bind 2 others receptors which are GPR1 (G protein coupled receptor 1) and CCRL2 (CC motif chemokine receptor like 2)(Marchese et al., 1994; Shimada et al., 1998).
In birds, few data are available about chemerin's role in metabolism and reproduction. In turkeys, chemerin mRNA is mainly found in the liver compared to the heart and muscles, while CMKLR1 and GPR1 mRNAs are ubiquitous (Diot et al., 2015). The messenger of CCRL2 is strongly present in the pectoralis muscle compared to the liver, heart, and leg muscle. Chicken chemerin, CMKLR1, and GPR1 have an amino acid identity of 36, 56, and 64% with human chemerin and its receptors, respectively. These results suggest that the chemerin system could act on the avian carbohydrate and lipid metabolism. At the metabolic level, a recent finding from our laboratory demonstrates that chemerin could be a peripheral appetite-regulating signal through modulation of hypothalamic peptides expression in chicken (Estienne et al., 2021), like leptin in mammals. Moreover, this hormone is also involved in bird reproduction. In males chicken, our laboratory determined that chemerin could negatively influence steroidogenesis and sperm motility and so impair eggs fertility rate (Estienne et al. 2020). In female, chemerin concentrations in plasma are negatively correlated with egg hatchability, suggesting a potential role of this adipokine on egg (Mellouk et al., 2018). In turkey, chemerin is expressed by granulosa cells and increases in the mature follicle before ovulation, thus, potentially influencing the Early Embryonic Development (EED)(Diot et al., 2015). Finally, our last results in chicken demonstrated that chemerin is locally secreted by the magnum of the hen reproductive tract and accumulates in egg albumen where it could positively influence embryo development through the promotion of angiogenesis and antibacterial defenses (Estienne et al., 2022). Chemerin homology between chicken (Gallus gallus domesticus) and pheasant (Phasianus colchicus), these 2 species belonging to the gallinacean family, is very high with 98% of identity. Having developed molecular tools (antibodies and recombinant proteins) specifically for the study of the chicken (Mellouk et al., 2018), a species of agronomical interest, we were able to conduct similar investigations in different pheasant breeds. Indeed, we took advantages of that the pheasant is a game species widely bred in the world and that its fine selection by breeding companies, depending on its behavior or reproductive abilities, has led to the appearance of different breeds with specific characteristics. Actually, even in this wild species, metabolism and nutrition can have a huge influence on reproduction in female (Kullu et al., 2017; Gugała et al., 2019). We have to our disposal two pheasant breeds from the same species (Phasianus Colchicus), showing different phenotypes and reproductive abilities. Our hypothesis is that domestication and selection, based on reproductive performances, have an impact on chemerin expression and that this adipokine could play a role in fertility and embryo development in pheasants, like in chicken. So, the aim of this study was to investigate the effect of domestic selection on reproductive parameters, linked to chemerin expression within the female reproductive tract, its accumulation in eggs and its potential link with fertility parameters including on embryo development in these two pheasant breeds.
MATERIALS AND METHODS
Ethical Issues
All Experiments have been conducted in accordance with the principles and specific ARRIVE guidelines. Plasma and tissues have been collected during meat processing as abattoir by-products by highly qualified and experienced laboratory staff. Thus, according to the ethical issues for the protection of animals, this project does not require the consent of the competent ethics committee for animal experiments.
Animals
Fifteen pheasant hens of F11 breed and 15 pheasant hens of F22 breed (Phasianus colchicus) from Gibo'Vendée (Chambretaud, France) were studied during their first laying period in 2019 and 2020 (total n = 30, n = 15 F11 and n = 15 F12 each year). Both breeds originate from the wild pheasant (Phasianus colchicus) species; The F11 breed was selected for its ability to gain weight and obtain heavier animals, while the F22 breed was selected for its ability to lay more eggs per female compared to its wild counterparts. The two strains diverged in the early 1980s. For all analyzed parameters, we did not observe any significant effect between the 2 yr studied, so we pooled data for 2019 and 2020 for each breed (F11 and F22). The animals were reared at Gibo'Vendée breeding facilities (Chambretaud, France) according to the traditional conditions of breeding: 8 h of light per day on arrival in November, followed by a gradual increase until reaching 14 h of light per day at the time of laying at the end of January and for 20 wk. All data about reproductive parameters were recorded and provided by the breeder (Gibo'Vendée, Chambretaud, France).
Fertility Parameters
As previously described (Mellouk et al., 2018; Barbe et al., 2020), the different percentages (hatchability, hatchability of fertile eggs, and fertility) were calculated using the following formula:
Biological Samples
During the laying period, eggs were collected daily and stored in a room at 15 to 16°C and 80 to 85% humidity for one week before sample collection. Around 1 mL of albumen was collected per egg and stored at −20°C before use. At the end of the reproduction period, certain animals intended for sale as meat were selected for the study and killed by electrical stunning and bled out as recommended by the ethical committee. Blood samples were collected after the killing into heparin tubes and plasma was recovered after centrifugation (5,000 g for 10 min at 4°C) and then stored at −20°C until use. Blood amounts of chemerin (n = 60) were analyzed by western blotting. Reproductive tracts samples have been collected (infundibulum, magnum, isthmus, shell gland, and vagina) by dissection after animal slaughter. All the samples have been stored at −80°C before use.
mRNA Expression of Chemerin and Its Receptors in Oviduct
Total RNA was extracted from the infundibulum, magnum, isthmus, shell gland, and vagina (6 animals per stage) by homogenization of 1mg of tissue in the lysis buffer reagent of a total RNA extraction kit according to the manufacturer's recommendations (NucleoSpin RNAMacherey-Nagel). The cDNA was generated by reverse transcription (RT) of total RNA (1 μg) in a mixture comprising 0.5 mM of each deoxyribonucleotide triphosphate (dATP, dGTP, dCTP, and DTTP), 2 M of RT buffer, 15 μg/μL of oligodT, 0.125 U of ribonuclease inhibitor, and 0.05 U of Moloney murine leukemia virus reverse transcriptase (MMLV) for 1 h at 37°C. Real-time PCR was performed using the MyiQ Cycle device (Bio-Rad, Marnes-la-Coquette, France), in a mixture containing SYBR Green Supermix 1X reagent (Bio-Rad), 250 nM specific primers (Invitrogen by Life Technologies, Villebon sur Yvette, France) (Table 1) and 5 μL of cDNA (diluted 5-fold) for a total volume of 20 μL. The samples were duplicated on the same plate and the following PCR procedure used: after an incubation of 2 min at 50°C and a denaturation step of 10 min at 95°C, samples were subjected to 40 cycles (30 s at 95°C, 30 s at 60°C and 30 s at 72°C). The levels of expression of messenger RNA were standardized to the geometric mean of three reference genes (EEF1, GAPDH, and βActin). The use of the geometric mean of several reference genes has already been validated (Vandesompele et al., 2002). For each gene (Chemerin, CMKLR1, GPR1, and CCRL2), expression was calculated according to primer efficiency (E) and quantification cycle (Cq), where expression = E-Cq. Then, relative expression of the target gene to the 3 reference genes was analyzed.
Table 1.
List of primers used for qRT-PCR.
| Gene | Primer forward | Primer reverse | Efficiency |
|---|---|---|---|
| RARRES2 | CGCGTGGTGAAGGATGTG | CGACTGCTCCCTAAAGAGAACT | 1.90 |
| CMKLR1 | CGGTCAACGCCATTTGGT | GGGTAGGAAGATGTTGAAGGAA | 1.95 |
| GPR1 | ACCTGCCTGAGGAAGAAGAA | AAAGGCCAGTGGAAGCCCAT | 2.00 |
| CCRL2 | CACGCAGTGTTTGCTTTAAAAGC | CAACAGCCCACGTGACAATG | 1.92 |
| GAPDH | TGCTGCCCAGAACATCATCC | ATCAGCAGCAGCCTTCACTACC | 1.98 |
| EEF1a | AGCAGACTTTGTGACCTTGCC | TGACATGAGACAGACGGTTGC | 1.95 |
| B ACTIN | ACGGAACCACAGTTTATCATC | GTCCCAGTCTTCAACTATACC | 2.02 |
Western Blot
Infundibulum, magnum, isthmus, shell gland, and vagina tissues from 6 animals from the F11 breed and 12 magnum samples from the F11 and the F22 breeds were lysed using an Ultraturax (Invitrogen by Life Technologies, Villebon sur Yvette, France) in lysis buffer. The lysates were centrifuged for 20 min at 17,000 g at 4°C and the supernatant containing proteins was collected and kept on ice. The protein concentration was measured using the bicinchoninic acid (BCA) protein assay (Interchim, Montluçon, France). Lysate protein (60 μg) was mixed with Laemmli buffer 5 X and proteins were denatured for 5 min by heat shock at 95°C. Proteins were loaded in an electrophoresis sodium dodecyl sulfate-polyacrylamide gel (15% for low protein weight [<20 kDa]). Then, proteins were transferred to a nitrocellulose membrane. Membranes were blocked with Tris-Buffered Saline Tween buffer containing 0.05% of Tween 20 and 5% of milk for 30 min at room temperature. Membranes were incubated overnight at 4°C with the appropriate primary antibody. Then, membranes were incubated 90 min at room temperature with a Horse Radish Peroxidase-conjugated anti-rabbit or anti-mouse IgG. Proteins of interest were detected by enhanced chemiluminescence (Western Lightning Plus-ECL, Perkin Elmer, Villebon-sur-Yvette, France) with a G-box SynGene (Ozyme, St Quentin en Yvelines, France) and GeneSnap software (Ozyme). Then, proteins were quantified with GeneTools software. The results were expressed as the intensity signal in arbitrary units after normalization.
Immunodetection of Chemerin System Within the Allantoic and Amniotic Cells
Pheasant eggs were conventionally incubated at the laboratory (INRAe, Centre Val de Loire, Nouzilly, France) according to the following protocol. Eggs were stored in a room at 15 to 16°C and 80 to 85% humidity for 1 wk. Then, all eggs were placed in alternative rows on each shelf of the incubator. They were maintained at 37.8°C and 56% relative humidity and automatically turned every hour. At d 7 of incubation, all eggs were candled, and infertile eggs, along with dead embryo eggs, were eliminated. Five fertile eggs were retrieved at 11 (ED11) days of incubation. Embryos were taken and sacrificed by decapitation, and the annexes were immediately sampled (amniotic membrane, chorioallantoic membrane) by dissection. After collection, tissues were washed three times in PBS and then digested with collagenase A 0.3% (Roche Diagnostic, Mannheim, Germany) for 30 min in a warm bath at 37°C. To remove most of the red blood cells, the pellet was centrifuged (400 g, 20 min) on a 2-layer discontinuous Percoll gradient (40%, 60% in Ham medium, GibcoBRL; Life Technologies, Cergy Pontoise, France). The 40% fraction was collected and washed with fresh Dulbecco's Modified Eagle Medium (DMEM), cells were counted in a hemocytometer, and cell viability was determined using Trypan Blue dye exclusion (Sigma). Cells were cultured in DMEM medium supplemented with 20 mmol/L Hepes, penicillin (100 U/mL), streptomycin (100 mg/L), and L-glutamine (3 mmol/L). The cells were cultured for 24 h under a water-saturated atmosphere of 95% air, 5% CO2 at 37°C in Lab-Tek chamber slides (ThermoFisher, Courtaboeuf, France) at 1 × 105 cells/well in 0.5 mL. After platting, cells were washed tree times with PBS and fixed in 10% formalin. Antigen retrieval was performed by steaming the labtecks in a microwave in citrate buffer (0.01 M) with a pH of 6.0 for 5 min, then cooling for 20 min after two 5-min washes in PBS. Labtecks were incubated at 4°C overnight with the rabbit anti-chicken chemerin, CMKLR1, GPR1, and CCRL2 antibodies diluted 1:200 in PBS-Bovine Serum Albumin (BSA). After washes in PBS–0.05% Tween 20 and PBS, slides were incubated in goat anti-rabbit Alexa 488 (diluted at 1:500 in PBS for 1 h). After 3 to 5-min washes in PBS, labteks were mounted with Vectashield. Negative controls were performed by replacing antibody with rabbit IgG (Sigma-Aldrich, l'Isle d'Abeau Chesnes, France).
Source of Antibodies
Monoclonal chicken chemerin antibodies and polyclonal chicken CMKLR1 and GPR1 were produced by AgroBio (La Ferté Saint Aubin, France) and their specificities were tested as previously described by (Mellouk et al., 2018) and (Estienne et al., 2022). Mouse monoclonal antibodies to vinculin (hVIN-1) and polyclonal CCRL2 antibodies (Sigma [SAB2100371]) was obtained from Sigma. All antibodies were used at 1/1,000 dilution in western blotting.
Statistical Analysis
The GraphPad Prism software (version 8) was used for all analyses. All data are reported as means ± standard error of mean (SEM). We performed one t test or one-way analysis of variance (ANOVA) to compare the different means, when appropriates. When the ANOVA indicated significant effects, the means were analyzed by using the Fisher's test. The relationships between quantitative parameters (chemerin protein amounts vs. reproductive parameters) were investigated by Pearson's correlation analysis.
RESULTS
Characteristic of Pheasant Hens and Reproductive Parameters
Morphometric data of F11 (n = 30) and F22 (n = 30) pheasant hens and measures obtained on their respective eggs are resumed in Table 2. We did not find any significant differences between the two breeds for the body weight of females, the weight of eggs, albumen and yolk, and the albumen / yolk ratio. However, we found a significantly higher number of eggs per female during the laying period in breed F22 compared to breed F11 (P = 0.04). Furthermore, focusing on reproductive parameters, we observed a significantly higher fertility rate (P = 0.04), hatchability rate (P = 0.04) and hatched / incubated eggs rate (P = 0.03) in the F11 breed compared to the F22 breed. When we focused on chemerin concentration within the blood plasma, we didn't find any significant difference between the 2 breeds (Table 2).
Table 2.
Phenotypic and reproductive parameters for F11 and F22 female pheasants.
| Parameter | F11 breed | F22 breed |
|---|---|---|
| Female body weight (g) (n = 30) | 1467 ± 36.93 | 1422 ± 37.41 |
| Egg weight (g) (n = 60) | 30.50 ± 0.44 | 29.87 ± 0.33 |
| Albumen weight (g) (n = 60) | 15.00 ± 0.33 | 14.84 ± 0.24 |
| Yolk weight (g) (n = 60) | 10.83 ± 0.18 | 10.63 ± 0.14 |
| Ratio albumen/yolk (n = 60) | 1.39 ± 0.03 | 1.41 ± 0.03 |
| Egg number per female | 84.79 ± 2.45 | 90.92 ± 1.44* (P = 0.04) |
| Fertility rate (%) | 88.35 ± 0.77 | 85.47 ± 1.13* (P = 0.04) |
| Hatchability rate (%) | 82.30 ± 1.77 | 77.60 ± 1.33* (P = 0.04) |
| Hatched/incubated eggs rate (%) | 72.66 ± 1.31 | 65.58 ± 1.25* (P = 0.03) |
| Plasma chemerin (n = 30) | 0.009 ± 0.0008 | 0.010 ± 0.0008 |
The body weight of female pheasants, their total eggs, the yolk, and the albumen were weighted. The ratio between albumen and yolk weight was calculated.
The total number of eggs laid by female for the studied reproductive season was determined. The percentage of fertilized eggs, the hatchability of total eggs and the ratio between hatched and incubated eggs were calculated.
Plasma chemerin amounts were determined by western blotting.
Results are presented as means ± S.E.M.
P-values of the effect of the breed (F11 or F22) were considered as significant if P < 0.05 (t test) and asterisks * indicate significant differences.
Chemerin System Within the Reproductive Tract and Eggs
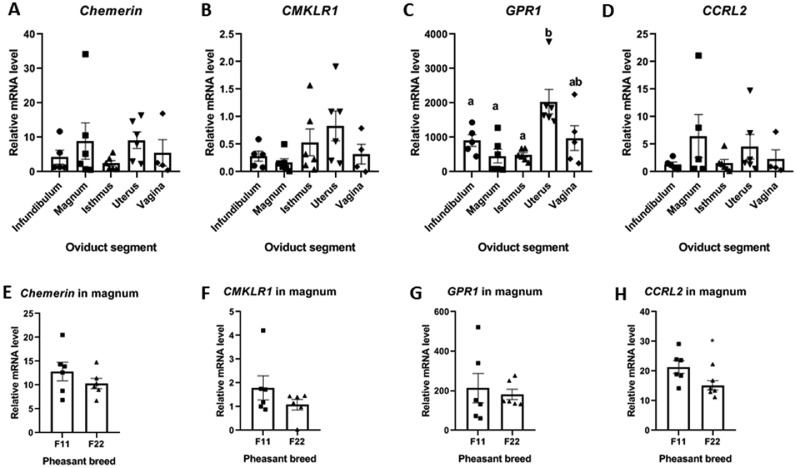
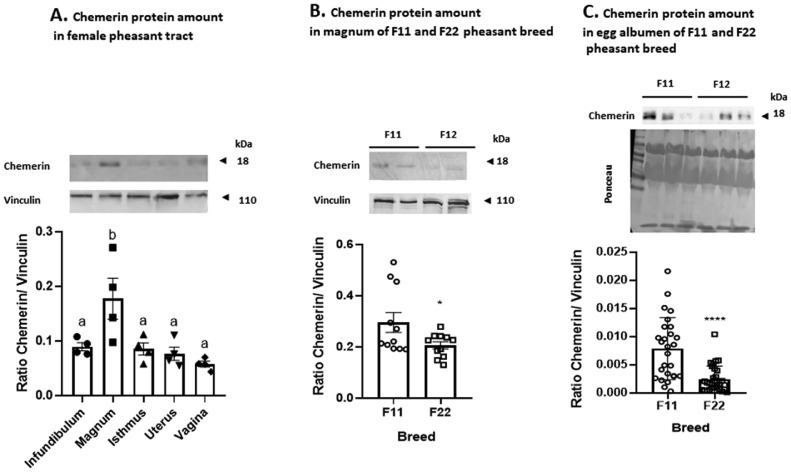
By qPCR analysis in F11 breed only, we found that chemerin is expressed by the different parts of the reproductive tract in female pheasants, without significant variations between the infundibulum, the magnum, the isthmus, the shell gland, and the vagina (Figure 1A). Similar results were obtained for CMKLR1 (Figure 1B) and CCRL2 (Figure 1D). However, results showed a significant increase of GPR1 expression within the shell gland compare to the other parts of the reproductive tract (Figure 1C). Then, we compared the expression of the very same genes between the magnums of the F11 and the F22 breeds. Here again, we didn't find any difference of expression between the F11 and the F22 magnums for chemerin, CMKLR1, GPR1, and CCRL2 (Figures 1E–1H). However, when focusing on chemerin protein expression, western blotting analysis highlighted a significant increase of the protein amount in the magnum compared to the other parts of the reproductive tract (Figure 2A). We decided to go further by comparing the protein amount of chemerin within the magnum of the breeds and found that F22 pheasant hens exhibit significantly less chemerin within their magnum compared to the F11 hens (Figure 2B). We also obtained a similar result for the amount of chemerin in the albumen of the eggs from the 2 breeds with a reduced chemerin amount in the albumen of eggs from F22 hens compared to the F11 hens (Figure 2C).
Figure 1.
The chemerin system is expressed within the female pheasant reproductive tract. A total of 6 pheasant hens were randomly selected during the laying period and euthanized to collect samples of their oviducts. (A) Chemerin, (B) CMKLR1, (C) GPR1, and (D) CCRL2 relative expression in the different parts of the oviduct of hens (infundibulum, magnum, isthmus, shell gland, and vagina) quantified by RT-qPCR (n = 6). (E) Chemerin, (F) CMKLR1, (G) GPR1, and (H) CCRL2 relative expression in the magnum of F11 (n = 6) and F22 (n = 6) pheasant hens quantified by RT-qPCR (n = 6). Values are expressed as mean ± standard errors of means. Different letters indicate significant differences at P < 0.05, and * indicates significant differences between the F11 and F22 breeds at P < 0.05.
Figure 2.
Chemerin is mostly secreted by the magnum of female pheasants, and differentially expressed by the F11 and the F22 breeds magnums leading to different amounts of the protein within the albumen of eggs. A total of 6 pheasant F11 and F22 hens were randomly selected during the laying period and euthanized to collect samples of their oviducts. (A) Protein abundance of chemerin detected by western blotting within the infundibulum, magnum, isthmus, uterus, and vagina of F11 hens (n = 6). (B) Protein abundance of chemerin detected by western blotting within the magnum of F11 and F22 hens (n = 6 for each breeds). (C) Protein abundance of chemerin detected by western blotting within the albumen of eggs from F11 and F22 hens (n = 30 for each breeds). Values are expressed as mean ± standard errors of means. Different asterisks * indicate significant differences between the F11 and F22 breeds at P < 0.05.
Correlations Between Chemerin and Reproductive Parameters
Finally, we determined correlations between the chemerin amount in the blood plasma and in the albumen and the reproductive parameters obtained from the 2 breeds. Indeed, we found a negative correlation between blood plasma chemerin in F11 breed and the egg hatchability (r = −0.48; P < 0.05). We also found a positive correlation between the albumen chemerin in F11 breed and the rate of hatched eggs (r = 0.5; P < 0.05). In addition, we observed negative correlations between the albumen chemerin in F22 breed and the rate of infertile eggs (r = −0.37; P < 0.05; Table 3).
Table 3.
Phenotypic and reproductive parameters for F11 and F22 female pheasants.
| Unfertile egg rate (F11) | Unfertile egg rate (F22) | Hatching rate (F11) |
Hatching rate (F22) |
|
|---|---|---|---|---|
| Chemerin plasma (F11, n = 30) |
r = −0.093 P = 0.742 |
- |
r = −0.48 P = 0.033* |
- |
| Chemerin plasma (F22, n = 30) |
- |
r = −0.375 P = 0.927 |
- | r = 0.113 P = 0.218 |
| Chemerin albumen (F11, n = 30) | r = 0.375 P = 0.569 |
- |
r = 0.50 P = 0.04* |
- |
| Chemerin albumen (F22, n = 30) | - |
r = −0.37 P = 0.04* |
- | r = 0.123 P = 0.407 |
Correlations between chemerin amounts in blood plasma or in albumen and fertility parameters.
Pearson's correlation analysis is presented below.
Asterisks (*) and red text identify correlations that are statistically significant.
Chemerin System Within the Allantoic and Amniotic Cells
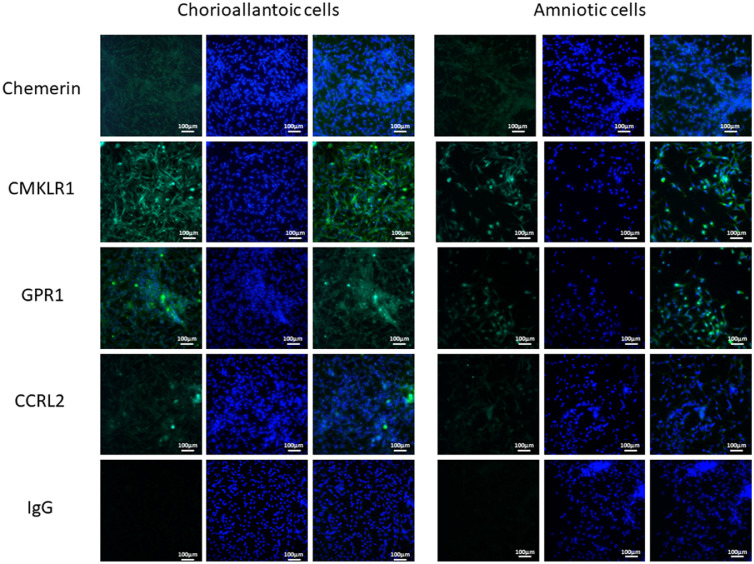
Immunocytofluorescence experiments made it possible to qualitatively assess the presence of proteins of the chemerin system in the allantoic and amniotic cells. Indeed, samples of allantoic and amniotic membranes taken from ED11 incubated eggs were digested enzymatically and put in short culture for 24 h on labteck slides to allow the detection of target proteins. Chemerin was detected in allantoic and amniotic cells with weak staining. For CMKLR1, staining was stronger in both cell types. GPR1 and CCRL2 were also detected in both cell types with less staining than CMKLR1. Finally, the negative control with anti-rabbit IgG showed no staining. This result demonstrates that chemerin and its 3 receptors are present in the allantoic and amniotic membrane, at least at ED11 in the pheasant (Figure 3).
Figure 3.
Chemerin system is expressed by the chorioallantoic and the amniotic membranes of pheasant eggs at ED11 during incubation. Immunofluorescence was in vitro performed for chemerin, CMKLR1, GPR1, and CCRL2 qualitative detection in chorioallantoic and amniotic cells of pheasant eggs at ED11 (scale 100 μm). In each culture, chorioallantoic and amniotic cells were obtained from 5 fertilized eggs. The pictures are representative of 5 cultures.
DISCUSSION
In the present study, we showed for the first time that chemerin and its 3 receptors CMKLR1, GPR1, and CCRL2 are expressed in the pheasant oviduct and chemerin is highly present in magnum and consequently accumulates in egg albumen as previously shown in chicken. In addition, we analyzed 2 lines of pheasants (F11 and F22) with different fertility parameters, and we demonstrated that F22 animals that laid more eggs but had low fertility exhibited a lower amount of chemerin protein in their magnum and in the albumen of their eggs. We also showed that chemerin amount in the albumen was positively and negatively correlated with the hatching rate and the rate of unfertilized eggs, respectively, suggesting that it could be a physiological parameter associated to avian female fertility.
The involvement of chemerin in reproductive function, both in male and in female, is well-known in mammals (Estienne et al., 2020). But until now, there are few data about its implication within bird's reproduction. Our laboratory has already obtained interesting results in bird species of agronomical interests. Indeed, we demonstrated that in turkey, chemerin is expressed by granulosa cells and increases in the mature follicle before ovulation, thus, potentially influencing the EED (Diot et al., 2015). In male chicken, this hormone has a negative impact on steroidogenesis and sperm function in rooster, leading to a decreased fertility (Estienne et al., 2020). We also demonstrated that plasma chemerin concentrations are negatively correlated with egg hatchability, suggesting a potential role of this adipokine on EED here again (Mellouk et al., 2018). We found the same result in the present study with a negative correlation between blood plasma chemerin in F11 breed and the egg hatchability (r = −0.48; P < 0.05). More recently, our laboratory showed that in hens, chemerin is expressed locally within the reproductive tract, more particularly within the magnum (Estienne et al., 2022). The magnum is the region of the avian oviduct where the synthesis and secretion of the egg white albumen occurs (Wyburn et al., 1970). The secretion normally takes place around 0.6 to 4 h after the previous laying, when the yolk passes along the magnum. We found a similar result in pheasants with a more important amount of chemerin protein in the magnum compare to the other oviduct parts. In chicken, chemerin is expressed by the magnum during albumen secretion, accumulates within the egg albumen and plays an important role during embryo development (Estienne et al., 2022). Our investigations on pheasant eggs revealed a similar observation with important amount of chemerin in the albumen of eggs. We also found a difference between the 2 studied breeds with higher amount of chemerin in magnums and egg albumens of F11 hens compare to the F22 hens exhibiting lower fertility and hatchability rates. The differential selection between the 2 breeds, based on a metabolic trait for the F11 breed, and on a reproductive trait for the F22 breed, could explain this difference. The F22 breed lays more eggs but these are less fertile. This last result suggests, as in the chicken hen, that this adipokine could play an important role in embryonic development during incubation. Indeed, in (Estienne et al., 2022), we demonstrated that chemerin present an antibacterial activity that could protect the egg from bacterial development. As chemerin is well conserved between the species (Bernardi et al., 2021), we hypothesize that this hormone, due to its huge accumulation within the egg, could play a similar role in pheasant species. Moreover, we found that the chemerin system, ligand plus receptors, is expressed within the embryo annexes in pheasant during egg incubation. This result is similar to the observation we've made in chicken (Estienne et al., 2022). In this last study, we showed that chemerin and its 3 receptors are expressed in chicken allantoic and amniotic membranes and that chicken recombinant chemerin has proliferative effect on these cells cultured in vitro. Thus, we hypothesize that chemerin system in allantoic and amniotic membranes in pheasant is necessary for annexes function and so, embryo development, like in chicken. Finally, when focusing on correlations between chemerin and reproductive parameters, we found that chemerin amount in the albumen is positively correlated with the rate of hatched eggs in pheasant F11 breed and it is negatively correlated with the rate of infertile eggs in pheasant F22 breed. All these results were obtained with biological samples collected at the end of the first period of reproduction of the pheasant hens, the conclusions of this study can, therefore, only be representative of the 1-yr-old pheasant at this precise period. But in view of our previous study in chicken hens, we can hypothesize that these interesting results could also indicate that, in general, chemerin is beneficial for fertility and egg hatch in all gallinaceans, throughout the breeding season.
CONCLUSIONS
Taken together, we demonstrated that in pheasant oviduct, chemerin is predominantly present in magnum and accumulates in egg albumen. Moreover, in fertilized eggs, chemerin and its three receptor, CMKLR1, GPR1, and CCRL2 are found in allantoic and amniotic cells. In addition, by using two pheasant lines we showed that chemerin protein amount in egg albumen was significantly positively and negatively correlated with the rate of hatched eggs and the rate of infertile eggs, respectively. Thus, chemerin in egg albumen could play a role in embryo annexes and development and it could be used as a marker for selection in avian species in terms of fertility and hatchability. More investigations are needed to validate these hypotheses.
ACKNOWLEDGMENTS
The authors greatly thank to Gen’éthic filiale sélection Gibovendée for access to pheasants and the data associated to these animals. This work was supported by the SYSAAF (FertiliChem grant number 3440).
TAuthor contributions: J.D., A.E., S.T., and M.R. conceived the project. O.B., A.E., P.F. and C.R. performed RTqPCR and Western-blot. A.E realized in vitro culture and immunodetection of chemerin system within the allantoic and amniotic cells and performed all statisticak analyses. A.E. and J.D. wrote the manuscript. All authors reviewed the manuscript.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Barbe A., Mellouk N., Ramé C., Grandhaye J., Staub C., Venturi E., Cirot M., Petit A., Anger K., Chahnamian M., Ganier P., Callut O., Cailleau-Audouin E., Metayer-Coustard S., Riva A., Froment P., Dupont J. A grape seed extract maternal dietary supplementation in reproductive hens reduces oxidative stress associated to modulation of plasma and tissue adipokines expression and improves viability of offsprings. PLoS One. 2020;15 doi: 10.1371/journal.pone.0231131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi O., Estienne A., Reverchon M., Bigot Y., Froment P., Dupont J. Adipokines in metabolic and reproductive functions in birds: an overview of current knowns and unknowns. Mol. Cell. Endocrinol. 2021;534 doi: 10.1016/j.mce.2021.111370. [DOI] [PubMed] [Google Scholar]
- Bondue B., Wittamer V., Parmentier M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 2011;22:331‑38. doi: 10.1016/j.cytogfr.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Bozaoglu K., Bolton K., McMillan J., Zimmet P., Jowett J., Collier G., Walder K., Segal D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148:4687‑94. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- Diot M., Reverchon M., Rame C., Froment P., Brillard J.-P., Brière S., Levêque G., Guillaume D., Dupont J. Expression of adiponectin, chemerin and visfatin in plasma and different tissues during a laying season in Turkeys. Reprod. Biol. Endocrinol. 2015;13:81. doi: 10.1186/s12958-015-0081-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estienne A., Brossaud A., Ramé C., Bernardi O., Reverchon M., Rat C., Delaveau J., Chambellon E., Helloin E., Froment P., Dupont J. Chemerin is secreted by the chicken oviduct, accumulates in egg albumen and could promote embryo development. Sci. Rep. 2022;12:8989. doi: 10.1038/s41598-022-12961-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estienne A., Brossaud A., Reverchon M., Ramé C., Froment P., Dupont J. Adipokines expression and effects in oocyte maturation, fertilization and early embryo development: lessons from mammals and birds. Int. J. Mol. Sci. 2020;21:3581. doi: 10.3390/ijms21103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estienne A., Ramé C., Ganier P., Chahnamian M., Barbe Alix., Grandhaye J., Dubois J.-P., Batailler M., Migaud M., Lecompte F., Adriaensen H., Froment P., Dupont J. Chemerin impairs food intake and body weight in chicken: focus on hypothalamic neuropeptides gene expression and AMPK signaling pathway. Gen. Comp. Endocrinol. 2021;304 doi: 10.1016/j.ygcen.2021.113721. [DOI] [PubMed] [Google Scholar]
- Gantz I., Konda Y., Yang Y.-K., Miller D.E., Dierick H.A., Yamada T. Molecular cloning of a novel receptor (CMKLR1) with homology to the chemotactic factor receptors. Cytogenet. Genome Res. 1996;74:286‑90. doi: 10.1159/000134436. [DOI] [PubMed] [Google Scholar]
- Gugała D., Flis M., Grela E.R. The effect of zinc, iron, calcium, and copper from organic sources in pheasant diet on the performance, hatching, minerals, and fatty acid composition of eggs. Poult. Sci. 2019;98:4640‑47. doi: 10.3382/ps/pez162. [DOI] [PubMed] [Google Scholar]
- Helfer G., Wu Q.-F. Chemerin: a multifaceted adipokine involved in metabolic disorders. J. Endocrinol. 2018;238:R79‑94. doi: 10.1530/JOE-18-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst Gerhard van der., Maree L. Origin, migration, and reproduction of indigenous domestic animals with special reference to their sperm quality. Animals. 2022;12:657. doi: 10.3390/ani12050657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A.J., Davenport A.P. International union of basic and clinical pharmacology CIII: chemerin receptors CMKLR1 (Chemerin 1) and GPR1 (Chemerin 2) nomenclature, pharmacology, and function. Pharmacol. Rev. 2018;70:174‑96. doi: 10.1124/pr.116.013177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullu S.S., Das A., Bajpai S.K., Garg A.K., Yogi R.K., Saini M., Sharma A.K. Egg production performance, egg yolk antioxidant profile and excreta concentration of corticosterone in golden pheasants (Chrysolophus Pictus) fed diets containing different levels of green vegetables. J. Anim. Physiol. Anim. Nutr. (Berl.) 2017;101:e31‑42. doi: 10.1111/jpn.12555. [DOI] [PubMed] [Google Scholar]
- Marchese A., Cheng R., Lee M.C., Porter C.A., Heiber M., Goodman M., George S.R., O'Dowd B.F. Mapping studies of two G protein-coupled receptor genes: an amino acid difference may confer a functional variation between a human and rodent receptor. Biochem. Biophys. Res. Commun. 1994;205:1952‑58. doi: 10.1006/bbrc.1994.2899. [DOI] [PubMed] [Google Scholar]
- Mattern A., Zellmann T., Beck-Sickinger A.G. Processing, signaling, and physiological function of chemerin: processing, signaling, and physiological function of chemerin. IUBMB Life. 2014;66:19‑26. doi: 10.1002/iub.1242. [DOI] [PubMed] [Google Scholar]
- Mellouk N., Ramé C., Delaveau J., Rat C., Marchand M., Mercerand F., Travel A., Brionne A., Chartrin P., Linlin M., Froment P., Dupont J. Food restriction but not fish oil increases fertility in hens: role of RARRES2? Reproduction. 2018;155:321‑31. doi: 10.1530/REP-17-0678. [DOI] [PubMed] [Google Scholar]
- Nynca J., Żarski D., Bobe J., Ciereszko A. Domestication is associated with differential expression of pikeperch egg proteins involved in metabolism, immune response and protein folding. Animal. 2020;14:2336‑50. doi: 10.1017/S1751731120001184. [DOI] [PubMed] [Google Scholar]
- Pérez-Enciso M., de los Campos G., Hudson N., Kijas J., Reverter A. The ‘heritability’ of domestication and its functional partitioning in the pig. Heredity. 2017;118:160‑68. doi: 10.1038/hdy.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourke J.L., Dranse H.J., Sinal C.J. Towards an integrative approach to understanding the role of chemerin in human health and disease: chemerin in human health and disease. Obes. Rev. 2013;14:245‑62. doi: 10.1111/obr.12009. [DOI] [PubMed] [Google Scholar]
- Shimada T., Matsumoto M., Tatsumi Y., Kanamaru A., Akira S. A novel lipopolysaccharide inducible C-C chemokine receptor related gene in murine macrophages. FEBS Lett. 1998;425:490‑94. doi: 10.1016/s0014-5793(98)00299-3. [DOI] [PubMed] [Google Scholar]
- Sleeman M.A., Fraser J.K., Murison J.G., Kelly S.L., Prestidge R.L., Palmer D.J., Watson J.D., Kumble K.D. B Cell- and monocyte-activating chemokine (BMAC), a novel non-ELR α-chemokine. Int. Immunol. 2000;12:677‑89. doi: 10.1093/intimm/12.5.677. [DOI] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe Bruce., Van Roy N., De Paepe A., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyburn G.M., Johnston H.S., Draper M.H., Davidson Maida F. The fine structure of the infundibulum and magnum of the oviduct of gallus domesticus. Q. J. Exp. Physiol. Cogn. Med. Sci. 1970;55:213‑32. doi: 10.1113/expphysiol.1970.sp002071. [DOI] [PubMed] [Google Scholar]
- Zabel B.A., Allen S.J., Kulig P., Allen J.A., Cichy J., Handel T.M., Butcher E.C. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J. Biol. Chem. 2005;280:34661‑66. doi: 10.1074/jbc.M504868200. [DOI] [PubMed] [Google Scholar]