Abstract
Objectives
To evaluate whether SARS-CoV-2 infection in residents of long-term care (LTC) facilities is associated with higher mortality after the acute phase of infection, and to estimate survival in uninfected residents.
Design
Extended follow-up of a previous, propensity score-matched, retrospective cohort study based on the Swedish Senior Alert register.
Setting
LTC facilities in Sweden.
Participants
n=3604 LTC residents with documented SARS-CoV-2 until 15 September 2020 matched to 3604 uninfected controls using time-dependent propensity scores on age, sex, health status, comorbidities, prescription medications, geographical region and Senior Alert registration time. In a secondary analysis (n=3731 in each group), geographical region and Senior Alert registration time were not matched for in order to increase the follow-up time in controls and allow for an estimation of median survival.
Primary outcome measures
All-cause mortality until 24 October 2020, tracked using the National Cause of Death Register.
Results
Median age was 87 years and 65% were women. Excess mortality peaked at 5 days after documented SARS-CoV-2-infection (HR 21.5, 95% CI 15.9 to 29.2), after which excess mortality decreased. From the second month onwards, mortality rate became lower in infected residents than controls. The HR for death during days 61–210 of follow-up was 0.76 (95% CI 0.62 to 0.93). The median survival of uninfected controls was 1.6 years, which was much lower than the national life expectancy in Sweden at age 87 (5.05 years in men, 6.07 years in women).
Conclusions
The risk of death after SARS-CoV-2 infection in LTC residents peaked after 5 days and decreased after 2 months, probably because the frailest residents died during the acute phase, leaving healthier residents remaining. The limited life expectancy in this population suggests that LTC resident status should be accounted for when estimating years of life lost due to COVID-19.
Keywords: COVID-19, EPIDEMIOLOGY, GERIATRIC MEDICINE
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study leveraged data from large databases with nationwide coverage.
We were unable to match infected residents with uninfected residents from the same facility.
Our data pertain to fatalities during the first wave of COVID-19 and until the fall of 2020.
Some controls may have been asymptomatically infected, but the infection remained unnoticed due to limited testing.
Introduction
During the COVID-19 pandemic, many of the COVID-19 deaths that occurred in high-income countries were seen in long-term care (LTC) facilities,1 where case fatality rates were 10%–40% or even higher.2 3 We have previously reported that 30-day mortality in Swedish LTC was 40% in residents infected with SARS-CoV-2 versus 6% in matched, non-infected controls in the first wave of the pandemic.4 A natural follow-up question to ask is whether SARS-CoV-2 also increases the risk of death beyond the acute period of 30 days, that is, whether it has long-term effects on mortality in LTC residents who recover from infection. A major concern is that LTC residents who recover from SARS-CoV-2 infection may have residual debilitation caused by the infection. If so, this may affect also their life expectancy beyond the acute phase of the infection. Moreover, it would be interesting to estimate the loss of life expectancy in LTC residents infected with SARS-CoV-2. The current study set out to answer these two questions by (1) extending the follow-up period in our previous analysis from 30 days to 8 months, and (2) by estimating survival in LTC residents not infected with SARS-CoV-2.
Methods
Patient and public involvement
No patients or members of the public were involved in the study design, data collection, data analysis, interpretation of the results, decision to publish the paper or preparation of the manuscript.
Study design and population
The present study offers extended follow-up on a propensity score-matched retrospective cohort study.4 The basic study design and selection of exposed (infected) and unexposed (uninfected control) residents has been described in detail previously in the publication presenting 30 days of follow-up.4 In brief, data on Swedish LTC residents were obtained from Senior Alert, a database of health assessments performed in older adults aged ≥65 years.5 All residents of LTC facilities in Sweden registered in Senior Alert were eligible to be considered. Senior Alert collects health data on various conditions in adults aged ≥65 years. Senior Alert captures an estimated 73% of all Swedish LTC facility residents. In Sweden, LTC facilities include several types of permanent housing that have been adapted for older adults in need of 24-hour care.
Cohort construction and matching
Individuals considered for inclusion were LTC residents who had a record in Senior Alert from 2019 or 2020; the latest record during these years was used, whenever there were multiple records. Among these, 3731 LTC residents with a documented SARS-CoV-2 infection until 15 September 2020 were identified. Infected residents who did not have a record in Senior Alert within a year prior to date of testing or confirmed infection (whichever came first or was available) were excluded, as were those where dates of testing and confirmed infection were both unavailable. Each infected resident was matched 1:1 to a control resident on age, sex, body mass index, health status, comorbidity and prescription medication use (all variables listed in table 1), using time-dependent propensity scores. This enables matching when the exposure (date of documented SARS-CoV-2 infection) does not coincide with the time of cohort entry (date of Senior Alert record). The matching variables were selected in the previous study,4 where the intention was to identify both known and suspected risk factors for mortality after SARS-CoV-2 infection. With time starting at the date of the Senior Alert record, a Cox model calculated a propensity score for the propensity to contract SARS-CoV-2 based on all the matching variables. Each infected resident was matched to the control with the closest propensity score among those who were still alive when the SARS-CoV-2 case occurred (counting time since the Senior Alert date). Matching was done sequentially, starting with the first case (smaller number of days since cohort entry) and proceeding with cases with increasingly larger number of days since cohort entry. Diagnoses and medications were used as time-varying covariates in the Cox regression model. Thus, 3731 cases were matched to 3731 controls. Next, an additional matching was performed where also the geographical region (21 categories) and the date (year and month) of Senior Alert registration were included. This was done to account for potential confounding by secular trends and geographic variations in mortality. As a result, 3604 cases could be matched to 3604 controls. In the present study, this cohort was used in the main analysis to assess time-varying mortality after SARS-CoV-2. The cohort of 3731 cases and 3731 controls was used in a secondary analysis to estimate median survival in uninfected controls, which was made possible given that follow-up time in this cohort was much longer because of not matching on Senior Alert registration date.
Table 1.
Baseline characteristics
| Variables | SARS-CoV-2-infected residents (main analysis) (n=3604) | Uninfected controls (main analysis) (n=3604) | SARS-CoV-2-infected residents (secondary analysis) (n=3731) | Uninfected controls (secondary analysis) (n=3731) |
| Median (IQR) days between Senior Alert registration and baseline* | 118 (59–184) | 118 (59–184) | 120 (60–188) | 120 (60–188) |
| Male sex | 1278 (35.5) | 1233 (34.2) | 1325 (35.5) | 1318 (35.3) |
| Age, median (IQR), years | 86 (80–91) | 87 (81–92) | 87 (81–92) | 87 (81–92) |
| Age group (years) | ||||
| <70 | 152 (4.2) | 166 (4.6) | 140 (3.8) | 164 (4.4) |
| 70–74 | 251 (7.0) | 302 (8.4) | 249 (6.7) | 238 (6.4) |
| 75–79 | 465 (12.9) | 438 (12.2) | 456 (12.2) | 431 (11.6) |
| 80–84 | 965 (19.3) | 688 (19.1) | 706 (18.9) | 693 (18.6) |
| 85–89 | 911 (25.3) | 829 (23.0) | 938 (25.1) | 927 (24.9) |
| ≥90 | 1130 (31.4) | 1181 (32.8) | 1242 (33.3) | 1278 (34.3) |
| BMI, kg/m2, mean (SD) | 25.4 (5.1) | 25.0 (5.0) | 25.4 (5.0) | 25.6 (5.3) |
| BMI categories | ||||
| Underweight (<18.5 kg/m2) | 233 (6.5) | 264 (7.3) | 240 (6.4) | 258 (6.9) |
| Normal weight (18.5–24.99 kg/m2) | 1614 (44.8) | 1701 (47.2) | 1672 (44.8) | 1604 (43.0) |
| Overweight (25.0–29.99 kg/m2) | 1108 (30.7) | 1160 (32.2) | 1196 (32.1) | 1182 (31.7) |
| Obesity (≥30 kg/m2) | 597 (14.7) | 531 (16.6) | 623 (16.7) | 687 (18.4) |
| Neuropsychological conditions | ||||
| None | 858 (23.8) | 906 (25.1) | 886 (23.8) | 884 (23.7) |
| Mild dementia or depression | 1755 (48.7) | 1724 (47.8) | 1822 (48.8) | 1805 (48.4) |
| Severe dementia or depression | 991 (27.5) | 974 (27.0) | 1023 (27.4) | 1042 (27.9) |
| Known previous falls | 1893 (52.5) | 1884 (52.3) | 1970 (52.8) | 1950 (52.3) |
| Walking ability | ||||
| Safe with or without walking aids | 1467 (40.7) | 1458 (40.5) | 1513 (40.6) | 1492 (40.0) |
| Unsafe walk | 1309 (36.3) | 1306 (36.2) | 1367 (36.6) | 1379 (37.0) |
| Unable to walk | 828 (23.0) | 840 (23.3) | 851 (22.8) | 860 (23.1) |
| Fluid intake (mL/day) | ||||
| >1000 | 2118 (58.8) | 2189 (60.7) | 2191 (58.7) | 2182 (58.5) |
| 700–1000 | 1292 (35.9) | 1237 (34.3) | 1327 (35.6) | 1312 (35.2) |
| 500–700 | 180 (5.0) | 161 (4.5) | 196 (5.3) | 210 (5.6) |
| <500 | 14 (0.4) | 17 (0.5) | 17 (0.5) | 27 (0.7) |
| Food intake | ||||
| Normal serving | 2523 (70.0) | 2486 (69.0) | 2597 (69.6) | 2.587 (69.3) |
| ¾ serving | 654 (18.2) | 686 (19.9) | 686 (18.4) | 699 (18.7) |
| ½ serving | 334 (9.3) | 339 (9.4) | 350 (9.4) | 341 (9.1) |
| <½ serving | 93 (2.6) | 93 (2.6) | 98 (2.6) | 104 (2.8) |
| General physical condition | ||||
| Good | 2020 (56.1) | 2002 (55.6) | 2077 (55.7) | 2034 (54.5) |
| Fair | 1463 (40.6) | 1471 (40.8) | 1524 (40.9) | 1545 (41.4) |
| Poor | 113 (3.1) | 128 (3.6) | 121 (3.2) | 142 (3.8) |
| Very bad | 8 (0.2) | 3 (0.1) | 9 (0.2) | 10 (0.3) |
| Incontinence | ||||
| No | 952 (26.4) | 962 (26.7) | 986 (26.4) | 989 (26.5) |
| Temporarily but unusual | 542 (15.0) | 482 (13.4) | 565 (15.1) | 556 (14.9) |
| Urinary or bowel | 881 (24.5) | 940 (26.1) | 906 (24.3) | 890 (23.9) |
| Urinary and bowel | 1229 (34.1) | 1220 (33.9) | 1274 (34.2) | 1296 (34.7) |
| Comorbidities | ||||
| Stroke | 911 (25.3) | 940 (26.1) | 942 (25.3) | 940 (25.2) |
| Myocardial infarction | 428 (11.9) | 408 (11.3) | 446 (12.0) | 431 (11.6) |
| Angina pectoris | 553 (15.3) | 550 (15.3) | 576 (15.4) | 574 (14.4) |
| Heart failure | 733 (20.3) | 721 (20.0) | 771 (20.7) | 776 (20.8) |
| Atrial fibrillation | 963 (26.7) | 936 (26.0) | 997 (26.7) | 971 (26.0) |
| Autoimmune disease | 454 (12.6) | 446 (12.4) | 487 (13.1) | 491 (13.2) |
| Diabetes | 791 (22.0) | 765 (21.2) | 825 (22.1) | 845 (22.7) |
| COPD | 454 (12.6) | 459 (12.7) | 483 (13.0) | 486 (13.0) |
| Asthma | 258 (7.2) | 247 (6.9) | 275 (7.4) | 242 (6.5) |
| Cancer | 1630 (45.2) | 1623 (45.0) | 1687 (45.2) | 1661 (44.5) |
| Renal failure/CKD | 479 (13.3) | 505 (14.0) | 521 (14.0) | 536 (14.4) |
| Liver disease | 65 (1.8) | 62 (1.7) | 72 (1.9) | 75 (2.0) |
| Sepsis | 298 (8.3) | 296 (8.2) | 316 (8.5) | 309 (8.3) |
| Influenza | 172 (4.8) | 174 (4.8) | 184 (4.9) | 193 (5.2) |
| Pneumonia | 870 (24.1) | 895 (24.8) | 915 (24.5) | 923 (24.7) |
| Alcohol intoxication | 226 (6.3) | 250 (6.9) | 233 (6.2) | 221 (5.9) |
| Medications | ||||
| Antithrombotics | 2122 (58.9) | 2102 (58.3) | 2205 (59.1) | 2253 (60.4) |
| Antihypertensives (non-diuretic) | 2174 (60.3) | 2150 (59.7) | 2257 (60.5) | 2271 (60.9) |
| Diuretics | 1537 (42.7) | 1453 (40.3) | 1611 (43.2) | 1608 (43.1) |
| Antidepressants | 2100 (58.3) | 2078 (57.7) | 2178 (58.4) | 2140 (57.4) |
| Psycholeptics | 2556 (70.9) | 2553 (70.8) | 2649 (71.0) | 2648 (71.0) |
The data are displayed as n (%) unless stated otherwise. The data in the last two columns are the same as those presented in our previous publication.4
*Baseline was the date of SARS-CoV-2 test/date of confirmed SARS-CoV-2 and the corresponding date in matched controls.
BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease.
The data on SARS-CoV-2 infections were obtained from the national SmiNet register, managed by the Public Health Agency of Sweden. According to Swedish law, all healthcare providers were legally required to report confirmed cases of SARS-CoV-2 infection to this register. Information on comorbidities was obtained from the National Patient Register and for cancer from the Swedish Cancer Register. Information on recent use of medications (prescriptions in 2019–2020) came from Senior Alert and the Prescribed Drug Register. Data linkage across registers was performed using the Swedish personal identification number, which is unique to each individual.
Outcome
The study outcome was all-cause mortality (until 24 October 2020). These data were obtained from the Cause of Death Register.6 While our previous study only assessed 30-day mortality,4 the extended follow-up period considered in the present study allows to get a more complete picture of the mortality risk of this frail population, while at the same time it largely excludes the subsequent waves and also the COVID-19 vaccination period which may have further affected mortality risk in this population.
Statistical analysis
In both the main and secondary analyses, starting date for follow-up was the date of documented SARS-CoV-2 documentation date in infected residents (exposed) and the corresponding date (in days since cohort entry) in uninfected controls. Follow-up time in days was calculated as censor date (24 October 2020 or death whichever came first) minus baseline date+1 day. This was done so that the baseline date could also be included in the follow-up time and analysis (thus, a person would be able to die on the same date as they were documented to be infected). The HR for death among infected compared with uninfected residents was plotted over time using flexible parametric models with restricted cubic splines (four knots in default positions). HRs and 95% CIs were also estimated using Cox regression for time intervals of follow-up until 210 days. To adjust for matching, 95% CIs in the Cox models and the flexible parametric models were calculated using robust SEs. The absolute risk of death was examined using the Kaplan-Meier plots. From these, the median survival of uninfected residents was estimated in the secondary analysis, and survival of uninfected residents at 210 days of follow-up was estimated in both the main and secondary analyses. All analyses were performed using Stata MP V.16.1 for Mac (StataCorp, College Station, Texas).
Results
Baseline characteristics
Baseline characteristics are shown in table 1. The median age was 87 years, 65% were women and comorbidities were common. In the main analysis, median (IQR) baseline date for infected residents was 26 April 2020 (IQR 10 April to 21 May), median (maximum) follow-up was 130 (246) days and there were 1640 deaths. For controls, median (IQR) baseline date was 28 April 2020 (9 April to 23 May), median (maximum) follow-up was 173 (249) days and there were 536 deaths. In the secondary analysis, median (IQR) baseline date for infected residents was 27 April 2020 (10 April to 22 May), median (maximum) follow-up was 129 (246) days and there were 1713 deaths. For controls, median (IQR) baseline date was 12 April 2020 (16 December 2019 to 30 June 2020), median (maximum) follow-up was 146 (641) days and there were 899 deaths.
Time-varying mortality analyses
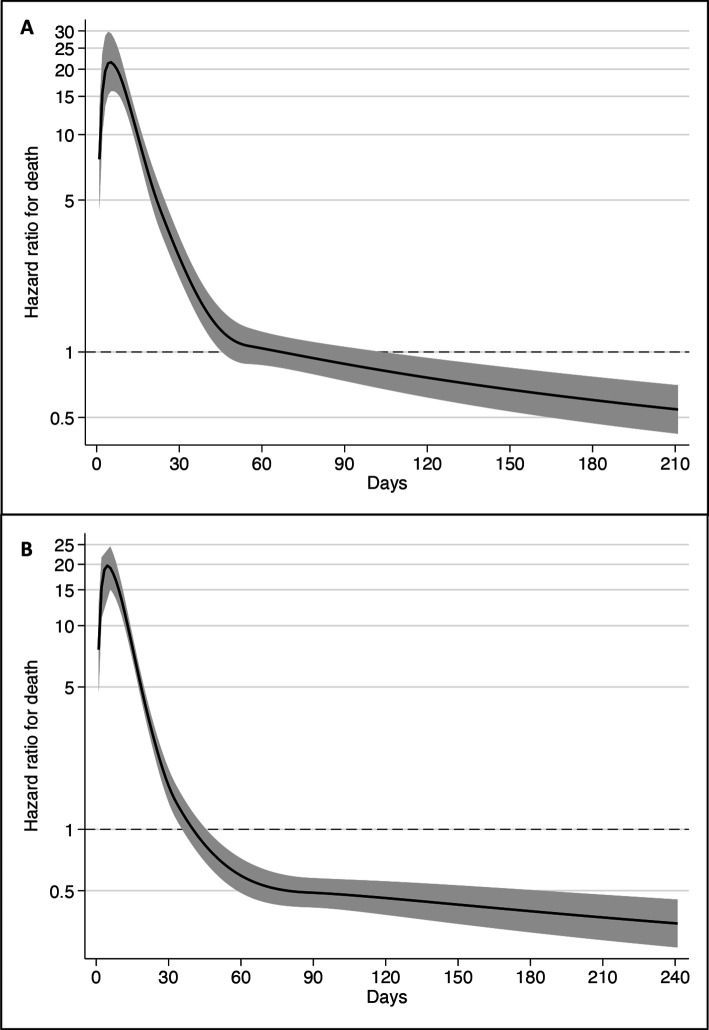
In the main analysis, SARS-CoV-2 was associated with a sharp, early increased risk of death: 39% vs 4% within 30 days (1414/3604 vs 152/3604). However, extending the follow-up period showed that the risk soon plateaued (figure 1A). Similar results were seen in the secondary analysis (figure 1B). Peak HR (21.5, 95% CI 15.9 to 29.2) occurred at 5 days after documented infection. The HR was high in the first month, decreased below 1.0 after the second month and remained below 1.0 for the remaining duration of follow-up (figure 2A). During days 0–30, there were 1414 deaths among infected residents (17.69 per 1000 person-days) versus 152 among controls (1.44 per 1000 person-days), resulting in an HR of 11.54 (95% CI 9.78 to 13.63). For 31–60 days, there were 93 (1.46 per 1000 person-days) versus 104 deaths (1.02 per 1000 person-days) (HR 1.42, 95% CI 1.07 to 1.88). From 61 to 210 days, there were 131 (0.59 per 1000 person-days) versus 278 deaths (0.78 per 1000 person-days), with the HR being 0.76 (95% CI 0.62 to 0.93). In the secondary analysis, peak HR was 19.1 (95% CI 14.6 to 24.8) and occurred after 5 days. Again, HR decreased sharply but dropped below 1.0 after around the first month (figure 2B). The HR for death by time of follow-up was 8.81 (95% CI 7.64 to 10.15) for days 0–30; for 31–60 days the HR was 0.38 (95% CI 0.26 to 0.55); and for 61–210 days the HR was 0.41 (95% CI 0.34 to 0.50).
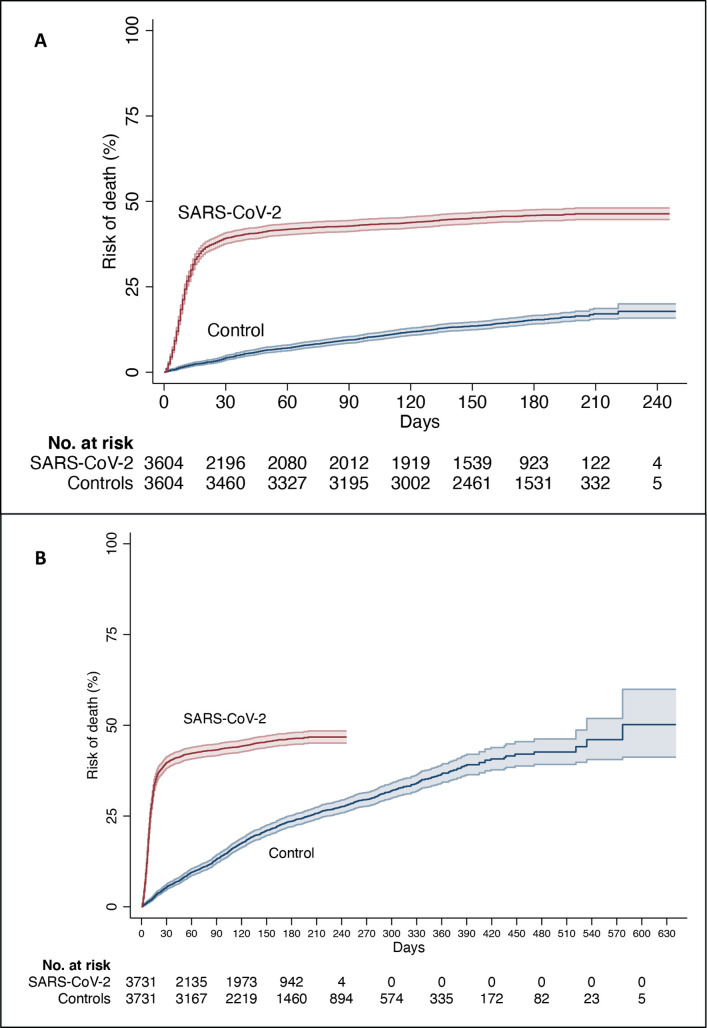
Figure 1.
Risk of death in residents with SARS-CoV-2 and controls in (A) the main analysis and (B) in the secondary analysis, estimated using the Kaplan-Meier method. The coloured areas show the 95% CI.
Figure 2.
HR for death over time in residents with SARS-CoV-2, as compared with controls in (A) the main analysis and (B) in the secondary analysis. Estimates were obtained using flexible parametric models with restricted cubic splines (four knots in default positions). The shaded areas show the 95% CI.
Survival in uninfected residents
In the main analysis, survival of controls at 210 days was 82.9% (81.3%–84.4%), and in the secondary analysis it was 74.3% (72.6%–75.9%).
The median survival of uninfected controls, as determined in the secondary analysis, was 577 days (~1.6 years). Similarly, median survival was 577 days among the 1487 uninfected controls who were matched to the 1487 infected residents who died in the first month. Survival of these 1487 controls was also similar to the survival of the remaining 2244 controls, for example, their survival at 210 days was 72.5% (69.8–75.1) vs 75.4% (73.3–77.4).
Discussion
In this extended follow-up analysis of mortality in SARS-CoV-2-infected versus uninfected control LTC residents, the risk of death peaked during the first week of documented infection, after which it decreased. Mortality remained elevated for 2 months after infection, but then reverted back to baseline levels (ie, control levels) before it dropped below baseline levels, where it remained for the remaining duration of follow-up (up to 8 months). Despite concerns that infected residents who survive may have persistent residual debilitation that might increase their subsequent risk of death, the results suggest that SARS-CoV-2 does not reduce the life expectancy of LTC residents who survive the acute period of the disease. However, it is important to note that the lower risk of death after the acute phase should not be interpreted as a sign that SARS-CoV-2 infection causally decreases the risk of death during long-term follow-up, as it probably reflects mostly a selection process, where residents who died early were probably sicker and debilitated prior to infection, while those surviving probably had better life expectancy.
Furthermore, the estimates of median survival among uninfected controls suggest that deaths due to COVID-19 in LTC facilities in Sweden during the first wave probably resulted in an average loss of life expectancy of less than 1.6 years on average. This figure is much lower than the life expectancy in the general Swedish population, which in 2019 was 5.05 years for men and 6.07 years for women at the age of 87 (the median age in our study).7 The estimates of limited median survival in uninfected residents agree with prepandemic estimates from previous studies, for example, 541 days in one study in the UK8 and 2 years in a study in New Zealand.9 Similarly, previous data from Sweden10 suggested that, on average, median survival after moving to institutionalised care declined from 764 to 595 days between 2006 and 2012. For the lower percentiles, the decrease was very large, for example, for the 30th percentile, the length of stay declined from 335 days in 2006 to 119 days in 2012, and in 2012 10% died within 8 days. A widening survival gap (due to shortening survival in nursing home residents) versus community-dwelling older people has also been documented in a 10-year study in England.11 Another study12 evaluated all deaths in people >67 years old in November 2015 in Sweden and focused on the 2 years prior to death. Women used LTC for 15.6 months and men for 14.1 months out of these 24 months. The length of stay in institutional care was 7.2 and 6.2 months, respectively. Taken together, these survival data are in line with the estimated median survival of controls in the present study, validating that survival in residents of LTC facilities is generally very limited.
Calculations of burden of disease due to COVID-19 typically do not account for LTC residence and general health and thus can yield massively inflated estimates.13 Adjustment for comorbidities has been shown to decrease years of life lost estimates.14–16 However, the change is typically modest (eg, ~1 year) and much smaller than what was observed in the present LTC resident population. Possibly, in most studies, information on comorbidities may not be available in sufficient granularity and accuracy regarding severity. Thus, as LTC resident status is a surrogate for increased frequency and severity of comorbidities and overall frailty, it should be accounted for when estimating years of life lost. ‘Aspirational life table’ approaches such as the Global Burden of Disease Reference Life Table17 (aka Theoretical Minimum Risk Life Table) can be particularly misleading. In an effort to standardise comparisons across countries, aspirational life tables assume idealised populations with optimised life expectancy: 88.9 years at birth; 9.99 years at age 85; 7.62 years at age 90; and 5.92 years at age 95.18 Using these popular aspirational life tables would overestimate by 5-fold to 10-fold the years of life lost for SARS-CoV-2-deceased residents in LTC facilities.
Limitations and strengths
We should acknowledge that there can be large heterogeneity in survival in different LTC facilities. Some LTC facilities admit mostly residents with known limited life expectancy, while others may be institutions that admit mostly older adults who are quite healthy or have limited health problems with substantial life expectancy. A systematic review has found that across six cohort studies, the mortality rate within 6 months of admission to a nursing home ranged from 0% to 34% (median 20.2%).19 In our analysis, we could not include data on the features of each LTC facility and we could not match infected residents with uninfected residents from the same facility. Nevertheless, the control groups both in the main and secondary analyses seem to have median survival that is compatible with the literature on LTC residents and their overall limited expected survival, on average.
Furthermore, the current data pertain to fatalities during the first wave of COVID-19 and until the fall of 2020. The first wave was the most devastating in most high-income countries, with a few exceptions (eg, Australia).20 21 The relatively lower proportion of fatalities in LTC residents in subsequent waves may reflect a combination of multiple factors: high levels of prior infection (seroprevalence studies have found 5–10 times higher infection rates in LTC facilities than in the general population in the first wave),22–24 better protection of nursing homes, more extensive testing, widespread use of vaccination in 202121 and the possibility that the sickest individuals were the first to succumb.25 Moreover, as stated earlier, the lower risk of death during long-term follow-up is probably due to a selection process, where residents who died early were probably frailer, as shown in the previous study based on the same population, where short-term mortality in infected residents was higher, for example, among those with neuropsychological conditions, incontinence and previous pneumonia.4 We also cannot exclude the possibility that some controls may have been asymptomatically infected but the infection remained unnoticed due to limited testing, especially in the early weeks of the pandemic. If so, this would mean that survival rates among uninfected controls in our study are underestimated. Yet, with more systematic testing after the end of the first wave and with limited epidemic activity during the late spring and summer of 2020, it is unlikely that infections in controls were missed in that period, let alone that these infections would shorten the survival of the control groups. Similarly, survival rates in this group may have been negatively affected and thereby underestimated due to pandemic-related factors and restrictions affecting all LTC residents, such as isolations, visit restrictions, cancelled activities, staff shortages and limited hospital transfers. However, we are inclined to believe that this did not have a major influence during the follow-up period in the present study, because as described previously, the observed limited survival of the control group (1.6 years) is in line with data on residents of LTC facilities in the absence of COVID-19 from Sweden and elsewhere. In further support, excess death calculations for Sweden for 2020 and also for the entire pandemic period to end of 2021 and early 2022 show a substantial burden of excess deaths in the first wave, but overall very limited excess deaths or even a death deficit, when the full duration of the pandemic is considered.26–29 This pattern is congruent with the possibility that many residents who died of SARS-CoV-2 in the first wave had very limited life expectancy. Therefore, they would not contribute to excess death calculations if excess deaths were assessed over 1–2 years downstream. Accordingly, Aburto and colleagues28 estimated that life expectancy at birth fell by 0.59 year for women and 0.87 year for men between 2019 and 2020, and estimates of excess deaths for the year 2020 alone in Sweden suggest a substantial impact.27 However, when both 2020 and 2021 combined are considered, age-adjusted estimates of excess deaths suggest a death deficit (367 fewer deaths compared with what would be expected based on 2017–2019 mortality patterns)29 and the same applies to the first 19 weeks of 2022 for which data are available (a further death deficit of 244 deaths).29
Allowing for these caveats, a major strength of the present study is that it was based on data from large databases with nationwide coverage, which enabled matching on a large number of characteristics. Even so, similar analyses should also be performed in other countries because the health status of LTC residents may be different and with assessments covering also the vaccination period for a complete picture of the COVID-19 pandemic.30 This will allow to obtain more solid evidence on both the years of life lost over 2020–2022, as well as insights about the long-term outcomes of SARS-CoV-2-infected residents of various types of LTC facilities who survived and recovered from the acute infection.
Conclusions
In this matched cohort study of LTC residents, the risk of death after SARS-CoV-2 infection peaked during the first week after infection, and decreased after 2 months, with the latter decrease probably because the frailest residents died during the acute phase, leaving healthier residents remaining. The limited life expectancy in this population suggests that LTC resident status should be accounted for when estimating years of life lost due to COVID-19.
Supplementary Material
Footnotes
Twitter: @Marcel_Ballin
Contributors: MB, JPI, JB and PN conceived and designed the study. PN acquired the data. MB and JB analysed the data. MB, JPI, JB, MK, AN and PN interpreted the data. MB and JPI drafted the manuscript. MB, JPI, JB, MK, AN and PN critically revised the manuscript for important intellectual content. PN and JPI supervised the work. MB and JPI contributed equally to this work. MB is the guarantor and accepts full responsibility for the work and conduct of the study and had access to the data.
Funding: The authors received funding used for salaries from Foundation Stockholms Sjukhem (MK), Academy of Finland (MK), Läkaresällskapet (MK) and the Swedish Research Council (MK, AN, PN).
Disclaimer: The funders had no role in any part of this manuscript or the decision to publish.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data may be obtained from a third party and are not publicly available. The data files used for the present study are publicly unavailable according to regulations under Swedish law. However, all data used for the present study can be applied for from the National Board of Health and Welfare, Statistics Sweden and the Public Health Agency of Sweden. Statistical code underlying the results of the present analysis may be available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the Swedish Ethical Review Authority (2020-02552). The Swedish Ethical Review Authority waived the informed consent requirement given the retrospective nature of the study.
References
- 1.Comas-Herrera A, Zalakaín J, Lemmon E. Mortality associated with COVID-19 in care homes: international evidence. International Long-Term Care Policy Network, CPEC-LSE, 2020. LTCcovid.org [Google Scholar]
- 2.Panagiotou OA, Kosar CM, White EM, et al. Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Intern Med 2021;181:439–48. 10.1001/jamainternmed.2020.7968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murti M, Goetz M, Saunders A, et al. Investigation of a severe SARS-CoV-2 outbreak in a long-term care home early in the pandemic. Can Med Assoc J 2021;193:E681–8. 10.1503/cmaj.202485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballin M, Bergman J, Kivipelto M, et al. Excess mortality after COVID-19 in Swedish long-term care facilities. J Am Med Dir Assoc 2021;22:1574–80. 10.1016/j.jamda.2021.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edvinsson J, Rahm M, Trinks A, et al. Senior alert: a quality registry to support a standardized, structured, and systematic preventive care process for older adults. Qual Manag Health Care 2015;24:96–101. 10.1097/QMH.0000000000000058 [DOI] [PubMed] [Google Scholar]
- 6.Brooke HL, Talbäck M, Hörnblad J, et al. The Swedish cause of death register. Eur J Epidemiol 2017;32:765–73. 10.1007/s10654-017-0316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Statistical Database . Available: https://www.statistikdatabasen.scb.se/pxweb/en/ssd/START__BE__BE0101__BE0101I/LivslangdEttariga/ [Accessed 03 Mar 2022].
- 8.Rothera IC, Jones R, Harwood R, et al. Survival in a cohort of social services placements in nursing and residential homes: factors associated with life expectancy and mortality. Public Health 2002;116:160–5. 10.1016/S0033-3506(02)90005-3 [DOI] [PubMed] [Google Scholar]
- 9.Broad JB, Lumley T, Ashton T, et al. Transitions to and from long-term care facilities and length of completed stay: reuse of population-based survey data. Australas J Ageing 2017;36:E1–7. 10.1111/ajag.12378 [DOI] [PubMed] [Google Scholar]
- 10.Schön P, Lagergren M, Kåreholt I. Rapid decrease in length of stay in institutional care for older people in Sweden between 2006 and 2012: results from a population-based study. Health Soc Care Community 2016;24:631–8. 10.1111/hsc.12237 [DOI] [PubMed] [Google Scholar]
- 11.Espuny Pujol F, Hancock R, Morciano M. Trends in survival of older care home residents in England: a 10-year multi-cohort study. Soc Sci Med 2021;282:113883. 10.1016/j.socscimed.2021.113883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meinow B, Wastesson JW, Kåreholt I, et al. Long-term care use during the last 2 years of life in Sweden: implications for policy to address increased population aging. J Am Med Dir Assoc 2020;21:799–805. 10.1016/j.jamda.2020.01.003 [DOI] [PubMed] [Google Scholar]
- 13.Ioannidis JPA. Over- and under-estimation of COVID-19 deaths. Eur J Epidemiol 2021;36:581–8. 10.1007/s10654-021-00787-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferenci T. Different approaches to quantify years of life lost from COVID-19. Eur J Epidemiol 2021;36:589–97. 10.1007/s10654-021-00774-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von der Lippe E, Devleesschauwer B, Gourley M, et al. Reflections on key methodological decisions in national burden of disease assessments. Arch Public Health 2020;78:137. 10.1186/s13690-020-00519-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanlon P, Chadwick F, Shah A, et al. COVID-19 – exploring the implications of long-term condition type and extent of multimorbidity on years of life lost: a modelling study. Wellcome Open Res 2021;5:75. 10.12688/wellcomeopenres.15849.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020;396:1204–22. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Global Burden of Disease Study . Reference life table, 2019. Available: http://ghdx.healthdata.org/record/ihme-data/global-burden-disease-study-2019-gbd-2019-reference-life-table [Accessed 08 Mar 2022].
- 19.Ferrah N, Ibrahim JE, Kipsaina C, et al. Death following recent admission into nursing home from community living: a systematic review into the transition process. J Aging Health 2018;30:584–604. 10.1177/0898264316686575 [DOI] [PubMed] [Google Scholar]
- 20.Ioannidis JPA, Axfors C, Contopoulos-Ioannidis DG. Second versus first wave of COVID-19 deaths: shifts in age distribution and in nursing home fatalities. Environ Res 2021;195:110856. 10.1016/j.envres.2021.110856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pastorino R, Pezzullo AM, Villani L, et al. Change in age distribution of COVID-19 deaths with the introduction of COVID-19 vaccination. Environ Res 2022;204:112342. 10.1016/j.envres.2021.112342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Candel FJ, Barreiro P, San Román J, et al. The demography and characteristics of SARS-CoV-2 seropositive residents and staff of nursing homes for older adults in the community of Madrid: the SeroSOS study. Age Ageing 2021;50:1038–47. 10.1093/ageing/afab096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vena A, Berruti M, Adessi A, et al. Prevalence of antibodies to SARS-CoV-2 in Italian adults and associated risk factors. J Clin Med 2020;9:2780. 10.3390/jcm9092780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krutikov M, Palmer T, Tut G, et al. Incidence of SARS-CoV-2 infection according to baseline antibody status in staff and residents of 100 long-term care facilities (VIVALDI): a prospective cohort study. Lancet Healthy Longevity 2021;2:e362–70. 10.1016/S2666-7568(21)00093-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levi M, Cipriani F, Romeo G, et al. Analysis of the excess mortality and factors associated with deaths from COVID-19 versus other causes in central Tuscany (Italy) in 2020. Epidemiol Prev 2021;45:496–503. 10.19191/EP21.6.123 [DOI] [PubMed] [Google Scholar]
- 26.Kowall B, Standl F, Oesterling F, et al. Excess mortality due to Covid-19? A comparison of total mortality in 2020 with total mortality in 2016 to 2019 in Germany, Sweden and Spain. PLoS One 2021;16:e0255540. 10.1371/journal.pone.0255540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Human mortality database. Available: mortality.org [Accessed 15 Aug 2022].
- 28.Aburto JM, Schöley J, Kashnitsky I, et al. Quantifying impacts of the COVID-19 pandemic through life-expectancy losses: a population-level study of 29 countries. Int J Epidemiol 2022;51:63–74. 10.1093/ije/dyab207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levitt M, Zonta F, Ioannidis JPA. Comparison of pandemic excess mortality in 2020–2021 across different empirical calculations. Environ Res 2022;213:113754. 10.1016/j.envres.2022.113754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ioannidis JPA. The end of the COVID-19 pandemic. Eur J Clin Invest 2022;52:e13782. 10.1111/eci.13782 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The data files used for the present study are publicly unavailable according to regulations under Swedish law. However, all data used for the present study can be applied for from the National Board of Health and Welfare, Statistics Sweden and the Public Health Agency of Sweden. Statistical code underlying the results of the present analysis may be available from the corresponding author upon reasonable request.