Abstract
Objective: With the global epidemic of coronavirus disease 2019 (COVID-19), vaccination rates are increasing globally. This study evaluated the relevant clinical manifestations of vaccinated COVID-19 patients. Methods: We searched carefully in 11 databases such as PubMed, Embase, Scopus, Cochrane Library, Web of Science, Ovid, China National Knowledge Infrastructure Database, Wan Fang Data, Sinomed, VIP Database, and Reading Showing Database up to 26 March 2022. To search for articles that have described the characteristics of vaccinated patients including epidemiological and clinical symptoms. Statistical analysis of the extracted data using STATA 14.0. Results: A total of 58 articles and 263,708 laboratory-confirmed COVID-19 patients were included. Most of the patients in the vaccinated group had more asymptomatic infection and fewer severe illnesses. There were significant differences in ethnicity, and strain infected with COVID-19, and comorbidities (hyperlipidemia, diabetes, obesity, kidney disease, immunocompromised, cardiovascular disease, and tumor) and symptoms (fever, cough, gastrointestinal symptoms, neurological symptoms, and dysgeusia/anosmia) between vaccinated group and unvaccinated group. Oxygen support, use of steroid, days in hospital, hospital treatment, ICU treatment, death, and poor prognosis were also significantly different. Conclusion: Compared with the vaccinated group, patients in the unvaccinated group had a more severe clinical manifestations. Vaccines are also protective for infected people.
Keywords: COVID-19, vaccine, epidemiological characteristics, clinical characteristics, meta-analysis
Introduction
Coronavirus disease 2019 (COVID-19) first appeared in China in 2019 and was defined as a global pandemic in March 2020, which was a major global public health problem. As of 24 August 2022, there have been 595,219,966 confirmed cases of COVID-19, including 6,453,458 deaths.1 In response to the spread of COVID-19, vaccines are rapidly developed and administered. The World Health Organization also has approved 7 vaccines for emergency use.2 Various types of vaccines including mRNA vaccines, viral vector, inactivated vaccine, protein subunit, and DNA vaccines are being developed to control the spread of COVID-19.3 In December 2020, the mass vaccination program was launched,4 and as of 1 August, 2022, a total of 12,308,330,588 doses of vaccine have been administered worldwide.
Vaccination was significantly effective in reducing viral infections as well as hospitalizations and deaths.5–7 But with the emergence of variants of concern (VOCs), viruses with multiple clusters of mutations in the genome with higher infection rates, vaccines cannot play a 100% preventive effect8 and there are many patients infected with COVID-19 after being vaccinated. “Breakthrough infection” was defined as a patient who was fully vaccinated with two doses of the vaccine for more than 14 days.9 There are still some people who are hesitant to receive the COVID-19 vaccine, especially those living in developing countries.10 Residents' misunderstanding and distrust of vaccines may also lead to blind anti-vaccine movements.11
Unfortunately, it is not known whether VOCs can identify antibodies accurately, and whether mutations can evade vaccine-induced immunity and reduce vaccine activity is also of concern. According to some studies, vaccinated patients have lower risk factors for hospitalization, infectious diseases, and death.12,13 However, other studies have indicated that VOCs can evade antibody responses14 and there is a similar Cycle threshold (Ct) value15 between infected individuals who have not been vaccinated and have been vaccinated.
We conducted this study to assess whether vaccines were protective in COVID-19 patients. We would conduct this research by evaluating the characteristics of vaccinated COVID-19 patients from the epidemiological history, clinical symptoms, treatments, and prognosis of the patients.
Methods
This is a systematic review and meta-analysis following the international prospective register of systematic reviews. We obtained the registration number which was CRD42021293918. Follow the principles of PICOS (Population, Intervention, Comparison, Outcome, Study type). Populations were confirmed COVID-19 patients; intervention means patients had been vaccinated before COVID-19 infection; comparison of general data and clinical data between vaccinated and unvaccinated patients; outcome was to explore whether clinical data and general data were statistically different between two groups; and study types were case-control studies and cohort studies.
Search strategy
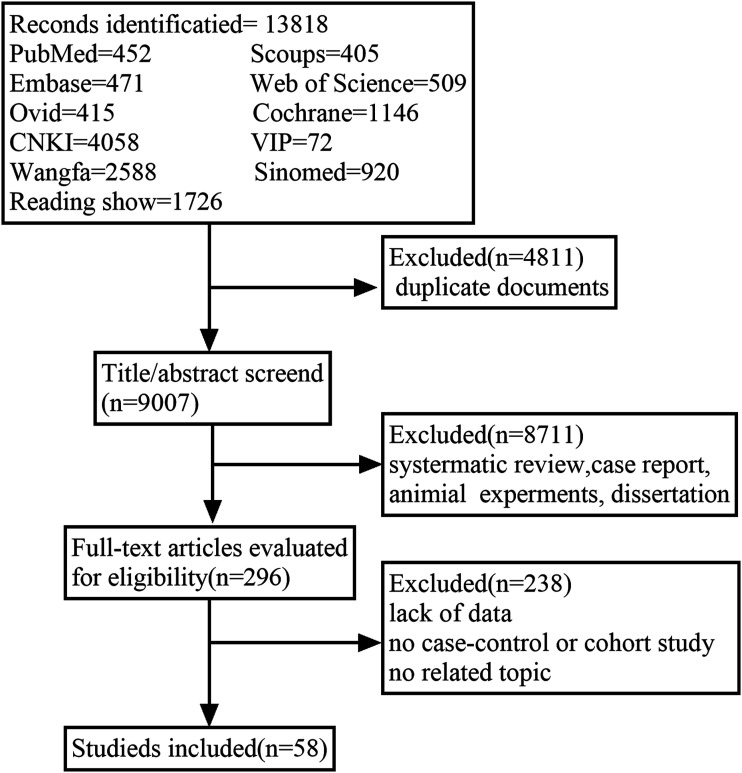
We had made a complete search plan and fully searched 11 databases including PubMed, Embase, Scopus, Cochrane Library, Web of Science, Ovid, China National Knowledge Infrastructure Database, Wan Fang Data, Sinomed, VIP Database, and Reading Showing Database from January 1, 2020 to March 26, 2022. We conducted a comprehensive search for relevant research in relevant fields, not specific countries, regions, or continents. We searched by using “COVID-19,” “COVID-19 Virus,” “SARS-CoV-2,” “coronavirus disease 2019,” “2019-nCoV,” “severe acute respiratory syndrome coronavirus 2 infection,” “Wuhan seafood market pneumonia virus,” “vaccine,” “vaccines,” “vaccination,” “breakthrough infections,” and “breakthrough.” The flow chart of the process and results of literature retrieval was shown in Figure 1.
Figure 1.
Flowchart of the retrieval process.
Study selection
The literature included in this systematic review and meta-analysis included studies with the following characteristics. (1) Patients need to be clearly diagnosed of COVID-19 such as COVID-19 RNA was detected by PCR, isolate the virus or IGM/IGG antibodies from the patient’s serum; (2) the study must be a case-control or cohort study; (3) the study must be including the vaccinated and unvaccinated COVID-19 patients; (4) the patient’s clinical characteristics including gender, age, symptoms, treatments, prognosis, and other relevant information should be exhibited.
Exclusion criteria were the following items: (1) duplicate literatures, (2) non‐Chinese or non-English researches, (3) lack of data or failing to extract data, and (4) meta-analysis and reviews.
Two review authors (Tian and Ren) independently selected articles for initial screening by reading the title and abstract of each article retrieved and then determined whether the full text should be read carefully according to inclusion or exclusion criteria. The final inclusion or exclusion of studies was discussed in consultation between two reviewers after reading the full text. If there is any disagreement, it will be discussed with the third reviewer.
Data extraction
We would extract the following information from the included studies including authors, publication information, sample size, gender, age, comorbidities such as diabetes, obesity, liver and kidney disease, clinical treatment such as antiviral drugs, steroid and supplementary oxygen required, prognosis, and clinical outcomes. It would be discussed with a third author when data extraction was in dispute.
Quality assessment
Each study we included would be assessed for quality by two reviewers using the Newcastle–Ottawa Scale (NOS).16
Meta-analysis
Statistical analysis was performed using Stata14.0. We assessed the heterogeneity of each study by using Cochran’s Q test and I2 test, if p-value > 0.1 and I2 < 50%, using a fixed-effects model, otherwise a random-effects model would be used. The possible sources of heterogeneity would be explored through subgroup analysis. A funnel plot was used to evaluate the publication bias of these studies.
Results
Literature search, basic information, and quality assessments
A total of 13,818 literatures were searched. After removing duplicate and did not meet the inclusion criteria literatures and reading 296 full-text articles, 58 literatures were included finally. The detail of the study was shown in Figure 1. The basic characteristics of each study were shown in Table 1. We used the NOS scale to evaluate the quality of each included study, and all of the studies were rated 6 stars or above.
Table 1.
Basic information and quality evaluation of included studies.
| Study | Type | Vaccinated | Unvaccinated | NOS | |||
|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome | Scores | ||||
| Li17 | Case-control study | 44 | 67 | ☆☆☆ | ☆☆ | ☆☆ | 7☆ |
| Balachandran12 | Case-control study | 346 | 1100 | ☆☆☆ | ☆☆ | ☆☆ | 7☆ |
| Wi18 | Case-control study | 142 | 20 | ☆☆ | ☆☆ | ☆☆ | 6☆ |
| Tenforde13 | Case-control study | 314 | 1669 | ☆☆☆ | ☆☆ | ☆☆ | 7☆ |
| Hsu19 | Case-control study | 85 | 85 | ☆☆ | ☆☆ | ☆☆☆ | 7☆ |
| Blanquart20 | Case-control study | 724 | 5459 | ☆☆☆ | ☆☆ | ☆☆ | 7☆ |
| Bollineni21 | Case-control study | 14 | 56 | ☆ | ☆☆ | ☆☆☆ | 6☆ |
| Marincu22 | Case-control study | 62 | 62 | ☆☆ | ☆☆ | ☆☆☆ | 7☆ |
| Wolff23 | Case-control study | 260 | 507 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Kalligeros24 | Case-control study | 91 | 824 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Fragoulis25 | Cohort study | 101 | 60 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Trunfio26 | Cohort study | 55 | 110 | ☆☆☆ | ☆☆ | ☆☆ | 7☆ |
| Safdar27 | Cohort study | 45 | 65 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Bouton28 | Cohort study | 96 | 329 | ☆☆ | ☆☆ | ☆☆☆ | 7☆ |
| Bosch29 | Case-control study | 126 | 839 | ☆☆ | ☆☆ | ☆☆☆ | 7☆ |
| Christensen30 | Case-control study | 3346 | 13,619 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Yu31 | Case-control study | 23 | 50 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Bayhan32 | Case-control study | 38 | 190 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Kustin33 | Case-control study | 396 | 396 | ☆☆ | ☆☆ | ☆☆ | 6☆ |
| Toda34 | Case-control study | 11 | 15 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Tian4 | Case-control study | 88 | 41 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Sayed35 | Case-control study | 50 | 70 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Lee36 | Case-control study | 174 | 587 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Luo37 | Case-control study | 484 | 1782 | ☆☆☆ | ☆☆ | ☆☆ | 7☆ |
| Puhach38 | Case-control study | 139 | 245 | ☆☆ | ☆☆ | ☆☆ | 6☆ |
| Whittaker39 | Case-control study | 716 | 2487 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Sanghavi40 | Case-control study | 149 | 478 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Butt (1)41 | Case-control study | 250 | 250 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Butt (2)42 | Case-control study | 456 | 456 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Spiera43 | Case-control study | 88 | 2317 | ☆☆ | ☆☆ | ☆☆☆ | 7☆ |
| Baltas44 | Case-control study | 119 | 476 | ☆☆☆ | ☆☆ | ☆☆ | 7☆ |
| John45 | Case-control study | 254 | 508 | ☆☆ | ☆☆ | ☆☆ | 6☆ |
| Jacobson46 | Case-control study | 189 | 471 | ☆☆ | ☆☆ | ☆☆ | 6☆ |
| Estofolete47 | Case-control study | 259 | 2518 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Servellita48 | Case-control study | 39 | 433 | ☆☆☆ | ☆ | ☆☆☆ | 7☆ |
| Thangaraj49 | Case-control study | 376 | 183 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Galan-huerta50 | Case-control study | 53 | 19 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Butt (3)51 | Case-control study | 2332 | 40,540 | ☆☆ | ☆☆ | ☆☆☆ | 7☆ |
| Romano52 | Case-control study | 34 | 86 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Basso53 | Case-control study | 155 | 81 | ☆☆ | ☆☆ | ☆☆☆ | 7☆ |
| Griffin54 | Case-control study | 12,179 | 29,989 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Padovani55 | Case-control study | 234 | 50 | ☆☆☆ | ☆☆ | ☆☆ | 7☆ |
| Anand56 | Case-control study | 3240 | 1551 | ☆☆ | ☆☆ | ☆☆☆ | 7☆ |
| Rovida57 | Case-control study | 33 | 20 | ☆☆ | ☆☆ | ☆☆☆ | 7☆ |
| Taquet58 | Case-control study | 10,024 | 83,957 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Cocchio59 | Case-control study | 773 | 12,499 | ☆☆ | ☆☆ | ☆☆☆ | 7☆ |
| Stupica60 | Case-control study | 175 | 354 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Ioannou61 | Cohort study | 24 | 31 | ☆☆ | ☆☆ | ☆☆☆ | 7☆ |
| Bahl62 | Cohort study | 954 | 10,880 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Muthukrishnan63 | Cohort study | 450 | 718 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Chia64 | Case-control study | 84 | 130 | ☆☆ | ☆☆ | ☆☆ | 6☆ |
| Zheng65 | Case-control study | 39 | 127 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Chen66 | Case-control study | 38 | 38 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Ma67 | Case-control study | 46 | 43 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Jiang68 | Case-control study | 85 | 362 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Yue69 | Case-control study | 20 | 55 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Papagoras70 | Case-control study | 48 | 147 | ☆☆ | ☆☆ | ☆☆☆ | 7☆ |
| Naik71 | Case-control study | 1010 | 1003 | ☆☆ | ☆☆ | ☆☆ | 7☆ |
Gender
A total of 44 articles were included in the study of gender. After the heterogeneity test, we found that I2 = 37.4% and p < 0.1, indicating that there was low heterogeneity, therefore a random-effect model was used. We found that OR = 0.995 (95% CI: 0.942–1.05, p = 0.849, Figure S1(a)), showing no statistical difference in gender between vaccinated and unvaccinated patients. Through subgroup analysis, we found heterogeneity of studies derived from studies published in 2022 (Figure S1(b)). The funnel plot of this study was symmetric by drawing a funnel plot (Figure S1(c)) and Egger’s test (p = 0.47).
Age
A total of 14 studies were included to analyze the effect of age. We found no statistical difference in the age of the two groups (SMD = 0.085, 95% CI = −0.073–0.189, p = 0.388, Figure S2(a)). Through subgroup analysis, we found that heterogeneity was mainly present in groups with more than 300 people in the population (Figure S2(b)).
Severity of disease
We would assess the severity of the disease, asymptomatic infection, and disease activity. We found that the severe patients in the vaccinated group were 0.247 times lower than those in the unvaccinated group (OR = 0.247, 95% CI: 0.128–0.474, p < 0.001, Figure S3(a)), and subgroup analysis found heterogeneity only present in studies with a population of 1000 (Figure S3(b)). The patients with asymptomatic infection were 1.686 times higher than those who were in the unvaccinated group (OR = 1.686, 95% CI: 1.277–2.225, p < 0.001, Figure S3(c)), and heterogeneity existed in studies with a vaccinated population of 30 and 1000 (Figure S3(d)). There was no statistical difference between the two groups in terms of disease activity (OR = 1.266, 95% CI: 0.598–2.68, p = 0.538, Figure S3(e)).
Epidemiology and personal history
The studies would be conducted from race, lineage, occupation, smoking history, and pregnancy history. We found noted that the vaccinated group being a Caucasian was higher than the unvaccinated group (OR = 1.52, 95% CI: 1.255–1.887, p < 0.001, Figure S4(a)); through subgroup analysis, we found that the source of heterogeneity came from studies with a total population more of than 10,000 (Figure S4(b)). For Black or African American (OR = 0.761, 95% CI: 0.517–1.121, p = 0.167, Figure S4(c)) and Asian (OR = 1.269, 95% CI: 0.65–2.476, p = 0.486, Figure S4(d)), there were no statistical difference between the two groups of patients. In vaccine variants, B.1.1.7 (OR = 1.609, 95% CI: 1.04–2.488, p = 0.032, Figure S4(e)) in the vaccinated group was 1.609 times higher than the unvaccinated group. And there were no statistically significant differences in occupational (doctors or non–doctors) (OR = 1.874, 95% CI: 0.835–4.202, p = 0.128, Figure S4(f)), pregnancy history (OR = 0.314, 95% CI: 0.086–1.15, p = 0.08, Figure S4(g)), and smoking (OR = 0.824, 95% CI: 0.668–1.071, p = 0.071, Figure S4(h)) between the two groups.
Comorbidity
We would analyze whether the presence of comorbidities and the different types of comorbidities. Including 8 literature studies, we found no difference in the presence of comorbidities between the vaccinated and unvaccinated groups (OR = 1.75, 95% CI: 0.936–3.273, p = 0.08, Figure S5(a)). Diabetes in the vaccine group was higher than in the unvaccinated group (OR = 1.407, 95% CI: 1.028–1.925, p = 0.033, Figure S5(b)). By subgroup analysis, heterogeneity was only present in studies with vaccinated groups of 2000 (Figure S5(c)). More patients with hyperlipidemia (OR = 1.806, 95% CI: 1.316–2.478, p < 0.001, Figure S5(d)) in the vaccinate group. However, obesity in the vaccinated group was lower than those in the vaccine group (OR = 0.873, 95% CI: 0.843–0.905, p < 0.001, Figure S5(e)). By subgroup analysis, heterogeneity exists only in studies with a total population of 10,000 (Figure S5(f)).
There were also more kidney disease (OR = 1.827, 95% CI: 1.182–2.823, p = 0.007, Figure S6(a)), immunocompromised patients (OR = 2.615, 95% CI: 1.671–4.092, p < 0.001, Figure S6(b)), and organ transplants (OR = 3.727, 95% CI: 2.682–5.18, p < 0.001, Figure S6(c)), and cardiovascular (OR = 1.667, 95% CI: 1.076–2.582, p = 0.022, Figure S6(d)), and tumors (OR = 1.296, 95% CI: 1.249–1.344, p < 0.001, Figure S6(e)) in the vaccinated group than the unvaccinated group.
There were no statistical differentials in liver disease (OR = 0.939, 95% CI: 0.871–1.012, p = 0.101, Figure S7(a)), lung disease (OR = 1.054, 95% CI: 0.673–1.65, p = 0.818, Figure S7(b)), hypertension (OR = 1.439, 95% CI: 0.878–2.356, p = 0.149, Figure S7(c)), and cerebrovascular disease (OR = 1.551, 95% CI: 0.74–3.25, p = 0.245, Figure S7(d)).
Symptom
Among the symptom of fever, the morbidity rate of patients in the vaccinated group was lower (OR = 0.583, 95% CI: 0.421–0.808, p = 0.001, Figure S8(a)). Patients had lower cough (OR = 0.718, 95% CI: 0.597–0.865, p < 0.001, Figure S8(b)), and gastrointestinal symptoms (OR = 0.743, 95% CI: 0.585–0.944, p = 0.015, Figure S8(c)) and dysgeusia/anosmia (OR = 0.449, 95% CI: 0.24–0.84, p = 0.012, Figure S8(d)) in the vaccinated group. However, among patients with neurological symptoms (OR = 1.950, 95% CI: 1.053–3.61, p = 0.034, Figure S8(e)), it was higher.
There was no statistical difference between the two groups of patients in the symptoms of anemia (OR = 2.183, 95% CI: 0.951–5.013, p = 0.066, Figure S9(a)), sore throat (OR = 0.785, 95% CI: 0.591–1.041, p = 0.093, Figure S9(b)), muscle and joint pain (OR = 0.56, 95% CI: 0.276–1.138, p = 0.109, Figure S9(c)), headache (OR = 0.77, 95% CI: 0.467–1.269, p = 0.305, Figure S9(d)), fatigue (OR = 0.362, 95% CI: 0.059–2.215, p = 0.271, Figure S9(e)), nausea/vomiting (OR = 1.106, 95% CI: 0.729–1.679, p = 0.636, Figure S9(f)), low oxygen saturation (OR = 0.79, 95% CI: 0.358–1.744, p = 0.559, Figure S9(g)), dyspnea (OR = 0.682, 95% CI: 0.483–1.021, p = 0.063, Figure S9(h)) and nasal congestion and runny nose (OR = 1.929, 95% CI: 0.85–4.379, p = 0.116, Figure S9(i)).
Treatment
We would analyze two aspects of supportive care and drug treatment. Patients in the vaccinated group required less oxygen support than those in the unvaccinated group (OR = 0.579, 95% CI: 0.418–0.801, p = 0.001, Figure S10(a)). Steroid use was lower in the vaccinated group than in the unvaccinated group (OR = 0.679, 95% CI: 0.496–0.929, p = 0.015, Figure S10(b)). There was no statistical difference in the use of antiviral drugs (OR = 0.896, 95% CI: 0.595–1.348, p = 0.597, Figure S10(c)) between the two groups of patients.
Hospitalization
A total of 4 studies compared the days of hospitalization and we found the duration in days in the vaccinated group was 0.79 times lower than the unvaccinated group (SMD = −0.790, 95% CI: −1.123 to −0.458, p < 0.001, Figure S11(a)). Fewer patients in the vaccinated group required hospitalization (OR = 0.660, 95% CI: 0.489–0.889, p = 0.006, Figure S11(b)), and ICU treatment (OR = 0.648, 95% CI: 0.479–0.876, p = 0.005, Figure S11(c)). There was no statistically significant difference between the two groups of patients who lived in a nursing facility for a long time (OR = 1.773, 95% CI: 0.571–5.5, p = 0.322, Figure S11(d)).
Outcome and prognosis
A total of 31 studies were included to investigate the prognosis of patients. And we found that the mortality rate of patients in the vaccinated group was 0.659 times lower than that of patients in the unvaccinated group (OR = 0.659, 95% CI: 0.462–0.94, p = 0.021, Figure S12(a)). In the study of patients with poor prognosis, we found that the poor prognosis of patients in the vaccinated group was 0.315 times lower than that of patients in the unvaccinated group (OR = 0.315, 95% CI: 0.148–0.668, p = 0.003, Figure S12(b)).
Discussion
We found that vaccination could reduce the severity of disease, which was consistent with the study of Giuseppe,72 and more patients with asymptomatic infection were found in the vaccinated group in our study. What plays a central role in vaccines are the antigens.73 Vaccines can stimulate the patient’s humoral and cellular immune systems. They play a role similar to live viruses,74 and stimulate the body to retain relevant stimulatory T cells and related memory B cells. Studies have shown that T cell responses play an important role in maintaining long-term immunity.75 When exposed to the virus, the body can quickly initiate immunity, reduce immune damage and the severity of the disease. Therefore, severe patients in the vaccine group were fewer, mostly asymptomatic infections, in line with global data.76–78
Statistical results show that vaccinated patients were more Caucasian than unvaccinated patients, which may be due to the uneven distribution of vaccines. Studies have shown that 4.2 billion doses of vaccines have been ordered by high-income countries, and 70% of vaccine candidates have been purchased.79 The B.1.1.7 strain, which was one of the four major mutants, with more infections in patients in the vaccine group. VOCs have more evasion capabilities for vaccines, changing the efficacy of neutralizing antibodies to affect the efficacy and effectiveness of vaccines. Animal models have demonstrated that the lack of neutralizing antibodies can lead to increased inflammation and poor clinical outcomes.80,81 Clinical studies have also shown that neutralizing antibodies are associated with faster virus clearance responses.9 Neutralizing antibodies in patients with vaccine breakthrough infection are significantly lower than those without breakthrough infection of patients.
For COVID-19 patients with pregnancy, there was no difference in infection between the two groups of patients, indicating that pregnancy did not increase the susceptibility of COVID-19’s infection. We discovered more comorbidities in the vaccine group than in the unvaccinated group. In a study by Kertes,82 it was found that patients with comorbidities had lower levels of antibodies than healthy individuals. Vaccination is more beneficial for patients with comorbidities and immunocompromised patients.83 Studies have shown that vaccinated patients have lower levels of autoimmune IG receptor antibodies and ACE2 receptor antibodies in transplant patients, kidney disease, malignancies, and autoimmune diseases, so they are more susceptible to infection.84
In our study, fever and cough, typical symptoms of COVID-19 patients, were lower in the vaccine group. Nevertheless, neurological symptoms and taste/smell disturbance symptoms were higher in the vaccine group, which was consistent with Sagar.85 This may be due to the lack of protection of the olfactory cleft and oral cavity by circulating antibodies.
We also found that patients in the vaccine group required less oxygen support and hormone steroids. Due to the characteristics of viral pneumonia, COVID-19 shows symptoms such as abnormal hemorrhagic gas, alveolar exudation, and accumulation of inflammatory markers of inflammatory factors. So oxygen therapy and symptomatic treatment are often used.86,87 For antiviral therapeutic drugs, such as Remdesivir, an RNA polymerase (RdRp) blocker, can inhibit the synthesis of viral nucleic acid, and favipiravir inhibits the synthesis of viral genomic RNA to achieve the effect of antiviral therapy.88 For immunosuppressants, studies have shown that such drugs can be used to block the effects of inflammatory cytokine storms.89
The results showed that patients in the vaccine group required fewer hospitalizations and ICU treatment, and the incidence of death or poor prognosis was lower, indicating that the vaccine was very important for the reduction of hospitalization and mortality. Which was consistent with real-world studies90 but inconsistent with research of Lee.91
Despite the fact that patients are still at risk of contracting COVID-19 after vaccination, our study indicates that vaccination provides a protective effect on us. Vaccines are the pillars of public health, and policy makers should encourage vaccination of people, especially occupational health service workers, whose occupational risk assessment plays an important role in preventing and combating COVID-19.92,93 And it is also suggested that for the emergence of different VOCs, the research of vaccines should be increased to maintain the effectiveness of vaccines in the population.
There are, however, limitations to this study as well. Laboratory data and antibodies of the two groups of patients are lacking, imaging characteristics of the different patients are lacking, and comparative research on cytokines has not been conducted. There are few research records on the Ct value and viral load of the two groups of patients, which cannot be included in this study. Therefore, we were unable to assess relative differences in viral load across groups of patients. Due to the different regions and sample sizes included in this study, the heterogeneity of the results of this study is also quite different, which may affect the accuracy of the results. More studies are expected to be added in the future to address these limitations.
Conclusion
Based on our study, the vaccine appears to provide a protective effect for patients with COVID-19, with significant improvements in symptoms, hospitalizations, and patient outcomes. Similarly, we also observed that Caucasians were more likely to be vaccinated, and that those who were vaccinated were more likely to be exposed to VOCs. Our research shows that vaccination is an effective tool for combating COVID-19.
Supplemental Material
Supplemental Material for Epidemiological and clinical characteristics of vaccinated COVID-19 patients: A meta-analysis and systematic review by Wen Tian, Xingxiang Ren, Mei Han, Yuanyuan Zhang, Xu Gao, Zhihai Chen, and Wei Zhang in International Journal of Immunopathology and Pharmacology
Acknowledgements
This manuscript has been approved for publication by all authors. This manuscript represents original research that has not been published elsewhere. A consensus has been reached among the authors regarding the content of this manuscript.
Author contribution: Research design, literature search, and article preparation were conducted by Wen Tian and Xingxiang Ren, statistical methods were contributed by Mei Han, data extraction and quality evaluation were completed by Xu Gao, Yuanyuan Zhang, and Zhihai Chen. Wei Zhang contributed research ideas and reviewed the manuscript. The final manuscript has been approved by all authors.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by National Key Research and Development Plan ( 2021YFC1712901), Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-C-202006), Beijing Hospitals Authority’s Ascent Plan (DFL20221601), and Changjiang Scholar Program of Chinese Ministry of Education
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Xingxiang Ren https://orcid.org/0000-0002-5140-1256
Wei Zhang https://orcid.org/0000-0001-7148-4776
References
- 1.WHO Coronavirus (COVID-19) Dashboard . (2022), https://covid19.who.int (acceseed 2022-8-25).
- 2.Chirico F, Teixeira Da Silva J, Tsigaris P, et al. (2022) Safety and effectiveness of COVID-19 vaccines: a narrative review. Indian J Med Res 155: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang M, Liang Y, Yu D, et al. (2022) A systematic review of vaccine breakthrough infections by SARS-CoV-2 delta variant. Int J Biol Sci 18: 889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian D, Song Y, Zhang M, et al. (2022) Genomic, immunological, and clinical analysis of COVID-19 vaccine breakthrough infections in Beijing, China. J Med Virol 94: 2237–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagan N, Barda N, Kepten E, et al. (2021) BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 384: 1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chemaitelly H, Yassine HM, Benslimane FM, et al. (2021) mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med 27: 1614–1621. [DOI] [PubMed] [Google Scholar]
- 7.Abu-Raddad LJ, Chemaitelly H, Butt AA. (2021) Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med 385: 187–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chirico F, Nucera G, Ilesanmi O, et al. (2022) Identifying asymptomatic cases during the mass COVID-19 vaccination campaign: insights and implications for policy makers. Future Virol 17: 141–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergwerk M, Gonen T, Lustig Y, et al. (2021) Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med 385: 1474–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Achrekar GC, Batra K, Urankar Y, et al. (2022) Assessing COVID-19 booster hesitancy and its correlates: an early evidence from India. Vaccines 10: 1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chirico F. (2018) The new Italian mandatory vaccine law as a health policy instrument against the anti-vaccination movement. Ann Ig 30: 251–256. [DOI] [PubMed] [Google Scholar]
- 12.Balachandran S, Moni M, Sathyapalan DT, et al. (2022) A comparison of clinical outcomes between vaccinated and vaccine-naive patients of COVID-19, in four tertiary care hospitals of Kerala, South India. Clin Epidemiol Glob Health 13: 100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tenforde MW, Self WH, Adams K, et al. (2021) Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA 326: 2043–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdool KS, de Oliveira T. (2021) New SARS-CoV-2 variants - clinical, public health, and vaccine implications. N Engl J Med 384: 1866–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown CM, Vostok J, Johnson H, et al. (2021) Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings - Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep 70: 1059–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stang A. (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Xu Y, Wang H, et al. (2022) Epidemiological and molecular characteristics of SARS-CoV-2 delta variant-caused pneumonia in Henan province in 2021. Chin J Microbiol Immunol 42: 11–15. [Google Scholar]
- 18.Wi YM, Kim S, Peck KR. (2022) An outbreak of breakthrough infections by the SARS-CoV-2 delta variant in a psychiatric closed ward. J Kor Med Sci 37: e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu L, Hurraß J, Kossow A, et al. (2022) Breakthrough infections with the SARS-CoV-2 delta variant: vaccinations halved transmission risk. Publ Health 204: 40–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanquart F, Abad C, Ambroise J, et al. (2021) Characterisation of vaccine breakthrough infections of SARS-CoV-2 delta and alpha variants and within-host viral load dynamics in the community, France, June to July 2021. Euro Surveill 26: 2100824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bollineni S, Mahan LD, Duncan P, et al. Characteristics and outcomes among vaccinated lung transplant patients with breakthrough COVID-19. Transpl Infect Dis 2021: e13784. [DOI] [PubMed] [Google Scholar]
- 22.Marincu I, Citu C, Bratosin F, et al. (2022) Clinical characteristics and outcomes of COVID-19 hospitalized patients: a comparison between complete mRNA vaccination profile and natural immunity. J Personalized Med: 12, 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolff M, Gilabert M, Hernandez R. (2022) Clinical outcomes in hospitalized vaccine-breakthrough COVID-19 cases compared with contemporary unvaccinated hospitalized adults. Open Forum Infect Di 9(4): ofac122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalligeros M, Shehadeh F, Mylona EK, et al. (2021) Clinical outcomes of adult patients hospitalized with COVID-19 after vaccination. Tropical Medicine and Infectious Disease 6(4): 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fragoulis GE, Karamanakos A, Arida A, et al. (2022) Clinical outcomes of breakthrough COVID-19 after booster vaccination in patients with systemic rheumatic diseases. RMD Open 8(1): e002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trunfio M, Verga F, Ghisetti V, et al. (2021) Clinical Phenotype and Contagiousness of Early Breakthrough SARS-CoV-2 Infections after BNT162b2 COVID-19 mRNA Vaccine: A Parallel Cohort Study in Healthcare Workers. Basel: Vaccines, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Safdar T, Shafqat F, Ansari AM, et al. (2021) Comparison of outcomes between vaccinated versus non-vaccinated COVID-19 patients, 15. [Google Scholar]
- 28.Bouton TC, Lodi S, Turcinovic J, et al. (2021) Coronavirus disease 2019 vaccine impact on rates of severe acute respiratory syndrome coronavirus 2 cases and postvaccination strain sequences among health care workers at an urban academic medical center: a prospective cohort study. Open Forum Infect Di 8(10): ofab465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosch W, Cowart JB, Bhakta S, et al. (2021) COVID-19 vaccine-breakthrough infections requiring hospitalization in mayo clinic Florida through August 2021. Clin Infect Dis 75(1): e892–e894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christensen PA, Olsen RJ, Long SW, et al. (2022) Delta variants of SARS-CoV-2 cause significantly increased vaccine breakthrough COVID-19 cases in Houston, Texas. Am J Pathol 192: 320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu ED, Wang E, Garrigan E, et al. (2021) Distinguishing COVID-19 Infection and Vaccination History by T Cell Reactivity. [Google Scholar]
- 32.Bayhan GI, Guner R. (2022) Effectiveness of CoronaVac in preventing COVID-19 in healthcare workers. Hum Vaccines Immunother 18(1): 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kustin T, Harel N, Finkel U, et al. (2021) Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med 27: 1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toda M, Yoshifuji A, Kikuchi K, et al. (2022) Factors associated with SARS-CoV-2 antibody titers and prognosis of breakthrough infection in hemodialysis patients. Clin Exp Nephrol 26(6): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sayed TM, Arshad SH, Aalam M, et al. (2021) ICU stay and mortality between vaccinated and non-vaccinated patients of Covid-19; a comparative study. Pakistan Journal of Medical and Health Sciences 15: 2789–2792. [Google Scholar]
- 36.Lee JE, Hwang M, Kim YH, et al. Imaging and clinical features of COVID-19 breakthrough infections: a multicenter study. Radiology 2022: 213072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo CH, Morris CP, Sachithanandham J, et al. (2022) Infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) delta variant is associated with higher recovery of infectious virus compared to the alpha variant in both unvaccinated and vaccinated individuals. Clin Infect Dis 75: e715–e725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puhach O, Adea K, Hulo N, et al. (2022) Infectious Viral Load in Unvaccinated and Vaccinated Patients Infected with SARS-CoV-2 WT. Delta and Omicron. [DOI] [PubMed] [Google Scholar]
- 39.Whittaker R, Kristofferson AB, Salamanca BV, et al. (2022) Length of hospital stay and risk of intensive care admission and in-hospital death among COVID-19 patients in Norway: a register-based cohort study comparing patients fully vaccinated with an mRNA vaccine to unvaccinated patients. Clin Microbiol Infect 28(6): 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanghavi DK, Bhakta S, Wadei HM, et al. (2022) Low anti-spike antibody levels correlate with poor outcomes in COVID-19 breakthrough hospitalizations. J Intern Med 292(1): 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butt AA, Nafady-Hego H, Chemaitelly H, et al. (2021) Outcomes among patients with breakthrough SARS-CoV-2 infection after vaccination. Int J Infect Dis 110: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butt AA, Yan P, Shaikh OS, et al. (2021) Outcomes among patients with breakthrough SARS-CoV-2 infection after vaccination in a high-risk national population. EClinicalMedicine 40: 101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spiera E, Ganjian DY, Zhang X, et al. (2022) Outcomes of COVID-19 infections in vaccinated patients with inflammatory bowel disease: data from an international registry. Inflamm Bowel Dis 28(7): 1126–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baltas I, Boshier FAT, Williams CA, et al. (2021) Post-vaccination coronavirus disease 2019: a case-control study and genomic analysis of 119 breakthrough infections in partially vaccinated individuals. Clin Infect Dis 75(2): 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.John BV, Deng Y, Schwartz KB, et al. (2022) Postvaccination COVID-19 infection is associated with reduced mortality in patients with cirrhosis. Hepatology 76(1): 126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacobson KB, Pinsky BA, Rath MEM, et al. (2021) Post-vaccination severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections and incidence of the presumptive B.1.427/B.1.429 variant among healthcare personnel at a northern California academic medical center. Clin Infect Dis 74(5): 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Estofolete CF, Fares GF, Banho CA, et al. (2022) Predictors of death in COVID-19 vaccine breakthrough infections in Brazil. J Infect 84(4): e22–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Servellita V, Morris MK, Sotomayor-Gonzalez A, et al. (2022) Predominance of antibody-resistant SARS-CoV-2 variants in vaccine breakthrough cases from the San Francisco Bay Area, California. Nat Microbiol 7: 277–288. [DOI] [PubMed] [Google Scholar]
- 49.Thangaraj J, Yadav P, Kumar CG, et al. (2022) Predominance of delta variant among the COVID-19 vaccinated and unvaccinated individuals, India, May 2021. J Infect 84: 94–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galan-Huerta KA, Flores-Trevino S, Salas-Trevino D, et al. (2022) Prevalence of SARS-CoV-2 variants of concern and variants of interest in COVID-19 breakthrough infections in a hospital in Monterrey, Mexico. Viruses 14(1): 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Butt AA, Yan P, Shaikh OS, et al. (2021) Rate and risk factors for severe/critical disease among fully vaccinated persons with breakthrough SARS-CoV-2 infection in a high-risk national population. Clin Infect Dis 75(1): e849–e856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romano A, Cerchione C, Conticello C, et al. (2022) Reduced absolute count of monocytes in patients carrying hematological neoplasms and SARS-CoV2 infection. Cancers 14(5): 1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basso P, Negro C, Cegolon L, et al. (2022) Risk of vaccine breakthrough SARS-CoV-2 infection and associated factors in healthcare workers of Trieste teaching hospitals (North-Eastern Italy). Viruses-Basel 14(2): 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Griffin JB, Haddix M, Danza P, et al. (2021) SARS-CoV-2 infections and hospitalizations among persons aged ≥16 years, by vaccination status — Los Angeles county, California, May 1–July 25, 2021. MMWR. Morbidity and Mortality Weekly Report 70: 1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Padovani A, Cristillo V, Tomasoni D, et al. (2021) SARS-CoV-2 Vaccination Predicts COVID-19 Progression and Outcomes in Hospitalized Patients. [Google Scholar]
- 56.Anand S, Montez-Rath ME, Han J, et al. (2021) SARS-CoV-2 Vaccine Antibody Response and Breakthrough Infection in Dialysis.medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rovida F, Cassaniti I, Paolucci S, et al. (2021) SARS-CoV-2 vaccine breakthrough infections with the alpha variant are asymptomatic or mildly symptomatic among health care workers. Nat Commun 12: 6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taquet M, Dercon Q, Harrison PJ. (2021) Six-month Sequelae of Post-vaccination SARS-CoV-2 Infection: A Retrospective Cohort Study of 10,024 Breakthrough Infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cocchio S, Zabeo F, Facchin G, et al. (2022) The Effectiveness of a Diverse COVID-19 Vaccine Portfolio and Its Impact on the Persistence of Positivity and Length of Hospital Stays: The Veneto Region's Experience. Basel: Vaccines, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stupica D, Collinet-Adler S, Kejžar N, et al. (2022) The impact of SARS-CoV-2 primary vaccination in a cohort of patients hospitalized for acute COVID-19 during delta variant predominance. J Clin Med 11(5): 1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ioannou P, Karakonstantis S, Astrinaki E, et al. (2021) Transmission of SARS-CoV-2 variant B.1.1.7 among vaccinated health care workers. Infect Dis (Lond) 53: 876–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bahl A, Johnson S, Maine G, et al. (2021) Vaccination reduces need for emergency care in breakthrough COVID-19 infections: a multicenter cohort study. Lancet Reg Health Am 4: 100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muthukrishnan J, Vardhan V, Mangalesh S, et al. (2021) Vaccination status and COVID-19 related mortality: a hospital based cross sectional study. Med J Armed Forces India 77: S278–S282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chia PY, Ong S, Chiew CJ, et al. (2021) Virological and serological kinetics of SARS-CoV-2 delta variant vaccine breakthrough infections: a multicentre cohort study. Clin Microbiol Infect 28(4): 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng D, Weng H, Liu Y, et al. (2021) Clinical characteristics of coronavirus disease 2019 infected with delta variant in guangzhou: a real-world study. Chin J Emerg Med 30: 1220–1228. [Google Scholar]
- 66.Chen M, Zhou Y, Peng H, et al. (2021) Clinical characteristics of imported COVID-19 patients after inoculating inactivated vaccine. Chin J Infect Control 20: 586–591. [Google Scholar]
- 67.Ma N, Zhang Y, Han H, et al. (2022) Analysis in antibody levels in blood of patients infected with coronavirus disease 2019 after inoculation with novel coronavirus inactivated vaccine and disease status. Journal of Clinical Medicine in Practice 26: 117–119. [Google Scholar]
- 68.Jiang X, Yue Y, Li M, et al. (2022) Effects of SARS-CoV-2 vaccination on clinical and immune features of imported COVID-19 cases in Chengdu. China Preventive Medicine 23: 32–36. [Google Scholar]
- 69.Yue Y, Liang X, Mao Y, et al. (2021) Influence of SARS-CoV-2 vaccination on the epidemiological and clinical characteristics of imported COVID-19 cases in Chengdu. Chin J Epidemiol 42: 1365–1370. [DOI] [PubMed] [Google Scholar]
- 70.Papagoras C, Fragoulis GE, Zioga N, et al. (2022) Better outcomes of COVID-19 in vaccinated compared to unvaccinated patients with systemic rheumatic diseases. Ann Rheum Dis 81: 1013–1016. [DOI] [PubMed] [Google Scholar]
- 71.Naik BR, Kumar SA, Rachegowda N, et al. (2022) Severity of COVID-19 infection using chest computed tomography severity score index among vaccinated and unvaccinated COVID-19-positive healthcare workers: an analytical cross-sectional study. Cureus 14: e22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Novelli G, Biancolella M, Mehrian-Shai R, et al. (2021) COVID-19 one year into the pandemic: from genetics and genomics to therapy, vaccination, and policy. Hum Genomics 15: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mattoo SS, Myoung J. (2021) A promising vaccination strategy against COVID-19 on the horizon: heterologous immunization. J Microbiol Biotechnol 31: 1601–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sahin U, Derhovanessian E, Miller M, et al. (2017) Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547: 222–226. [DOI] [PubMed] [Google Scholar]
- 75.Zhao J, Zhao J, Mangalam AK, et al. (2016) Airway memory CD4(+) T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity 44: 1379–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao J, Zhao J, Perlman S. (2010) T cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus-infected mice. J Virol 84: 9318–9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Channappanavar R, Fett C, Zhao J, et al. (2014) Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J Virol 88: 11034–11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.https://www.cdc.gov/coronavirus/2019-ncov/ (2022).
- 79.Wouters OJ, Shadlen KC, Salcher-Konrad M, et al. (2021) Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet 397: 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Houser KV, Broadbent AJ, Gretebeck L, et al. (2017) Enhanced inflammation in New Zealand white rabbits when MERS-CoV reinfection occurs in the absence of neutralizing antibody. PLoS Pathog 13: e1006565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiao AT, Gao C, Zhang S. (2020) Profile of specific antibodies to SARS-CoV-2: The first report. J Infect 81: 147–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kertes J, Gez SB, Saciuk Y, et al. (2021) The BNT162b2 Vaccine Was Found to Be Less Effective in Protecting against Covid-19 Infection after Six Months, and Vaccination with a Third Dose Is Indicated.medRxiv. [Google Scholar]
- 83.Munro C. (2021) Covid-19: 40% of patients with weakened immune system mount lower response to vaccines. BMJ 374: n2098. [DOI] [PubMed] [Google Scholar]
- 84.Sogaard OS, Reekie J, Johansen IS, et al. (2022) Characteristics associated with serological COVID-19 vaccine response and durability in an older population with significant comorbidity: the Danish Nationwide ENFORCE Study. Clin Microbiol Infect 28(8): 1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sagar P, Kumar R, Thakar A. (2021) Archetype of olfactory and gustatory dysfunction in breakthrough COVID-19 illness. Indian J Otolaryngol Head Neck Surg 25: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Velavan TP, Meyer CG. (2020) The COVID-19 epidemic. Trop Med Int Health 25: 278–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cao X. (2020) COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol 20: 269–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu C, Liu Y, Yang Y, et al. (2020) Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B 10: 766–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Levy G, Guglielmelli P, Langmuir P, et al. (2022) JAK inhibitors and COVID-19. J. Immunother Cancer 10: e002838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tartof SY, Slezak JM, Fischer H, et al. (2021) Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet 398: 1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee CJ, Woo W, Kim AY, et al. (2022) Clinical manifestations of COVID-19 breakthrough infections: a systematic review and meta-analysis. J Med Virol 94: 4234–4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chirico F, Sacco A. (2022) Enhancing the role of occupational health services in the battle against Corona virus disease 2019. Ann Ig 34: 537–541. [DOI] [PubMed] [Google Scholar]
- 93.Chirico F, Nucera G, Sacco A, et al. (2022) Protecting hospitals from SARS-CoV-2 infection: a review-based comprehensive strategy for COVID-19 prevention and control. G Ital Med Lav Erg 1: 32–40. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Epidemiological and clinical characteristics of vaccinated COVID-19 patients: A meta-analysis and systematic review by Wen Tian, Xingxiang Ren, Mei Han, Yuanyuan Zhang, Xu Gao, Zhihai Chen, and Wei Zhang in International Journal of Immunopathology and Pharmacology