Abstract
We have shown that injury to alveolar epithelial type I cells may account, in part, for damage to the air-blood barrier of the lung in a rat model of Staphylococcus aureus pneumonia. We have also shown that alpha-toxin is an important cause of damage to the air-blood barrier; however, our data suggest that the toxin is not acting directly on alveolar type I cells.
Although Staphylococcus aureus is a significant cause of nosocomial pneumonia (19), little is known about the role of specific virulence factors for the induction of lung injury. Alpha-toxin is an important virulence factor in experimental models of mastitis (4), peritonitis (14), and corneal keratitis (13). Alpha-toxin monomers bind cell membranes and then associate in a heptameric complex to form a pore (17). The effects of alpha-toxin are both concentration and cell-type dependent and include cell lysis (1, 2), release of proinflammatory mediators and cytokines (6), and induction of apoptosis (7).
Purified alpha-toxin, administered through the vasculature, causes injury to the air-blood barrier in isolated perfused lungs (16). Specifically, alpha-toxin increases the permeability of the lung to fluid and causes necrosis of capillary endothelial cells (16). In this study, we determined the role of alpha-toxin in vivo by developing a rat model of S. aureus-induced pneumonia.
Alveolar epithelial type I cells cover 95% of the lungs’ surface and are part of a tight epithelial barrier that is vital for maintaining a dry alveolus (8). Until recently, it was difficult to assess the extent of injury to type I cells other than by quantitative morphologic analysis at the electron microscopic level. However, we have recently demonstrated that the content of a type I cell-specific protein, rTI40 (5, 15), in bronchoalveolar lavage (BAL) fluid is associated with morphologic injury to type I cells (9–11). In this study, we used the rTI40 assay to determine the extent of damage to alveolar epithelial type I cells in S. aureus-induced pneumonia.
Bacterial strains 8325-4 and DU1090 were used to establish pneumonia. 8325-4 is an hla-positive strain derived from NCTC 8325. DU1090 is an alpha-toxin-defective mutant of 8325-4 constructed by allelic replacement (14). For inoculations, cultures of S. aureus were grown for 18 h with aeration in Todd-Hewitt broth (Oxoid). S. aureus was washed twice in sterile phosphate-buffered saline (PBS) before finally being resuspended in PBS. The number of viable bacterial cells was measured by colony counts. Production of alpha-toxin by S. aureus isolated from BAL fluid samples was confirmed by culture on 5% calf blood agar plates (4).
Rat model.
Rats (Sprague-Dawley, male, 300 to 350 g; University College, Dublin, Ireland) were anesthetized with 4% halothane. Rats were then intubated through a tracheotomy with a blunt 16-gauge needle and ventilated with 100% oxygen at a rate of 70 breaths per min, a tidal volume of 3.5 ml, and positive end expiratory pressure of 1.5 cm of H2O. Anesthesia was maintained with halothane (0.5 to 1.0%). Mean arterial pressure was measured with a carotid arterial catheter. The neuromuscular response was blocked with pancuronium (0.3 mg per kg of body weight).
After a baseline period of 1 h, S. aureus was instilled into the left lung of the anesthetized, ventilated rats, as described previously (9). At the end of the experimental period (4 h from the beginning of the instillation), rats were killed by exsanguination. In the experimental groups, control rats received 1 ml of PBS. 8325-4-infected rats received two different doses: (6.1 ± 1.1) × 108 or (3.53 ± 0.36) × 109 CFU. DU1090-infected rats received a single dose of (4.04 ± 0.87) × 109 CFU.
BAL fluid analysis.
The left bronchus was intubated, and the lung was lavaged with a total of 7 ml of PBS (4 ml and then 3 ml). The returned BAL fluid was pooled, and the total number of leukocytes was counted. The amount of rTI40 in BAL fluid was determined with an enzyme-linked immunosorbent assay (ELISA)-based assay, as previously described (9–11). Data are presented in relative densitometry units (RDU). The molecular weight of rTI40 was determined in BAL fluid by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting by standard methods (9, 11). The protein concentration of BAL fluid was determined by using the Bradford assay. The hemoglobin content was determined by measuring the oxidation of 3,3′5,5′-tetramethylbenzidine (Sigma diagnostic kit). Alpha-toxin protein was detected by Western and ELISA-based dot blot analyses.
Histology.
Lungs were stained with hematoxylin and eosin to determine the overall extent of leukocyte recruitment to the airspaces or Gram stain to confirm the location of S. aureus. For detection of rTI40, lungs were fixed with 4% paraformaldehyde and frozen; thin sections were then cut (5 μm). Lung sections were incubated with anti-rTI40 hybridoma supernatant (5), followed by fluorescein isothiocyanate-linked antimouse immunoglobulin (Organon Teknika, West Chester, Pa.) sections were viewed in a Zeiss fluorescent microscope.
Alveolar epithelial cell isolation.
Alveolar type II cells were isolated from rat lungs by using elastase (5). Type II cells were plated into 12-well plates and grown at 37°C in 5% CO2, in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum. Alveolar epithelial cells acquire a type I cell-like phenotype after 7 days in culture, including the expression of rTI40 (3, 5). Day 7 cells were exposed to purified alpha-toxin (0.01 to 10 μg/ml) for 4 h in serum-free medium at 37°C. Cell necrosis was determined by measuring lactate dehydrogenase activity and rTI40 content in cells and culture medium.
Statistical analysis of the differences between means was carried out by one-way analysis of variance, and Bonferroni’s multiple comparison test was performed when appropriate. Difference levels of P < 0.05 were considered significant.
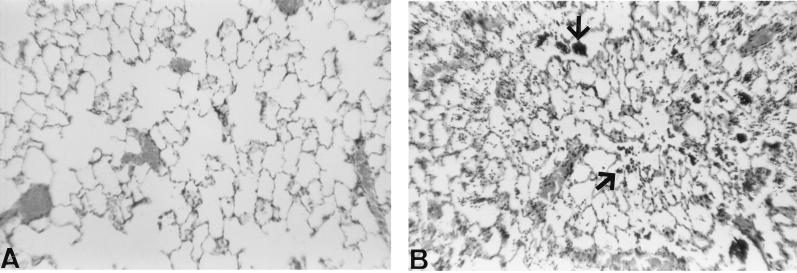
There were numerous leukocytes (predominately macrophages and neutrophils) and bacteria in the airspaces of lungs from 8325-4-infected rats in comparison with control rats (Fig. 1). Gram-stained lung sections demonstrated that gram-positive cocci were predominately associated with leukocytes in the airspaces (data not shown). In addition, both 8325-4 and DU1090 stimulated an inflammatory response, as assessed by the increased number of leukocytes recovered in BAL fluid (Table 1). However, there was no significant difference in the number of leukocytes recovered in BAL fluid between 8325-4- and DU1090-infected rats.
FIG. 1.
Representative photographs of hematoxylin-and-eosin-stained lung 4 h after distal airway instillation of PBS (A) or S. aureus 8325-4 (B). Strain 8325-4 caused an influx of inflammatory cells into the airspaces (arrows) in comparison with the section from control lung. Original magnification, ×200.
TABLE 1.
Effect of S. aureus 8325-4 and DU1090 on the total amount of protein, rTI40, number of leukocytes and hemoglobin recovered in BAL fluid
| Treatment group | Innoculum size (CFU) | n | Amt of protein (mg)a | Amt of rTI40 (RDU)a | No. of leukocytes (106)a | Amt of hemoglobin (μg)a |
|---|---|---|---|---|---|---|
| Control | None | 5 | 2.04 ± 0.86 | 31.8 ± 7.45 | 0.19 ± 0.06 | 8.87 ± 5.60 |
| 8325-4 | 6 × 108 | 4 | 7.07 ± 3.40b | 35.6 ± 7.02 | 3.14 ± 1.30 | 127 ± 51.4 |
| 4 × 109 | 5 | 24.6 ± 5.18c | 92.8 ± 19.8b | 2.84 ± 1.70 | 1,160 ± 372c | |
| DU1090 | 4 × 109 | 5 | 7.88 ± 1.46d | 33.9 ± 9.36d | 1.84 ± 0.35 | 43.4 ± 15.6d |
Values are means ± standard errors.
P < 0.05 for 8325-4 versus control.
P < 0.01 for 8325-4 versus control.
P < 0.05 for 8325-4 (109 CFU) versus DU1090 (109 CFU).
We were unable to detect alpha-toxin in BAL fluid by Western blot or ELISA-based dot blot analysis (although we could detect nanogram levels of the purified toxin) (data not shown). However, the total amount of hemoglobin (a measure of erythrocyte lysis) recovered in BAL fluid from 8325-4-infected rats was elevated above values obtained from both control and DU1090-infected rats (Table 1). Erythrocytes could be lysed by either S. aureus alpha- or beta-toxin. However, since the extent of beta-toxin production is the same in 8325-4 and DU1090 strains (12), the difference in BAL hemoglobin content is likely to be due to alpha-toxin activity. These data therefore suggest that alpha-toxin is produced in vivo within our experimental time frame (4 h).
The total amount of protein recovered in BAL fluid from 8325-4-infected rats (3.5 × 109 CFU) was 12-fold higher than the control values (Table 1), while the amount of protein recovered in BAL fluid from DU1090-infected rats (4 × 109 CFU) was elevated only 4-fold (Table 1). In rats that received a lower innoculum of 8325-4, BAL protein was elevated only 2.5-fold above control values (Table 1). These data demonstrate that strain 8325-4 impaired the function of the lung’s air-blood barrier and that alpha-toxin contributed significantly to this injury.
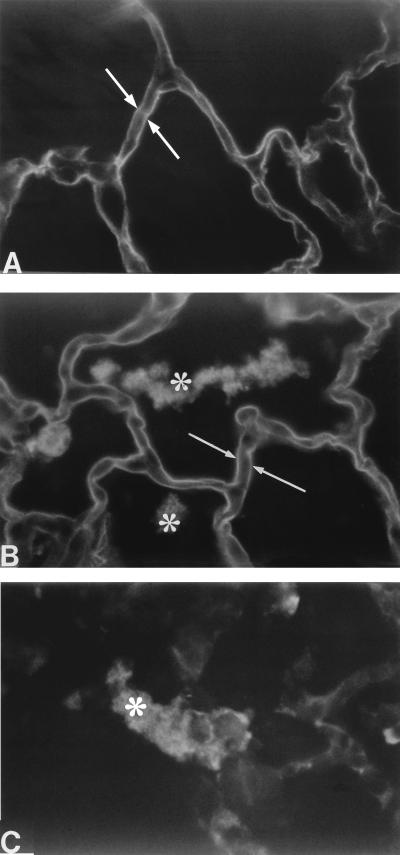
To determine whether damage to alveolar epithelial type I cells was responsible for the influx of protein into the airspaces, we measured the amount of rTI40 in BAL fluid. The amount of rTI40 recovered in BAL fluid from 8325-4-infected rats (3.5 × 109 CFU) was elevated threefold above control values (Table 1). However, the amount of rTI40 in BAL fluid from DU1090-infected rats was not significantly different from control values (Table 1). The molecular mass of rTI40 in BAL fluid from all groups was determined to be 42 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting (data not shown). Immunofluorescence analysis showed binding of the anti-rTI40 monoclonal antibody to the apical surface of alveolar epithelial type I cells in lung sections from both 8325-5-infected and control rats (Fig. 2A and B). In agreement with the relatively small increase in BAL fluid levels of rTI40 from 8325-4-infected rats, there was no qualitative difference in the binding of anti-rTI40 monoclonal antibody between 8325-4-infected and control lung sections (Fig. 2A).
FIG. 2.
Immunofluorescence detection of rTI40 4 h after distal airway instillation of either PBS (A) or S. aureus 8325-4 (B). White arrows show binding of anti-rTI40 monoclonal antibody to alveolar type I cells. Stars show binding of secondary antibody to S. aureus in the airspaces (B and C) (probably due to antibody binding to protein A in the cell wall of S. aureus). DU1090-infected lungs were not different from control lungs (data not shown). Original magnification, ×500.
Previous studies have shown that the amount of rTI40 recovered in BAL fluid is associated with the extent of morphologic injury to type I cells (9–11). For example, nitrogen dioxide and hyperoxic lung injury induced an approximately twofold increase in levels of rTI40 in BAL fluid (10, 11). Both of these lung injury models are characterized by limited alveolar epithelial type I cell damage, although the location of type I cell injury is distinct between the models (10, 11). On the other hand, in a rat model of Pseudomonas aeruginosa-induced lung injury, the content of rTI40 in distal airway fluid was elevated 40-fold after 4 h (9). In addition, there was major alveolar epithelial type I cell necrosis in the P. aeruginosa-infected lungs (9). Since the total amount of rTI40 recovered in BAL fluid was elevated only by threefold above control values, our data suggest that the extent of type I cell necrosis in S. aureus-induced acute pneumonia is minimal.
In contrast to 8325-4-infected rats, levels of rTI40 in BAL fluid from DU1090-infected rats were similar to control values. These data suggest that alpha-toxin is responsible for the increased levels of rTI40 recovered in BAL fluid from 8325-4-infected rats. However, relatively high concentrations of purified alpha-toxin (10 μg/ml) over a 4-h incubation period did not cause necrosis of alveolar epithelial cells in culture; the percentages of lactate dehydrogenase and rTI40 released were not different from control values (data not shown). Cultured endothelial cells are lethally injured by alpha-toxin at 1 μg/ml (18). Furthermore, purified alpha-toxin causes morphologic injury to endothelial cells and fluid accumulation in the alveolar wall and airspaces in an isolated lung model (16). Therefore, type I cell damage may be secondary to fluid accumulation in the airspaces. The precise mechanism of alpha-toxin injury to alveolar epithelial type I cells will be investigated in future work.
In summary, our data demonstrate that the function of the air-blood barrier is impaired in S. aureus-induced pneumonia, which is, in part, accounted for by damage to alveolar epithelial type I cells. However, although our data demonstrate that alpha-toxin is an important cause of damage to the air-blood barrier in vivo, our data suggest that the toxin is probably not acting directly on type I cells.
Acknowledgments
We thank Leland Dobbs for the anti-rTI40 monoclonal antibody. We also thank Sucharit Bhakdi for purified alpha-toxin, for a polyclonal antibody against alpha-toxin, and for helpful comments on our manuscript. We are grateful for the technical assistance of Philipa Marks.
This research was supported by the Provost’s fund, Trinity College Dublin, The Health Research Board of Ireland, the MRC, and the Wellcome Trust. M.C.M. is a Faculty of Medicine Fellow (University of Edinburgh).
REFERENCES
- 1.Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991;55:733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhakdi S, Walev I, Jonas D, Palmer M, Weller U, Suttorp N, Grimminger F, Seeger W. Pathogenesis of sepsis syndrome: possible relevance of pore-forming bacterial toxins. Curr Top Microbiol Immunol. 1996;216:101–118. doi: 10.1007/978-3-642-80186-0_5. [DOI] [PubMed] [Google Scholar]
- 3.Borok Z, Danto S I, Lubman R L, Cao Y, Williams M C, Crandall E D. Modulation of TI alpha expression with alveolar epithelial cell phenotype in vitro. Am J Physiol. 1998;275:L155–L164. doi: 10.1152/ajplung.1998.275.1.L155. [DOI] [PubMed] [Google Scholar]
- 4.Bramley A J, Patel A H, O’Reilly M, Foster R, Foster T J. Roles of alpha-toxin and beta-toxin in virulence of Staphylococcus aureus for the mouse mammary gland. Infect Immun. 1989;57:2489–2494. doi: 10.1128/iai.57.8.2489-2494.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobbs L G, Williams M C, Gonzalez R. Monoclonal antibodies specific to the apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated type II cells. Biochim Biophys Acta. 1988;970:146–156. doi: 10.1016/0167-4889(88)90173-5. [DOI] [PubMed] [Google Scholar]
- 6.Grimminger F F, Rose U, Sibelius U, Meinhardt M, Potzsch B, Spriestersbach R, Bhakdi S, Suttorp N, Seeger W. Human endothelial cell activation and mediator release in response to the bacterial exotoxins Escherichia coli hemolysin and staphylococcal alpha-toxin. J Immunol. 1997;159:1909–1916. [PubMed] [Google Scholar]
- 7.Jonas D, Walev I, Berger T, Liebetrau M, Palmer M, Bhakdi S. Novel path to apoptosis: small transmembrane pores created by staphylococcal alpha-toxin in T lymphocytes evoke internucleosomal DNA degradation. Infect Immun. 1994;62:1304–1312. doi: 10.1128/iai.62.4.1304-1312.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthay M A, Folkesson H G, Verkman A S. Salt and water transport across alveolar and distal airway epithelia in the adult lung. Am J Physiol. 1996;270:L487–L503. doi: 10.1152/ajplung.1996.270.4.L487. [DOI] [PubMed] [Google Scholar]
- 9.McElroy M C, Pittet J F, Hashimoto S, Allen L, Wiener-Kronish J P, Dobbs L G. A type I cell-specific marker is a biochemical marker of epithelial injury in a rat model of pneumonia. Am J Physiol. 1995;268:L181–L186. doi: 10.1152/ajplung.1995.268.2.L181. [DOI] [PubMed] [Google Scholar]
- 10.McElroy M C, Wiener-Kronish J P, Miyazaki H, Sawa T, Modelska K, Dobbs L G, Pittet J F. Nitric oxide attenuates lung endothelial injury caused by sublethal hyperoxia in rats. Am J Physiol. 1997;272:L631–L638. doi: 10.1152/ajplung.1997.272.4.L631. [DOI] [PubMed] [Google Scholar]
- 11.McElroy M C, Pittet J F, Allen L, Wiener-Kronish J P, Dobbs L G. Biochemical detection of type I cell damage after nitrogen dioxide-induced lung injury in rats. Am J Physiol. 1997;273:L1228–L1234. doi: 10.1152/ajplung.1997.273.6.L1228. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson I-M, Hartford O, Foster T, Tarkowski A. Alpha-toxin and gamma-toxin jointly promote Staphylococcus aureus virulence in murine septic arthritis. Infect Immun. 1999;67:1045–1049. doi: 10.1128/iai.67.3.1045-1049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Callaghan R J, Callegan M C, Moreau J M, Green L C, Foster T J, Hartford O M, Engel L S, Hill J M. Specific roles of alpha-toxin and beta-toxin during Staphylococcus aureus corneal infection. Infect Immun. 1997;65:1571–1578. doi: 10.1128/iai.65.5.1571-1578.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel A H, Nowlan P, Weavers E D, Foster T. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect Immun. 1987;55:3103–3110. doi: 10.1128/iai.55.12.3103-3110.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rishi A K, Joyce-Brady M, Fisher J, Dobbs L G, Floros J, VanderSpek J, Brody J S, Williams M C. Cloning, characterization, and development expression of a rat lung alveolar type I cell gene in embryonic endodermal and neural derivatives. Dev Biol. 1995;167:294–306. doi: 10.1006/dbio.1995.1024. [DOI] [PubMed] [Google Scholar]
- 16.Seeger W, Birkemeyer R G, Ermert N, Suttorp N, Bhakdi S, Duncker H-R. Staphylococcal alpha-toxin-induced vascular leakage in isolated perfused rabbit lungs. Lab Investig. 1990;63:341–349. [PubMed] [Google Scholar]
- 17.Song L, Hobaugh M R, Shustak C, Cheley S, Bayley H, Gouaux J E. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science. 1996;274:1859–1866. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 18.Suttorp N, Hessz T, Seeger W, Wilke A, Koob R, Lutz F, Drenckhahn D. Bacterial exotoxins and endothelial permeability for water and albumin in vitro. Am J Physiol. 1988;255:C368–C376. doi: 10.1152/ajpcell.1988.255.3.C368. [DOI] [PubMed] [Google Scholar]
- 19.Touchie C, Marrie T J. Respiratory tract infections. In: Crossley K B, Archer G L, editors. The staphylococci in human disease. Edinburgh, United Kingdom: Churchill Livingstone; 1997. pp. 475–492. [Google Scholar]