Abstract
Background
Patients admitted with acute decompensated heart failure (ADHF) have a poor prognosis and poor quality of life due to dyspnea and edema. Tolvaptan, a vasopressin V2 receptor antagonist, is an effective water diuretic. This study aimed to evaluate the efficacy and safety of a short course of tolvaptan to treat volume overload in patients with ADHF.
Methods
We conducted a phase III, multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of a short course of tolvaptan (15 mg/day for 4 days) in hospitalized ADHF patients with volume overload despite the use of conventional diuretics. The primary end-point was the change in body weight after 4 days of treatment. The secondary end-points were the change in intake/output balance, change in serum sodium/potassium concentrations, physician/patient assessed signs and symptoms of heart failure after 4 days of treatment, and all-cause mortality in 1 month.
Results
A total of 110 patients were screened, and 91 were randomized to receive 15 mg/day of tolvaptan for 4 days (n = 46) or matching placebo (n = 45). Compared to the placebo-treated patients, tolvaptan significantly reduced body weight (-1.36 ± 2.13 kg in the tolvaptan group vs. -0.59 ± 1.27 kg in the placebo group, p = 0.0394). The tolvaptan group also had a negative intake/urine volume balance compared to the placebo group (-509.3 ± 2788.2 ml vs. 975.5 ± 1903.1 ml, p = 0.0059). The safety profile of tolvaptan was acceptable.
Conclusions
Tolvaptan significantly reduced volume overload in hospitalized ADHF patients with volume overload despite the use of conventional diuretics.
Keywords: ADHF, Diuretic, Heart failure, Tolvaptan
INTRODUCTION
Heart failure (HF) is a disease which causes high morbidity and mortality despite current state of the art therapies.1 In a recent registry of patients with HF reduced ejection fraction in Taiwan, the in-hospital mortality rate was 2.4%.2 However, at 1 year after hospital discharge, the all-cause mortality and cardiovascular mortality rates were 15.9% and 10.5%, respectively, and the rehospitalization rate was 38.5%.3 In addition, HF is associated with high healthcare expenditure, with hospitalizations for HF costing over $20 billion each year in the USA.4
During admission, patients with acute decompensated heart failure (ADHF) usually display signs and symptoms of vascular and interstitial congestion, such as jugular venous distention, ascites, dyspnea, orthopnea, pulmonary and peripheral edema. Consequently, fluid removal is one of the major therapies to relieve symptoms and improve oxygenation. To achieve this, diuretic therapy should be initiated without delay, and early diuretic interventions have been associated with better symptom relief for patients hospitalized with ADHF5 and improved outcomes.6 Loop diuretics such as furosemide act as venodilators and diuretic agents and are first-line treatments. In addition, they inhibit sodium–potassium–chloride cotransport in the thick ascending limb of Henle’s loop, and induce natriuresis, chloruresis and kaliuresis.7 Therefore, loop diuretics stimulate water loss by producing hypo to isotonic urine, and may induce serum electrolyte imbalance such as hyponatremia and hypokalemia.7,8 In addition, diuretic resistance is common in patients with ADHF, and it may be associated with adverse outcomes in this population.7
Tolvaptan is an orally active selective arginine vasopressin (AVP)-receptor antagonist which acts by inhibiting the action of vasopressin V2 receptors in renal collecting ducts to induce aquaresis (free water clearance).9 By promoting aquaresis, tolvaptan has been shown to increase urine output and serum sodium concentration in a variety of hyponatremic conditions including syndrome of inappropriate antidiuretic hormone, liver cirrhosis and chronic HF.10 In patients with ADHF, tolvaptan has been shown to be beneficial in reducing body weight and improving congestive symptoms.11
This study aimed to evaluate the efficacy and safety of a short course of tolvaptan to treat volume overload in patients with ADHF. The primary objective of the study was to evaluate the efficacy of tolvaptan in stabilized ADHF patients through fluid removal and body weight reduction compared to placebo-treated patients.
MATERIAL AND METHODS
This was a randomized, multicenter, parallel-group, placebo-controlled and double-blind study. Patients were observed for 3 days following a 4-day treatment period and 1-month follow-up period. This study was approved by the Institutional Review Board of each study site prior to initiation.
Patients
Eligible patients were aged from 20 to 85 years with a history of chronic HF who had been hospitalized due to worsening HF with signs or symptoms of volume congestion. Other inclusion criteria were having HF symptoms at rest or during minimal exertion and signs of congestion (ex. lower limb edema, jugular venous distention, or pulmonary congestion) at the time of randomization, and receiving any of the following oral diuretic therapies without any changes in the dose or mode of administration during the observation period: an oral loop diuretic at a daily dosage equivalent to ≥ 40 mg of oral form furosemide; concomitant administration of an oral loop diuretic and a thiazide diuretic (at any dose); and the concomitant administration of an oral loop diuretic and mineralocorticoid receptor antagonist (at any dose). In addition, the patients had to keep variations in their body weight to within 1.0 kg during the 2 days prior to starting treatment. The study protocol and informed consent documents for this study were approved by an appropriate Institutional Review Board for each participating center. This study was registered at ClinicalTrials.gov (Identifier: NCT01618448). Written informed consent to participate in the study was obtained from all patients.
Patients with any of the following were excluded from this study: (1) cardiac surgery within 60 days of enrollment; (2) with an assisted cardiac mechanical device; (3) receiving cardiac resynchronization therapy within 60 days of enrollment; (4) suspected of having a decrease in circulatory blood flow; (5) refractory end-stage HF (patients considered to require mechanical circulatory support, continuous intravenous positive inotropic therapy, referral for cardiac transplantation, or hospice care); (6) cardiac valvular disease with significant heart valve stenosis, sustained ventricular tachycardia or ventricular fibrillation within 30 days prior to the screening examination; (7) acute myocardial infarction within 30 days prior to the screening examination; (8) cerebrovascular disorders within 6 months prior to the screening examination (other than asymptomatic cerebral infarction); (9) with a definite diagnosis of active myocarditis or amyloid cardiomyopathy; (10) poorly controlled diabetes mellitus (HbA1c ≥ 10%); (11) anuria (urinary output < 100 ml per day); (12) history of hyperthyroidism, impaired urination due to urinary tract stricture, urinary calculus, tumor in the urinary tract, or other cause, hemofiltration or dialysis; (13) unable to sense thirst, inappropriate response to thirst or impaired oral fluid intake; (14) with a history of hypersensitivity or idiosyncratic reaction to benzazepine derivatives such as mozavaptan hydrochloride or benazepril hydrochloride; (15) severely obese patients [body mass index (BMI) > 35 kg/m2]; (16) with systolic blood pressure in the decubitus position < 90 mmHg; (17) with any of following abnormal laboratory values: total bilirubin > 3.0 mg/dL, hemoglobin < 9 g/dL, serum creatinine > 3.0 mg/dL, serum sodium > 147 mEq/L, or serum potassium > 5.5 mEq/L; (18) female patients who were pregnant, possibly pregnant, or lactating, or who planned to become pregnant; (19) who received any investigational drug other than tolvaptan within 30 days prior to the screening examination; and (20) with a general physical condition which may have confounded the results of the study, posed additional risks or precluded evaluations and assessments in this study.
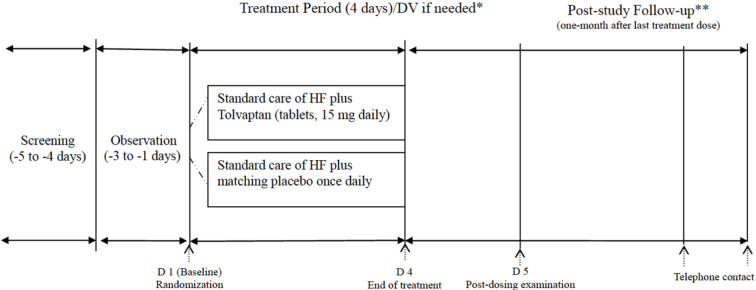
Study protocol (Figure 1)
Figure 1.
The flow chart of the study. * DV: protocol deviation. Only for patients who were withdrawn early from study treatment prior to day 4. Examinations were performed at any time but no later than 3 days after the last dose of the investigational drug. ** Follow-up for the occurrence of serious adverse events 14 (±3) days and 28 (±3) days after receiving the final study drug dose by telephone contact. HF, heart failure.
The subjects underwent screening tests, and the eligible subjects were enrolled for a 3-day observation period (Day -3 to -1). After being evaluated during the observation period, the subjects who met the entry criteria were randomized to receive either tolvaptan (15 mg) or a placebo, once daily after breakfast for 4 consecutive days (Day 1-4). Drug efficacy was assessed on Day 5 by using body weight as the primary endpoint. Post-study follow-up examinations on adverse events and/or death were also performed on Day 15-21 and Day 29-35. The patients received post-treatment follow-up for 1 month (Figure 1).
Endpoints and criteria for evaluation
The primary endpoint was the body weight change from Day 1 (baseline) to Day 5 (after 4 days of treatment, measured at the post-dosing examination visit). The secondary endpoints were the change in intake/output balance, serum sodium concentration, serum potassium concentration from Day 1 to Day 5, change in physician-assessed signs and symptoms of HF after 4 days of treatment, change in patient-assessed global clinical status after 4 days of treatment, change in patient-assessed dyspnea status after 4 days of treatment, and all-cause mortality rate in the 1 month after last treatment dose.
The physician-assessed signs and symptoms of HF at baseline and 4 days after treatment including jugular venous distention, lower limb edema, pulmonary congestion, and pulmonary rales were determined and compared between the two groups using a proportional odds model. The distribution of jugular venous distension was graded as 0: absent; 1: < 6 cm; 2: 6-9 cm; 3: 10-15 cm; and 4: > 15 cm. The severity of lower limb edema was graded as 0: absent (no pitting), 1: slight (very slight pitting), 2: moderate (definite pitting), and 3: marked (considerable pitting) as judged by the physician. The distribution of pulmonary congestion severity was graded as 0: absent; 1: slight; 2: moderate; and 3: marked. Pulmonary rales were assessed by auscultation and were graded as 0: no rales, 1: rales only in bases of lungs, 2: bases to 50% way up the lungs, and 3: bases to > 50% way up the lungs. Changes in these variables from baseline to post-treatment day were determined and compared between the two groups using a proportional odds model. The improvement rate (percentage of patients with an improvement by one grade based on all patients) and the resolution rate (percentage of patients with a grade of 0 after treatment based on patients with higher grades between baseline and the post-dosing examination) were determined and compared between the two groups using Fisher’s exact test.
Safety was assessed by evaluating the incidence of treatment-emergent adverse events (TEAEs), laboratory data, vital signs, and electrocardiograms. A TEAE was defined as a new adverse event experienced by a study subject which occurred after the initiation of the investigational medicinal product administration; an event or pre-existing medical problem that changed adversely in nature or severity from baseline in a study subject while receiving the investigational medicinal product.
Statistical analysis
In the analyzed population, the intent-to-treat (ITT) population was defined as all subjects who were randomized to receive treatment and took at least one dose of the study medication (tolvaptan or placebo), and had at least one follow-up efficacy endpoint evaluation regardless of their compliance with the protocol. This was considered to be the primary analysis population. The per-protocol (PP) population was defined as all subjects who underwent any study treatment and had no major protocol violations affecting their efficacy assessments. The safety population included all randomized subjects who received at least one dose of the study medication.
Sample size calculation was performed using a test for superiority based on the mean change from baseline in body weight. At least 74 evaluable ITT patients were required to detect a difference of -1 kg in the change in body weight from baseline between two groups under 80% statistical power and two-sided type I error rate of 0.05. Assuming a drop-out rate of 15% who may not satisfy the ITT definition, approximately 88 patients were required.
The primary endpoint was the change in body weight from Day 1 (baseline) to Day 5 (defined as the end of the study: the post-dosing examination visit after 4 days of treatment). The full analysis set (ITT group) was used to study drug efficacy. The last observation carried forward (LOCF) approach was used to impute missing data at the end of the study. Changes from baseline were compared between two groups using ANCOVA, with treatment as the main effect and baseline body weight as a covariate; 95% confidence intervals were also calculated. Other secondary endpoints in terms of changes in severity from baseline for HF symptoms were compared between two groups using a proportional odds model with treatment as the main effect and baseline as a covariate, or using Fisher’s exact test to compare differences in proportions and incidence of mortality between two groups. For safety, Fisher’s exact test was used to test for between-treatment group differences for each TEAE coded according to the Medical Dictionary for Regulatory Activities (MedDRA version 17.1). For chemistry, hematology, and vital sign variables, group changes from baseline to end of treatment were analyzed using ANCOVA with treatment as the factor and baseline (pre-treatment) level as the covariate. For baseline data, Fisher’s exact test and two sample t-tests were used for categorical and continuous data, respectively.
RESULTS
Patients’ characteristics
This study was conducted from 12 July 2012 to 05 May 2014. A total of 110 HF subjects were screened and provided informed consent, of whom 103 were eligible to proceed to the pretreatment observation period while receiving standard HF therapy. Of the 103 subjects, 12 were withdrawn from the study during the pretreatment observation period. The remaining 91 subjects were then randomized into the tolvaptan group (46 subjects) and the placebo group (45 subjects). A total of 85 subjects completed the treatment phase, including 44 in the tolvaptan group and 41 in the placebo group. All 91 randomized subjects (ITT group) were followed for 4 weeks after the final dose of the study drug. The baseline characteristics of the 91 patients are listed in Table 1. Two subjects in the placebo group died during the post-study follow-up period. According to the definition of the PP population, 10 subjects were excluded from the ITT population. The PP population consisted of 81 subjects (42 in the tolvaptan group, 39 in the placebo group). The details of the 10 randomized subjects excluded from the ITT population are shown in Supplement Tables 1 and 2. The use of diuretics and dose of oral loop diuretics were balanced between the two groups on the first day of trial drug administration (Table 2).
Table 1. Summary of baseline characteristics.
| Item/category | Tolvaptan (N = 46) | Placebo (N = 45) | p value |
| Demographic characteristics | |||
| Age, years | 68.0 (12.3) | 65.6 (15.6) | 0.4030 |
| Male | 33 (71.7%) | 33 (73.3%) | 1.0000 |
| Hypertension | 23 (50.0%) | 28 (62.2%) | 0.2930 |
| Diabetes mellitus | 26 (56.5%) | 26 (57.8%) | 1.0000 |
| Coronary artery disease | 16 (34.8%) | 20 (44.4%) | 0.7116 |
| Pacemaker | 0 (0%) | 0 (0%) | 1.0000 |
| Implanted cardiac defibrillator | 0 (0%) | 0 (0%) | 1.0000 |
| Valvular heart disease | 11 (23.9%) | 12 (26.7%) | 0.9276 |
| Arrhythmia | 18 (39.1%) | 15 (33.3%) | 0.6641 |
| Weight at screening, kg | 65.37 (14.68) | 68.93 (14.59) | 0.2495 |
| Weight at baseline, kg | 64.26 (14.65) | 68.03 (14.48) | 0.2206 |
| Height, cm | 162.77 (8.51) | 164.46 (7.84) | 0.3261 |
| Body mass index (kg/m2) | 24.58 (4.72) | 25.37 (4.52) | 0.4219 |
| HbA1c (%) | 6.68 (1.15) | 6.74 (1.11) | 0.8008 |
| Creatinine (mg/dL) | 1.44 (0.55) | 1.41 (0.61) | 0.8425 |
| Serum sodium (mmol/L) | 137.0 (4.8) | 137.5 (3.7) | 0.5618 |
| Serum potassium (mmol/L) | 3.95 (0.81) | 4.16 (0.57) | 0.1720 |
| Congestive symptoms and signs at baseline | |||
| New York Heart Association | 0.1811 | ||
| Class II | 20 (43.5%) | 22 (48.9%) | |
| Class III | 22 (47.8%) | 23 (51.1%) | |
| Class IV | 4 (8.7%) | 0 (0.0%) | |
| Jugular venous distention | 0.6990 | ||
| Absent | 18 (39.1%) | 17 (37.8%) | |
| < 6 cm | 10 (21.7%) | 15 (33.3%) | |
| 6-9 cm | 9 (19.6%) | 8 (17.8%) | |
| 10-15 cm | 6 (13.0%) | 3 (6.7%) | |
| > 15 cm | 3 (6.5%) | 2 (4.4%) | |
| Lower limb edema | 0.5098 | ||
| Absent | 9 (19.6%) | 15 (33.3%) | |
| Slight | 23 (50.0%) | 18 (40.0%) | |
| Moderate | 10 (21.7%) | 9 (20.0%) | |
| Marked | 4 (8.7%) | 3 (6.7%) | |
| Pulmonary congestion | 0.1891 | ||
| Absent | 7 (15.2%) | 8 (17.8%) | |
| Slight | 16 (34.8%) | 24 (53.3%) | |
| Moderate | 19 (41.3%) | 12 (26.7%) | |
| Marked | 4 (8.7%) | 1 (2.2%) | |
| Dyspnea | 0.2436 | ||
| None | 5 (10.9%) | 1 (2.2%) | |
| Seldom | 27 (58.7%) | 32 (71.1%) | |
| Frequent | 13 (28.3%) | 12 (26.7%) | |
| Continuous | 1 (2.2%) | 0 (0.0%) | |
| Pulmonary rales | 0.6831 | ||
| No rales | 20 (43.5%) | 22 (48.9%) | |
| Bases | 23 (50.0%) | 22 (48.9%) | |
| Bases to 50% way up | 3 (6.5%) | 1 (2.2%) |
Supplement Table 1. List the reason for withdrawal.
| Patient No. | Group | Comment |
| S02D02 | Placebo | Protocol compliance becomes impossible due to a newly emergent disease or symptom or worsening of clinical laboratory test findings. (Diuretic IV injection received before withdrawal) |
| S17L05 | Tolvaptan | Compliance with the study protocol becomes impossible or the investigator judge withdrawal to be necessary. (Adverse Event: Pneumonia) |
| S20L07 | Placebo | Compliance with the study protocol becomes impossible or the investigator judge withdrawal to be necessary. (Non-compliance the standard control during the treatment period) |
| S03F03 | Tolvaptan | Protocol compliance becomes impossible due to a newly emergent disease or symptom or worsening of clinical laboratory test findings. (Serious Adverse Event: Ischaemic Hepatitis) |
| S02N02 | Placebo | A major deviation is discovered. |
| S04K04 | Placebo | The patient requested to withdraw from the study. |
Supplement Table 2. List of major deviations in the trial.
| Subject No. | Group | Comment |
| S25I08 | Tolvaptan | Violated exclusion criteria #8 (hemoglobin was 8.7 g/dL at screening visit). |
| S05B03 | Tolvaptan | Perform the thoracentesis during the treatment period. |
| S13B10 | Placebo | Perform the thoracentesis during the treatment period. |
| S02D02 | Placebo | Diuretic IV injection during the treatment period. |
| S20L07 | Placebo | Violated exclusion criteria #4 (HbA1c at screening period was 11.8%). Non-compliance the standard control during the treatment period. |
| S02H02 | Placebo | Violated inclusion criteria #3 (no heart failure symptoms at rest or signs of congestion). Violated exclusion criteria #8 (all laboratory tests were not performed). |
Table 2. Diuretics used on the First Day of Trial Drug Administration.
| Item/category | Tolvaptan (N = 46) | Placebo (N = 45) | p value |
| Use of diuretic | 0.6216 | ||
| Loop diuretic alone | 21 (45.7%) | 18 (40.0%) | |
| Loop + Spironolactone | 19 (41.3%) | 24 (53.3%) | |
| Loop + Spironolactone + Thiazide | 3 (6.5%) | 2 (4.4%) | |
| Loop + Thiazide | 3 (6.5%) | 1 (2.2%) | |
| Dose of loop diuretic* | 0.3585 | ||
| < 40 mg/day | 6 (13.0%) | 9 (20.0%) | |
| 40 mg/day-80 mg/day | 36 (78.3%) | 29 (64.4%) | |
| ≥ 80 mg/day | 4 (8.7%) | 7 (15.6%) |
* Flurosemide oral form equivalence.
Efficacy evaluation
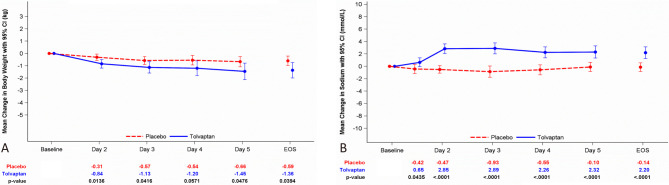
Of the 91 subjects randomized into the 4-day daily treatment period (ITT group), there were no significant differences in age, sex, BMI, causes of HF, types of HF, or distribution of New York Heart Association class between the two groups. After 4 days of treatment, a significantly greater body weight reduction was observed in the tolvaptan group (-1.45 ± 2.16 kg) than in the placebo group (-0.66 ± 1.31 kg), group difference: -0.81 kg, 95% confidence interval (CI): -1.62 to -0.01 kg, p = 0.0476. In LOCF analysis, a significantly greater body weight reduction was still observed in the tolvaptan group (-1.36 ± 2.13 kg) than in the placebo group (-0.59 ± 1.27 kg) after 4 days of treatment, group difference: -0.78 kg, 95% CI: -1.52 to -0.04 kg, p = 0.0394 (Figure 2A, Table 3). In the PP group (n = 81), the trend in body weight reduction was similar to the ITT group; however, the p value was non-significant (p = 0.0614) (Supplement Table 3). The urine volume increased daily compared with baseline in the tolvaptan group, and most of the patients achieved a significant difference (Table 4). A significant trend of an increase in daily urine volume was observed in the tolvaptan group compared with the placebo group in both cumulative value (p = 0.0036) and mean daily urine output (p = 0.0041). The cumulative change in input/output balance between the two groups was significant starting from Day 1 (tolvaptan: -450.7 ± 1167.2 mL vs. placebo: 277.1 ± 749.4 mL; p = 0.0015) to the end of the study (tolvaptan: -509.3 ± 2788.2 mL vs. placebo: 975.5 ± 1903.1 mL; p = 0.0059). The mean daily fluid intake/urine volume balance was -97.5 ± 748.8 mL in the tolvaptan group and 262.1 ± 517.7 mL in the placebo group (p = 0.0131).
Figure 2.
The efficacy of tolvaptan for fluid management. (A) Body weight change. (B) Serum sodium concentration change in the two groups. Data are expressed as means with 95% confidence intervals. EOS, end of study, the data represented with the result after 4-day tolvaptan treatment analyzed with the last observation carried forward (LOCF) method.
Table 3. Mean change in body weight from baseline to each post-baseline observation (ITT population).
| Treatment day, Unit: kg | Tolvaptan (N = 46) | Placebo (N = 45) | Adjust group difference (95% CI) | p value | ||||
| N | Mean (SD) | Change (SD) | N | Mean (SD) | Change (SD) | |||
| Day 1 (baseline) | 46 | 64.3 (14.7) | -- | 45 | 68.0 (14.5) | -- | -3.77 (-9.84, 2.30)- | 0.2206 |
| Day 2 | 46 | 63.4 (14.7) | -0.84 (1.17)* | 45 | 67.7 (14.3) | -0.31 (0.86)* | -0.55 (-0.98, -0.12) | 0.0136 |
| Day 3 | 45 | 62.6 (14.2) | -1.13 (1.52)* | 43 | 67.9 (14.7) | -0.57 (1.06)* | -0.59 (-1.15, -0.02) | 0.0416 |
| Day 4 | 42 | 62.9 (14.2) | -1.20 (1.92)* | 43 | 67.9 (14.7) | -0.54 (1.26)* | -0.69 (-1.40, -0.02) | 0.0571 |
| Post-dosing Day 5 | 43 | 62.1 (14.7) | -1.45 (2.16)* | 40 | 67.8 (15.0) | -0.66 (1.31)* | -0.81 (-1.62, -0.01) | 0.0476 |
| EOS | 46 | 62.9 (14.8) | -1.36 (2.13)* | 45 | 67.4 (14.4) | -0.59 (1.27)* | -0.78 (-1.52, -0.04) | 0.0394 |
Definition: EOS, end of study, the data represented with the result after 4-day tolvaptan treatment analyzed with the last observation carried forward (LOCF) method. 95% CI, confidence interval; SD, standard deviation.
p value: pair t-test for intragroup comparison; Post-Baseline ANCOVA Model: outcome = treatment + baseline level.
* With significant mean change compared to baseline value (intra p value < 0.05).
Supplement Table 3. Mean change in body weight from baseline to each post-baseline observation (PP population).
| Treatment day, Unit: kg | Tolvaptan (N = 42) | Placebo (N = 39) | Adjust group difference (95% CI) | p value | ||||
| N | Mean (SD) | Change (SD) | N | Mean (SD) | Change (SD) | |||
| Day 1 (baseline) | 42 | 64.3 (14.5) | -- | 39 | 68.0 (15.0) | -- | -4.00 (-10.51, 2.52) | 0.2254 |
| Day 2 | 42 | 63.1 (14.5) | -0.87 (1.20)* | 39 | 67.6 (14.8) | -0.36 (0.88)* | -0.53 (-1.00, -0.06) | 0.0292 |
| Day 3 | 42 | 62.8 (14.3) | -1.18 (1.54)* | 39 | 67.4 (15.0) | -0.58 (1.07)* | -0.63 (-1.23, -0.04) | 0.0379 |
| Day 4 | 41 | 63.3 (14.1) | -1.20 (1.94)* | 39 | 67.4 (15.0) | -0.60 (1.29)* | -0.64 (-1.38, -0.11) | 0.0918 |
| Post-dosing Day 5 | 42 | 62.5 (14.6) | -1.45 (2.19)* | 39 | 67.3 (14.9) | -0.69 (1.31)* | -0.78 (-1.60, 0.04) | 0.0614 |
| EOS | 42 | 62.5 (14.6) | -1.45 (2.19)* | 39 | 67.3 (14.9) | -0.69 (1.31)* | -0.78 (-1.60, 0.04) | 0.0614 |
Definition: Post-Dosing Day 5: treatment measurement at post Day 4 dosing examination visit. EOS, end of study, the data represented with the result of post-dosing day 5 analyzed with the last observation carried forward (LOCF) method.
p value: pair t-test for intragroup comparison; Post-Baseline ANCOVA Model: outcome = treatment + baseline level.
* With significant mean change compared to baseline value (intra p value < 0.05).
Table 4. Mean daily urine volume changed from baseline (ITT population).
| Treatment period, Unit: mL | Tolvaptan (N = 46) | Placebo (N = 45) | Adjust group difference (95% CI) | p value | ||||
| N | Mean (SD) | Change (SD) | N | Mean (SD) | Change (SD) | |||
| Day -1 to Day 1* | 45 | 1682.7 (861.3) | -- | 45 | 1568.2 (666.7) | -- | 114.53 (-208.13, 437.18) | 0.4824 |
| Day 1 to Day 2 | 45 | 2386.4# (1252.7) | 703.65 (1325.39) | 45 | 1671.6 (619.6) | 103.35 (605.37) | 665.77 (272.08, 1059.45) | 0.0012 |
| Day 2 to Day 3 | 44 | 2268.8# (1005.1) | 565.55 (1169.79) | 43 | 1689.8 (572.3) | 158.27 (602.44) | 522.21 (185.44, 858.99) | 0.0028 |
| Day 3 to Day 4 | 42 | 1988.4 (908.3) | 297.91 (1133.40) | 43 | 1673.4 (579.2) | 141.87 (606.76) | 267.79 (-48.33, 583.91) | 0.0958 |
| Day 4 to Day 5 | 42 | 1992.9# (748.8) | 302.48 (894.75) | 40 | 1654.9 (548.6) | 115.34 (708.68) | 289.64 (18.89, 560.38) | 0.0363 |
| EOS | 45 | 1902.1 (800.6) | 219.32 (928.74) | 45 | 1632.5 (539.9) | 64.23 (697.82) | 232.49 (-36.07, 501.05) | 0.0889 |
| Cumulative value (Day 1 to EOS) | 45 | 8320.7 (3690.0) | -- | 45 | 6356.3 (2141.1) | -- | 1820.5 (612.95, 3027.98) | 0.0036 |
| Mean daily urine | 45 | 2109.4# (871.8) | 426.61 (1020.07) | 45 | 1668.4 (451.9) | 100.23 (525.93) | 400.68 (130.41, 670.95) | 0.0041 |
Definition: EOS, end of study, the data represented with the urine volume from Day 4 to Day 5 analyzed with the last observation carried forward (LOCF) method. 95% CI, confidence interval; SD, standard deviation.
* The urine volume collected from the day before treatment Day 1 was defined as baseline. # With significant mean change compared to baseline value (intra p value < 0.05).
p value: pair t-test for intragroup comparison; Post-Baseline ANCOVA Model: outcome = treatment + baseline level.
Improvements in physician-assessed congestive symptoms and signs and patient-assessed global clinical status are shown in Table 5, Supplement Tables 4 and 5. Global clinical status score was based on a visual analog scale.12 There was no significant difference in the percentage of improvement in physician-assessed congestive symptoms and signs between the two groups. Patients in both groups had significant improvements in mean scores of self-assessed global clinical status in both groups after 4 days of treatment (tolvaptan: 18.26 vs. placebo: 23.32). No significant difference between groups was observed at baseline (tolvaptan: 51.4 ± 23.6 score and placebo: 50.0 ± 21.1 scores) or at end of the study. None of the subjects in the ITT population died during the treatment period; a total of two subjects (4.4%) in the placebo group died during the follow-up period (p = 0.2418).
Table 5. Improvements in physician assessed congestive symptoms and signs and patient self-assessed global clinical status after 4-day of treatment.
| Tolvaptan (N = 46) | Placebo (N = 45) | p value* | |
| Physician assessed heart failure symptoms and signs | |||
| Jugular venous distension | 14 (30.4%) | 11 (24.4%) | 0.6398 |
| Lower limb edema | 31 (67.4%) | 26 (57.8%) | 0.3905 |
| Pulmonary congestion | 22 (47.8%) | 19 (42.2%) | 0.6751 |
| Pulmonary rales | 17 (37.0%) | 14 (31.1%) | 0.6595 |
| Physician assessed dyspnea | 34 (73.9%) | 30 (66.7%) | 0.2087 |
| Patient self-assessed heart failure symptoms | |||
| Mean change of global clinical status score form baseline# | 18.26 ± 23.89 | 23.32 ± 25.42 | 0.3565 |
| Patient self-assessed dyspnea | 41 (89.1%) | 36 (80.0%) | 0.7717 |
* The distribution of congestive symptoms severity grading at baseline and at the end of study, and corresponding changes from baseline were determined and compared between the two groups by proportional odds model. # Global clinical status score was based on a visual analog scale.
Supplement Table 4. Severity change in physician assessed congestive symptoms and signs after 4-day of treatment.
| Tolvaptan (N = 46) | Placebo (N = 45) | p value | |
| Jugular venous distension | |||
| Severity at baseline, n (%) | 0.5694 | ||
| Absent | 18 (39.1%) | 17 (37.8%) | |
| < 6 cm | 10 (21.7%) | 15 (33.3%) | |
| 6-9 cm | 9 (19.6%) | 8 (17.8%) | |
| 10-15 cm | 6 (13.0%) | 3 (6.7%) | |
| > 15 cm | 3 (6.5%) | 2 (4.4%) | |
| Change from baseline at end of study, n (%) | 0.9493 | ||
| 3-level improved | 0 (0.0%) | 1 (2.2%) | |
| 2-level improved | 4 (8.7%) | 2 (4.4%) | |
| 1-level improved | 10 (21.7%) | 8 (17.8%) | |
| No change | 31 (67.4%) | 34 (75.6%) | |
| 1-level worsened | 1 (2.2%) | 0 (0.0%) | |
| Lower limb edema | |||
| Severity at baseline, n (%) | 0.2560 | ||
| Absent | 9 (19.6%) | 15 (33.3%) | |
| Slight | 23 (50.0%) | 18 (40.0%) | |
| Moderate | 1 (21.7%) | 9 (20.0%) | |
| Marked | 4 (8.7%) | 3 (6.7%) | |
| Change from baseline at end of study, n (%) | 0.5164 | ||
| 2-level improved | 5 (10.9%) | 3 (6.7%) | |
| 1-level improved | 26 (56.5%) | 23 (51.1%) | |
| No change | 15 (32.6%) | 19 (42.2%) | |
| Pulmonary congestion | |||
| Severity at baseline, n (%) | 0.0689 | ||
| Absent | 7 (15.2%) | 8 (17.8%) | |
| Slight | 16 (34.8%) | 24 (53.3%) | |
| Moderate | 19 (41.3%) | 12 (26.7%) | |
| Marked | 4 (8.7%) | 1 (2.2%) | |
| Change from baseline at end of study, n (%) | 0.8638 | ||
| 3-level improved | 1 (2.2%) | 0 (0.0%) | |
| 2-level improved | 5 (10.9%) | 1 (2.2%) | |
| 1-level improved | 16 (34.8%) | 18 (40.0%) | |
| No change | 22 (47.8%) | 24 (53.3%) | |
| 1-level worsened | 2 (4.4%) | 1 (2.2%) | |
| 2-level worsened | 0 (0.0%) | 1 (2.2%) | |
| Pulmonary rales | |||
| Severity at baseline, n (%) | 0.4844 | ||
| No rales | 20 (43.5%) | 22 (48.9%) | |
| Bases | 23 (50.0%) | 22 (48.9%) | |
| Bases to 50% way up | 3 (6.5%) | 1 (2.2%) | |
| Change from baseline at end of study, n (%) | 0.8386 | ||
| 2-level improved | 2 (4.4%) | 0 (0.0%) | |
| 1-level improved | 15 (32.6%) | 14 (31.1%) | |
| No change | 28 (60.9%) | 31 (68.9%) | |
| 1-level worsened | 1 (2.2%) | 0 (0.0%) | |
| 2-level worsened | 0 (0.0%) | 1 (2.2%) | |
| Physician assessed dyspnea | |||
| Severity at baseline, n (%) | 0.8384 | ||
| None | 5 (10.9%) | 1 (2.2%) | |
| Seldom | 27 (58.7%) | 32 (71.1%) | |
| Frequent | 13 (28.3%) | 12 (26.7%) | |
| Continuous | 1 (2.2%) | 0 (0.0%) | |
| Change from baseline at end of study, n (%) | 0.2087 | ||
| 3-level improved | 1 (2.2%) | 0 (0.0%) | |
| 2-level improved | 4 (8.7%) | 4 (8.9%) | |
| 1-level improved | 29 (63.0%) | 26 (57.8%) | |
| No change | 10 (21.7%) | 13 (28.9%) | |
| 1-level worsened | 2 (4.4%) | 2 (4.4%) |
Definition: End of study, the data measured at the post-dosing examination visit after 4-day treatment.
Proportional odds model at Post-Baseline Visit: outcome = treatment + baseline severity (ordinal).
Supplement Table 5. Severity change in patient self-assessed global clinical status after 4-day of treatment.
| Tolvaptan (N = 46) | Placebo (N = 45) | p value | |
| Patient self-assessed global clinical status | |||
| Baseline | |||
| Mean | 51.4 ± 23.6 | 50.0 ± 21.1 | 0.7789 |
| End of study | |||
| Mean | 69.8 ± 28.2 | 73.3 ± 19.5 | |
| Mean change from baseline | 18.26 ± 23.89 | 23.32 ± 25.42 | 0.3536 |
| Patient self-assessed dyspnea | |||
| Status at baseline, n (%) | 0.8243* | ||
| Yes | 30 (65.2%) | 31 (68.9%) | |
| No | 16 (34.8%) | 14 (31.1%) | |
| Change from baseline at end of study, n (%) | 0.7717 | ||
| Markedly better | 9 (19.6%) | 8 (17.8%) | |
| Moderately better | 19 (41.3%) | 19 (42.2%) | |
| Minimally better | 13 (28.3%) | 9 (20.0%) | |
| No change | 3 (6.5%) | 9 (20.0%) | |
| Moderately worse | 2 (4.3%) | 0 (0.0%) |
Definition: End of study, the data measured at the post-dosing examination visit after 4-day treatment.
p value: pair t-test for intragroup comparison; Post-Baseline ANCOVA Model was used in patient self-assessed global clinical status: outcome = treatment + baseline level; proportional odds model at Post-Baseline Visit was used in patient self-assessed dyspnea: outcome = treatment + baseline level (ordinal).
* The p value was tested by Fisher’s Exact Test.
Safety evaluation
In the tolvaptan group, a significant increase in serum sodium concentration from baseline was noted starting from Day 2 (mean change: 2.85 ± 2.62 mEq/L) to the end of study (mean change: 2.20 ± 3.18 mEq/L, Figure 2B). All increases in serum sodium concentration remained within the normal range, and the biggest change from baseline to each post-baseline value in the tolvaptan group was 11 mEq/L. A significant difference in serum sodium concentration was observed at the end of the study between the two groups (mean group difference: 2.16 mEq/L, p < 0.001). For serum potassium concentration, neither intragroup nor intergroup analysis revealed a significant difference at baseline or the end of the study.
A total of 123 adverse events including 27 adverse events occurred during the screening/observation period, and 96 TEAEs occurred after study drug administration (Supplement Table 6). There were 23 serious adverse events, one of which occurred during the screening/observation period, and 22 serious TEAEs occurred after study drug administration. Two patients died in the placebo group, and none died in the tolvaptan group. The incidence rate of TEAEs was non-significantly higher in the tolvaptan group (p = 0.0590), and most included mild TEAEs. Only 19.7% (12/61) of the TEAEs in the tolvaptan group and 13.3% (6/45) of those in the placebo group were considered to be study drug related.
Supplement Table 6. Overview of treatment emergent adverse events (safety population).
| Tolvaptan (N = 46) | Placebo (N = 45) | p value | |||
| No. of events | Subjects (%) | No. of events | Subjects (%) | ||
| Treatment emergent adverse event (TEAE)* | 61 | 30 (65.2%) | 35 | 20 (44.4%) | 0.0590 |
| Serious TEAE | 12 | 8 (17.4%) | 10 | 8 (17.8%) | 1.0000 |
| Mild TEAE | 35 | 22 (47.8%) | 18 | 12 (26.7%) | 0.0511 |
| Moderate TEAE | 17 | 12 (26.1%) | 8 | 8 (17.8%) | 0.4489 |
| Severe TEAE | 9 | 5 (10.9%) | 9 | 6 (13.3%) | 0.7582 |
| TEAE related to study drug | 12 | 8 (17.4%) | 6 | 4 (8.9%) | 0.3538 |
| TEAE leading to discontinuation | 2 | 2 (4.3%) | 0 | 0 (0.0%) | 0.4945 |
| TEAE resulted to death | 0 | 0 (0.0%) | 3 | 2 (4.4%) | 0.2418 |
* Definition of TEAE (treatment emergent adverse event): TEAE is a new AE experienced by a study subject that occurs after initiation of investigational medicinal product administration; an event or pre-existing medical problem that has changed adversely in nature or severity from baseline in subject while receiving investigational medicinal products.
DISCUSSION
In this study, we demonstrated that 4 days of tolvaptan treatment in ADHF patients with persistent volume overload despite treatment with conventional diuretics significantly reduced body weight. However, there was no significant difference in congestive symptoms and signs between the patients who received tolvaptan and a placebo, which may be due to the small number of patients in this clinical trial.
Patients admitted due to ADHF often have a poor prognosis.2 In ADHF, there are two categories of HF symptoms according to their etiology; fluid retention ("wet" presentation), and low cardiac output ("cold" presentation).2,13 On admission, clinically wet presentations are much more common than cold presentations. This is supported by a recent registry in Taiwan enrolling 1509 ADHF patients with reduced ejection fraction (TSOC-HFrEF Registry), in which wet presentations were common and included pulmonary congestion and pulmonary rales in 63.5% of the patients, peripheral edema in 49.3%, pleural effusion in 28.8% and an engorged jugular vein in 23.9% of the cases.2 Therefore, to treat congestive symptoms, the use of diuretics to remove fluid is essential in the management of ADHF.
In the TSOC-HFrEF Registry, intravenous diuretics were used in 62.6% of the patients. The median duration of intravenous diuretics therapy was 4 days, and the patients had a median body weight change of -2.1 kg.2 Loop diuretics inhibit sodium–potassium–chloride cotransport in the thick ascending limb of Henle’s loop and stimulate water loss by producing hypo to isotonic urine, and they may induce conditions that affect serum electrolytes such as hyponatremia and hypokalemia.7,8 The 2021 European Society of Cardiology (ESC) heart failure guidelines suggest using loop diuretic as first-line management for acute HF patients with fluid overload and congestion.14 Aggressive diuresis with loop diuretics is frequently needed during the initial management of ADHF regardless of the left ventricular ejection fraction (LVEF), however the optimal dosing, timing, and method of administration are still unclear.14 However, diuretic resistance, which is common in patients with ADHF, may limit the effect of loop diuretics, and it is also associated with worse outcomes.7,15 Overcoming loop diuretic resistance may require escalating the dose of diuretics, the addition of a thiazide diuretic, or the use of ultrafiltration.16,17 However, thiazide diuretics may worsen hyponatremia. Compared with loop diuretics, tolvaptan is a selective, competitive vasopressin receptor 2 (V2) antagonist that inhibits inappropriate elevation of vasopressin, and it thus has emerged as a promising agent to mediate fluid retention.9,18,19 Tolvaptan was initially used in the treatment of euvolemic or hypervolemic hyponatremia, and it has been shown to be safe and effective at promoting aquaresis and raising serum sodium levels.10,20
Congestive HF is a common cause of hyponatremia with elevated plasma AVP levels.21 AVP stimulates both V1A and V2 receptors. V1A receptors are expressed in vascular smooth muscle cells and lead to vasocontraction, and V2 receptors are expressed on the basolateral side of the principal cells in cortical collecting ducts. In addition, activated V2 receptors will increase aquaporin-2 channels to facilitate free water absorption in collecting tubules, a process which is blocked by the competitive V2 receptor antagonist, tolvaptan.22,23 Several clinical trials have been conducted to evaluate the safety and efficacy of tolvaptan in HF patients.11,24,25 In 2004, the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Congestive Heart Failure (ACTIV in CHF) trial demonstrated the short- and intermediate-term effects of tolvaptan in decreasing body weight without inducing hypokalemia or worsening renal function.24 In that study, the median body weight changes were -1.8 (-3.85 to -0.5), -2.1 (-3.10 to -0.85) and -0.60 (-1.60 to 0.00) kg at Day 1 in patients receiving 30, 60 and 90 mg of tolvaptan, respectively. In the current study, we used a lower dose of tolvaptan (15 mg), and the mean body weight change was about -0.84 kg at Day 1 after receiving tolvaptan. In 2007, the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) study showed that tolvaptan significantly improved congestive symptoms including patient-assessed dyspnea and edema. The congestive symptoms and signs were also improved in our study but did not reaching statistical significance, possibly due to the small number of cases. However, there was no beneficial effect on long-term mortality or HF-related morbidity in patients receiving tolvaptan for ADHF in the EVEREST study.26 In addition, the Targeting Acute Congestion with Tolvaptan in Congestive Heart Failure randomized control trial demonstrated that treatment with 30 mg tolvaptan in ADHF patients in the United States did not improve dyspnea but resulted in greater weight loss and net fluid loss compared with placebo at 24 hours after medication use.27 These results would limit its use in long-term HF management. However, in the hyponatremia subgroup analysis of the EVEREST study, tolvaptan was associated with more favorable outcomes in ADHF patients with pronounced hyponatremia (Na < 130 mEq/L) compared with standard therapy.28 The 2021 ESC guidelines suggest that tolvaptan can be considered to increase serum sodium and diuresis in patients with persistent hyponatremia and congestion.14 Consequently, tolvaptan may still play a role in the initial stage of ADHF management, especially in selected patients with fluid overload, hyponatremia, and diuretic resistance.
Our study demonstrated that tolvaptan significantly improved fluid overload in hospitalized ADHF patients with volume overload despite the use of conventional diuretics. A recent meta-analysis also concluded that adding tolvaptan to standard care therapy could benefit hospitalized patients with ADHF by reducing body weight and improving serum sodium levels.29 In the current study, we enrolled ADHF patients regardless of their LVEF, which is a confounder, especially for those who had HF with preserved ejection fraction. Recently, Kinugawa et al. reported a prospective, multicenter, post-marketing surveillance study of tolvaptan which showed that tolvaptan was effective and safe for treating fluid retention in patients with HF with preserved ejection fraction, as well as HF with midrange ejection fraction and HF with reduced ejection fraction.30 Tamaki et al. also demonstrated that adjunctive tolvaptan use may provide rapid decongestion without worsening sympathetic nerve activity as with loop diuretics in patients with acute decompensated HF with preserved ejection fraction.31 In the current study, we demonstrated that low dose tolvaptan 15 mg was safe and effective in hospitalized ADHF patients regardless of LVEF. Even for extremely old patients, tolvaptan has been shown to be safe and effective in the management of ADHF as a complementary therapeutic option.32,33
There are some limitations to the study. First, we did not have LVEF data in this study. Therefore, we do not know how many of the patients had a reduced or preserved ejection fraction, which may limit the analysis. Second, the follow-up period was 1 month, so we could not assess the long-term safety of tolvaptan. However, the safety of long-term usage has already been demonstrated in a previous study.26 Third, the study was performed in an inpatient setting, and further studies are needed to evaluate the safety of tolvaptan in an outpatient setting.
CONCLUSIONS
In this randomized control trial in Taiwanese ADHF patients, daily 15 mg tolvaptan use could significantly improve volume overload despite the use of conventional diuretics. In ADHF patients with diuretic resistance or hyponatremia under conventional diuretic treatment, tolvaptan can be an alternative option to improve volume overload.
DECLARATION OF CONFLICT OF INTEREST
This study was funded by Otsuka Pharmaceutical Co., Ltd. The funding source had no role in data interpretation and manuscript preparation. All the authors declared no other competing interests.
REFERENCES
- 1.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29:2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 2.Wang CC, Chang HY, Yin WH, et al. TSOC-HFrEF Registry: a registry of hospitalized patients with decompensated systolic heart failure: description of population and management. Acta Cardiol Sin. 2016;32:400–411. doi: 10.6515/ACS20160704A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang HY, Wang CC, Wu YW, et al. One-year outcomes of acute decompensated systolic heart failure in Taiwan: lessons from TSOC-HFrEF registry. Acta Cardiol Sin. 2017;33:127–138. doi: 10.6515/ACS20170202A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mebazaa A, Tolppanen H, Mueller C, et al. Acute heart failure and cardiogenic shock: a multidisciplinary practical guidance. Intensive Care Med. 2016;42:147–163. doi: 10.1007/s00134-015-4041-5. [DOI] [PubMed] [Google Scholar]
- 6.Matsue Y, Damman K, Voors AA, et al. Time-to-furosemide treatment and mortality in patients hospitalized with acute heart failure. J Am Coll Cardiol. 2017;69:3042–3051. doi: 10.1016/j.jacc.2017.04.042. [DOI] [PubMed] [Google Scholar]
- 7.Verbrugge FH. Editor’s choice-diuretic resistance in acute heart failure. Eur Heart J Acute Cardiovasc Care. 2018;7:379–389. doi: 10.1177/2048872618768488. [DOI] [PubMed] [Google Scholar]
- 8.Verbrugge FH, Steels P, Grieten L, et al. Hyponatremia in acute decompensated heart failure: depletion versus dilution. J Am Coll Cardiol. 2015;65:480–492. doi: 10.1016/j.jacc.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Gheorghiade M, Niazi I, Ouyang J, et al. Vasopressin V2-receptor blockade with tolvaptan in patients with chronic heart failure: results from a double-blind, randomized trial. Circulation. 2003;107:2690–2696. doi: 10.1161/01.CIR.0000070422.41439.04. [DOI] [PubMed] [Google Scholar]
- 10.Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099–2112. doi: 10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]
- 11.Matsuzaki M, Hori M, Izumi T, et al. Efficacy and safety of tolvaptan in heart failure patients with volume overload despite the standard treatment with conventional diuretics: a phase III, randomized, double-blind, placebo-controlled study (QUEST study). Cardiovasc Drugs Ther. 2011;25 Suppl 1:S33–S45. doi: 10.1007/s10557-011-6304-x. [DOI] [PubMed] [Google Scholar]
- 12.Gheorghiade M, Konstam MA, Burnett JC, Jr., et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 13.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 14.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 15.Neuberg GW, Miller AB, O'Connor CM, et al. Diuretic resistance predicts mortality in patients with advanced heart failure. Am Heart J. 2002;144:31–38. doi: 10.1067/mhj.2002.123144. [DOI] [PubMed] [Google Scholar]
- 16.Costanzo MR, Saltzberg M, O'Sullivan J, Sobotka P. Early ultrafiltration in patients with decompensated heart failure and diuretic resistance. J Am Coll Cardiol. 2005;46:2047–2051. doi: 10.1016/j.jacc.2005.05.099. [DOI] [PubMed] [Google Scholar]
- 17.Kramer BK, Schweda F, Riegger GA. Diuretic treatment and diuretic resistance in heart failure. Am J Med. 1999;106:90–96. doi: 10.1016/s0002-9343(98)00365-9. [DOI] [PubMed] [Google Scholar]
- 18.Goldsmith SR. Vasopressin: a therapeutic target in congestive heart failure? J Card Fail. 1999;5:347–356. doi: 10.1016/s1071-9164(99)91339-8. [DOI] [PubMed] [Google Scholar]
- 19.Ghali JK, Hamad B, Yasothan U, Kirkpatrick P. Tolvaptan. Nat Rev Drug Discov. 2009;8:611–612. doi: 10.1038/nrd2946. [DOI] [PubMed] [Google Scholar]
- 20.Gheorghiade M, Gottlieb SS, Udelson JE, et al. Vasopressin v(2) receptor blockade with tolvaptan versus fluid restriction in the treatment of hyponatremia. Am J Cardiol. 2006;97:1064–1067. doi: 10.1016/j.amjcard.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 21.Goldsmith SR, Francis GS, Cowley AW, Jr., et al. Increased plasma arginine vasopressin levels in patients with congestive heart failure. J Am Coll Cardiol. 1983;1:1385–1390. doi: 10.1016/s0735-1097(83)80040-0. [DOI] [PubMed] [Google Scholar]
- 22.Lien YH, Shapiro JI. Hyponatremia: clinical diagnosis and management. Am J Med. 2007;120:653–658. doi: 10.1016/j.amjmed.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 23.Dixon MB, Lien YH. Tolvaptan and its potential in the treatment of hyponatremia. Ther Clin Risk Manag. 2008;4:1149–1155. doi: 10.2147/tcrm.s3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gheorghiade M, Gattis WA, O'Connor CM, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA. 2004;291:1963–1971. doi: 10.1001/jama.291.16.1963. [DOI] [PubMed] [Google Scholar]
- 25.Konstam MA, Kiernan M, Chandler A, et al. Short-term effects of tolvaptan in patients with acute heart failure and volume overload. J Am Coll Cardiol. 2017;69:1409–1419. doi: 10.1016/j.jacc.2016.12.035. [DOI] [PubMed] [Google Scholar]
- 26.Konstam MA, Gheorghiade M, Burnett JC, Jr., et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 27.Felker GM, Mentz RJ, Cole RT, et al. Efficacy and safety of tolvaptan in patients hospitalized with acute heart failure. J Am Coll Cardiol. 2017;69:1399–1406. doi: 10.1016/j.jacc.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Hauptman PJ, Burnett J, Gheorghiade M, et al. Clinical course of patients with hyponatremia and decompensated systolic heart failure and the effect of vasopressin receptor antagonism with tolvaptan. J Card Fail. 2013;19:390–397. doi: 10.1016/j.cardfail.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Zhang Q, Liu M, et al. Tolvaptan in reversing worsening acute heart failure: a systematic review and meta-analysis. J Int Med Res. 2019;47:5414–5425. doi: 10.1177/0300060519882221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinugawa K, Sato N, Inomata T, et al. A prospective, multicenter, post-marketing surveillance study to evaluate the safety and effectiveness of tolvaptan in patients with reduced, preserved, and mid-range ejection fraction heart failure. Int Heart J. 2019;60:1123–1130. doi: 10.1536/ihj.18-671. [DOI] [PubMed] [Google Scholar]
- 31.Tamaki S, Yamada T, Morita T, et al. Impact of adjunctive tolvaptan on sympathetic activity in acute heart failure with preserved ejection fraction. ESC Heart Fail. 2020;7:933–937. doi: 10.1002/ehf2.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato Y, Uzui H, Mukai M, et al. Efficacy and safety of tolvaptan in patients more than 90 years old with acute heart failure. J Cardiovasc Pharmacol Ther. 2020;25:47–56. doi: 10.1177/1074248419861962. [DOI] [PubMed] [Google Scholar]
- 33.Kinugawa K, Inomata T, Sato N, et al. Effectiveness and adverse events of tolvaptan in octogenarians with heart failure. Interim analyses of Samsca Post-Marketing Surveillance In Heart faiLurE (SMILE study). Int Heart J. 2015;56:137–143. doi: 10.1536/ihj.14-332. [DOI] [PubMed] [Google Scholar]