Abstract
Maize is a staple food of smallholder farmers living in highland regions up to 4,000 m above sea level worldwide. Mexican and South American highlands are two major highland maize growing regions, and population genetic data suggest the maize's adaptation to these regions occurred largely independently, providing a case study for convergent evolution. To better understand the mechanistic basis of highland adaptation, we crossed maize landraces from 108 highland and lowland sites of Mexico and South America with the inbred line B73 to produce F1 hybrids and grew them in both highland and lowland sites in Mexico. We identified thousands of genes with divergent expression between highland and lowland populations. Hundreds of these genes show patterns of convergent evolution between Mexico and South America. To dissect the genetic architecture of the divergent gene expression, we developed a novel allele–specific expression analysis pipeline to detect genes with divergent functional cis-regulatory variation between highland and lowland populations. We identified hundreds of genes with divergent cis-regulation between highland and lowland landrace alleles, with 20 in common between regions, further suggesting convergence in the genes underlying highland adaptation. Further analyses suggest multiple mechanisms contribute to this convergence in gene regulation. Although the vast majority of evolutionary changes associated with highland adaptation were region specific, our findings highlight an important role for convergence at the gene expression and gene regulation levels as well.
Keywords: highland adaptation, allele-specific expression, convergent evolution, flowering time, maize
Introduction
Highland maize is cultivated in cold, mountainous regions worldwide, at altitudes of up to 4,000 m above sea level (masl) and with mean growing season temperatures below 20 °C (Lothrop 1994; Hartkamp et al. 2000). The International Maize and Wheat Improvement Center (CIMMYT) estimates that >6 million hectares (Mha) are used for highland maize production worldwide, mainly in developing countries where it is grown by smallholder farmers as one of the main sources of calories in their diet (Lothrop 1994; Zambrano et al. 2021). Mexico (∼2.9 Mha, 46.6%) and South America (∼0.6 Mha, 9.4%) are two major highland maize-producing regions and are geographically separated from each other. Highland maize landraces (open-pollinated traditional varieties) in central Mexico and South America have distinct morphologic characteristics from lowland tropical or temperate germplasm (Janzen et al. 2022), including purple stems, drooping leathery leaves, weak roots, tassels with few branches, conical-shaped ears (Anderson and Cutler 1942), and a changed biochemical response to UV radiation (Casati and Walbot 2005). They also have other specific characteristics that make them suitable to live in high-elevation climates, including frost tolerance and improved seedling emergence, growth, and grain filling at low temperatures (Eagles and Lothrop 1994).
These consistent differences between highland and lowland landraces indicate that highland maize has undergone considerable local adaptation since its introduction to highland environments in the past 6,200 years (Piperno and Flannery 2001). However, we still know little about the genetic basis of highland adaptation in maize: What genes were involved? Was adaptation driven by standing genetic variation or novel alleles? Is the genetic basis of adaptation independent between populations from different geographic regions? Recent population genetic studies have begun to paint a complex and divergent picture of highland adaptation between Mexican and South American maize. Genome-wide single-nucleotide polymorphism (SNP) data show strong population structure in maize landraces from Mesoamerica and South America (Van Heerwaarden et al. 2011; Takuno et al. 2015). Several studies using population genetic data (Hufford et al. 2013; Pyhäjärvi et al. 2013; Calfee et al. 2021; Barnes et al. 2022) identified genomic loci that were introgressed from a wild ancestor of maize, Zea mays ssp. mexicana (hereafter mexicana) found exclusively in the highlands of central and northern Mexico (De Jesús Sánchez González et al. 2018), suggesting that alleles contributing to highland adaptation may have been acquired by crossing with pre-adapted relatives. Three of these loci have been well characterized: Inv4m (Hufford et al. 2013; Crow et al. 2020), mhl1 (Hufford et al. 2013; Calfee et al. 2021), and HPC1 (Barnes et al. 2022), and the mexicana alleles are found almost exclusively in landraces from the Mexican highlands. Wang et al. (2017) found no evidence for substantial spread of mexicana haplotypes to South America and Takuno et al. (2015) found <1.8% of SNPs and 2.1% of genes showing evidence for convergent evolution between Mesoamerican and South American highland populations. However, in a recent genome-wide scan with high-density SNPs, Wang et al. (2021) identified 10,199–11,345 SNPs and 1,651–2,015 genes with evidence for population divergence between highland and counterpart lowland populations in Central America and South America, respectively, including 10.7% of SNPs, 15.0% of genes, and flowering time pathway showing evidence of convergent adaptation between Andes and Mexican highland landrace populations. The extent of convergence in adaptation to highlands is important because it can indicate whether alleles beneficial for highland adaptation in one geographic region are likely to also be beneficial in another or whether adaptation is likely constrained by a limited set of possible loci or if multiple different adaptive paths are available (Lee and Coop 2017; Wang et al. 2021).
Population genetic scans using SNP markers can efficiently discover loci that have diverged between populations, indicating a potential role in adaptation. However, discovering mechanisms controlled by these loci remains a challenge. Although predicting the function of protein-coding variants is possible, we have little ability to predict the function of non-coding variants, including those affecting gene regulation. For example, although the 13 Mb Inv4m locus has been known about for more than a decade (Hufford et al. 2013) and appears to play a role in flowering time (Romero Navarro et al. 2017), the mechanisms underlying its role remained unclear. Gene expression analysis can provide a link between sequence variation and molecular mechanisms, particularly by discovering expression patterns of groups of genes that share common biologic functions or attributes (Maleki et al. 2020). Crow et al. (2020) developed two populations segregating for highland and lowland alleles at this locus and measured gene expression effects of the locus across nine tissues. They identified 39–607 genes per tissue that were consistently regulated by Inv4m in both families, and gene set enrichment analyses suggested a role of the locus in the regulation of photosynthesis and several other biologic processes. Other studies have begun to use gene expression to study the process of highland adaptation in maize as well. Kost et al. (2017) measured expression variation among landraces from three distinct elevational zones (highland, midland, and lowland) and identified two co-expression modules correlated with temperature-related environmental parameters. Aguilar-Rangel et al. (2017) used allele-specific expression (ASE) to study cis-regulatory divergence between the highland landrace Palomero Toluqueño and the modern inbred B73 and identified 2,386 genes with divergent expression caused by the different genotypes. These expression studies are limited, however, in their ability to describe the complexity and genetic architecture of gene regulatory adaptation at the population level where evolution occurs.
In this study, we used population-level -ASE analyses to identify gene expression traits that have diverged between highland and lowland populations of Mexican and South American maize landraces. We selected maize landraces from 108 highland and lowland sites that cover broad growing regions of highland maize in Mexico and South America and crossed them with a common inbred line B73 to produce F1 hybrids (F1s). We planted the F1 families at two locations that represented highland and lowland environments in Mexico. Our primary objectives were to (1) identify genes that show evidence for adaptive divergence in cis-regulation between high and low elevation landraces in Mexico and South America; (2) identify candidate gene pathways and functional groups that underwent directional selection for gene regulation during adaptation to highland climates; and (3) gain insights into the convergent evolutionary patterns of highland adaptation between populations in Mexico and South America. We first identified thousands of genes with divergent expression between highland and lowland populations, of which hundreds show patterns of convergent evolution between Mexico and South America. We then differentiated the two alleles of each gene using ASE and identified genes with divergent cis-regulation between highland and lowland alleles. We used the term convergence to define the repeated evolution of similar phenotypic differences between highland and lowland populations in both Mexico and South America (Arendt and Reznick 2008). To achieve the population-level ASE analysis, we developed a novel analysis pipeline that can accurately measure the ASE of each individual at the gene level using RNAseq data. We discovered hundreds of genes with divergent cis-regulation between highland and lowland landrace alleles in the Mexican and South American populations, respectively. Of these, 20 genes were in common between populations, suggesting a low level of convergence at the gene regulation level underlies highland adaptation in maize.
New Approaches
Allelic read counts are the starting point of all ASE analyses (Castel et al. 2015). Most ASE analyses have been done either based on individual SNPs (Shao et al. 2019; Zhou et al. 2019; Li et al. 2021) or by integrating allelic read counts across SNPs within a gene (Lemmon et al. 2014; Fan et al. 2020). Gene-level ASE ratios are more robust because they are based on more total reads, and in a population sample, SNP-level ASE ratios cannot reliably be compared across individuals because many SNPs are individual specific. Therefore, most existing studies using ASE have been based on a single F1 individual (Aguilar-Rangel et al. 2017; Shao et al. 2019; Zhou et al. 2019; but see Lemmon et al. 2014 who used 29 F1s from different maize and teosinte parents to study the genetics of maize domestication), so the generality of the discoveries to whole populations was unclear.
We have developed a novel analysis pipeline that can accurately measure ASE of each individual at the gene level using RNAseq data alone, and efficiently detect genes with common functional variation in cis-regulatory regions that have diverged between populations. First, we crossed maize landraces from 108 highland and lowland sites in Mexico and South America with a common inbred line B73 to produce F1 hybrids and took advantage of this genetic design to phase heterozygous SNPs of each F1 sample based on the B73 reference genome. Then, we extracted reads that were assigned to either of the two parental origins at all overlapping loci with heterozygous SNPs into separate BAM files and counted the reads overlapping each gene feature in each BAM file. These gene counts are the allelic expressions of the maternal and paternal alleles of each gene, respectively. Finally, we tested for cis-regulatory divergence between highland and lowland populations in the Mexican and South American populations by analyzing the average difference in landrace ASE (relative to B73 ASE) between F1s derived from highland and lowland landraces.
Our methodology can efficiently detect genes showing cis-regulatory divergence between populations. In addition, gene-level ASE ratios estimated with our method can be used to identify gene–trait relationships relevant to hybrid breeding through transcriptome-wide association studies (TWASs). In such programs, candidate lines are evaluated by crossing to common testers. TWAS using ASE can pinpoint causal gene regulatory traits underlying key performance traits of interest, enabling further targeted gene editing for genetic improvement.
Results
Geographical Origins and Population Structure of Maize Landraces
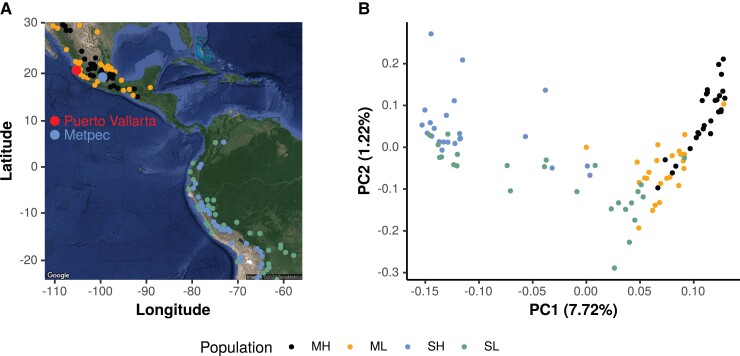
We selected 108 maize landraces from CIMMYT's germplasm bank representing highland and lowland sites (one landrace accession per site) across broad geographical regions of Mexico and South America where maize landraces are cultivated (fig. 1A; supplementary table S1, Supplementary Material online). Individuals from highland (>2,000 masl) and lowland (<1,000 masl) sites were paired latitudinally (within 1° latitude) and chosen such that all pairwise distances were >50 km (fig. 1A).
Fig. 1.
The Geographical origins (A) and genomic relationships (B) of the 108 maize landraces used as paternal parents of the F1 populations. MH, Mexican Highland; ML, Mexican Lowland; SH, South American Highland; SL, South American Lowland. In (A), the larger dots represent physical positions of the two field trials, and the smaller dots represent physical positions where the 108 maize landraces were collected.
We did whole-genome skim sequencing of a single plant of each of these 108 landraces and performed a principal component (PC) analysis (PCA, fig. 1B) to study the genetic structure of the landraces. The first two PCs separated the landraces into four populations (Mexican Highland, Mexican Lowland, South American Highland, South American Lowland). The genomic relationships of the 108 maize landraces estimated here were consistent with Janzen et al. (2022) who used a different individual from each of the same landrace populations genotyped with DArTseq-Based SNP markers (Wenzl et al. 2004). Our results were also consistent with patterns of genetic structure reported by Van Heerwaarden et al. (2011) using a small SNP panel of 1,127 accessions of maize landraces.
Highland and Lowland Landraces Show Widespread Divergences in Gene Expression
We measured gene expression (total expression of both alleles) in F1 hybrids derived from the 108 landraces described above in two leaf-derived tissues sampled from two locations: leaf tip and leaf base samples from a fully expanded leaf of a V4 plant from each F1 family in each of two field blocks at the highland site in Metepec, Mexico at 2,620 masl, and leaf tip samples from a comparably staged leaf from a single plant from each F1 family in a single field block at the lowland site in Puerto Vallarta, Mexico at 7 masl. These tissues (hereafter site:tissues) are labeled MetLeaftip, MetLeafbase, and PvLeaftip below. In each of these three site:tissues, we tested for differences in the expression of each expressed gene between highland- and lowland-derived F1s separately for the Mexican and South American populations, accounting for sampling effects due to time of collection and collection team, and leveraging shared signals across site:tissues using multivariate adaptive shrinkage (mash; Urbut et al. 2019). In total, we discovered 4,432 and 1,816 (supplementary tables S2 and S3, Supplementary Material online, ) genes with differential expression between highland- and lowland-derived F1 plants from the Mexican and South American continents, respectively, using a 5% local false sign rate (lfsr) threshold for declaring significance. Breaking these lists down by site:tissue, we discovered 1,278, 3,716, and 319 genes with divergent highland expression in the Mexican F1 families in MetLeaftip, MetLeafbase, and PvLeaftip, and 715, 1,626, and 368 genes with divergent highland expression in the South American F1 families (fig. 2A, supplementary fig. S1, Supplementary Material online). We detected many more genes with differential expression between highland and lowland landraces on each continent than between the Mexican and South American populations on average (total of 124 genes, supplementary table S3, Supplementary Material online), or that were associated with latitude on either continent (total of 60 and 131 genes in the Mexican and South American populations, respectively, supplementary table S3, Supplementary Material online). However, many more genes showed significant changes in expression during the ∼1.5 h sampling window within each site:tissue (a total of 18,844 out of the 21,599 genes assayed across the 3 site:tissues, supplementary table S3 and fig. S2, Supplementary Material online), suggesting that the transcriptome-wide consequences of elevation adaptation were smaller than diel expression variation during the course of a morning.
Fig. 2.
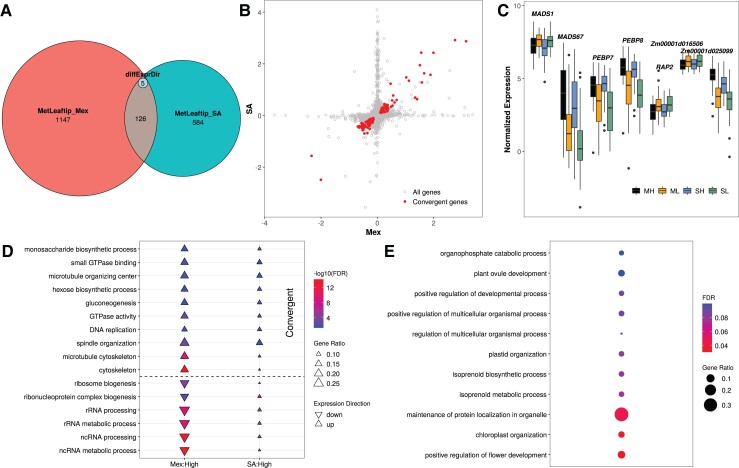
Results of gene expression analyses. (A) Numbers of differentially expressed genes between highland and lowland populations from Mexico and South America and common genes detected in both continents in the MetLeaftip tissue. The small inset in the overlapping region shows genes significant in both populations, but with opposite directions of expression change. (B) Correlation of Posterior Mean highland effects between Mexican and South American population for all genes measured for gene expression and a subset of genes showing evidence of convergent evolution (highlighted in red) in the MetLeaftip tissue. (C) Expression of flowering-related genes in the Mexican Highland (ML), Mexican Lowland (ML), South American Highland (SA), and South American Lowland (SL) populations in the MetLeaftip tissue. These flowering-related genes are identified by looking for overlapping between the convergent genes and maize flowering time candidate genes aggregated by Li et al. (2016) and Swarts et al. (2016). (D) FDR of 16 GO terms that are significant in both Mexican and South American populations across three site:tissue. The size of each triangle indicates the enrichment ratio of this GO term, defined as ratio of number of differentially expressed genes in a GO category divided by the size of the category. We tested up-regulated and down-regulated differentially expressed genes separately and triangles and upside-down triangles represent up-regulated and down-regulated GO categories, respectively. (E) GO categorical enrichments of the genes individually classified as having convergent expression evolution in MetLeaftip and MetLeafbase.
Among these genes with differential expression in highland populations, a small minority were significantly associated with elevation in the F1 families of both continents. One-hundred and thirty-one, 429, and 30 were detected in both continents per site:tissue, representing 18%, 26%, and 8% of the lesser of the number of significant genes from either continent (fig. 2A, supplementary fig. S1 and table S4, Supplementary Material online). However, despite being a relatively small overlap, this is many more than expected by chance (P = 2.74 × 10−25, 1.12 × 10−17, and 3.28 × 10−12 per site:tissue, respectively), and if we relax the significance threshold, the overlap percentage grows considerably larger. Furthermore, of the genes with significant responses to elevation on both continents, both the direction and magnitude of expression difference between highland and lowland populations was highly correlated (fig. 2B, supplementary fig. S3, Supplementary Material online). Although the estimated highland effects were positively correlated for all genes (r = 0.22, 0.26, and 0.20), the effects of genes with significant effects in both populations were much higher (r = 0.96, 0.94, and 0.97). We thus considered the 126, 411, and 30 genes exhibiting identical directional change of expression as having convergent evolution of gene expression between the two continents.
Because previous studies of highland adaptation in maize have described earlier flowering as a characteristic of highland landraces (Romero Navarro et al. 2017; Wang et al. 2021; Janzen et al. 2022), we inspected a list of maize of 886 flowering time genes and candidates aggregated by Li et al. (2016) and Swarts et al. (2016). Of these, 17 showed convergent expression differences in F1 families from both continents (table 1, fig. 2C, supplementary fig. S4, Supplementary Material online), including four well-known transcription factors and ZCN8 that contributes to early flowering during highland adaptation (Guo et al. 2018). Additionally, phosphatidylglycerols have been linked to the regulation of flowering through the sequestration of florigen in phloem cells (Susila et al. 2021), and we found 31 (false discovery rate [FDR] = 2.4 × 10−4) and 12 (FDR = 0.27) differentially expressed genes (supplementary table S5, Supplementary Material online) labeled with the Gene Ontology (GO) term “phosphatidylglycerol biosynthetic process” (GO:0006655) associated with elevation from the Mexican and South American continents, respectively, using a 5% lfsr to declare differentially expressed genes. All of these differentially expressed genes were down-regulated in the highlands in both populations, consistent with earlier flowering. If we relax the significance threshold, for example, lfsr = 0.2, the differentially expressed genes mapped to GO:0006655 and down-regulated in the highlands in both populations grow to 50 (FDR = 7.8 × 10−7) and 36 (FDR = 5.9 × 10−6) with 30 in common (supplementary table S5, Supplementary Material online). These results further support Wang et al. (2021) finding of convergent evolution of flowering regulation along elevational gradients in Mexico and South America.
Table 1.
Seventeen Flowering-related Genes that Showed Convergent Expression Differences Between Highland- and Lowland-derived F1 Families From Mexican and South American Populations.
| Gene ID | Gene Name | Chr | Position (bp) | Description | Expression changes in highland genotypes | References |
|---|---|---|---|---|---|---|
| Zm00001d022088 | MADS67 | 7 | 169,844,061 | MADS-transcription factor 67 | Up | Li et al. (2016) |
| Zm00001d010752 | PEBP8/ZCN8 | 8 | 126,880,531 | Phosphatidylethanolamine-binding protein8 | Up | Swarts et al. (2016) |
| Zm00001d038725 | PEBP7/ZCN7 | 6 | 163,368,049 | phosphatidylethanolamine-binding protein7 | Up | Swarts et al. (2016) |
| Zm00001d010987 | RAP2 | 8 | 136,009,216 | rap2—rap2.7 orthologue (transcription factor) | Down | Swarts et al. (2016) |
| Zm00001d025099 | 10 | 103,947,429 | Up | Li et al. (2016) | ||
| Zm00001d016506 | cl27878_1 | 5 | 165,302,124 | Down | Li et al. (2016) | |
| Zm00001d048474 | MADS1/ZMM5 | 9 | 156,960,598 | transcription factor | Down | Swarts et al. (2016) |
| Zm00001d049543 | CCA1 | 4 | 34,070,590 | Down | Swarts et al. (2016) | |
| Zm00001d051951 | 4 | 175,147,743 | Down | Li et al. (2016) | ||
| Zm00001d014990 | RUP1 | 5 | 71,267,717 | repressor of UV-B photomorphogenesis homolog1 | Down | Li et al. (2016) |
| Zm00001d015293 | 5 | 82,992,330 | Up | Li et al. (2016) | ||
| Zm00001d005814 | 2 | 189,518,235 | Down | Li et al. (2016) | ||
| Zm00001d040323 | CAL2 | 3 | 38,197,170 | calmodulin2 | Up | Li et al. (2016) |
| Zm00001d022558 | 7 | 180,004,346 | Up | Li et al. (2016) | ||
| Zm00001d023833 | 10 | 23,764,459 | Down | Li et al. (2016) | ||
| Zm00001d046935 | 9 | 111,766,412 | Down | Li et al. (2016) | ||
| Zm00001d012119 | JMJ11 | 8 | 168,442,999 | JUMONJI-transcription factor 11 | Up | Li et al. (2016) |
Note.—Position (bp) represents starting physical position of a gene (bp; B73 AGPv4).
Beyond flowering regulation, the long lists of differentially expressed genes (supplementary table S2, Supplementary Material online) themselves are difficult to parse for insights into highland elevation. Therefore, to summarize these results, we tested for enrichment of GO categories (Wimalanathan et al. 2018) and KEGG (Kanehisa et al. 2021) and CornCyc (Hawkins et al. 2021) pathways among the lists of significant genes, measuring enrichment separately for up-regulated and down-regulated highland genes in each site:tissue. A total of 763 GO categories, 38 KEGG pathways and 3 CornCyc pathways were significantly enriched in at least one site:tissue at a 5% FDR (supplementary table S6, Supplementary Material online). The most significant GO terms were thylakoid (GO:0009579), plastid envelope (GO:0009526), chloroplast envelope (GO:0009941).
Of these functional GO categories, 16 were identified in F1 families from both continents, and 10 of them were similarly enriched with up-regulated or down-regulated genes on both continents suggesting that the evolutionary changes were convergent (fig. 2D). Confirming the results above, categorical enrichments of the genes individually declared to show convergent expression evolution identified 6 and 15 terms in MetLeaftip and MetLeafbase (fig. 2E, supplementary table S7, Supplementary Material online), respectively, including the terms positive regulation of flower development (GO:0009911) and chloroplast organization (GO:0009658), and also including endoplasmic reticulum (ER) retention sequence binding (GO:0046923).
To explore whether the gene expression changes could be partially explained by alterations in cell-type compositions of leaf tissues, we used a set of marker genes for seven cell populations identified by single-cell sequencing of a maize leaf (Bezrutczyk et al. 2021) to estimate relative cell population sizes in each sample. The first two PCs of our cell population scores clearly separated the three site:tissues (fig. 3A), and the scores explained significantly more variation among samples than expected from random subsets of genes (fig. 3B), suggesting that these gene sets captured meaningful variation, even if the precise identities of the cell populations are not clear. The first PC of the cell population scores of the MetLeafbase sample were also unevenly distributed across the field, suggesting spatial variation in leaf anatomy or developmental stage. However, within each range of the field the highland and lowland samples from the Mexican population were clearly differentiated, and highland and lowland samples from the South American population were also clearly differentiated across 3/5 of the field (fig. 3C), suggesting that there were consistent anatomical differences between highland and lowland leaves. These anatomical differences likely cause the appearance of differential expression because different cell populations express genes at different levels.
Fig. 3.
Cell type proportion inference. (A) Each point represents a single RNA sample, colored by the site:tissue and positioned according to its coordinates on the first two PC axes of the projections onto seven sets of cell-type-specific genes identified by Bezrutczyk et al. (2021) in maize leaves. (B) The highest point in each column shows the standard deviation of the cell-type projection scores within each tissue. Box plots show the distribution of 200 randomized projection scores based on random sets of genes. (C) Distributions of the PC1 coordinates for the MetLeafbase samples, separated by population and range of the field.
We attempted to control for these anatomical differences when testing for differential expression between highland and lowland accessions by including the cell population scores as covariates. In these models, the number of differentially expressed genes and enriched GO terms dropped significantly (a total of 648 genes and 0 GO terms were significant for elevation in the Mexican population, and a total of 1,182 genes and 68 GO terms were significant for elevation in the South American population, supplementary table S8, Supplementary Material online) suggesting that anatomical differences were the primary driver of expression differences observed above, at least for the Mexican population. However, the differential expression of flowering-related genes remained significant even after accounting for these anatomical differences.
Development of a Novel Allele–Specific Expression Analysis Pipeline to Identify Genetic Loci Underlying Morphologic and/or Transcriptomic Differences Between Highland and Lowland Landraces
The gene expression analysis results above point to a diverse set of expression traits associated with highland adaptation in Mexican and South American landraces; however, the genetic architecture of these differences remains unclear. Although differential gene expression analyses can detect differences in thousands of expression traits, it remains possible that a small number of genetic loci might be responsible for most of these changes (Crow et al. 2020). On the other hand, differences in expression between the two allelic copies of each gene in each F1 individual can only be caused by differences in the local cis-regulatory region around each gene (Sun and Hu 2013), as trans-acting regulatory elements will impact both alleles. Therefore, we used ASE (defined as the ratio of landrace allelic count to B73 allelic count) to scan the genome for genes that have undergone divergence in the cis-control of gene expression between highland and lowland landraces.
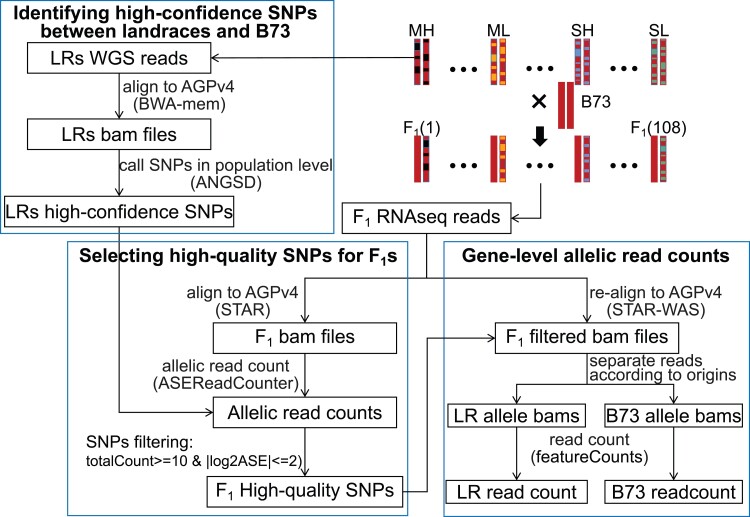
To resolve major challenges (supplementary text, Supplementary Material online) for ASE detection across individuals at the gene level when only RNAseq data are available, we took advantage of our genetic design involving the 108 F1 hybrids all crossed to the same tester line B73 (fig. 4). We developed a novel analysis pipeline for directly counting allelic reads at the gene level in each F1 individual. Briefly, our pipeline included three parts: First, we identified a set of high-confidence SNPs between any of the landrace parents and B73 from our low-coverage whole-genome sequencing (WGS) data. Next, we used the RNAseq data to genotype and phase these SNPs within each F1 sample. Finally, we counted the number of reads confidently assigned to either the B73 reference or landrace genome, accounting for allelic mapping bias using the WASP algorithm (Van De Geijn et al. 2015). Full details are available in the Materials and Methods.
Fig. 4.
The analysis pipeline for gene-level allelic read count. AGPv4, B73 reference genome version 4; bams, bam files; LR, landrace; MH, Mexican Highland; ML, Mexican Lowland; SH, South American Highland; SL, South American Lowland; WGS, whole-genome sequencing.
To assess the reliability of our pipeline, we performed three validation analyses. First, the distribution of log2ASE ratios across all genes was approximately symmetric around zero for each sample, suggesting that we did not have strong reference bias toward the B73 allele (supplementary fig. S5A, Supplementary Material online). In contrast, less stringent filtering of SNPs led to strong reference allele bias (supplementary fig. S5B, Supplementary Material online). Second, the ASE values from our real data had much more variation than expected by counting variance alone, suggesting the observed variation is due to biology (supplementary fig. S6, Supplementary Material online). Finally, the correlation of ASE between samples collected from two different individuals from the same F1 family was high for genes in genomic regions where the two individuals shared the same haplotype but much lower for genes in genomic regions where the two individuals did not share the same haplotype (supplementary fig. S7, Supplementary Material online). Full details are available in the supplementary results, Supplementary Material online.
Detection of Differential cis-regulation of Landrace Alleles Between Highland and Lowland Landrace Populations
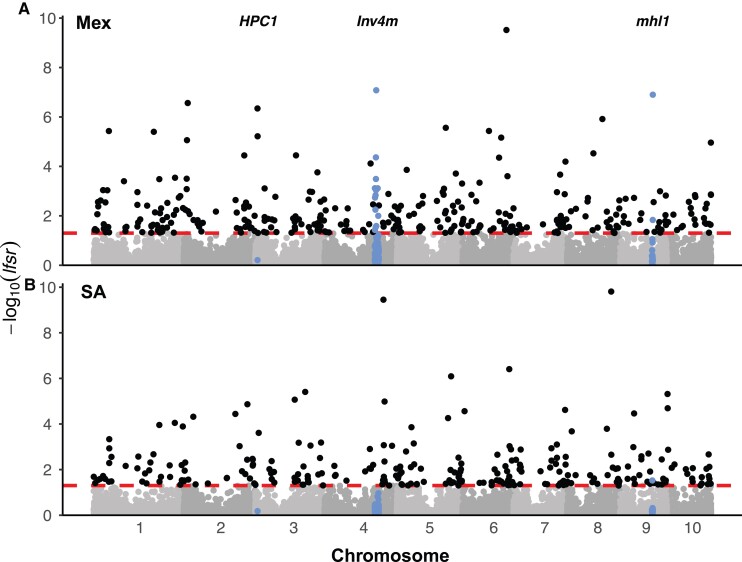
We tested for cis-regulatory divergence at the population level between highland and lowland alleles in the Mexican and South American populations by comparing ASE ratios among samples for each gene. We refer to this as differential ASE (DASE) analysis. In total, we identified 341 and 260 genes (fig. 5, supplementary tables S9 and S10, Supplementary Material online) with DASE between highland- and lowland-derived F1 plants from the Mexican and South American continents, respectively, in at least one site:tissue by meta-analysis using a 5% lfsr threshold. The number of genes that were significantly differentiated in ASE between highland and lowland landraces on each continent was slightly higher than the number of genes that were differentially expressed between the Mexican and South American populations on average (249, supplementary table S10, Supplementary Material online) and was much higher than the number of genes that were associated with latitude on either continent (17 and 23 in the Mexican and South American populations, respectively, supplementary table S10, Supplementary Material online). However, more genes showed significant changes in ASE during the ∼1.5 h sampling window within each site:tissue (760 genes across the 3 site:tissues, supplementary table S10, Supplementary Material online), which was consistent with our observations in the gene expression analysis above.
Fig. 5.
Manhattan plots showing the local false sign rate (lfsr) of the meta-analysis with mash for detecting differential ASE between highland and lowland landraces in the (A) Mexican and (B) South American F1 populations, expressed as −log 10 (lfsr). The lfsr is analogous to a FDR but more stringent (Stephens 2017). Each dot represents a gene. The dashed line in each plot indicates the significant level at lfsr < 0.05. Blue dots under each locus name highlight genes within the three prior-identified loci: HPC1, Inv4m and mhl1. Mex, Mexican F1 population; SA, South American F1 population.
Subsequently, we inspected the three loci with known genetic differentiation between highland and lowland landraces in Mexico: Inv4m, mhl1, and HPC1. There were 13 and 2 DASE genes detected inside the Inv4m and mhl1 regions in the Mexican population, but only one gene with weak evidence (lfsr = 0.03) in the mhl1 region in the South American population (fig. 5). This is consistent with our knowledge that the mexicana-to-maize introgression mainly happened in Mexican highlands (Hufford et al. 2013; Pyhäjärvi et al. 2013; Wang et al. 2017; Crow et al. 2020; Calfee et al. 2021). The differences of landrace ASE were not significant in the HPC1 gene in either population.
Beyond the genes detected in the genomic regions that have been characterized (Hufford et al. 2013; Pyhäjärvi et al. 2013; Crow et al. 2020; Barnes et al. 2022), the remaining genes with differentiated cis-regulation between highland and lowland landrace alleles had not been reported in previous studies and were distributed through all ten chromosomes with no obvious clustering (fig. 5). We compared this list of genes (i.e., DASE), to the genes previously identified with differential gene expression (i.e., DE), between highland and lowland landraces. Of the 4,432 and 1,816 DE genes detected in the Mexican and South American populations, respectively, roughly 70% (3,364 and 1,235) were successfully assayed for ASE (supplementary fig. S8A, Supplementary Material online). One-hundred and sixty-eight and 91 genes were detected in both differential gene expression analysis and differential allele-specific analysis (supplementary fig. S8B, Supplementary Material online), which account for 50% and 35% of the total numbers of DASE genes detected in the two populations and are many more than expected by chance (P = 8.67 × 10−49, 2.27 × 10−47 for Mexican and South American populations, respectively).
Convergent cis-regulatory Evolution Between the Mexican and South American Populations
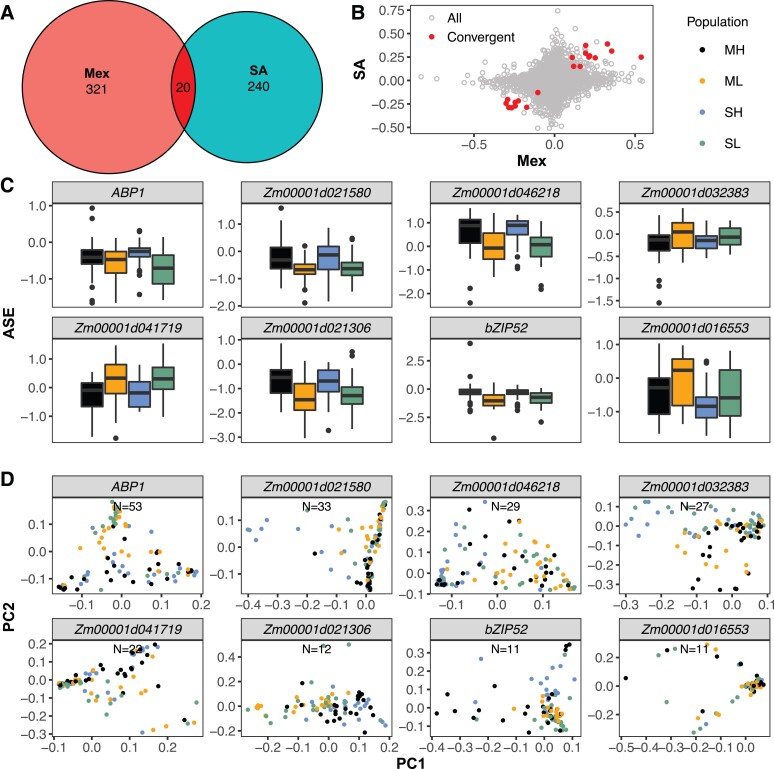
Among these genes that were significantly differentiated in ASE between highland and lowland populations (fig. 5, supplementary table S9, Supplementary Material online), 20 were significantly associated with elevation in the F1 families of both continents, representing 8% of the lesser of the number of significant genes from either continent (fig. 6A, table 2). However, despite being a relatively small overlap, this is many more than expected by chance (P = 8.74 × 10−6). In addition, each of the 20 genes showed the same direction of changes of ASE between highland and lowland populations in both continents and the estimated highland effects of the 20 genes (r = 0.93) were much more highly correlated between continents than that of all measured genes (r = 0.12, fig. 6B). Therefore, we classified these 20 genes as showing convergent cis-regulatory evolution between the two continents.
Fig. 6.
Results of ASE analyses. (A) Numbers of genes showing cis-regulatory divergence between highland and lowland populations from Mexico and South America and common genes detected in both continents. (B) Correlation of estimated highland effects between Mexican and South American populations for all genes measured for ASE and a subset of 20 genes showing evidence of convergent evolution (highlighted in red). (C) ASE values of 8 of the 20 convergent genes in the Mexican Highland (ML), Mexican Lowland (ML), South American Highland (SA), and South American Lowland (SL) populations. The 8 genes were selected based on a threshold of >10 SNPs from the landrace parents in each of the 20 convergent genes. (D) PC analysis of the landraces based on SNPs called from the whole-genome sequencing data for each of eight genes with more than ten SNPs.
Table 2.
Twenty Genes with Convergent Highland cis-regulatory Evolution in Both the Mexican and South American Populations.
| Gene Model | Gene Name | Chr | Position (bp) | Description |
|---|---|---|---|---|
| Zm00001d032370 | 1 | 224,157,746 | Co-chaperone protein p23-1 | |
| Zm00001d021306 | 7 | 148,361,780 | ER lumen protein retaining receptor B | |
| Zm00001d010995 | 8 | 136,175,479 | Thylakoid membrane protein TERC, chloroplastic | |
| Zm00001d046218 | 9 | 72,602,369 | Protein NDL1 | |
| Zm00001d030623 | 1 | 149,354,547 | Solute carrier family 40 member 3, chloroplastic | |
| Zm00001d016736 | 5 | 174,721,846 | 2-Cys peroxiredoxin BAS1-like, chloroplastic | |
| Zm00001d041711 | ABP1 | 3 | 134,550,012 | Auxin binding protein1 |
| Zm00001d021580 | 7 | 156,778,841 | Transducin/WD40 repeat-like superfamily protein | |
| Zm00001d027874 | NFYA1 | 1 | 16,038,734 | Nuclear transcription factor y subunit a1 |
| Zm00001d052769 | 4 | 200,157,142 | Thioredoxin H-type 5 | |
| Zm00001d050238 | 4 | 75,293,161 | Unknown | |
| Zm00001d028936 | bZIP52 | 1 | 52,167,612 | bZIP-transcription factor 52 |
| Zm00001d041719 | 3 | 134,955,964 | Heat shock protein 90-6 mitochondrial | |
| Zm00001d040775 | GATA27 | 3 | 64,946,021 | C2C2-GATA-transcription factor 27 |
| Zm00001d021654 | 7 | 159,175,708 | Unknown | |
| Zm00001d016553 | 5 | 167,128,735 | F-box/kelch-repeat protein | |
| Zm00001d043070 | MAGI104405 | 3 | 188,315,697 | Ubiquitin-conjugating enzyme E2-17 kDa like |
| Zm00001d032383 | 1 | 224,766,461 | Phosphoenolpyruvate/phosphate translocator 2, chloroplastic | |
| Zm00001d030892 | 1 | 166,128,618 | Unknown | |
| Zm00001d026326 | 10 | 143,599,140 | F-BOX PROTEIN 2 |
Note.—Position (bp) represents starting physical position of a gene (bp; B73 AGPv4).
To understand the biologic functions of the 20 DASE genes that were significantly associated with elevation in both continents, we searched their annotation from the Gramene database (Tello-ruiz et al. 2022) and their characterized function from maizeGDB (Woodhouse et al. 2021). Five of them have gene names from the maizeGDB, and at least three of them are transcription factors (table 2). The gene Zm00001d041711 encodes auxin binding protein 1 (ZmABP1), which binds auxin and is a receptor for a number of auxin responses (Sauer and Kleine-Vehn 2011). The genes Zm00001d027874, Zm00001d028936, and Zm00001d040775 encode nuclear transcription factor Y subunit A1 (NFYA1), bZIP-transcription factor bZIP52, and GATA-transcription factor GATA27, respectively. These transcription factors and transcription factor families play important roles in plant development, growth, and abiotic stress responses (Zhang et al. 2016; Guo et al. 2021; Zhang et al. 2021; Li et al. 2022).
For each of the 20 genes that showed consistency in both expression scales and directions in the two continents (fig. 6C, table 2), we performed a PC analysis of the landraces based on SNPs called from the WGS data. We analyzed eight genes with more than ten SNPs each and found that landraces were separated by elevation for at least six genes. Highland landraces from Mexico and South America were clustered together for ABP1, Zm00001d046218, Zm00001d041719, and Zm00001d021306 and showed divergence for Zm00001d021580 and bZIP52 (fig. 6D).
Identifying Links Between DASE and DE
While cis-regulatory variation should contribute to the total gene expression variation among samples, other sources of variation due to developmental, environmental, or trans-regulatory variation may dominate the gene expression variation for many genes (Liu et al. 2019). We observed generally positive correlations between log2ASE and log2Expression for most genes in each site:tissue (supplementary fig. S9, Supplementary Material online). The correlation between log2ASE and log2Expression increased when we accounted for technical factors (sampling group, order of sampling, and block), and cell type composition. However, for the majority of genes, log2ASE only explained a few percent of the total expression variation.
As several of our candidate genes for cis-regulatory adaptation are transcription factors, we used the MaizeGRN data set (Zhou et al. 2020) which contains predicted gene regulatory networks for ∼2,000 transcription factors based on co-expression results across multiple maize data sets. For each transcription factor network, we used goseq as described above to test whether the network was enriched for up- or down-regulated genes between highland and lowland populations. A total of 216 networks were significantly enriched in the Mexican population and 55 in the South American population in at least one site:tissue (supplementary table S11, Supplementary Material online). However, we did not find any examples of these networks with transcription factors for which we observed significant divergence in cis-regulatory alleles in either population.
Discussion
Complex Process of Maize High-elevation Adaptation
Previous studies have demonstrated substantial differences in phenotype (Anderson and Cutler 1942; Eagles and Lothrop 1994; Janzen et al. 2022) and gene expression (Kost et al. 2017; Aguilar-Rangel et al. 2017; Crow et al. 2020) between highland and lowland maize. However, the genetic architecture of regulatory variants that control these phenotypic and expression traits is still unclear and cannot be directly determined either with analyses of sequence variation or differential gene expression analysis. Differential gene expression studies cannot identify how many independent loci across the genome control expression of these genes because a single locus could plausibly affect the expression of every other gene in the genome by altering processes like cellular physiology, tissue anatomy, or organismal level development. ASE, in contrast, as studied in the maize highland adaptation context by Aguilar-Rangel et al. (2017), is not sensitive to these trans-regulatory mechanisms because the two alleles of a gene are always observed in the same cellular environment. Therefore, most genes identified by Aguilar-Rangel et al. (2017) are likely controlled by distinct functional variants in cis to each gene. However, as this study used only a single highland and a single lowland genotype, it is unclear which of the cis-regulatory differences they observed are common in highland populations and which may be unique to this particular lineage.
Therefore, we used population-level ASE analysis, which allows us to count at least a lower bound of the number of independent genetic loci that have diverged between highland and lowland populations. Of the 13,632 genes we successfully assayed for ASE in at least one site:tissue, 341 and 260 genes (fig. 6A) showed significantly differential allele-specific regulation between highland and lowland populations in Mexico and South America, respectively, and these genes were distributed across all 10 chromosomes with no obvious clustering (fig. 5). It is reasonable to expect more DASE genes would be detected if all the 36,207 expressed genes in maize (Hoopes et al. 2019) were analyzed across multiple tissues. Moreover, all leaf tissues were sampled ∼4 h after sunrise in the morning, and other genes might show divergent patterns at other times of the day, or under different weather conditions. Furthermore, as our DASE analysis cannot detect functional variants in protein sequence or activity, for example, transcription factor DNA-binding affinity or other trans-regulatory variants, our list of candidate regulatory variants is clearly an underestimate of the total genetic architecture underlying highland adaptation. For example, recent studies have estimated that 70% or more of total expression variation in any gene is caused by trans-effects, not cis-effects (Liu et al. 2019). While some of these trans-effects may be caused by cis-effects on upstream genes, we have likely underestimated the number of functional variants that differ between highland and lowland maize populations.
Evolutionary Patterns of Maize Highland Adaptation in Mexico and South America
We found a small proportion of genes for which differential gene expression and/or ASE were detected in both the Mexican and South American populations (fig. 2A, supplementary fig. S1, Supplementary Material online). Even when assaying higher level processes through GO categories or KEGG pathways, we found little evidence of shared patterns among the loci with gene expression divergence. Takuno et al. (2015) investigated the molecular basis of convergent adaptation in maize to highland climates in Mesoamerica and South America and found limited evidence for convergent evolution at the nucleotide level. Using high-depth resequencing data to investigate demographic change during highland adaptation, Wang et al. (2017) detected introgression from mexicana-to-maize landraces in the highlands of Mexico, Guatemala, and the southwestern United States, but found no evidence for substantial spread of mexicana haplotypes to South America. Consistent with these results, our analysis of two loci shown to have adaptively introgressed from mexicana into highland Mexican maize, Inv4m and mhl1, finds evidence of DASE in the Mexican population but not in the South American population (except one gene with very weak evidence detected in mhl1, fig. 5). Together, both our new results and previous studies suggest that the loci underlying adaptations to highlands were largely distinct and support the model of predominantly independent evolution to the highlands in Mexican and South American maize landraces.
Nonetheless, the small but significant overlap of convergent genes detected from either differential gene expression or differential ASE in both continents suggests convergent evolution plays a non-negligible role in highland adaptation. While the genetic basis of convergence at the expression level is not clear from differential expression data alone, convergence at the cis-regulatory level implies functionally similar local regulatory alleles differentiating highland and lowland accessions on both continents. There are three possible mechanisms of convergent adaptation: independent mutation, shared ancestral standing variation, or spread throughout subpopulations via geneflow (Lee and Coop 2017). Of the eight genes that showed convergent cis-regulatory evolution between the two continents based on differential ASE analysis and of which we had sufficient data from the low-coverage genome sequencing to measure local genetic relationships among samples, at least four clustered by elevation with no clear separation between Mexican highland and South American highland individuals (fig. 6D), suggesting a potential homogenization of the two highland populations through gene flow, consistent with observations of (Wang et al. 2021) where the majority of shared loci between Mexican and Andes highland landraces were due to migration. In addition, we also found at least two genes for which accessions clustered by elevation (fig. 6D), but Mexican highland and South American highland individuals clustered separately from each other. This suggests different haplotypes have arisen and/or spread independently in the two highland populations but that these two haplotypes likely have a similar biologic function in each continent. However, our data cannot distinguish whether these haplotypes contain independent causal mutations, or both have captured the same variant segregating in the ancestral population. Therefore, both our results and those of Wang et al. (2021) suggest convergent evolution plays a role in maize highland adaptation, and that this adaptation likely occurred through a combination of migration or the repeated recruitment of standing variants, and new mutations.
Applications and Limitations of Population-level ASE Analyses in Evolutionary Genetics and Plant Science
Most prior studies of ASE have been based on SNP-level allelic counts in single individuals (Aguilar-Rangel et al. 2017; Shao et al. 2019; Zhou et al. 2019). Although observing ASE in an individual demonstrates that two cis-regulatory alleles differ functionally from each other, we cannot conclude from one individual that the populations that these individuals came from have diverged in cis-regulatory function until we have replicated the ASE results across multiple independently derived F1s. Lemmon et al. (2014) pioneered this approach in maize, demonstrating cis-regulatory divergence in many genes relative to its wild relative teosinte. Our experimental design was similar to Lemmon et al. (2014), except we used many more parental lines and crossed each to a common tester genotype (B73) to facilitate comparisons among all landrace alleles. In an ASE analysis, the tester allele is effectively a blocking factor used to control for all trans-regulatory variation and so any reference should be approximately equivalent unless cis-trans interactions differ greatly among populations. As in this earlier study, we did not focus on discovering all functionally variable cis-regulatory alleles, but instead on identifying alleles with large changes in frequency between highland and lowland populations, as a signature of selection on gene regulation at this locus. In some cases, the divergence may represent a sweep of a particular haplotype (e.g., Inv4m, mhl1 are candidates for this), in other cases divergence may be more polygenic even for a single gene, with an increase in frequency of multiple (potentially unrelated) haplotypes with similar cis-regulatory function. Detailed investigation of these alternatives will require a closer look at individual samples with higher coverage genome sequencing.
Although our experimental design was optimized for discovering loci with divergent cis-regulatory activity between populations, it lacks power to describe the downstream effects of these loci on other traits. As the functional alleles are necessarily in a heterozygous state in each F1 plant (because all landraces were crossed to a common tester), for any locus, we only observe individuals that are either heterozygous or homozygous for one allele—we never observe individuals homozygous for both allelic states, and therefore cannot observe the full phenotypic effect of substituting alleles. The phenotypic differences that we observe are expected to be half of the effect we could see if the loci were homozygous, but may be much less if the landrace allele is recessive. This likely explains why we do not see strong correlations between ASE and phenotypic traits. Even for the expression of a gene itself, cis-regulatory haplotypes often explain only a small percentage of the expression variation (Liu et al. 2019) due to the large number of sources of trans-regulatory effects. This is likely true in our study as well. We see evidence of large trans-effects caused by the time of day and changes in tissue composition across samples (fig. 3A, B), and after correcting for these sources of variation the correlations between ASE and gene expression do increase (supplementary fig. S9, Supplementary Material online). Many of these trans-effects may ultimately be caused by cis-effects on other genes, potentially at other times or stages of development, but those effects cannot be discovered in our experiment itself. Further study of the biologic roles of the cis-regulatory alleles we discovered here will require isolating them in other genomic backgrounds and replicating their effects in homozygous states.
Finally, although we have designed our experiment to answer questions about regulatory divergence among populations, we believe similar strategies could be used to identify gene–trait relationships relevant to hybrid breeding schemes. Hybrids dominate many key crops including maize. In such programs, candidate lines are evaluated by crossing to common testers. Experimental methods for assaying gene-level ASE as we have used here could be used for TWASs in such hybrid populations. TWAS using ASE can pinpoint causal gene regulatory traits underlying key performance metrics, enabling further targeted gene editing work and breeding.
Materials and Methods
Plant Materials
One-hundred and eight maize landraces (supplementary table S1, Supplementary Material online) from highland and lowland sites of Mexico and South America were chosen from the CIMMYT's germplasm bank: 28 accessions from high-elevation sites (>2,000 masl) and 28 accessions from low elevation sites (<1,000 masl) of Mexico, and 26 accessions from high-elevation sites (>2,000 masl) and 26 accessions from low elevation sites (<1,000 masl) of South America. The landraces were paired latitudinally and East-West of the continental divide (fig. 1A), such that both landrace accessions of a pair collected from the same 1° of latitude bin and all pairwise distances between accessions were >50 km. Each of the 108 maize landraces was used as a pollinator to cross with the inbred line B73 to produce 108 F1 families. Crosses were performed at Curtiss Farm at Iowa State University and in Columbia, Missouri, and an approximately balanced set of successful F1 families of each type (Highland/Lowland and Mexico/South America) were chosen from each site.
Field Experimental Design and Leaf Sample Collection
The F1 families were planted at two locations in Mexico: Puerto Vallarta and Metepec. Puerto Vallarta is located at 20°40’N 105°16’W and represents a lowland environment at ∼7 masl. Over the course of the year, the temperature typically varies from 16 °C to 32 °C. Metepec is located at 19°14'N 99°35'W and represents a highland environment at ∼2,620 masl. Over the course of the year, the temperature typically varies from 7 °C to 27 °C. At each of the two locations, a randomized complete block design with two replications was used for the field trial design. The two landraces from the same latitudinal band were planted in consecutive 20 kernel rows.
Leaf tissue was sampled at the V4 developmental stage (collar of the fourth leaf became visible) from within 5 cm of the tip of the leaf blade (leaf tip) and within 5 cm of the leaf blade base (leaf base) at both locations from a randomly selected healthy-looking plant in the interior of each row. Both fields were sampled 4 h after sunrise and all samples were taken within 90 min. Approximately 20 mg of tissue was sampled, placed into a 2-ml centrifuge tube, flash frozen in liquid nitrogen, and stored at −80 °C until RNA extraction. Leaf tissues of the 108 landrace parents were collected, placed on ice, and transported to the laboratory where tissue was lyophilized and ground through bead beating or mortar and pestle prior to DNA isolation.
RNA Extraction, Library Preparation and Illumina Sequencing of F1 Hybrids
Leaf tissue was ground using stainless steel beads in a SPEX Geno/Grinder (Metuchen, NJ, USA). mRNA was extracted using oligo (dT) beads (DYNABEADS direct) to extract polyadenylated mRNA using the double-elution protocol. We prepared strand-specific mRNA-seq libraries using the Breath Adapter Directional sequencing (BrAD-seq) protocol (Townsley et al. 2015) with random priming and 14 polymerase chain reaction cycles. Samples were quantified using the Quant-iTTM PicoGreen dsDNA kit, and then normalized to 1 ng/μl. We multiplexed 96 samples for sequencing and sequenced each on 2–4 lanes of an Illumina HiSeq X platform generating 150 nucleotides (nts) paired-end (PE) sequences. Trimmomatic version 0.39 (Bolger et al. 2014) was used to remove the BrAD-seq adapters remnants and bases with an average base quality value below 15 within 4-bp sliding windows of each read. Entire reads were removed if the remaining length was <36 nt.
Differential Gene Expression Analysis in Gene Expression Data
RNAseq reads of the F1 families were aligned to B73 AGPv4 using the STAR software version 2.7.2a (Dobin et al. 2013) and the STAR 2-pass method with default parameters (Engström et al. 2013). We counted reads at each locus using featureCounts v2.0.1(Liao et al. 2014) with default parameters. We filtered the raw count matrix separately for each tissue and estimated effect sizes for elevation of origin in each tissue separately, then combined evidence across three single-tissue analyses by meta-analysis to identify the union set of genes differentially expressed in at least one tissue. In detail: First, in each single-tissue analysis, we removed F1 samples with fewer than 2 million mapped reads filtered genes using the filterByExpr function from EdgeR (Robinson et al. 2010), requiring at least 10 samples in one population-by-elevation class group to have at least 32 reads. This reduced the gene expression matrices of MetLeaftip, MetLeafbase, and PvLeaftip to 18,369 genes × 160 samples, 20,401 genes × 164 samples, and 18,079 genes × 110 samples, respectively. A total of 21,599 genes were assayed in at least one site:tissue, and 16,851 genes in common among all the three tissues after filtering.
Then, for each tissue separately, we calculated normalization factors using the calcNormFactors function in EdgeR, normalized to log2(counts per million) and estimated weighting factors with voom (Law et al. 2014). To perform voom processing, for each tissue, we specified a linear model accounting for Block (in Metepec samples only), the sampling team (three teams sampled tissue in parallel), sampling time (expressed as a cubic polynomial of the order in the field, separately for each of the three sampling teams), the interaction of Population (Mexico or South America) and Elevation class (Highland or Lowland parental landrace), and the interaction of Population and Latitude of the parental landrace.
Next, we re-fitted the linear model described above using lmFit in limma (Ritchie et al. 2015) taking the precision weights estimated by voom into account. We used the eBayes function to perform empirical Bayes moderation of the t-statistics. We extracted the estimated average difference in log2(counts per million) between highland- and lowland-derived F1s for each population separately from fit$coefficients and the standard errors of these estimates as sqrt(fit$s2.post)*fit$stdev.unscaled.
Finally, we performed a meta-analysis of the elevation effects of each gene across three tissues, accounting for correlations of measurements among conditions using the multivariate adaptive shrinkage (mash) method implemented in mashr package 0.2.50 (Urbut et al. 2019) on the estimated effect sizes and standard errors calculated above. This produced a union set of genes with evidence of a difference in the average expression between highland and lowland F1s in any condition. We used the 21,599 genes with estimated elevation effects in at least one site:tissue for the meta-analysis, setting input effect sizes and output results to NA for genes not assayed in a particular site:tissue. We ran mashr with the mash_estimate_corr_em to estimate a residual correlation matrix, passing both the canonical covariance matrices (cov_canonical) and data-driven covariance matrices (cov_ed, with inputs from cov_pca pasted on the genes significant at an lfsr of 0.05 in at least one condition).
Gene Set Analysis in Gene Expression Data
We ran gene set enrichment analyses on gene lists discovered by the meta-analysis across tissues, separately for the Mexican and South American populations, using the goseq function of the goseq R package (Young et al. 2010). We began with a list of 12,035 GO categories (Wimalanathan et al. 2018), 137 KEGG pathways (Kanehisa et al. 2021), and 556 CornCyc pathways (Hawkins et al. 2021), and then filtered for categories with between 10 and 1,000 assayed genes in a particular site:tissue. We ran the enrichment analyses separately for up- and down-regulated genes selected with by lfsr < 0.05 in each site:tissue. We accounted for biased probabilities of detection as a function of expression and gene length using the nullp function with bias.data set to the log of the average counts per gene across all samples in that site:tissue, including only genes that passed the expression filter described above.
We assessed convergence in each site:tissue at the gene level by selecting genes with lfsr < 0.05 for effects of elevation separately in the Mexican and South American populations and filtering for genes where the Posterior Mean effect size estimate had the same size in both populations. We assessed convergence at the gene set level based on Benjamini–Hochberg adjusted P-values <0.05 in the test of either up-regulated or down-regulated genes for both populations. A hypergeometric test was used for convergence assessment based on numbers of detected genes in each population and in both populations for each site:tissue.
Assessment of Cell Composition Variation Among Samples
We used single-cell expression data from Bezrutczyk et al. (2021) to estimate cell composition in each sample. This data set included 200–900 marker genes with enriched expression in 7 cell types (5 classified as mesophyll and 2 as bundle sheath). We calculated a projection score for each of our samples against each of the 7 cells as the weighted sum of mean-centered expression of the marker genes (weighted by the avg_log2FC in the specific cell population in the reference data set). This is closely related to the OLS method for estimating cell type proportions in single-cell expression data (Avila Cobos et al. 2020), but less restrictive because we do not assume that all cell populations in our samples are represented in the reference data set. We summarized variation in cell type composition across samples using a PCs analysis of the seven projection scores.
To assess the reliability of the projection scores, we re-calculated the scores 200 times after randomly assigning the marker gene identities to random expressed genes and measuring the total variation explained by the real or permuted scores across samples.
We assessed whether the projection scores representing cell composition variation could account for some of the differential expression observed between highland- and lowland-derived F1s by including the seven projection scores as additional covariates in the design matrices for the differential expression analyses derived above.
Whole-genome Sequencing and Variant Identification From the Landrace Parents
As variant calling from RNAseq libraries is notoriously difficult due to: (1) allelic imbalance, as most variant callers assume the true frequency of each allele is 50%, (2) highly variable sequencing coverage across loci, negating depth filters from variant calling software, and (3) mapping difficulties due to spliced reads, we used low-coverage WGS data of the landrace parents to identify a set of high-confidence genic SNPs to use for ASE quantification.
DNA was extracted from parental landrace leaf tissue using the CTAB method. The tissue was collected from the same male plant used to produce the F1s that were used for RNA sequencing. Sample concentrations were quantified using Qubit (Life Technologies), and 1 μg of DNA was fragmented using a bioruptor (Diagenode) with cycles of 30 s on, 30 s off. Fragments of DNA were then prepared for Illumina sequencing. (1) DNA fragments were repaired with the End-Repair enzyme mix (New England Biolabs). (2) A deoxyadenosine triphosphate was added at each 3′ end with the Klenow fragment (New England Biolabs), and (3) Illumina Truseq adapters (Affymetrix) were added with the Quick ligase kit (New England Biolabs). Between each enzymatic step, DNA was washed with sera-mags speed beads (Fisher Scientific). Finally, samples were multiplexed using Illumina compatible adapters with inline barcodes and libraries were sequenced with Illumina HiSeq X platform generating 150 nt PE sequences, resulting in an average of 9,862,996 properly paired reads/library, corresponding to an average of ∼1.2× coverage. Reads were aligned to version 4 of the B73 reference genome (Jiao et al. 2017) with BWA-MEM version 0.7.17 (Li and Durbin 2009). High-confidence SNPs between any landrace and B73 were identified with Analysis of Next Generation Sequencing Data (ANGSD) version 0.931-2 (Korneliussen et al. 2014) using the following parameters: angsd -GL 2 -P 20 -uniqueOnly 1 -remove_bads 1 -only_proper_pairs 1 -trim 0 -C 50 -minMapQ 20 -mminQ 20 -SNP_pval 1e-6 -doMaf 2 -doMajorMinor 4 -doSaf 1. SNPs outside of annotated exons in the B73 genome were excluded.
As the landrace parents were outbred, their genomes are heterozygous and the ∼1× WGS reads will likely not detect ∼50% of the SNPs carried by each parent and passed on to the F1 individuals. Given the size of the maize genome, achieving sufficiently high coverage for each individual for comprehensive SNP discovery would have been prohibitively expensive. However, SNPs relative to the reference genome (B73 AGPv4) that are relatively common in the population (e.g., >2% frequency) are likely to be sequenced by multiple reads across all 108 WGS libraries. This includes a large number of SNPs where the B73 allele is rare which will be observed in nearly every landrace. In total, we identified 53,891,495 high-confidence SNPs in exonic regions across the 108 landraces, providing a large set of candidate SNPs to test for ASE in the RNAseq data.
Per-sample Detection of ASE-tagging SNPs without Biasing ASE Ratios
Although the WGS-derived SNPs are likely real in the whole population, only SNPs that are heterozygous in a particular F1 individual are useful for ASE quantification. Including the same set of fixed loci in ASE counts across samples will severely bias allelic read counts for a gene because all reads from both alleles will be assigned to the same allele. We therefore used the RNAseq data to genotype each F1 individual at all WGS-derived SNPs.
Using WGS-derived SNPs alleviates the issue of confident SNP detection, but genotyping using RNAseq data for ASE applications still presents challenges:
When a small number of reads cover an SNP (e.g., when in a low-expressed gene) one allele will frequently drop-out due to sampling error even if there is no actual allelic imbalance. In our experimental design, we know that every locus contains at least one copy of the B73 allele (as B73 was the female parent). While loci where only the landrace allele was observed are almost certainly heterozygous and therefore informative for ASE, keeping these loci would bias the genes estimated ASE ratio toward the landrace allele, because the opposite loci (where only the B73 allele is detected) would be dismissed as apparently homozygous. We therefore kept only SNPs where both the B73 and the landrace allele were observed to prevent biased ASE ratios.
When a large number of reads covers an SNP (e.g., when in a high-expressed gene), the low rate of sequencing errors present in Illumina data can generate false-positive heterozygous calls. Including these loci in the ASE analysis will severely bias ASE ratios toward the B73 allele (because most sequencing errors will be away from the reference and therefore look like low-expressed non-B73 alleles.
Mismatches relative to the reference can cause ambiguous or incorrect read-mapping, biasing ASE ratios. We used the WASP algorithm (Van De Geijn et al. 2015) implemented in the STAR software version 2.7.2a to identify reliably mapped reads. WASP uses an allele swapping and RNAseq remapping strategy to filter out reads with mapping biases, and the STAR-WASP algorithm assigns a multilocus genotype to each individual read for all SNPs it overlaps.
RNAseq reads of the F1 families were aligned to B73 AGPv4 using the STAR software version 2.7.2a and the STAR 2-pass method was used with default parameters. For each F1 sample separately, alleles were counted at WGS-derived loci using ASEReadCounter from GATK version 4.0.11.0. To minimize the impact of the above issues on downstream ASE analyses, we kept only SNPs for each sample where both alleles were detected, the total number of reads covering the SNP was at least 10, and the absolute value of the log2ASE ratio: log2(ALT)-log2(REF) was <2. We applied these filters to each SNP in each RNAseq sample.
Identifying Regions of IBD Between Plants From the Same F1 Family
We used the heterozygous SNP calls from each RNAseq sample to identify regions of IBD between the three plants per F1 family (two plants from two blocks in Metepec and one plant from Puerta Vallarta). For each F1 family, we compared RNAseq samples of two tissues from the same plant in Metepec and of two plants from two blocks in Metepec/Puerta Vallarta for the same tissue. For each pair of RNAseq samples, we divided each chromosome into 20 blocks with equal numbers of SNPs from the WGS data, and in each bin counted the number of heterozygous sites identified in common between the two samples. We then divided this number by the minimum number of heterozygous sites identified in each sample separately. This percentage of common sites was generally bimodal across bins, reflecting the inheritance of the two paternal alleles in the sibling plants. We fit a Gaussian mixture distribution to these percentages for each sample with k = 2 using the normalmixEM function from the mixtools package (Benaglia et al. 2009) to classify each bin into either IBD (if the posterior probability of the bin being in the higher probability class was >90%), not-IBD (posterior probability < 10%), or ambiguous.
Gene-level Allelic Read Counts for F1 Samples
While SNP-level allelic expression counts can document allelic imbalance in a single sample, to identify genes with common allelic imbalance at the population level we combined the information across SNPs in the same gene into a single ASE ratio per gene per sample. Gene-level ASE ratios should be more robust because they are based on more total reads, and in a population sample SNP-level ASE ratios cannot reliably be compared across individuals because many SNPs are individual specific.
To combine SNP-level allelic expression counts into gene-level allelic expression, we used the WASP algorithm (Van De Geijn et al. 2015) implemented in STAR-WASP (Dobin et al. 2013). Therefore, we extracted reads that were assigned either REF or ALT genotypes at all overlapping loci into separate BAM files, and then counted the reads overlapping each gene feature in each BAM file using featureCounts v2.0.1 (Liao et al. 2014). These gene counts are the allelic expressions of the maternal and paternal alleles of each gene, respectively.
Differential Allele-specific Expression Analysis
Using the gene-level allelic read counts, we analyzed the average difference in landrace ASE (relative to B73 ASE) between F1s derived from highland and lowland landraces. We modeled this landrace elevation effect separately for three tissues: the leaf tip and leaf base tissues from the Metepec field (MetLeaftip, MetLeafbase), and the leaf tip samples from the Puerta Vallarta field (PvLeaftip). We then performed a meta-analysis across three tissues to identify the set of genes with divergent allelic expression between highland and lowland F1s in any condition.
First, in each single-tissue analysis, we removed F1 samples with fewer than 2 million mapped reads and genes in which fewer than 10 samples had at least 32 ASE-informative reads in each of the 4 populations. This stronger filter was necessary for the ASE analysis because genes with few reads are informative for total expression analyses (i.e., low expressed), but uninformative for ASE. For each gene in each F1 sample, we calculated the log2ASE ratio as log2(landrace counts)—log2(B73 counts), where landrace and B73 are actually paternal and maternal alleles, respectively. This resulted in data sets of size: 10,886 genes × 160 samples for MetLeaftip, 12,747 genes × 164 samples for MetLeafbase, and 9,178 genes × 110 samples for PvLeaftip. A total of 13,632 genes were assayed in at least one site:tissue, and 8,605 genes were in common among all the three tissues after filtering.
We expected that the precision of these log2ASE ratios would vary strongly among genes and samples due to the expression of each gene, the number of informative SNPs, and the sequencing depth of each sample. This heteroskedasticity would reduce the efficiency of standard tests for differential expression (similarly to the effect of counting variance on total expression in RNAseq samples). We therefore developed an adaptation of the voom algorithm for modeling the expected variance of each data point. For each tissue, we specified the same linear model accounting for Block, sampling group, order in the field, the interaction of Population and Elevation class, and the interaction of Population and Latitude of the parental landrace as described above in the total expression analysis. We used the lmFit function in limma version 3.42.2 (Ritchie et al. 2015) to fit this model to the log2ASE ratios of each gene and extracted the estimate of the residual standard deviation of each gene. In this step, all genes with zero counts from either allele were set to missing (given zero weights) because a zero log2ASE ratios implies equal allelic expression, whereas zero counts is a complete lack of information about the actual allelic ratio. Next, we used the lowess function to fit a smoothed trend to the square root of residual standard deviations extracted above as a function of an average normalized total count of each gene (in log2 scale). Finally, we used this trend line to predict the variance of each observation in the data matrix as a function of the total read count (landrace + B73) of that gene in that sample.
Next, we re-fitted the linear model above using lmFit, this time including the inverse of the estimated variance matrix as precision weights, again setting the weights of points with zero total counts to zero. We used the eBayes function to perform empirical Bayes moderation of the t-statistics. We extracted the estimated average difference in log2ASE between highland- and lowland-derived F1s for each population separately from fit$coefficients and the standard errors of these estimates as sqrt(fit$s2.post)*fit$stdev.unscaled.
Finally, based on the observed effect sizes and corresponding standard errors of each gene of three single-tissue analyses, we performed a meta-analysis using mashr (Urbut et al. 2019) to identify a union set of genes with evidence of a difference in the average landrace ASE between highland and lowland F1s in any condition following the same procedure of total expression analysis. In this analysis, the mash results suggested the correlation in true effect sizes was close to 1 across all three site:tissues. We therefore used the overall lfsr across all three site:tissues as a measure of significance, and did not break results down by site:tissue. We assessed convergence in each site:tissue at the gene level using the same method as described in the gene expression analysis.
Supplementary Material
Acknowledgments
This study was supported by the National Science Foundation (grant number 1546719). The authors thank Dr Graham McVicker at The Salk Institute, Dr Alexander Dobin at Cold Spring Harbor Laboratory, and Mr Arya Massarat at Harvey Mudd College for their valuable advice on building the allelic read counts pipeline. The authors also thank Dr Garrett Janzen for his valuable advice and discussion on analysis of Mexican and South American maize landrace populations.
Contributor Information
Haixiao Hu, Department of Plant Sciences, University of California, Davis, CA.
Taylor Crow, Department of Plant Sciences, University of California, Davis, CA.
Saghi Nojoomi, Department of Plant Sciences, University of California, Davis, CA.
Aimee J Schulz, Department of Ecology, Evolution, and Organismal Biology, Iowa State University, Ames, IA.
Juan M Estévez-Palmas, National Laboratory of Genomics for Biodiversity, Irapuato, México.
Matthew B Hufford, Department of Ecology, Evolution, and Organismal Biology, Iowa State University, Ames, IA.
Sherry Flint-Garcia, United States Department of Agriculture—Agricultural Research Service, Columbia, MO; Division of Plant Sciences, University of Missouri, Columbia, MO.
Ruairidh Sawers, Department of Plant Science, The Pennsylvania State University, State College, PA.
Rubén Rellán-Álvarez, National Laboratory of Genomics for Biodiversity, Irapuato, México; Department of Molecular and Structural Biochemistry, North Carolina State University, Raleigh, NC.
Jeffrey Ross-Ibarra, Department of Evolution and Ecology, Center for Population Biology, and Genome Center, University of California, Davis, CA.
Daniel E Runcie, Department of Plant Sciences, University of California, Davis, CA.
Supplementary Material
Supplementary data are available at Molecular Biology and Evolution online.
Data Availability
The pipeline and custom scripts utilized in this study are documented in the following GitHub repository: https://github.com/hh622/Maize_Highland_Adaptation_allele_specific_expression. The RNA sequencing (PRJNA796614) and the whole-genome sequencing (PRJNA799784) raw reads have been deposited in NCBI SRA.
References
- Aguilar-Rangel MR, Montes RAC, González-Segovia E, Ross-Ibarra J, Simpson JK, Sawers RJH. 2017. Allele specific expression analysis identifies regulatory variation associated with stress-related genes in the Mexican highland maize landrace Palomero Toluqueño. PeerJ 5:e3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E, Cutler HC. 1942. Races of Zea Mays: I. Their recognition and classification. Ann Mo Bot Gard. 29:69–89. [Google Scholar]
- Arendt J, Reznick D. 2008. Convergence and parallelism reconsidered: what have we learned about the genetics of adaptation? Trends Ecol Evol. 23:26–32. [DOI] [PubMed] [Google Scholar]
- Avila Cobos F, Alquicira-Hernandez J, Powell JE, Mestdagh P, De Preter K.. 2020. Benchmarking of cell type deconvolution pipelines for transcriptomics data. Nat Commun. 11(1):5650.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes AC, Rodríguez-Zapata F, Juárez-Núñez KA, Gates DJ, Janzen GM, Kur A, Wang L, Jensen SE, Estévez-Palmas JM, Crow TM, et al. 2022. An adaptive teosinte mexicana introgression modulates phosphatidylcholine levels and is associated with maize flowering time. Proc Natl Acad Sci U S A. 119:e2100036119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benaglia T, Chauveau D, Hunter DR, Young DS. 2009. Mixtools: an R package for analyzing finite mixture models. J Stat Softw. 32:1–29. [Google Scholar]
- Bezrutczyk M, Zöllner NR, Kruse CPS, Hartwig T, Lautwein T, Köhrer K, Frommer WB, Kim JY. 2021. Evidence for phloem loading via the abaxial bundle sheath cells in maize leaves. Plant Cell. 33:531–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics 30:2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfee E, Gates D, Lorant A, Perkins MT, Coop G, Ross-Ibarra J. 2021. Selective sorting of ancestral introgression in maize and teosinte along an elevational cline. PLoS Genet. 17(10):e1009810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casati P, Walbot V. 2005. Differential accumulation of maysin and rhamnosylisoorientin in leaves of high-altitude landraces of maize after UV-B exposure. Plant Cell Environ. 28:788–799. [Google Scholar]
- Castel SE, Levy-Moonshine A, Mohammadi P, Banks E, Lappalainen T. 2015. Tools and best practices for data processing in allelic expression analysis. Genome Biol. 16:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T, Ta J, Nojoomi S, Aguilar-Rangel MR, Rodriguez JVT, Gates D, Rellan-Alvarez R. 2020. Gene regulatory effects of a large chromosomal inversion in highland maize. PLoS Genet. 16:e1009213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jesús Sánchez González J, Corral JAR, García GM, Ojeda GR, De La Cruz Larios L, Holland JB, Medrano RM, Romero GEG. 2018. Ecogeography of teosinte. PLoS One 13:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagles HA, Lothrop JE. 1994. Highland maize from central Mexico - its origin, characteristics, and use in breeding programs. Crop Sci. 34:11–19. [Google Scholar]
- Engström PG, Steijger T, Sipos B, Grant GR, Kahles A, Rätsch G, Goldman N, Hubbard TJ, Harrow J, Guigó R, et al. 2013. Systematic evaluation of spliced alignment programs for RNA-seq data. Nat Methods. 10:1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Hu J, Xue C, Zhang H, Susztak K, Reilly MP, Reilly MP, Xiao R, Li M. 2020. ASEP: gene-based detection of allele-specific expression across individuals in a population by RNA sequencing. PLoS Genet. 16:1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Bai X, Dai K, Yuan X, Guo P, Zhou M, Shi W, Hao C. 2021. Identification of GATA transcription factors in Brachypodium distachyon and functional characterization of BdGATA13 in drought tolerance and response to gibberellins. Front Plant Sci. 12:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Wang X, Zhao M, Huang C, Li C, Li D, Yang CJ, York AM, Xue W, Xu G, et al. 2018. Stepwise cis-regulatory changes in ZCN8 contribute to maize flowering-time adaptation. Curr Biol. 28:3005–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartkamp AD, White JW, Rodríguez Aguilar A, Bänziger M, Srinivasan G, Granados G, Crossa J. 2000. Maize production environments revisited: a GIS-based approach. Mexico: CIMMYT. [Google Scholar]
- Hawkins C, Ginzburg D, Zhao K, Dwyer W, Xue B, Xu A, Rice S, Cole B, Paley S, Karp P, et al. 2021. Plant metabolic network 15: a resource of genome-wide metabolism databases for 126 plants and algae. J Integr Plant Biol. 63:1888–1905. [DOI] [PubMed] [Google Scholar]
- Hoopes GM, Hamilton JP, Wood JC, Esteban E, Pasha A, Vaillancourt B, Provart NJ, Buell CR. 2019. An updated gene atlas for maize reveals organ-specific and stress-induced genes. Plant J. 97:1154–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufford MB, Lubinksy P, Pyhäjärvi T, Devengenzo MT, Ellstrand NC, Ross-Ibarra J. 2013. The genomic signature of crop-wild introgression in maize. PLoS Genet. 9:e1003477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen GM, Aguilar-Rangel MR, Cíntora-Martínez C, Blöcher-Juárez KA, González-Segovia E, Studer AJ, Runcie DE, Flint-Garcia SA, Rellán-Álvarez R, Sawers RJH, et al. 2022. Demonstration of local adaptation in maize landraces by reciprocal transplantation. Evol Appl. 15: 817–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Peluso P, Shi J, Liang T, Stitzer MC, Wang B, Campbell MS, Stein JC, Wei X, Chin CS, et al. 2017. Improved maize reference genome with single-molecule technologies. Nature 546:524–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. 2021. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 49: D545–D551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneliussen TS, Albrechtsen A, Nielsen R. 2014. ANGSD: analysis of next generation sequencing data. BMC Bioinf. 15:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost MA, Perales HR, Wijeratne S, Wijeratne AJ, Stockinger E, Mercer KL.. 2017. Differentiated transcriptional signatures in the maize landraces of Chiapas, Mexico. BMC Genomics 18:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law CW, Chen Y, Shi W, Smyth GK. 2014. Voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coop G. 2017. Distinguishing among modes of convergent adaptation using population genomic data. Genetics 207:1591–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon ZH, Bukowski R, Sun Q, Doebley JF. 2014. The role of cis regulatory evolution in maize domestication. PLoS Genet. 10:e1004745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Fu D, Wang X, Zeng R, Zhang X, Tian J, Zhang S, Yang X, Tian F, Lai J, et al. 2022. The transcription factor bZIP68 negatively regulates cold tolerance in maize. Plant Cell. 34:2833–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YX, Li C, Bradbury PJ, Liu X, Lu F, Romay CM, Glaubitz JC, Wu X, Peng B, Shi Y, et al. 2016. Identification of genetic variants associated with maize flowering time using an extremely large multi-genetic background population. Plant J. 86:391–402. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhou P, Della Coletta R, Zhang T, Brohammer AB, O’Connor CH, Vaillancourt B, Lipzen A, Daum C, Barry K, et al. 2021. Single-parent expression drives dynamic gene expression complementation in maize hybrids. Plant J. 105:93–107. [DOI] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W. 2014. Featurecounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30:923–930. [DOI] [PubMed] [Google Scholar]
- Liu X, Li YI, Pritchard JK. 2019. Trans effects on gene expression can drive omnigenic inheritance. Cell 177:1022–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothrop JE. 1994. Research on maize for highland regions. In: Bjarnason M, editor. The subtropical, midaltitude, and highland maize subprogram. Maize Program Special Report. Mexico: CIMMYT. p. 19–42. [Google Scholar]
- Maleki F, Ovens K, Hogan DJ, Kusalik AJ. 2020. Gene set analysis: challenges, opportunities, and future research. Front Genet. 11:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno DR, Flannery K V. 2001. The earliest archaeological maize (Zea mays L.) from highland Mexico: new accelerator mass spectrometry dates and their implications. Proc Natl Acad Sci U S A. 98:2101–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyhäjärvi T, Hufford MB, Mezmouk S, Ross-Ibarra J. 2013. Complex patterns of local adaptation in teosinte. Genome Biol Evol. 5:1594–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. 2015. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. 2010. Edger: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero Navarro JA, Willcox M, Burgueño J, Romay C, Swarts K, Trachsel S, Preciado E, Terron A, Delgado HV, Vidal V, et al. 2017. A study of allelic diversity underlying flowering-time adaptation in maize landraces. Nat Genet. 49:476–480. [DOI] [PubMed] [Google Scholar]
- Sauer M, Kleine-Vehn J. 2011. Auxin binding protein1: the outsider. Plant Cell. 23:2033–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Xing F, Xu C, Qinghua Zhang, Che J, Wang X, Song J, Li X, Xiao J, Chen LL, et al. 2019. Patterns of genome-wide allele-specific expression in hybrid rice and the implications on the genetic basis of heterosis. Proc Natl Acad Sci U S A. 116:5653–5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M. 2017. False discovery rates: a new deal. Biostatistics 18:275–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Hu Y. 2013. eQTL mapping using RNA-seq data. Stat Biosci. 5:198–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susila H, Jurić S, Liu L, Gawarecka K, Chung KS, Jin S, Kim SJ, Nasim Z, Youn G, Suh MC, et al. 2021. Florigen sequestration in cellular membranes modulates temperature-responsive flowering. Science 373:1137–1142. [DOI] [PubMed] [Google Scholar]
- Swarts K, Bauer E, Glaubitz JC, Ho T, Johnson L, Li Y, Li Y, Miller Z, Romay C, Schöen C-C, et al. 2016. A large scale joint analysis of flowering time reveals independent temperate adaptations in maize. BioRxiv 086082. 10.1101/086082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuno S, Ralph P, Swart K, Elshire RJ, Glaubitz JC, Buckler ES, Hufford MB, Ross-Ibarra J. 2015. Independent molecular basis of convergent highland adaptation in maize. Genetics 200:1297–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tello-ruiz MK, Jaiswal P, Ware D. 2022. Gramene: a resource for comparative analysis of plants genomes and pathways. Methods Mol Biol. 2443:101–131. [DOI] [PubMed] [Google Scholar]
- Townsley BT, Covington MF, Ichihashi Y, Zumstein K, Sinha NR. 2015. BrAD-seq: breath adapter directional sequencing: a streamlined, ultra-simple and fast library preparation protocol for strand specific mRNA library construction. Front Plant Sci. 6:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbut SM, Wang G, Carbonetto P, Stephens M. 2019. Flexible statistical methods for estimating and testing effects in genomic studies with multiple conditions. Nat Genet. 51:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Geijn B, Mcvicker G, Gilad Y, Pritchard JK. 2015. WASP: allele-specific software for robust molecular quantitative trait locus discovery. Nat Methods. 12:1061–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heerwaarden J, Doebley J, Briggs WH, Glaubitz JC, Goodman MM, Gonzalez JDJS, Ross-Ibarra J.. 2011. Genetic signals of origin, spread, and introgression in a large sample of maize landraces. Proc Natl Acad Sci U S A. 108:1088–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Beissinger TM, Lorant A, Ross-Ibarra C, Ross-Ibarra J, Hufford MB. 2017. The interplay of demography and selection during maize domestication and expansion. Genome Biol. 18:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Josephs EB, Lee KM, Roberts LM, Rellán-Álvarez R, Ross-Ibarra J, Hufford MB. 2021. Molecular parallelism underlies convergent highland adaptation of maize landraces. Mol Biol Evol. 38:3567–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzl P, Carling J, Kudrna D, Jaccoud D, Huttner E, Kleinhofs A, Kilian A. 2004. Diversity arrays technology (DArT) for whole-genome profiling of barley. Proc Natl Acad Sci U S A. 101:9915–9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimalanathan K, Friedberg I, Andorf CM, Lawrence-Dill CJ. 2018. Maize GO annotation—methods. Evaluation, and review (maize-GAMER). Plant Direct. 2:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhouse MR, Cannon EK, Portwood JL, Harper LC, Gardiner JM, Schaeffer ML, Andorf CM. 2021. A pan-genomic approach to genome databases using maize as a model system. BMC Plant Biol. 21:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MD, Wakefield MJ, Smyth GK, Oshlack A. 2010. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 11:R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano JL, Yánez CF, Sangoquiza CA. 2021. Maize breeding in the highlands of Ecuador, Peru, and Bolivia: a review. Agronomy 11:1–9. [Google Scholar]
- Zhang Z, Li X, Zhang C, Zou H, Wu Z. 2016. Isolation, structural analysis, and expression characteristics of the maize nuclear factor Y gene families. Biochem Biophys Res Commun. 478:752–758. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wu T, Li Z, Huang K, Kim NE, Ma Z, Kwon SW, Jiang W, Du X. 2021. OsGATA16, a GATA transcription factor. Confers cold tolerance by repressing OsWRKY:45–1. At the seedling stage in rice. Rice 14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Hirsch CN, Briggs SP, Springer NM. 2019. Dynamic patterns of gene expression additivity and regulatory variation throughout maize development. Mol Plant. 12:410–425. [DOI] [PubMed] [Google Scholar]
- Zhou P, Li Z, Magnusson E, Cano FG, Crisp PA, Noshay JM, Grotewold E, Hirsch CN, Briggs SP, Springer NM. 2020. Meta gene regulatory networks in maize highlight functionally relevant regulatory interactions. Plant Cell. 32:1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The pipeline and custom scripts utilized in this study are documented in the following GitHub repository: https://github.com/hh622/Maize_Highland_Adaptation_allele_specific_expression. The RNA sequencing (PRJNA796614) and the whole-genome sequencing (PRJNA799784) raw reads have been deposited in NCBI SRA.