Abstract
Pregnancy is characterized by immunological alterations in pregnant women that permit the growth of a semi-allogenic fetus, resulting in greater susceptibility of childbearing women to infections. Furthermore, due to the immaturity of the immune system of neonates, a protection gap is present in early life, leaving neonates and infants vulnerable to infectious diseases with increased morbidity and mortality. Maternal immunization against influenza, pertussis, and, in the context of the COVID-19 pandemic, SARS-CoV-2 has been implemented in several countries, with beneficial effects on both the mother and the offspring. The main protective mechanism of vaccination during pregnancy is transplacental transfer of maternal antibodies. However, recent evidence has implied that the fetal immune system may be influenced beyond passive immunity. This review sheds light on the current status of the routinely administered vaccinations during pregnancy, focusing on the impact of maternal immunization on the priming of the fetal immune system and suggesting future perspectives for the optimization of vaccination strategies.
Keywords: maternal immunization, influenza, pertussis, SARS-CoV-2, fetal immune system, neonates, infants, immune system priming
1. Introduction
Pregnant women are more susceptible to common infections. This is due to attenuated immune responses to antigens, driven by the immunological changes occurring during gestation, in order to support pregnancy and tolerance of a semi-allogenic developing fetus [1]. Consequently, infections during pregnancy often result in severe maternal disease, increased maternal mortality, and associated pregnancy complications, i.e., spontaneous abortion and pre-term birth [2,3]. Neonates are also vulnerable to certain infections due to their naive immune system [4]. Furthermore, vaccination of full-term and preterm neonates is challenging due to their immature immune system and inefficient immune response to vaccine antigens [5].
Vaccination during pregnancy is a well-established strategy to protect both the mother and the developing fetus from the corresponding infections. Currently, vaccines routinely administered during pregnancy include the inactivated influenza vaccine and the combined tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap). The World Health Organization (WHO), the Advisory Committee on Immunization Practices (ACIP), and the American College of Obstetricians and Gynecologists (ACOG) recommend immunization with inactivated influenza vaccine for all pregnant women regardless of the stage of pregnancy, as well as for women of childbearing age [6,7]. In 2011, the ACIP recommended Tdap vaccination in the last trimester of pregnancy (27–36 weeks of gestation) for women that had never been vaccinated with Tdap before pregnancy [8]. This recommendation was extended in 2012 to all women of childbearing age, regardless of previous vaccination status, and has been implemented ever since [9,10]. Following the pertussis epidemic in 2012 in the UK, a temporary maternal vaccination program was launched in 2012 and is still ongoing due to continuing high numbers of pertussis cases [11]. In addition to the US and UK, maternal Tdap vaccination is currently recommended and government-funded in many countries globally [12,13,14,15]. Remarkably, in the setting of COVID-19 pandemic during the last 2 years, pregnant women are currently strongly advised to get vaccinated against SARS-CoV-2, to protect both themselves and their infants [16]. Except for the routinely administered vaccines, several vaccines can be safely used in pregnancy under specific circumstances. In the context of an epidemic, before traveling or after exposure, pregnant women can be vaccinated against hepatitis B, Neisseria meningitidis with the meningococcal polysaccharide vaccines, and polio with the inactivated virus vaccine (IPV) [17].
Beneficiary effects of maternal immunization for both mothers and fetuses have been thoroughly described. Maternal immunization confers protection to both mothers and fetuses by augmenting concentrations of maternal antibodies [18]. Thus, the quantity of antibodies transferred through the placenta to the fetus is enhanced, leading to more effective protection of the neonate during the period of the highest vulnerability until routine infant vaccinations are initiated [19]. Several factors may affect this process. Firstly, the timing and quality of the IgG transplacental transfer are of paramount importance for the development of maternal immunization strategies. While maternal IgG antibodies are transplacentally transferred throughout pregnancy, the majority of the transfer occurs in the third trimester of gestation. This is possibly due to an increase in the surface area of IgG uptake in placenta with higher gestational age [18]. It has been established that the transplacental transfer of maternally derived antibodies already begins from the first trimester of gestation and increases longitudinally, where the maximum IgG transfer occurs after the 28th gestational week [20]. In addition, maternally derived IgA and IgG are also transferred after birth in the colostrum and the breastmilk, enhancing the immunological response of the offspring in the first months of life [21,22].
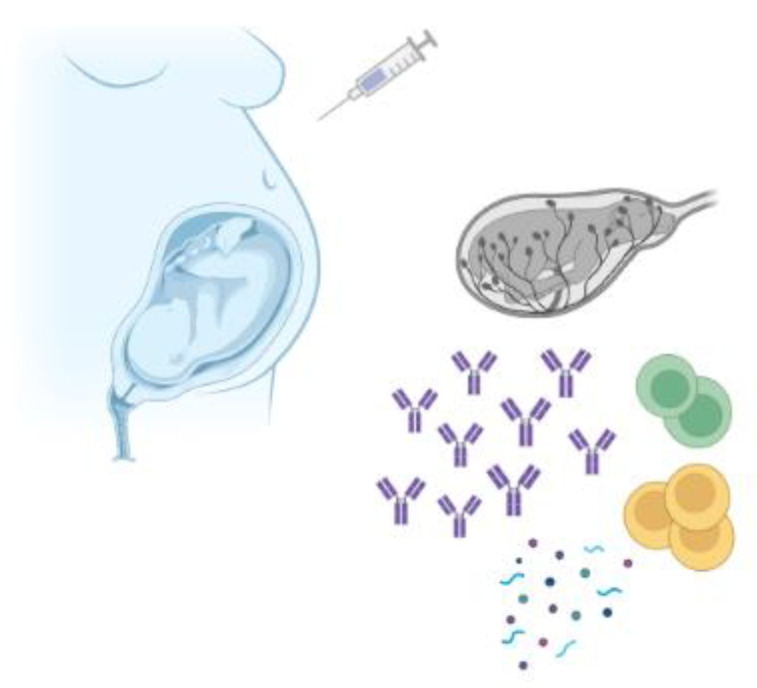
Importantly, it has been lately reported that the fetal and neonatal immune system may be affected beyond the passive immunity induced through IgG transfer; transfer of immune cells from the mother to the fetus has been proposed, as shown in Figure 1 [23,24]. It is possible that in utero priming following maternal immunization may provide additional, antibody-independent protection to the neonate. Current evidence suggests that fetal and neonatal immune system may be primed in response to maternal vaccinations, with confounding effect on subsequent neonate and infant B-cell repertoire [25]. Maternal cells have been identified in fetal tissues already from the second trimester of gestation. These cells are transferred via the placenta and promote the generation of fetal T regulatory cells against certain maternal antigens, somehow priming the fetal immune system [24]. In support of this, fetal memory T cells against human immunodeficiency virus (HIV), hepatitis B, and hepatitis C have been detected in uninfected children of mothers with wildtype infection. The aforementioned findings suggest in utero exposure to maternally derived infectious antigens, during maternal wildtype infection, implying that a similar mechanism may occur following maternal vaccination [26].
Figure 1.
Maternal vaccination may influence the fetal immune system beyond passive transplacental transfer of vaccine-specific antibodies. Evidence suggests that vaccination during pregnancy could prime fetal B cells and T cells. The role of cytokines is currently under investigation. Created with BioRender.com.
Nonetheless, several issues remain to be elucidated. Although the aim of maternal vaccination is the increase in maternal antigen-specific antibody titers and antibodies transferred to the infant through the placenta, maternal antibodies could interfere with offspring immune response to subsequent routine immunizations. This so-called “immunological blunting effect” results in attenuated humoral responses in early or late infancy [27]. However, the clinical significance of blunting remains unclear. Furthermore, vaccines during pregnancy may have “nonspecific effects”, by inducing modifications in the fetal immune system in an independent way to antigen-specific adaptive responses [28].
In this review, we aim to summarize current data on routinely administered immunizations during pregnancy and their effect on early life immune responses. In-depth understanding is crucial to achieve better infant survival rates and further optimize maternal and infant vaccination strategies.
2. Maternal Immunization against Influenza
Influenza infections are more severe during pregnancy due to changes in cardiorespiratory function of pregnant women, leading to high rates of morbidity and mortality among this vulnerable population, which progressively increase during pregnancy [29]. Notably, mortality rates surged among pregnant women (up to 45%) amidst major global influenza outbreaks [30]. Infection-related adverse events also occur among fetuses and neonates, with increased risk of miscarriage, preterm birth, low-birthweight neonates, and neonatal death [31]. Furthermore, both inactivated and live influenza vaccines are not currently indicated for children below 6 months due to safety reasons and impaired immunogenicity [32]. Thus, young infants are left unprotected during a period when they are more susceptible to severe complications. Currently, the absence of influenza epidemic during COVID-19 pandemics is expected to negatively affect infants in the following influenza seasons. Mothers most likely would not be capable of transferring antibodies to their offspring, as they have neither been exposed to strains of the virus, nor been routinely immunized. Hence, the launch of strategies that promote maternal vaccination against influenza worldwide is of paramount importance.
2.1. Maternal Immunization Effectiveness and Immunogenicity in Animals and Humans
Maternal immunization is critical to protect embryos in utero or newborns postnatally from adverse effects of influenza infection [33]. Immunization of gestating mice and ferrets with an inactivated vaccine containing H3N2, H2N2, H1N1, or H5N1 antigens provided enhanced passive protection to neonate animals [34,35]. Prospective studies in humans with laboratory-confirmed influenza showed that maternal vaccination not only reduces risk of influenza infection in infants (up to 63%), but also protects infected infants from severe disease [36].
Taking into consideration the frequent genetic modifications of the influenza virus, the WHO, the ACIP, and the ACOG recommended annual immunization with a quadrivalent inactivated influenza vaccine for all pregnant women regardless of pregnancy trimester, as well as for women of childbearing age or in the postpartum phase [37,38,39]. Nevertheless, only 61.2% of pregnant women in the US were vaccinated in 2020, and this percentage ranged globally from 1.7% to 95% [17]. Pregnancy is a state of relative immune suppression; thus, immunization during pregnancy has been estimated at 41–91% effectiveness in protecting newborns against influenza [40]. These results may be overestimated considering the protective impact of breastfeeding, as well as reduced risk of exposure to the influenza virus achieved by cocooning practices. Importantly, although a single dose of H1N1pdm09 vaccine was proven to induce seroprotection in most subjects, vaccines against pandemic viruses such as H5N1 display low immunogenicity. Thus, multiple immunizations with high antigen doses or addition of adjuvants are required to achieve seroprotective levels [41]. The development of influenza vaccines that are not affected by pregnancy-associated immune suppression is an urgent priority.
2.2. When to Vaccinate Mothers
Pregnant women are encouraged to be vaccinated against influenza during any trimester. However, flu vaccine administered to pregnant women in the late second or third trimester (after 20 weeks gestation) may offer two significant benefits. Firstly, the current subunit or split influenza vaccines induce a short-lived humoral immunity, which wanes at 6–7 months post vaccination, whereby a late vaccination would efficiently protect the mother until labor. Secondly, since infants are not vaccinated against influenza before 6 months of age, it is crucial to provide them with a robust passive immunity via transplacental transfer of maternal antibodies in utero or by breastmilk during infancy. Consistent with this, a meta-analysis documented that women vaccinated earlier in pregnancy had a greater increase in antibody titers compared to those vaccinated later [42]. Μοreover,, the first and early second trimesters of pregnancy, including implantation and placentation, are characterized by a proinflammatory environment, with potentially increased risk of adverse events to infectious and vaccine antigens [43,44,45]. Later, during the second trimester, the maternal immune system is predominated by T regulatory (Treg) cells in order to tolerate the semi-allogenic developing fetus [46]. Treg cells regulate exuberant immune responses and may inhibit the protective immunity induced by infection and vaccination [47]. During the late second and third trimester, the period of rapid fetal growth and development, hormonal changes, and exposure to fetal antigens, maternal immunity moves toward a more anti-inflammatory setting; thus, a more effective response to vaccine antigens is expected [1,48]. Nevertheless, infants of mothers who were vaccinated just 2 weeks before labor were not seroprotected against influenza [33,49]. This finding implies that the influenza vaccine should be administered to pregnant women at least 15 days before labor to achieve maximum neonatal protection. In a study conducted in Mali during three influenza seasons, the overall vaccine effectiveness (VE) of trivalent vaccine for infants against influenza was 70.2% in the first 4 months of age but reduced to 37.3% in the fifth and sixth months of age [50]. Similarly, in a trial from South Africa in 2011–2012, the VE was 85.6% in infants aged below 8 weeks, 25.5% in infants aged 8–16 weeks, and 30.3% in infants 16–24 weeks of age [51]. Hence, further research is required to define the optimal timing to achieve the highest maternal vaccine-derived IgG antibodies.
2.3. Passive Immunity and in Utero Priming
The primary mechanism of transferred passive immunity during gestation is an interplay between the Fc part of IgG antibodies and the neonatal Fc-receptor (FcRn), expressed on placental syncytiotrophoblasts. FcRn is prone to binding more favorably to IgG1 compared to other subclasses, mainly during the last 4 weeks of the pregnancy, by better promoting natural killer function [52]. Notably, antigen-specific antibodies against influenza are mainly ΙgG1, effectively protecting the infant in the first months of life. Remarkably, Pou et al. showed that the concentration of IgG specific antibodies was not correlated with factors such as gestational age, maternal IgG concentration, and birthweight [53]. Thus, the role of other important determinants for IgG transfer, such as isotype differences, glycosylation patterns, or binding capacity to the FcRn, remains to be studied, to further optimize maternal and infant immunization strategies.
Although transplacental transfer of IgG during pregnancy is crucial for the protection of the newborn against influenza infection, evidence suggests that the fetal immune system may be influenced by maternal vaccination in more ways than the passive immunity provided through IgG transfer. As with infectious disease antigens [25], the fetal immune system may also be primed in utero to vaccine antigens to which the mother was vaccinated during pregnancy; however, research in this area is still limited. In a case series, influenza-specific IgM antibodies were detected in the cord blood of one out of eight infants born to immunized mothers [54]. Given that IgM cannot cross the placenta, such findings imply that fetal B lymphocyte sensitization may have occurred. More recently, Rastogi et al. reported that the influenza-specific cord T cells were mainly CD45RO+ [55]. However, it has been previously demonstrated that cord-blood T cells are usually predominantly naïve, due to their low expression of CD45RO [56,57]. Therefore, Rastogi et al.’s findings may suggest activation of the adaptive immunity in the fetus following maternal immunization against influenza, considering that these compartments of immune response present features of immunological effector memory. Nevertheless, it remains unclear whether fetal priming, to the extent that it occurs, may shape the subsequent postnatal vaccine or clinical response.
2.4. Blunting Effect
Despite the benefits of maternal antibodies transferred to the fetus, a blunting effect has been described [26]. Mechanisms of interference between maternal antibodies and the infant’s antibody titers in response to active vaccination and the duration of this effect remain unclear. It has been suggested that B-cell responses are mainly inhibited by the following: (i) antigen neutralization (live replicating vaccines) [58]; (ii) epitope masking inhibiting antigen binding and, thus, infant B-cell priming (as in antibody feedback regulation studies) [22,59]; (iii) active inhibition of infant B-cell activation by Fcg receptor IIB (FcgRIIB)-mediated signaling [18,60]; (iv) clearance of complexes of maternally derived antibodies and vaccine antigens through Fc-dependent phagocytosis [58,61]. It is widely considered that immunization in the presence of maternal antibodies essentially leaves CD4+ T cells unaffected but prevents B-cell activation and, thus, antibody responses [62].
Assessing the dampening or inhibition of antibody response following the flu vaccine in infants of immunized mothers is quite challenging, as no vaccine is recommended for infants <6 months of age; thus, studies on the safety and immunogenicity of influenza vaccines on this vulnerable population are limited. In general, the majority of infants <6 months with high maternal antibody titers who received influenza vaccine did not significantly increase antibody titers [63,64,65]. This antibody suppression was not validated by Groothuis et al., as all infants enrolled had low titers to the antigens tested before vaccination. Nonetheless, this study included many patients with bronchopulmonary dysplasia who were born prematurely and had limited transplacental transfer of maternal antibodies [66].
However, a recent study investigated long-term influence of maternal antibodies against influenza on infant response to influenza vaccination using several neonatal and infant immunization models. It was demonstrated that, in spite of intact CD4+ T effector cell responses, T follicular helper cells prematurely decline. Of note, the presence of high titers of maternal antibodies did not dampen B-cell activation, germinal center (GC) B-cell differentiation, and bona fide GC responses to neonatal immunization [67]. Therefore, successful activation and differentiation of neonatal and infant B cells into GC B cells do occur, even in the presence of very high titers of maternal antibodies. Maternally derived antibodies essentially control the B-cell repertoire leading the infant B cells to express distinct BCRs, binding to distinct epitopes of vaccine antigens, most likely non-immunodominant ones. Similarly, Zimmermann et al. concluded that there is no effect of maternal vaccination with a trivalent inactivated influenza vaccine on the antibody concentrations and the seroprotection rates at 7 and 13 months of age after the completion of the primary series and the 12 month vaccination [68].
3. Maternal Immunization against Pertussis
Bordetella pertussis is the causative pathogen of pertussis disease. Very young infants are at disproportional risk of severe complications, including secondary bacterial pneumonia, apnea, pneumothorax, dehydration, seizures, encephalitis, and death [69]. Therefore, maternal immunization is of great importance to protect the offspring from pertussis and its complications [70]. Pertussis-containing vaccines include whole-cell pertussis vaccines and acellular pertussis vaccines, which contain one or more pertussis antigens, including pertussis toxoid (PT), filamentous hemagglutinin (FHA), pertactin (PRN), and fimbrial proteins 2 (FIM2) and 3 (FIM3) [71]. Currently, acellular pertussis vaccines are recommended in most developed countries, since whole-cell pertussis-containing vaccines were associated with increased frequency of local and systemic adverse events. The use of whole-cell pertussis vaccines is limited to some low- and middle-income countries [72]. As the pertussis vaccine is not administered mono-component, but only combined with diphtheria and tetanus toxoids, vaccination in pregnancy is performed with the Tdap vaccine. Infants also receive the DTaP vaccine, containing the same antigens as Tdap but in different quantities [73].
3.1. Maternal Immunization Immunogenicity and Effectiveness in Animals and Humans
Maternal antibodies effectively protected their offspring from B. pertussis in animal studies [74,75]. The humoral response after single Tdap immunization among pregnant women is evanescent and may not confer protection in subsequent pregnancies [76,77,78,79]; therefore, according to the WHO and the ACIP, the Tdap vaccine should be administered to all pregnant women in every pregnancy, regardless of the previous vaccination status [10]. Notably, repeated Tdap immunizations in consecutive pregnancies have been well tolerated [80]. Following the recommendations for antenatal vaccination, an increasing trend of maternal vaccination coverage against pertussis has been reported. In 2020–2021, 53.5% of pregnant women were immunized with Tdap in the USA [81]. Uptake of Tdap vaccine depends on various factors, such as sociodemographic factors, access to healthcare providers, and maternal comorbidities [82]
Tdap immunization results in the production of mostly IgG1 immunoglobulin [83], which can be transferred easily through the placenta from the mother to the fetus; thus, neonates display higher B. pertussis-specific antibody titers than their mothers [84]. The effectiveness of maternal immunization against pertussis has been thoroughly described in the literature in high- and middle/low-income countries, reaching 91% [85,86,87,88,89,90].
The safety of pertussis vaccination during pregnancy has been well documented. Although pregnant women displayed an increased risk for fever and chorioamnionitis following pertussis vaccination, there was no association with an increased frequency of clinically related events for the baby or for the mother [91,92,93,94,95,96,97].
3.2. When to Vaccinate Mothers
Pregnant women are advised to receive the Tdap vaccine during the last trimester of gestation, due to the highest maternal IgG levels and the maximum transplacental transport of maternal immunoglobulins to the fetus after the 34th gestational week [61]. However, the optimal timing of maternal vaccination remains to be defined, while it seems that the sooner a pregnant woman gets vaccinated, the better the neonatal protection. The latter potentially implies that, in addition to a more robust maternal immunological response, the fetal immune system might have a longer period of time for stimulation [98,99,100,101,102]. Regarding antibody avidity, two studies reported higher avidity when pregnant women were immunized during the early third trimester than later [103,104], although this finding was not further validated [105].
3.3. In Utero Priming
Similarly to maternal influenza vaccination, the fetal immune response following tetanus and pertussis vaccination in pregnancy appears to expand beyond the transplacental maternal IgG transfer. In detail, studies have shown that, following maternal vaccination with tetanus toxoid vaccine, tetanus IgM was identified in their offspring, while IgM levels were correlated with the timing of maternal immunization, implying fetal B lymphocyte sensitization [106,107].
With regard to cellular immunity, data remain scarce. Lima et al. found that maternal Tdap immunization and higher pertussis-specific IgG antibody levels did not affect the cellular immune response to B. pertussis at birth, as no differences in the neonatal ex vivo challenge of B and T cells were observed [108]. However, this study did not investigate cellular responses at later timepoints after birth. Rice et al. demonstrated that Tdap vaccination induced distinct cytokine profiles in infants of immunized mothers against B. pertussis [109]. At birth, offspring of immunized women had elevated IL-2 and IL-12 whole-blood levels, increased classical monocyte frequencies, and reduced monocyte and NK cell cytokines. These findings suggest the possibility of in utero priming of the fetal immune system beyond IgG transplacental transfer. At 7 weeks of age, IL-2 remained elevated, while lower IL-10 and IL-13 responses were observed. Following primary DTaP series vaccination, babies of immunized women still displayed lower IL-10 and IL-4 responses. Persistently high levels of IL-2 could affect cellular immune response, while the reduction in Th2 cytokines in older infants may play a role in the attenuated humoral response following primary DTaP immunization [109]. Vaccine-induced maternal cytokines could be transferred via the placenta and directly modify the fetal and neonatal immune response [25,109,110].
3.4. Blunting Effect
Previous studies have shown that the immune response induced post primary series of pertussis vaccination among infants of vaccinated pregnant women is lower compared to infants of unvaccinated mothers and varies among different pertussis antibodies (PT, FHA, and PRN); however, this effect seems to diminish following the booster dose in the second year of age [13,111,112,113,114]. Remarkably, surveillance data from the United Kingdom did not report any resurgence of pertussis cases in the last months on infancy [115]. Therefore, this blunting effect may lack any clinical implications, although continuous monitoring is required.
4. Maternal Immunization against SARS-CoV-2
Since the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in December 2019 and the subsequent coronavirus disease (COVID-19), a new challenge has arisen for the public health globally, declared as the COVID-19 pandemic. Soon it became apparent that vaccination would be the most effective way to mitigate the burden of severe COVID-19. COVID-19 vaccines have received an emergency use authorization, including pregnant women, albeit initially excluded from clinical trials. Pregnant women present a higher risk of severe COVID-19 compared to their non-pregnant counterparts [62,116,117], mainly those with pre-existing conditions such as diabetes or hypertension, with higher rates of intensive care unit admission, invasive ventilation, and mortality [118,119]. The risk of perinatal complications such as preeclampsia, preterm labor, and fetus growth restriction is also increased [120]. Intrauterine transmission of the virus to the fetus is rare but postpartum neonatal infections are more prevalent, mainly presenting with respiratory distress, low oxygen saturation, and cough, with 38% of previously infected neonates requiring intensive care [121,122]. Thus, COVID-19 vaccination before or during pregnancy is crucial for the protection of both mothers and their offspring. Even during the era of the less severe Omicron variant disease, compared to previous variants, studies on the outcome of Omicron infection during pregnancy reported that moderate to severe disease can occur especially among those who are not vaccinated, highlighting the importance of vaccination, especially among the vulnerable pregnant women [123].
The Centers for Disease Control and Prevention and other professional organizations such as the ACOG and the Royal College of Obstetricians and Gynecologists (RCOG) highly recommend that pregnant and lactating women should receive immunization against COVID-19 when the benefits outweigh the potential risks [124,125,126,127]. According to the current recommendation, the available vaccines include Pfizer/BioNTech BNT162b2, Moderna mRNA-1273, AstraZeneca AZD1222, Johnson and Johnson/Janssen Ad26.COV2.S, Novavax NVX-Co2373, Sinopharm BIBP, Sinovac CoronaVac, and Bharat Biotech BBV152. In the USA, mRNA vaccines (Pfizer-BioNTech and Moderna) are preferred for primary vaccination and additional doses (for immunocompromised individuals), as well as booster vaccination. Thrombosis with thrombocytopenia syndrome (TTS) has been reported as an uncommon complication post immunization with the adenovirus-vectored vaccines AstraZeneca and Janssen. Nonetheless, there are currently no data supporting that pregnant women should be considered as a high-risk population for TTS compared to nonpregnant ones [128]. COVID-19 vaccines may be administered simultaneously with other vaccines such as influenza vaccine and Tdap which are routinely administered during pregnancy.
A meta-analysis for the uptake of COVID-19 vaccines in pregnant women among five countries covering the period from December 2020 to October 2021 reported that the overall percentage of vaccinated women did not exceed 27.5% [129]. Several factors, i.e., age, demographic and socioeconomic characteristics, and the fear for severe disease may contribute to vaccine hesitancy. Given the low prevalence of vaccine uptake against COVID-19 among pregnant women, it is imperative for governments to foster the trust in the vaccines and surpass the hurdle of vaccine hesitancy.
Maternal Immunization Immunogenicity in Humans
Collier et al. were among the first to demonstrate that two doses of mRNA vaccine (BNT162b2 or mRNA-1273) received 3–4 weeks apart at various timepoints during pregnancy were immunogenic in pregnant women, while vaccine-elicited antibodies were detected in infant cord blood and mother’s breastmilk [130]. Another study showed that mRNA COVID-19 vaccines in pregnant women lead to antibody production in 5 days post first dose and transplacental transfer of IgG antibodies as early as 16 days post first dose [131]. Several subsequent studies confirmed the presence of maternal antibodies in cord blood, breast milk, and serum in infants of vaccinated mothers [132].
An anamnestic dose is now recommended for several high-risk populations, including pregnant women [133,134,135,136]. Individuals who received a third vaccine dose during the last trimester of pregnancy displayed higher levels of anti-spike IgG antibodies in maternal and cord blood [137]. This difference indicates that women with a primary series of vaccination followed by a booster dose transfer higher levels of antibodies to the neonate compared to those who received a two-dose schedule Interestingly, anti-spike IgG antibodies persist through the first 6 months of life, while IgG titers are higher at delivery, as well as 2 and 6 months postpartum, among infants born to mothers who were vaccinated at 20–32 weeks of gestation compared to those infants whose mothers were infected by SARS-CoV-2 during pregnancy at the same time interval [132]. More prospective and longitudinal studies are required in order to enlighten aspects such as the mechanisms of passive immunity after maternal vaccination, the exact duration of the provided immunity, and whether the antibodies passed by the mother have an adverse impact on the developing neonatal immune response [138].
Real-world data have confirmed vaccine effectiveness against hospitalization and admission to intensive care unit for COVID-19 among infants born to vaccinated mothers, most of them suggesting a stronger level of protection following the booster dose [139,140]. Interestingly, effectiveness was lower during the predominance of the omicron variant than during the delta circulation period as the omicron variant evades neutralizing antibodies induced by vaccination. Importantly, vaccine effectiveness against either variant was greater when the second dose of COVID-19 vaccine was administered after the 20th gestational week compared to earlier administration.
Further research is required to optimize maternal vaccination schedule, mainly regarding the booster dose, as well as define the level of subsequent protection in newborns. The main challenge remains to determine until when maternal vaccination may be postponed, considering the higher risk of severe COVID-19 during gestation.
5. Future Perspectives
Immunization against influenza, pertussis, and COVID-19 during pregnancy has been demonstrated to confer significant protection to both mothers [16,96,141] and young infants, and it should be offered to all pregnant women. Nonetheless, there are still issues to be addressed in this field. Immunization strategies should focus on maximizing the protective efficacy of maternal immunization while minimizing their inhibitory influence on infant B-cell responses. Further clinical trials are required to evaluate the long-term impact of maternal antibodies on memory B-cell induction and the potential skew of infant B-cell responses from immunodominant to non-immunodominant epitopes. Another promising hypothesis is the use of distinct vaccines in mothers and infants, for the recruitment of distinct B cells into the immunodominant response. Furthermore, it appears wise not to aim to higher maternal antibody titers than required for offspring protection. To this direction, the use of non-adjuvanted vaccines in pregnant women may be considered as an alternative [67]. Delaying immunization of infants of immunized mothers or offering additional/booster doses at an age at which maternal antibodies have declined below the threshold may be also considered.
Furthermore, in utero priming following maternal vaccination, if confirmed, could benefit the neonate, beyond protection through antibody-mediated passive immunity. This may be of great importance for cell-mediated infections, such as respiratory syncytial virus (RSV) [142]. RSV disease is responsible for severe respiratory distress among infants and young children, otherwise healthy, associated with high rates of hospital admissions and visits at the emergency departments [143]. Currently, there is only a monoclonal antibody, palivizumab, for the prevention of RSV disease among very preterm infants or those with specific comorbidities such as congenital heart disease, congenital respiratory abnormalities, or neuromuscular disease [144]. Maternal vaccination during pregnancy and infant vaccination with monoclonal antibodies may protect this vulnerable population against RSV disease [145]. Several RSV vaccines are currently under phase 3 clinical trials. A maternal vaccine formulation using the pre-fusion conformation of RSV protein (pre-F) seems to be the most promising one, eliciting high titers of RSV neutralizing antibodies, protecting the offspring from severe disease [146]. In a phase 2b trial, it was shown to be well tolerated for the mother and produced robust neutralizing antibody responses in pregnant women with adequate transplacental transfer [147]. Nevertheless, the clinical impact of the elevation of neutralizing antibody titers is under investigation. Additionally, the question of whether this vaccine may induce in utero priming of the fetus and affect the cellular immune response of the offspring is yet to be deciphered. Outside of RSV, other vaccines candidates are currently under investigation for the protection against group B streptococcus (GBS) and cytomegalovirus (CMV) infection, as phase 2 trial results and enrollment in phase 3 trial are soon expected [17].
Lastly, one should consider that protection of the mother and the offspring starts long before pregnancy, through maintaining high levels of active and herd immunity against significant vaccine-preventable infectious diseases. Pregnant women represent a vulnerable population to several infections, especially considering that many of the corresponding vaccines are contraindicated during pregnancy. A representative example of the significance of vaccination prior to gestation is the devastating outcome of congenital rubella syndrome, which causes serious birth abnormalities. This highlights the importance of active immunity before pregnancy and herd immunity. However, currently, the latter is undoubtedly threatened by a period of general vaccine hesitancy and major backsliding on childhood vaccinations due to the COVID-19 pandemic. Completed immunization with all the recommended vaccines is of paramount significance to sustain herd immunity. In the context of resurgence of previously eradicated diseases, such as measles and polio [148,149,150], healthcare providers should focus on ensuring high vaccination rates and offering any missed vaccinations in order to maintain antibody titers above herd immunity levels.
6. Conclusions
Maternal vaccination is a successful but still underutilized way for the prevention of infectious diseases among childbearing women and their offspring. The importance of informing pregnant women of the potential benefits of their timely vaccination for both themselves and their offspring cannot be emphasized enough.
Remarkably, increasing evidence supports the significance of maternal immunization not only through passive immunity, but also through in utero priming of the developing immune system. However, further in-depth research is required to shed light on the underlying mechanisms and possible clinical implications. Recent advances suggest that in utero fetal priming following maternal vaccination could benefit the newborn by offering protection in ways beyond antibody-mediated passive immunity. Nevertheless, several issues remain to be elucidated regarding maternal vaccination i.e., the possible impact on subsequent infant vaccinations, their potential “nonspecific” effects, and how the design and timing of immunization may dictate prenatal priming.
As data accumulate on how and via which mechanisms maternal vaccination affects fetal immune system, other possible methods for measuring antigen-specific T cells should be considered. This could aid in interpreting priming following vaccination, as it is debatable whether the observed cellular responses necessarily mirror in utero sensitization. Improving our understanding of the perinatal and neonatal immune systems is essential for improving infant survival rates and the optimization of vaccination in pregnancy and in early life, especially in developing countries, where the burden of infectious diseases is the highest.
Author Contributions
Conceptualization, T.L., D.G. and V.S.; writing—original draft preparation, T.L., D.G., M.G. and P.T.; writing—review and editing, T.L., D.G., M.G., P.T. and V.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
As this is a review article, no data were collected from patients.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This review received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Robinson D.P., Klein S.L. Pregnancy and Pregnancy-Associated Hormones Alter Immune Responses and Disease Pathogenesis. Horm. Behav. 2012;62:263–271. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kourtis A.P., Read J.S., Jamieson D.J. Pregnancy and Severity of Infection. N. Engl. J. Med. 2014;370:2211–2218. doi: 10.1056/NEJMra1213566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson A.N., Pollak E.A., Williams D., Smith M.A. Intrauterine Infection. Compr. Toxicol. Second Ed. 2010;12:239–258. doi: 10.1016/B978-0-08-046884-6.01523-2. [DOI] [Google Scholar]
- 4.Lawn J.E., Blencowe H., Oza S., You D., Lee A.C.C., Waiswa P., Lalli M., Bhutta Z., Barros A.J.D., Christian P., et al. Every Newborn: Progress, Priorities, and Potential beyond Survival. Lancet. 2014;384:189–205. doi: 10.1016/S0140-6736(14)60496-7. [DOI] [PubMed] [Google Scholar]
- 5.Faucette A.N., Pawlitz M.D., Pei B., Yao F., Chen K. Immunization of Pregnant Women: Future of Early Infant Protection. Hum. Vaccines Immunother. 2015;11:2549–2555. doi: 10.1080/21645515.2015.1070984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walensky R.P., Jernigan D.B., Bunnell R., Layden J., Kent C.K., Gottardy A.J., Leahy M.A., Martinroe J.C., Spriggs S.R., Yang T., et al. Morbidity and Mortality Weekly Report Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021–2022 Influenza Season Centers for Disease Control and Prevention MMWR. Recomm. Rep. 2021;70:1–28. [Google Scholar]
- 7.American College of Obstetricians and Gynecologists Influenza Vaccination during Pregnancy: Committee Opinion No 732, 2018. [(accessed on 5 October 2020)]. Available online: https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Influenza-Vaccination-During-Pregnancy.
- 8.Sawyer M., Liang J., Messonier N., Clark T., Centers for Disease Control and Prevention (CDC) Updated Recommendations for Use of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine (Tdap) in Pregnant Women and Persons Who Have or Anticipate Having Close Contact with an Infant Aged <12 Months—Advisory Committee on Immuniza. MMWR Morb. Mortal. Wkly. Rep. 2011;60:1424–1426. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) Updated Recommendations for Use of Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis Vaccine (Tdap) in Pregnant Women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb. Mortal. Wkly. Rep. 2013;62:131–135. doi: 10.15585/mmwr.mm6907a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Use of Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis Vaccines: Updated Recommendations of the Advisory Committee on Immunization Practices—United States, 2019. MMWR Morb. Mortal. Wkly. Rep. 2020;69:77–83. doi: 10.15585/mmwr.mm6903a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Public Health England Guidelines for the Public Health Management of Pertussis in England. [(accessed on 19 September 2022)]; Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/762766/Guidelines_for_the_Public_Health_management_of_Pertussis_in_England.pdf.
- 12.Vizzotti C., Neyro S., Katz N., Juárez M.V., Perez Carrega M.E., Aquino A., Kaski Fullone F. Maternal Immunization in Argentina: A Storyline from the Prospective of a Middle Income Country. Vaccine. 2015;33:6413–6419. doi: 10.1016/j.vaccine.2015.07.109. [DOI] [PubMed] [Google Scholar]
- 13.Maertens K., Caboré R.N., Huygen K., Vermeiren S., Hens N., Van Damme P., Leuridan E. Pertussis Vaccination during Pregnancy in Belgium: Follow-up of Infants until 1 Month after the Fourth Infant Pertussis Vaccination at 15 Months of Age. Vaccine. 2016;34:3613–3619. doi: 10.1016/j.vaccine.2016.04.066. [DOI] [PubMed] [Google Scholar]
- 14.Moreno-Pérez D., Álvarez García F.J., Arístegui Fernández J., Cilleruelo Ortega M.J., Corretger Rauet J.M., García Sánchez N., Hernández Merino A., Hernández-Sampelayo Matos T., Merino Moína M., Ortigosa Del Castillo L., et al. Immunisation Schedule of the Spanish Association of Paediatrics: 2015 Recommendations. An. Pediatr. 2014;82:44.e1–44.e12. doi: 10.1016/j.anpedi.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 15.National Immunization Schedule for Adults. [(accessed on 8 October 2022)]; Available online: https://www.moh.gov.gr/articles/health/dieythynsh-dhmosias-ygieinhs/emboliasmoi/ethniko-programma-emboliasmwn-epe-enhlikwn/9968-ethniko-programma-emboliasmwn-enhlikwn-2022.
- 16.Badell M.L., Dude C.M., Rasmussen S.A., Jamieson D.J. Covid-19 Vaccination in Pregnancy. BMJ. 2022;378:e069741. doi: 10.1136/bmj-2021-069741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etti M., Calvert A., Galiza E., Lim S., Khalil A., Le Doare K., Heath P.T. Maternal Vaccination: A Review of Current Evidence and Recommendations. Am. J. Obstet. Gynecol. 2022;226:459–474. doi: 10.1016/j.ajog.2021.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards K.M. Maternal Antibodies and Infant Immune Responses to Vaccines. Vaccine. 2015;33:6469–6472. doi: 10.1016/j.vaccine.2015.07.085. [DOI] [PubMed] [Google Scholar]
- 19.Wilcox C.R., Holder B., Jones C.E. Factors Affecting the FcRn-Mediated Transplacental Transfer of Antibodies and Implications for Vaccination in Pregnancy. Front. Immunol. 2017;8:1294. doi: 10.3389/fimmu.2017.01294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malek A., Sager R., Kuhn P., Nicolaides K.H., Schneider H. Evolution of Maternofetal Transport of Immunoglobulins during Human Pregnancy. Am. J. Reprod. Immunol. 1996;36:248–255. doi: 10.1111/j.1600-0897.1996.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 21.Marshall H., McMillan M., Andrews R.M., Macartney K., Edwards K. Vaccines in Pregnancy: The Dual Benefit for Pregnant Women and Infants. Hum. Vaccines Immunother. 2016;12:848–856. doi: 10.1080/21645515.2015.1127485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niewiesk S. Maternal Antibodies: Clinical Significance, Mechanism of Interference with Immune Responses, and Possible Vaccination Strategies. Front. Immunol. 2014;5:446. doi: 10.3389/fimmu.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jonsson A.M., Uzunel M., Götherström C., Papadogiannakis N., Westgren M. Maternal Microchimerism in Human Fetal Tissues. Am. J. Obstet. Gynecol. 2008;198:325.e1–325.e6. doi: 10.1016/j.ajog.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 24.Mold J.E., Michaëlsson J., Burt T.D., Muench M.O., Beckerman K.P., Busch M.P., Lee T.H., Nixon D.F., McCune J.M. Maternal Alloantigens Promote the Development of Tolerogenic Fetal Regulatory T Cells in Utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilcox C.R., Jones C.E. Beyond Passive Immunity: Is There Priming of the Fetal Immune System Following Vaccination in Pregnancy and What Are the Potential Clinical Implications? Front. Immunol. 2018;9:1548. doi: 10.3389/fimmu.2018.01548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cinicola B., Conti M.G., Terrin G., Sgrulletti M., Elfeky R., Carsetti R., Fernandez Salinas A., Piano Mortari E., Brindisi G., De Curtis M., et al. The Protective Role of Maternal Immunization in Early Life. Front. Pediatr. 2021;9:332. doi: 10.3389/fped.2021.638871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vojtek I., Dieussaert I., Doherty T.M., Franck V., Hanssens L., Miller J., Bekkat-Berkani R., Kandeil W., Prado-Cohrs D., Vyse A. Maternal Immunization: Where Are We Now and How to Move Forward? Ann. Med. 2018;50:193–208. doi: 10.1080/07853890.2017.1421320. [DOI] [PubMed] [Google Scholar]
- 28.Aaby P., Kollmann T.R., Benn C.S. Nonspecific Effects of Neonatal and Infant Vaccination: Public-Health, Immunological and Conceptual Challenges. Nat. Immunol. 2014;15:895–899. doi: 10.1038/ni.2961. [DOI] [PubMed] [Google Scholar]
- 29.Sakala I.G., Honda-Okubo Y., Fung J., Petrovsky N. Influenza Immunization during Pregnancy: Benefits for Mother and Infant. Hum. Vaccines Immunother. 2016;12:3065–3071. doi: 10.1080/21645515.2016.1215392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bloom-Feshbach K., Simonsen L., Viboud C., Mølbak K., Miller M.A., Gottfredsson M., Andreasen V. Natality Decline and Miscarriages Associated with the 1918 Influenza Pandemic: The Scandinavian and United States Experiences. J. Infect. Dis. 2011;204:1157–1164. doi: 10.1093/infdis/jir510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasmussen S.A., Jamieson D.J., Uyeki T.M. Effects of Influenza on Pregnant Women and Infants. Am. J. Obstet. Gynecol. 2012;207:S3–S8. doi: 10.1016/j.ajog.2012.06.068. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention (CDC) Prevention and Control of Seasonal Influenza with Vaccines Recommendations of the Advisory Committee on Immunization Practices—United States, 2021–2022 Influenza Season. Volume 70. CDC; Atlanta, GA, USA: 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blanchard-Rohner G., Meier S., Bel M., Combescure C., Othenin-Girard V., Swali R.A., De Tejada B.M., Siegrist C.A. Influenza Vaccination given at Least 2 Weeks before Delivery to Pregnant Women Facilitates Transmission of Seroprotective Influenza-Specific Antibodies to the Newborn. Pediatr. Infect. Dis. J. 2013;32:1374–1380. doi: 10.1097/01.inf.0000437066.40840.c4. [DOI] [PubMed] [Google Scholar]
- 34.Mbawuike I.N., Six H.R., Cate T.R., Couch R.B. Vaccination with Inactivated Influenza A Virus during Pregnancy Protects Neonatal Mice against Lethal Challenge by Influenza A Viruses Representing Three Subtypes. J. Virol. 1990;64:1370–1374. doi: 10.1128/jvi.64.3.1370-1374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Do Hwang S., Shin J.S., Ku K.B., Kim H.S., Cho S.W., Seo S.H. Protection of Pregnant Mice, Fetuses and Neonates from Lethality of H5N1 Influenza Viruses by Maternal Vaccination. Vaccine. 2010;28:2957–2964. doi: 10.1016/j.vaccine.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Christian L.M., Beverly C., Mitchell A.M., Karlsson E., Porter K., Schultz-Cherry S., Ramilo O. Effects of Prior Influenza Virus Vaccination on Maternal Antibody Responses: Implications for Achieving Protection in the Newborns. Vaccine. 2017;35:5283–5290. doi: 10.1016/j.vaccine.2017.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization . How to Implement Influenza Vaccination of Pregnant Women. WHO; Geneva, Switzerland: 2017. [Google Scholar]
- 38.ACOG . ACOG Committee Opinion—Influenza Vaccination during Pregnancy. Volume 131 ACOG; Waltham, MA, USA: 2018. [Google Scholar]
- 39.Tenforde M.W., Kondor R.J.G., Chung J.R., Zimmerman R.K., Nowalk M.P., Jackson M.L., Jackson L.A., Monto A.S., Martin E.T., Belongia E.A., et al. Effect of Antigenic Drift on Influenza Vaccine Effectiveness in the United States-2019–2020. Clin. Infect. Dis. 2021;73:e4244–e4250. doi: 10.1093/cid/ciaa1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manske J.M. Efficacy and Effectiveness of Maternal Influenza Vaccination During Pregnancy: A Review of the Evidence. Matern. Child Health J. 2014;18:1599–1609. doi: 10.1007/s10995-013-1399-2. [DOI] [PubMed] [Google Scholar]
- 41.Manzoli L., Ioannidis J.P.A., Flacco M.E., De Vito C., Villari P. Effectiveness and Harms of Seasonal and Pandemic Influenza Vaccines in Children, Adults and Elderly: A Critical Review and Re-Analysis of 15 Meta-Analyses. Hum. Vaccines Immunother. 2012;8:851–862. doi: 10.4161/hv.19917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cuningham W., Geard N., Fielding J.E., Braat S., Madhi S.A., Nunes M.C., Christian L.M., Lin S.Y., Lee C.N., Yamaguchi K., et al. Optimal Timing of Influenza Vaccine during Pregnancy: A Systematic Review and Meta-Analysis. Influenza Other Respi. Viruses. 2019;13:438–452. doi: 10.1111/irv.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borzychowski A.M., Croy B.A., Chan W.L., Redman C.W.G., Sargent I.L. Changes in Systemic Type 1 and Type 2 Immunity in Normal Pregnancy and Pre-Eclampsia May Be Mediated by Natural Killer Cells. Eur. J. Immunol. 2005;35:3054–3063. doi: 10.1002/eji.200425929. [DOI] [PubMed] [Google Scholar]
- 44.Borzychowski A.M., Sargent I.L., Redman C.W.G. Inflammation and Pre-Eclampsia. Semin. Fetal Neonatal Med. 2006;11:309–316. doi: 10.1016/j.siny.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Sargent I.L., Borzychowski A.M., Redman C.W.G. NK Cells and Human Pregnancy—An Inflammatory View. Trends Immunol. 2006;27:399–404. doi: 10.1016/j.it.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 46.Kollmann T.R., Kampmann B., Mazmanian S.K., Marchant A., Levy O. Protecting the Newborn and Young Infant from Infectious Diseases: Lessons from Immune Ontogeny. Immunity. 2017;46:350–363. doi: 10.1016/j.immuni.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 47.Lin P.H., Wong W.I., Wang Y.L., Hsieh M.P., Lu C.W., Liang C.Y., Jui S.H., Wu F.Y., Chen P.J., Yang H.C. Vaccine-Induced Antigen-Specific Regulatory T Cells Attenuate the Antiviral Immunity against Acute Influenza Virus Infection. Mucosal Immunol. 2018;11:1239–1253. doi: 10.1038/s41385-018-0004-9. [DOI] [PubMed] [Google Scholar]
- 48.Gaunt G., Ramin K. Immunological Tolerance of the Human Fetus. Am. J. Perinatol. 2001;18:299–312. doi: 10.1055/s-2001-17861. [DOI] [PubMed] [Google Scholar]
- 49.Poehling K.A., Szilagyi P.G., Staat M.A., Snively B.M., Payne D.C., Bridges C.B., Chu S.Y., Light L.S., Prill M.M., Finelli L., et al. Impact of Maternal Immunization on Influenza Hospitalizations in Infants. Am. J. Obstet. Gynecol. 2011;204:S141–S148. doi: 10.1016/j.ajog.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tapia M.D., Sow S.O., Tamboura B., Tégueté I., Pasetti M.F., Kodio M., Onwuchekwa U., Tennant S.M., Blackwelder W.C., Coulibaly F., et al. Maternal Immunisation with Trivalent Inactivated Influenza Vaccine for Prevention of Influenza in Infants in Mali: A Prospective, Active-Controlled, Observer-Blind, Randomised Phase 4 Trial. Lancet Infect. Dis. 2016;16:1026–1035. doi: 10.1016/S1473-3099(16)30054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nunes M.C., Cutland C.L., Jones S., Hugo A., Madimabe R., Simões E.A.F., Weinberg A., Madhi S.A. Duration of Infant Protection against Influenza Illness Conferred by Maternal Immunization Secondary Analysis of a Randomized Clinical Trial. JAMA Pediatr. 2016;170:840–847. doi: 10.1001/jamapediatrics.2016.0921. [DOI] [PubMed] [Google Scholar]
- 52.Jennewein M.F., Goldfarb I., Dolatshahi S., Cosgrove C., Noelette F.J., Krykbaeva M., Das J., Sarkar A., Gorman M.J., Fischinger S., et al. Fc Glycan-Mediated Regulation of Placental Antibody Transfer. Cell. 2019;178:202–215.e14. doi: 10.1016/j.cell.2019.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pou C., Nkulikiyimfura D., Henckel E., Olin A., Lakshmikanth T., Mikes J., Wang J., Chen Y., Bernhardsson A.K., Gustafsson A., et al. The Repertoire of Maternal Anti-Viral Antibodies in Human Newborns. Nat. Med. 2019;25:591–596. doi: 10.1038/s41591-019-0392-8. [DOI] [PubMed] [Google Scholar]
- 54.Englund J.A., Mbawuike I.N., Hammill H., Holleman M.C., Baxter B.D., Glezen W.P. Maternal Immunization with Influenza or Tetanus Toxoid Vaccine for Passive Antibody Protection in Young Infants. J. Infect. Dis. 1993;168:647–656. doi: 10.1093/infdis/168.3.647. [DOI] [PubMed] [Google Scholar]
- 55.Rastogi D., Wang C., Mao X., Lendor C., Rothman P.B., Miller R.L. Antigen-Specific Immune Responses to Influenza Vaccine in Utero. J. Clin. Investig. 2007;117:1637–1646. doi: 10.1172/JCI29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Vries E., De Bruin-Versteeg S., Comans-Bitter M.M., De Groot R., Hop W.C.J., Boerma G.J.M., Lotgering F.K., Van Dongen J.J.M. Longitudinal Survey of Lymphocyte Subpopulations in the First Year of Life. Pediatr. Res. 2000;47:528–537. doi: 10.1203/00006450-200004000-00019. [DOI] [PubMed] [Google Scholar]
- 57.Tosato F., Bucciol G., Pantano G., Putti M.C., Sanzari M.C., Basso G., Plebani M. Lymphocytes Subsets Reference Values in Childhood. Cytom. Part A. 2015;87:81–85. doi: 10.1002/cyto.a.22520. [DOI] [PubMed] [Google Scholar]
- 58.Siegrist C.A. Mechanisms by Which Maternal Antibodies Influence Infant Vaccine Responses: Review of Hypotheses and Definition of Main Determinants. Vaccine. 2003;21:3406–3412. doi: 10.1016/S0264-410X(03)00342-6. [DOI] [PubMed] [Google Scholar]
- 59.Brüggemann M., Rajewsky K. Regulation of the Antibody Response against Hapten-Coupled Erythrocytes by Monoclonal Antihapten Antibodies of Various Isotypes. Cell. Immunol. 1982;71:365–373. doi: 10.1016/0008-8749(82)90270-2. [DOI] [PubMed] [Google Scholar]
- 60.Kim D., Huey D., Oglesbee M., Niewiesk S. Insights into the Regulatory Mechanism Controlling the Inhibition of Vaccine-Induced Seroconversion by Maternal Antibodies. Blood. 2011;117:6143–6151. doi: 10.1182/blood-2010-11-320317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abu-Raya B., Maertens K., Edwards K.M., Omer S.B., Englund J.A., Flanagan K.L., Snape M.D., Amirthalingam G., Leuridan E., Van Damme P., et al. Global Perspectives on Immunization During Pregnancy and Priorities for Future Research and Development: An International Consensus Statement. Front. Immunol. 2020;11:1282. doi: 10.3389/fimmu.2020.01282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Orije M.R.P., Maertens K., Corbière V., Wanlapakorn N., Van Damme P., Leuridan E., Mascart F. The Effect of Maternal Antibodies on the Cellular Immune Response after Infant Vaccination: A Review. Vaccine. 2020;38:20–28. doi: 10.1016/j.vaccine.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 63.Halasa N.B., Gerber M.A., Chen Q., Wright P.F., Edwards K.M. Safety and Immunogenicity of Trivalent Inactivated Influenza Vaccine in Infants. J. Infect. Dis. 2008;197:1448–1454. doi: 10.1086/587643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gruber W.C., Darden P.M., Still J.G., Lohr J., Reed G., Wright P.F. Evaluation of Bivalent Live Attenuated Influenza A Vaccines in Children 2 Months to 3 Years of Age: Safety, Immunogenicity and Dose-Response. Vaccine. 1997;15:1379–1384. doi: 10.1016/S0264-410X(97)00032-7. [DOI] [PubMed] [Google Scholar]
- 65.Walter E.B., Englund J.A., Blatter M., Nyberg J., Ruben F.L., Decker M.D. Trivalent Inactivated Influenza Virus Vaccine given to Two-Month-Old Children: An off-Season Pilot Study. Pediatr. Infect. Dis. J. 2009;28:1099–1104. doi: 10.1097/INF.0b013e3181b0c0ca. [DOI] [PubMed] [Google Scholar]
- 66.Groothuis J.R., Levin M.J., Rabalais G.P., Meiklejohn G., Lauer B.A. Immunization of High-Risk Infants Younger than 18 Months of Age with Split-Product Influenza Vaccine. Pediatrics. 1991;87:823–828. doi: 10.1542/peds.87.6.823. [DOI] [PubMed] [Google Scholar]
- 67.Vono M., Eberhardt C.S., Auderset F., Mastelic-Gavillet B., Lemeille S., Christensen D., Andersen P., Lambert P.H., Siegrist C.A. Maternal Antibodies Inhibit Neonatal and Infant Responses to Vaccination by Shaping the Early-Life B Cell Repertoire within Germinal Centers. Cell Rep. 2019;28:1773–1784.e5. doi: 10.1016/j.celrep.2019.07.047. [DOI] [PubMed] [Google Scholar]
- 68.Zimmermann P., Perrett K.P., Messina N.L., Donath S., Ritz N., van der Klis F.R.M., Curtis N. The Effect of Maternal Immunisation During Pregnancy on Infant Vaccine Responses. EClinicalMedicine. 2019;13:21–30. doi: 10.1016/j.eclinm.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cherry J.D. Pertussis in Young Infants throughout the World. Clin. Infect. Dis. 2016;63:S119–S122. doi: 10.1093/cid/ciw550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Locht C. The Path to New Pediatric Vaccines against Pertussis. Vaccines. 2021;9:228. doi: 10.3390/vaccines9030228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dewan K.K., Linz B., Derocco S.E., Harvill E.T. Acellular Pertussis Vaccine Components: Today and Tomorrow. Vaccines. 2020;8:217. doi: 10.3390/vaccines8020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Esposito S., Stefanelli P., Fry N.K., Fedele G., He Q., Paterson P., Tan T., Knuf M., Rodrigo C., Olivier C.W., et al. Pertussis Prevention: Reasons for Resurgence, and Differences in the Current Acellular Pertussis Vaccines. Front. Immunol. 2019;10:1344. doi: 10.3389/fimmu.2019.01344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nguyen V.T.N., Simon L. Pertussis: The Whooping Cough. Prim. Care Clin. Off. Pract. 2018;45:423–431. doi: 10.1016/j.pop.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 74.Nguyen A.W., Wagner E.K., Laber J.R., Goodfield L.L., Smallridge W.E., Harvill E.T., Papin J.F., Wolf R.F., Padlan E.A., Bristol A., et al. A Cocktail of Humanized Anti-Pertussis Toxin Antibodies Limits Disease in Murine and Baboon Models of Whooping Cough. Sci. Transl. Med. 2015;7:316ra195. doi: 10.1126/scitranslmed.aad0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kapil P., Papin J.F., Wolf R.F., Zimmerman L.I., Wagner L.D., Merkel T.J. Maternal Vaccination with a Monocomponent Pertussis Toxoid Vaccine Is Sufficient to Protect Infants in a Baboon Model of Whooping Cough. J. Infect. Dis. 2018;217:1231–1236. doi: 10.1093/infdis/jiy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Storsaeter J., Hallander H.O., Gustafsson L., Olin P. Low Levels of Antipertussis Antibodies plus Lack of History of Pertussis Correlate with Susceptibility after Household Exposure to Bordetella Pertussis. Vaccine. 2003;21:3542–3549. doi: 10.1016/S0264-410X(03)00407-9. [DOI] [PubMed] [Google Scholar]
- 77.Halperin B.A., Morris A., MacKinnon-Cameron D., Mutch J., Langley J.M., McNeil S.A., MacDougall D., Halperin S.A. Kinetics of the Antibody Response to Tetanus-Diphtheria-Acellular Pertussis Vaccine in Women of Childbearing Age and Postpartum Women. Clin. Infect. Dis. 2011;53:885–892. doi: 10.1093/cid/cir538. [DOI] [PubMed] [Google Scholar]
- 78.Raya B.A., Srugo I., Kessel A., Peterman M., Vaknin A., Bamberger E. The Decline of Pertussis-Specific Antibodies after Tetanus, Diphtheria, and Acellular Pertussis Immunization in Late Pregnancy. J. Infect. Dis. 2015;212:1869–1873. doi: 10.1093/infdis/jiv324. [DOI] [PubMed] [Google Scholar]
- 79.Maertens K., Tran T.M.P., Hens N., Van Damme P., Leuridan E. Effect of Prepregnancy Pertussis Vaccination in Young Infants. J. Infect. Dis. 2017;215:1855–1861. doi: 10.1093/infdis/jix176. [DOI] [PubMed] [Google Scholar]
- 80.Sukumaran L., McCarthy N.L., Kharbanda E.O., McNeil M.M., Naleway A.L., Klein N.P., Jackson M.L., Hambidge S.J., Lugg M.M., Li R., et al. Association of Tdap Vaccination with Acute Events and Adverse Birth Outcomes among Pregnant Women with Prior Tetanuscontaining Immunizations. JAMA J. Am. Med. Assoc. 2015;314:1581–1587. doi: 10.1001/jama.2015.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Centers for Disease Control and Prevention (CDC) Flu and Tdap Vaccination Coverage among Pregnant Women—United States, April 2021. CDC; Atlanta, GA, USA: 2021. [Google Scholar]
- 82.Fernández-Cano M.I., Arreciado Marañón A., Reyes-Lacalle A., Feijoo-Cid M., Manresa-Domínguez J.M., Montero-Pons L., Cabedo-Ferreiro R.M., Toran-Monserrat P., Falguera-Puig G. Influenza and Pertussis Maternal Vaccination Coverage and Influencing Factors in Spain: A Study Based on Primary Care Records Registry. Int. J. Environ. Res. Public Health. 2022;19:4391. doi: 10.3390/ijerph19074391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hendrikx L.H., Schure R.M., Öztürk K., de Rond L.G.H., de Greeff S.C., Sanders E.A.M., Berbers G.A.M., Buisman A.M. Different IgG-Subclass Distributions after Whole-Cell and Acellular Pertussis Infant Primary Vaccinations in Healthy and Pertussis Infected Children. Vaccine. 2011;29:6874–6880. doi: 10.1016/j.vaccine.2011.07.055. [DOI] [PubMed] [Google Scholar]
- 84.Munoz F.M., Bond N.H., Maccato M., Pinell P., Hammill H.A., Swamy G.K., Walter E.B., Jackson L.A., Englund J.A., Edwards M.S., et al. Safety and Immunogenicity of Tetanus Diphtheria and Acellular Pertussis (Tdap) Immunization during Pregnancy in Mothers and Infants: A Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2014;311:1760–1769. doi: 10.1001/jama.2014.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Winter K., Nickell S., Powell M., Harriman K. Effectiveness of Prenatal versus Postpartum Tetanus, Diphtheria, and Acellular Pertussis Vaccination in Preventing Infant Pertussis. Clin. Infect. Dis. 2017;64:3–8. doi: 10.1093/cid/ciw634. [DOI] [PubMed] [Google Scholar]
- 86.Winter K., Cherry J.D., Harriman K. Effectiveness of Prenatal Tetanus, Diphtheria, and Acellular Pertussis Vaccination on Pertussis Severity in Infants. Clin. Infect. Dis. 2017;64:9–14. doi: 10.1093/cid/ciw633. [DOI] [PubMed] [Google Scholar]
- 87.Dabrera G., Amirthalingam G., Andrews N., Campbell H., Ribeiro S., Kara E., Fry N.K., Ramsay M. A Case-Control Study to Estimate the Effectiveness of Maternal Pertussis Vaccination in Protecting Newborn Infants in England and Wales, 2012–2013. Clin. Infect. Dis. 2015;60:333–337. doi: 10.1093/cid/ciu821. [DOI] [PubMed] [Google Scholar]
- 88.Bellido-Blasco J., Guiral-Rodrigo S., Míguez-Santiyán A., Salazar-Cifre A., González-Morán F. A Case–Control Study to Assess the Effectiveness of Pertussis Vaccination during Pregnancy on Newborns, Valencian Community, Spain, 1 March 2015 to 29 February 2016. Eurosurveillance. 2017;22:30545. doi: 10.2807/1560-7917.ES.2017.22.22.30545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saul N., Wang K., Bag S., Baldwin H., Alexander K., Chandra M., Thomas J., Quinn H., Sheppeard V., Conaty S. Effectiveness of Maternal Pertussis Vaccination in Preventing Infection and Disease in Infants: The NSW Public Health Network Case-Control Study. Vaccine. 2018;36:1887–1892. doi: 10.1016/j.vaccine.2018.02.047. [DOI] [PubMed] [Google Scholar]
- 90.Fernandes E.G., Sato A.P.S., Vaz-de-Lima L.R.A., Rodrigues M., Leite D., de Brito C.A., Luna E.J.A., Carvalhanas T.R.M.P., Ramos M.L.B.N., Sato H.K., et al. The Effectiveness of Maternal Pertussis Vaccination in Protecting Newborn Infants in Brazil: A Case-Control Study. Vaccine. 2019;37:5481–5484. doi: 10.1016/j.vaccine.2019.03.049. [DOI] [PubMed] [Google Scholar]
- 91.Vygen-Bonnet S., Hellenbrand W., Garbe E., Von Kries R., Bogdan C., Heininger U., Röbl-Mathieu M., Harder T. Safety and Effectiveness of Acellular Pertussis Vaccination during Pregnancy: A Systematic Review. BMC Infect. Dis. 2020;20:136. doi: 10.1186/s12879-020-4824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kharbanda E.O., Vazquez-Benitez G., Lipkind H.S., Klein N.P., Cheetham T.C., Naleway A., Omer S.B., Hambidge S.J., Lee G.M., Jackson M.L., et al. Evaluation of the Association of Maternal Pertussis Vaccination with Obstetric Events and Birth Outcomes. JAMA J. Am. Med. Assoc. 2014;312:1897–1904. doi: 10.1001/jama.2014.14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shakib J.H., Korgenski K., Sheng X., Varner M.W., Pavia A.T., Byington C.L. Tetanus, Diphtheria, Acellular Pertussis Vaccine during Pregnancy: Pregnancy and Infant Health Outcomes. J. Pediatr. 2013;163:1422–1426.e4. doi: 10.1016/j.jpeds.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Walls T., Graham P., Petousis-Harris H., Hill L., Austin N. Infant Outcomes after Exposure to Tdap Vaccine in Pregnancy: An Observational Study. BMJ Open. 2016;6:e009536. doi: 10.1136/bmjopen-2015-009536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Villarreal Pérez J.Z., Ramírez Aranda J.M., de la OCavazos M., Zamudio Osuna M.D.J., Perales Dávila J., Ballesteros Elizondo M.R., Gómez Meza M.V., García Elizondo F.J., Rodríguez González A.M. Randomized Clinical Trial of the Safety and Immunogenicity of the Tdap Vaccine in Pregnant Mexican Women. Hum. Vaccines Immunother. 2017;13:128–135. doi: 10.1080/21645515.2016.1232786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.D’Heilly C., Switzer C., Macina D. Safety of Maternal Immunization Against Pertussis: A Systematic Review. Infect. Dis. Ther. 2019;8:543–568. doi: 10.1007/s40121-019-00265-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tseng H.F., Sy L.S., Ackerson B.K., Lee G.S., Luo Y., Florea A., Becerra-Culqui T., Tartof S.Y., Tian Y., Taylor C., et al. Safety of Tetanus, Diphtheria, Acellular Pertussis (Tdap) Vaccination during Pregnancy. Vaccine. 2022;40:4503–4512. doi: 10.1016/j.vaccine.2022.06.009. [DOI] [PubMed] [Google Scholar]
- 98.Abu Raya B., Srugo I., Kessel A., Peterman M., Bader D., Gonen R., Bamberger E. The Effect of Timing of Maternal Tetanus, Diphtheria, and Acellular Pertussis (Tdap) Immunization during Pregnancy on Newborn Pertussis Antibody Levels—A Prospective Study. Vaccine. 2014;32:5787–5793. doi: 10.1016/j.vaccine.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 99.Naidu M.A., Muljadi R., Davies-Tuck M.L., Wallace E.M., Giles M.L. The Optimal Gestation for Pertussis Vaccination during Pregnancy: A Prospective Cohort Study. Am. J. Obstet. Gynecol. 2016;215:237.e1–237.e6. doi: 10.1016/j.ajog.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 100.Wanlapakorn N., Maertens K., Chaithongwongwatthana S., Srimuan D., Suratannon N., Vongpunsawad S., Mai Phuong Tran T., Hens N., Van Damme P., Locht C., et al. Assessing the Reactogenicity of Tdap Vaccine Administered during Pregnancy and Antibodies to Bordetella Pertussis Antigens in Maternal and Cord Sera of Thai Women. Vaccine. 2018;36:1453–1459. doi: 10.1016/j.vaccine.2018.01.059. [DOI] [PubMed] [Google Scholar]
- 101.Eberhardt C.S., Blanchard-Rohner G., Lemaître B., Combescure C., Othenin-Girard V., Chilin A., Petre J., De Tejada B.M., Iegrist C.A. Pertussis Antibody Transfer to Preterm Neonates after Second-versus Third-Trimester Maternal Immunization. Clin. Infect. Dis. 2017;64:1129–1132. doi: 10.1093/cid/cix046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gomme J., Wanlapakorn N., Thi H., Ha T., Leuridan E., Herzog S.A., Maertens K. The Impact of Timing of Pertussis Vaccination During Pregnancy on Infant Antibody Levels at Birth: A Multi-Country Analysis. Front. Immunol. 2022;13:913922. doi: 10.3389/fimmu.2022.913922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abu Raya B., Bamberger E., Almog M., Peri R., Srugo I., Kessel A. Immunization of Pregnant Women against Pertussis: The Effect of Timing on Antibody Avidity. Vaccine. 2015;33:1948–1952. doi: 10.1016/j.vaccine.2015.02.059. [DOI] [PubMed] [Google Scholar]
- 104.Abu-Raya B., Giles M.L., Kollmann T.R., Sadarangani M. The Effect of Timing of Tetanus-Diphtheria-Acellular Pertussis Vaccine Administration in Pregnancy on the Avidity of Pertussis Antibodies. Front. Immunol. 2019;10:2423. doi: 10.3389/fimmu.2019.02423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maertens K., Hoang T.H.T., Caboré R.N., Leuridan E. Avidity of Maternal Pertussis Antibodies after Vaccination during Pregnancy. Vaccine. 2015;33:5489. doi: 10.1016/j.vaccine.2015.05.075. [DOI] [PubMed] [Google Scholar]
- 106.Gill T.J., Repetti C.F., Metlay L.A., Rabin B.S., Taylor F.H., Thompson D.S., Cortese A.L. Transplacental Immunization of the Human Fetus to Tetanus by Immunization of the Mother. J. Clin. Investig. 1983;72:987–996. doi: 10.1172/JCI111071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vanderbeeken Y., Sarfati M., Bose R., Delespesse G. In Utero Immunization of the Fetus to Tetanus by Maternal Vaccination During Pregnancy. Am. J. Reprod. Immunol. Microbiol. 1985;8:39–42. doi: 10.1111/j.1600-0897.1985.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 108.Lima L., Molina M.D.G.F., Pereira B.S., Nadaf M.L.A., Nadaf M.I.V., Takano O.A., Carneiro-Sampaio M., Palmeira P. Acquisition of Specific Antibodies and Their Influence on Cell-Mediated Immune Response in Neonatal Cord Blood after Maternal Pertussis Vaccination during Pregnancy. Vaccine. 2019;37:2569–2579. doi: 10.1016/j.vaccine.2019.03.070. [DOI] [PubMed] [Google Scholar]
- 109.Rice T.F., Diavatopoulos D.A., Guo Y., Donaldson B., Bouqueau M., Bosanquet A., Barnett S., Holder B., Kampmann B. Modification of Innate Immune Responses to Bordetella Pertussis in Babies from Pertussis Vaccinated Pregnancies. EBioMedicine. 2021;72:103612. doi: 10.1016/j.ebiom.2021.103612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.May K., Grube M., Malhotra I., Long C.A., Singh S., Mandaliya K., Siegmund W., Fusch C., Schneider H., King C.L. Antibody-Dependent Transplacental Transfer of Malaria Blood-Stage Antigen Using a Human Ex Vivo Placental Perfusion Model. PLoS ONE. 2009;4:e7986. doi: 10.1371/journal.pone.0007986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ladhani S.N., Andrews N.J., Southern J., Jones C.E., Amirthalingam G., Waight P.A., England A., Matheson M., Bai X., Findlow H., et al. Antibody Responses after Primary Immunization in Infants Born to Women Receiving a Pertussis-Containing Vaccine during Pregnancy: Single Arm Observational Study with a Historical Comparator. Clin. Infect. Dis. 2015;61:1637–1644. doi: 10.1093/cid/civ695. [DOI] [PubMed] [Google Scholar]
- 112.Maertens K., Hoang T.T.H., Nguyen T.D., Caboré R.N., Duong T.H., Huygen K., Hens N., Van Damme P., Dang D.A., Leuridan E. The Effect of Maternal Pertussis Immunization on Infant Vaccine Responses to a Booster Pertussis-Containing Vaccine in Vietnam. Clin. Infect. Dis. 2016;63:S197–S204. doi: 10.1093/cid/ciw551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hoang H.T.T., Leuridan E., Maertens K., Nguyen T.D., Hens N., Vu N.H., Caboré R.N., Duong H.T., Huygen K., Van Damme P., et al. Pertussis Vaccination during Pregnancy in Vietnam: Results of a Randomized Controlled Trial Pertussis Vaccination during Pregnancy. Vaccine. 2016;34:151–159. doi: 10.1016/j.vaccine.2015.10.098. [DOI] [PubMed] [Google Scholar]
- 114.Gkentzi D., Katsakiori P., Marangos M., Hsia Y., Amirthalingam G., Heath P.T., Ladhani S. Maternal Vaccination against Pertussis: A Systematic Review of the Recent Literature. Arch. Dis. Child. Fetal Neonatal Ed. 2017;102:F456–F463. doi: 10.1136/archdischild-2016-312341. [DOI] [PubMed] [Google Scholar]
- 115.Amirthalingam G., Andrews N., Campbell H., Ribeiro S., Kara E., Donegan K., Fry N.K., Miller E., Ramsay M. Effectiveness of Maternal Pertussis Vaccination in England: An Observational Study. Lancet. 2014;384:1521–1528. doi: 10.1016/S0140-6736(14)60686-3. [DOI] [PubMed] [Google Scholar]
- 116.Zambrano L.D., Ellington S., Strid P., Galang R.R., Oduyebo T., Tong V.T., Woodworth K.R., Nahabedian J.F., Azziz-Baumgartner E., Gilboa S.M., et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status—United States, January 22–October 3, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Villar J., Ariff S., Gunier R.B., Thiruvengadam R., Rauch S., Kholin A., Roggero P., Prefumo F., Do Vale M.S., Cardona-Perez J.A., et al. Maternal and Neonatal Morbidity and Mortality among Pregnant Women with and without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021;175:817–826. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eskenazi B., Rauch S., Iurlaro E., Gunier R.B., Rego A., Gravett M.G., Cavoretto P.I., Deruelle P., García-May P.K., Mhatre M., et al. Diabetes Mellitus, Maternal Adiposity, and Insulin-Dependent Gestational Diabetes Are Associated with COVID-19 in Pregnancy: The INTERCOVID Study. Am. J. Obstet. Gynecol. 2022;227:e1–e16. doi: 10.1016/j.ajog.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Knight M., Bunch K., Vousden N., Morris E., Simpson N., Gale C., Obrien P., Quigley M., Brocklehurst P., Kurinczuk J.J. Characteristics and Outcomes of Pregnant Women Admitted to Hospital with Confirmed SARS-CoV-2 Infection in UK: National Population Based Cohort Study. BMJ. 2020;369:m2107. doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dang D., Wang L., Zhang C., Li Z., Wu H. Potential Effects of SARS-CoV-2 Infection during Pregnancy on Fetuses and Newborns Are Worthy of Attention. J. Obstet. Gynaecol. Res. 2020;46:1951–1957. doi: 10.1111/jog.14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dhir S.K., Kumar J., Meena J., Kumar P. Clinical Features and Outcome of SARS-CoV-2 Infection in Neonates: A Systematic Review. J. Trop. Pediatr. 2021;67:fmaa059. doi: 10.1093/tropej/fmaa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jamieson D.J., Rasmussen S.A. An Update on COVID-19 and Pregnancy. Am. J. Obstet. Gynecol. 2022;226:177–186. doi: 10.1016/j.ajog.2021.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Birol Ilter P., Prasad S., Mutlu M.A., Tekin A.B., O’Brien P., von Dadelszen P., Magee L.A., Tekin S., Tug N., Kalafat E., et al. Maternal and Perinatal Outcomes of SARS-CoV-2 Infection in Unvaccinated Pregnancies during Delta and Omicron Waves. Ultrasound Obstet. Gynecol. 2022;60:96–102. doi: 10.1002/uog.24916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.COVID-19 Vaccines While Pregnant or Breastfeeding. [(accessed on 8 September 2022)]; Available online: https://www.cdc.gov/coronavirus/%0A2019-ncov/vaccines/recommendations/pregnancy.%0Ahtm%0A.
- 125.Facciola A., Micali C., Visalli G., Venanzi Rullo E., Russotto Y., Lagana P., Lagana A., Nunnari G., Di Pietro A. COVID-19 and Pregnancy: Clinical Outcomes and Scientific Evidence about Vaccination. Eur. Rev. Med. Pharmacol. Sci. 2022;26:2610–2626. doi: 10.26355/eurrev_202204_28499. [DOI] [PubMed] [Google Scholar]
- 126.Sadarangani M., Soe P., Shulha H., Valiquette L., Vanderkooi O.G., Kellner J.D., Muller M.P., Top K.A., Isenor J.E., McGeer A., et al. Safety of COVID-19 Vaccines in Pregnancy: A Canadian National Vaccine Safety (CANVAS) Network Study. Lancet Infect. Dis. 2022;11:1553–1564. doi: 10.1016/S1473-3099(22)00426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ciapponi A., Bardach A., Mazzoni A., Alconada T., Anderson S.A., Argento F.J., Ballivian J., Bok K., Comandé D., Erbelding E., et al. Safety of Components and Platforms of COVID-19 Vaccines Considered for Use in Pregnancy: A Rapid Review. Vaccine. 2021;39:5891–5908. doi: 10.1016/j.vaccine.2021.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pischel L., Patel K.M., Goshua G., Omer S.B. Adenovirus-Based Vaccines and Thrombosis in Pregnancy: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2022;2483:1179–1186. doi: 10.1093/cid/ciac080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Galanis P., Vraka I., Siskou O., Konstantakopoulou O., Katsiroumpa A., Kaitelidou D. Uptake of COVID-19 Vaccines among Pregnant Women: A Systematic Review and Meta-Analysis. Vaccines. 2022;10:766. doi: 10.3390/vaccines10050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Collier A., McMahan K., Yu J., Tostanoski L., Aguayo R., Ansel J., Chandrashekar A., Patel S., Apraku Bondzie E., Sellers D., et al. Immunogenicity of COVID-19 MRNA Vaccines in Pregnant and Lactating Women. JAMA. 2021;325:2370–2380. doi: 10.1001/jama.2021.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Prabhu M., Murphy E.A., Sukhu A.C., Yee J., Singh S., Eng D., Zhao Z., Riley L.E., Yang Y.J. Antibody Response to Coronavirus Disease 2019 (COVID-19) Messenger RNA Vaccination in Pregnant Women and Transplacental Passage Into Cord Blood. Obstet. Gynecol. 2021;138:278–280. doi: 10.1097/AOG.0000000000004438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shook L.L., Atyeo C.G., Yonker L.M., Fasano A., Gray K.J., Alter G., Edlow A.G. Durability of Anti-Spike Antibodies in Infants After Maternal COVID-19 Vaccination or Natural Infection. JAMA. 2022;327:1087–1089. doi: 10.1001/jama.2022.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Laguila Altoé A., Marques Mambriz A.P., Cardozo D.M., Valentini Zacarias J.M., Laguila Visentainer J.E., Bahls-Pinto L.D. Vaccine Protection Through Placenta and Breastfeeding: The Unmet Topic in COVID-19 Pandemic. Front. Immunol. 2022;13:910138. doi: 10.3389/fimmu.2022.910138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., Frankland T.B., Ogun O.A., Zamparo J.M., Gray S., et al. Effectiveness of MRNA BNT162b2 COVID-19 Vaccine up to 6 Months in a Large Integrated Health System in the USA: A Retrospective Cohort Study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thomas S.J., Moreira E.D., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Polack F.P., Zerbini C., et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kalafat E., Magee L.A., von Dadelszen P., Heath P., Khalil A. COVID-19 Booster Doses in Pregnancy and Global Vaccine Equity. Lancet. 2022;399:907–908. doi: 10.1016/S0140-6736(22)00166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang Y.J., Murphy E.A., Singh S., Sukhu A.C., Wolfe I., Adurty S., Eng D., Yee J., Mohammed I., Zhao Z., et al. Association of Gestational Age at Coronavirus Disease 2019 (COVID-19) Vaccination, History of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection, and a Vaccine Booster Dose with Maternal and Umbilical Cord Antibody Levels at Delivery. Obstet. Gynecol. 2022;139:373–380. doi: 10.1097/AOG.0000000000004693. [DOI] [PubMed] [Google Scholar]
- 138.Liu S., Zhong J., Zhang D. Transplacental Transfer of Maternal Antibody against SARS-CoV-2 and Its Influencing Factors: A Review. Vaccines. 2022;10:1083. doi: 10.3390/vaccines10071083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Carlsen E.Ø., Magnus M.C., Oakley L., Fell D.B., Greve-Isdahl M., Kinge J.M., Håberg S.E. Association of COVID-19 Vaccination During Pregnancy with Incidence of SARS-CoV-2 Infection in Infants. JAMA Intern. Med. 2022;182:825–831. doi: 10.1001/jamainternmed.2022.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Halasa N.B., Olson S.M., Staat M.A., Newhams M.M., Price A.M., Pannaraj P.S., Boom J.A., Sahni L.C., Chiotos K., Cameron M.A., et al. Maternal Vaccination and Risk of Hospitalization for Covid-19 among Infants. N. Engl. J. Med. 2022;387:109–119. doi: 10.1056/NEJMoa2204399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Madhi S.A., Cutland C.L., Kuwanda L., Weinberg A., Hugo A., Jones S., Adrian P.V., van Niekerk N., Treurnicht F., Ortiz J.R., et al. Influenza Vaccination of Pregnant Women and Protection of Their Infants. N. Engl. J. Med. 2014;371:918–931. doi: 10.1056/NEJMoa1401480. [DOI] [PubMed] [Google Scholar]
- 142.Castilow E.M., Varga S.M. Overcoming T-Cell-Mediated Immunopathology to Achieve Safe Respiratory Syncytial Virus Vaccination. Future Virol. 2008;3:445–454. doi: 10.2217/17460794.3.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shi T., McAllister D.A., O’Brien K.L., Simoes E.A.F., Madhi S.A., Gessner B.D., Polack F.P., Balsells E., Acacio S., Aguayo C., et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children in 2015: A Systematic Review and Modelling Study. Lancet. 2017;390:946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brady M.T., Byington C.L., Davies H.D., Edwards K.M., Jackson M.A., Maldonado Y.A., Murray D.L., Orenstein W.A., Rathore M.H., Sawyer M.H., et al. Updated Guidance for Palivizumab Prophylaxis among Infants and Young Children at Increased Risk of Hospitalization for Respiratory Syncytial Virus Infection. Pediatrics. 2014;134:e620–e638. doi: 10.1542/peds.2014-1666. [DOI] [PubMed] [Google Scholar]
- 145.Esposito S., Abu Raya B., Baraldi E., Flanagan K., Martinon Torres F., Tsolia M., Zielen S. RSV Prevention in All Infants: Which Is the Most Preferable Strategy? Front. Immunol. 2022;13:880368. doi: 10.3389/fimmu.2022.880368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Eichinger K.M., Kosanovich J.L., Lipp M., Empey K.M., Petrovsky N. Strategies for Active and Passive Pediatric RSV Immunization. Ther. Adv. Vaccines Immunother. 2021;9:2515135520981516. doi: 10.1177/2515135520981516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Simões E.A.F., Center K.J., Tita A.T.N., Swanson K.A., Radley D., Houghton J., McGrory S.B., Gomme E., Anderson M., Roberts J.P., et al. Prefusion F Protein–Based Respiratory Syncytial Virus Immunization in Pregnancy. N. Engl. J. Med. 2022;386:1615–1626. doi: 10.1056/NEJMoa2106062. [DOI] [PubMed] [Google Scholar]
- 148.Tseha S.T. Polio: The Disease That Reemerged after Six Years in Ethiopia. Ethiop. J. Health Sci. 2020;31:897–902. doi: 10.4314/ejhs.v31i4.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Feldman A.G., O’Leary S.T., Danzifer Isakov L. The Risk of Resurgence in Vaccine Preventable Infections Due to COVID-Related Gaps in Immunization. Clin. Infect. Dis. 2021;76:1920–1923. doi: 10.1093/cid/ciab127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hübschen J.M., Gouandjika-vasilache I., Dina J. Measles. Lancet. 2022;399:678–690. doi: 10.1016/S0140-6736(21)02004-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As this is a review article, no data were collected from patients.