Abstract
Acanthamoeba is a ubiquitous free-living amoeba capable of being an opportunistic pathogen in humans and animals. A critical step in infection is the adhesion of the amoeba to host cells and tissues, and two major parasite adhesins, mannose-binding protein (MBP) and laminin-binding protein (LBP), are known to recognize the cell surface glycoproteins and those of the extracellular matrix, respectively. In this study, the available genomes of Acanthamoeba were analysed to recover the sequences of MBP and LBP using previously published genetic data. Genes for both proteins were successfully obtained from strains belonging to various genotypes (T4A, T4D, T4G, T4F, T2, T5, T10, T22, T7 and T18), resulting in a single gene for LBP but identifying two types of MBP, MBP1 and MBP2. Phylogenetic analysis based on deduced amino acid sequences shows that both MBP and LBP have a branching pattern that is consistent with that based on 18S rDNA, indicating that changes in both proteins occurred during diversification of Acanthamoeba lines. Notably, all MBPs possess a conserved motif, shared with some bacterial C-type lectins, which could be the recognition site for mannose binding.
Keywords: Acanthamoeba, mannose-binding protein, laminin-binding protein, variability, phylogenesis
1. Introduction
Free-living amoebae of the genus Acanthamoeba (Amoebozoa, Discosea, Centramoebida) are ubiquitous worldwide in any type of natural or man-made environment. The active stage, the trophozoite, feeds on bacteria and other microbial organisms, while the dormant cyst is highly resistant to many physical and chemical stresses. Acanthamoeba can, however, occasionally infect humans and other animals, causing two main diseases [1]: an often-fatal granulomatous amoebic encephalitis (GAE) which follows invasion of the central nervous system [2], and a sight-threatening amoebic keratitis (AK) due to infection of the surface of the eye [3]. Once inside the host, the amoebae adhere to its cells and trigger a series of cascading reactions involving both the release of parasite proteases and the activation of host cell factors, leading to the destruction of epithelium and extracellular matrix and the progression of invasion [4,5].
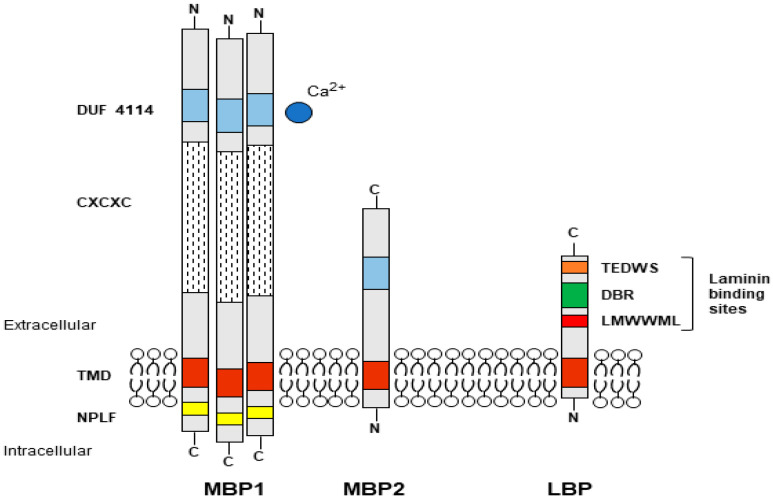
Adhesion is, therefore, a critical step in infection, and one of the main Acanthamoeba adhesins identified is the mannose-binding protein (MBP1), a lectin-like glycoprotein located on the surface of trophozoites, which recognizes mannose residues of glycoproteins of host cells [6,7,8]. MBP1 is a protein of approximately 400 kDa composed of several 130 kDa subunits. It has a long extracellular N-terminal part, a transmembrane domain and a short C-terminal part containing the NPLF motif known to participate in intracellular signalling events. The mannose-specific recognition domain is expected to be located in the extracellular part, but it has not yet been identified [7,9]. Another important Acanthamoeba adhesin is the laminin-binding protein (LBP), which allows further progression of infected tissues [10,11], as laminin is a major glycoprotein of the extracellular matrix separating epithelia from other tissues. Acanthamoeba LBP belongs to the family of non-integrin 37/67-kDa laminin receptors (37/67LR), also involved as receptors for viruses and other pathogens as well as in other cellular processes such as motility and differentiation [12]. LBP homologs are present in all organisms including prokaryotes as this adhesin derives from a 40S ribosomal protein which acquired the ability to bind laminin with evolution [13]. Overall, LBPs have a short transmembrane domain at the N-terminal, and three recognition domains for laminin on the extracellular C-terminal domain, comprising a palindromic LMWWML motif located in the peptide G [14], a direct binding region (DBR), and TEDWS motif repeats (Figure 1).
Figure 1.
Schematic drawings of Acanthamoeba adhesins. Mannose binding protein 1 (MBP1) and 2 (MBP2); Laminin binding protein (LBP). The putative site for mannose binding in both MBP1 and MBP2 should be in the DUF 4114 region and involve a divalent cation. For LBP, the laminin binding sites correspond to the palindromic motif, the direct binding region (DBR), and the TEDWS motif. See text for more details and explanation.
The sequences of the two adhesins were obtained by cDNA cloning, from a strain attributed to A. castellanii for MBP1 [7], and from A. healyi for LBP [10], two very distant species, belonging to different morphological groups and genetic lines. Indeed, according to the cyst shapes and the variations in the nuclear small subunit (SSU) of the ribosomal RNA gene (18S rDNA), A. castellanii is placed in group 2, genotype T4, while A. healyi is in group 3, genotype T12 [15,16,17,18]. Biomolecular studies based on the 18S rDNA revealed high diversity within Acanthamoeba, currently comprising at least 23 genotypes, T1-T23 [18,19,20], which partly correspond to the traditional species [21]. In this study the available data are analysed in order to assess if and how MBP and LBP are present and vary in the different lines of Acanthamoeba.
2. Materials and Methods
Acanthamoeba genomes available on the NCBI portal were analysed by BLAST using as query sequences the complete MBP1 gene from the AK strain MEEI 0184 (GenBank ID AY604039) [7] and the complete LBP gene from the environmental strain OC-3A (ATCC 30866) of A. healyi (GenBank ID AY351649) [10]. The genomic regions obtained were analysed with Genscan [22] and Augustus [23] to generate coding fragments (exons), and further verified and if necessary corrected, using as guides the known amino acid sequences of MBP1 (GenBank ID AAT37864) and LBP (GenBank ID AAQ63482).
Amino acid sequences were separately aligned with COBALT [24] and phylogenetic trees (1000 bootstraps) were inferred with protein maximum likelihood (ML, JTT + G:4 model) using TREEFINDER [25], and distance (Minimum Evolution, ME) and Maximum Parsimony (MP) using MEGA7 [26]. As an outgroup, the sequences hereinafter referred to as MBP2 (see below) were used for MBP, whereas for LBP, the trees were rooted on the sequence of Balamuthia mandrillaris, recovered from its genome as described above. Similarity and identity values were estimated with MatGat [27] using Blocks Substitution Matrix 50 (BLOSUM50). Molecular weights were predicted by using BioEdit [28], and putative N-glycosylation sites were identified by using NetNGlyc 1.0 [29].
A reference 18S rDNA tree Iding the sequences of the studied strains and representatives of all genotypes was constructed with ML (GTR G + I:4; 1000 bootstraps) after MAFFT alignment and manual editing, as previously described [19,30].
3. Results
3.1. 18S rDNA Phylogeny
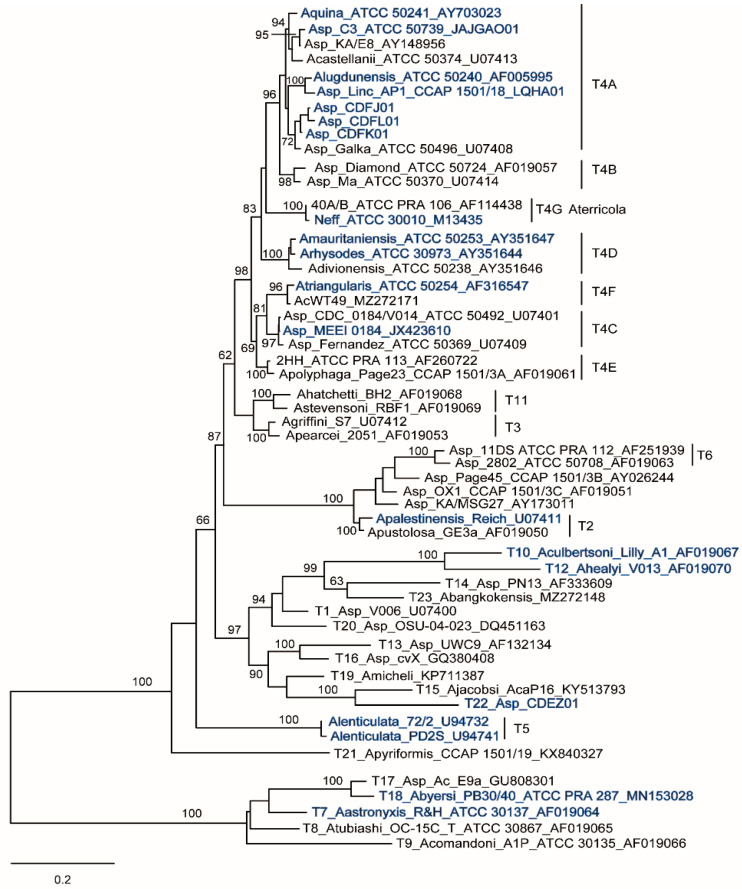
An 18S tree was constructed including members of all Acanthamoeba genotypes as well as the strains analysed here for the genes of the two adhesins (Figure 2).
Figure 2.
The 18S rDNA phylogeny. Maximum likelihood tree (1000 bootstraps) based on complete 18S rDNA sequences including representatives of all Acanthamoeba genotypes, rooted on members of morphological group 1. Strains analysed herein are highlighted in blue.
As expected, the resulting tree is consistent with results obtained in previous studies including a larger number of strains and based on both nuclear and mitochondrial SSU rDNA [21] as well as internal transcribed spacer (ITS) region and the large subunit (LSU) of the nuclear rDNA operon [31]. The analysis places the MEEI 0184 strain in the T4C lineage, which includes various isolates from around the world implicated in AK, probably forming one or more species, but distinct from A. castellanii (T4A) and closer to A. triangularis (T4F) [21]. For A. lenticulata (T5), 18S sequences from only two strains were used here since in the third strain, PT14, the likely presence of a group 1 intron prevented complete sequencing of the gene [31]. It should be noted that for A. healyi (T12), the 18S rDNA was from the strain type CDC:1283:V013 (=V013) [17], isolated from a non-fatal case of brain granuloma, while LBP was from the freshwater strain OC-3A [32], deposited in the American Type Culture Collection (ATCC 30866). There is often confusion between these two strains of A. healyi, mistakenly assuming that ATCC 30866 is from GAE. Studies including this species but using the ATCC strain have actually analysed the freshwater strain OC-3A. Nuclear and mitochondrial SSU rDNA sequences are available only for V013 [18,33], and their deduced restriction profiles are different, although very similar from those reported for OC-3A [34,35].
3.2. Recovery of Mannose and Laminin Binding Protein Genes: General Features
The genes for MBP1 from strain MEEI (T4C) and LBP from A. healyi OC-3A (T12) were used to search for respective orthologs in publicly available genomes. The LBP gene was successfully recovered from all analysed genomes including that of sister species Balamuthia mandrillaris, while the MBP1 gene appears to be missing not only in Balamuthia, but also in group 1 species, A. astronyxis (T7) and A. byersi (T18). The two genes both differ by their length and their structure (number of exon/intron) giving proteins of different sizes depending on the genotype, and all genes have U2-type GT/AG spliceosomal introns, 1 to 3 for LBP, and 3 to 8 for MBP1, except for the LBP gene of A. healyi which codes for a single transcript. Despite these variations, the deduced amino acids give for the two proteins well-conserved overall structures congruent with the literature data (Table 1) (Table S1).
Table 1.
Summary of Acanthamoeba binding proteins data.
| GT | Species | Strain | Sequence Source 1 | Mannose Binding Protein | Laminin Binding Protein | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Protein | Gene | Protein | ||||||||
| nt 2 | exons | aa | kDa 3 | nt 2 | exons | aa | kDa 3 | ||||
| T4A | A. quina | Vil3 | CDFN01 | 3104 | 6 | 833 | 85.2 | 1084 | 3 | 265 | 29.3 |
| T4A | Acanthamoeba sp. | undet. | CDFL01 | 3106 | 6 | 833 | 85.3 | 1090 | 3 | 266 | 29.3 |
| T4A | Acanthamoeba sp. | undet. | CDFJ01 | 3105 | 6 | 833 | 85.4 | 1090 | 3 | 266 | 29.3 |
| T4A | Acanthamoeba sp. | undet. | CDFK01 | 3120 | 6 | 833 | 85.5 | 1092 | 3 | 248 | 27.2 |
| T4A | A. lugdunensis | L3a | CDFB01 | 3112 | 6 | 833 | 85.1 | 1085 | 3 | 266 | 29.3 |
| T4A | Acanthamoeba sp. | C3 | JAJGAO01 | 3151 | 6 | 833 | 85.2 | 1082 | 3 | 266 | 29.4 |
| T4A | Acanthamoeba sp. | Linc-AP1 | LQHA01 | 3118 | 6 | 833 | 85.0 | 1085 | 3 | 266 | 29.3 |
| T4C | Acanthamoeba sp. | MEEI 0184 | AY604039 1 | 3156 | 6 | 833 | 85.2 | not available | |||
| T4D | A. rhysodes | Singh | CDFC01 | 3168 | 6 | 833 | 85.1 | 1066 | 3 | 266 | 29.3 |
| T4D | A. mauritaniensis | 1652 | CDFE01 | 3218 | 6 | 834 | 84.9 | 1077 | 3 | 266 | 29.3 |
| T4G | A. terricola | Neff | JAJGAP01 | 3159 | 6 | 834 | 85.1 | 1082 | 3 | 264 | 29.1 |
| T4G | A. terricola | Neff | AEYA01 | 3159 | 6 | 834 | 85.1 | 1082 | 3 | 264 | 29.1 |
| T4G | A. terricola | Neff | AHJI01 | no results 4 | 1082 | 3 | 264 | 29.1 | |||
| T4F | A. triangularis | SH621 | CDFD01 | 3507 | 6 | 928 | 95.0 | 1063 | 3 | 265 | 29.3 |
| T2 | A. palestinensis | Reich | CDFA01 | 3365 | 7 | 844 | 87.0 | 1043 | 2 | 265 | 29.4 |
| T10 | A. culbertsoni | Lilly A1 | CDFF01 | 2520 | 5 | 716 | 74.5 | 1100 | 3 | 260 | 28.8 |
| T12 | A. healyi | OC-3A | AY351649 1 | not available | 771 | 1 | 252 | 28.3 | |||
| T22 | Acanthamoeba sp. | undet. | CDEZ01 | 3440 | 9 | 747 | 76.4 | 967 | 2 | 260 | 29.5 |
| T5 | A. lenticulata | PD2S | CDFG01 | 3138 | 4 | 956 | 97.7 | 967 | 2 | 260 | 28.9 |
| T5 | A. lenticulata | 72/2 | MSTW01 | 3152 | 4 | 956 | 97.8 | 967 | 2 | 260 | 28.9 |
| T5 | A. lenticulata | PT14 | NAVB01 | 3150 | 4 | 956 | 97.8 | 967 | 2 | 260 | 28.9 |
| T7 | A. astronyxis | undet. | CDFI01 | no results 4 | 1071 | 4 | 233 | 25.9 | |||
| T7 | A. astronyxis | R&H | CDFH01 | no results 4 | 1071 | 4 | 233 | 25.9 | |||
| T18 | A. byersi | Pb30/40 | MRZZ01 | no results 4 | 1072 | 4 | 232 | 25.9 | |||
| Balamuthia mandrillaris | 2046 | LEOU01 | no results | 871 | 2 | 264 | 28.8 | ||||
1 Sequences from T4C and T12 are the original query sequences for MBP1 and LBP; 2 Length spanning start/stop codons; 3 Predicted molecular weight of non-glycosylated mature protein; 4 Analysis recovered MBP2.
All LBPs present the recognition sites for laminin, including an LMFWLL motif or more rarely LLYWLL (Group 1 species and Balamuthia) corresponding to the palindromic LMWWML motif of the peptide G, but a single complete TEDWS element (as TEEWG), and all have at the N-terminus a short sequence of hydrophobic residues corresponding to the transmembrane domain. Notably, as expected, an identical gene for LBP, consisting of three exons and two introns, is present in all three available genomes of A. terricola (strain Neff, T4G), but it is annotated as a pseudogene in the AHJI01 genome (ACA1_385450) because the introns are not recognized. Furthermore, the multiple alignment of LBPs suggests that the C-terminus of the original sequence of A. healyi might be incomplete, and the first four amino acids at the N-terminus might not actually be part of the protein (Figure S1). LBP sequences are highly conserved, with identity/similarity values >80/90% for those of group 2 and 3 species, and around 60/70% between these and those of group 1 species (Table S2).
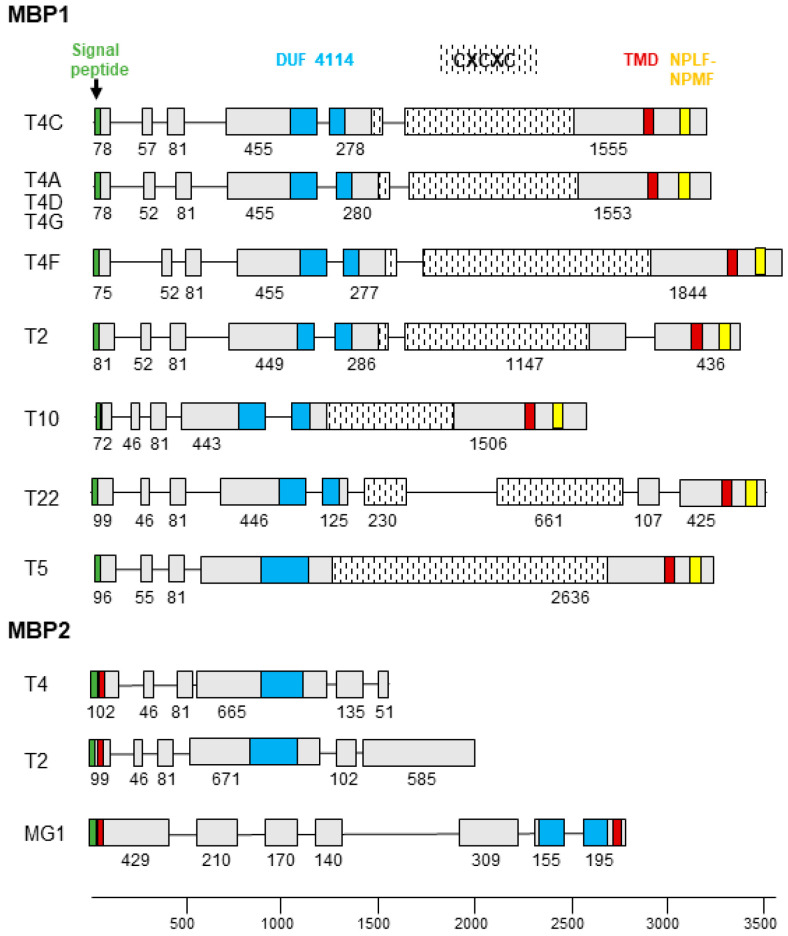
MBP1 is a conventional membrane protein with a signal peptide at the N-terminus and a transmembrane domain located at the C-terminus. The extracellular portion contains a Cys-rich repetitive motif (CXCXC) and a domain of unknown function (DUF 4114), while two NPLF motifs involved in intracellular signalling are located in the intracytoplasmic region [7,9]. MBP1 appears to be specific only to Acanthamoeba species of groups 2 and 3, with different gene structure and amino acid sequence depending on the genotype (Table 1) (Figure S2), while shorter MBP-like sequences could be identified in the group 1 species (A. astronyxis T7 and A. byersi T18), as well as in T4 and T2 genotypes. The resulting protein, labelled MBP2, covers the N-terminal part containing DUF 4114 but lacks the Cys-rich repetitive elements (usually only a single CXCXC motif is present), as well as the intracytoplasmic domain. MBP2 has a signal peptide at the N-terminal followed by a transmembrane motif, although a second short transmembrane motif is predicted at the C-terminus for group 1 species (Figure 3).
Figure 3.
MBP gene structure. Schematic drawing of the exon/intron structure of the MBP1 and MBP2 genes of Acanthamoeba. Exons are shown as boxes with the number of nucleotides below, introns as a line. The signal peptide, DUF 4114, CXCXC repeat coding region, transmembrane domain (TMD), and cell signalling NPLF motif are colour coded and shown at the top.
It is this short MBP2 which is present in the AHJI01 genome of Neff strain and incorrectly annotated MBP (ACA1_248600; L8GXW7), whereas the true MBP1 is missing, although it is present in the two other Neff genomes. MBP1 sequences from different genotypes are variable, with identity/similarity values <60/75% (Table S3). Moreover, values between MBP1 and MBP2 are even lower (between 25% and 35%), the most conserved part being the DUF 4114 domain (approximately 65% of identical sites).
3.3. Molecular Phylogeny of Binding Proteins
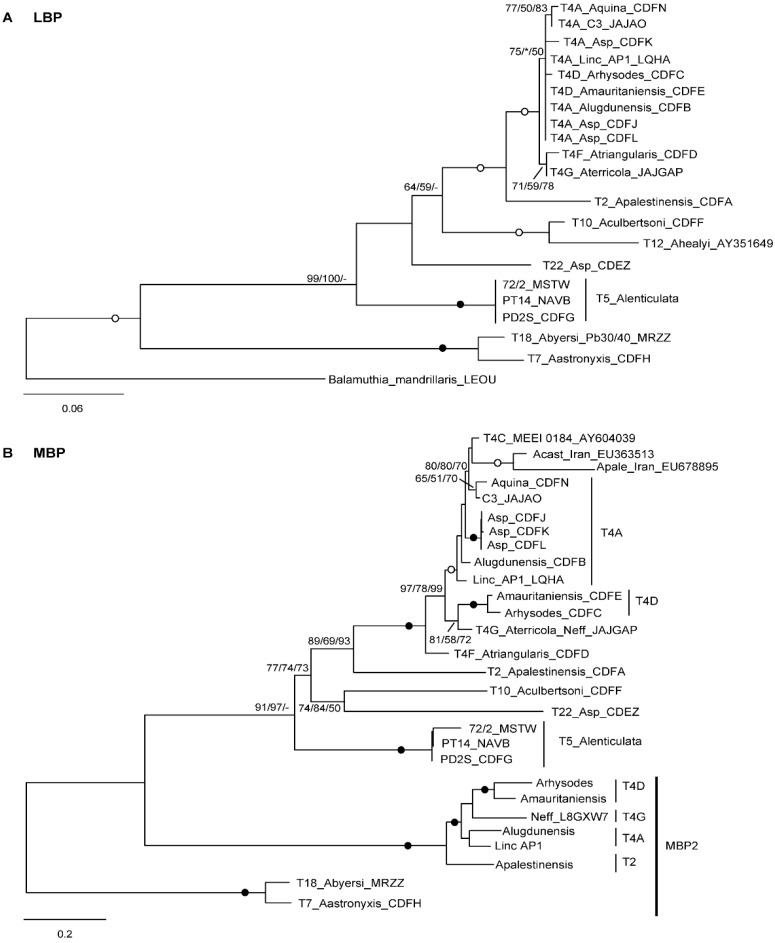
To assess the evolutionary relationships of LBP and MBP from the different Acanthamoeba strains and genotypes, phylogenetic trees were constructed separately for the two proteins from the inferred amino acid sequences (Figure 4).
Figure 4.
Molecular phylogeny of laminin (A) and mannose (B) binding proteins. Trees based on amino acid sequences, rooted on the sequence of Balamuthia mandrillaris for LBP (A), and on MBP2 for MBP (B). At the nodes, bootstrap values (1000 replicates) for ML/ME/MP are shown, with filled and open circles for values 100 or >95% with all methods. *, node recovered but support <50%; -, node not recovered.
It is noteworthy that the resulting tree topologies almost perfectly mirrors that obtained using the 18S rDNA (Figure 2), with several nodes strongly supported, indicating that the divergences within LBP and MBP occurred by following the diversification of Acanthamoeba lineages (Figure 4). This scenario is clearly evident for LBPs, where homologous sequences are available not only for group 1 species (A. astronyxis T7 and A. byersi T18), but also for Balamuthia which can serve as an outgroup (Figure 4A). On the other hand, the picture for MBP is more complicated by the presence of two types of proteins, MBP1 and MBP2, and the absence of a reliable outgroup. However, MBP1 and MBP2 could be homologous, as they show notable sequence conservation in the shared part (see below, Figure 5), and A. lenticulata MBP1 clusters with MBP2 in the maximum parsimony tree, albeit with weak support. In any case, while the putative MBP found in group 1 species appears to be the least derived type, MBP1 exhibits a branching pattern consistent with that produced by the 18S rDNA for group 2 and 3 species, spanning down to the T4 subtypes. The same relationships are also recovered for MBP2 from T2 and T4 (Figure 4B).
In the present analysis, MBP1 from strain MEEI (T4C) clusters tightly with MBP1 detected from strain ATCC 30234 which is derived from the strain type of A. castellanii ATCC 30011 (T4A). Surprisingly, the MBP1 of A. castellanii (Q6J288) is identical to that of MEEI (AAT37864), and the two sequences are even treated as equivalent, i.e., MBP1 of A. castellanii, in protein databases. MBP1, as well as MBP2, have been identified in A. castellanii ATCC 30234 (T4A) by mass spectrometry [36]. This allowed them to be recognized as such for their overall similarity with MBP1 from MEEI (T4C) and MBP2 (L8GXW7; ACA1_248600) from A. terricola (T4G), respectively, but it is unlikely that the sequences of A. castellanii could be sequentially identical to the two original proteins. They were therefore excluded from the final analysis.
Another puzzling feature is the branching with MEEI of two partial MBP1 sequences reported as obtained from isolates of A. castellanii and A. palestinensis (Figure 4B), presumably of genotype T4 and T2, respectively [37,38]. This grouping could be an artefact due to the incompleteness of these sequences, also explaining the relatively long branch. Nevertheless, it is unlikely that either of these sequences could have originated from A. palestinensis, and the MBP1 primers developed by the authors have exact matches only in a subset of the T4 strains.
4. Discussion
The analysis presented herein reveals that two of the main adhesins of Acanthamoeba, MBP and LBP, are constitutive of the amoeba genome, their genes being also present in non-pathogenic strains/species such as A. terricola Neff (T4G) (Table 1). The variations observed within the two adhesins, in terms of gene structure and amino acid sequence, appear specific to each Acanthamoeba line, reflecting the evolutionary history of the species (Figure 4). This seems congruent when we consider that Acanthamoeba is, above all, a free-living amoeba grazing on bacteria, algae, yeasts and small protozoa, and that the different adhesins present on the trophozoite serve primarily to recognize the surface glycoproteins of the prey that will be phagocytized [36,39,40].
High Acanthamoeba antibody seroprevalence, even in apparently healthy subjects, suggests wide exposure to the amoeba [41], favoured by its environmental ubiquity. However, not all Acanthamoeba strains are capable of infecting and causing disease in humans and other animals. Pathogenic strains are likely those able to adapt to vertebrate tissue which is ultimately an accidental environment, requiring probably various quantitative and qualitative factors, such as the increased expression of adhesins with good affinity for animal tissue glycoproteins, the overproduction of several types of enzymes, or different sensitivities to tissue temperature or osmotic pressure. The pathogenic potential of a strain is in fact often evaluated by plating tests for thermo-tolerance and osmo-tolerance [42,43,44,45], as well as its ability to induce a cytopathic effect (CPE) on cell monolayers [43,45,46], i.e., the successful adhesion and secretion of cytolytic enzymes.
Expression levels of both MBP1 and LBP vary between Acanthamoeba strains and correlate with pathogenicity [47,48], as does the diversity of proteases produced. Serine proteases, in particular, have been identified playing a critical role in pathogenicity, such as a 133 kDa mannose-induced protein (MIP-133) which has a cytopathic effect on host cells, and other proteins of different molecular sizes, which attack various components of the extracellular matrix. These proteases are poorly or not expressed in non-pathogenic strains/species [49,50,51,52,53,54].
Group 1 species are of particular interest because they are generally considered non-pathogenic, and therefore used as a negative control. LBP and MBP were not detected [11,55], despite the presence of the corresponding genes, and the inability to induce CPE appears to be due to lack of MIP-133 [50]. However, some data indicate that group 1 species are also potentially pathogenic [56], and furthermore, the evolutionary distance that separates them from other Acanthamoeba suggests that some difference might be expected. For example, it is possible that group 1 species use other adhesins to capture their prey since a putative MBP2 and not a true MBP1 could be detected in the analysed genomes. In addition, the total N-glycosylation pattern differs from that of group 2 and 3 species [57], implying recognition changes at the molecular level.
Recently, molecular mimicry with Acanthamoeba MBP, possibly resulting from convergent evolution, has been reported for mammalian macrophage receptors involved in the antifungal immune response, which recognize mannosylated cell wall proteins of various fungi [58]. Sequence analysis performed herein using more MBP1 and MBP2 sequences confirms affinity with C-type lectin domains, although stronger identity was found with mannose-binding lectins from the opportunistic pathogen Burkholderia cenocepacia (Proteobacteria). Notably, the ED(xx)GxDxDYND motif of bacterial lectins involved in calcium and mannose binding in dimeric/trimeric organisation [59,60] is present in the DUF 4114 domain of all Acanthamoeba MBP1 and MBP2 (Figure 5).
Figure 5.
Multiple alignment of Acanthamoeba MBP1 and MBP2 with other C-type lectins. Only part of the alignment is shown here, focusing on the DUF 4114 domain, marked with a light blue rectangle in the consensus sequence. The following sequences were used: Concanavalin A (ConA; PDB: 1JBC_A), Dioclea lasiophylla lectin (DlyL; Uniprot C0HK27; PDB: 6CJ9_A), BclA (PDB: 2WR9_A) and BclC (PDB: 2XR4_A) lectins from Burkholderia cenocepacia, and macrophage receptors Mrc1 (Q61830) and Mrc2 (Q64449). The Bcl ED(xx)GxDxDYND motif, highlighted by a red rectangle, is well conserved in all Acanthamoeba MBPs and includes the binding sites for calcium (blue circle) and mannose (hexagon) as shown on consensus sequence histogram. Note that MBP2, except those of group 1 (T7, T18), have a single CXCXC motif corresponding to the first repeat in MBP1 (grey rectangle).
This suggests that the DUF 4114 domain could participate in the formation of the mannose binding site, possibly by including a divalent cation, in a multimeric complex, which is congruent with a 400 kDa MBP1 composed of three 130 kDa subunits.
The enzymatic treatment of MBP1 from the MEEI strain (T4C) made it possible to estimate that approximately 15% of its apparent mass is due to N-linked oligosaccharides. The de-glycosylated protein shifts from 130 to 110 kDa, the difference with the predicted mass of 85 kDa being probably due to abnormal electrophoretic mobility induced by folding modifications [8]. Interestingly, the 83 kDa MBP1 from A. culbertsoni (T10) reported by Kang et al. [61] is congruent not with the predicted molecular mass of the MEEI gene as stated by the authors, but with the fact that the sequence retrieved herein produces a mature protein with a predicted mass of 74.5 kDa having only two N-glycosylation sites, compared with six in that of MEEI. Accordingly, the MBP1 from A. lenticulata (T5) is expected to have a larger molecular mass given the predicted size of 97.8 kDa (Table 1) and ten N-glycosylation sites.
For LBP, the general structure and recognition sites are easily deducible from the conservation of the molecule in all eukaryotes; however, this is not the case for MBP which appears specific to Acanthamoeba. It seems that Acanthamoeba has indeed developed a new type of lectin to bind the mannose of the surface glycoproteins of the different hunted preys, consisting of the N-terminal region comprising DUF 4114 shared by MBP1 and MBP2, which likely belong to the same protein class. The mannose recognition site would be located in DUF 4114 in all MBPs. The fact that only simpler MBPs seem to be present in group 1 species suggests that the C-terminal part of MBP1, including in particular the CXCXC repeating units, developed in group 2 and 3 species to form a more complex and perhaps more efficient adhesin. The persistence of MBP2 at least in the T4 and T2 strains, and possibly of similar molecules in other genotypes, would indicate the presence of a diversified arsenal of adhesins that may interact with different target glycoproteins.
The presence of genes for two of the main Acanthamoeba adhesins, MBP and LBP, in a subset of distinct genotypes suggests their ubiquity within the genus. It is likely that both MBP and LBP serve primarily as ligands to catch microbial prey in the environment and only incidentally as virulence factors by recognizing glycoproteins from animal tissues. The observed variations are largely consistent with the branching of 18S lineages, indicating that the changes within the two adhesins are mainly due to speciation within Acanthamoeba. It is obviously essential to insist on the importance of properly recognizing the different lineages of Acanthamoeba, in order to better appreciate these differences and also to explain certain previous conflicting results, while avoiding persistent confusion on misattributed strains.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10112162/s1, Figure S1: Multiple alignment of full-length laminin-binding proteins (LBPs) from Acanthamoeba and Balamuthia; Figure S2: Multiple alignment of Acanthamoeba full-length mannose-binding proteins (MBP1); Table S1: List of Acanthamoeba binding proteins accession numbers; Table S2: Identity and similarity percentage values for LBP; Table S3: Identity and similarity percentage values for MBP.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Supplementary Material.
Conflicts of Interest
The author declares no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marciano-Cabral F., Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 2003;16:273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalra S.K., Sharma P., Shyam K., Tejan N., Ghoshal U. Acanthamoeba and its pathogenic role in granulomatous amebic encephalitis. Exp. Parasitol. 2020;208:107788. doi: 10.1016/j.exppara.2019.107788. [DOI] [PubMed] [Google Scholar]
- 3.Niederkorn J.Y. The biology of Acanthamoeba keratitis. Exp. Eye Res. 2021;202:108365. doi: 10.1016/j.exer.2020.108365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke D.W., Niederkorn J.Y. The pathophysiology of Acanthamoeba keratitis. Trends Parasitol. 2006;22:175–180. doi: 10.1016/j.pt.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Siddiqui R., Emes R., Elsheikha H., Khan N.A. Area 51: How do Acanthamoeba invade the central nervous system? Trends Parasitol. 2011;27:185–189. doi: 10.1016/j.pt.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Yang Z., Cao Z., Panjwani N. Pathogenesis of Acanthamoeba keratitis: Carbohydrate-mediated host-parasite interactions. Infect. Immun. 1997;65:439–445. doi: 10.1128/iai.65.2.439-445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garate M., Cao Z., Bateman E., Panjwani N. Cloning and characterization of a novel mannose-binding protein of Acanthamoeba. J. Biol. Chem. 2004;279:29849–29856. doi: 10.1074/jbc.M402334200. [DOI] [PubMed] [Google Scholar]
- 8.Garate M., Cubillos I., Marchant J., Panjwani N. Biochemical characterization and functional studies of Acanthamoeba mannose-binding protein. Infect. Immun. 2005;73:5775–5781. doi: 10.1128/IAI.73.9.5775-5781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panjwani N. Pathogenesis of Acanthamoeba keratitis. Ocul. Surf. 2010;8:70–79. doi: 10.1016/S1542-0124(12)70071-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong Y.-C., Lee W.-M., Kong H.-H., Jeong H.-J., Chung D.-I. Molecular cloning and characterization of a cDNA encoding a laminin-binding protein (AhLBP) from Acanthamoeba healyi. Exp. Parasitol. 2004;106:95–102. doi: 10.1016/j.exppara.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Rocha-Azevedo B.D., Jamerson M., Cabral G.A., Silva-Filho F.C., Marciano-Cabral F. Acanthamoeba interaction with extracellular matrix glycoproteins: Biological and biochemical characterization and role in cytotoxicity and invasiveness. J. Eukaryot. Microbiol. 2009;56:270–278. doi: 10.1111/j.1550-7408.2009.00399.x. [DOI] [PubMed] [Google Scholar]
- 12.DiGiacomo V., Meruelo D. Looking into laminin receptor: Critical discussion regarding the non-integrin 37/67-kDa laminin receptor/RPSA protein. Biol. Rev. 2016;91:288–310. doi: 10.1111/brv.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ardini E., Pesole G., Tagliabue E., Magnifico A., Castronovo V., Sobel M.E., Colnaghi M.I., Ménard S. The 67-kDa laminin receptor originated from a ribosomal protein that acquired a dual function during evolution. Mol. Biol. Evol. 1998;15:1017–1025. doi: 10.1093/oxfordjournals.molbev.a026000. [DOI] [PubMed] [Google Scholar]
- 14.Castronovo V., Taraboletti G., Sobel M.E. Functional domains of the 67-kDa laminin receptor precursor. J. Biol. Chem. 1991;266:20440–20446. doi: 10.1016/S0021-9258(18)54943-7. [DOI] [PubMed] [Google Scholar]
- 15.Pussard M., Pons R. Morphologie de la paroi kystique et taxonomie du genre Acanthamoeba (Protozoa, Amoebida) Protistologica. 1977;13:557–598. [Google Scholar]
- 16.Page F.C. A New Key to Freshwater and Soil Gymnamoebae. Freshwater Biological Association; Ambleside, UK: 1988. pp. 92–97. [Google Scholar]
- 17.Moura H., Wallace S., Visvesvara G.S. Acanthamoeba healyi n. sp. and the isozyme and immunoblot profiles of Acanthamoeba spp. Groups 1 and 3. J. Protozool. 1992;39:573–583. doi: 10.1111/j.1550-7408.1992.tb04853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stothard D.R., Schroeder-Diedrich J.M., Awwad M.H., Gast R.J., Ledee D.R., Rodriguez-Zaragoza S., Dean C.L., Fuerst P.A., Byers T.J. The evolutionary history of the genus Acanthamoeba and the identification of eight new 18S rRNA gene sequence types. J. Eukaryot. Microbiol. 1998;45:45–54. doi: 10.1111/j.1550-7408.1998.tb05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corsaro D., Walochnik J., Köhsler M., Rott M.B. Acanthamoeba misidentification and multiple labels: Redefining genotypes T16, T19 and T20, and proposal for Acanthamoeba micheli sp. nov. (genotype T19) Parasitol. Res. 2015;114:2481–2490. doi: 10.1007/s00436-015-4445-8. [DOI] [PubMed] [Google Scholar]
- 20.Putaporntip C., Kuamsab N., Nuprasert W., Rojrung R., Pattanawong U., Tia T., Yanmanee S., Jongwutiwes S. Analysis of Acanthamoeba genotypes from public freshwater sources in Thailand reveals a new genotype, T23 Acanthamoeba bangkokensis sp. nov. Sci. Rep. 2021;11:17290. doi: 10.1038/s41598-021-96690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corsaro D. Update on Acanthamoeba phylogeny. Parasitol. Res. 2020;119:3327–3338. doi: 10.1007/s00436-020-06843-9. [DOI] [PubMed] [Google Scholar]
- 22.Burge C., Karlin S. Prediction of complete gene structures in human genomic DNA. J. Mol. Biol. 1997;268:78–94. doi: 10.1006/jmbi.1997.0951. [DOI] [PubMed] [Google Scholar]
- 23.Stanke M., Waack S. Gene prediction with a hidden Markov model and a new intron submodel. Bioinformatics. 2003;19:215–225. doi: 10.1093/bioinformatics/btg1080. [DOI] [PubMed] [Google Scholar]
- 24.Papadopoulos J.S., Agarwala R. COBALT: Constraint-based alignment tool for multiple protein sequences. Bioinformatics. 2007;23:1073–1079. doi: 10.1093/bioinformatics/btm076. [DOI] [PubMed] [Google Scholar]
- 25.Jobb G., von Haeseler A., Strimmer K. TREEFINDER: A powerful graphical analysis environment for molecular phylogenetics. BMC Evol. Biol. 2004;4:18. doi: 10.1186/1471-2148-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campanella J.J., Bitincka L., Smalley J. MatGAT: An application that generates similarity/identity matrices using protein or DNA sequences. BMC Bioinform. 2003;4:29. doi: 10.1186/1471-2105-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- 29.Gupta R., Brunak S. Prediction of glycosylation across the human proteome and the correlation to protein function. Pac. Symp. Biocomput. 2002;7:310–322. [PubMed] [Google Scholar]
- 30.Corsaro D., Venditti D. Phylogenetic evidence for a new genotype of Acanthamoeba (Amoebozoa, Acanthamoebida) Parasitol. Res. 2010;107:233–238. doi: 10.1007/s00436-010-1870-6. [DOI] [PubMed] [Google Scholar]
- 31.Corsaro D. Exploring LSU and ITS rDNA sequences for Acanthamoeba identification and phylogeny. Microorganisms. 2022;10:1776. doi: 10.3390/microorganisms10091776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis E.J., Sawyer T.K. Identificationoffree-living amoebae (Protozoa: Acanthamoebidae) from a fresh- to salt-water gradient in the St. Martin River, Ocean City, Maryland (Abstr) Trans. Am. Microsc. Soc. 1979;98:152–153. [Google Scholar]
- 33.Ledee D.R., Booton G.C., Awwad M.H., Sharma S., Aggarwal R.K., Niszl I.A., Markus M.B., Fuerst P.A., Byers T.J. Advantages of using mitochondrial 16S rDNA sequences to classify clinical isolates of Acanthamoeba. Investig. Ophthalmol. Vis. Sci. 2003;44:1142–1149. doi: 10.1167/iovs.02-0485. [DOI] [PubMed] [Google Scholar]
- 34.Chung D.I., Yu H.S., Hwang M.Y., Kim T.H., Kim T.O., Yun H.C., Kong H.H. Subgenus classification of Acanthamoeba by riboprinting. Korean J. Parasitol. 1998;36:69–80. doi: 10.3347/kjp.1998.36.2.69. [DOI] [PubMed] [Google Scholar]
- 35.Yu H.S., Hwang M.Y., Kim T.O., Yun H.C., Kim T.H., Kong H.H., Chung D.I. Phylogenetic relationships among Acanthamoeba spp. based on PCR-RFLP analyses of mitochondrial small subunit rRNA gene. Korean J. Parasitol. 1999;37:181–188. doi: 10.3347/kjp.1999.37.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonçalves D.S., Ferreira M.D.S., Gomes K.X., Rodríguez-de La Noval C., Liedke S.C., da Costa G.C.V., Albuquerque P., Cortines J.R., Saramago Peralta R.H., Peralta J.M., et al. Unravelling the interactions of the environmental host Acanthamoeba castellanii with fungi through the recognition by mannose-binding proteins. Cell. Microbiol. 2019;21:e13066. doi: 10.1111/cmi.13066. [DOI] [PubMed] [Google Scholar]
- 37.Niyyati M., Rezaie S., Rahimi F., Mohebali M., Maghsood A.H., Farnia S.H., Rezaeian M. Molecular characterization and sequencing of a gene encoding Mannose Binding Protein in an Iranian isolate of Acanthamoeba castellanii as a major agent of Acanthamoeba keratitis. Iran. J. Public Health. 2008;37:9–14. [Google Scholar]
- 38.Niyyati M., Rezaie S., Babaei Z., Rezaeian M. Molecular identification and sequencing of Mannose Binding Protein (MBP) gene of Acanthamoeba palestinensis. Iran. J. Parasitol. 2010;5:1–5. [PMC free article] [PubMed] [Google Scholar]
- 39.Allen P.G., Dawidowicz E.A. Phagocytosis in Acanthamoeba: I. A mannose receptor is responsible for the binding and phagocytosis of yeast. J. Cell. Physiol. 1990;145:508–513. doi: 10.1002/jcp.1041450317. [DOI] [PubMed] [Google Scholar]
- 40.Alsam S., Sissons J., Dudley R., Khan N.A. Mechanisms associated with Acanthamoeba castellanii (T4) phagocytosis. Parasitol. Res. 2005;96:402–409. doi: 10.1007/s00436-005-1401-z. [DOI] [PubMed] [Google Scholar]
- 41.Brindley N., Matin A., Khan N.A. Acanthamoeba castellanii: High antibody prevalence in racially and ethnically diverse populations. Exp. Parasitol. 2009;121:254–256. doi: 10.1016/j.exppara.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Griffin J.L. Temperature tolerance of pathogenic and nonpathogenic free-living amoebas. Science. 1972;178:869–870. doi: 10.1126/science.178.4063.869. [DOI] [PubMed] [Google Scholar]
- 43.De Jonckheere J.F. Growth characteristics, cytopathic effect in cell culture, and virulence in mice of 36 type strains belonging to 19 different Acanthamoeba spp. Appl. Environ. Microbiol. 1980;39:681–685. doi: 10.1128/aem.39.4.681-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan N.A., Jarroll E., Paget T. Acanthamoeba can be differentiated by the Polymerase Chain Reaction and simple plating assays. Curr. Microbiol. 2001;43:204–208. doi: 10.1007/s002840010288. [DOI] [PubMed] [Google Scholar]
- 45.Walochnik J., Obwaller A., Aspöck H. Correlations between morphological, molecular biological, and physiological characteristics in clinical and nonclinical isolates of Acanthamoeba spp. Appl. Environ. Microbiol. 2000;66:4408–4413. doi: 10.1128/AEM.66.10.4408-4413.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cursons R.T., Brown T.J. Use of cell cultures as an indicator of pathogenicity of free-living amoebae. J. Clin. Pathol. 1978;31:1–11. doi: 10.1136/jcp.31.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garate M., Marchant J., Cubillos I., Cao Z., Khan N.A., Panjwani N. In vitro pathogenicity of Acanthamoeba is associated with the expression of the mannose-binding protein. Investig. Ophthalmol. Vis. Sci. 2006;47:1056–1062. doi: 10.1167/iovs.05-0477. [DOI] [PubMed] [Google Scholar]
- 48.Ng S.L., Nordin A., Abd Ghafar N., Suboh Y., Ab Rahim N., Chua K.H. Acanthamoeba-mediated cytopathic effect correlates with MBP and AhLBP mRNA expression. Parasit. Vectors. 2017;10:625. doi: 10.1186/s13071-017-2547-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hong Y.C., Kong H.H., Ock M.S., Kim I.S., Chung D.I. Isolation and characterization of a cDNA encoding a subtilisin-like serine proteinase (ahSUB) from Acanthamoeba healyi. Mol. Biochem. Parasitol. 2000;111:441–446. doi: 10.1016/S0166-6851(00)00326-1. [DOI] [PubMed] [Google Scholar]
- 50.Hurt M., Neelam S., Niederkorn J., Alizadeh H. Pathogenic Acanthamoeba spp. secrete a mannose-induced cytolytic protein that correlates with the ability to cause disease. Infect. Immun. 2003;71:6243–6255. doi: 10.1128/IAI.71.11.6243-6255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blaschitz M., Köhsler M., Aspöck H., Walochnik J. Detection of a serine proteinase gene in Acanthamoeba genotype T6 (Amoebozoa: Lobosea) Exp. Parasitol. 2006;114:26–33. doi: 10.1016/j.exppara.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Kim W.T., Kong H.H., Ha Y.R., Hong Y.C., Jeong H.J., Yu H.S., Chung D.I. Comparison of specific activity and cytopathic effects of purified 33 kDa serine proteinase from Acanthamoeba strains with different degree of virulence. Korean J. Parasitol. 2006;44:321–330. doi: 10.3347/kjp.2006.44.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sissons J., Alsam S., Goldsworthy G., Lightfoot M., Jarroll E.L., Khan N.A. Identification and properties of proteases from an Acanthamoeba isolate capable of producing granulomatous encephalitis. BMC Microbiol. 2006;6:42. doi: 10.1186/1471-2180-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tripathi T., Smith A.D., Abdi M., Alizadeh H. Acanthamoeba-cytopathic protein induces apoptosis and proinflammatory cytokines in human corneal epithelial cells by cPLA2a activation. Investig. Ophthalmol. Vis. Sci. 2012;53:7973–7982. doi: 10.1167/iovs.12-10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huth S., Reverey J.F., Leippe M., Selhuber-Unkel C. Adhesion forces and mechanics in mannose-mediated Acanthamoeba interactions. PLoS ONE. 2017;12:e0176207. doi: 10.1371/journal.pone.0176207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corsaro D. On the diversity and clinical importance of Acanthamoeba spp. from Group 1. Parasitol. Res. 2021;120:2057–2064. doi: 10.1007/s00436-021-07171-2. [DOI] [PubMed] [Google Scholar]
- 57.Schiller B., Makrypidi G., Razzazi-Fazeli E., Paschinger K., Walochnik J., Wilson I.B. Exploring the unique N-glycome of the opportunistic human pathogen Acanthamoeba. J. Biol. Chem. 2012;287:43191–43204. doi: 10.1074/jbc.M112.418095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferreira M.D.S., Mendoza S.R., Gonçalves D.S., Rodríguez-de la Noval C., Honorato L., Nimrichter L., Ramos L.F.C., Nogueira F.C.S., Domont G.B., Peralta J.M., et al. Recognition of cell wall mannosylated components as a conserved feature for fungal entrance, adaptation and survival within trophozoites of Acanthamoeba castellanii and murine macrophages. Front. Cell. Infect. Microbiol. 2022;12:858979. doi: 10.3389/fcimb.2022.858979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lameignere E., Malinovská L., Sláviková M., Duchaud E., Mitchell E.P., Varrot A., Sedo O., Imberty A., Wimmerová M. Structural basis for mannose recognition by a lectin from opportunistic bacteria Burkholderia cenocepacia. Biochem. J. 2008;411:307–318. doi: 10.1042/BJ20071276. [DOI] [PubMed] [Google Scholar]
- 60.Sulák O., Cioci G., Lameignère E., Balloy V., Round A., Gutsche I., Malinovská L., Chignard M., Kosma P., Aubert D.F., et al. Burkholderia cenocepacia BC2L-C is a super lectin with dual specificity and proinflammatory activity. PLoS Pathog. 2011;7:e1002238. doi: 10.1371/journal.ppat.1002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang A.Y., Park A.Y., Shin H.J., Khan N.A., Maciver S.K., Jung S.Y. Production of a monoclonal antibody against a mannose-binding protein of Acanthamoeba culbertsoni and its localization. Exp. Parasitol. 2018;192:19–24. doi: 10.1016/j.exppara.2018.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in Supplementary Material.