Abstract
We have recently cloned a new protein, recombinant P40 (rP40). When tested in vivo after conjugation to a B-cell epitope, rP40 induces an important antibody response without the need for adjuvant. To characterize its potency, this carrier protein was coupled to a peptide derived from respiratory syncytial virus attachment G protein (G1′). After immunization of mice with the rP40-G1′ conjugate, strong antipeptide antibodies were detected, whereas peptide alone was not immunogenic. To emphasize the carrier properties of rP40, a polysaccharide derived from Haemophilus influenzae type b (Hib) was coupled to it. Immunoglobulin G responses against the Hib polysaccharide were observed after coupling to rP40. Interestingly, an antipeptide antibody response was observed despite preexisting anti-rP40 antibodies generated by preimmunization with rP40. In addition, rP40 compares well with the reference carrier protein, tetanus toxoid (TT), since antibody responses of equal intensity were observed when a peptide or a polysaccharide was coupled to TT and rP40. Moreover, rP40 had advantages compared to TT; e.g., it induced a mixed Th1/Th2 response, whereas TT induced only a Th2 profile. Together, the results indicate that rP40 is a novel carrier protein with potential for use as an alternative carrier for human vaccination.
Most conventional vaccines consist of killed organisms or purified antigenic proteins derived from these organisms. However, this approach to vaccine development has several limitations. First, the large-scale growth of certain pathogenic organisms may be difficult to achieve and is not completely free of risk; second, it may be difficult to establish whether some vaccine preparations are completely killed and free of contaminants. A safer method is the use of synthetic peptides corresponding to immunogenic epitopes of pathogens for vaccination. However, such peptides are usually not immunogenic by themselves. Coupling such antigens to carrier protein has been reported to increase their immunogenicity. The choice of appropriate carriers is of primary importance for designing synthetic vaccines for human use. Tetanus toxoid (TT) has been used in most studies because it has been used for human vaccination for many years without untoward side effects (17, 34). More recently, the outer membrane protein complex of Neisseria meningitidis has been shown to be effective in humans as a conjugate vaccine with Haemophilus influenzae along with pneumococcal and meningococcal capsular polysaccharides (1, 15, 18). The carrier molecules are particularly useful for polysaccharide vaccines. The coupling of polysaccharides to a carrier molecule converts a T-independent immune response to T-dependent one with the production of immunoglobulin G (IgG) antibodies recognizing polysaccharides and the generation of B-cell memory (3).
A major drawback for the use of carrier proteins is the observation that the antibody response to an antigen coupled to a carrier protein can be decreased in individuals previously immunized with the carrier. This carrier-induced epitope-specific suppression was first described as a general regulatory process found among different hapten carrier systems and mouse strains. Preimmunization with a carrier can impair the antibody response, mostly for the IgG2a isotype, to a hapten coupled to the same carrier (11, 12). The same phenomenon was observed with TT as a carrier, with a strong suppression of the IgG1 response against two different coupled antigens (6, 24, 27, 28). The effect has been further found in humans (4). Possible mechanisms responsible for the observed suppression have been investigated (11, 25, 28, 29). However, in some cases preimmunization with the carrier protein led to enhancement of the response to the coupled antigen rather than suppression (19). Nevertheless, the majority of subjects have probably been vaccinated against tetanus in early childhood; therefore, the phenomenon of epitope-specific suppression through preimmunization with carrier could be a drawback to the use of this system in human vaccination programs. An alternative which may resolve this type of problem could be the use of multiple carrier proteins as suggested in a recent publication (7).
A protein corresponding to the Klebsiella pneumoniae I-145 outer membrane protein A (OmpA) has been identified, cloned, and expressed in Escherichia coli as a recombinant molecule rP40 (21). Purified rP40 was analyzed to verify purity and structural integrity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrospray mass spectrometry (10). Preliminary results showed that rP40 expresses at least one T-cell epitope and that when coupled to a peptide, it increased the antipeptide antibody response.
To validate rP40 among the other carrier molecules, the first aim of this study was to compare the efficacy of this new carrier protein rP40 to TT, a carrier protein currently used for human vaccination. In addition, the type of immune response generated after immunization either with rP40 or TT coupled to peptide was investigated. A pure B-cell epitope derived from the attachment G protein of the respiratory syncytial virus (RSV) was used as a model. The use of carrier molecules is critical to obtain an IgG immune response with polysaccharides. The second aim of this study was to extend carrier-related properties of rP40 to polysaccharides. Finally, we investigated the effect of preexisting anti-rP40 antibodies on the antipeptide responses.
We found that rP40 induces antipeptide and antipolysaccharide antibody responses that compare well with the ones obtained with TT. In contrast to TT, immunization with rP40 conjugate leads to a mixed Th1/Th2 pathway. Interestingly, carrier priming with rP40 does not affect the antibody response to a peptide-rP40 conjugate.
MATERIALS AND METHODS
Proteins, peptide, and polysaccharide.
Methods for high-level expression in E. coli, isolation, refolding, and purification of rP40, the recombinant K. pneumoniae I-145 OmpA, have been previously described (10, 21). TT was purchased from SBL Vaccin AB (Stockholm, Sweden). Peptide G1′ (SIDSNNPTOWAISCK), purchased from Neosystem Laboratoire (Strasbourg, France), corresponded to amino acid residues 174 to 187 of the attachment G protein of the RSV-A subgroup, to which a C-terminal cysteine residue has been added for coupling purposes (10). Cys186 found in native G protein was replaced by a serine residue, and Cys residues 176 and 182 were replaced by aspartic acid and ornithine, respectively, to provide a lactame bridge mimicking the disulfide bridge found in natural G protein (33). H. influenzae type b (Hib) polysaccharide was kindly provided by Rino Rappuoli, Chiron Biocine Immunobiological Research Institute, Siena, Italy.
Preparation of peptide-protein conjugates.
Conjugates rP40-G1′, TT-G1′, and bovine serum albumin (BSA)-G1′ were prepared as previously described (10). Briefly, proteins were activated by bromoacetylation with a method that employs bromoacetic acid N-hydroxysuccinimide ester (Sigma, Saint Quentin Fallavier, France) before peptide G1′ was coupled to bromoacetylated proteins. Conjugates were then extensively dialyzed against phosphate-buffered saline (PBS) and kept at −20°C until use. They were analyzed by SDS-PAGE by using the Mini Protean II gel system (Bio-Rad, Ivry Sur Seine, France). The degree of coupling reaction (peptide G1′/protein molar ratio) was determined by amino acid analysis quantifying the amount of S-carboxymethylcysteine released after conjugate acid hydrolysis (16). G1′/protein conjugate molar ratios were estimated to be 10 for rP40-G1′ and 12 for TT-G1′.
Preparation of polysaccharide-protein conjugates.
Conjugation of the Hib polysaccharide to rP40 and TT was carried out as described by Chu et al. (2). The polysaccharide was activated with cyanogen bromide (Sigma) at pH 10.5 for 6 min and reacted with 0.1 M adipic acid dihydrazide (Sigma) at pH 8.5 overnight at room temperature. After dialysis and purification by gel filtration on a Sephadex G-100 column (2.6 by 61 cm; Amersham Pharmacia Biotech, Saclay, France), the polysaccharide was freeze-dried. The percentage of hydrazide on the polysaccharide was measured by the 1,3,5-trinitrobenzenesulfonic assay with adipic acid dihydrazide as a standard (26). The resultant polysaccharide contained about 0.9 to 1.5% (wt/wt) adipic hydrazide. For conjugation, this adipic hydrazide polysaccharide derivative and proteins were used at 10 mg/ml each, coupling was done with 0.1 M 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (Sigma) at pH 5 for 3 h at 4°C. The conjugates were purified from the reaction by a Sepharose CL-4B column (1.5 by 62 cm; Amersham Pharmacia Biotech) equilibrated with PBS. Protein fractions eluted in the void volume, as measured by their optical density at 280 nm were combined, concentrated by ultrafiltration, and stored at 4°C. Conjugates were analyzed for carbohydrate with the orcinol-ferric chloride-HCl assay by using ribose as a standard (23) and for protein with the bicinchoninic acid assay (Pierce, Rockford, Ill.) by using BSA as a standard. Polysaccharide/protein (wt/wt) ratios were determined to be 0.7, 1.1, and 1.0 for rP40-Hib, TT-Hib, and BSA-Hib conjugates, respectively. All conjugates were also characterized by gel filtration and SDS-PAGE analyses to demonstrate the covalence of the polysaccharide-protein linkage and the absence of uncoupled protein.
Mouse strains.
Six-week-old pathogen-free BALB/c female mice were purchased from IFFA CREDO (L'Arbresle, France) and kept under specific-pathogen-free conditions.
Immunization of mice.
Groups of five BALB/c mice were immunized subcutaneously on days 0, 10, and 20 with 5 μg of G1′ peptide equivalent, conjugated with rP40 or TT, and suspended in PBS, with or without aluminum hydroxide (20%, vol/vol; Superfos Biosector a/s, Vedbaek, Denmark). Mice were bled from the retro-orbital venous plexus at regular intervals to determine anti-G1′ serum antibody titers.
Groups of five BALB/c mice were immunized subcutaneously on days 0, 14, and 28 with 10 μg of Hib polysaccharide equivalent, alone or conjugated with rP40 or TT, in aluminum hydroxide (20%, vol/vol; Superfos Biosector). Control groups included mice immunized with PBS plus adjuvant alone. Mice were bled from the retroorbital venous plexus at regular intervals to determine anti-Hib serum antibody titers.
Epitopic suppression.
Groups of five animals were presensitized subcutaneously with 100 μg of either rP40 or TT adsorbed on aluminum hydroxide. Mice in the control group received aluminum hydroxide and saline only. After 30 days, control and experimental animals were immunized by the same route with 0.1-μg equivalent of G1′ conjugated to rP40 or TT adsorbed on aluminum hydroxide. This was followed by booster immunizations with the same preparation 60 and 90 days after the initial presensitization dose of carrier. Animals were bled on days 67 and 97, and anti-G1′ antibody titers were measured.
ELISA.
Enzyme-linked immunosorbent assays (ELISA) were performed essentially as described elsewhere (32). Briefly, microtiter plates (Immulon 2; Dynatech, Chantilly, Va.) were coated overnight at 4°C with BSA-G1′ (1 μg of peptide/ml) in carbonate buffer (pH 9.8) when anti-G1′ peptide antibodies were analyzed. When antibodies against the carrier protein rP40 or TT were analyzed, a concentration of 2 μg of antigen/ml was used for coating the plates. For anti-Hib antibodies, plates were coated with BSA-Hib (25 μg of Hib/ml) in carbonate buffer (pH 9.8). Nonspecific reactions were blocked with 0.5% gelatin (Serva, Heidelberg, Germany). The antibody samples were serially diluted down the plate, which was then incubated for 2 h at room temperature and subjected to extensive washing. Peroxidase-conjugated goat anti-mouse IgG (Pierce) or goat anti-mouse IgG1 or IgG2a (Southern Biotechnology Associates, Birmingham, Ala.) was reacted with each well for 1 h at 37°C. After washing, a solution of 3,3′,5,5′-tetramethylbenzidine (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) was added to each well. The reaction proceeded for 10 min and was stopped by the addition of 1 M H2SO4. A450 was determined with a Labsystems IEMS reader (Labsystems, Helsinki, Finland). Titers were defined as the reciprocal of the serum dilution which gave an A450 of >2 standard deviations (SD) above the value for a negative control serum.
Cytokine measurements.
Groups of five mice were immunized by intraperitoneal injection of 100 μg of rP40, rP40-G1′, or PBS per mouse. Ten days after immunization, spleens were removed and perfused with RPMI 1640 (Gibco, Cergy Pontoise, France). Spleen cells were pooled within experimental groups, washed, and resuspended in RPMI 1640 (Gibco) containing 1% fetal calf serum (Costar, Brumath, France), 1% glutamine (Gibco), and 50 IU of antibiotics (Gibco, Cergy Pontoise, France)/ml. Cells were restimulated in vitro by incubating 5 × 105 cells/ml in 96 round-bottom plates (Costar) in triplicate with various concentrations of rP40. Cultures were incubated for either 48 h (for gamma interferon [IFN-γ] and interleukin-2 [IL-2] detection) or 96 h (for IL-5 and IL-10 detection) at 37°C in 5% CO2. Supernatants were collected, and cytokines were measured in duplicate by ELISA with commercially available kits (R&D System [Minneapolis, Minn.] for IFN-γ, IL-2, and IL-10; Endogen [Woburn, Mass.] for IL-5) according to the manufacturers' instructions.
Statistical analyses.
Statistical analyses were performed by using an analysis of variance with P > 0.05, using the Statgraphic program (Manugistics, Rockville, Md.). For analysis of experiments using Hib as the antigen, statistical analyses were performed by using either an analysis of variance with P > 0.05 or the Kolmogorov-Smirnov t test of the statistic software program (Manugistics). Probability values greater than 0.05 were considered nonsignificant.
RESULTS
Anti-G1′ peptide antibody responses after subcutaneous immunization with the conjugates.
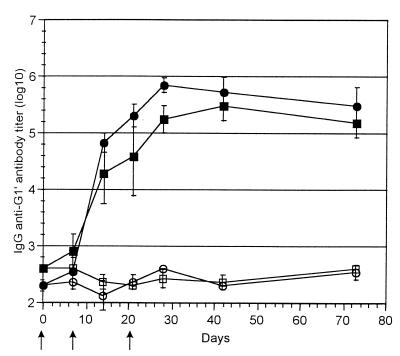
Mice immunized with 5 μg of rP40-G1′ (equivalent peptide) without adjuvant developed an antibody response on day 10 that increased over time and was maximal on day 28. The response reached 5.5 log10 after three immunizations. The titer was maintained over time (Fig. 1). Anti-G1′ antibodies were detected after one immunization with 5 μg of rP40-G1′. Addition of aluminum hydroxide did not significantly increase the anti-G1′ antibody response observed after immunization with 5 μg of rP40-G1′ (data not shown). The same profile of antibody production was observed for mice immunized with TT-G1′ conjugates without adjuvant (Fig. 1). The anti-G1′ antibody response generated after three immunizations with 5 μg of TT-G1′ was not significantly different from that obtained with rP40-G1′ immunizations. No antibody response was detected after immunization either with G1′ alone or with PBS (data not shown). Interestingly, these results indicate that rP40 and TT generated comparable profiles for antibody responses when coupled to the peptide G1′.
FIG. 1.
Anti-G1′ antibody responses. Mice were immunized three times with 5 μg of equivalent G1′ conjugated to rP40 (■) or with 5 μg of equivalent G1′ conjugated to TT (●). Several days after the immunizations, sera were collected and antipeptide IgG antibody content was measured by ELISA. Results are expressed as means ± SD (n = 5). Group of mice (n = 5) immunized with rP40 (□) or TT (○) alone were used as controls.
Comparative analysis of distribution of the IgG1 and IgG2a subclasses of G1′-specific antibodies, selected as indirect markers of a Th1/Th2 type of response, indicated that mice immunized with rP40-G1′ raised a mixed antibody pattern consisting of G1′-specific antibodies of both IgG1 and IgG2a isotypes (5.4 ± 0.34 and 4.1 ± 0.21 log10, respectively). In contrast, mice immunized with TT-G1′ expressed primarily IgG1 and lower G1′-specific IgG2a antibodies, with heterogeneous IgG2a response (5.72 ± 0.16 and 2.66 ± 0.96 log10, respectively). Similar results were observed irrespective of the number of immunizations or the doses of G1′ used for immunization (data not shown). These results indicated that rP40-G1′ generated a more mixed immune response compared to TT-G1′.
To confirm the ability of rP40 to direct the immune response toward a Th0 response, splenocytes from rP40-G1′- and rP40-immunized mice were reactivated in vitro with several concentrations of rP40. Results for 1 μg of rP40, which gave the highest production of IL-2, IL-5, IL-10, and IFN-γ, are presented in Table 1. Activation of splenocytes with rP40 induced a strong production of IFN-γ in the supernatants, with some IL-2, IL-5, and IL-10. Surprisingly, activation with rP40 of splenocytes from rP40-G1′-immunized mice induced the production of higher amounts of IL-2 and IFN-γ and lower amounts of IL-5. No cytokine was detected in medium from naive splenocytes activated with rP40 or from rP40-G1′-immunized splenocytes activated with G1′ (data not shown).
TABLE 1.
Cytokine determination after rP40 immunizationa
| Immunization/in vitro stimulation | Content (ng/ml)
|
|||
|---|---|---|---|---|
| IL-10 | IL-5 | IL-2 | IFN-γ | |
| PBS/rP40 | 7.2 | 0 | 8.7 | 0 |
| rP40/rP40 | 90 | 55 | 28 | 398 |
| rP40-G1′/rP40 | 103 | 1.6 | 108 | 888 |
Mice were immunized with rP40. Ten days after immunization, splenocytes were removed and activated with rP40 at a final concentration of 1 μg/ml. Supernatants were collected, and IFN-γ, IL-2, IL-10, and IL-5 contents were measured by ELISA. Results are representative of one experiment (n = 2).
Effect of carrier preimmunization on the antibody response to conjugates.
To test whether preimmunization with the carrier protein influenced a subsequent response to the conjugate, we compared the responses to peptide G1′ or carrier protein in mice presensitized with rP40. The presence of anti-rP40 in the latter group was determined before immunization with rP40-G1′.
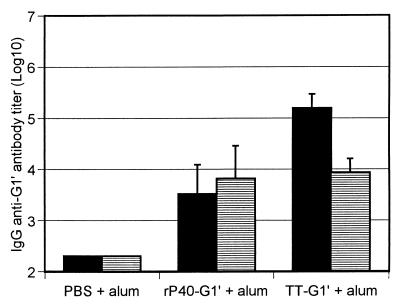
After immunization with 0.1 μg of rP40-G1′, an anti-G1′ antibody response was observed. Interestingly, mice preimmunized with rP40 before the administration of rP40-G1′ had the same antipeptide antibody titers (Fig. 2), in contrast to TT, where the anti-G1′ antibody titer was significantly decreased after sensitization with 100 μg of TT. In addition, this phenomenon was observed, whereas the level of anticarrier antibody was the same, in rP40 or TT-sensitized mice (5.19 ± 0.4 and 5.85 ± 0.33 log10, respectively). The results indicate that preexisting anti-rP40 antibodies were not inhibiting the antipeptide antibody response.
FIG. 2.
Effect of carrier preimmunization. Mice were preimmunized with 100 μg of each carrier in aluminum hydroxide (alum; striped bar) or with PBS in aluminum hydroxide (black bar) and then immunized twice with 0.1 μg of equivalent G1′ conjugated to rP40 or TT in the presence of aluminum hydroxide. On day 67, sera were collected and antipeptide IgG antibody content was measured by ELISA. Results are expressed as means ± (n = 5).
Anti-Hib antibody responses after subcutaneous immunization with the conjugates.
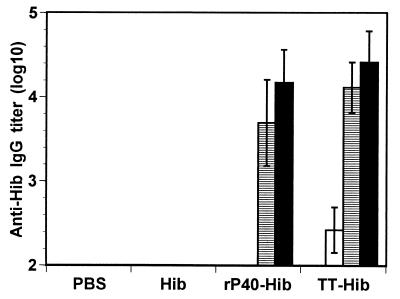
To confirm the carrier properties of rP40, the polysaccharide of Hib was coupled to rP40 and TT. Mice immunized twice with 10 μg of Hib conjugated to rP40 in aluminum hydroxide developed an IgG antibody response against Hib. A third injection induced an increase in the IgG titer which reached 4.2 log10 (Fig. 3). In contrast, no IgG response was observed with uncoupled polysaccharide even when it was administered with aluminum hydroxide. When mice were immunized with TT-Hib conjugate, a slight IgG antibody response against Hib was observed after the first immunization. After two or three injections, no significant differences (P < 0.05) were observed in the titers obtained after immunization with TT-Hib or rP40-Hib, using either a parametric or a nonparametric statistical test. The results indicate that in addition to peptides, rP40 is able to induce antipolysaccharide antibody response of the IgG type.
FIG. 3.
Antipolysaccharide antibody responses. Mice were immunized three times with 10 μg of Hib polysaccharide conjugated to rP40 or TT in presence of aluminum hydroxide. After one (white bars), two (horizontal bars), or three (black bars) immunizations, sera were collected and polysaccharide-specific IgG antibody content was measured by ELISA. Results are expressed as means ± SD (n = 5).
DISCUSSION
In an earlier study, we have demonstrated that immunization with a peptide conjugated to the carrier protein rP40 induces a strong immune response against the peptide (10). To demonstrate the advantage of using this new carrier protein in a future conjugate vaccine, we have compared the antipeptide antibody titers elicited in mice immunized with rP40-G1′ conjugates and with TT-G1′ conjugates.
The same intensity in the antipeptide antibody immune responses were observed independently of the carrier used. Similarly, anti-Hib antibodies were not significantly different after immunization with TT-Hib or rP40-Hib. Altogether, these results indicated that rP40 is as effective as TT in inducing antibodies and could be considered as an alternative carrier for human vaccination.
Previous studies (6, 11, 27, 29) have extensively demonstrated that preimmunization with TT can affect responses to haptens linked to TT. The phenomenon of epitope-specific suppression through preimmunization with carrier protein could be a drawback to the use of this system in human vaccination programs, given that the majority of subjects would probably have been vaccinated against tetanus in early childhood. The mechanism responsible for suppression of the antihapten antibody response to hapten-carrier conjugates upon high-dose carrier priming is still unclear. Involvement of both T suppressor cells and carrier-specific B memory cells (8, 11, 29) has been reported. Another possible explanation might be that circulating antibodies against the carrier protein are responsible for scavenging the antigen or induce the formation of immune complexes which inhibit the immune response (22). Interestingly, preimmunization with rP40 and immunization with rP40 conjugated to G1′ did not lead to specific epitopic suppression, in contrast to what was observed with TT, confirming that rP40 could be a good alternative carrier.
It has been demonstrated (29) that the induction of suppression can depend on the ratio between the concentration of carrier and epitope on the conjugate: suppression induced with a low concentration of the conjugated molecule could be abrogated by using higher concentrations. This hypothesis could not explain the absence of epitopic suppression with the carrier protein rP40 in our experiments since we deliberately chose a low concentration of rP40-G1′. In addition, no epitopic suppression was observed with a higher concentration of rP40-G1′ (data not shown).
In contrast to TT, which usually is formulated in adjuvant for human use, outer proteins like the one from Borrelia burgdorferi are administered and efficient in human without the need for adjuvant. As already demonstrated, rP40 is a carrier protein that elicits high antibody titers without the need for adjuvant. This is an important point to consider since there are many of problems with aluminum hydroxide, the most widely used adjuvant and the only one licensed for human use (20): first, not all proteins and peptides are equally well adsorbed (30); second, there are strict conditions for the storage of vaccines adsorbed onto aluminum hydroxide; third, aluminum hydroxide-associated pathology and vaccine-specific IgE production have been reported (9).
We have not directly examined the Th cell responses induced by the conjugates; however, production of high serum levels of antigen-specific IgG1 may be indicative of a Th2-type response, whereas high serum levels of IgG2a may reflect a Th1-type response (13). We demonstrated that the new carrier protein rP40 induces a mixed antibody pattern characteristic of a Th1/Th2 immune response. Orientation of the immune response with rP40 toward a Th0 profile was confirmed by analysis of rP40- and rP40-G1′-immunized splenocytes, where Th1 and Th2 cytokines were produced. In contrast, the reference carrier protein TT induces only IgG1 antibodies, characteristic of a Th2 immune response. This last result is in contradiction with the results of el Ghazali et al. (5), who showed that TT induced both IL-4 and IFN-γ, characteristic of a Th1/Th2 profile. However, the immunity to tetanus is an antibody-dependent Th2-type response. These contradictory results could be explained by the fact that G1′ derived from the RSV G protein may orient the immune response toward the Th2 pathway (33).
The carrier protein rP40 could be used to induce an immune response against many diseases by the induction of a Th1/Th2 profile. Indeed, resistance to many intracellular pathogens, including bacteria, protozoa, and fungi, is linked to the induction of Th1 responses (14, 31). In contrast, extracellular pathogens and particularly parasitic helminths typically trigger Th2-dominated responses (31).
In conclusion, these studies have shown that the new carrier protein rP40 is an efficient carrier since after immunization with peptide- or polysaccharide-rP40 conjugates, we observed antibody responses similar to the response obtained in presence of the TT conjugates. Moreover, rP40 induces a mixed Th1/Th2 response. Finally, preexisting anti-rP40 antibodies do not inhibit further immunization using the same carrier. This last result is of importance since in humans, anti-rP40 antibodies have been detected (data not shown). Therefore, rP40 is a good alternative to the existing carrier proteins for use in future conjugate vaccines.
ACKNOWLEDGMENTS
We thank F. Derouet, J. Challier, and L. Zanna for excellent technical assistance; we thank C. Libon for critical review of the manuscript.
REFERENCES
- 1.Ahonkhai V I, Lukacs L J, Jonas L C, Matthews H, Vella P P, Ellis R W, Staub J M, Dolan K T, Rusk C M, Calandra G B, Gerety R J. Haemophilus influenzae type b conjugate vaccine (meningococcal protein conjugate) (PedvaxHIB): clinical evaluation. Pediatrics. 1990;85:676–681. [PubMed] [Google Scholar]
- 2.Chu C, Schneerson R, Robbins J B, Rastogi S C. Further studies on the immunogenicity of Haemophilus influenzae type b and pneumococcal type 6A polysaccharide-protein conjugates. Infect Immun. 1983;40:245–256. doi: 10.1128/iai.40.1.245-256.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Guidice G. Hsp70: a carrier molecule with built-in adjuvanticity. Experiencia. 1994;50:1061–1066. doi: 10.1007/BF01923462. [DOI] [PubMed] [Google Scholar]
- 4.Di John D, Wasserman S S, Torres J R, Cortesia M J, Murillo J, Losonsky G A, Herrington D A, Sturcher D, Levine M H. Effect of priming with carrier on response to conjugate vaccine. Lancet. 1989;16:1415–1418. doi: 10.1016/s0140-6736(89)92033-3. [DOI] [PubMed] [Google Scholar]
- 5.el Ghazali G E, Paulie S, Andersson G, Hansson Y, Holmquist G, Sun J B, Olsson T, Ekre H P, Troye-Blomberg M. Number of interleukin-4 and interferon-gamma secreting human T cells reactive with tetanus toxoid and the mycobacterial antigen PPD or phytohemagglutinin: distinct response profiles depending on the type of antigen used for activation. Eur J Immunol. 1993;23:2740–2745. doi: 10.1002/eji.1830231103. [DOI] [PubMed] [Google Scholar]
- 6.Etlinger H M, Gillessen D, Lahm H W, Matile H, Schönfeld H J, Trzeciak A. Use of prior vaccinations for the development of new vaccines. Science. 1990;249:423–425. doi: 10.1126/science.1696030. [DOI] [PubMed] [Google Scholar]
- 7.Fattom A, Cho Y H, Chu C Y, Fuller S, Fries L, Naso R. Epitopic overload at the site of injection may result in suppression of the immune response to combined capsular polysaccharide conjugate vaccines. Vaccine. 1990;17:126–133. doi: 10.1016/s0264-410x(98)00162-5. [DOI] [PubMed] [Google Scholar]
- 8.Galleli A, Charlot B. Clonal anergy of memory B cells in epitope specific regulation. J Immunol. 1990;145:2397–2405. [PubMed] [Google Scholar]
- 9.Gupta R K, Relyveld E H. Adverse reactions after injection of adsorbed diphtheria pertussis tetanus (DPT) vaccines are not only due to pertussis organisms or pertussis components in the vaccine. Vaccine. 1991;9:699–702. doi: 10.1016/0264-410x(91)90283-c. [DOI] [PubMed] [Google Scholar]
- 10.Haeuw J F, Rauly I, Zanna L, Libon C, Andréoni C, Nguyen T, Baussant T, Bonnefoy J Y, Beck A. The recombinant Klebsiella pneumoniae outer membrane protein OmpA has carrier properties for conjugated antigenic peptides. Eur J Chem. 1998;255:446–454. doi: 10.1046/j.1432-1327.1998.2550446.x. [DOI] [PubMed] [Google Scholar]
- 11.Herzenberg L A, Tokuhisa T. Epitope-specific regulation. Carrier-specific induction of suppression for IgG anti-hapten antibody responses. J Exp Med. 1982;155:1730–1740. doi: 10.1084/jem.155.6.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herzenberg L A, Tokuhisa T. Carrier-priming leads to hapten-specific suppression. Nature. 1980;285:664–667. doi: 10.1038/285664a0. [DOI] [PubMed] [Google Scholar]
- 13.Jackson R J, Staats H F, Xu-Amano J, Takahashi I, Kiyono H, Hudson M E, Gilley R M, Chatfield S N, McGhee J R. Oral vaccine models: multiple delivery systems employing tetanus toxoid. Ann N Y Acad Sci. 1994;730:217–234. doi: 10.1111/j.1749-6632.1994.tb44251.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann S H. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 15.Käyhty H, Ahman H, Rönnberg P R, Tillikainen R, Eskola J. Pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine is immunogenic in infants and children. J Infect Dis. 1995;172:1273–1278. doi: 10.1093/infdis/172.5.1273. [DOI] [PubMed] [Google Scholar]
- 16.Kolodny N, Robey F A. Conjugation of synthetic peptides to proteins: quantitation from S-carboxymethylcysteine released upon acid hydrolysis. Anal Biochem. 1990;187:136–140. doi: 10.1016/0003-2697(90)90431-8. [DOI] [PubMed] [Google Scholar]
- 17.Kurikka S, Käyhty H, Peltola H, Saarinen L, Eskola J, Mäkelä H. Neonatal immunization: response to Haemophilus influenzae type b-tetanus toxoid conjugate vaccine. Pediatrics. 1995;95:815–822. [PubMed] [Google Scholar]
- 18.Lieberman J M, Chiu S S, Wong V K, Partidge S, Chang S J, Chiu C Y, Gheesling L L, Carlone G M, Ward J I. Safety and immunogenicity of a serogroups A/C Neisseria meningitidis oligosaccharide-protein conjugate vaccine in young children. JAMA. 1996;275:1499–1503. [PubMed] [Google Scholar]
- 19.Lise L D, Mazier D, Jolivet M, Audibert F, Chedid L, Schlesinger D. Enhanced epitopic response to a synthetic human malarial peptide by preimmunization with tetanus toxoid carrier. Infect Immun. 1987;55:2658–2661. doi: 10.1128/iai.55.11.2658-2661.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mannhalter J W, Neychev H O, Zlabinger G J, Ahmad R, Eibl M M. Modulation of the human immune response by the non-toxic and non-pyrogenic adjuvant aluminium hydroxide: effect on antigen uptake and antigen presentation. Clin Exp Immunol. 1985;61:141–151. [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen T, Samuelson P, Sterky F, Merle-Poitte C, Robert A, Baussant T, Haeuw J F, Uhlen M, Binz H, Stahl S. Chromosomal sequencing using a PCR-based biotin-capture method allowed isolation of the complete gene for the outer membrane protein A of Klebsiella pneumoniae. Gene. 1998;210:93–101. doi: 10.1016/s0378-1119(98)00060-2. [DOI] [PubMed] [Google Scholar]
- 22.Peeters C C A M, Tenbergen-Meekes A M, Poolman J T, Beurret M, Zegers B J M, Rijkers G T. Effect of carrier priming on immunogenicity of saccharide-protein conjugate vaccines. Infect Immun. 1991;59:3504–3510. doi: 10.1128/iai.59.10.3504-3510.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reuter G, Schauer R. Determination of sialic acids. Methods Enzymol. 1994;230:168–199. doi: 10.1016/0076-6879(94)30012-7. [DOI] [PubMed] [Google Scholar]
- 24.Sad S, Gupta H M, Talwar G P, Raghupathy R. Carrier-induced suppression of the antibody response to a “self” hapten. Immunology. 1991;74:223–227. [PMC free article] [PubMed] [Google Scholar]
- 25.Sad S, Rao K, Arora R, Talwar J P, Raghupathy R. Bypass of carrier-induced epitope-specific suppression using a T-helper epitope. Immunology. 1992;76:599–603. [PMC free article] [PubMed] [Google Scholar]
- 26.Schneerson R, Barrera O, Sutton A, Robbins J B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980;152:361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schutze M P, Leclerc C, Jolivet M, Audibert F, Chedid L. Carrier-induced epitopic suppression, a major issue for future synthetic vaccines. J Immunol. 1985;135:2319–2322. [PubMed] [Google Scholar]
- 28.Schutze M P, Leclerc C, Vogel F R, Chedid L. Epitopic suppression in synthetic vaccine models: analysis of the effector mechanisms. Cell Immunol. 1987;104:79–90. doi: 10.1016/0008-8749(87)90008-6. [DOI] [PubMed] [Google Scholar]
- 29.Schutze M P, Deriaud E, Przewlocki G, Leclerc C. Carrier-induced epitopic suppression is initiated through clonal dominance. J Immunol. 1989;142:2635–2640. [PubMed] [Google Scholar]
- 30.Seeber S J, White J L, Hem S L. Predicting the adsorption of proteins by aluminium containing adjuvants. Vaccine. 1991;9:201–203. doi: 10.1016/0264-410x(91)90154-x. [DOI] [PubMed] [Google Scholar]
- 31.Sher A, Coffman R L. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- 32.Ternynck T, Avrameas S. Techniques immuno-enzymatiques. Paris, France: Edn. INSERM; 1987. [Google Scholar]
- 33.Trudel M, Nadon F, Seguin C, Binz H. Protection of BALB/c mice from respiratory syncytial virus infection by immunization with a synthetic peptide derived from the G glycoprotein. Virology. 1991;185:749–757. doi: 10.1016/0042-6822(91)90546-n. [DOI] [PubMed] [Google Scholar]
- 34.Valmori D, Pessi A, Bianchi E, Corradin G. Use of human universally antigenic tetanus toxin T cell epitopes as carriers for human vaccination. J Immunol. 1992;149:717–721. [PubMed] [Google Scholar]