Abstract
Mycobacterium tuberculosis GroES was purified from culture filtrate, and its identity was confirmed by immunoblot analysis and N-terminal sequencing. Comparing the immunological recognition of native and recombinant GroES, we found that whereas native GroES elicited a strong proliferative response and release of gamma interferon-γ by peripheral blood mononuclear cells from healthy tuberculin reactors, the recombinant protein failed to do so. The same difference in immunological recognition was observed in a mouse model of TB infection. Both the native and recombinant preparations were recognized by mice immunized with the recombinant protein. Biochemical characterization including sodium dodecyl sulfate-polyacrylamide gel electrophoresis, two-dimensional electrophoresis, and mass spectrometry analysis of both proteins demonstrated no differences between the native and recombinant forms of GroES except for the eight additional N-terminal amino acids derived from the fusion partner in recombinant GroES. The recombinant fusion protein, still tagged with the maltose binding protein, was recognized by T cells isolated from TB-infected mice if mixed with culture filtrate before affinity purification on an amylose column. The maltose binding protein treated in the same manner as a control preparation was not recognized. Based on the data presented, we suggest that the association of biologically active molecules from culture filtrate with the chaperone GroES may be responsible for the observed T-cell recognition of the native preparation.
Characterization of antigens which are recognized during natural infection in tuberculosis (TB) patients as well as in animal models of the disease is important in the selection of antigens to be included in new TB vaccines and diagnostics. Several immunodominant antigens have been described (as reviewed in reference 46 and recently in reference 2). Some of these antigens have been produced as recombinant proteins and are distributed through the WHO IMMYC Recombinant Protein Bank. This important initiative has allowed standardized large-scale studies of T-cell recognition in human patients and in animal models. It is of critical importance, however, to ensure that the activities of the recombinant and native preparations are comparable. We have previously found that antigen recognition of T cells isolated from patients with minimal TB is directed against a wide range of different-molecular-mass antigens (15). In this study, the low-molecular-mass fraction enriched in GroES (also called cpn10, hsp10, and 10-kDa antigen) was frequently recognized and gave high levels of gamma interferon (IFN-γ) production in patients with minimal TB (15). The same fraction elicited high levels of IFN-γ production from memory immune lymphocytes in the mouse model of TB infection (3). Mycobacterial GroES has previously been described as a major T-cell antigen strongly recognized in TB patients and infected animals (12, 28, 34). However, several studies have tested recombinant Mycobacterium tuberculosis GroES and reported only limited T-cell responses in TB-infected mice and patients (3, 29, 37, 42). This discrepancy together with recent reports of different immunological activities of native and recombinant mycobacterial antigen preparations (13, 23, 39, 44) have stimulated our interest in performing immunological and biochemical comparisons of native and recombinant GroES.
In this study, we purified native M. tuberculosis GroES (nGroES) from short-term culture filtrate (ST-CF) and compared the T-cell recognition of recombinant and native antigens. We have demonstrated that in BCG-vaccinated human donors as well as in TB-infected mice, recombinant GroES (rGroES) induced almost no cellular immune responses whereas nGroES was highly stimulatory. No biochemical differences between the two molecules could be detected by a range of techniques. Interestingly, the recombinant molecule was biologically active after contact with culture filtrate, suggesting that antigen moieties present in the culture filtrate are associated with nGroES and may be responsible for the T-cell recognition.
MATERIALS AND METHODS
Antigens and mitogens.
ST-CF was produced as described previously (5). Briefly, M. tuberculosis H37Rv was grown in modified Sauton medium without Tween 80 on an orbital shaker for 7 days at 37°C. The bacteria were removed by filtration and the filtrate was concentrated 100-fold on an Amicon (Danvers, Mass.) YM3 membrane.
Purification of nGroES. (i) Preparative electrophoresis.
ST-CF (200 mg) was precipitated with ammonium sulfate to a final saturation of 80%. The precipitate was resuspended in distilled water and washed three times with 8 M urea on a Centriprep 3 unit (Amicon). ST-CF proteins were fractionated on a Rotofor preparative isoelectric focusing cell (Bio-Rad, Richmond, Calif.) as described elsewhere (45). The collected fractions were analyzed by silver-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the GroES-containing fractions were pooled and refractionated. The pooled protein was further purified by whole-gel elution as described in reference 7. Briefly, SDS-PAGE sample buffer was added to the sample, which was boiled for 5 min and separated by standard SDS-PAGE. After termination of electrophoresis, the gel was equilibrated in 2 mM phosphate buffer and electroeluted in the whole-gel eluter (Bio-Rad). The obtained fractions were aspirated, adjusted to isotonia with concentrated phosphate-buffered saline (PBS; pH 7.4), and analyzed by silver-stained SDS-PAGE and immunoblot analysis using the M. tuberculosis GroES monoclonal antibody (MAb) HYB 76-1.
(ii) Column chromatography.
Ammonium sulfate was added to ST-CF to obtain a final concentration of 1.5 M ammonium sulfate. The soluble proteins were subsequently subjected to thiophilic adsorption chromatography (36) on an Affi-T gel column (Kem-En-Tec, Copenhagen, Denmark), and proteins were eluted by a linear 1.5 to 0 M gradient of ammonium sulfate. The fractions were analyzed by silver-stained SDS-PAGE, fractions containing nGroES were concentrated, and the buffer was changed to 10 mM Tris-HCl (pH 8.5) by ultrafiltration. Further purification was performed on a Mono Q HR 5/5 column (Pharmacia, Uppsala, Sweden) equilibrated with 10 mM Tris-HCl (pH 8.5) and eluted with a linear gradient of sodium chloride (0 to 0.75 M). Fractions were analyzed by silver-stained SDS-PAGE and immunoblot analysis with HYB 76-1, fractions of pure nGroES were pooled, and the buffer was changed to PBS (pH 7.4).
Recombinant proteins.
M. tuberculosis and M. leprae GroES were expressed in Escherichia coli as fusion proteins with the maltose binding protein, purified on an amylose affinity column, cleaved with factor Xa, and further purified by anion exchange as described previously (28). M. tuberculosis GroES batches Mt 10-1B and Mt 10-2 and M. leprae GroES batches Ml 10-1B and Ml 10-2 were supplied by the WHO Recombinant Protein Bank. The maltose binding protein was obtained from the amylose affinity column after factor Xa cleavage of the MPT64 fusion protein (32). The expression and purification of M. tuberculosis GroES in E. coli with no additional amino acids (Mtcpn10) have previously been described (21, 22).
For affinity chromatography, 0.5 mg of the M. tuberculosis GroES fusion protein or the maltose binding protein was mixed with 10 mg of ST-CF for 1 h at room temperature in PBS (pH 7.4). The fusion protein or the maltose binding protein was thereafter purified on the amylose affinity column as recommended by the manufacturer (New England Biolabs, Beverly, Mass.). Fractions eluted by maltose were analyzed by silver-stained SDS-PAGE and immunoblot analysis with either the maltose binding protein antiserum or HYB 76-1.
Other antigens and mitogens.
Synthetic M. tuberculosis GroES was produced and purified as described previously (26). Ag85B was kindly provided by S. Nagai (Osaka City University Medical School, Osaka, Japan). Phytohemagglutinin (HA 17) was from Wellcome (Beckenham, United Kingdom), concanavalin A was from Sigma Chemical Co. (St. Louis, Mo.), and purified protein derivative (PPD; RT47) was from Statens Serum Institut (Copenhagen, Denmark).
Analyses of antigen preparations.
Protein concentrations were determined by the Micro BCA (bicinchoninic acid) method (Pierce, Oud-Beijerland, The Netherlands).
SDS-PAGE and two-dimensional PAGE (2-DE) were performed in the Protean IIxi system (Bio-Rad). Standard SDS-PAGE in 10 to 20% gradient gels, 2-DE, and Tricine SDS-PAGE were performed as previously described (41).
Lipopolysaccharide (LPS) content was determined by the Limulus amoebocyte lysate clot test described in reference 10. Recombinant antigen preparations were passaged through endotoxin-removing columns (Detoxi-Gel, 20344; Pierce) as recommended by the manufacturer. The LPS content in the rGroES preparations after removal of endotoxin was 0.06 to 0.6 ng of endotoxin/μg of protein. nGroES preparations contained 0.08 to 0.8 ng of endotoxin/μg of protein.
Antibodies.
HYB 76-1, the MAb reacting with GroES, was produced by immunization of BCG-primed BALB/c mice with PPD (25) and was kindly provided by C. Koch, Statens Serum Institut. The maltose binding protein rabbit antiserum was from New England Biolabs.
N-terminal sequence analysis.
nGroES, purified by preparative electrophoresis, was subjected to reversed-phase high-performance liquid chromatography on a Vydac (Hesperia, Calif.) 214TP52 C4 column (2.1 by 25 mm) equilibrated with 9.5% isopropanol in 0.1% trifluoroacetic acid. Elution was performed with a 9.5 to 76% linear gradient of isopropanol in 0.1% trifluoroacetic acid. The collected peak material was analyzed by silver-stained SDS-PAGE, and N-terminal sequence analysis was performed on a Procise sequencer (Applied Biosystems, Foster City, Calif.) as described by the manufacturer.
MS analysis.
nGroES purified by preparative electrophoresis and rGroES (batch Mt 10-2) were subjected to 16% Tricine SDS-PAGE and blotted onto a polyvinylidene difluoride membrane, which was stained with Coomassie blue, as previously described (41). The bands were excised and digested in situ with trypsin, and the total mixtures were analyzed by matrix-assisted laser desorption ionization–time-of-flight (MALDI-TOF) mass spectrometry (MS) (20). The fragments were identified by matching the experimental masses with the calculated masses of the expected tryptic peptides of nGroES and rGroES, using the GPMAW program (Lighthouse Data, Odense, Denmark). The mass of intact nGroES was determined by MALDI-TOF MS after dialysis of nGroES against 0.1 M ammonium bicarbonate buffer in a Tube-O-Dialyser unit (cutoff, 1,000 Da; Chemicon, Temecula, Calif.).
Experimental infection/immunization and mouse lymphocyte cultures.
M. tuberculosis H37Rv, used for infection experiments, was grown at 37°C on Löwenstein-Jensen medium or in suspension in modified Sauton medium enriched with 0.5% sodium pyrovate and 0.5% glucose. The animals, female 6- to 12-week-old C57BL/6J mice (Bomholtegaard, Ry, Denmark), were housed in cages contained within laminar flow safety enclosure for the infection experiments. Intravenous infections were administered via the lateral tail vein with an inoculum of 5 × 104 M. tuberculosis suspended in PBS (pH 7.4) in a volume of 0.1 ml. Memory immune mice were obtained by clearing the primary infection by chemotherapy as described previously (8). This group of mice received isoniazid (Merck, Rathway, N.J.) and rifabutin (Farmatalia Carlo Erba, Milano, Italy) in their drinking water for 2 months. The mice were rested for 4 to 6 months before being used for experiments. For the study of the recall reaction, animals were infected with an inoculum of 106 bacteria and sacrificed at day 4.
Naive C57BL/6J mice were immunized subcutaneously into the footpads with 10 μg of rGroES (batch Mt 10-2) or PBS in a volume of 0.2 ml emulsified in incomplete Freund's adjuvant (IFA; Statens Serum Institut). Twelve days after immunization, animals were sacrificed.
Spleen cells and lymph node cells were obtained by preparing single-cell suspensions as described previously (6). Lymphocytes were cultured in microtiter wells (Nunc, Roskilde, Denmark) containing 2 × 105 cells in a volume of 200 μl of RPMI 1640 supplemented with 5 × 10−5 M 2-mercaptoethanol, penicillin-streptomycin, 1 mM glutamine, and 5% (vol/vol) fetal calf serum. Cellular proliferation was investigated by pulsing cultures after 48 h of incubation (1 μCi of [3H]thymidine per well), and after further 22 h of incubation, plates were harvested and processed for liquid scintillation counting as previously described (16). All tests were carried out in triplicate, and all counts-per-minute values are given as geometric means. Supernatants for the investigation of IFN-γ were harvested after 48 h of incubation. Culture supernatants were tested in triplicate, and IFN-γ was detected by enzyme-linked immunosorbent assay (ELISA) with biotin-labeled rat anti-murine IFN-γ MAb (16). Concanavalin A (1 μg/ml) was used in all experiments as a positive control for cell viability.
Isolation and in vitro culture of PBMC from human donors.
Peripheral blood mononuclear cells (PBMC) were obtained from healthy BCG-vaccinated Danish donors. PBMC were freshly isolated by gradient centrifugation of heparinized blood on Lymphoprep (Nycomed, Oslo, Norway). The cells were resuspended in RPMI 1640 supplemented with 5 × 10−5 M 2-mercaptoethanol, 100 IU of penicillin per ml, 50 μg of streptomycin per ml, nonessential amino acids, 1 mM glutamine, and 10% human ABO serum. PBMC (105) were added to triplicate wells in round-bottom microtiter plates (Nunc) and stimulated for 3 days with mitogens and 5 or 7 days with antigens; 24 h before harvesting, 0.25 μCi of [3H]thymidine (TRA 120; Radiochemical Centre, Amersham, United Kingdom) was added to triplicate wells, and [3H]thymidine incorporation was measured by liquid scintillation counting. IFN-γ was detected on duplicate supernatants by ELISA (MABTECH, Stockholm, Sweden) according to the manufacturer's instructions. Recombinant human IFN-γ (Gibco BRL, Rockville, Md.) was used as a standard. Phytohemagglutinin (1 μg/ml) was used in all experiments as a positive control for cell viability.
Statistical analysis.
A paired t test was used to compare the difference in proliferative or IFN-γ response between native and recombinant antigens, and P < 0.05 was considered significant.
RESULTS
Purification of nGroES.
The 10-kDa M. tuberculosis homologue of the heat shock protein GroES was purified from ST-CF by preparative 2-DE with isoelectric focusing as the first step followed by size separation in SDS-PAGE and electroelution as described in Materials and Methods. The combination of these methods gave a nontoxic, pure preparation of nGroES, which could be used directly in immunological assays.
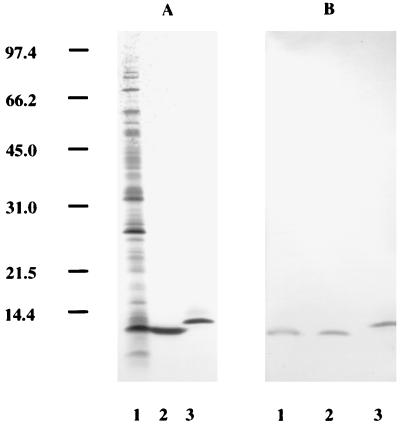
nGroES was analyzed by SDS-PAGE (Fig. 1A) and compared with the rGroES supplied by the WHO Recombinant Protein Bank. Both proteins gave one band in SDS-PAGE, and for rGroES the observed mobility was slightly lower than that for the nGroES protein, in accordance with the presence of eight additional amino acids in rGroES derived from the construction of the fusion protein in the pMAL expression system. As judged from SDS-PAGE, nGroES was purified to homogeneity; no contaminating bands were observed (Fig. 1A). Immunoblot analysis with MAb HYB 76-1 showed reactivity to nGroES as well as rGroES (Fig. 1B).
FIG. 1.
Analysis of native and recombinant GroES. Different preparations of M. tuberculosis GroES were analyzed by silver-stained SDS-PAGE (A) and immunoblot analysis with MAb HYB 76-1 (B). Lanes: 1, ST-CF; 2, biochemically purified nGroES; 3, rGroES. Numbers at the left indicate molecular mass markers in kilodaltons.
T-cell responses to nGroES and rGroES.
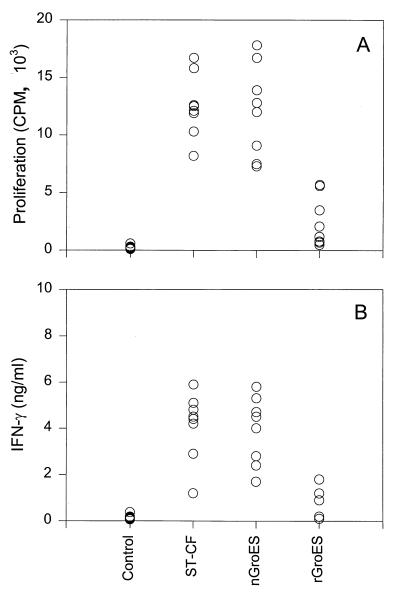
PBMC from two healthy PPD-positive individuals were stimulated with different concentrations of the recombinant and native GroES preparations. Stimulation with nGroES induced significant proliferative and IFN-γ response in all concentrations above 0.1 μg/ml, whereas neither proliferation nor IFN-γ release was observed after stimulation with the same concentrations of rGroES (results not shown). To confirm and extend these observations, a comparison of nGroES and rGroES at the concentration 5 μg/ml was done on eight PPD-responsive, healthy Danish individuals (Fig. 2). All donors recognized the nGroES, whereas only very limited response to rGroES was found. This difference was pronounced for both proliferation and IFN-γ release: the median value of the proliferative responses of eight donors to nGroES was 12.4 × 103 cpm, whereas the median value of the responses to rGroES was 1.65 × 103 cpm; for IFN-γ release, the median values of the responses to nGroES and rGroES were 4.45 and 0.55 ng/ml, respectively. These differences in the cellular responses to the two preparations were highly significant (P < 0.02).
FIG. 2.
Human T-cell responses to nGroES and rGroES. PBMC from eight healthy, BCG-vaccinated individuals were stimulated with no antigen (control) or with ST-CF, nGroES, and rGroES at the concentration 5 μg/ml. The results are shown as individual proliferative (A) and IFN-γ (B) responses after stimulation. The variation between individual wells was less than 10% of the mean.
We continued by investigating the recognition of nGroES and rGroES in the mouse model of TB infection. Mice rendered memory immune to TB by a primary infection followed by chemotherapy were tested 4 days after challenge with M. tuberculosis. Splenic lymphocytes were isolated, and cellular responses to ST-CF, nGroES, rGroES, and antigen 85B (Ag85B) were investigated (Table 1, experiment I). In this experiment, two different batches of rGroES were compared to check for potential batch-to-batch variation. Furthermore, two preparations of M. leprae rGroES were included. None of the mycobacterial rGroES batches induced measurable levels of IFN-γ, whereas the nGroES was strongly recognized. Stimulation with ST-CF and Ag85B gave a prominent IFN-γ release, as expected (3). Thereafter, we investigated a different nGroES preparation (purified by a combination of hydrophobic interaction chromatography and anion exchange [see Materials and Methods]), a different rGroES preparation (expressed in a different expression system), and a chemically synthesized version of the GroES molecule (Table 1, experiment II). Among the M. tuberculosis GroES samples tested, only nGroES induced a T-cell response, demonstrating that only the native preparation is a source of the epitope(s) which are recognized by splenocytes from memory immune mice.
TABLE 1.
Recognition of mycobacterial antigens by mouse memory effector cellsa
| Expt | Antigenb | Mean IFN-γ releasec (ng/ml) ± SE |
|---|---|---|
| I | ST-CF | 21.4 ± 0.9 |
| Ag85B | 6.5 ± 0.2 | |
| nGroES (I) | 6.5 ± 0.2 | |
| rGroES (Mt 10-2) | 0.4 ± 0.1 | |
| rGroES (Mt 10-1B) | 0.3 ± 0.1 | |
| rGroES (Ml 10-2) | 0.3 ± 0.1 | |
| rGroES (Ml 10-1B) | 0.4 ± 0.2 | |
| II | ST-CF | 22.9 ± 2.7 |
| nGroES (II) | 3.2 ± 0.3 | |
| rGroES (Mt 10-2) | 0.4 ± 0.1 | |
| rGroES (Mtcpn10) | 0.5 ± 0.0 | |
| sGroES | 0.4 ± 0.0 |
Splenic lymphocytes were isolated 4 days after reinfection with 106 M. tuberculosis and stimulated in vitro.
All antigens were used at a concentration of 5 μg/ml. nGroES (I) was purified by preparative electrophoresis; nGroES (II) was purified by column chromatography; rGroES (M. tuberculosis) was expressed in E. coli either in the pMAL system (Mt 10-2 and Mt 10-1B) or in the T7 expression system (Mtcpn10); rGroES (M. leprae) was expressed in the pMAL system (Ml 10-2 and Ml 10-1B); sGroES was the synthetic version of M. tuberculosis GroES.
Stimulation without antigen gave IFN-γ values of <0.05 ng/ml.
The presence of potentially toxic substances in the rGroES preparation (batch Mt 10-2) causing inhibition of the murine and human T-cell responses was also investigated. Lymphocytes from non-mycobacterium-reactive human donors or naive mice were stimulated with suboptimal concentrations of mitogen plus different concentrations of rGroES. No inhibitory effect on the proliferative responses were detected in either model (data not shown).
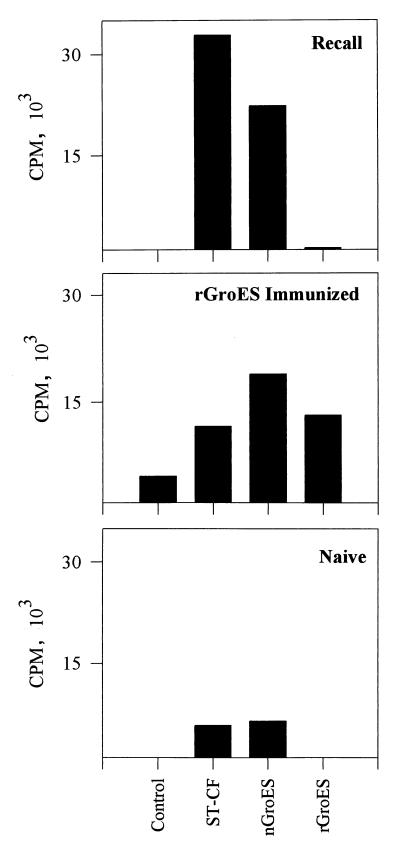
To investigate if rGroES can induce a recall response to the homologous preparation, the recognition of nGroES and rGroES was investigated after immunization with rGroES and compared to responses induced by TB infection. C57BL/6J mice were immunized with 10 μg of rGroES in IFA; 12 days later, the draining lymph node cells were isolated and stimulated with ST-CF, nGroES, and rGroES (Fig. 3). In mice immunized with rGroES, we observed in vitro recall responses to the homologous preparation as well as to nGroES and ST-CF. This demonstrates that the recombinant preparation contains T-cell epitopes and that these epitopes are found in the native version of the molecule. Splenocytes from M. tuberculosis-infected mice recognized ST-CF and nGroES but not rGroES. In naive mice, only a low level of proliferation was observed to any of the antigens, excluding the presence of nonspecific stimulatory compounds in the preparation.
FIG. 3.
Priming for T-cell recognition of nGroES and rGroES by immunization and TB infection. Lymphocytes were isolated from the spleens of memory immune mice 4 days after reinfection with M. tuberculosis (top) from naive mice (bottom), or from the lymph nodes of mice immunized with rGroES in IFA (middle). The lymphocytes were stimulated in vitro with ST-CF (4.0 μg/ml), nGroES (10 μg/ml), and rGroES (10 μg/ml). Control, no antigen added. Standard errors were less than 15% of the mean.
Biochemical characterization of nGroES and rGroES.
To identify possible differences between the nGroES and rGroES molecules, several biochemical analyses were undertaken.
The purity of the nGroES preparation was evaluated by 2-DE analysis and silver staining, which demonstrated three individual spots (Fig. 4). All three spots were recognized by HYB 76-1 after immunoblot analysis (data not shown), indicating that no other proteins were copurified with GroES. Similar results were obtained for nGroES purified by column chromatography (data not shown).
FIG. 4.
Silver-stained 2-DE gel of biochemically purified nGroES. The three individual spots reacting with HYB 76-1 are marked by arrowheads. Molecular masses in kilodaltons are indicated on the left.
The N-terminal amino acid sequence of nGroES was determined, and one sequence was obtained (Table 2). This sequence is in agreement with the published amino acid sequence of M. tuberculosis GroES (11, 30). As previously reported by Nagai and coworkers (30), the N-terminal methionine is cleaved off in the mature protein. In rGroES, the first seven amino acids are derived from the fusion partner; in total, rGroES contains eight additional amino acids compared to the mature, native protein. This analysis confirmed the identity of the purified nGroES and revealed no other proteins or peptides present in this preparation.
TABLE 2.
N-terminal amino acid sequences of nGroES and rGroES
| Protein | Sequence |
|---|---|
| nGroES | X X V N I K P L E Da |
| GroES | M A K V N I K P L E Db |
| rGroES | L R P Q P E F V A Kc |
Sequence of purified nGroES from ST-CF (this study).
Deduced from the published DNA sequence (11).
As stated in the data sheet accompanying the batch Mt 10-2 from the WHO Recombinant Protein Bank.
A recent study reports that delayed-type hypersensitivity activity of the Brucella L7/L12 ribosomal proteins is dependent on lipidation which occurs, in brucellae but not in E. coli (9). An MS analysis was performed after tryptic digestion of nGroEs and rGroES to determine whether any posttranslational modifications are present in either preparation (Table 3). The measured masses of the peptides obtained were compared with the calculated masses; these values were identical for all peptides within experimental error, indicating that no posttranslational modifications are found in nGroES or rGroES; 90 and 93% sequence coverage was observed for nGroES and rGroES, respectively. The mass of intact nGroES was determined by MS to be 10,678.5 (±10) Da, which is in full agreement with the calculated mass of 10,673 Da. In this analysis, peaks corresponding to masses of ca. 3,000 Da were also observed. However, attempts to identify the origins of these peptides or potential fragments of nGroES were not successful.
TABLE 3.
MS tryptic peptide data
| Amino acid residues | Avg molecular mass (Da)
|
||
|---|---|---|---|
| Calculated | nGroES | rGroES | |
| −8–2 | 1,185.4 | 1,185.5 | |
| 3–11 | 1,056.3 | 1,056.7 | 1,056.0 |
| 12–34 | 2,343.6 | 2,344.6 | 2,344.6 |
| 35–49 | 1,524.7 | 1,525.1 | 1,524.9 |
| 35–56 | 2,384.6 | 2,385.4 | 2,385.0 |
| 35–57 | 2,540.8 | 2,541.7 | 2,541.5 |
| 50–57 | 1,035.1 | 1,035.1 | 1,035.0 |
| 57–72 | 1,777.0 | 1,777.4 | 1,777.4 |
| 58–72 | 1,620.8 | 1,621.0 | 1,620.7 |
| 73–91 | 2,177.4 | 2,179.1 | 2,178.7 |
| 80–91 | 1,428.6 | 1,429.5 | 1,429.4 |
Murine recognition of the recombinant GroES fusion protein.
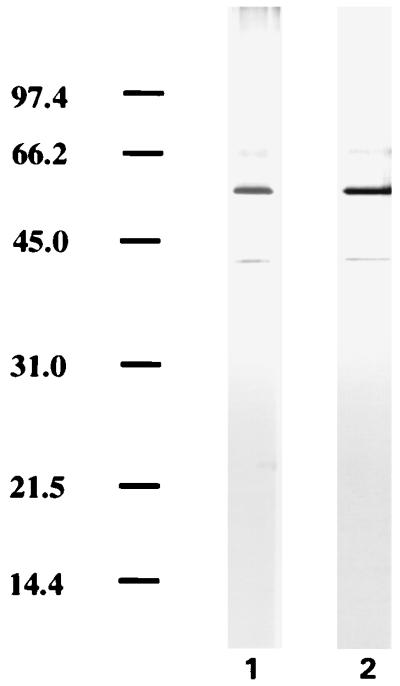
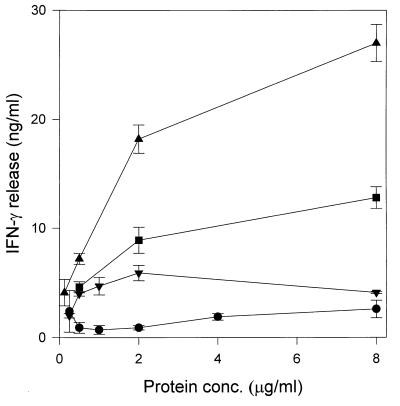
Although the purity of the GroES preparations has been checked rigorously, the above results raised the possibility that small amounts of molecules derived from culture filtrate associate with the GroES protein in a noncovalent manner and are responsible for the observed activity of nGroES. We addressed this possibility by mixing the recombinant M. tuberculosis GroES protein still fused to the maltose binding protein with ST-CF. This was followed by affinity purification of the GroES fusion protein on an amylose column. SDS-PAGE analysis of the two preparations did not reveal any additional bands in the GroES fusion protein preparation after contact with ST-CF (Fig. 5). T cells isolated from memory immune mice 4 days after reinfection with M. tuberculosis were thereafter stimulated with the two preparations of the GroES fusion protein. In confirmation of our earlier findings, no induction of IFN-γ was observed in response to the GroES fusion protein in the dose range of 0.25 to 8 μg/ml, but the fusion protein which had been mixed with culture filtrate induced a significant release of IFN-γ with a peak at 2 μg/ml, although the levels of IFN-γ induced were lower than those observed for nGroES (Fig. 6). The same experiment was performed with the maltose binding protein alone, but at a concentration of 2.0 μg/ml no detectable IFN-γ was released neither before or after treatment with ST-CF (data not shown). These data suggest that the GroES part of the fusion protein binds the moieties from ST-CF responsible for the immunological recognition.
FIG. 5.
Analysis of ST-CF-treated rGroES. The recombinant fusion protein of GroES and the maltose binding protein was mixed with ST-CF followed by affinity chromatography on an amylose column. The preparation was analyzed before (lane 1) and after (lane 2) this treatment by SDS-PAGE and silver staining. Molecular masses in kilodaltons are indicated on the left.
FIG. 6.
Recognition of mycobacterial antigens by mouse memory effector cells. Splenic lymphocytes were isolated from three memory immune mice 4 days after reinfection with 106 M. tuberculosis. The lymphocytes were stimulated in vitro with ST-CF (▴), nGroES purified by column chromatography (■), the recombinant fusion protein of the maltose binding protein and M. tuberculosis GroES (●), and the recombinant fusion protein treated with ST-CF (▾). The levels of IFN-γ were measured in culture supernatants; each point represents the mean of triplicate values ± standard error of the mean. Stimulation without antigen gave IFN-γ values below 0.05 ng/ml. This experiment was repeated twice with the same overall results.
DISCUSSION
M. tuberculosis GroES was originally identified as a major T-cell antigen responsible for the strong human and murine T-cell recognition of antigen fractions with a molecular mass of around 10 kDa (12, 34). The strong reactivity to low-mass antigens has been confirmed in other studies of TB patients and BCG-vaccinated human donors (15, 38) and is also a characteristic of early disease in several animal species (16, 24, 35). However, data from a number of more recent studies collectively suggested that the same high level of responses cannot be induced by the recombinant version of GroES (3, 29, 37, 42).
Intrigued by these findings, we purified M. tuberculosis GroES by classical chromatographic methods as well as by preparative 2-DE and conducted a comparative study of nGroES and rGroES. The nGroES molecule was purified to homogeneity as evaluated by SDS-PAGE, 2-DE, MS, and N-terminal sequence analysis. The present study demonstrated a marked difference in the T-cell recognition of these preparations, and the lack of reactivity to the recombinant preparations was reproduced with different batches of rGroES from the WHO Recombinant Protein Bank. The rGroES preparations tested all had a low content of LPS and were nontoxic in cell cultures.
Mycobacteria are phylogenetically different from E. coli and may therefore handle many posttranslational modifications differently. This may directly influence the immunogenicity of lipidated or glycosylated proteins expressed in E. coli. It is widely believed that this limitation may impose severe constraints on the use of E. coli for the expression of mycobacterial proteins. In this regard, two recent reports have described immunological differences between the mycobacterial protein MPT64 and the 35-kDa M. leprae protein when expressed in M. smegmatis instead of E. coli (39, 44). A conformation-dependent MAb recognized the M. smegmatis-derived 35-kDa protein but not the E. coli-derived form of the protein, suggesting a different folding of the proteins (44). The 19-kDa M. tuberculosis glycosylated lipoprotein has in a similar way been expressed in E. coli and M. vaccae, and stronger IFN-γ responses to the M. vaccae-derived protein were observed. After immunization with M. vaccae, splenocytes recognized the recombinant 19-kDa protein purified from M. vaccae but not the equivalent E. coli protein. This suggested that posttranslational modifications are important for the processing and presentation of the 19-kDa protein or that the M. vaccae-derived protein contains noncovalently associated contaminants which influence the immune response (1). In the present study, MS analysis of tryptic peptides from nGroES and rGroES demonstrated no posttranslational modifications of either protein, and the two molecules had identical mass spectra, the only difference being, as expected, the eight additional amino acids in the recombinant molecule. However, the extension itself is not likely to explain the lack of T-cell stimulatory properties, as neither rGroES with no extra amino acids nor a synthetic version of the entire M. tuberculosis GroES molecule is recognized in the mouse model of TB infection. Interestingly, both rGroES and nGroES were recognized by T cells after immunization with the recombinant molecule, indicating that the recombinant preparation contains T-cell epitopes also present in the native version of the molecule. Having demonstrated that nGroES and rGroES are biochemically identical, we see two different possible explanations for the observed differences in biological activity. It has been reported that M. tuberculosis GroES exists as a tetramer in lysates and dilute solutions and forms a heptamer only at high protein concentrations or in the presence of divalent ions. E. coli GroES, in contrast, exists predominantly as a heptamer (21). At present, the influence of different oligomeric forms on antigen processing, presentation, and recognition is an unexplored field, but from a theoretical point of view such differences may influence the accessibility of proteolytic cleavage sites. Alternatively, it is possible that monomeric rGroES is misfolded, which might conceal T-cell epitopes, and incubation with ST-CF may lead to a correctly folded molecule which stimulates T cells. However, the hypothesis that we find most likely is that the differences in T-cell recognition may be due to the presence of trace amounts of biologically active contaminants in the nGroES preparation not detectable by conventional analytical methods. By mixing the uncleaved recombinant fusion protein, still containing the maltose binding protein, with ST-CF followed by repurification on an amylose column, the recombinant preparation was modified such that it was recognized by mouse T cells. The molecule still appeared pure, and no visible differences were observed in a silver-stained SDS-PAGE analysis. One possibility that cannot be rigorously ruled out by our analysis is that trace amounts of the nGroES were copurified with the recombinant molecule, but given the high sensitivity of silver staining, our data suggest that such low concentrations of nGroES would be much below the minimal stimulatory concentration of this molecule. However, highly stimulatory mycobacterial antigen fractions such as Ag85 and ESAT-6 give detectable responses at very low concentrations (4, 33). We therefore hypothesize that the observed differences are due to presence of small amounts of highly reactive molecules bound to GroES. This hypothesis could help explain a number of unexpected observations. For example, Barnes and coworkers (12) showed that nGroES purified from culture filtrate stimulated a prominent proliferative response in PBMC from healthy PPD-positive human donors. Ten nGroES-reactive T-cell clones were established from these donors, but it was reported that only some of them seemed to recognize epitopes on the GroES molecule when they were screened by overlapping peptides (12). Similarly, Ben-Nun et al. identified and investigated a T-cell line specific for nGroES purified from PPD by preparative electrophoresis (13). This line was found totally nonresponsive to the recombinant version of GroES.
Interestingly, the suggested binding of ST-CF molecules to the fusion protein exhibited some specificity since the fusion partner, the maltose binding protein, coincubated and purified in a similar way, had no immunological activity. Although the chaperone function of M. tuberculosis GroES has not been demonstrated, it is well documented that E. coli GroES binds to the heat shock protein GroEL, forming a complex involved in proper folding of proteins intracellularly (19). In this complex, GroEL is involved in substrate binding by hydrophobic interactions. However, M. leprae GroES has a polar inner surface in the complex, and it has been hypothesized that M. leprae GroES also may play an active role in protein folding by binding to hydrophilic parts of the substrate molecules (27). This hypothesis is supported by the study of Bochkareva and Girshovich which demonstrated interaction of newly synthesized proteins with E. coli GroES (14). The binding sites of hsp70, another chaperone, have been mapped on three proteins from M. tuberculosis, and these were predominantly regions with clusters of aliphatic amino acids (40). Recent findings from our laboratories indicate a markedly different activity also of native and recombinant M. tuberculosis hsp70 (37), in contrast to a number of recently expressed antigens such as ESAT-6, which has been demonstrated to have similar stimulatory activities in the native and recombinant forms (43). The proposed association of culture filtrate molecules with GroES and hsp70 must be quite resistant, as it is retained at least partially after SDS-PAGE. The observed differences in immunological activity may be related to the biological function of GroES and hsp70 as heat shock proteins, and of interest for this discussion are experiments showing induction of antitumor immunity after immunization with the heat shock proteins hsp70 and gp96 purified from cancer cells, presumably due to association of tumor-derived peptides with the heat shock proteins (17, 31). Future work in our laboratories will be directed to establishing whether peptides bind in the same way to M. tuberculosis GroES.
The future selection of antigens for novel TB vaccines will have to be based on large-scale evaluation of antigen recognition in different populations. The WHO antigen bank is an important initiative which allows comparative studies to be conducted in several laboratories. However, the present study emphasizes that a careful evaluation of native as well as recombinant proteins is of crucial importance for the successful establishment of such standard panels. The access to recombinant M. tuberculosis proteins is facilitated by the complete genomic sequence of M. tuberculosis (18). In many cases, the corresponding native protein will not be purified, and therefore the choice of the host expression system is of major importance. In the present study, E. coli was demonstrated to have the advantage that no contaminant of mycobacterial origin was copurified. In other cases, M. smegmatis or other mycobacterial expression systems will be needed to ensure proper processing of the recombinant protein; in this case, new sensitive methods for the detection of trace amounts of mycobacterial contaminants may be needed.
ACKNOWLEDGMENTS
This investigation was supported by the Danish National Association against Lung Diseases, the University of Copenhagen, UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, and the Danish Research Center for Biotechnology.
We thank T. Oettinger for provision of the maltose binding protein and Bente Lund Sørensen, Birgitte Smedegård, Annette Hansen, Tove Slotved Simonsen, and Marie Olesen for excellent laboratory assistance.
REFERENCES
- 1.Abou-Zeid C, Gares M P, Inwald J, Janssen R, Zhang Y, Young D B, Hetzel C, Lamb J R, Baldwin S L, Orme I M, Yeremeev V, Nikonenko B V, Apt A S. Induction of a type 1 immune response to a recombinant antigen from Mycobacterium tuberculosis expressed in Mycobacterium vaccae. Infect Immun. 1997;65:1856–1862. doi: 10.1128/iai.65.5.1856-1862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen P. Host responses and antigens involved in protective immunity to Mycobacterium tuberculosis. Scand J Immunol. 1997;45:115–131. doi: 10.1046/j.1365-3083.1997.d01-380.x. [DOI] [PubMed] [Google Scholar]
- 3.Andersen P, Andersen Å B, Sørensen A L, Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol. 1995;154:3359–3372. [PubMed] [Google Scholar]
- 4.Andersen P, Askgaard D, Gottschau A, Bennedsen J, Nagai S, Heron I. Identification of immunodominant antigens during infection with Mycobacterium tuberculosis. Scand J Immunol. 1992;36:823–831. doi: 10.1111/j.1365-3083.1992.tb03144.x. [DOI] [PubMed] [Google Scholar]
- 5.Andersen P, Askgaard D, Ljungqvist L, Bennedsen J, Heron I. Proteins released from Mycobacterium tuberculosis during growth. Infect Immun. 1991;59:1905–1910. doi: 10.1128/iai.59.6.1905-1910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen P, Askgaard D, Ljungqvist L, Bentzon M W, Heron I. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect Immun. 1991;59:1558–1563. doi: 10.1128/iai.59.4.1558-1563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen P, Heron I. Simultaneous electroelution of whole SDS-polyacrylamide gels for the direct cellular analysis of complex protein mixtures. J Immunol Methods. 1993;161:29–39. doi: 10.1016/0022-1759(93)90195-d. [DOI] [PubMed] [Google Scholar]
- 8.Andersen P, Heron I. Specificity of a protective memory immune response against Mycobacterium tuberculosis. Infect Immun. 1993;61:844–851. doi: 10.1128/iai.61.3.844-851.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachrach G, Banai M, Fishman Y, Bercovier H. Delayed-type hypersensitivity activity of the Brucella L7/L12 ribosomal protein depends on posttranslational modification. Infect Immun. 1997;65:267–271. doi: 10.1128/iai.65.1.267-271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baek L. New, sensitive rocket immunoelectrophoretic assay for measurement of the reaction between endotoxin and Limulus amoebocyte lysate. J Clin Microbiol. 1983;17:1013–1020. doi: 10.1128/jcm.17.6.1013-1020.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baird P N, Hall L M C, Coates A R M. Cloning and sequence analysis of the 10 kDa antigen gene of Mycobacterium tuberculosis. J Gen Microbiol. 1989;135:931–939. doi: 10.1099/00221287-135-4-931. [DOI] [PubMed] [Google Scholar]
- 12.Barnes P F, Mehra V, Rivoire B, Fong S J, Brennan P J, Voegtline M S, Minden P, Houghten R A, Bloom B R, Modlin R L. Immunoreactivity of a 10-kDa antigen of Mycobacterium tuberculosis. J Immunol. 1992;148:1835–1840. [PubMed] [Google Scholar]
- 13.Ben-Nun A, Mendel I, Sappler G, Kerlero de Rosbo N. A 12-kDa protein of Mycobacterium tuberculosis protects mice against experimental autoimmune encephalomyelitis. J Immunol. 1995;154:2939–2948. [PubMed] [Google Scholar]
- 14.Bochkareva E S, Girshovich A S. A newly synthesized protein interacts with GroES on the surface of chaperonin GroEL. J Biol Chem. 1992;267:25672–25675. [PubMed] [Google Scholar]
- 15.Boesen H, Jensen B N, Wilcke T, Andersen P. Human T-cell responses to secreted antigen fractions of Mycobacterium tuberculosis. Infect Immun. 1995;63:1491–1497. doi: 10.1128/iai.63.4.1491-1497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandt L, Oettinger T, Holm Å, Andersen A B, Andersen P. Key epitopes on the ESAT-6 antigen recognized in mice during the recall of protective immunity to Mycobacterium tuberculosis. J Immunol. 1996;157:3527–3533. [PubMed] [Google Scholar]
- 17.Ciupitu A M, Petersson M, O'Donnell C L, Williams K, Jindal S, Kiessling R, Welsh R M. Immunization with a lymphocytic choriomeningitis virus peptide mixed with heat shock protein 70 results in protective antiviral immunity and specific cytotoxic T lymphocytes. J Exp Med. 1998;187:685–691. doi: 10.1084/jem.187.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 19.Ellis R J, van-der-Vies S M. Molecular chaperones. Annu Rev Biochem. 1991;60:321–347. doi: 10.1146/annurev.bi.60.070191.001541. [DOI] [PubMed] [Google Scholar]
- 20.Fey S J, Nawrocki A, Larsen M R, Gorg A, Roepstorff P, Skews G N, Williams R, Larsen P M. Proteome analysis of Saccharomyces cerevisiae: a methodological outline. Electrophoresis. 1997;18:1361–1372. doi: 10.1002/elps.1150180811. [DOI] [PubMed] [Google Scholar]
- 21.Fossati G, Lucietto P, Giuliani P, Coates A R, Harding S, Colfen H, Legname G, Chan E, Zaliani A, Mascagni P. Mycobacterium tuberculosis chaperonin 10 forms stable tetrameric and heptameric structures. Implications for its diverse biological activities. J Biol Chem. 1995;270:26159–26167. doi: 10.1074/jbc.270.44.26159. [DOI] [PubMed] [Google Scholar]
- 22.Fossati, G., P. Lucietto, G. Legname, P. Giuliani, H. L. Ball, A. R. M. Coates, F. Leoni, D. Modena, and P. Mascagni. Efficient chemical and recombinant synthesis of Mycobacterium tuberculosis chaperonin 10 an important antigen with pleiotropic biological activities. Submitted for publication.
- 23.Haga S, Yamaguchi R, Nagai S, Matsuo K, Yamazaki A, Nakamura R M. Delayed-type hypersensitivity to a recombinant mycobacterial antigen, MPB64, in guinea pigs sensitized to Mycobacterium tuberculosis or Mycobacterium bovis BCG. J Leukoc Biol. 1995;57:221–225. doi: 10.1002/jlb.57.2.221. [DOI] [PubMed] [Google Scholar]
- 24.Hasløv K, Andersen A, Nagai S, Gottschau A, Sørensen T, Andersen P. Guinea pig cellular immune responses to proteins secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:804–810. doi: 10.1128/iai.63.3.804-810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klausen J, Magnusson M, Andersen Å B, Koch C. Characterization of purified protein derivative of tuberculin by use of monoclonal antibodies: isolation of a delayed-type hypersensitivity reactive component from M. tuberculosis culture filtrate. Scand J Immunol. 1994;40:345–349. doi: 10.1111/j.1365-3083.1994.tb03471.x. [DOI] [PubMed] [Google Scholar]
- 26.Lucietto P, Fossati G, Ball H L, Giuliani P, Mascagni P. Mycobacterium tuberculosis chaperonin 10 and N-truncated fragments. Their synthesis and purification by the isoelectric focusing technique carried out in solution. J Pept Res. 1997;49:308–323. doi: 10.1111/j.1399-3011.1997.tb01131.x. [DOI] [PubMed] [Google Scholar]
- 27.Mande S C, Mehra V, Bloom B R, Hol W G J. Structure of the heat shock protein chaperonin-10 of Mycobacterium leprae. Science. 1996;271:203–207. doi: 10.1126/science.271.5246.203. [DOI] [PubMed] [Google Scholar]
- 28.Mehra V, Bloom B R, Bajardi A C, Grisso C L, Sieling P A, Alland D, Convit J, Fan X D, Hunter S W, Brennan P J, et al. A major T cell antigen of Mycobacterium leprae is a 10-kD heat-shock cognate protein. J Exp Med. 1992;175:275–284. doi: 10.1084/jem.175.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehra V, Gong J, Ilyer D, Lin Y, Boylen C T, Bloom B R, Barnes P F. Immune response to recombinant mycobacterial proteins in patients with tuberculosis infection and disease. J Infect Dis. 1996;174:431–434. doi: 10.1093/infdis/174.2.431. [DOI] [PubMed] [Google Scholar]
- 30.Nagai S, Wiker H G, Harboe M, Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991;59:372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nieland T J, Tan M C, Monne-van Muijen M, Koning F, Kruisbeek A M, van Bleek G M. Isolation of an immunodominant viral peptide that is endogenously bound to the stress protein GP96/GRP94. Proc Natl Acad Sci USA. 1996;93:6135–6139. doi: 10.1073/pnas.93.12.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oettinger T, Holm A, Mtoni I M, Andersen Å B, Hasløv K. Mapping of the delayed-type hypersensitivity-inducing epitope of secreted MPT64 from Mycobacterium tuberculosis. Infect Immun. 1995;63:4613–4618. doi: 10.1128/iai.63.12.4613-4618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olsen, A., and P. Andersen. Unpublished data.
- 34.Orme I M, Miller E S, Roberts A D, Furney S K, Griffin J P, Dobos K M, Chi D, Rivoire B, Brennan P J. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992;148:189–196. [PubMed] [Google Scholar]
- 35.Pollock J M, Andersen P. Predominant recognition of the ESAT-6 protein in the first phase of infection with Mycobacterium bovis in cattle. Infect Immun. 1997;65:2587–2592. doi: 10.1128/iai.65.7.2587-2592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porath J, Maisano F, Belew M. Thiophilic adsorption—a new method for fractionation. FEBS Lett. 1985;185:306–310. doi: 10.1016/0014-5793(85)80928-5. [DOI] [PubMed] [Google Scholar]
- 37.Ravn, P. Unpublished data.
- 38.Ravn P, Boesen H, Pedersen B K, Andersen P. Human T cell responses induced by vaccination with Mycobacterium bovis bacillus Calmette-Guérin. J Immunol. 1997;158:1949–1955. [PubMed] [Google Scholar]
- 39.Roche P W, Winter N, Triccas J A, Feng C G, Britton W J. Expression of Mycobacterium tuberculosis MPT64 in recombinant Myco. smegmatis: purification, immunogenicity and application to skin tests for tuberculosis. Clin Exp Immunol. 1996;103:226–232. doi: 10.1046/j.1365-2249.1996.d01-613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roman E, Moreno C, Young D. Mapping of Hsp70-binding sites on protein antigens. Eur J Biochem. 1994;222:65–73. doi: 10.1111/j.1432-1033.1994.tb18842.x. [DOI] [PubMed] [Google Scholar]
- 41.Rosenkrands I, Rasmussen P B, Carnio M, Jacobsen S, Theisen M, Andersen P. Identification and characterization of a 29-kilodalton protein from Mycobacterium tuberculosis culture filtrate recognized by mouse memory effector cells. Infect Immun. 1998;66:2728–2735. doi: 10.1128/iai.66.6.2728-2735.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silveira H, Ordway D, Dockrell H, Jackson M, Ventura F. Cell-mediated immune responses to mycobacterial antigens in patients with pulmonary tuberculosis and HIV infection. Clin Exp Immunol. 1997;110:26–34. doi: 10.1046/j.1365-2249.1997.5091407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sørensen A L, Nagai S, Houen G, Andersen P, Andersen Å B. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:1710–1717. doi: 10.1128/iai.63.5.1710-1717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Triccas J A, Roche P W, Winter N, Feng C G, Butlin C R, Britton W J. A 35-kilodalton protein is a major target of the human immune response to Mycobacterium leprae. Infect Immun. 1996;64:5171–5177. doi: 10.1128/iai.64.12.5171-5177.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weldingh K, Rosenkrands I, Jacobsen S, Rasmussen P B, Elhay M J, Andersen P. Two-dimensional electrophoresis for analysis of Mycobacterium tuberculosis culture filtrate and purification and characterization of six novel proteins. Infect Immun. 1998;66:3492–3500. doi: 10.1128/iai.66.8.3492-3500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young D B, Kaufmann S H E, Hermans P W M, Thole J E R. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992;6:133–145. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]