Abstract
Visceral leishmaniasis is a severe and lethal disease caused by the protozoan parasites of the genus Leishmania. In areas where leishmaniasis is endemic, most infected individuals control the infection and remain asymptomatic; chemotherapy of visceral leishmaniasis restores some immunity which protects against relapses. In the present study, Leishmania-specific T-cell clones were established from six asymptomatic and five cured patients. Cytokines production by these clones was analyzed. A large fraction of the parasite-specific T-cell clones from asymptomatic patients were CD8+ and produced high amounts of gamma interferon (IFN-γ). Most CD4+ T-cell clones from two asymptomatic subjects exhibited an unusual phenotype: production of high levels of IFN-γ low levels of interleukin-4, (IL-4), but high levels of IL-5. In contrast, only few parasite-specific CD8+ T-cell clones were obtained from cured patients after chemotherapy; moreover, CD4+ T-cell clones from these patients exhibited an heterogeneous profile of cytokines from Th1-like to Th2-like phenotypes. These results point to CD8+ T cells and to IL-5- and IFN-γ-producing CD4+ T cells as possible contributors to human resistance to Leishmania infection. They should stimulate new immunological approaches in the control of this disease.
Visceral leishmaniasis (VL) is a disease caused by the intracellular parasite Leishmania donovani (L. donovani donovani, L. donovani infantum, and L. donovani chagasi), characterized by fever, hepatosplenomegaly and anemia; if left untreated, the disease is lethal within weeks or months. Most studies to identify the immunological factors that determine visceral disease were performed with samples from cured patients (4). In regions where VL is endemic, such as the Mediterranean area, severe disease occurs only in certain subjects whereas a majority of infected individuals show no clinical symptoms (2, 18); previous infections in asymptomatic subjects can be demonstrated by immunological means: skin tests performed with Leishmania lysates are positive and partially correlate with detection of specific antibodies (Abs) by Western blot analysis, and blastogenesis assays using peripheral blood mononuclear cells (PBMC) show a T-cell proliferative response (16, 17). These results indicate a persistent immune response in such individuals. The immunological mechanisms that control parasite multiplication in asymptomatic subjects are not well defined.
Experimental models indicate that parasite-specific CD4+ Th1 cells are critical for the control of primary infection by C57BL/6 mice infected with L. major (26), whereas control of infection by BALB/c infected with L. donovani has been partially associated with the expansion of parasite-specific CD8+ lymphocytes (29).
The aim of the present work was to characterize at the single-cell level the parasite-specific T-lymphocyte response in asymptomatic subjects who spontaneously control the infection by comparison to VL patients who control the infection after chemotherapy. To this end, we derived Leishmania-specific T-cell clones (TCC) from the blood T lymphocytes and analyzed their phenotypes. The results of this analysis strongly suggest a role of CD8+ T cells in the control of infection in asymptomatic subjects. It also uncovers a new CD4+ T-cell subpopulation producing large amounts of both gamma interferon (IFN-γ) and interleukin-5 (IL-5).
MATERIALS AND METHODS
Subjects.
All study subjects were Europeans living in the south of France; seven were male, and five were female (Table 1). Five patients had VL; the diagnosis of VL was based on clinical examination and on the demonstration of Leishmania in blood smears or in bone marrow aspirates. Two patients (Cu1 and Cu2) had completed 1 month of Ambisome treatment. Both recovered from VL after that treatment and have not relapsed since. Three subjects (Cu3, Cu4, and Cu5) had completed their treatment with Glucantime 2, 4, and 7 years ago. Subjects Cu3 and Cu4 were cured, while Cu5 relapsed; definitive cure of Cu5 was obtained only after splenectomy and pentamidine (Lomidine) treatment. Six healthy adults, considered asymptomatic, exhibited immunological signs of previous infection: positive delayed-type hypersensitivity reaction with L. infantum antigens and a specific recognition of the 14- and/or 16-kDa Leishmania antigens on immunoblots (17). These individuals never exhibited clinical manifestations of leishmaniasis. It is likely that all subjects have been exposed to Leishmania in the Mediterranean area. None of the study subjects had active human immunodeficiency virus infection or Abs to the virus in their sera.
TABLE 1.
L. infantum-specific CD4+ and CD8+ TCC derived from study subjects according to clinical status of the T-cell donor
| Subject | Clinical status | Gender | Age (yr) | No. of clones
|
|||
|---|---|---|---|---|---|---|---|
| Total | Specific | CD4+ | CD8+ | ||||
| Cu1 | Cured 1 month | F | 3 | 20 | 12 | 11 | 1 |
| Cu2 | Cured 1 month | F | 2 | 46 | 23 | 23 | 0 |
| Cu3 | Cured 2 years | M | 45 | 17 | 11 | 11 | 0 |
| Cu4 | Cured 4 years | F | 33 | 8 | 6 | 4 | 2 |
| Cu5 | Cured 7 years | M | 42 | 16 | 14 | 6 | 8 |
| Asy1 | Asymptomatic | M | 30 | 9 | 2 | 2 | 0 |
| Asy2 | Asymptomatic | F | 51 | 7 | 1 | 0 | 1 |
| Asy3 | Asymptomatic | M | 43 | 17 | 4 | 4 | 0 |
| Asy4 | Asymptomatic | F | 35 | 125 | 41 | 28 | 13 |
| Asy5 | Asymptomatic | M | 47 | 89 | 68 | 54 | 14 |
| Asy6 | Asymptomatic | M | 38 | 16 | 12 | 4 | 8 |
F, female; M, male.
All subjects gave informed consent for inclusion in the study.
Parasites and antigens.
L. infantum MCAN/82/GR/MON497 was maintained as promastigotes by in vitro culture at 24°C in RPMI 1640 medium (Gibco Life Technologies, Paris, France) supplemented with 10% fetal calf serum (Eurobio, Paris, France), 2 mM l-glutamine, and 50 μg of gentamicin (Gibco Life Technologies) per ml.
Leishmania soluble antigen (LSA) was prepared from stationary-phase promastigotes. Cells were harvested by centrifugation and washed twice in RPMI 1640 medium; the pellet was suspended at 107 cells/ml in the same medium and sonicated at 4°C for 1 min at 12-μm peak amplitude. After centrifugation at 15,000 × g for 15 min, the supernatant was sterilized by filtration through a 0.22-μm-pore-size membrane and frozen as 1-mg/ml aliquots at −80°C until use.
Cell separation and T-cell cloning.
TCC were derived from PBMC that were restimulated in vitro with LSA plus IL-2 before the cloning. Cloning was performed by the limiting dilution method using phytohemagglutinin A (PHA; 2 μg/ml) and allogeneic irradiated (2,500 rads) PBMC as feeder cells. Briefly, 106 PBMC, isolated by centrifugation on Ficoll density gradient, were cultured with LSA (50 μg/ml) in 1 ml of RPMI 1640 medium supplemented with l-glutamine (2 mM), gentamicin (5 μg/ml), sodium pyruvate (1 mM), nonessential amino acids, 2-mercaptoethanol (50 μM) (all from Gibco Life Technologies), and 10% AB human serum. Recombinant human IL-2 (rhIL-2; 10 U/ml Proleukin; Chiron, Paris, France) was added on day 3. On day 7, T-cell cloning was carried out by the limiting dilution (0, 3, 1, 3 and cells per well) method. T cells were grown on irradiated allogeneic PBMC (5.104/well), rhIL-2 (10 U/ml), and PHA (2 μg/ml). After verification that the number of positive wells fit with statistical criteria of clonality (less than 30% growing cultures), TCC clones were transferred and fed weekly with medium, irradiated feeder cells, rhIL-2, and PHA as described above. Clonality of the cell lines was assessed by analysis of the T-cell receptor Vβ chains as described by Choi (5) on 24 cultures. Each culture was found to express one and only one of five Vβ genes. Thus, one cell line expressed one Vβ chain and the 24 cultures expressed altogether five different Vβ chains.
Anti-Leishmania T-cell response in vitro.
Proliferative response to leishmania antigens was first checked on PBMC to evaluate the immune status of study subjects. PBMC were tested in triplicate at a final concentration of 5 × 104/well. LSA (10 and 50 μg/ml) and PHA (positive control; 1 μg/ml) were added. After 96 h of incubation, cultures were pulsed with bromodeoxyuridine (BrdU) for 18 h, and DNA synthesis was measured by enzyme-linked immunosorbent assay (ELISA) (cell proliferation kit; Boehringer, Meylan, France). The threshold value for significant proliferation test (mean + 3 standard deviations obtained from PBMC of 25 healthy subjects not exposed to Leishmania cultured with 50 μg of LSA per ml) was 0.12.
Specificity of the TCC was analyzed by the same methodology by culturing 5 × 104 T cells with 5 × 104 irradiated autologous PBMC in 96-well flat-bottom microplates. Tests were performed in triplicate and specific clones gave optical density (OD) values ranging from 0.12 to 1.60 (mean = 0.60; standard deviation = 0.32). Stimulation of the proliferation with 1 μg of PHA per ml yielded OD values between 1.6 and 1.8.
Flow cytometry analysis.
T cells were stained with fluorescein-labeled Ab by standard procedures (27). Mouse monoclonal Abs (MAbs) to CD3, CD4, and CD8 were obtained from Immunotech (Marseille, France). Phenotyping was performed by using a fluorescence-activated cell sorter (Becton Dickinson, Paris, France).
Cytokine production and quantification.
TCC were cultured at a density of 2 × 105 cells per well in 96-well microplates with anti-CD3 MAb (200 ng/ml; Immunotech) and Phorbol myristate acetate (PMA; 10 ng/ml; Sigma, Meylan, France). After 24 h of incubation, cells were harvested and supernatants were collected and stored at −20°C. IFN-γ, IL-4, and IL-5 were measured in the supernatants by ELISA according to standard procedures (6). Briefly, ELISA used capture and detection MAbs from Mabtech for IFN-γ and IL-4 assays and from Pharmingen for IL-5 assays and the corresponding standards. After coating with capture Ab, serial dilutions (in culture medium) of supernatants and standards (10 to 1,000 pg/ml) were incubated overnight; after washing, biotinylated Ab (diluted in phosphate-buffered saline–0.3% bovine serum albumin) were added for 3 h. Fixation of biotinylated Abs was measured by means of streptavidin-alkaline phosphatase, and assays for alkaline phosphatase activity were performed with p-nitrophenyl phosphate as the chromogenic substrate.
RESULTS
Isolation of specific TCC from asymptomatic subjects and from VL patients after parasitological cure by chemotherapy.
Eleven subjects were studied (Table 1); six individuals were asymptomatic carriers, two were VL patients and had just completed the treatment, and three had been cured 2 to 7 years before the study.
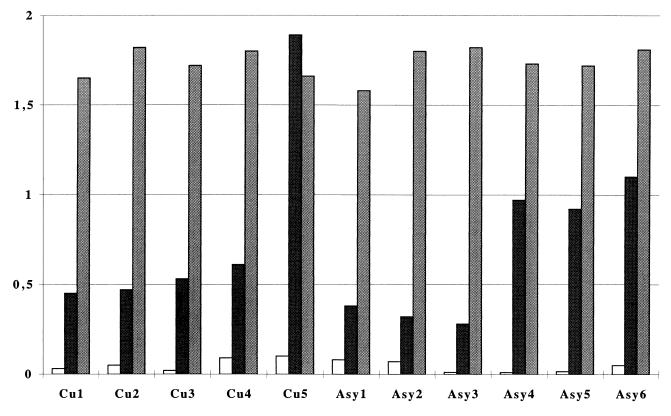
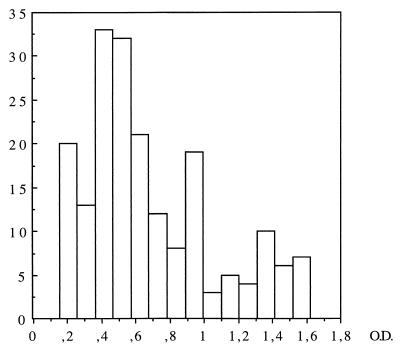
PBMC from three asymptomatic subjects (Asy4, Asy5, and Asy6) exhibited a strong proliferative response to LSA (Fig. 1), and 121 specific TCC were derived from them. In contrast, PBMC from the three remaining asymptomatic subjects (Asy1, Asy2, and Asy3) yielded a weak proliferation to LSA and only seven specific TCC (14 to 23% of all TCC isolated from these subjects). PBMC obtained from VL patients as soon as 1 month after Ambisome treatment proliferated well to LSA; 53% of the clones isolated from these patients (Cu1 and Cu2) were specific for LSA. Likewise, 75% of TCC obtained 2 to 7 years after treatment from subjects Cu3, Cu4, and Cu5 were parasite specific, and this correlated with a good PBMC reactivity to LSA in the blastogenesis assay. Figure 2 shows the OD values obtained after BrdU proliferation assays of the specific clones in presence of LSA (50 μg/ml). In the same conditions, no specific clones were isolated from four subjects whose PBMC did not show a proliferative response in vitro.
FIG. 1.
Proliferative response to LSA of PBMC from study subjects. Proliferation was measured by ELISA after BrdU incorporation at the fifth day of incubation. The negative control (open bars) was ovalbumin (20 μg/ml); LSA (black bars) and PHA (grey bars) were used at final concentrations of 50 and 2 μg/ml, respectively, after titration.
FIG. 2.
Distribution of OD values obtained for proliferation tests of Leishmania-specific clones in the presence of LSA (50 μg/ml).
Characterization of TCC. (i) Twenty to 66% of specific TCC from asymptomatic subjects were CD8+ whereas most TCC obtained from VL patients after chemotherapy were CD4+.
The phenotypes of L. infantum-specific TCC from asymptomatic and from cured patients were assessed by flow cytometry. We found that 20 to 66% of TCC from asymptomatic subjects Asy4, Asy5, and Asy6 were CD8+ (Table 1). The number of specific clones obtained from the other asymptomatic subjects (Asy1, Asy2, and Asy3) was too low to allow conclusions on the relative importance of each subset. In contrast, almost all TCC from cured patients Cu1 through Cu4 were CD4+, and only 3 of 52 clones (5.7%) were CD8+. The observations made with cells from Cu5 are discussed below. The total number of CD8+-specific TCC correlated positively with the proliferative response in the blastogenesis assay performed with PBMC and LSA (r = 0.67, P = 0.2), suggesting that CD8+ T cells could be the major subset involved in Leishamania-specific proliferative response in these asymptomatic individuals.
(ii) L. infantum-specific CD8+ TCC isolated from asymptomatic subjects displayed a Tc1 phenotype.
IL-4 and IFN-γ production was measured in culture supernatants of CD8+ TCC stimulated by anti-CD3 plus PMA. These stimulation conditions were used rather than stimulation of TCC with LSA-sensitized autologous antigen-presenting cells because PBMC were not available in sufficient quantity for cytokine production study. Although these conditions do not represent the physiological mode of activation, we have obtained the same results with PHA-PMA stimulation (data not shown), and other studies have shown that the cytokine profile of TCC is not modified when polyclonal stimulation is used (7).
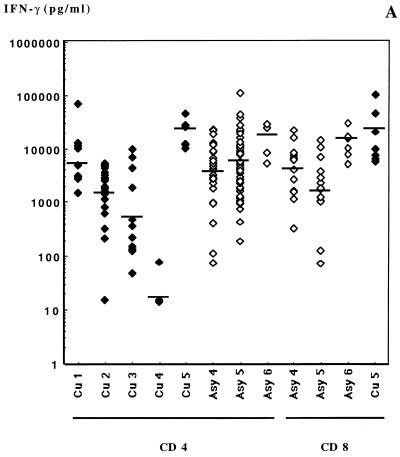
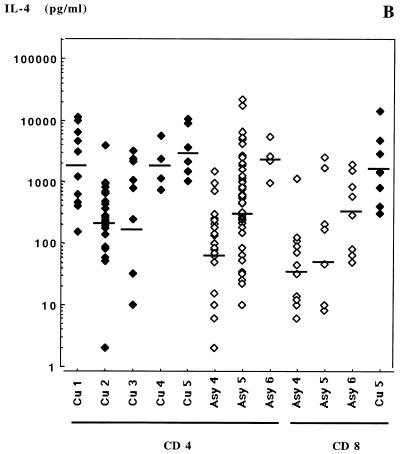
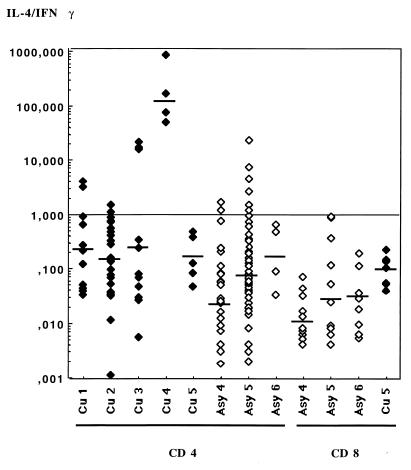
Cytokine levels in supernatants are presented in Fig. 3. All clones produced detectable amounts of IFN-γ and IL-4. The amounts of IL-4 produced by clones of each subject varied on a large (3-log) scale, while IFN-γ production was much less variable among clones. The average geometric mean concentrations of IFN-γ in supernatants were 40- to 100-fold greater than IL-4 concentrations produced in the same culture. Average IL-4/IFN-γ ratios were 0.009, 0.023, and 0.024 for CD8+ TCC from subjects Asy4, Asy5, and Asy6, respectively and indicated a clear Tc1 phenotype (Fig. 4).
FIG. 3.
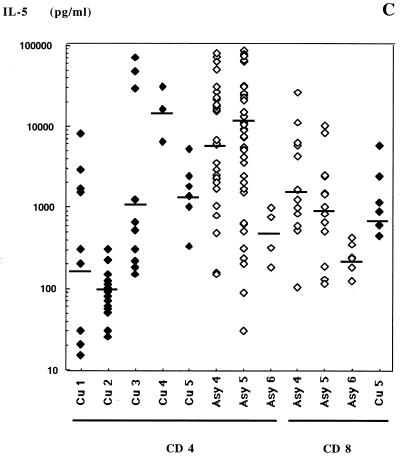
IFN-γ (A), IL-4 (B), and IL-5 (C) production after anti-CD3-PMA stimulation by Leishmania-specific TCC from subjects Cu1 through Cu5 and Asy4 through Asy6. Bars indicate the geometric means obtained from the clones from each subject.
FIG. 4.
IL-4/IFN-γ ratios for specific TCC isolated from cured subjects Cu1 through Cu5 and asymptomatic subjects Asy4 through Asy6. Bars represent the geometric means calculated for the CD4+ and CD8+ clones from each subject.
(iii) L. infantum-specific CD4+ TCC from most asymptomatic subjects produced large amounts of IFN-γ and IL-5.
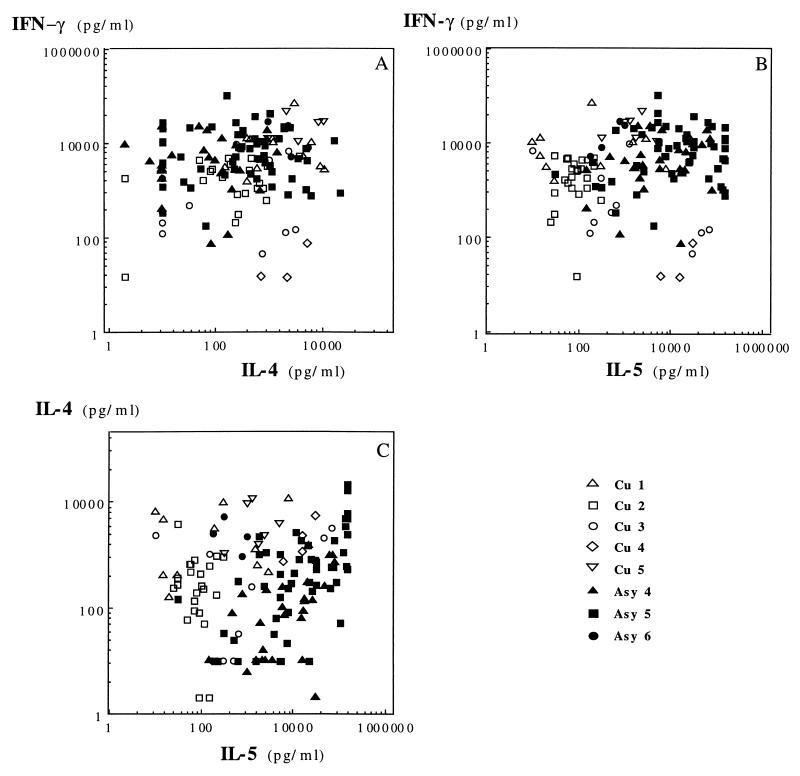
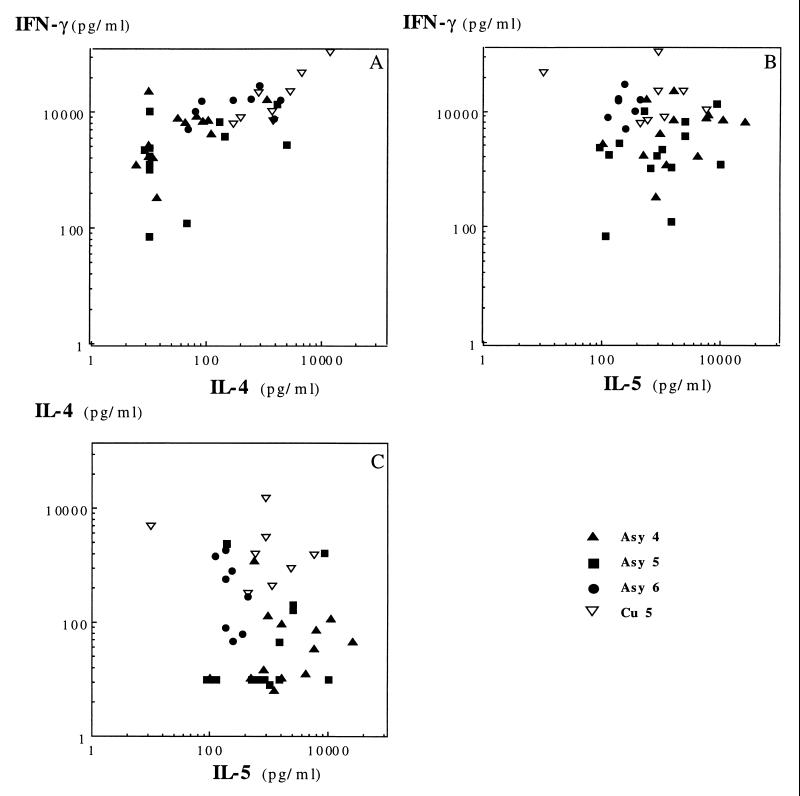
Results of cytokine assays with respect to dual cytokine production are presented in Fig. 5 for the CD4+ TCC and Fig. 6 for the CD8+ TCC. Specific CD4+ TCC from asymptomatic subjects exhibited an IL-4 and IFN-γ secretion profile comparable to that of CD8+ TCC from the same patients: they produced low levels of IL-4 and high levels of IFN-γ, with average IL-4/IFN-γ ratios of 0.02 (Asy4), 0.062 (Asy5), and 0.17 (Asy6) (Fig. 4). This suggested that most specific CD4+ TCC of asymptomatic subjects had a Th1-like phenotype. Unexpectedly, CD4+ TCC from asymptomatic subjects Asy4 and Asy5 also secreted high amounts of IL-5 (Fig. 5), indicating that they should not be considered Th1 cells. Geometric means of IL-5 amounts produced in culture supernatants of CD4+ TCC were 310 pg/ml for cured subjects and 6,072, 11,227, and 453 pg/ml for asymptomatic subjects Asy4 (P = 0.0012), Asy5 (P < 0.001), and Asy6 (P = 0.534), respectively. There was no correlation between IL-4 and IL-5 production by these TCC.
FIG. 5.
Distribution of CD4+ clones with respect to dual cytokine production: IFN-γ plus IL-4 (A), IFN-γ plus IL-5 (B), and IL-4 plus IL-5 (C).
FIG. 6.
Distribution of CD8+ clones with respect to dual cytokine production: IFN-γ plus IL-4 (A), IFN-γ plus IL-5 (B), and IL-4 plus IL-5 (C).
(iv) Specific CD4+ TCC isolated from treated VL patients displayed a Th0/1 or Th2 phenotype.
Specific TCC from three of four patients cured after chemotherapy produced more IFN-γ than IL-4 but threefold less IFN-γ than specific CD4+ TCC from asymptomatic individuals; therefore, they present a Th0/1 phenotype (Fig. 3 and 4). These clones produced much less IL-5 (average mean = 310 pg/ml) than the CD4+ TCC from asymptomatic subjects (Asy4 and Asy5; average mean = 9,128 pg/ml, P < 0.0001).
CD4+ TCC from the fourth cured subject (Cu4, treated 4 years before) produced large amounts of IL-4 and IL-5 and low quantities of IFN-γ, consistent with a Th2 phenotype. Interestingly, though this latter subject lives in an area where VL is endemic, he has never relapsed.
(v) Specific TCC from the splenectomized VL patient displayed characteristics comparable to those of TCC from asymptomatic subjects.
Patient Cu5 had an acute visceral leishmaniasis that was treated with Glucantime and relapsed 10 months later. He was definitively cured after splenectomy and Lomidine treatment. Six of 14 (43%) of the specific clones isolated from this subject were CD4+ with a Th0 phenotype (average IL-4/IFN-γ ratio = 0.167), while 57% of the TCC were CD8+ T cells secreting large amounts of IFN-γ (average IL-4/IFN-γ ratio = 0.095). A second cloning experiment, performed 6 months later, showed identical results (of 65 TCC, 32 were parasite specific, 13 were CD4+ and 19 were CD8+, most of them displaying a Th1 functional pattern).
DISCUSSION
To our knowledge, only studies on the immune response of VL subjects after chemotherapy have been reported so far; these studies have shown that VL develops in subjects with a high Ab response and low cellular immunity against Leishmania (9) and that recovery from disease is associated with a marked increase of the parasite-specific cellular immunity and with a fall in Ab production (4, 17). These observations supported the view that cell-mediated immunity, regulated by Th1 CD4+ lymphocytes, was required for the destruction of Leishmania parasites which grow and multiply in macrophage phagolysosomes. These studies analyzed the whole PBMC proliferation and bulk cytokine production.
The aim of this work was to characterize at the single-cell level the T-lymphocyte subpopulations with regard to cytokine production associated with the control of L. infantum infection in asymptomatic subjects and in individuals who recovered from visceral disease after chemotherapy.
The most remarkable and new finding of the present work is the high proportion of parasite-specific CD8+ T lymphocytes among TCC isolated from asymptomatic subjects, representing 20 to 66% of Leishmania-specific TCC of these subjects. L. infantum-specific CD8+ cells have been found in asymptomatic dogs experimentally infected with low doses of L. infantum (25), but not in dogs that did not control the infection and developed severe clinical disease. L. major-specific major histocompatibility complex class I-restricted CD8+ T cells, cytotoxic for Leishmania-infected macrophages, were also shown to contribute to anti-Leishmania protective immunity in C57BL/6 and BALB/c mice (19–21) which had resolved a primary infection with a small number of parasites. These cells appeared 3 to 4 months after the infection. CD8+ T cells, in association with CD4+ T cells, were also essential for the resolution of primary infection and resistance to reinfection (29) in BALB/c mice infected with L. donovani. Taken together, these results show an association between the presence of specific CD8+ T cells and the control of infection by Leishmania in the asymptomatic host.
The role of CD8+ T cells in protection could be mediated by the large amounts of IFN-γ that are secreted by these cells. IFN-γ is critical for the expression of macrophage leishmanicidal activity; an in vitro study in a mouse model indicated that CD8+ T cells can be cytotoxic for infected macrophages, releasing live parasites which are in turn phagocytized by bystander macrophages activated by IFN-γ produced by CD8+ T cells (28). Along the same lines, the production of IFN-γ by splenic cells from L. amazonensis-infected mice was markedly reduced after depletion of CD8+ T cells (15).
In marked contrast with the observations for asymptomatic subjects, CD8+ T cells were absent among the clones derived from VL subjects (if we except the splenectomized one) after these individuals had just recovered from disease or several years after infection. This suggests that a defect in the CD8+ T-cell differentiation pathway might be associated with susceptibility to VL. This study also shows that 57% of the specific TCC isolated from the VL subject who was splenectomized were CD8+. This observation together with the fact that relapsing VL patients can be cured by splenectomy suggests that the spleen may have a facilitating effect on disease development. Suppressive activity, mediated by CD4+ T cells, has been reported for susceptible BALB/c infected with L. major (11, 21). L. major-specific Th1 CD4+ TCC isolated from sensitized mice exacerbated disease development when adoptively transferred to syngeneic animals (30).
While an elevated Ab response is observed in the acute phase of the disease, cure by chemotherapy has been associated with the development of anti-Leishmania cellular immunity, suggesting the development of Th1-mediated immunity (1, 14). The results presented here show that TCC isolated from treated and from asymptomatic subjects exhibited cytokine secretion profiles from Th1 to Th2 phenotypes. Most TCC, however, produced IL-4, IFN-γ, and IL-5, indicating that they do not exhibit a fully polarized Th1 or Th2 phenotype; in previous studies, these clones were referred to as Th0/1 and Th0/2 (7). These results are consistent with those of Kemp et al. (13) for 22 CD4+ TCC isolated from cured patients (2 Th2, 8 Th1, and 7 Th0 clones and 5 TCC did not secrete IL-4 and IFN-γ). These findings may indicate that a fully polarized immune response is not best for the control of L. infantum infection in humans and that, unlike observations for L. major-infected mice, Th2 cytokines may also play a beneficial role in control of the disease. This view is also supported by another finding in this study, high IL-5 production by TCC from asymptomatic subjects. An alternative interpretation of these findings is that the cloning procedure would not be suitable for the isolation of fully differentiated Th1 or Th2 clones. This is, however, unlikely because we obtained a few Th1 and Th2 TCC in the same experiments.
A striking observation in this study was that CD4+-specific T cells isolated from two asymptomatic subjects produce amounts of IL-5 10-fold higher than those found in the culture supernatants of CD4+ TCC from cured patients. These persons, who were born and lived in the south of France, had neither allergic manifestations, helminth infections, nor hypereosinophilia. The same clones produce also large quantities of IFN-γ and low amounts of IL-4. The dissociation between IL-4 and IL-5 expression by human lymphocytes has been documented for asthma (8). Jung et al. showed at the single-cell level that these two cytokines are rarely coexpressed (12). Our observation raises the possibility that IL-5 is involved in control of the infection in certain asymptomatic subjects. IL-5 promotes the differentiation and activation of eosinophils and enhances the generation of cytotoxic effector cells from thymocytes. IL-5 also enhances activation of specific cytotoxic T lymphocytes (22, 23). Eosinophils represent up to 15% of the cellular infiltrate at the site of infection in mice resistant (C57BL/6) to L. major infection, while they are lacking in tissues of infected susceptible BALB/c mice (3). Furthermore, the number of amastigotes in cutaneous lesions caused by L. braziliensis in humans was found to be inversely related to the presence of eosinophils (10). Pimenta and colleagues (24) showed by electron microscopy that interaction between rat eosinophils and L. mexicana caused the degranulation of the cells and the release of granule contents onto the parasite, causing extracellular parasite killing. If such mechanisms of Ab-dependent cellular cytoxicity play a significant role in parasite destruction, then, the control of infection in certain subjects is likely to involve Ab.
In conclusion, this study provides the first demonstration that CD8+ T cells may play a significant role in protection against L. infantum in asymptomatic subjects. The results also show that control of the disease is associated in two of three asymptomatic individuals with an unusual CD4+ T-cell subset secreting IFN-γ and IL-5.
ACKNOWLEDGMENTS
We thank F. Haas and R. Piarroux for providing samples from two patients, L. Reininger for very helpful suggestions on the manuscript, and A. Bourgois and M. Morton for reading the manuscript.
REFERENCES
- 1.Bacellar O, Brodskyn C, Guerreiro J, Barral-Netto M, Henrique Costa C, Coffman R L, Johnson W D, Carvalho E M. Interleukin-12 restores interferon γ production and cytotoxic responses in visceral leishmaniasis. J Infect Dis. 1996;173:1515–1518. doi: 10.1093/infdis/173.6.1515. [DOI] [PubMed] [Google Scholar]
- 2.Badaro R, Jones T C, Carvalho E M, Sampaio D, Reed S G, Barral A, Teixeira R, Johnson W D., Jr New perspectives on a subclinical form of visceral leishmaniasis. J Infect Dis. 1986;154:1003–1011. doi: 10.1093/infdis/154.6.1003. [DOI] [PubMed] [Google Scholar]
- 3.Beil W J, Meinardus-Hager G, Neugebauer D C, Sorg C. Differences in the onset of the inflammatory response to cutaneous leishmaniasis in resistant and susceptible mice. J Leukoc Biol. 1992;52:135–142. doi: 10.1002/jlb.52.2.135. [DOI] [PubMed] [Google Scholar]
- 4.Carvahlo E M, Bacellar O, Brownell C, Regis T, Coffman R L, Reed S. Restoration of IFN-γ production and lymphocyte proliferation in visceral leishmaniasis. J Immunol. 1994;152:5949–5956. [PubMed] [Google Scholar]
- 5.Choi Y, Kotzin B, Herron L, Callahan J, Marrack P, Kappler J. Interaction of Staphylococcus aureus toxin superantigens with human T cells. Proc Natl Acad Sci USA. 1989;86:8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coligan J E, Kruissbeek A M, Margulies D H, Shevach E M, Strober W, editors. Currents protocols in immunology. New York, N.Y: John Wiley & Sons; 1995. [Google Scholar]
- 7.Couissinier-Paris P, Dessein A J. Schistosoma-specific helper T cell clones from subjects resistant to infection by Schistosoma mansoni are Th0:2. Eur J Immunol. 1995;25:2295–2302. doi: 10.1002/eji.1830250827. [DOI] [PubMed] [Google Scholar]
- 8.Doi S, Gemou-Engesaeth V, Kay A B, Corrigan C J. Polymerase chain reaction quantification of cytokine messenger RNA expression in peripheral blood mononuclear cells of patients with acute exacerbations of asthma: effect of glucocorticoid therapy. Clin Exp Allergy. 1994;24:854–867. doi: 10.1111/j.1365-2222.1994.tb01808.x. [DOI] [PubMed] [Google Scholar]
- 9.Ghalib H W, Piuvezam M R, Skeiky Y A W, Siddig M, Hashim F A, El Hassan A M, Russo D M, Reed S G. Interleukin 10 production correlates with pathology in human Leishmania donovani infections. J Clin Investig. 1993;92:324–329. doi: 10.1172/JCI116570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutierrez Y, Salinas G H, Palma G, Valderrama L B, Santrich C V, Saravia N G. Correlation between histopathology, immune response, clinical presentation and evolution in Leishmania braziliensis infection. Am J Trop Med Hyg. 1991;45:281–289. doi: 10.4269/ajtmh.1991.45.281. [DOI] [PubMed] [Google Scholar]
- 11.Hill J O. Reduced numbers of CD4+ suppressor cells with subsequent expansion of CD8+ protective T cells as an explanation for the paradoxical state of enhanced resistance to Leishmania in T-cell deficient BALB/c mice. Immunology. 1991;72:282–286. [PMC free article] [PubMed] [Google Scholar]
- 12.Jung T, Schauer U, Rieger C, Wagner K, Einsle K, Neumann C, Heuuser C. Interleukin-4 and interleukin-5 are rarely co-expressed by human T cells. Eur J Immunol. 1995;25:2413–2416. doi: 10.1002/eji.1830250843. [DOI] [PubMed] [Google Scholar]
- 13.Kemp M, Kurtzhals J A, Bendtzen K, Poulsen L K, Hansen M B, Koech D K, Kharazmi A, Theander T G. Leishmania donovani-reactive Th1 and Th2-like T-cell clones from individuals who have recovered from visceral leishmaniasis. Infect Immun. 1993;61:1069–1073. doi: 10.1128/iai.61.3.1069-1073.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Locksley R M, Louis J A. Immunology of leishmaniasis. Curr Opin Immunol. 1992;4:413–418. doi: 10.1016/s0952-7915(06)80032-4. [DOI] [PubMed] [Google Scholar]
- 15.Man-Ying Chan M. T cell response in murine Leishmania mexicana amazonensis infection: production of interferon γ by CD8+ cells. Eur J Immunol. 1993;23:1181–1184. doi: 10.1002/eji.1830230532. [DOI] [PubMed] [Google Scholar]
- 16.Marty P, Lelievre A, Quaranta J F, Rahal A, Gari-Toussaint M, Le Fichoux Y. Use of the leishmanin skin test and Western blot analysis for epidemiological studies in visceral leishmaniasis areas: experience in a highly endemic focus in Alpes-Maritimes (France) Trans R Soc Trop Med Hyg. 1994;88:658–659. doi: 10.1016/0035-9203(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 17.Mary C, Lamouroux D, Dunan S, Quilici M. Western blot analysis of antibodies to Leishmania infantum antigens: potential of the 14-kD and 16-kD antigens for diagnosis and epidemiologic purposes. Am J Trop Med Hyg. 1992;47:764–771. doi: 10.4269/ajtmh.1992.47.764. [DOI] [PubMed] [Google Scholar]
- 18.Meller-Melloul C, Farnarier C, Dunan S, Faugère B, Franck J, Mary C, Bongrand P, Quilici M, Kaplanski S. Evidence of subjects sensitized to L. infantum on the French Mediterraneen Coast: differences in γIFN production between this population and visceral leishmaniasis patients. Parasite Immunol. 1991;13:531–536. doi: 10.1111/j.1365-3024.1991.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 19.Müller I. Role of T cell subsets during the recall of immunologic memory to Leishmania major. Eur J Immunol. 1992;22:3063–3069. doi: 10.1002/eji.1830221206. [DOI] [PubMed] [Google Scholar]
- 20.Müller I, Kropf P, Etges R J, Louis J A. Gamma interferon responses in secondary Leishmania major infection: role of CD8+ T cells. Infect Immun. 1993;61:3730–3738. doi: 10.1128/iai.61.9.3730-3738.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller I, Pedrazzini T, Kropf P, Louis J A, Milon G. Establishment of resistance to Leishmania major infection in susceptible BALB/c mice requires parasite specific CD8+ T cells. Int Immunol. 1991;3:587–597. doi: 10.1093/intimm/3.6.587. [DOI] [PubMed] [Google Scholar]
- 22.Nagasawa M, Ohshiba A, Yata J. Effect of recombinant Interleukin 5 on the generation of cytotoxic T cells (CTL) Cell Immunol. 1991;133:317–326. doi: 10.1016/0008-8749(91)90107-m. [DOI] [PubMed] [Google Scholar]
- 23.Ohbo K, Asao H, Kouro T, Nakamura M, Takaki S, Kikuchi Y, Hirokawa K, Tominaga A, Takatsu K, Sugamura K. Demonstration of a cross-talk between IL-2 and IL-5 in phosphorylation of IL-2 and IL-5 receptor beta chains. Int Immunol. 1996;8:951–960. doi: 10.1093/intimm/8.6.951. [DOI] [PubMed] [Google Scholar]
- 24.Pimenta P F, Dos Santos M A, De Souza W. Fine structure and cytochemistry of the interaction between Leishmania mexicana amazonensis and rat neutrophils and eosinophils. J Submicrosc Cytol. 1987;19:387–395. [PubMed] [Google Scholar]
- 25.Pinelli E, Gonzalo R M, Boog C J P, Rutten V P M G, Gebhard D, del Real G, Ruitenberg E J. Leishmania infantum-specific T cell lines derived from asymptomatic dogs that lyse infected macrophages in a major histocompatibility complex-restricted manner. Eur J Immunol. 1995;25:1594–1600. doi: 10.1002/eji.1830250619. [DOI] [PubMed] [Google Scholar]
- 26.Reiner S L, Locksley R M. Cytokines in the differenciation of Th1/Th2 CD4+ subsets in leishmaniasis. J Cell Biochem. 1993;53:323–328. doi: 10.1002/jcb.240530409. [DOI] [PubMed] [Google Scholar]
- 27.Robinson J P, editor. Handbook of flow cytometry methods. New York, N.Y: Wiley-Liss; 1993. [Google Scholar]
- 28.Smith L E, Rodrigues M, Russell D G. The interaction between CD8+ cytotoxic T cells and Leishmania-infected macrophages. J Exp Med. 1991;174:499–505. doi: 10.1084/jem.174.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stern J J, Oca M J, Rubin B Y, Anderson S L, Murray H W. Role of L3T4 and LyT-2+ cells in experimental visceral leishmaniasis. J Immunol. 1988;140:3971–3977. [PubMed] [Google Scholar]
- 30.Titus R G, Muller I, Kimsey P, Cerny A, Behin R, Zinkernagel R M, Louis J A. Exacerbation of experimental murine cutaneous leishmaniasis with CD4+ T cell lines or clones which secrete interferon gamma and mediate parasite specific delayed-type hypersensitivity. Eur J Immunol. 1991;21:559–567. doi: 10.1002/eji.1830210305. [DOI] [PubMed] [Google Scholar]