Abstract
A protection against a challenge with Mycobacterium tuberculosis is induced by previous immunization with living attenuated mycobacteria, usually bacillus Calmette-Guérin (BCG). The 45/47-kDa antigen complex (Apa) present in culture filtrates of BCG of M. tuberculosis has been identified and isolated based on its ability to interact mainly with T lymphocytes and/or antibodies induced by immunization with living bacteria. The protein is glycosylated. A large batch of Apa was purified from M. tuberculosis culture filtrate to determine the extent of glycosylation and its role on the expression of the immune responses. Mass spectrometry revealed a spectrum of glycosylated molecules, with the majority of species bearing six, seven, or eight mannose residues (22, 24, and 17%, respectively), while others three, four, or five mannoses (5, 9, and 14%, respectively). Molecules with one, two, or nine mannoses were rare (1.5, 3, and 3%, respectively), as were unglycosylated species (in the range of 1%). To eliminate the mannose residues linked to the protein, the glycosylated Apa molecules were chemically or enzymatically treated. The deglycosylated antigen was 10-fold less active than native molecules in eliciting delayed-type hypersensitivity reactions in guinea pigs immunized with BCG. It was 30-fold less active than native molecules when assayed in vitro for its capacity to stimulate T lymphocytes primed in vivo. The presence of the mannose residues on the Apa protein was essential for the antigenicity of the molecules in T-cell-dependent immune responses in vitro and in vivo.
An important goal in the immunology of tuberculosis concerns the control of the infection during its initial stages, i.e., prevention of the multiplication of bacilli and their secondary dissemination. Prior vaccination with bacillus Calmette-Guérin (BCG) enhances cell-mediated immunity and induces a delayed-type hypersensitivity (DTH) to mycobacterial proteins. These two immune responses are mediated by mycobacterium-reactive T lymphocytes, and it was thought that vaccines able to stimulate and/or increase this cell population would result in better control of Mycobacterium tuberculosis infection.
In experimental models, increased resistance to challenge with virulent M. tuberculosis is obtained after previous immunization with living attenuated mycobacterial strains like BCG (8, 20, 27), in which case the two T-lymphocyte responses (cell-mediated immunity and DTH) develop simultaneously. Conversely, when killed bacteria of the same strains are injected alone or with potent adjuvant, no or marginal protection is detectable despite high DTH reactivity toward crude mycobacterial extracts, tuberculin, or purified protein derivative (PPD).
The 45/47-kDa antigen complex (Apa) was selected based on its ability to be recognized by antibodies raised in guinea pigs immunized with living bacteria (28). The complex is encoded by the apa gene (or the modD gene according to the recent annotation of the complete M. tuberculosis genome sequence) (4, 24). The molecules were also found to elicit potent DTH responses only in living-BCG-immunized guinea pigs. The proteins were approximately 40-fold more potent when assayed intradermally (i.d.) on guinea pigs immunized with living BCG than on heat-killed-BCG-immunized guinea pigs (28).
Evidence for glycosylation of Apa was reported (7, 10), along with details of the nature and location of the glycosyl residues on the peptide (6). To define the role of the different molecules in the immune response, a large batch of Apa was purified starting from M. tuberculosis culture filtrate. Mass spectrometry showed that the extent of mannosylation of Apa molecules was more variable than was previously reported (6). While a small number of molecules were not glycosylated and others had up to nine mannose residues, the most frequently observed molecular species bore seven mannoses. When tested on two T-lymphocyte-dependent immune responses, the deglycosylated molecules had a significantly lower capacity to elicit a DTH reaction in vivo or to activate specific T lymphocytes in vitro.
MATERIALS AND METHODS
Characterization of Apa molecules. (i) Competitive ELISA.
A competitive enzyme-linked immunosorbent assay (ELISA) was used to detect and to measure the concentration of Apa present in the crude and purified preparations as previously described (28). In brief, a potent polyclonal rabbit immune serum was obtained against the antigen of the Apa complex by using a classical immunization procedure: an injection of 50 μg of the complex mixed with incomplete Freund adjuvant, followed by an injection of 25 μg of the mixture 1 month later. The purified antigen complex was immobilized on a plastic surface (100 μl at 1 mg/ml in carbonate buffer). The optimal dilution of rabbit serum (1/8,000) was chosen in preliminary experiments as the last dilution of the plateau, just before the beginning of the decreasing slope. After their incubation for 1 h at 37°C with the fraction to be assayed, the amounts of the remaining antibodies were measured on a plate coated with purified Apa complex. Known amounts of the Apa complex were included in each assay to determine the 50% value of remaining antibodies. Phosphatase-labelled antibodies directed against rabbit immunoglobulin G (IgG) were used to determine the amounts of bound anti-Apa antibodies, resulting in a sensitivity of 2 ng/ml.
(ii) Immunoblotting.
The antigens present in each crude or purified preparation were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 10% polyacrylamide gels by the Laemmli method (22). Samples containing between 0.25 μg (purified complex) and 20 μg (crude freeze-dried culture filtrate) of proteins were loaded onto the wells of the SDS-containing gel in 10 μl of buffer containing 5% β2-mercaptoethanol, 3% SDS, and a trace of bromophenol blue.
After electrophoresis, the antigens present in each lane were transferred to a polyvinylidene difluoride (PVDF) sheet (Millipore) by wet electrophoretic transfer. Immunodetection was performed as previously described, with a specific rabbit antiserum (28). A twin gel was also transferred onto a PVDF sheet and stained with Aurodye (Pharmacia) as specified by the manufacturer. This staining procedure was chosen for its superior sensitivity, up to 20 times that of classical Coomassie blue staining.
Crude culture filtrate.
M. tuberculosis H37Rv was cultured at 37°C on synthetic Sauton medium and harvested after 20 days. Molecules above 10 kDa excreted into the culture medium were concentrated on a molecular filtration membrane (cutoff, 10 kDa) and freeze-dried as previously described (28). Starting from 32 liters of Sauton medium, 4.2 g of crude culture filtrate was obtained.
Purification procedure. (i) Ion-exchange column.
A preparative 24- by 250-mm column was packed with 120 ml of the ion-exchange gel Source 15Q (Pharmacia). The column was equilibrated with buffer at low ionic concentration (20 mM Tris-HCl [pH 8]) containing butanol (4%). The flow rate was 5 ml/min, with a maximum pressure of 13 × 105 Pa. A linear gradient of NaCl (0 to 0.5 M) into the same buffer was applied after injection of 2 ml containing 500 mg/ml of the crude culture filtrate diluted in the starting buffer. Four successive injections were performed. Eight fractions were determined by measurement of the optical density at 220 nm and collected. Fraction 5, eluted at 0.032 M NaCl, was selected for its high concentration of antigenic molecules, which were detected by competitive ELISA and immunoblotting. The fraction was extensively dialyzed against deionized water containing 4% butanol and freeze-dried, and 256 mg of material was recovered.
(ii) Molecular sieve column.
A semipreparative TSK G2000 (21.5- by 600-mm) column (LKB) was equilibrated with a saline buffer (50 mM Na2HPO4, adjusted to pH 7.5 with KH2PO4) containing 4% butanol. The flow rate through the column was 0.25 ml/min at 3 × 105 Pa. The samples (70 to 100 mg in 2 ml of buffer) were loaded onto the column, and four different fractions were collected as determined by measurement of the optical density at 220 nm. Fraction 1, containing the highest concentration of antigenic molecules, was chosen for loading onto the next column. The fraction was extensively dialyzed against deionized water and freeze-dried, and 34 mg of material was recovered.
(iii) Reverse-phase column.
An RPC 1-ml column (Pharmacia) was equilibrated with 0.1% trifluoroacetic acid (TFA) in water, at a flow rate of 1 ml/min with maximum pressure at 3 × 105 Pa. The 34 mg obtained from the preceding column was dissolved in 4 ml of 0.1% TFA. Four successive injections of 1 ml were performed. The elution gradient, acetonitrile containing 0.1% TFA, allowed the separation of three fractions as determined by measurement of the optical density at 220 nm. Acetonitrile was eliminated by concentration under vacuum at 40°C before freeze-drying of the molecules present in the fraction. They were assayed for their concentration in antigenic molecules in the competitive ELISA. The immunoblotting assay comparing the Aurodye stain with the specific antiserum stain allowed evaluation of the degree of purification obtained.
The molecules present in fraction 3 (13.8 mg) were eluted at 35% acetonitrile. They were N-terminally sequenced on 330 pmol (10 μg) by automatic Edman degradation (Applied Biosystems 470A).
Treatment with α-mannosidase.
A sample of Apa (0.4 mg) was solubilized in 0.4 ml of 0.1 M CH3COONa (pH 4.5) and incubated at 37°C for 24 h with 80 μl of α-mannosidase from Canavalia ensiformis supplied as a 5-mg/ml suspension (Boehringer Mannheim). The mixture was extensively dialyzed against 50 mM sodium citrate (pH 3.5), filtered on a 0.22-μm-pore-size filter, and loaded onto a Resource S column (Pharmacia). The α-mannosidase-treated proteins were eluted with a linear gradient of sodium citrate buffer containing 0.15 M NaCl. The treated molecules were recovered at the same concentration of NaCl (0.050 M) as the native Apa. The α-mannosidase was eluted at 0.15 M NaCl. The global amino acid composition and the N-terminal sequence for 10 amino acids were determined to confirm the purity of the molecules which had been α-mannosidase treated. These chemical analyses were used to measure the quantity of α-mannosidase-treated molecules.
Treatment with TFMS.
Apa was deglycosylated by the trifluoromethanesulfonic acid (TFMS) method (9). A 0.1-mg sample of the purified molecules was treated by the procedure described in the GlycoFree kit (Oxford Glyco Systems). After treatment, the molecules were extensively dialyzed and freeze-dried. They were chemically analyzed to measure their concentration.
Mass spectrometry determinations.
Samples dissolved in water-methanol-formic acid (50:50:5, vol/vol/vol) were introduced into an API 365 triple-quadrupole mass spectrometer (Perkin-Elmer-Sciex, Thornill, Canada) at 5 μl/min with a syringe pump (Harvard Apparatus, South Natick, Mass.). The device was equipped with an atmospheric pressure ion source used to sample positive ions produced from a pneumatically assisted electrospray interface. The ion spray probe tip was held at 4.5 kV, and the orifice voltage was set at 14 V. The mass spectrometer was scanned continuously from m/z 900 to 1,700 with a scan step of 0.1 and a dwell time per step of 2.0 ms, resulting in a scan duration of 16.0 s. Ten scans were averaged for each analysis. The instrument was mass calibrated by matching ions of polypropylene glycol to known reference masses stored in the mass calibration table of the mass spectrometer. Data were collected on a Power Macintosh 8600/200 and processed through the Biotoolbox 2.2 software from Sciex.
Carbohydrate composition analysis.
Samples (20 μg) of Apa (native or after α-mannosidase or TFMS treatment) were hydrolyzed in 100 μl of 4 M TFA at 100°C for 4 h in Teflon-capped vials. The hydrolysates, dried under vacuum in a SpeedVac, were dissolved in 100 μl of deionized water. A sample of 10 μl was loaded onto a high-pH anion-exchange column (CarboPak PA1). The elution was run at 1 ml/min with 20 mM NaOH for 25 min on a Dionex high-pressure liquid chromatography system equipped with a pulsed amperometric detector. The injections of each sample were done in triplicate, and the monosaccharides were identified in extra parallel runs by coinjection of samples to which a known amount of standard sugars had been added.
Guinea pigs.
Three groups of guinea pigs were used. (i) Groups of 10 female guinea pigs (outbred Hartley guinea pigs weighing 250 or 300 g at the beginning of the experiments) were injected once with living BCG. The living BCG, 1.2 × 107 viable units in 0.2 ml of saline (dead bacilli, <10%), were injected i.d. into two sites on the flanks. The DTH reactivity of the animals was fully expressed 1 month after the BCG injection and remained unchanged during 6 months. (ii) Small groups of three to five inbred BioAD guinea pigs of either sex were injected once with living BCG. The draining lymph nodes were collected on day 28, and the cells were assayed for their ability to be stimulated in vitro by the different antigens, native, α-mannosidase, and TFMS-treated Apa molecules. (iii) A group of three inbred S13 male guinea pigs were injected once with living BCG. The draining lymph nodes were collected on day 15, and the CD4+ and CD8+ cells were separated by using specific monoclonal antibodies and magnetic beads (Miltenyi Biotec) before their stimulation by Apa molecules.
Measurement of DTH reactions.
The tuberculin-type tests were performed on the flanks of guinea pigs 24 h after depilation. Four i.d. injections were performed on each flank to compare different dilutions of the experimental material with different dilutions of a standard PPD (0.25 μg corresponding to 10 tuberculin units [TU]). The procedure assesses the reactivity of experimental preparations against the control standard PPD, and it could permit the expression of the potency of the tested preparation in TU per milligram. All dilutions and injections (0.1 ml injected) were performed in phosphate-buffered saline containing Tween 80 (0.05%) to avoid adsorption of the tested material onto the vials, syringes, etc. (23).
DTH reactions were measured 28 h after the i.d. injections, with the degree of erythema and induration being noted. The longitudinal and transverse diameters were recorded in millimeters, and the mean values were plotted against the logarithm of the concentrations of tested material. A curve was drawn by using classical regression analysis for each tested material and compared with the values observed for the standard PPD administration, allowing transformation of the results into conventional TU per milligram (32).
Lymphocyte proliferation assay.
BioAD guinea pigs immunized with living BCG were sacrificed by carbon dioxide inhalation. The lymph nodes draining the sites of BCG injection were collected. Dissociated cells were adjusted at 107 cells/ml in RPMI 1640 (Seromed) supplemented with glutamine, β2-mercaptoethanol (5 × 10−5 M) and 10% heat-inactivated fetal calf serum. One volume of cell suspension (50 μl) was added to 50 μl of culture medium containing crude culture filtrate, native purified Apa, α-mannosidase- or TFMS-treated Apa, or control medium alone, in flat-bottom microwell plates (Corning). Parallel experiments were performed in the presence of monoclonal antibodies (supernatant of the H155 hybridoma kindly provided by R. Burger [2]) directed toward CD4+ lymphocytes.
In preliminary experiments, the crude supernatant of the H155 hybridoma was used at different dilutions (1/10, 1/50, and 1/100 final concentrations) on lymph node cells stimulated with concanavalin A (2 μg/ml). The thymidine incorporation was decreased less than twofold in the presence of the higher concentration (1/10) of the hybridoma supernatant. The dilution (1/10) was chosen to assay the responses of lymphocytes with or without H155 treatment. The plates were incubated at 37°C in a humidified air-CO2 incubator for 3 days, and 10 μl of [3H]thymidine solution in RPMI at 50 μCi/ml was added for an overnight incubation. The labeled cells were harvested onto fiberglass filters for liquid scintillation counting.
To separate the CD4+ and the CD8+ cells, dead cells were removed from lymph node cells (LNC) by using 40% Percoll (Pharmacia). Then 108 LNC were incubated for 30 min at 4°C with appropriate dilutions of monoclonal antibodies (MAb) raised against CD4+ (H155) or CD8+ (ascite of the CT6 hybridoma purchased from Harlan) guinea pig T cells. After washes in phosphate-buffered saline containing 5% fetal calf serum, LNC were incubated for 45 min at 4°C with goat anti-mouse IgG microbeads (Miltenyi Biotec) for cells treated with the anti-CD8+ MAb or with goat anti-rat IgG microbeads (Miltenyi Biotec) for those treated with the anti-CD4+ MAb. Magnetic separations were performed with MS+ columns as specified by the manufacturer (Miltenyi Biotec). The efficacy of the depletion (approximately 99%) was controlled after labelling the cells with fluorescein isothiocyanate-conjugated F(ab′)2 (Caltag Laboratories) directed against mouse or rat IgG by using a flow cytometer (FACScan; Becton Dickinson).
RESULTS
Purification of Apa molecules.
To determine the extent of glycosylation on Apa molecules and the role of the glycosylation on the immune responses, a large batch of Apa was prepared. The freeze-dried material (4.2 g) of M. tuberculosis H37Rv culture filtrate was separated on three successive semipreparative columns. The concentration of Apa in the different fractions was assayed by using a liquid competitive ELISA and immunoblotting as summarized in Materials and Methods.
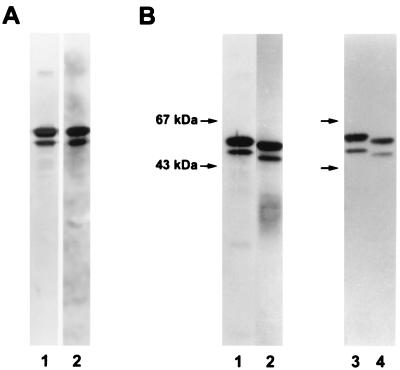
The batch of Apa obtained under semipreparative conditions, a total of 13.8 mg, was analyzed for its purity by different chemical or immunochemical assays. Western immunoblot assays of Apa stained with a rabbit antiserum directed against crude M. tuberculosis culture filtrate detected only the purified molecules (Fig. 1A, lane 1). A sensitive staining procedure (AuroDye) used to stain proteins after SDS-gel analysis and transfer to PVDF sheets also revealed only the expected molecules (lane 2). The N-terminal sequence obtained for the first 18 amino acids was in agreement with that deduced from the nucleotide sequence (GenBank accession no. X80268). Similarly, the amino acid composition was identical to the calculated composition deduced from the nucleotide sequence (data not shown). According to these methods, the batch of Apa was considered to be of adequate purity for further physicochemical or biochemical analysis.
FIG. 1.
SDS-PAGE analysis of the native and deglycosylated Apa molecules. (A) Lanes: 1, immunoblot analysis of the complex with a rabbit antiserum directed against a crude filtrate of M. tuberculosis; 2, Aurodye staining after SDS-PAGE and transfer to PVDF. (B) Slight decrease of the apparent molecular mass after deglycosylation of the molecules (immunoblot staining with MAb A5A3). Lanes: 1, no treatment; 2, treatment with α-mannosidase; 3, no treatment; 4, treatment with TFMS.
Deglycosylation of Apa molecules.
Previous results demonstrated the presence of glycosyl residues on the Apa molecules (6). To elucidate the role of the glycosyl residues in order to detect the immune system effectors, deglycosylated molecules were prepared.
After treatment with α-mannosidase as indicated in Materials and Methods, the mixture of Apa and enzyme was extensively dialyzed against a sodium citrate buffer at low ionic concentration and loaded onto a Resource S column. The 45/47-kDa molecules were eluted at the same NaCl concentration (50 mM) as the native molecules. The peptide chains of the enzymatically treated Apa molecules were apparently unaltered when loaded, run on SDS-PAGE, and immunodetected (Fig. 1B). A slight decrease in the apparent molecular mass, related to the loss of glycosyl residues, was observed only when samples were loaded on parallel lanes.
To confirm the absence of alteration of the protein core by the enzymatic treatment, a sample (100 μg) of the native molecules was treated with TFMS at −80°C for 2 h to eliminate glycosyl residues while preserving the peptidic chain. Native and TFMS-treated proteins were loaded on parallel lanes of the SDS-gel and were transferred onto twin PVDF sheets after electrophoresis. Staining with either AuroDye or a specific MAb (A5A3) (17) showed only the slight decrease in molecular mass related to loss of glycosyl residues (Fig. 1B).
Chemical analysis of the glycosyl residues.
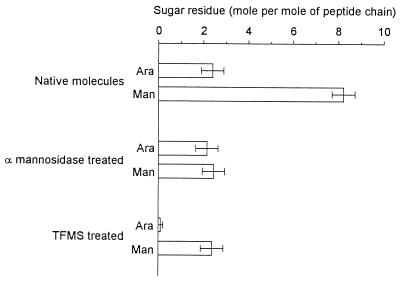
The Apa molecules (native, α-mannosidase treated, or TFMS treated) were analyzed for their global monosaccharide composition. This study shows the presence in the Apa batch of two types of monosaccharides assigned to mannose and arabinose. Their relative concentrations were approximately 8 mol of mannose and 2 mol of arabinose per mol of native 45/47-kDa molecules (Fig. 2). After the α-mannosidase treatment, 2 mol of arabinose and 2 mol of mannose per mol of 45/47-kDa proteins were found. The arabinose disappeared after the TFMS treatment. However 2 mol of mannose per mol of Apa molecules remained (Fig. 2). Such results are in agreement with previous reports demonstrating the difficulty of eliminating the contaminating arabinose molecules (7). The mass-spectrometric analysis of the native glycoprotein, indicating no peak related to the addition of the arabinose moiety on the protein molecules, confirmed that the arabinose was a contaminant arising from the well-known and highly frequent arabinomannan mycobacterial polysaccharides (31).
FIG. 2.
Global analysis of sugar residues present in native or α-mannosidase- or TFMS-treated Apa molecules. Ara, arabinose; Man, mannose.
Electrospray ionization mass spectrometry of Apa.
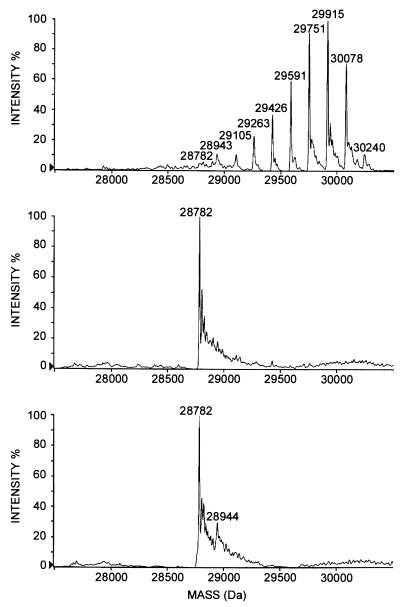
The mass spectrum of the native antigen showed the presence of nine species, whose measured mass differed by 162 mass units, corresponding to a hexose moiety (Fig. 3, top). Similar analysis of α-mannosidase- or TFMS-treated molecules showed a single major peak whose molecular mass (28,782.0 ± 1.6) was in agreement with the mass deduced from the nucleotide sequence (Fig. 3, middle and bottom). Assuming that ionization of various mannosylated species was similar regardless of the number of sugar residues linked to the protein, the percentage of each type of molecule in the mixture might be estimated. The most abundant molecules bore six, seven, or eight mannoses, representing, respectively, 22, 24, and 17% of the population. The molecules with three, four, or five mannoses were numerous (5, 9, and 14%, respectively). The molecules with one, two, or nine mannoses were rare (1.5, 3, and 3%, respectively). The nonglycosylated molecules were the least common, in the range of 1%.
FIG. 3.
Electrospray ionization mass spectra of native or α-mannosidase- or TFMS-treated Apa molecules (reconstructed molecular mass profiles). (Top) No treatment. (Middle) α-Mannosidase treatment. (Bottom) TFMS treatment.
Critical role of glycosylation in antigenicity. (i) DTH reaction.
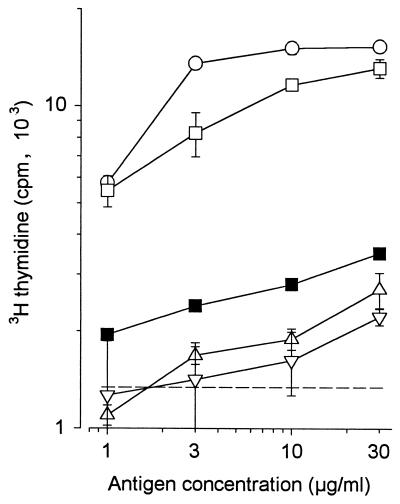
The Apa molecules were characterized and purified initially based on their ability to interact with unique antibodies present in the sera of guinea pigs immunized with living BCG; these antibodies were rare or absent after immunization with dead BCG. The purified molecules were also found to be potent antigens for a T-lymphocyte-dependent immune response, the DTH reaction. The native molecules were approximately 40-fold more potent in eliciting DTH reactions in guinea pigs immunized with living BCG than in those immunized with dead BCG (28).
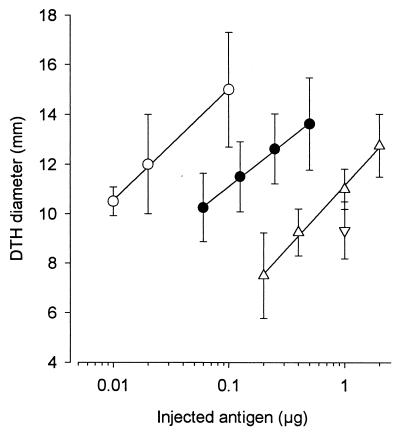
Guinea pigs immunized with living BCG were i.d. injected with native, α-mannosidase-, or TFMS-treated Apa molecules. The potency of native Apa molecules was approximately 200,000.00 TU/mg, i.e., fivefold more potent than control PPD. However, the relative potency of the enzymatically treated proteins was decreased 50-fold. After treatment with TFMS, the potency of eliciting a DTH reaction was decreased approximately 100-fold (Fig. 4). Because the amount of deglycosylated molecules recovered after TFMS treatment was small, only one concentration (1 μg in 100 μl) was assayed. The DTH reactions elicited by different doses of a standard PPD are shown in the figure to indicate the level of sensitization of guinea pigs against this positive control.
FIG. 4.
Decreased ability of deglycosylated molecules to elicit a DTH reaction in guinea pigs immunized with living BCG. Different concentrations of proteins were i.d. injected into immunized guinea pigs in a volume of 0.1 ml. The erythema diameters measured 28 h later were reported. The assays were performed on four guinea pigs for native Apa molecules (○) and α-mannosidase-treated (▵) and TFMS-treated (▿) Apa molecules and on a total of eight guinea pigs for PPD (mean ± SD). (●). At least three experimental groups of guinea pigs (two to four guinea pigs per group) were assayed, with similar results.
(ii) T-lymphocyte proliferation.
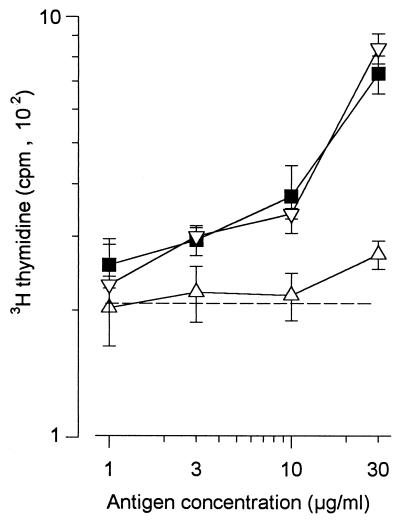
The lymphocytes were isolated from the draining lymph nodes of a cutaneous site in which living BCG had been injected 28 days previously. The native Apa molecules were potent antigens, able to stimulate the cells in vitro (Fig. 5). In contrast, the α-mannosidase- and TFMS-treated molecules were very weak antigens. The dose-response curves determined for lymphocytes obtained from different guinea pigs allowed us to conclude that there was a 30-fold decrease in the potency of α-mannosidase- and TFMS-treated molecules compared to native Apa molecules (Fig. 5). The responder cells in this assay were CD4+ lymphocytes. Indeed, proliferation was abolished when the culture was performed in the presence of the MAb (H155) directed against the CD4 molecule (Fig. 5).
FIG. 5.
Decreased ability of deglycosylated Apa molecules to mediate in vitro stimulation of T lymphocytes collected from guinea pigs immunized with living BCG. Draining LNC were collected 28 days after i.d. injection of living BCG in inbred BioAD guinea pigs. A total of 5 × 105 cells were incubated in vitro with different antigens: M. tuberculosis crude culture filtrate (○) and native (□) or α-mannosidase-treated (▵) or TFMS-treated (▿) Apa molecules. The [3H]thymidine incorporation was measured 4 days later. The cell stimulation observed with native Apa molecules was decreased in the presence of the anti-CD4 MAb (H155) (■). The reported results are representative of a series of three similar experiments performed on three to five guinea pigs (mean ± SD). The dotted line indicates the level of [3H]thymidine incorporation without antigen.
(iii) The CD4+ T-cell population supports in vitro stimulation.
To confirm the preceding result indicating that CD4+ T cells were the responder cells, separations of CD4+ or CD8+ T cells were performed with magnetic beads. The response of the cell populations depleted of CD8+ T cells was unmodified. However, after depletion of the CD4+ T cells, the response was depressed (Fig. 6).
FIG. 6.
The CD4+-T-cell population supports in vitro stimulation. Draining LNC were collected 15 days after i.d. injection of living BCG into three inbred S13 guinea pigs. The cells were treated with MAb against CD4+ or CD8+ T cells and separated with magnetic beads. The recovered cells (5 × 105) were incubated in vitro with the native Apa molecules. The cell stimulations judged on the basis of [3H]thymidine incorporation were measured on crude LNC (■), on a CD8+-T cell-depleted population (▿) and on a CD4+-T-cell-depleted population (▵) (values are means ± SD). The dotted line indicates the level of [3H]thymidine incorporation without antigen.
DISCUSSION
M. tuberculosis has been reported to acylate and glycosylate some proteins (14, 16). The identification of lipoproteins was not surprising, given the large distribution of such molecules in the bacterial kingdom (33). In contrast, a bona fide glycosylation process of bacterial proteins was considered to be rare. The first glycosylated proteins described were mainly those present in the crystalline S surface layer on the surface of some archaebacteria (26). Similarly, the presence of glycosylated proteins in eubacteria was linked to their location on the cell surface (25).
Concerning mycobacteria, the presence of glycosyl units linked to some particular proteins was suggested, but the covalent binding between glycosyl units and peptide chains was not demonstrated (10–12). Recently, glycosylation sites for the 45/47-kDa antigen complex were reported (6). Four sites of glycosylation were determined in proline-rich domains located at the N-terminal and C-terminal regions of the protein chain. Thr10 and Thr18 were reported to be linked to a mannobiose; Thr27 was linked to a single mannose; and Thr277 was linked to either a mannose, a mannobiose, or a mannotriose. However, this analytical approach did not allow us to determine the different glycoforms and more particularly the overall extent of mannosylation.
In our report, by mass spectrometry of the native proteins, the molecular mass of each glycoform were determined for the first time. From these values, we found that the so-called 47-kDa proteins have a molecular mass of 28,780 Da for the protein part, in agreement with the sequence. Moreover, nine glycoforms of the 47-kDa glycoprotein were characterized, among which those containing five, six, seven, and eight mannose residues were the most abundant.
The relative percentage of each glycoform was determined from the peak intensities. Such evaluation was supported by electrospray ionization and MALDI-TOF mass spectra (results not reported), which showed similar relative abundance of the 47-kDa glycoforms. Indeed, these results were in agreement with the linkage of the mannose residues to threonine, which does not change the number of charges of the protein. To support this view, chemically or enzymatically deglycosylated proteins were found to bear the same number of charges as native molecules after electrospray ionization (Fig. 3).
The band observed at 45 kDa after SDS-gel analysis was related to truncated molecules copurified with the 47-kDa molecules. The 45-kDa molecules were below the level of detection by mass spectrometry in the present batch; the mass spectra of these 45-kDa molecules were obtained in other purified samples, with the corresponding peaks being very small. They were revealed in the present batch only by highly amplified, nonquantitative methods such as Aurodye staining and immunoblotting.
The important difference between the molecular masses evaluated by SDS-PAGE and their exact measurement by mass spectrometry was certainly related to the high percentage of proline (20%), as reported for collagen peptides (13). Indeed, the Apa proteins have molecular masses of 28,780 Da for the protein core. Therefore, it is ambiguous to continue to name these proteins the 45/47-kDa complex.
The T lymphocytes recognize short antigenic peptides bound to either major histocompatibility complex class I or II molecules. Recent results with model peptides recognized by cloned T lymphocytes, demonstrated that glycosylation of the peptide can modulate (down regulate) the interaction of the T lymphocyte receptors with the peptide bound on major histocompatibility complex molecules (5, 15). Glycopeptide-specific responses have been identified when appropriate immunizations with glycopeptides have been performed. In these models, the glycopeptide-specific T-lymphocyte responses implied that both glycan and peptide make contact with the T lymphocyte receptor binding site (3, 5, 15).
In parallel with the possible modification of the binding capacity of the peptides by their glycosylation, another important role for the glycosylation by mannose of the bacterial proteins could exist. In an extension of the Janeway concept (18) for the existence of nonspecific receptors for pathogens, Stahl and Ezekowitz have proposed that the macrophage and dendritic cell mannose receptors (29), which are able to recognize molecular structures shared by large groups of pathogens, could be such pattern recognition receptors.
The human dendritic cell receptor (DEC205) has been recently cloned (21) based on its similarities to mouse DEC205 (19). The two DEC205 molecules showed 77% identity and have been predicted to have marked similarities to the macrophage mannose receptors. The similarities or identities suggest that a macrophage mannose receptor and/or a dendritic cell receptor could also exist on guinea pig cells with equivalent properties. The large decrease in the potency of demannosylated Apa molecules to stimulate specific T lymphocytes in vivo (DTH reaction) or in vitro (proliferation assay) could be related to a change in the uptake and/or processing of molecules by macrophages and/or dendritic cells. It has been demonstrated that mannosylation of antigen leads to selective targeting and subsequent greater presentation by dendritic cells (30). Thus, the “immunodominance” of Apa, which represents less than 2% of the molecules secreted by M. tuberculosis during its growth, could be related to the physiologic mannosylation of the molecule. A precise characterization of the immunogenicity of the different glycosylated molecules secreted by M. tuberculosis during its growth must be performed. These glycosylated molecules, which represent a small percentage (5 to 10%) of the secreted molecules, may be the most important molecules for immunization and/or vaccination by their targeting of the dendritic cells (1).
ACKNOWLEDGMENTS
This work was supported by Institut Pasteur, Ministère de la Défense (DGA contract 95-146), Ministère de l'Enseignement Supérieur et de la Recherche (ACC. SV 14.1995), and WHO grants (V25/181/119). Cynthia Horn was the recipient of a fellowship from the Parasitic Biology Course of Oswaldo Cruz Institute, Rio de Janeiro, Brazil.
We thank Pierre Chavarot for technical assistance and Fabienne Courmarcel for secretarial assistance.
REFERENCES
- 1.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:127–157. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Burger R, Schrod L, Schäfer H. Functionally relevant membrane proteins of human and guinea-pig T lymphocytes. Mol Immunol. 1986;23:1149–1156. doi: 10.1016/0161-5890(86)90145-8. [DOI] [PubMed] [Google Scholar]
- 3.Carbone F R, Gleeson P A. Carbohydrates and antigen recognition by T cells. Glycobiology. 1997;7:725–730. doi: 10.1093/glycob/7.6.725-d. [DOI] [PubMed] [Google Scholar]
- 4.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 5.Deck B, Elofsson M, Kihlberg J, Unanue E R. Specificity of glycopeptide-specific T cells. J Immunol. 1995;155:1074–1078. [PubMed] [Google Scholar]
- 6.Dobos K M, Khoo K H, Swiderek K M, Brennan P J, Belisle J T. Definition of the full extent of glycosylation of the 45-kilodalton glycoprotein of Mycobacterium tuberculosis. J Bacteriol. 1996;178:2498–2506. doi: 10.1128/jb.178.9.2498-2506.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobos K M, Swiderek K, Khoo K H, Brennan P J, Belisle J T. Evidence for glycosylation sites on the 45-kilodalton glycoprotein of Mycobacterium tuberculosis. Infect Immun. 1995;63:2846–2853. doi: 10.1128/iai.63.8.2846-2853.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubos R J, Pierce C H, Schaefer W B. Antituberculous immunity induced in mice by vaccination with living cultures of attenuated tubercle bacilli. J Exp Med. 1953;97:207–220. doi: 10.1084/jem.97.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edge A S B, Faltynek C R, Hof L, Reichert L E J, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981;118:131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- 10.Espitia C, Espinosa R, Saavedra R, Mancilla R, Romain F, Laqueyrerie A, Moreno C. Antigenic and structural similarities between Mycobacterium tuberculosis 50- to 55-kilodalton and Mycobacterium bovis BCG 45- to 47-kilodalton antigens. Infect Immun. 1995;63:580–584. doi: 10.1128/iai.63.2.580-584.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espitia C, Mancilla R. Identification, isolation and partial characterization of Mycobacterium tuberculosis glycoprotein antigens. Clin Exp Immunol. 1989;77:378–383. [PMC free article] [PubMed] [Google Scholar]
- 12.Fifis T, Costopoulos C, Radford A J, Bacic A, Wood P R. Purification and characterization of major antigens from a Mycobacterium bovis culture filtrate. Infect Immun. 1991;59:800–807. doi: 10.1128/iai.59.3.800-807.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furthmayer H, Timpl R. Characterization of collagen peptides by sodium dodecylsulfate-polyacrylamide electrophoresis. Anal Biochem. 1971;41:510–516. doi: 10.1016/0003-2697(71)90173-4. [DOI] [PubMed] [Google Scholar]
- 14.Garbe T, Harris D, Vordermeier M, Lathigra R, Ivanyi J, Young D. Expression of the Mycobacterium tuberculosis 19-kilodalton antigen in Mycobacterium smegmatis: immunological analysis and evidence of glycosylation. Infect Immun. 1993;61:260–267. doi: 10.1128/iai.61.1.260-267.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haurum J S, Arsequell G, Lellouch A C, Wong S Y, Dwek R A, McMichael A J, Elliott T. Recognition of carbohydrate by major histocompatibility complex class I-restricted glycopeptide-specific cytotoxic T lymphocytes. J Exp Med. 1994;180:739–744. doi: 10.1084/jem.180.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrmann J L, O'Gaora P, Gallagher A, Thole J E R, Young D. Bacterial glycoproteins: a link between glycosylation and proteolytic cleavage of a 19 kDa antigen from Mycobacterium tuberculosis. EMBO J. 1996;15:3547–3554. [PMC free article] [PubMed] [Google Scholar]
- 17.Horn C, Pescher P, Romain F, Marchal G. Characterization of murine monoclonal antibodies specific for the 45/47 kDa antigen complex (APA) of Mycobacterium tuberculosis, M. bovis and BCG. J Immunol Methods. 1996;197:151–159. doi: 10.1016/0022-1759(96)00141-x. [DOI] [PubMed] [Google Scholar]
- 18.Janeway C A. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 19.Jiang W, Swiggard W J, Heufler C, Peng M, Mirza A, Steinman R M, Nussenzweig M C. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 20.Kanaï K. Experimental studies on host-parasite equilibrium in tuberculous infection, in relation to vaccination and chemotherapy. Jpn J Med Sci Biol. 1966;19:181–199. doi: 10.7883/yoken1952.19.181. [DOI] [PubMed] [Google Scholar]
- 21.Kato M, Neil T K, Clark G J, Morris C M, Sorg R V, Hart D N J. cDNA cloning of human DEC-205, a putative antigen-uptake receptor on dendritic cells. Immunogenetics. 1998;47:442–450. doi: 10.1007/s002510050381. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Landi S, Held H R, Gupta K C. The multi-facets of tuberculin standardization. Dev Biol Stand. 1975;29:393–411. [PubMed] [Google Scholar]
- 24.Laqueyrerie A, Militzer P, Romain F, Eiglmeier K, Cole S, Marchal G. Cloning, sequencing and expression of the apa gene coding for the Mycobacterium tuberculosis 45/47-kilodalton secreted antigen complex. Infect Immun. 1995;63:4003–4010. doi: 10.1128/iai.63.10.4003-4010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lechner J, Wieland F. Structure and biosynthesis of prokaryotic glycoproteins. Annu Rev Biochem. 1989;58:173–194. doi: 10.1146/annurev.bi.58.070189.001133. [DOI] [PubMed] [Google Scholar]
- 26.Messner P, Sleytr U B. Crystalline bacterial cell-surface layers. Adv Microb Physiol. 1992;33:213–275. doi: 10.1016/s0065-2911(08)60218-0. [DOI] [PubMed] [Google Scholar]
- 27.Orme I M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance, in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988;56:3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romain F, Laqueyrerie A, Militzer P, Pescher P, Chavarot P, Lagranderie M, Auregan G, Gheorghiu M, Marchal G. Identification of a Mycobacterium bovis BCG 45/47-kilodalton antigen complex, an immunodominant target for antibody response after immunization with living bacteria. Infect Immun. 1993;61:742–750. doi: 10.1128/iai.61.2.742-750.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stahl P D, Ezekowitz R A B. The mannose receptor is a pattern recognition receptor involved in host defense. Curr Opin Immunol. 1998;10:50–55. doi: 10.1016/s0952-7915(98)80031-9. [DOI] [PubMed] [Google Scholar]
- 30.Tan M C, Mommaas A M, Drijfhout J W, Jordens R, Onderwater J J M, Verwoerd D, Mulder A A, van der Heiden A N, Scheidegger D, Oomen L C J, Ottenhoff T H, Tulp A, Neefjes J J, Koning F. Mannose receptor-mediated uptake of antigens strongly enhances HLA class II-restricted antigen presentation by cultured dendritic cells. Eur J Immunol. 1997;27:2426–2435. doi: 10.1002/eji.1830270942. [DOI] [PubMed] [Google Scholar]
- 31.Vercellone A, Nigou J, Puzo G. Relationships between the structure and the role of lipoarabinomannans and related glycoconjugates in tuberculosis pathogenesis. Front Biosci. 1998;3:149–163. doi: 10.2741/a372. [DOI] [PubMed] [Google Scholar]
- 32.Wadley F M. The use of biometric methods in comparison of acid-fast allergens. Am Rev Tuberc. 1949;60:131–139. doi: 10.1164/art.1949.60.1.131. [DOI] [PubMed] [Google Scholar]
- 33.Wu H L, Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]