Abstract
This study aimed to verify the level of repellent and mortality effect of two chemical substances (DEET and 2-undecanone) and seven essential oils (EOs), Allium sativum, Artemisia annua, Ocimum basilicum, Lavandula angustifolia, Eucalyptus globulus, Pinus sylvestris, and Curcuma longa. The storage pests Tribolium confusum, Tenebrio molitor, and Acanthoscelides obtectus were exposed to various concentrations in an olfactometer-and-mortality test. The effects were recorded 24–48–72 h after the treatments were applied. A. sativum, E. globulus, and L. augustifolia were found to have significant repellence effects. A substantial lethal effect was observed for A. sativum, E. globulus, and O. basilicum. We also found that even if the most efficient EOs were diluted to low concentrations, they still produced repellent and mortality effects. The presented results indicate that A. sativum and O. basilicum were the most effective against T. confusum and T. molitor; simultaneously, L. angustifolia and C. longa showed high activity against A. obtectus. All of these efficient EOs could be applied as effective bio-control agents in various stored conditions.
Keywords: insect repellents, DEET, 2-undecanone, essential oils, Tribolium confusum, Tenebrio molitor, Acanthoscelides obtectus
1. Introduction
There are more than 20,000 species of field and storage pests, which destroy roughly one-third of the world’s food production annually [1]. Furthermore, storage insects can cause significant crop-production losses, ranging from 9% to 20%, or even higher, in developing countries [2]. The most common are (1) the yellow mealworm beetle, Tenebrio molitor (Linnaeus, 1758) (Coleoptera: Tenebrionidae)—a species of “darkling beetle” [3]; (2) the confused flour beetle, Tribolium confusum (Jacquelin du Val, 1863) (Coleoptera: Tenebrionidae)—a cosmopolitan pest occurring in many flour mills, warehouses, and grocery stores, whose ability to move quickly between patches, makes it one of the major pests in stored products [4]; and (3) the bean weevil Acanthoscelides obtectus (Say, 1831) (Coleoptera: Bruchidae), a Mesoamerican beetle known as a serious post-harvest and field pest of the common bean (Phaseolus vulgaris L.) [5].
Contemporary storage pests are eradicated by using fumigants. Considering the increasing application of commercially available synthetic fumigants and their negative impact on human health and the environment [6], together with pests’ resistance to them, finding a new alternative to toxic fumigants that is safe for the environment and non-target animals is critical [7].
Essential oils (EOs) are produced by 17,500 aromatic species of higher plants belonging primarily to the families of Myrtaceae, Lauraceae, Lamiaceae, and Asteraceae [8]. For example, EOs are extracted from eucalyptus leaves (Eucalyptus globulus) [9], garlic (Allium sativum) [10], basil (Ocimum basilicum) [11], turmeric (Curcuma longa) [12], lavender (Lavandula angustifolia) [13], Scot’s pine tree (Pinus sylvestris) [14], and sweet wormwood (Artemisia annua) [11].
Essential oils are complex mixtures of volatile secondary metabolites produced by aromatic plants; they are obtained by distillation and steam distillation. These volatile molecules include predominantly terpenes, monoterpenes, and sesquiterpenes, respectively. However, their chemical structures might be modified by oxidation, rearranging the skeletons, or, rarely, during biosynthesis. In this case, the specific subunits (i.e., alcohols, aldehydes, phenols, ethers, ketones) or functional groups (i.e., sulphur or nitrogen) are attached or integrated into the structure. These chemicals are known as terpenoids. Each EO comprises only two to five main components, which constitute up to 60% of the oil [15,16,17,18]. The most frequently mentioned substances in EOs are β-caryophyllene (CAS 87-44-5), D-limonene (CAS 138-86-3), α-pinene (CAS 80-56-8), α-terpineol (CAS 98-55-5), δ-cadinene (CAS 483-76-1), α-humulene (CAS 6753-98-6), and p-cymene (CAS 99-87-6) [17]. The toxicity of various EO constituents is well described [18].
No proven natural fumigants are currently employed to combat pests attacking grains, dry-stored food, or other agricultural products. Some of the fumigants that are frequently used to protect stored commodities are phosphine, methyl bromide, and DDVP (2,2-dichlorovinyl dimethyl phosphate) [19]. On the other hand, the most popular and effective natural-product-based insect-repellent substances are DEET (N,N-diethyl-3-methylbenzamide) and 2-undecanone (methyl nonyl ketone). These essences protect against stinging insects. Interestingly, 2-undecanone is a natural compound found in soybean, palm-kernel oil and a garden rue Ruta graveolens [20,21,22]. Unfortunately, the suitability of its use against storage pests is unknown. Furthermore, essential oils (EOs) appear to be feasible tools in pest management. This is especially due to their fungicidal, insecticidal, and herbicidal effects on pests.
From the current vantage point, the use of EOs is problematic in many aspects. Some arise from chemical characteristics, such as their volatility and high degradation rate. Specifically, the oily and volatile qualities and particular chemical compositions may change the food-packaging features and alter the material resistance. Therefore, only some specific EOs can be included in food packaging despite their environmentally friendly and non-toxic qualities [23].
The biological activities of EOs are also problematic as they depend on the chemical composition. This is affected by factors such as the extraction method, plant phenology, timing of harvest season, location and age of the plant, soil condition, and many more environmental factors. Thus, each plant’s combination of chemicals is unique [24]. Unstandardized amounts of EOs might hinder their use in organic farming, even though their potential is immense. Other problems lead to costs connected with approval and registration processes. However, there discussions are currently underway to create a streamlined registration process for low-risk products (i.e., products that must not be toxic to non-target organisms and have a low soil persistency) [25]. The use of EOs is constrained for a variety of reasons, including a lack of specific regulations, a lack of knowledge about their effectiveness and negative effects, and their economic, consumer, and environmental impact, despite efforts to register EOs as flavourings and classify them as “Generally Recognized as Safe” (GRAS) substances [23].
According to Directive 128/2009 [26], the EU member states are obliged to reduce the use of synthetic pesticides and promote alternative means of pest control. Therefore, considerable efforts have been expended over recent years to use plant-based products (botanicals) for insect control [27], including stored product pests.
Based on the previously mentioned considerations of the necessity of new bioactive pest-control compounds of botanical origin, our work’s objective was to investigate and compare chemical compounds of the most frequently used essential oils and their repellence against T. confusum, T. molitor, and A. obtectus and to choose the most effective.
2. Results
2.1. Gas Chromatography–Mass Spectrometry
The chemical profiles of the EOs consisted of three to four dominant compounds and several lesser compounds with different positions. A gas chromatography–mass spectrometry analysis (GC–MS) of the selected essential oils revealed 23 compounds in O. basilicum, 57 in A. sativum, 82 in C. longa, 9 in E. globulus, 63 in L. angustifolia, 33 in P. sylvestris, and 14 in A. annua. Monoterpenes, organic disulfides, or terpenes accounted for the majority of the constituents. Some components of EOs, such as eucalyptol, carveol, o-cymene, and D-limonene, were detected in more than one species. Other components, such as citral for O. basilicum, eucalyptol for E. globulus, and tumerone for C. longa, were found to be species-specific. Detailed results can be found in Supplementary Table S1.
In brief:
-
-
Ocinum basilicum: trans-dihydrocarveol (17.81%, CAS 18383-51-2) and α -bisabolene (2.3%, CAS 17627-44-0).
-
-
Allium sativum: diallyl disulphide (25.8%, CAS 2179-57-9), diallyl sulfide (11.66%, CAS 592-88-1), diallyl tetradulfide (5.15%, CAS 2444-49-7), and diallyl trisulfide (2.7%, CAS 2050-87-5).
-
-
Artemisia annua: eucalyptol (12.13%, CAS 470-82-6), trans-dihydrocarveol (7.11%, CAS 18383-51-2), and artemisia ketone (6.6%, CAS 546-49-6).
-
-
Eucalyptus globulus: eucalyptol (85.79%, CAS 470-82-6), alloocimene (7.55%, CAS 3016-19-1), and gamma-terpinene (3.67%, CAS 99-85-4).
-
-
Pinus sylvestris: norbornene (11.75%, CAS 497-32-5), D-limonene (11.65%, CAS 138-86-3), and β-pinene (5.03%, CAS 127-91-3).
-
-
Lavender angustifolia: β-ocimene (5.24%, CAS 3338-55-4), caryophyllene (4.11%, CAS 87-44-5), and lavandulyl acetate (3.24%, CAS 20777-39-3).
-
-
Curcuma longa: tumerone (26.15%, CAS 180315-67-7), quinoxaline (6.93%, CAS 7153-23-3).
2.2. Olfactometer Study
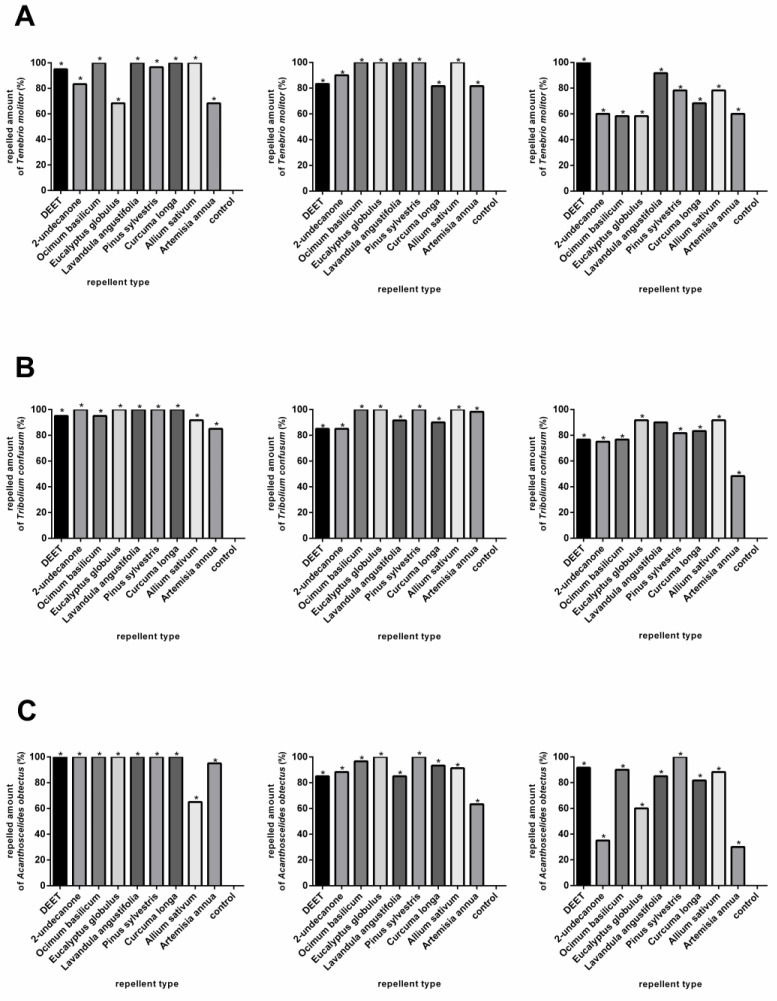
In the case of Tenebrio molitor, all the chemical substances and EOs were repellent at concentrations of 1% and 0.1%. At a concentration of 0.01%, only the EOs of A. sativum, L. augustifolia, P. sylvestris, and the chemical DEET caused significant repellence compared with the control variant (Figure 1A, Supplementary Table S2).
Figure 1.
The reactions of Tenebrio molitor (A), Tribolium confusum (B), and Acanthoscelides obtectus (C) to chemical products (DEET and 2-undecanone) and essential oils (Ocimum basilicum, Eucalyptus globulus, Lavandula angustifolia, Pinus sylvestris, Curcuma oblonga, Alium sativum, and Artemisia annua) in comparison to control group. Significant differences are marked with an asterisk (*). The order of the graphs determines the concentrations used in the experiments (1%; 0.1% and 0.01%). Means of attracted individuals are pictured.
The Tenebrio confusum showed a significant response to all repellent substances at all concentrations except A. annua at a concentration of 0.01% (Figure 1B, Supplementary Table S2). At a concentration of 0.1%, five EOs (A. sativum, A. annua, E. globulus, O. basilicum, P. sylvestris) showed significantly higher repellence than the chemical substances. The highest repellence was achieved at a concentration of 0.01% and was observed only in the EOs of A. sativum and E. globulus (Figure 1B, Supplementary Table S2). The repellent tests with A. obtectus showed that all the compound substances except the EO from A. annua were significantly effective at concentrations of 1% and 0.1%. The 2-undecanone and EOs from the A. annua and E. globulus showed little or no repellent activity at concentrations of 0.01% (Figure 1C, Supplementary Table S2). As shown in Figure 1A–C, the essential oils of A. sativum, E. globulus, and L. augustifolia were very effective against all three storage pests.
2.3. Mortality Study
Tenebrio molitor
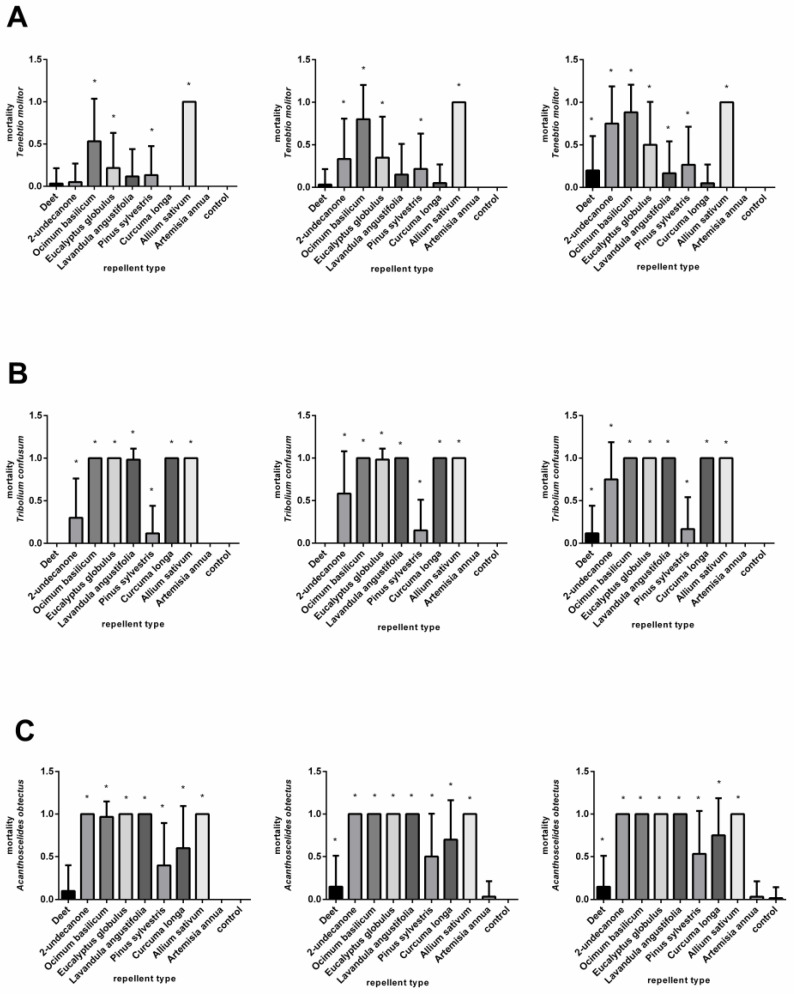
The mortality effects of the various compounds on the adult T. molitor after 24, 48, and 72 h are shown in Figure 2A and in Supplementary Table S3. At a concentration of 3.7 μL/cm2, all the substances except for the DEET and A. annua showed significant mortality. However, the highest mortality was obtained only after the application of the EOs from the A. sativum and O. basilicum.
Figure 2.
Mortality of Tenebrio molitor (A), Tribolium confusum (B), and Acanthoscelides obtectus (C) after using chemical products (DEET and 2-undecanone) and essential oils (Ocimum basilicum, Eucalyptus globulus, Lavandula angustifolia, Pinus sylvestris, Curcuma oblonga, Alium sativum, and Artemisia annua) at a concentration of 3.7 µL/cm2 in comparison to control group. Significant differences are marked with an asterisk (*). The order of the graphs determines the incubation time (24, 48, and 72 h) after application. Each column shows mean of dead individuals and SD.
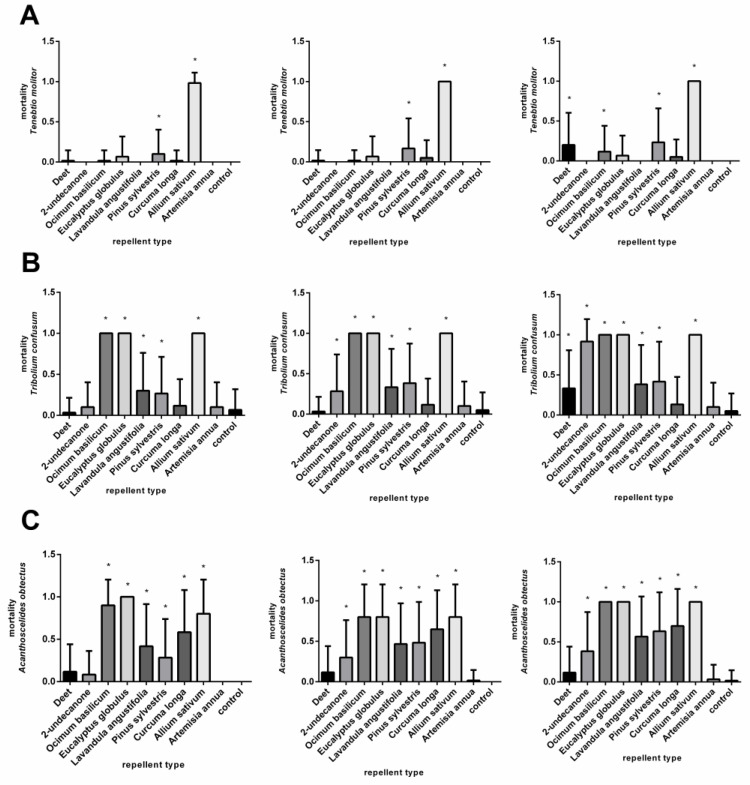
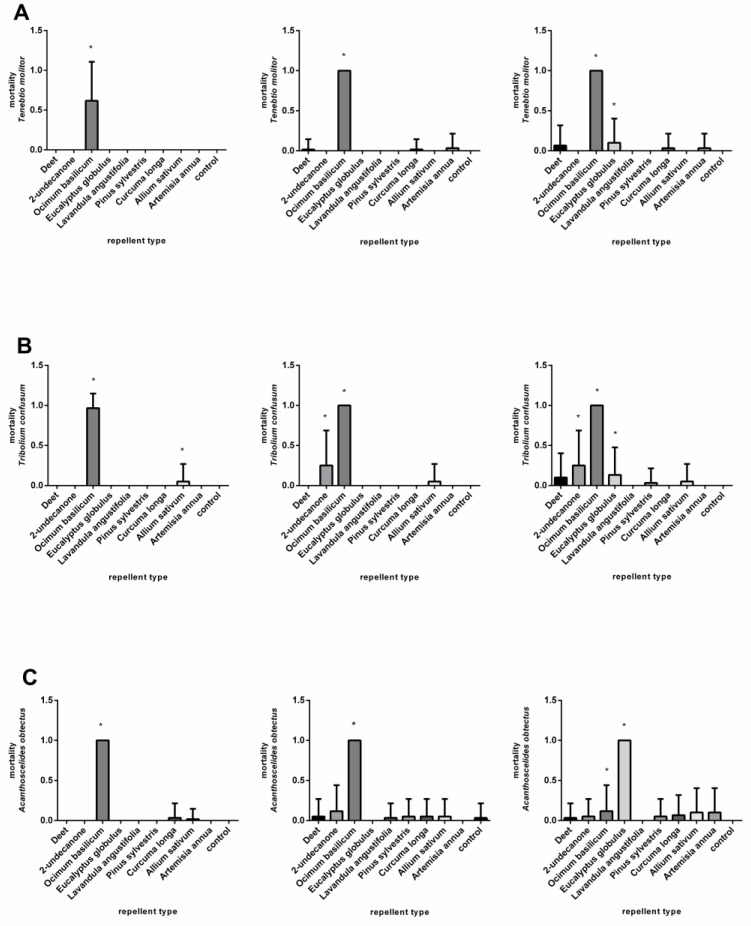
The application of the A. sativum EO at a medium concentration, 0.37 μL/cm2, resulted in death within 24 h for all the animals tested. A significant mortality effect was observed only after the application of DEET, P. sylvestris, and O. basilicum EOs 72 h after treatment (Figure 3A, Supplementary Table S3). At the lowest concentration, 0.07 μL/cm2, significantly high mortality was observed only after the use of the A. sativum EO (Figure 4A, Supplementary Table S3).
Figure 3.
Mortality of Tenebrio molitor (A), Tribolium confusum (B), and Acanthoscelides obtectus (C) after using chemical products (DEET and 2-undecanone) and essential oils (Ocimum basilicum, Eucalyptus globulus, Lavandula angustifolia, Pinus sylvestris, Curcuma oblonga, Alium sativum, and Artemisia annua) at a concentration of 0.37 µL/cm2 in comparison to control group. Significant differences are marked with an asterisk (*). The order of the graphs determines the incubation time (24, 48, and 72 h) after application. Each column shows mean of dead individuals and SD.
Figure 4.
Mortality of Tenebrio molitor (A), Tribolium confusum (B) and Acanthoscelides obtectus (C) after using chemical products (DEET and 2-undecanone) and essential oils (Ocimum basilicum, Eucalyptus globulus, Lavandula angustifolia, Pinus sylvestris, Curcuma oblonga, Alium sativum, and Artemisia annua) at a concentration of 0.07 µL/cm2 in comparison to control group. Significant differences are marked with an asterisk (*). The order of the graphs determines the incubation time (24, 48, and 72 h) after application. Each column shows mean of dead individuals and SD.
Tribolium confusum
Figure 2B and Supplementary Table S3 show the response of the T. confusum after the application of nine repellents at a concentration of 3.7 μL/cm2. The DEET and the EO of A. annua had minimal effects at 24, 48, and 72 h after treatment. By contrast, very high mortality was caused by the 2-undecanone. The essential oils of A. sativum, O. basilicum, C. longa, E. globulus, and L. augustifolia were found to be particularly lethal, as mortality reached 90%. The use of the EOs from A. sativum, O. basilicum, C. longa, and E. globulus at a concentration of 0.37 μL/cm2 caused extensive deaths of the adult T. confusum. By contrast, the EO of A. annua did not cause mortality. The remaining repellent substances significantly affected mortality after 24 h, except for the DEET, which did not show an effect until 72 h after treatment (Figure 3B, Supplementary Table S3). The lowest concentration, 0.07 μL/cm2, was lethal to 100% of the beetles tested after 24 h when the EOs from O. basilicum, A. sativum, and E. globulus were used. After the 48- and 72-h treatments, a significant mortality effect was also observed from the DEET, 2-undecanon, and EOs of L. angustifolia, P. sylvestris, and C. longa (Figure 4B, Supplementary Table S3).
Acanthoscelides obtectus
At a concentration of 3.7 μL/cm2, all the repellents, except the A. annua EO, caused the mortality of A. obtectus. The highest mortality was caused by the 2-undecanone and the EOs of A. sativum, L. augustifolia, E. globulus, and O. basilicum (Figure 2C, Supplementary Table S3). A similar result was also obtained after the application of repellents at a concentration of 0.37 μL/cm2, except for the maximum effect of the L. augustifolia (Figure 3C, Supplementary Table S3). At a concentration of 0.07 μL/cm2, the high toxicity of the 2-undecanone and the EO of A. sativum was confirmed at all the time points. Moreover, the EOs of the E. globulus and O. basilicum completely decreased the population after 72 h (Figure 4C, Supplementary Table S3). In general, the essential oils of the A. sativum, E. globulus, and O. basilicum had the highest mortality effect, while the mortality effect of the L. augustifolia EO decreased in line with the concentration.
3. Discussion
Currently, synthetic pesticides are still used to control stored-product pests. Synthetic pesticides are widely used in traditional, developing, and emerging countries [28]. With regard to the environment and the threat of rapidly growing resistance, there is an urgent need to identify new and safer options [29].
Recently, the use of repellents has attracted the interest of scientists and the crop-protection industry [30]. The most promising are DEET and 2-undecanone, which are already used as components of insect repellents [31]. A significant effect of DEET is well described for Lasioderma serricorne, Rhyzoptera dominica, Liposcelis bostrychophila, and Tribolium castaneum, for which two or three hours of treatment caused high mortality (78%, 94%, 96%, and 92%, respectively) in low concentrations [32,33]. However, in our experiment, the mortality increased in line with the concentration and the overall effect on the mortality rate was negligible for all the pests, contradicting the reports in previous studies. By contrast, the repellence effect of the 1% solution was surprisingly high (>97.5%). A similar trend was described by Brown and Hebert [34], who revealed the direct influence of DEET repellence and used the concentration on the most common mosquitoes, Aedes aegypti and Culex pipiens. However, they stated that a higher DEET content might affect the quality of impregnated goods and might extend the effect only by an hour. Further, 2-undecanone showed a significant repellence effect (78.5%) on Ahasverus advena [35]. Our olfactory test confirmed these results, as using 1% solution caused 100% repellence in T. molitor, T. confusum, and A. obtectus. The 2-undecanone showed an eliminating effect on A. obtectus in all the used concentrations; however, the other tested pests showed high survival, even after the highest concentrations were used. Interestingly, 2-undecanone is known to activate and inhibit mosquitos’ olfactory receptors. Therefore, its activity is modulated, which can have an agonistic or antagonistic effect [36]. This discovery might help to exploit 2-undecanone in developing biodegradable alternatives for synthetic pesticides against pests. Additionally, an antifeedant effect of 2-undecanone against Spodoptera frutiperda larvae was observed by Ayil-Gutiérrez et al. [37]. From the accessible results, we conclude that both chemicals might work more as preventative tools with a repellence effect rather than in the elimination already infected storages.
The other promising choices might be natural volatile extracts, as essential oils feature repellent and biological activity [29]. The chemical profiles of the EOs used in this study were analyzed, considering the dissimilarities between the chemical profiles according to geographical origin [24,38]. After comparing the chemical profiles used in our study with those in previous reports [16,39,40], no differences were revealed.
Allium sativum extracts have substantial acaricidal and insecticidal properties against coleopteran and dipteran pests [41]. As with the findings in [42,43,44], our results confirmed a high repellence ability (>90%) against Tenebrio molitor and a high affinity with A. obtectus and T. confusum. Strikingly, s high efficiency (more than 95%) was detected even in the lowest concentration (0.01%). Our data showed high toxicity (more than 95%) after using the EOs of Allium sativum. These results were condensed in [43,45], in which 50% mortality was reached in T. castaneum and Ephestia kuehniella after 24 h of treatment. Interestingly, the lethal effect decreased synergistically in line with the concentration. This pattern was also described in previous studies (i.e., [10,42,46,47]). Moreover, it seems that A. sativum extract has an inhibition effect on larvae development [48]. Combined with its long-lasting protection period of up to 135 days [10] and only negligible damage to stored crops [49], this means that EO appears to be the perfect tool for pest management.
The high toxicity to our pests was caused by EO extracted from O. basilicum – with a mortality rate > 98 % for T. confusum and A. obtectus and moderate for T. molitor. Our results are in accordance with those of Rodríguez-González et al. [50], who reported 33% mortality in 24–72 h experiments on A. obtectus. Furthermore, our experiments on T. molitor confirmed the synergistic effect of increasing effectiveness over time, previously described by Rodríguez-González et al. [50]. In addition, the repellence effect remained conspicuous, even though low concentrations were used.
The Eucalyptus globulus EO had a noticeable effect on the T. confusum and A. obtectus, which corresponded to the mortality previously obtained for E. kuehniella (80% within 24 h) [45]. Even though our mortality results for T. molitor were moderately low, the similarity to the results previously obtained for T. castaneum [51] is obvious. However, the A. obtectus mortality rate differed from those observed by Bittner et al. [52] and Papachristos et al. [53]. The higher concentration of EO used in our experiments may have been the cause.
The third EO to have previously shown promising toxicity results on T. confusum and A. obtectus was from L. angustifolia. Our pests, A. obtectus, Rhyzoptera dominica and Sitophilus granaries, also suffered from high mortality rates at low concentrations of L. angustifolia EOs (50% within 24 h) [53,54,55]. Interestingly, Germinara et al. [54] noted that the mortality effect of the EO might decrease by up to 10 times when the EO is used on infected grains. Furthermore, the repellence activity of L. angustifolia EO seems to be high (>80%) in all the studied pests. The effect was recognizable even at low concentrations, which was consistent with the findings on S. granaries [54].
Conversely, the lowest effect on our pests was observed in the extracts from C. longa and A. annua. In the case of C. longa, the mortality tests showed high toxicity for T. confusum and A. obtectus. By contrast, T. minor’s mortality reached only about 10%. This result supports the low efficiency of Tribolium castaneum adults reported by Ali et al. [48]. Surprisingly, no mortality caused by the A. annua EO was observed. This was inconsistent with the results obtained for all the adult pests of Solenopsis invicta and Callosobruchus maculatus [56,57]. C. longa and A. annua’s significant repellence effects were recognized in all the tested pests, as in [7]. However, the effect of the low EO concentrations is noteworthy. Even though our results showed minimal effects, their potential use might reside in their inhibition of egg hatchability and the reduction in pests’ developmental rate, as reported in [7].
Generally, in all our pests, the mortality increased over time and in line with the concentrations of the EOs. Furthermore, our tests suggested that eucalyptol, limonene, and O-cymene dispose of pests with high toxicity. In addition, the repellence effect was also significant, as is also acknowledged by different studies using various EOs [29,58,59,60,61,62,63]. Hence, we can conclude that these EOs might have significant potential against an extensive range of pests.
To summarize, synthetic insecticides kill target insect pests quickly and provide excellent control when applied. However, their applications leave toxic residues in stored grains, which are harmful to consumers. Therefore, a new and valuable alternative approach to reducing the use of insecticides in warehouses on stored grains should be found. One potential method uses the encapsulation and emulation of botanical EOs as surfactants in greenhouses [64]. The second is the direct application of EOs. Both approaches might be powerful tools for integrated storage-pest management. However, more research needs to be conducted in the laboratory and also in the field.
4. Materials and Methods
4.1. Storage Pests
Tribolium confusum, Tenebrio molitor, and Acanthoscelides obtectus were used in laboratory experiments and olfactometric tests with repellents. They were grown under controlled conditions at the Department of Plant Protection, Slovak University of Agriculture, Nitra, Slovakia. The culture medium consisted of 1000 g of whole-grain flour from Pohronský Ruskov a.s., 500 g of oat flakes from Štúrovo flour mill, and 20 g of dry yeast from AGROFORTEL, s.r.o. In the case of the bean weevil, the medium consisted of 100% beans (Phaseolus coccineus) of the Lady Di variety from Semena Online, s.r.o. The medium was mixed by hand and stored at a temperature of 25 °C for 24 h. Healthy beans were stored at 25 °C for 24 h after beetle release. Approximately 400 adult beetles were stored at 25 ± 2 °C and 50 ± 5% relative humidity at a 16:8 long-day cycle in plastic boxes (37 cm × 26 cm × 14 cm). Both sexes and approximately equal ages were used in the experiments.
4.2. Essential Oils and Chemical Substances
The oils were extracted as commercial essential oils by Mystic Moments (2020) Inc. Hampshire, (UK) and Forest Centre Herbs (2019) St. Louis, MO, (USA) from plant species from the families Amaryllidaceae (Allium sativum), Myrtaceae (Eucalyptus globulus), Lamiaceae (Ocimum basilicum, Lavandula angustifolia), Zingiberaceae (Curcuma longa), Asteraceae (Artemisia annua), and Pinaceae (Pinus sylvestris). Many foods are susceptible to microbial spoilage, with contamination involving high water activity. In addition, essential oils have low water solubility [65]. Therefore, the emulsifiers were used as antimicrobial agents. TWEEN 80 is a well-known food-grade emulsifier that is commonly used for this purpose [66]. The chemical substances DEET (N,N-diethyl-3-methylbenzamide; purity 97%, CAS 134-62-3) and 2-undecanone (methyl nonyl ketone; purity 99%, CAS 112-12-9) were obtained from Sigma-Aldrich (St. Louis, MO, USA) (2019).
4.3. Identification of Chemical Components by Gas Chromatography–Mass Spectrometry Analysis (GC/MS)
Prior to the experiment, the chemical constituents of plant EOs were identified by gas chromatography–mass spectrometry (GC/MS) analysis using GC Agilent 7890B and MS Agilent 5977A (Agilent Technologies Inc, Santa Clara, CA, USA at AgroBioTech at the Slovak University of Agriculture in Nitra. Essential oil samples were diluted in hexane (HPLC ≥ 97%, Sigma Aldrich GmbH, Darmstadt, Germany) at a concentration of 10 μL/mL. One microliter of the diluted sample was injected into the inlet (250 °C), which was operated in 1:10 split mode. Separation was performed using a HP-5 ms capillary column (30-m × 0.25-mm × 0.25-μm film; Agilent Technologies). The oven temperature was set at 50 °C for the first 5 min and then increased at a rate of 3 °C/min to 240 °C, after which it was held constant for 2 min. Helium was used as the carrier gas at a constant flow rate (1.2 mL/min). The mass detector had a filament-ionization energy of 70 eV, a transfer-line temperature of 250 °C, a MS source temperature of 230 °C, and a quadrupole temperature of 150 °C. The mass spectrometer was programmed under electron impact (EI) in full scan at m/z 40–350 with a scan rate of 2.4 scans/s. Compounds were identified by comparing the mass spectra (over 80% agreement) with a commercial database, NIST 2017, and the Wiley library of retention times of reference standards to compare occurrence data in EOs with those in the literature [67].
4.4. Olfactometer Studies
The behavior of stored-product pests used for repellent trials was examined using a Y-shaped glass-tube olfactometer modified from Sigma Scientific LLC of Florida (Micanopy, FL, USA). The Y-tube system included separate secondary filter cartridges for each channel of the system, a glass-threaded drain chamber with air-odor bypass, an air-supply system, and a vacuum-draw system. The inner diameter of the Y-pipe arms was 4 cm, the arm length was 10 cm, and the shaft length was 5 cm. The acute angle between the Y-tube and the horizontal plane was 45°. Ten beetles were released within the first centimeter of the base tube of the olfactometer. They ran up the tube. When the air supply was turned on, the recording of the experimental time began. It controlled the flow rate (volume) of air passing through the cabin arms of the Y-tube. The air was refuelled with a standard compressor and filtered with an air-delivery system. The total air flow for each chamber was 125 mL per minute. Arriving at the Y-junction, the beetles chose between the clean and the odor-laden airflow. Each beetle tested was considered to have made a choice if it moved halfway through the Y-tube arm toward the odor sources. If the beetles did not choose an arm within 10 min, a zero was recorded. The average duration of replicates was 10 min. The beetles that entered the arm, connected with the odor, and stayed for at least 10 s were recorded as attracted. The insects that entered the arm with the control and stayed for at least 10 min were recorded as repelled. Each experiment was performed in six replicates. For each bioassay, 100-milligram cotton swabs were dosed with 5 μL of the treatment solution and placed in one of the chambers of the olfactometer. The other arm received 5 μL TWEEN 80 as a control. Chemicals (DEET, 2-undecanone) and EOs (A. sativum, E. globulus, O. basilicum, C. longa, A. annua, P. sylvestris, and L. angustifolia) were prepared at three concentrations (1%, 0.1%, and 0.01%). The experiment in a Y-tube was conducted in a laboratory at a temperature of 25 °C and a relative humidity of 30%. After completion of the experiments, the entire Y-tube was cleaned with hot soapy water, wiped, and dried at the end of the day.
4.5. Evaluation of Toxicity
The Petri-dish method was used to evaluate toxicity. The filter paper was placed on the bottom of the petri dish (90 × 15 mm) (60 mm diameter, Whatman No.1). Adults of T. confusum, T. molitor and A. obtectus were used for the experiment. In the center of each Petri dish, one of the chemicals or EOs (doses of 3.7, 0.37, 0.07 L/cm2) enriched with a surface agent TWEEN 80 was applied [68,69]. TWEEN 80 (5 µL) was used as a control treatment. Six replicates of each treatment were performed. After evaporation (1 h), ten adults were placed in the center of the arena for each concentration and for the control. Adult mortality was observed and counted every 24, 48, and 72 h. Adults were considered dead if they did not move.
4.6. Analysis of the Data
Results were recorded using Prism graphing software (GraphPad Software 6, San Diego, CA, USA). One-way ANOVA and Dunnett’s multiple-comparison test were performed.
5. Conclusions
The repellent or toxic effects of the essential oils in this study were not based on specific chemicals common to all the essential oils. The essential oils tested had an unfavorable effect on the three storage pests—Tribolium confusum, Tenebrio molitor, and Acanthoscelides obtectus. Our results indicate that A. sativum and O. basilicum were the most effective against T. castaneum and T. molitor; at the same time, L. angustifolia and C. longa showed high activity against A. obtectus. We also found that the most effective EOs still exhibited remarkable repellent and mortality activity when diluted to low concentrations (0.01%, 0.07 μL/cm2). These EOs can be proposed as sufficient biological-control agents in different storage conditions.
Acknowledgments
The authors are grateful to Radka Tanzer Fabiánová for technical assistance and to Antonin Hlom for his support with manuscript preparation.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants11223077/s1. Table S1: The results of gas chromatography–mass spectrometry (GC–MS) analysis of selected EOs. Compounds whose content exceeded 0.1% in tested essential oils are listed. Table S2: The results of statistical tests of two chemical substances, seven essential oils (EOs), and three warehouse pests, Tenebrio molitor, Tribolium confusum, and Acanthoscelides obtectus to confirm the repellent effect. Statistically significant p values are marked with an asterisk. Dunnett’s multiple-comparison tests were used. Table S3: The results of statistical tests of two chemical substances and seven essential oils (EOs) and three warehouse pests, Tenebrio molitor, Tribolium confusum, and Acanthoscelides obtectus to confirm the mortality. Statistically significant p values are marked with an asterisk. Dunnett’s multiple-comparison tests were used.
Author Contributions
Conceptualization: Ľ.C., M.A.F., D.H. and O.S.H.; methodology: Ľ.C. and M.A.F.; software: D.H.; validation: Ľ.C., M.A.F., D.H. and O.S.H.; formal analysis: Ľ.C. and M.A.F.; investigation: Ľ.C., M.A.F., D.H. and O.S.H.; resources: Ľ.C.; data curation: Ľ.C., M.A.F., D.H. and O.S.H.; writing—original draft preparation: M.A.F.; writing—review and editing: Ľ.C., D.H. and O.S.H.; visualization: Ľ.C., M.A.F., D.H. and O.S.H.; supervision: Ľ.C.; project administration: Ľ.C. and O.S.H.; funding acquisition: Ľ.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All published data are available within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was financially supported by the Operational Programme of Integrated Infrastructure within the project, Sustainable smart farming systems taking into account the future challenges 313011W112, co-financed by the European Regional Development Fund. D.H. and O.S.H. were supported by Biology Centre CAS, Institute of Entomology (RVO 60077344).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Atwal A.S., Dhaliwal G.S. Agricultural Pests of South Asia and Their Management. 6th ed. Kalyani Publishers; New Delhi, India: Ludhiana, India: 2008. p. 487. [Google Scholar]
- 2.Geetanjly. Chandel R., Mishra V.K., Tiwari S.N. Comparative efficacy of eighteen essential oil against Rhyzopertha dominica (F.) Int. J. Agric. Environ. Biotechnol. 2016;9:253–360. doi: 10.5958/2230-732X.2016.00046.2. [DOI] [Google Scholar]
- 3.Wang L.P., Guo Y., Dong J.H. Design of Separating screen for Tenebrio molitor L. Appl. Mech. Mater. 2013;543:258–262. [Google Scholar]
- 4.Khemais A., Acheuk F., Miladi M., Omri G. Phytochemistry, biochemical and insecticidal activities of Ruta chalepensis essential oils on Tribolium confusum. Agric. For. 2018;64:31–45. doi: 10.17707/AgricultForest.64.3.03. [DOI] [Google Scholar]
- 5.Soares M.A., Dias Quintela E., Mascarin M., Paul Arthurs S. Effect of temperature on the development and feeding behavior of Acanthoscelides obtectus (Chrysomelidae: Bruchinae) on dry bean (Phaseolus vulgaris L.) J. Stored Prod. Res. 2014;61:90–96. doi: 10.1016/j.jspr.2014.12.005. [DOI] [Google Scholar]
- 6.Mutlu C., Teke A.M. Insecticidal and behavioural effects of some plant essential oils against Sitophilus granarius L. and Tribolium castaneum (Herbst) J. Plant Dis. Prot. 2020;30:50–52. doi: 10.1007/s41348-020-00377-z. [DOI] [Google Scholar]
- 7.Tripathi A.K., Prajapati V., Aggerwal K.K., Khanuja S.P.S., Kumar S. Repellency and toxicity of oil from Artemisia annua to certain stored-product beetles. Ecotoxicology. 2000;93:43–47. doi: 10.1603/0022-0493-93.1.43. [DOI] [PubMed] [Google Scholar]
- 8.Regnault-Roger C. The potential of botanical essential oils for insect pest control. Integr. Pest Manag. 1997;2:25–34. doi: 10.1023/A:1018472227889. [DOI] [Google Scholar]
- 9.Sousa J.P., Goncalves M.J., Da Luz T.N. Effect of Essential oils from Eucalyptus globulus leaves on soil organisms involved in leaf degradation. PLoS ONE. 2013;8:4. doi: 10.1371/journal.pone.0061233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deb-Kirtaniya S., Ghosh M.R., Adityachaudhury N., Chatterjee A. Extracts of garlic as possible source of insecticides. Indian J. Agric. Sci. 1980;50:507–510. [Google Scholar]
- 11.Blankson W.A., Johnson A.C., Gurr G.M. Natural enemy enhancement and botanical insecticide source: A review of dual use companion plants. App. Entomol. Zool. 2018;18:356–359. doi: 10.1007/s13355-018-00602-0. [DOI] [Google Scholar]
- 12.Chakira H., Long M., Liu S., Zhao J., He Y., Wagan T.A., Hua H. Repellency of essential oils against Nephotettix cincticeps: Laboratory and glasshouse assays. J. Appl. Entomol. 2017;141:708–720. doi: 10.1111/jen.12399. [DOI] [Google Scholar]
- 13.Cavanagh H.M.A., Wilkinson J.M. Biological activities of lavender essential oil. Phytother. Res. 2002;16:301–308. doi: 10.1002/ptr.1103. [DOI] [PubMed] [Google Scholar]
- 14.Ansari M.A., Mittal P.K., Razdana R.K., Sreeharia U. Larvicidal and mosquito repellent activities of Pine (Pinus longifolia, Family: Pinaceae) oil. J. Vector Borne Dis. 2005;42:95–99. [PubMed] [Google Scholar]
- 15.Gahukar R.T. Factors affecting content and bio efficacy of neem (Azadirachta indica A. Juss.) phytochemicals used in agricultural pest control. Crop Prot. 2014;62:93. doi: 10.1016/j.cropro.2014.04.014. [DOI] [Google Scholar]
- 16.Fongang F.Y.S., Bankeu K.J.J. Essential Oils-Bioactive Compounds, New Perspectives and Applications. IntechOpen; London, UK: 2020. Terpenoids as important bioactive constituents of essential oil. [DOI] [Google Scholar]
- 17.De Groot A.C., Schmidt E. Essential Oils, Part III. Dermatitis. 2016;27:161–169. doi: 10.1097/DER.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 18.Dima C., Dima S. Essential oils in foods: Extraction, stabilization, and toxicity. Curr. Opin. Food Sci. 2015;5:29–35. doi: 10.1016/j.cofs.2015.07.003. [DOI] [Google Scholar]
- 19.Tripathi A.K., Upadhyay S., Bhuiyan M., Bhattacharya P.R. A review on prospects of essential oils as biopesticides in insect pest managements. J. Pharmacogn. Phytother. 2009;1:52–53. [Google Scholar]
- 20.Maia M.F., Moore S. Plant-based insect repellents: A review of their efficacy, development and testing. Malar. J. 2011;10:11–15. doi: 10.1186/1475-2875-10-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanghong R., Junkum A., Chaithong U., Jitpakdi A., Riyong D., Tuetun B. Remarkable repellency of Ligusticum sinense (Umbelliferae), an herbal alternative against laboratory population of Anopheles minimus and Aedes aegypti (Diptera: Culicidae) Malar. J. 2015;14:307. doi: 10.1186/s12936-015-0816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nahar L., El-Seedi H.R., Khalifa S.A.M., Mohammadhosseini M., Sarker S.D. Ruta Essential Oils: Composition and Bioactivities. Molecules. 2021;26:4766. doi: 10.3390/molecules26164766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marsin A.M., Muhamad I.I., Anis S.N.S., Lazim N.A.M., Ching L.W., Dolhaji N.H. Essential oils as insect repellent agents in food packaging: A review. Eur. Food Res. Technol. 2020;246:1519–1532. doi: 10.1007/s00217-020-03511-1. [DOI] [Google Scholar]
- 24.Durán-Laura E., Valderrama A., Marican A. Natural organic compounds for application in organic farming. Agriculture. 2020;10:41. doi: 10.3390/agriculture10020041. [DOI] [Google Scholar]
- 25.Raveau R., Fontaine J., Hadj Sahraoui A.L. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A Review. Foods. 2020;9:365. doi: 10.3390/foods9030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Directive 128/2009/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides. [(accessed on 8 December 2021)]. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009L0128.
- 27.Liu H., Gui S.S., Lu L., Li D., Liang J., Huang Z.H., Du S. Essential oil from Artemisia annua aerial parts; composition and repellent activity against two storage pests. Nat. Prod. Res. 2019;35:822–825. doi: 10.1080/14786419.2019.1599887. [DOI] [PubMed] [Google Scholar]
- 28.FAO The Future of Food and Agriculture ã 2017. 2017. [(accessed on 3 January 2020)]. Available online: http://www.fao.org/3/a-i6583e.pdf.
- 29.Chaudhari A.K., Singh V.K., Kedia A., Das S., Dubey N.K. Essential oils and their bioactive compounds as eco-friendly novel green pesticides for management of storage insect pests: Prospects and retrospect. Environ. Sci. Poll. Res. 2021;29:18918–18940. doi: 10.1007/s11356-021-12841-w. [DOI] [PubMed] [Google Scholar]
- 30.Isman M.B. Plant essential oils for pest and disease management. Crop Prot. 2000;19:603–608. doi: 10.1016/S0261-2194(00)00079-X. [DOI] [Google Scholar]
- 31.Witting—Bissinger B.E., Stumpf C.F., Donohue K.V., Apperson C.S., Roe R.M. Novel Arthropod Repellent, BioUD, is an efficacious alternative to deet. J. Med. Entomol. 2008;45:891–898. doi: 10.1093/jmedent/45.5.891. [DOI] [PubMed] [Google Scholar]
- 32.Ramadan G.R.M., Abdelgaleil S.A.M., Shawir M.S., El-bakary A.S., Zhu K.Y., Philips T.W. Terpenoids, DEET and short chain fatty acids as toxicants and repellents for Rhyzoptera dominica (Coleoptera: Bostrichidae) and Lasioderma serricorne (Coleoptera: Ptinidae) J. Stored Prod. Res. 2020;87:101610. doi: 10.1016/j.jspr.2020.101610. [DOI] [Google Scholar]
- 33.Liang Y., Li J.L., Xu S., Zhao N.N., Zhou L., Cheng J., Liu Z.L. Evaluation of repellency of some chinese medicinal herbs essential oils against Liposcelis bostrychophila (Psocoptera: Liposcelidae) and Tribolium castaneum (Coleoptera: Tenebrionidae) J. Econom. Entomol. 2013;106:513–519. doi: 10.1603/EC12247. [DOI] [PubMed] [Google Scholar]
- 34.Brown M., Hebert A.A. Insect repellents: An overview. J. Am. Acad. Dermatol. 1997;36:243–349. doi: 10.1016/S0190-9622(97)70289-5. [DOI] [PubMed] [Google Scholar]
- 35.Wakefield M.E., Bryning G.P., Collins L.E., Chambers J. Identification of attractive components of carob volatiles for the foreigh grain beetle, Ahasverus advena (Walt): (Coleoptera: Cucujidae) J. Stored Prod. Res. 2005;41:239–253. doi: 10.1016/j.jspr.2004.03.005. [DOI] [Google Scholar]
- 36.Bohbot J.D., Dickens J.C. Insect repellents: Modulators of mosquito odorant receptor activity. PLoS ONE. 2010;5:e12138. doi: 10.1371/journal.pone.0012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ayil-Gutiérrez B.A., Villegas-Mendoza J.M., Santes-Hernández Z., Paz-González A.D., Mireles-Martínez M., Rosas-García N.M., Rivera G. Ruta graveolens extracts and metabolites against Spodoptera frugiperda. Nat. Prod. Commun. 2015;10:1955–1958. doi: 10.1177/1934578X1501001137. [DOI] [PubMed] [Google Scholar]
- 38.Salehi B., Valussi M., Bezerra Morais-Brage M.F., Pereira Carneiro J.N., Vitalini S., Kregiel D. Tagetes spp. essential oils and other extracts: Chemical characterization and biological activity. Molecules. 2018;23:1847. doi: 10.3390/molecules23112847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masango P. Cleaner production of essential oils by steam distillation. J. Clean. Prod. 2005;13:833–839. doi: 10.1016/j.jclepro.2004.02.039. [DOI] [Google Scholar]
- 40.Sell C.S. The Chemistry of Fragrances: From Perfume to Consumer. 2nd ed. Royal Society of Chemistry; London, UK: 2006. [Google Scholar]
- 41.Abdalla M.I., Abdelbagi A.O., Hamma A.M.A., Laing M.D. Use of volatile oils of garlic to control the cowpea weevil Callosobruchus maculatus (Bruchidae: Coleoptera) S. Afr. J. Plant Soil. 2017;34:185. doi: 10.1080/02571862.2016.1225232. [DOI] [Google Scholar]
- 42.Plata -Rueda A., Martínez L.C., Dos Santos M.H., Fernandes F.L., Wilcken C.F., Soares M.A., Serrão J.E., Zanuncio J.C. Insecticidal activity of garlic essential oil and their constituents against the mealworm beetle, Tenebrio molitor (Coleoptera: Tenebrionidae) Sci. Rep. 2017;7:46406. doi: 10.1038/srep46406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mobki M., Safavi S.A., Safaralizadeh M.H., Panahi O. Toxicity and repellency of garlic (Allium sativum L.) extract grown in Iran against Tribolium castaneum (Herbst) larvae and adults. Arch. Phytopathol. Plant Prot. 2014;47:59–68. doi: 10.1080/03235408.2013.802896. [DOI] [Google Scholar]
- 44.Moncada A., Obed C., Cruz N., Isabel X. Efecto del Tiempo y la dosis del Allium sativum L. en el Control Biologico del Acanthoscelides Obtectus. Ingeniero Tesis. Facultad de Ingeniería y Arquitectura, Escuela Professional de Ingeniería Ambiental. Universidad César Vallejo; Trujillo, Perú: 2021. [(accessed on 5 April 2021)]. p. 60. Available online: https://repositorio.ucv.edu.pe/bitstream/handle/20.500.12692/81158/Asencio_MCO_Navarro_CXI-SD.pdf?sequence=1&isAllowed=y. [Google Scholar]
- 45.Shahriari M., Zibaee A., Shamakhi L., Sahebzadeh N., Naseri D., Hoda H. Bio-efficacy and physiological effects of Eucalyptus globulus and Allium sativum essential oils against Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) Toxin Rev. 2019;39:422–433. doi: 10.1080/15569543.2018.1554588. [DOI] [Google Scholar]
- 46.Park I.I.K., Choi K.-S., Kim D.-H., Choi I.H., Kim L.-S., Bak W.-C., Choi J.-W., Shin S.-C. Fumigant activity of plant essential oils and components from horseradish (Armoracia rusticana), anise (Pimpinella anisum) and garlic (Allium sativum) oils against Lycoriella ingenua (Diptera: Sciaridae) Pest Manag. Sci. 2006;62:723–728. doi: 10.1002/ps.1228. [DOI] [PubMed] [Google Scholar]
- 47.Upadhyay S.A. Management strategies for control of stored grain insect pests in farmer stores and public ware house. World J. Agric. Sci. 2011;7:527–549. [Google Scholar]
- 48.Ali S., Sagheer M., Hassan M., Abbas M., Hafeez F., Farooq M., Hussain D., Saleem M., Ghaffar A. Insecticidal activity of turmeric (Curcuma longa) and garlic (Allium sativum) extracts against red flour beetle, Tribolium castaneum: A safe alternative to insecticides in stored commodities. J. Entomol. Zool. Stud. 2014;2:201–205. [Google Scholar]
- 49.Ho S.H., Koh L., Huang Y., Sim K.Y. The oil of garlic, Allium sativum L. (Amaryllidaceae), as a potential grain protectant against Tribolium castaneum (Herbs) and Sitophilus zeamais Motsch. Postharvest Biol. Technol. 2000;9:41–48. doi: 10.1016/0925-5214(96)00018-X. [DOI] [Google Scholar]
- 50.Rodríguez-González Á., Álvarez-García S., González-López Ó., Da Silva F., Casquero P.A. Insecticidal properties of Ocimum basilicum and Cymbopogon winterianus against Acanthoscelides obtectus, insect pest of the common bean (Phaseolus vulgaris, L.) Insects. 2019;10:151. doi: 10.3390/insects10050151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ebadollahi A. Antifeedant activity of essential oils from Eucalyptus globulus Labill and Lavandula stoechas L. on Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) Biharean Biol. 2011;5:8–10. [Google Scholar]
- 52.Bittner M., Casanueva M.E., Arbert C.C., Aguilera M.A., Hernández V.J., Becerra J.V. Effect of essential oils from five plants species against the granary weevils Sitophilus zeamais and Acanthoscelides obtectus (Coleoptera) J. Chil. Chem. Soc. 2008;53:1455–1459. doi: 10.4067/S0717-97072008000100026. [DOI] [Google Scholar]
- 53.Papachristos D.P., Karamanoli K.I., Stamopoulos D.C., Menkissoglu-Spiroudi U. The relationship between the chemical composition of three essential oils and their insecticidal activity against Acanthoscelides obtectus (Say) Pest. Manag. Sci. 2004;60:514–520. doi: 10.1002/ps.798. [DOI] [PubMed] [Google Scholar]
- 54.Germinara G.S., Di Stefano M.G., De Acutis L., Pati S., Delfine S., De Cristofaro A., Rotundo G. Bioactivities of Lavandula angustifolia essential oil against the stored grain pest Sitophilus granaries. Bull. Insectology. 2017;70:129–139. [Google Scholar]
- 55.Nardjis S., Samir T., Noureddine S. Toxicity and physiological effects of essential oil from Lavandula angustifolia (M.) against Rhyzoptera dominica (F.) (Coleoptera: Botrichidae) adults. J. Ent. Res. 2021;45:929–936. doi: 10.5958/0974-4576.2021.00144.4. [DOI] [Google Scholar]
- 56.Brisibe E.A., Adugbo S.E., Ekanem U., Brisibe F., Figueira G.M. Controlling bruchid pests of stored cowpea seeds with dried leaves of Artemisia annua and two other common botanicals. Afr. J. Biotechnol. 2011;10:9586–9592. doi: 10.5897/AJB10.2336. [DOI] [Google Scholar]
- 57.Tang L., Sun Y.-Y., Zhang Q.-P., Zhou Y., Zhang N., Zhang Z.-X. Fumigant activity of eight plant essential oils against workers of red imported fire ant, Solenopsis invicta. Sociobiology. 2013;60:35–40. doi: 10.13102/sociobiology.v60i1.35-40. [DOI] [Google Scholar]
- 58.Tu H., Qin Y. Repellent effect of different celery varieties in Bemisia tabaci (Hemiptera: Aleyrodidae) Biotype, Q. J. Econom. Entomol. 2017;110:1307–1316. doi: 10.1093/jee/tox110. [DOI] [PubMed] [Google Scholar]
- 59.Athanassiou C.G., Kavallieratos N.G., Evergetis E., Katsoula A.M., Haroutounian S.A. Insecticidal efficacy of silicagel with Juniperus oxycedrus (Pinales: Cupressaceae) essential oil against Sitophilus oryzae (Coleoptera: Curculionidae) and Tribolium confusum (Coleoptera: Tenebrionidae) J. Econom. Entomol. 2013;106:1902–1910. doi: 10.1603/EC12474. [DOI] [PubMed] [Google Scholar]
- 60.Campolo O., Malacrinò A., Zappalà L., Laudani F., Chiera E., Serra D., Russo M., Palmeri V. Fumigant bioactivity of five citrus essential oils against Tribolium confusum. Phytoparasitica. 2013;42:223–233. doi: 10.1007/s12600-013-0355-4. [DOI] [Google Scholar]
- 61.Tapondjou A.L., Adler C., Fontem D.A., Bouda H., Reichmuth C.H. Bioactivities of cymol and essential oils of Cupressus sempervirens and Eucalyptus saligna against Sitophilus zeamais Motschulsky and Tribolium confusum du Val. J. Stored Prod. Res. 2005;41:91–102. doi: 10.1016/j.jspr.2004.01.004. [DOI] [Google Scholar]
- 62.Li W.X., Zhang Z.J., Hafeez M., Huang J., Zhang J.M., Wang L.K., Lu Y.B. Rosmarinus officinalis L. (Lamiales: Lamiaceae), a promising repellent plant for thrips management. J. Econom. Entomol. 2021;114:131–141. doi: 10.1093/jee/toaa288. [DOI] [PubMed] [Google Scholar]
- 63.Conboy N.J.A., McDaniel T., George D., Ormerod A., Edwards M., Donohoe P., Gatehouse A.M.R., Tosh C.R. Volatile organic compounds as insect repellents and plant elicitors: An integrated pest management (IPM) strategy for glasshouse whitefly (Trialeurodes vaporariorum) J. Chem. Ecol. 2020;46:1090–1104. doi: 10.1007/s10886-020-01229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Oliveira J.L., Campos V.R., Camara M.C., Vechia J.F.D., De Matos S.T.S., De Andrade D.J., Gonçalves K.C., De Nascimento J., Polanczyk R.A., Araújp D.R., et al. Hydrogels containing botanicals repellents encapsulated in zein nanoparticles for crop protection. ASC Appl. Nano Mater. 2019;8:33. doi: 10.1021/acsanm.9b01917. [DOI] [Google Scholar]
- 65.Wishart D.S., Jewison T., Gui T., Wilson M., Knox C., Liu Y. HMDB 3.0- The human metabolome database in 2013. Nucleic Acids Res. 2013;41:801–807. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van De Vel E., Sampers I., De Neve L., Denon Q., Van Der Meeren P., Raes K. Emulsion Characteristics Explaining the Effect of Tween-80 on the Antimicrobial Activity of Essential Oil Compounds. Gent University; Ghent, Belgium: 2017. [(accessed on 1 November 2020)]. Available online: https://biblio.ugent.be/publication/8633474/file/8633475.pdf. [Google Scholar]
- 67.Kačániová M., Terentjeva M., Vukovic N., Puchalski C., Roychoudhury S., Kunová S., Klūga A., Tokár M., Kluz M., Ivanošová E. The antioxidant and antimicrobial activity of essential oils against Pseudomonas spp. isolated from fish. Saudi Pharm. J. 2017;25:1108–1116. doi: 10.1016/j.jsps.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abidi A., Sebaj E.D., Dhibi M., Alimi D., Rekik M., B’chir F., Maizels R.M., Akkri H. Chemical analyses and anthelmintic effects of Artemisia campestris essential oil. Vet. Parasitol. 2018;263:59–65. doi: 10.1016/j.vetpar.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 69.Łyczko J., Masztalrz K., Lipan L., Lech K., Carbonell Barrachina A.A., Szummy A. Chemical determinants of dried thai basil (O. basilicum var. thyrsiflora) Ind. Crop. Prod. 2020;155:27–169. doi: 10.1016/j.indcrop.2020.112769. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All published data are available within the article and Supplementary Materials.